Diversity and Ecology of Myxomycetes (Amoebozoa) Along a Vegetational Gradient in the Peruvian Andes
Abstract
1. Introduction
2. Materials and Methods
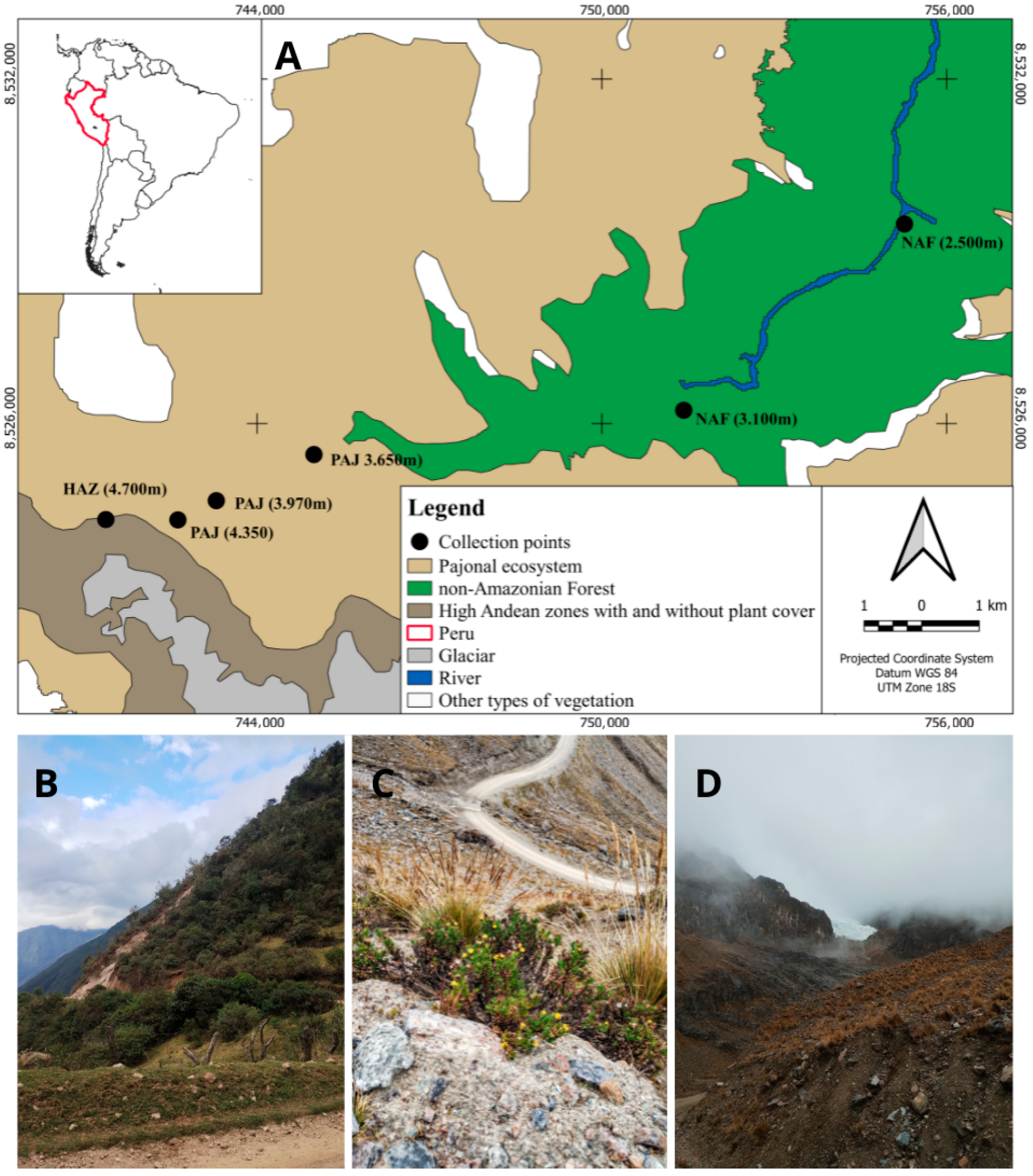
2.1. Study Area
2.2. Collection Methodology
2.3. Data Analysis
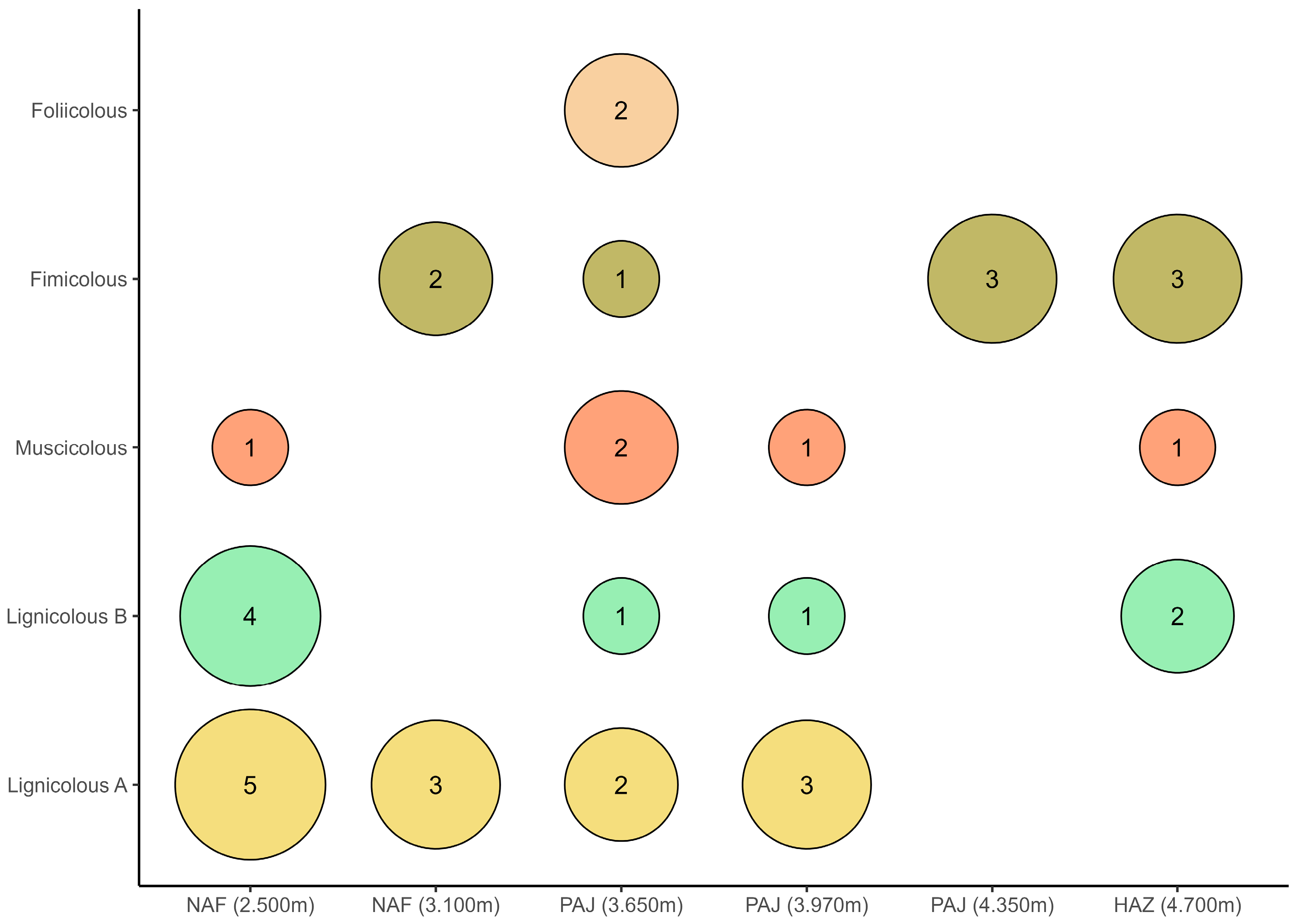
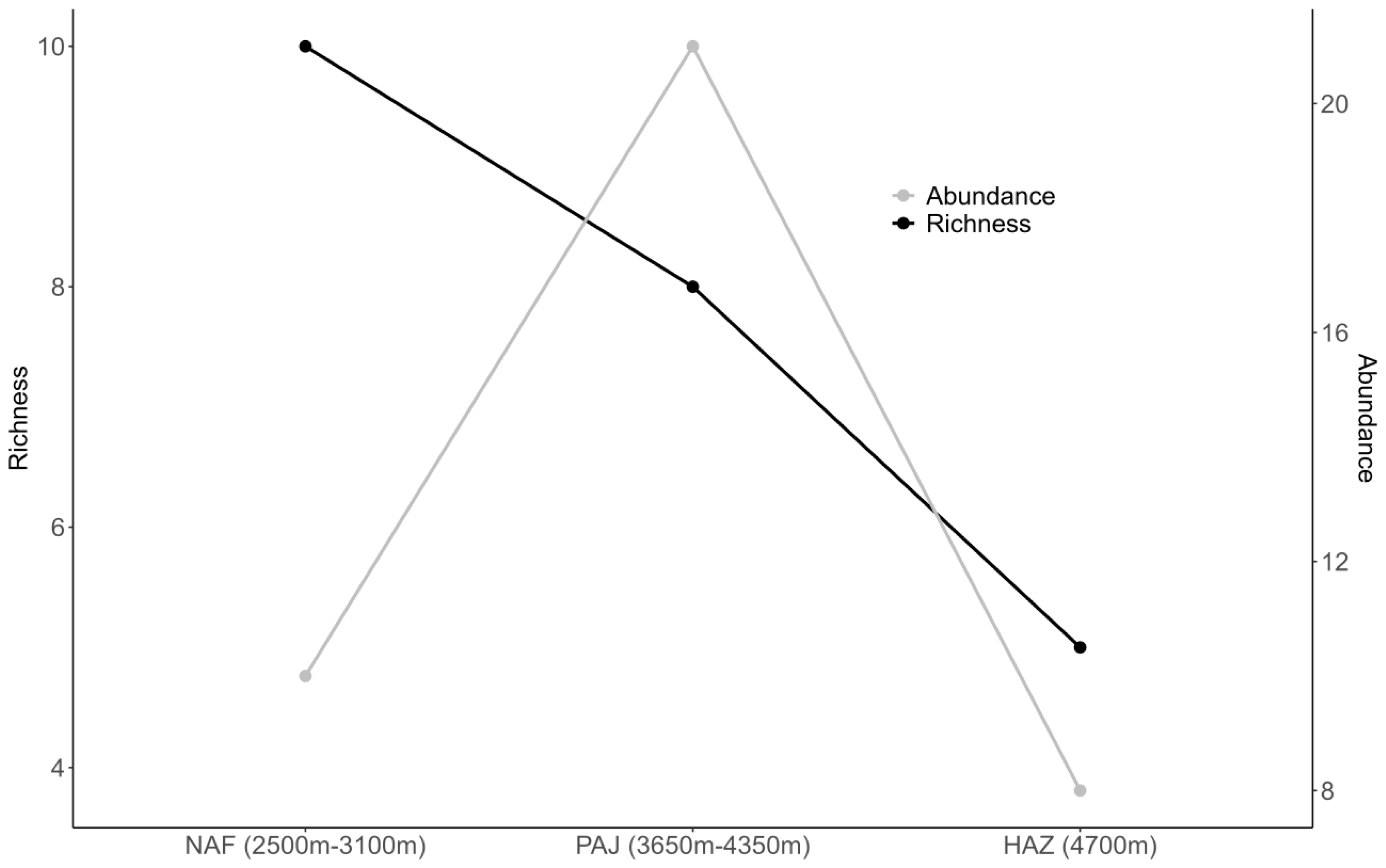
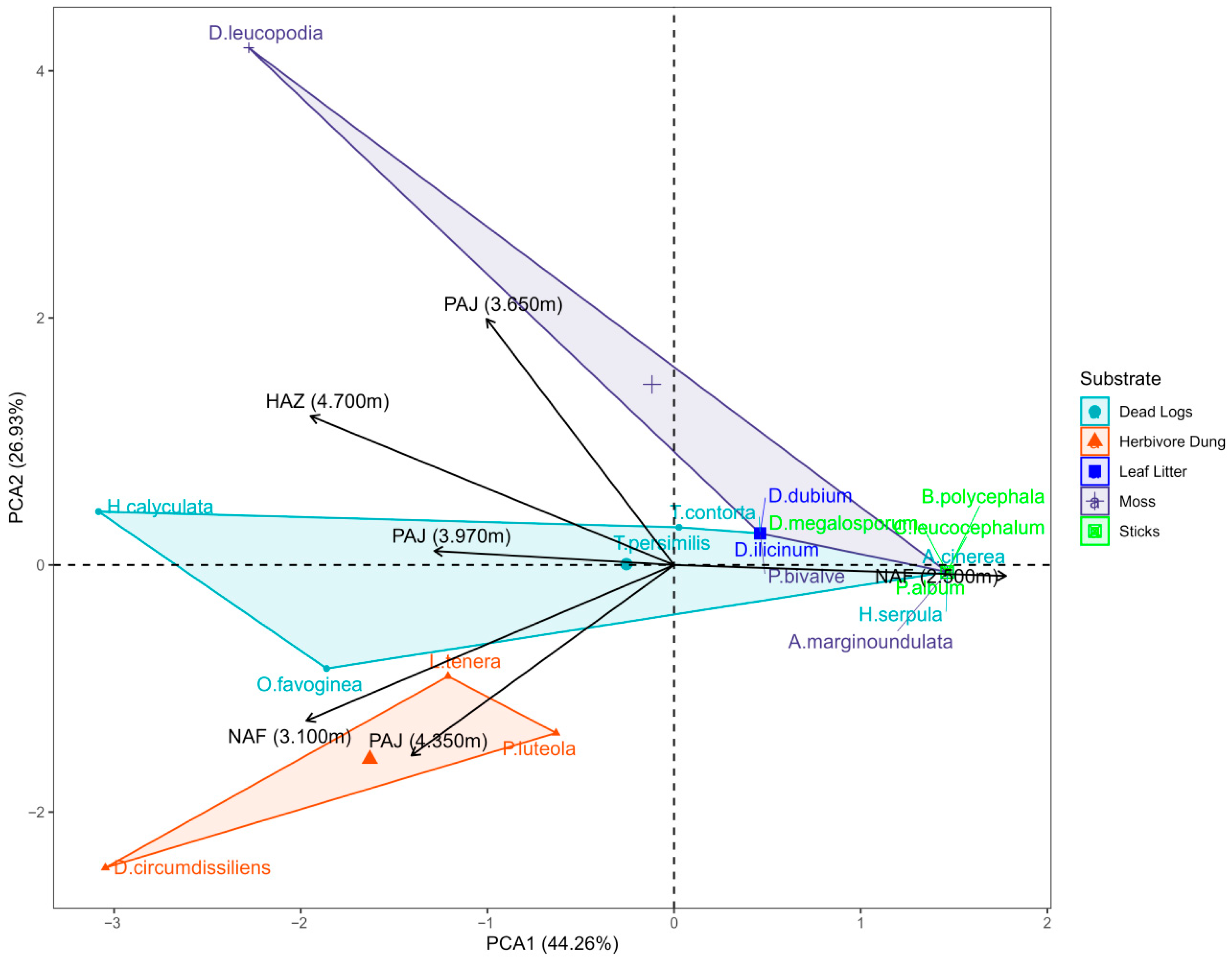
3. Results
New Occurrences for Peru

4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- MINAM. Mapa Nacional de Cobertura Vegetal: Memoria Descriptiva; Dirección General de Evaluación, Valoración y Financiamiento del Patrimonio Natural: Lima, Peru, 2015; 105p. [Google Scholar]
- Trevino-Zevallos, I.; Garcia-Cunchillos, I.; Lado, C. New records of Myxomycetes (Amoebozoa) from the tropical Andes. Phytotaxa 2021, 522, 231–239. [Google Scholar] [CrossRef]
- Wrigley de Basanta, D.; Lado, C.; García-Martín, J.; Estrada-Torres, A. Didymium xerophilum, a new myxomycete from the tropical Andes. Mycologia 2015, 107, 157–158. [Google Scholar] [CrossRef]
- Wrigley de Basanta, D.; Estrada-Torres, A.; García-Cunchillos, I.; Cano Echevarría, A.; Lado, C. Didymium azorellae, a new myxomycete from cushion plants of cold arid areas of South America. Mycologia 2018, 109, 993–1002. [Google Scholar] [CrossRef]
- Wrigley De Basanta, D.; Estrada-Torres, A.; Lado, C. Licea aurea a new Myxomycete from the Peruvian Andes. Phytotaxa 2019, 391, 218–224. [Google Scholar] [CrossRef]
- Treviño-Zevallos, I.F.; Lado, C. Myxomycete diversity in a humid montane forest on the eastern slopes of the Peruvian Andes. Plant Ecol. Evol. 2020, 153, 390–398. [Google Scholar] [CrossRef]
- Xavier de Lima, V.; Cavalcanti, L.D.H. Second world record of Metatrichia floripara (Trichiaceae, Myxomycetes), found in Brazil. Phytotaxa 2016, 247, 292–294. [Google Scholar] [CrossRef]
- Lado, C.; Treviño-Zevallos, I.; García-Martín, J.M.; Wrigley de Basanta, D. Diachea mitchellii: A new myxomycete species from high elevation forests in the tropical Andes of Peru. Mycologia 2022, 114, 798–811. [Google Scholar] [CrossRef]
- Treviño-Zevallos, I.; García-Cunchillos, I.; de Basanta, D.W.; Lado, C. Diversity of Myxomycetes from Peru part III: The high andes and the altiplano. Phytotaxa 2023, 624, 1–92. [Google Scholar] [CrossRef]
- Wrigley de Basanta, D.; Estrada-Torres, A. Techniques for Recording and Isolating Myxomycetes. In Myxomycetes. Biology, Systematics, Biogeography, and Ecology; Stephenson, S.L., Rojas, C., Eds.; Academic Press: London, UK, 2017; pp. 333–363. [Google Scholar] [CrossRef]
- Martin, G.W.; Alexopoulos, C.J. The Myxomycetes; University of Iowa Press: Iowa City, IA, USA, 1969; 560p. [Google Scholar]
- Farr, M.L. Myxomycetes; Rogerson, C.T., Ed.; Flora neotropica Monograph 16; New York Botanical Garden: New York, NY, USA, 1976; pp. 1–305. [Google Scholar]
- Poulain, M.; Meyer, M.; Bozonnet, J. Les Myxomycètes; Fédération Mycologique et Botanique Dauphiné-Savoie: Delémont, Switzerland, 2011; p. 1112. [Google Scholar]
- Lado, C. An Online Nomenclatural Information System of Eumycetozoa. 2005–2025. Available online: https://eumycetozoa.com (accessed on 15 May 2025).
- Wickham, H. dplyr: A Grammar of Data Manipulation. R Package Version 1.0.10. 2022. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 3 April 2025).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; Available online: https://CRAN.R-project.org/package=ggplot2 (accessed on 3 April 2025).
- Wickham, H.; Henry, L. tidyr: Tidy Messy Data. R Package Version 1.2.1. 2021. Available online: https://CRAN.R-project.org/package=tidyr (accessed on 3 April 2025).
- Lê, S.; Husson, F.; Pagès, J. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R Package Version 1.0.7. 2020. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 3 April 2025).
- Flatau, L.; Schinner, P. Diderma circundissilens, eine neue Art der Myxomyceten. Z. Mykol. 2002, 68, 73–80. [Google Scholar]
- GBIF.org. GBIF Occurrence Download—Licea tenera. Available online: https://doi.org/10.15468/dl.mqsyf4 (accessed on 16 May 2025).
- GBIF.org. GBIF Occurrence Download—Diderma circundissilens. Available online: https://doi.org/10.15468/dl.a4v5wf (accessed on 16 May 2025).
- GBIF.org. GBIF Occurrence Download—Perichaena luteola. Available online: https://doi.org/10.15468/dl.dc47up (accessed on 16 May 2025).
- Takahashi, K. Altitudinal distribution patterns of myxomycete species growing on bark of Cryptomeria japonica tree in warm temperate zone of Japan. Hikobia 2017, 17, 207–217. [Google Scholar]
- Takahashi, K.; Harakon, Y.; Degawa, Y. Myxomycetes distribution along an elevational gradient on coniferous woody debris in the mountains of Central Japan. Slime Molds 2024, 4, V4A1. [Google Scholar]
- Stephenson, S.L.; Landolt, J.C.; Moore, D.L. Protostelídeos, dictiostelídeos e mixomicetos no microhabitat da serapilheira da Floresta Experimental de Luquillo, Porto Rico. Mycol. Res. 1999, 103, 209–214. [Google Scholar] [CrossRef]
- Stephenson, S.L.; Schnittler, M.; Lado, C. Caracterização ecológica de uma assembleia tropical de mixomicetos: Reserva Florestal Nublada de Maquipucuna, Equador. Mycologia 2004, 96, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Schnittler, M.; Stephenson, S.L. Biodiversidade de mixomicetos em quatro diferentes tipos de floresta na Costa Rica. Mycologia 2000, 92, 626–637. [Google Scholar] [CrossRef]
- Rojas, C.; Stephenson, S.L. Myxomycete ecology along an elevation gradient on Cocos Island, Costa Rica. Fungal Divers. 2008, 29, 117–127. [Google Scholar]
- Rojas, C.; Valverde, R.; Stephenson, S.L.; Vargas, M.J. Padrões ecológicos de mixomicetos da Costa Rica. Fungal Ecol. 2010, 3, 139–147. [Google Scholar] [CrossRef]
- Rojas, C.; Valverde, R.; Calvo, E. Does elevation influence the distributional patterns of tropical myxomycetes? A case study in Costa Rica. Mycology 2016, 7, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, S.L. Distribution and ecology of myxomycetes in temperate forests. II. Patterns of occurence on bark surface of living trees, leaf litter, and dung. Mycologia 1989, 81, 608–621. [Google Scholar] [CrossRef]
- Novozhilov, Y.K.; Rollins, A.W.; Shchepin, O.N.; Schnittler, M. Ecology and distribution of Myxomycetes. In Myxomycetes; Academic Press: London, UK, 2022; pp. 325–376. [Google Scholar]
- Novozhilov, T.K.; Schnittler, M. Myxomycete diversity in arid regions of the Great Lake Basin of western Mongolia. Fungal Divers. 2008, 30, 97–119. [Google Scholar]
- Novozhilov, Y.K.; Zemlyanskaya, I.; Schnittler, M.; Stephenson, S.L. Myxomycete diversity and ecology in the arid regions of the Lower Volga River Basin (Russia). Fungal Divers. 2006, 23, 193–241. [Google Scholar]
- Novozhilov, Y.K.; Schnittler, M.; Vlasenko, A.V.; Fefelov, K.A. Myxomycete diversity of the Altay Mts. (southwestern Siberia, Russia). Mycotaxon 2010, 111, 91–94. [Google Scholar] [CrossRef]
- Schnittler, M.; Novozhilov, Y.K. Myxomycetes of the winter-cold desert in western Kazakhstan. Mycotaxon 2000, 74, 267–285. [Google Scholar] [CrossRef]
- Novozhilov, Y.K.; Rollins, A.W.; Schnittler, M. Ecology and Distribution of Myxomycetes. In Myxomycetes. Biology, Systematics, Biogeography, and Ecology; Stephenson, S.L., Rojas, C., Eds.; Academic Press: London, UK, 2017; pp. 253–297. [Google Scholar]
- Novozhilov, Y.K.; Schnittler, M.; Erastova, D.A.; Okun, M.V.; Schepin, O.N.; Stephenson, S.L. Altitudinal patterns of diversity of myxomycetes across tropical forests of Southern Vietnam. Protistology 2018, 12, 73–95. [Google Scholar] [CrossRef]
- Arenas-Taborda, J.; Schnittler, M.; Stephenson, S.L. Myxomycete assemblage turnover across a moisture and elevation gradient in Costa Rica. Slime Molds 2021, 1, V1A4. [Google Scholar] [CrossRef]
- Lado, C.; Wrigley de Basanta, D.; Estrada-Torres, A.; Stephenson, S.L. Myxomycete diversity in the coastal desert of Peru with emphasis on the lomas formations. An. Jard. Bot. Madr. 2016, 73, e032. [Google Scholar] [CrossRef]
- Lado, C.; Wrigley de Basanta, D.; Estrada-Torres, A.; Stephenson, S.L.; Treviño-Zevallos, I. Diversity of Myxomycetes in arid zones of Peru part II: The cactus belt and transition zones. An. Jard. Bot. Madr. 2019, 76, e083. [Google Scholar] [CrossRef]
- Novozhilov, Y.K.; Erastova, D.A.; Shchepin, O.N.; Schnittler, M.; Aleksandrova, A.V.; Popov, E.S.; Kuznetsov, A.N. Myxomycetes associated with monsoon lowland tropical for ests in southern Vietnam. Nova Hedwig. 2017, 104, 143–182. [Google Scholar] [CrossRef]
- Lado, C.; Wrigley de Basanta, D.; Estrada-Torres, A.; Stephenson, S.L. The biodiversity of myxomycetes in central Chile. Fungal Divers. 2013, 59, 3–32. [Google Scholar] [CrossRef]
- Wrigley de Basanta, D.; Lado, C.; Estrada-Torres, A.; Stephenson, S. Biodiversity of myxomycetes in subantarctic forests of Patagonia and Tierra del Fuego, Argentina. Nova Hedwig. 2010, 90, 45–79. [Google Scholar] [CrossRef]

| Taxa | DL | ST | DB | MO | DU | Vegetation Type |
|---|---|---|---|---|---|---|
| Liceales | ||||||
| Licea tenera * E. Jahn | x | HAZ, NAF, PAJ | ||||
| Physarales | ||||||
| Angioridium sinuosum (Bull.) Grev. | x | PAJ | ||||
| Diachea leucopodia (Bull.) Rostaf. | x | HAZ, PAJ | ||||
| Badhamia polycephala (Schwein.) JM García-Martín, JC Zamora & Lado | x | NAF | ||||
| Craterium leucocephalum (Pers. ex J.F. Gmel.) Ditmar | x | NAF | ||||
| Diderma circumdissiliens * Flatau & Schirmer | x | HAZ, NAF, PAJ | ||||
| Didymium dubium Rostaf. | x | PAJ | ||||
| Didymium ilicinum Ing | x | PAJ | ||||
| Didymium megalosporum Berk. & M.A. Curtis | x | NAF | ||||
| Physarum album (Bull.) Chevall. | x | NAF | ||||
| Trichiales | ||||||
| Arcyria cinerea (Bull.) Pers. | x | NAF | ||||
| Arcyria cf. marginoundulata Nann.-Bremek. & Y. Yamam. | x | NAF | ||||
| Hemitrichia calyculata (Ditmar) Rostaf. | x | HAZ, NAF, PAJ | ||||
| Hemitrichia serpula var. piauiensis L. H. Cavalc. & Mobin | x | NAF | ||||
| Oligonema favogineum (Batsch) García-Cunch., JC Zamora & Lado | x | HAZ, NAF, PAJ | ||||
| Oligonema persimile (P.Karst.) García-Cunch., J.C.Zamora & Lado | x | PAJ | ||||
| Perichaena luteola * (Kowalski) Gilert | x | HAZ, NAF, PAJ | ||||
| Trichia contorta (Ditmar) Rostaf. | x | PAJ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velloso, J.R.P.; Cavalcanti, L.d.H.; Treviño-Zevallos, I.; Schaefer, C.E.G.R.; Francelino, M.R.; Putzke, J. Diversity and Ecology of Myxomycetes (Amoebozoa) Along a Vegetational Gradient in the Peruvian Andes. Diversity 2025, 17, 745. https://doi.org/10.3390/d17110745
Velloso JRP, Cavalcanti LdH, Treviño-Zevallos I, Schaefer CEGR, Francelino MR, Putzke J. Diversity and Ecology of Myxomycetes (Amoebozoa) Along a Vegetational Gradient in the Peruvian Andes. Diversity. 2025; 17(11):745. https://doi.org/10.3390/d17110745
Chicago/Turabian StyleVelloso, Jorge Renato Pinheiro, Laise de Holanda Cavalcanti, Italo Treviño-Zevallos, Carlos Ernesto Gonçalves Reynaud Schaefer, Marcio Rocha Francelino, and Jair Putzke. 2025. "Diversity and Ecology of Myxomycetes (Amoebozoa) Along a Vegetational Gradient in the Peruvian Andes" Diversity 17, no. 11: 745. https://doi.org/10.3390/d17110745
APA StyleVelloso, J. R. P., Cavalcanti, L. d. H., Treviño-Zevallos, I., Schaefer, C. E. G. R., Francelino, M. R., & Putzke, J. (2025). Diversity and Ecology of Myxomycetes (Amoebozoa) Along a Vegetational Gradient in the Peruvian Andes. Diversity, 17(11), 745. https://doi.org/10.3390/d17110745






