Submarine Groundwater Discharge Alters Benthic Community Composition and Functional Diversity on Coral Reefs
Abstract
:1. Introduction
2. Materials and Methods
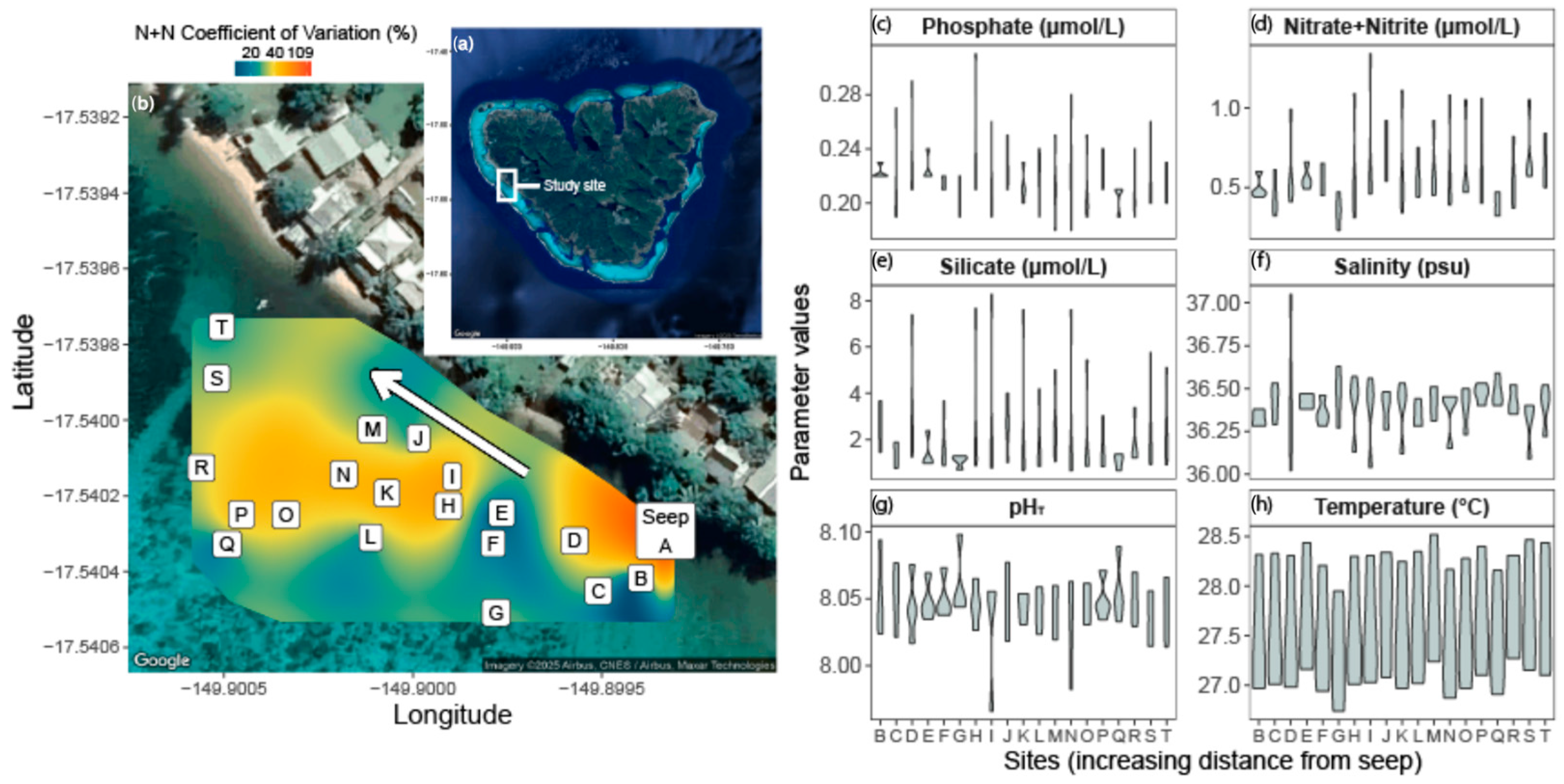
2.1. Study Site and Characterization
2.2. Community Surveys
2.3. Classification of Functional Traits
2.4. Taxonomic and Functional Diversity
2.5. Statistical Analyses
3. Results
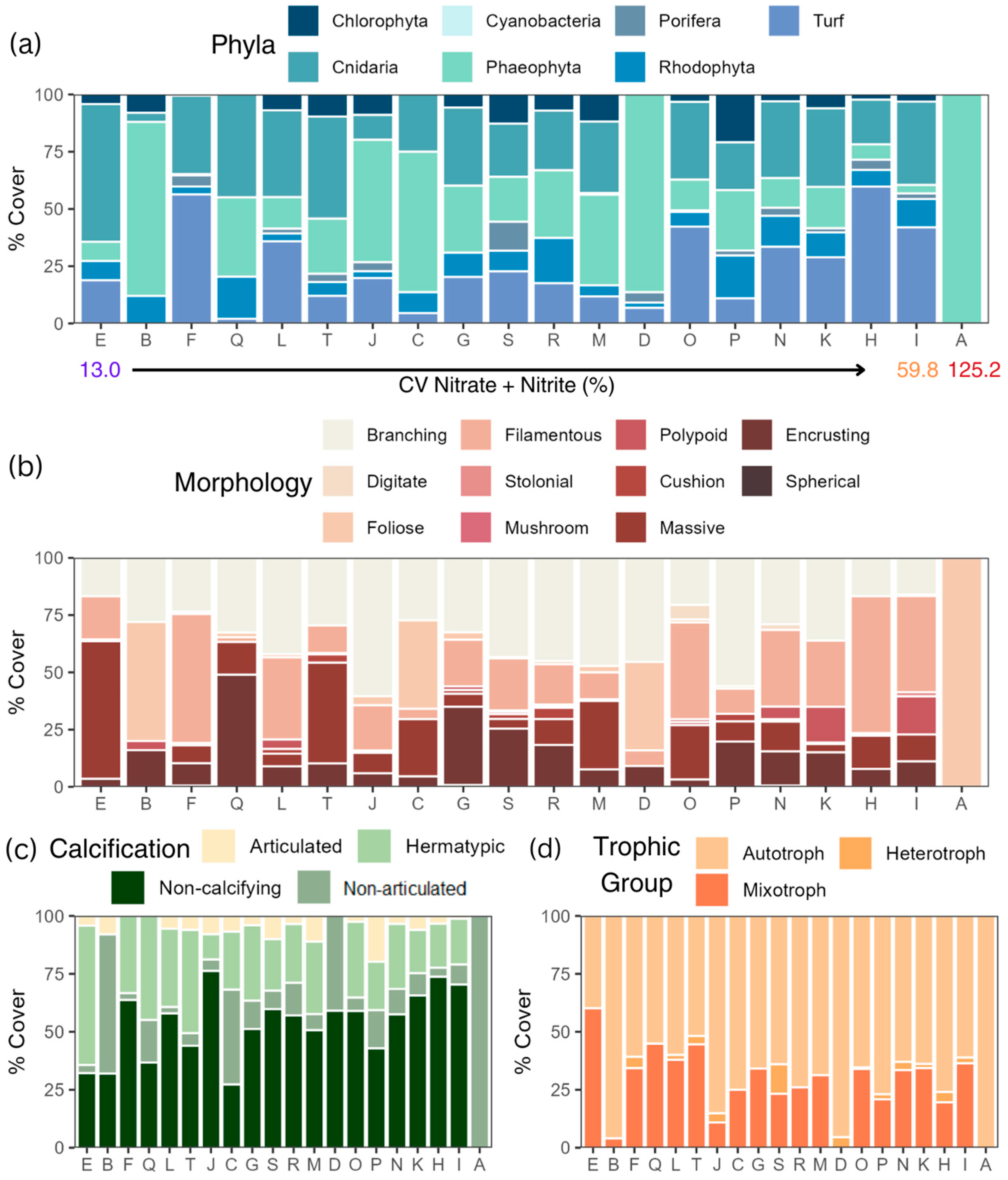
3.1. Community Composition
3.2. SGD Gradient Yields Variable Nutrients and Biogeochemistry
3.3. Model Selection
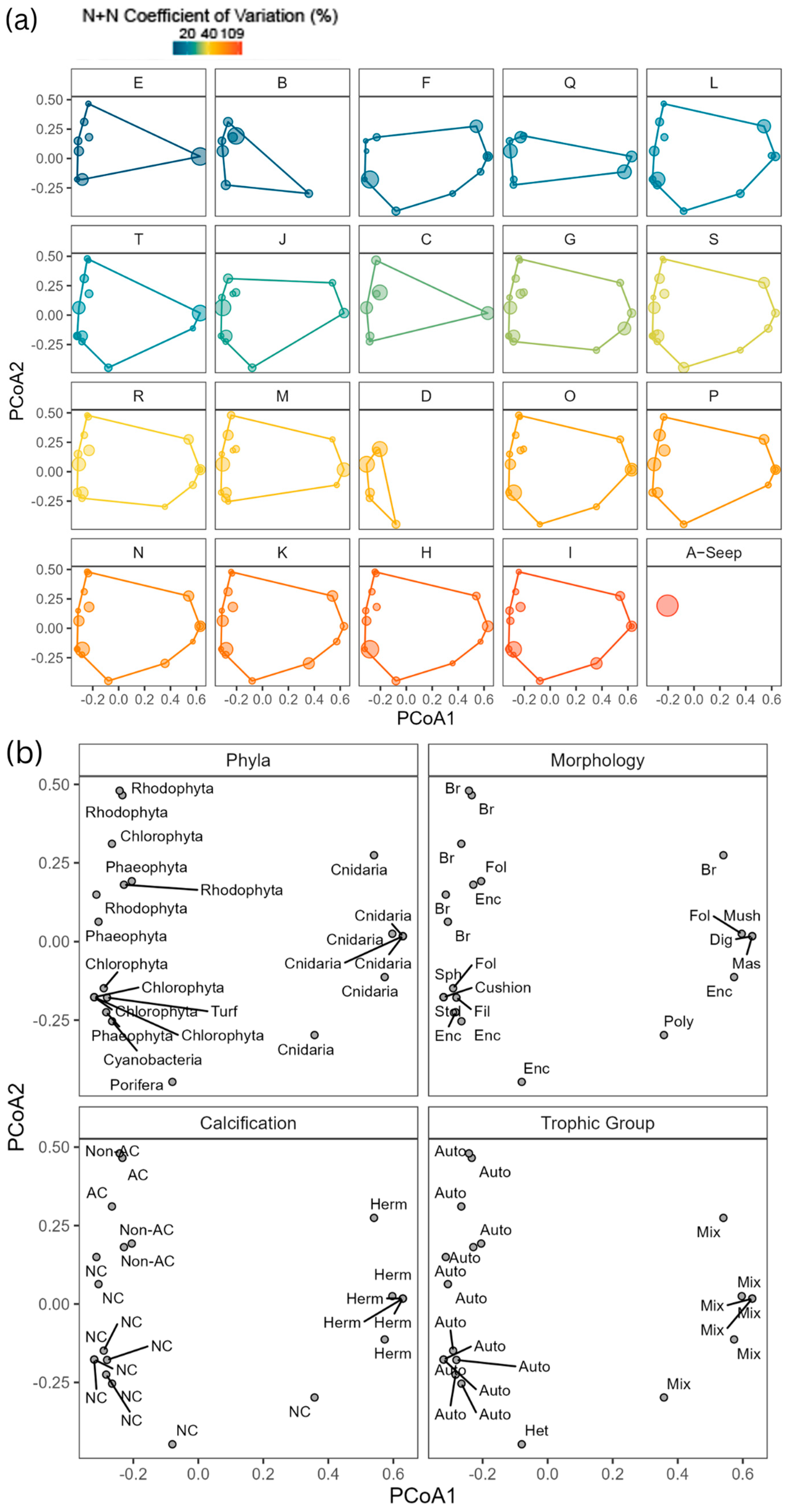
3.4. Diversity Exhibits a Unimodal Shift Along SGD Gradient
3.5. Taxonomic and Functional-Trait Patterns Shift Along the SGD Gradient
3.6. Dissimilarity of Functional Entities Along SGD
3.7. SGD Alters Community Taxonomic Composition
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chapin, F.S.; Walker, B.H.; Hobbs, R.J.; Hooper, D.U.; Lawton, J.H.; Sala, O.E.; Tilman, D. Biotic Control over the Functioning of Ecosystems. Science 1997, 277, 500–504. [Google Scholar] [CrossRef]
- Grime, J.P. Benefits of Plant Diversity to Ecosystems: Immediate, Filter and Founder Effects. J. Ecol. 1998, 86, 902–910. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Hooper, D.U. Biological Diversity and Terrestrial Ecosystem Biogeochemistry. In Biodiversity and Ecosystem Function; Schulze, E.-D., Mooney, H.A., Eds.; Springer: Berlin/Heidelberg, Germany, 1994; pp. 3–14. ISBN 9783642580017. [Google Scholar]
- Hooper, D.U.; Vitousek, P.M. The Effects of Plant Composition and Diversity on Ecosystem Processes. Science 1997, 277, 1302–1305. [Google Scholar] [CrossRef]
- Mouchet, M.A.; Villéger, S.; Mason, N.W.H.; Mouillot, D. Functional Diversity Measures: An Overview of Their Redundancy and Their Ability to Discriminate Community Assembly Rules. Funct. Ecol. 2010, 24, 867–876. [Google Scholar] [CrossRef]
- Cadotte, M.W.; Carscadden, K.; Mirotchnick, N. Beyond Species: Functional Diversity and the Maintenance of Ecological Processes and Services. J. Appl. Ecol. 2011, 48, 1079–1087. [Google Scholar] [CrossRef]
- Frederiksen, M.; Edwards, M.; Richardson, A.J.; Halliday, N.C.; Wanless, S. From Plankton to Top Predators: Bottom-up Control of a Marine Food Web across Four Trophic Levels. J. Anim. Ecol. 2006, 75, 1259–1268. [Google Scholar] [CrossRef]
- Funk, J.L.; Larson, J.E.; Ames, G.M.; Butterfield, B.J.; Cavender-Bares, J.; Firn, J.; Laughlin, D.C.; Sutton-Grier, A.E.; Williams, L.; Wright, J. Revisiting the Holy Grail: Using Plant Functional Traits to Understand Ecological Processes. Biol. Rev. Camb. Philos. Soc. 2017, 92, 1156–1173. [Google Scholar] [CrossRef]
- Zawada, K.J.A.; Madin, J.S.; Baird, A.H.; Bridge, T.C.; Dornelas, M. Morphological Traits Can Track Coral Reef Responses to the Anthropocene. Funct. Ecol. 2019, 33, 962–975. [Google Scholar] [CrossRef]
- den Haan, J.; Huisman, J.; Brocke, H.J.; Goehlich, H.; Latijnhouwers, K.R.W.; van Heeringen, S.; Honcoop, S.A.S.; Bleyenberg, T.E.; Schouten, S.; Cerli, C.; et al. Nitrogen and Phosphorus Uptake Rates of Different Species from a Coral Reef Community after a Nutrient Pulse. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef]
- Yachi, S.; Loreau, M. Biodiversity and Ecosystem Productivity in a Fluctuating Environment: The Insurance Hypothesis. Proc. Natl. Acad. Sci. USA 1999, 96, 1463–1468. [Google Scholar] [CrossRef]
- Naeem, S.; Wright, J.P. Disentangling Biodiversity Effects on Ecosystem Functioning: Deriving Solutions to a Seemingly Insurmountable Problem. Ecol. Lett. 2003, 6, 567–579. [Google Scholar] [CrossRef]
- Chesson, P. General Theory of Competitive Coexistence in Spatially-Varying Environments. Theor. Popul. Biol. 2000, 58, 211–237. [Google Scholar] [CrossRef]
- Tilman, D.; Knops, J.; Wedin, D.; Reich, P.; Ritchie, M.; Siemann, E. The Influence of Functional Diversity and Composition on Ecosystem Processes. Science 1997, 277, 1300–1302. [Google Scholar] [CrossRef]
- Brandl, S.J.; Rasher, D.B.; Côté, I.M.; Casey, J.M.; Darling, E.S.; Lefcheck, J.S.; Duffy, J.E. Coral Reef Ecosystem Functioning: Eight Core Processes and the Role of Biodiversity. Front. Ecol. Environ. 2019, 17, 445–454. [Google Scholar] [CrossRef]
- Loreau, M.; Naeem, S.; Inchausti, P.; Bengtsson, J.; Grime, J.P.; Hector, A.; Hooper, D.U.; Huston, M.A.; Raffaelli, D.; Schmid, B.; et al. Ecology: Biodiversity and Ecosystem Functioning: Current Knowledge and Future Challenges. Science 2001, 294, 804–808. [Google Scholar] [CrossRef]
- Goswami, M.; Bhattacharyya, P.; Mukherjee, I.; Tribedi, P. Functional Diversity: An Important Measure of Ecosystem Functioning. Adv. Microbiol. 2017, 7, 82–93. [Google Scholar] [CrossRef]
- McGill, B.J.; Enquist, B.J.; Weiher, E.; Westoby, M. Rebuilding Community Ecology from Functional Traits. Trends Ecol. Evol. 2006, 21, 178–185. [Google Scholar] [CrossRef]
- Vila-Concejo, A.; Kench, P. Storms in Coral Reefs. In Coastal Storms; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 127–149. ISBN 9781118937099. [Google Scholar]
- Goñi, R. Ecosystem Effects of Marine Fisheries: An Overview. Ocean Coast. Manag. 1998, 40, 37–64. [Google Scholar] [CrossRef]
- Lewin, W.C.; Weltersbach, M.S.; Ferter, K.; Hyder, K.; Mugerza, E.; Prellezo, R.; Radford, Z.; Zarauz, L.; Strehlow, H.V. Potential Environmental Impacts of Recreational Fishing on Marine Fish Stocks and Ecosystems. Rev. Fish. Sci. Aquac. 2019, 27, 287–330. [Google Scholar] [CrossRef]
- Fabricius, K.E. Effects of Terrestrial Runoff on the Ecology of Corals and Coral Reefs: Review and Synthesis. Mar. Pollut. Bull. 2005, 50, 125–146. [Google Scholar] [CrossRef]
- Lu, Y.; Yuan, J.; Lu, X.; Su, C.; Zhang, Y.; Wang, C.; Cao, X.; Li, Q.; Su, J.; Ittekkot, V.; et al. Major Threats of Pollution and Climate Change to Global Coastal Ecosystems and Enhanced Management for Sustainability. Environ. Pollut. 2018, 239, 670–680. [Google Scholar] [CrossRef]
- Doney, S.C.; Ruckelshaus, M.; Emmett Duffy, J.; Barry, J.P.; Chan, F.; English, C.A.; Galindo, H.M.; Grebmeier, J.M.; Hollowed, A.B.; Knowlton, N.; et al. Climate Change Impacts on Marine Ecosystems. Ann. Rev. Mar. Sci. 2012, 4, 11–37. [Google Scholar] [CrossRef] [PubMed]
- Done, T.J.; Moran, P.J.; de Vantier, L. Cyclone Winifred—Observations on Some Ecological and Geomorphological Effects. In The Offshore Effects of Cyclone Winifred. GBRMPA Workshop; Dutton, I.M., Ed.; Great Barrier Reef Marine Park Authority: Townsville, QLD, Australia, 1986; pp. 51–52. ISBN 9780642525291. [Google Scholar]
- Gouveia, N.A.; Gherardi, D.F.M.; Wagner, F.H.; Paes, E.T.; Coles, V.J.; Aragão, L.E.O.C. The Salinity Structure of the Amazon River Plume Drives Spatiotemporal Variation of Oceanic Primary Productivity. J. Geophys. Res. Biogeosci. 2019, 124, 147–165. [Google Scholar] [CrossRef]
- Petus, C.; Marieu, V.; Novoa, S.; Chust, G.; Bruneau, N.; Froidefond, J.-M. Monitoring Spatio-Temporal Variability of the Adour River Turbid Plume (Bay of Biscay, France) with MODIS 250-M Imagery. Cont. Shelf Res. 2014, 74, 35–49. [Google Scholar] [CrossRef]
- Golbuu, Y.; van Woesik, R.; Richmond, R.H.; Harrison, P.; Fabricius, K.E. River Discharge Reduces Reef Coral Diversity in Palau. Mar. Pollut. Bull. 2011, 62, 824–831. [Google Scholar] [CrossRef]
- Morris, L.J.; Hall, L.M.; Miller, J.D.; Lasi, M.A.; Chamberlain, R.H.; Virnstein, R.W.; Jacoby, C.A. Diversity and Distribution of Seagrasses as Related to Salinity, Temperature, and Availability of Light in the Indian River Lagoon, Florida. Fla. Sci. 2021, 84, 119–137. [Google Scholar]
- Neumann-Leitão, S.; Melo, P.A.M.C.; Schwamborn, R.; Diaz, X.F.G.; Figueiredo, L.G.P.; Silva, A.P.; Campelo, R.P.S.; de Melo Júnior, M.; Melo, N.F.A.C.; Costa, A.E.S.F.; et al. Zooplankton from a Reef System Under the Influence of the Amazon River Plume. Front. Microbiol. 2018, 9, 355. [Google Scholar] [CrossRef]
- Carlson, R.R.; Foo, S.A.; Asner, G.P. Land Use Impacts on Coral Reef Health: A Ridge-to-Reef Perspective. Front. Mar. Sci. 2019, 6, 562. [Google Scholar] [CrossRef]
- Baumann, K.A. Macroinvertebrate Community Responses to Hydrologic Extremes in a Divided River: Implications for Restoration Efforts. Master’s Thesis, Southern Illinois University, Carbondale, IL, USA, 2016. [Google Scholar]
- Teixidó, N.; Gambi, M.C.; Parravacini, V.; Kroeker, K.; Micheli, F.; Villéger, S.; Ballesteros, E. Functional Biodiversity Loss along Natural CO2 Gradients. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef]
- Johannes, R.E. The Ecological Significance of the Submarine Discharge of Groundwater. Mar. Ecol. Prog. Ser. 1980, 3, 365–373. [Google Scholar] [CrossRef]
- Sims, Z.C.; Cohen, A.L.; Luu, V.H.; Wang, X.T.; Sigman, D.M. Uptake of Groundwater Nitrogen by a near-Shore Coral Reef Community on Bermuda. Coral Reefs 2020, 39, 215–228. [Google Scholar] [CrossRef]
- Redding, J.E.; Myers-Miller, R.L.; Baker, D.M.; Fogel, M.; Raymundo, L.J.; Kim, K. Link between Sewage-Derived Nitrogen Pollution and Coral Disease Severity in Guam. Mar. Pollut. Bull. 2013, 73, 57–63. [Google Scholar] [CrossRef]
- Paytan, A.; Shellenbarger, G.G.; Street, J.H.; Gonneea, M.E.; Davis, K.; Young, M.B.; Moore, W.S. Submarine Groundwater Discharge: An Important Source of New Inorganic Nitrogen to Coral Reef Ecosystems. Limnol. Oceanogr. 2006, 51, 343–348. [Google Scholar] [CrossRef]
- Lecher, A.L.; Mackey, K.R.M. Synthesizing the Effects of Submarine Groundwater Discharge on Marine Biota. Hydrology 2018, 5, 60. [Google Scholar] [CrossRef]
- Santos, I.R.; Chen, X.; Lecher, A.L.; Sawyer, A.H.; Moosdorf, N.; Rodellas, V.; Tamborski, J.; Cho, H.M.; Dimova, N.; Sugimoto, R.; et al. Submarine Groundwater Discharge Impacts on Coastal Nutrient Biogeochemistry. Nat. Rev. Earth Environ. 2021, 2, 307–323. [Google Scholar] [CrossRef]
- Stoddart, D.R. Ecology and Morphology of Recent Coral Reefs. Biol. Rev. Camb. Philos. Soc. 1969, 44, 433–498. [Google Scholar] [CrossRef]
- Kirst, G.O. Salinity Tolerance of Eukaryotic Marine Algae. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1990, 41, 21–53. [Google Scholar] [CrossRef]
- La Valle, F.F.; Kantar, M.B.; Nelson, C.E. Coral Reef Benthic Community Structure Is Associated with the Spatiotemporal Dynamics of Submarine Groundwater Discharge Chemistry. Limnol. Oceanogr. 2021, 66, 188–200. [Google Scholar] [CrossRef]
- Amato, D.W.; Smith, C.M.; Duarte, T.K. Submarine Groundwater Discharge Differentially Modifies Photosynthesis, Growth, and Morphology for Two Contrasting Species of Gracilaria (Rhodophyta). Hydrology 2018, 5, 65. [Google Scholar] [CrossRef]
- Amato, D.W.; Bishop, J.M.; Glenn, C.R.; Dulai, H.; Smith, C.M. Impact of Submarine Groundwater Discharge on Marine Water Quality and Reef Biota of Maui. PLoS ONE 2016, 11, e0165825. [Google Scholar] [CrossRef]
- Gattuso, J.-P.; Frankignoulle, M.; Bourge, I.; Romaine, S.; Buddemeier, R.W. Effect of Calcium Carbonate Saturation of Seawater on Coral Calcification. Glob. Planet. Change 1998, 18, 37–46. [Google Scholar] [CrossRef]
- Adam, T.C.; Burkepile, D.E.; Holbrook, S.J.; Carpenter, R.C.; Claudet, J.; Loiseau, C.; Thiault, L.; Brooks, A.J.; Washburn, L.; Schmitt, R.J. Landscape-Scale Patterns of Nutrient Enrichment in a Coral Reef Ecosystem: Implications for Coral to Algae Phase Shifts. Ecol. Appl. 2020, 31, e2227. [Google Scholar] [CrossRef] [PubMed]
- Pisternick, T.; Lilkendey, J.; Audit-Manna, A.; Dumur Neelayya, D.; Neehaul, Y.; Moosdorf, N. Submarine Groundwater Springs Are Characterized by Distinct Fish Communities. Mar. Ecol. 2020, 41, 5. [Google Scholar] [CrossRef]
- Lubarsky, K.A.; Silbiger, N.J.; Donahue, M.J. Effects of Submarine Groundwater Discharge on Coral Accretion and Bioerosion on Two Shallow Reef Flats. Limnol. Oceanogr. 2018, 63, 1660–1676. [Google Scholar] [CrossRef]
- Harmelin-Vivien, M.L. The Effects of Storms and Cyclones on Coral Reefs: A Review. J. Coast. Res. 1994, 12, 211–231. [Google Scholar]
- Taniguchi, M.; Burnett, W.C.; Cable, J.E.; Turner, J.V. Investigation of Submarine Groundwater Discharge. Hydrol. Process. 2002, 16, 2115–2129. [Google Scholar] [CrossRef]
- Knee, K.L.; Crook, E.D.; Hench, J.L.; Leichter, J.J.; Paytan, A. Assessment of Submarine Groundwater Discharge (SGD) as a Source of Dissolved Radium and Nutrients to Moorea (French Polynesia) Coastal Waters. Estuaries Coasts 2016, 39, 1651–1668. [Google Scholar] [CrossRef]
- Hagedorn, B.; Becker, M.W.; Silbiger, N.J. Evidence of Freshened Groundwater below a Tropical Fringing Reef. Hydrogeol. J. 2020, 28, 2501–2517. [Google Scholar] [CrossRef]
- Hagedorn, B.; Becker, M.; Maine, B.; Justis, E.; Silbiger, N.; Barnas, D.; Zeff, M. Refining Submarine Groundwater Discharge Analysis Through Nonlinear Quantile Regression of Geochemical Time Series. J. Hydrol. 2024, 645, 132145. [Google Scholar] [CrossRef]
- Silbiger, N.; Donahue, M.; Hagedorn, B.; Barnas, D.; Jorissen, H.; Kerlin, J.; McClintock, R.; Nixon, E.; Sparagon, W.; Zeff, M.; et al. Nutrient Subsidies Restructure Coral Reef Dissolved Carbon Fluxes via Biogeochemical Cascades. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Burnett, W.C.; Aggarwal, P.K.; Aureli, A.; Bokuniewicz, H.; Cable, J.E.; Charette, M.A.; Kontar, E.; Krupa, S.; Kulkarni, K.M.; Loveless, A.; et al. Quantifying Submarine Groundwater Discharge in the Coastal Zone via Multiple Methods. Sci. Total Environ. 2006, 367, 498–543. [Google Scholar] [CrossRef] [PubMed]
- Payri, C.E.; N’Yeurt, A.D.R.; Orempuller, J. Algae of French Polynesia; Au Vent de Îles Editions: Tahiti, French Polynesia, 2000; ISBN 9782909790824. [Google Scholar]
- Bosserelle, C. Morphodynamics and Sand Transport on Perched Beaches. Ph.D. Thesis, University of Western Australia, Perth, WA, Australia, 2014. [Google Scholar]
- Risk, M.J. Fish Diversity on a Coral Reef in the Virgin Islands. Atoll Res. Bull. 1972, 153, 1–4. [Google Scholar] [CrossRef]
- Villéger, S.; Novack-Gottshall, P.M.; Mouillot, D. The Multidimensionality of the Niche Reveals Functional Diversity Changes in Benthic Marine Biotas across Geological Time. Ecol. Lett. 2011, 14, 561–568. [Google Scholar] [CrossRef]
- Chao, A.; Chiu, C.-H.; Jost, L. Unifying Species Diversity, Phylogenetic Diversity, Functional Diversity, and Related Similarity and Differentiation Measures Through Hill Numbers. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 297–324. [Google Scholar] [CrossRef]
- Alvarez-Filip, L.; Carricart-Ganivet, J.P.; Horta-Puga, G.; Iglesias-Prieto, R. Shifts in Coral-Assemblage Composition Do Not Ensure Persistence of Reef Functionality. Sci. Rep. 2013, 3, 3486. [Google Scholar] [CrossRef]
- Carlot, J.; Rouzé, H.; Barneche, D.R.; Mercière, A.; Espiau, B.; Cardini, U.; Brandl, S.J.; Casey, J.M.; Pérez-Rosales, G.; Adjeroud, M.; et al. Scaling up Calcification, Respiration, and Photosynthesis Rates of Six Prominent Coral Taxa. Ecol. Evol. 2022, 12, e8613. [Google Scholar] [CrossRef]
- Gattuso, J.-P.; Allemand, D.; Frankignoulle, M. Photosynthesis and Calcification at Cellular, Organismal and Community Levels in Coral Reefs: A Review on Interactions and Control by Carbonate Chemistry. Am. Zool. 1999, 39, 160–183. [Google Scholar] [CrossRef]
- Dennison, W.C.; Barnes, D.J. Effect of Water Motion on Coral Photosynthesis and Calcification. J. Exp. Mar. Biol. Ecol. 1988, 115, 67–77. [Google Scholar] [CrossRef]
- Madin, J.S.; Baird, A.H.; Baskett, M.L.; Connolly, S.R.; Dornelas, M.A. Partitioning Colony Size Variation into Growth and Partial Mortality. Biol. Lett. 2020, 16, 20190727. [Google Scholar] [CrossRef]
- Pentecost, A. Calcification Processes in Algae and Cyanobacteria. In Calcareous Algae and Stromatolites; Springer: Berlin/Heidelberg, Germany, 1991; pp. 3–20. ISBN 9783642523373. [Google Scholar]
- Mammola, S.; Carmona, C.P.; Guillerme, T.; Cardoso, P. Concepts and Applications in Functional Diversity. Funct. Ecol. 2021, 35, 1869–1885. [Google Scholar] [CrossRef]
- de Bello, F.; Carmona, C.P.; Mason, N.W.H.; Sebastià, M.-T.; Lepš, J. Which Trait Dissimilarity for Functional Diversity: Trait Means or Trait Overlap? J. Veg. Sci. 2013, 24, 807–819. [Google Scholar] [CrossRef]
- Maechler, M.; Rousseeuw, P.; Struyf, A.; Hubert, M.; Hornik, K. Cluster: Cluster Analysis Basics and Extensions. R Package Version 2.1.3. 2023. Available online: https://CRAN.R-project.org/package=cluster (accessed on 24 June 2023).
- Roussel, J.-R.; Barber, C.B.; Habel, K.; Grasman, R.; Gramacy, R.B.; Mozharovskyi, P.; Sterratt, D.C. The R Geometry Package: Mesh Generation and Surface Tessellation. Available online: https://davidcsterratt.github.io/geometry (accessed on 24 June 2023).
- Idjadi, J.A.; Edmunds, P.J. Scleractinian Corals as Facilitators for Other Invertebrates on a Caribbean Reef. Mar. Ecol. Prog. Ser. 2006, 319, 117–127. [Google Scholar] [CrossRef]
- Hatcher, B.G. Coral Reef Primary Productivity. A Hierarchy of Pattern and Process. Trends Ecol. Evol. 1990, 5, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Hoegh-Guldberg, O.; Mumby, P.J.; Hooten, A.J.; Steneck, R.S.; Greenfield, P.; Gomez, E.; Harvell, C.D.; Sale, P.F.; Edwards, A.J.; Caldeira, K.; et al. Coral Reefs under Rapid Climate Change and Ocean Acidification. Science 2007, 318, 1737–1742. [Google Scholar] [CrossRef]
- Taniguchi, M.; Dulai, H.; Burnett, K.M.; Santos, I.R.; Sugimoto, R.; Stieglitz, T.; Kim, G.; Moosdorf, N.; Burnett, W.C. Submarine Groundwater Discharge: Updates on Its Measurement Techniques, Geophysical Drivers, Magnitudes, and Effects. Front. Environ. Sci. 2019, 7, 141. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.; O’Hara, R.B.; Simpson, G.; Solymos, P. Vegan: Community Ecology Package, R Package Version 2.4-4.2. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computer; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- van Woesik, R.; van Woesik, K.; van Woesik, L.; van Woesik, S. Effects of Ocean Acidification on the Dissolution Rates of Reef-Coral Skeletons. PeerJ 2013, 1, e208. [Google Scholar] [CrossRef]
- Chan, B.K.K.; Wang, T.-W.; Chen, P.-C.; Lin, C.-W.; Chan, T.-Y.; Tsang, L.M. Community Structure of Macrobiota and Environmental Parameters in Shallow Water Hydrothermal Vents off Kueishan Island, Taiwan. PLoS ONE 2016, 11, e0148675. [Google Scholar] [CrossRef]
- La Valle, F.F.; Thomas, F.I.; Nelson, C.E. Macroalgal Biomass, Growth Rates, and Diversity Are Influenced by Submarine Groundwater Discharge and Local Hydrodynamics in Tropical Reefs. Mar. Ecol. Prog. Ser. 2019, 621, 51–67. [Google Scholar] [CrossRef]
- Hoffmann, R.; Bisson, M.A. Chara buckellii, a euryhaline charophyte from an unusual saline environment. I. Osmotic relations at steady state. Can. J. Bot. 1986, 64, 1599–1605. [Google Scholar] [CrossRef]
- Ritchie, R.J.; Larkum, A.W.D. Potassium Transport in Enteromorpha Intestinalis (L.) Link: II. Effects of Medium Composition and Metabolic Inhibitors. J. Exp. Bot. 1985, 36, 394–412. [Google Scholar] [CrossRef]
- Naim, O. Seasonal Responses of a Fringing Reef Community to Eutrophication (Reunion Island, Western Indian Ocean). Mar. Ecol. Prog. Ser. 1993, 99, 137–151. [Google Scholar]
- Carscadden, K.A.; Emery, N.C.; Arnillas, C.A.; Cadotte, M.W.; Afkhami, M.E.; Gravel, D.; Livingstone, S.W.; Wiens, J.J. Niche Breadth: Causes and Consequences for Ecology, Evolution, and Conservation. Q. Rev. Biol. 2020, 95, 179–214. [Google Scholar] [CrossRef]
- May, R.M.; MacArthur, R.H. Niche Overlap as a Function of Environmental Variability. Proc. Natl. Acad. Sci. USA 1972, 69, 1109–1113. [Google Scholar] [CrossRef] [PubMed]
- Chesson, P. Mechanisms of Maintenance of Species Diversity. Annu. Rev. Ecol. Syst. 2000, 31, 343–366. [Google Scholar] [CrossRef]
- Lapointe, B.E. Nutrient Thresholds for Bottom-up Control of Macroalgal Blooms on Coral Reefs in Jamaica and Southeast Florida. Limnol. Oceanogr. 1997, 42, 1119–1131. [Google Scholar] [CrossRef]
- Gil, M.A. Unity through Nonlinearity: A Unimodal Coral-Nutrient Interaction. Notes Ecol. 2013, 94, 1871–1877. [Google Scholar] [CrossRef]
- Silbiger, N.; Donahue, M.J.; Lubarsky, K. Submarine Groundwater Discharge Alters Coral Reef Ecosystem Metabolism: SGD Alters Ecosystem Metabolism. Proc. R. Soc. B Biol. Sci. 2020, 287, 20202743. [Google Scholar] [CrossRef]
- Bellwood, D.R.; Streit, R.P.; Brandl, S.J.; Tebbett, S.B. The Meaning of the Term “function” in Ecology: A Coral Reef Perspective. Funct. Ecol. 2019, 33, 948–961. [Google Scholar] [CrossRef]
- Mouillot, D.; Villéger, S.; Parravicini, V.; Kulbicki, M.; Arias-González, J.E.; Bender, M.; Chabanet, P.; Floeter, S.R.; Friedlander, A.; Vigliola, L.; et al. Functional Over-Redundancy and High Functional Vulnerability in Global Fish Faunas on Tropical Reefs. Proc. Natl. Acad. Sci. USA 2014, 111, 13757–13762. [Google Scholar] [CrossRef]
- Barnas, D.M.; Zeff, M.; Silbiger, N.J. Submarine Groundwater Discharge Alters Benthic Community Composition and Functional Diversity on Coral Reefs. Proc. B 2025, 292, 20241554. [Google Scholar] [CrossRef]


| Morphology | Phyla | Calcification | Trophic Group |
|---|---|---|---|
| Branched (Br) | Chlorophyta | Non-calcified (NC) | Autotrophy (Auto) |
| Cushion-like (Cushion) | Cnidaria | Articulated (AC) | Heterotrophy (Het) |
| Digitate (Dig) | Cyanobacteria | Non-articulated (Non-AC) | Mixotrophy (Mix) |
| Encrusting (Enc) | Phaeophyta | Hermatypic (Herm) | |
| Filamentous (Fil) | Porifera | ||
| Foliose (Fol) | Rhodophyta | ||
| Massive (Mas) | |||
| Mushroom (Mush) | |||
| Polypoid (Poly) | |||
| Spherical (Sph) | |||
| Stolonial (Stol) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barnas, D.M.; Zeff, M.; Silbiger, N.J. Submarine Groundwater Discharge Alters Benthic Community Composition and Functional Diversity on Coral Reefs. Diversity 2025, 17, 161. https://doi.org/10.3390/d17030161
Barnas DM, Zeff M, Silbiger NJ. Submarine Groundwater Discharge Alters Benthic Community Composition and Functional Diversity on Coral Reefs. Diversity. 2025; 17(3):161. https://doi.org/10.3390/d17030161
Chicago/Turabian StyleBarnas, Danielle M., Maya Zeff, and Nyssa J. Silbiger. 2025. "Submarine Groundwater Discharge Alters Benthic Community Composition and Functional Diversity on Coral Reefs" Diversity 17, no. 3: 161. https://doi.org/10.3390/d17030161
APA StyleBarnas, D. M., Zeff, M., & Silbiger, N. J. (2025). Submarine Groundwater Discharge Alters Benthic Community Composition and Functional Diversity on Coral Reefs. Diversity, 17(3), 161. https://doi.org/10.3390/d17030161






