Mosasaur Feeding Ecology from the Campanian Bearpaw Formation, Alberta, Canada: A Preliminary Multi-Proxy Approach
Abstract
:1. Introduction
2. Materials and Methods
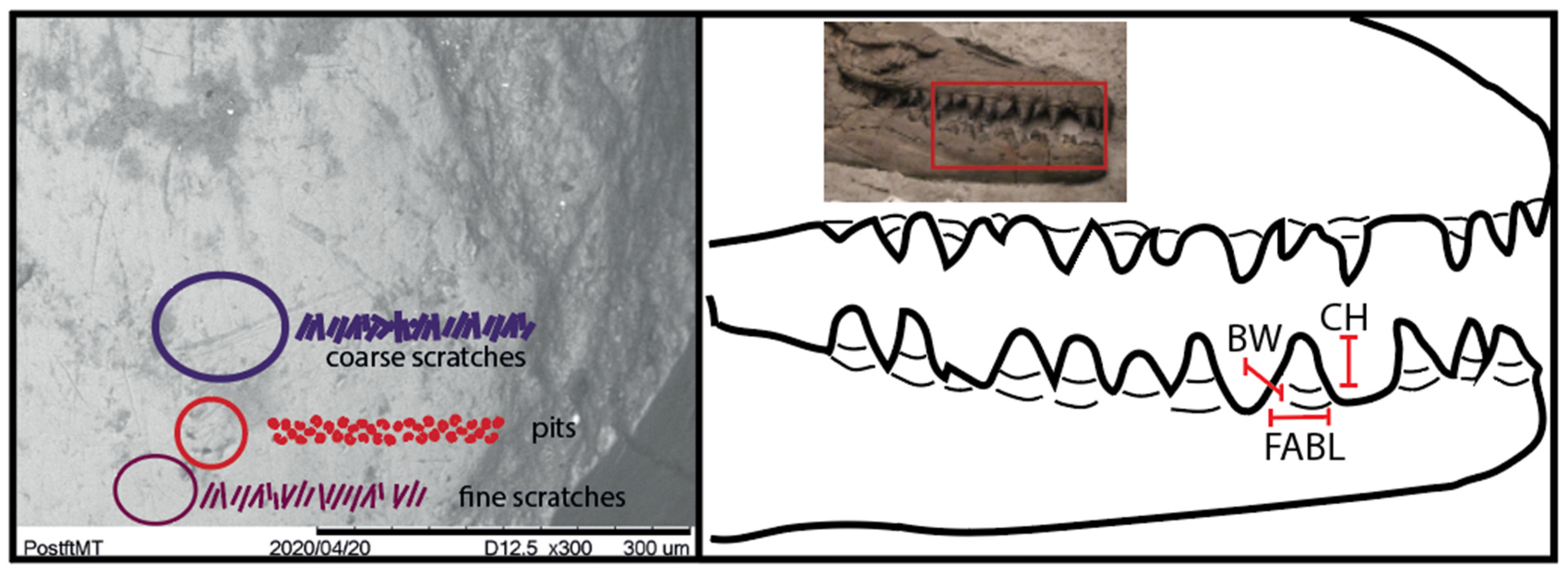
2.1. Microwear
2.2. EDX
2.3. Tooth Bending Strength
3. Results
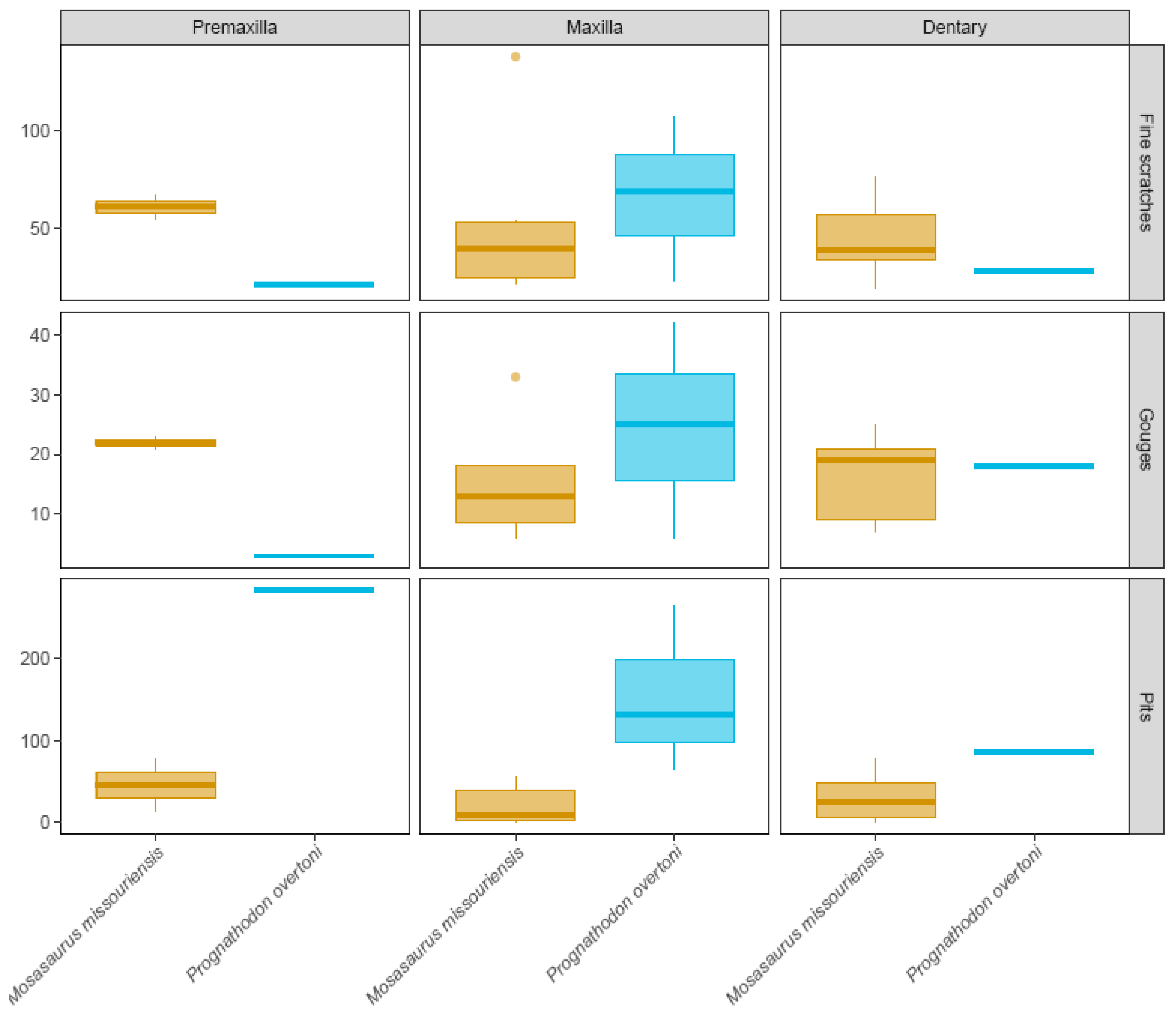
3.1. Microwear
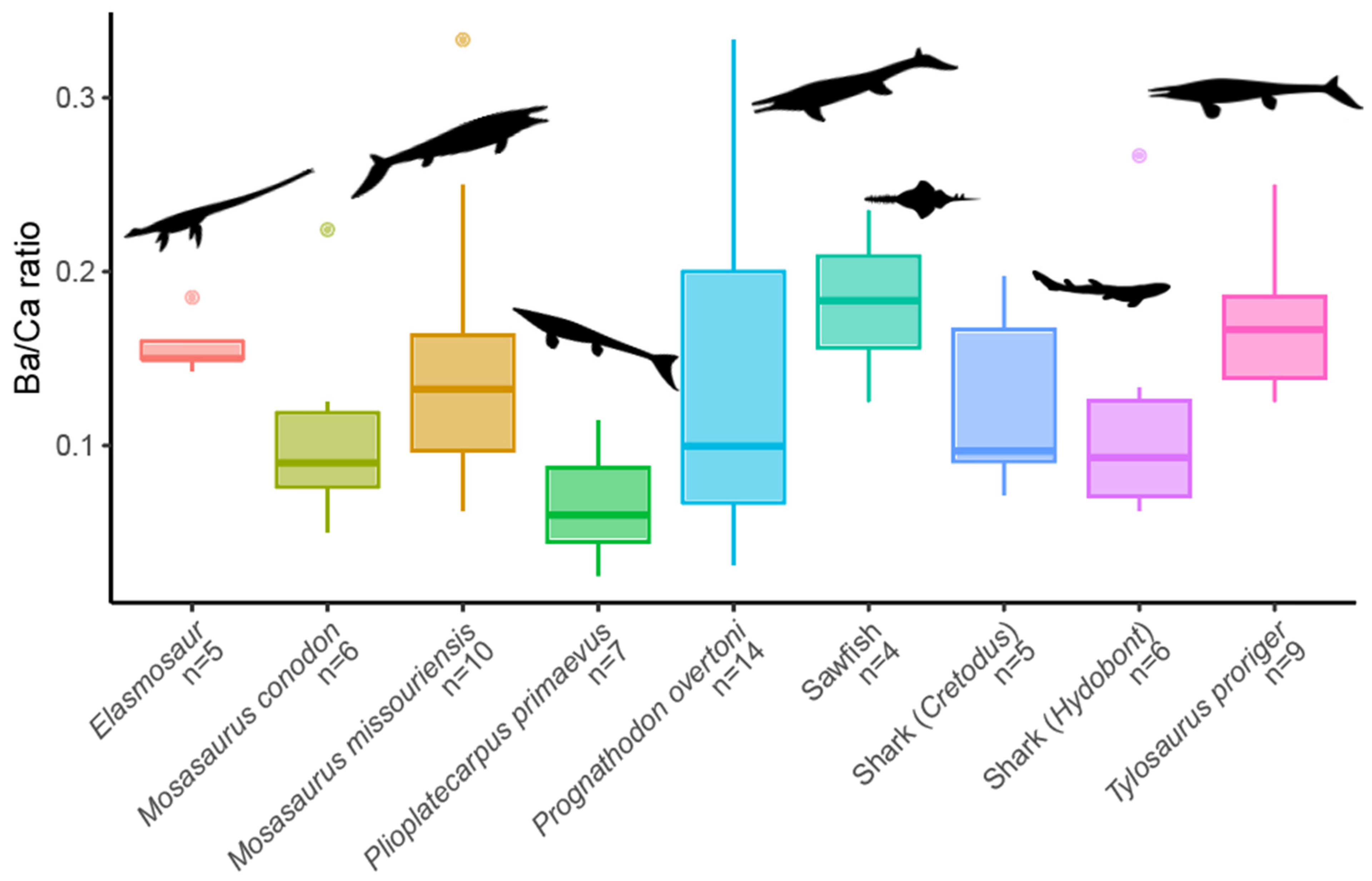
3.2. EDX Analysis
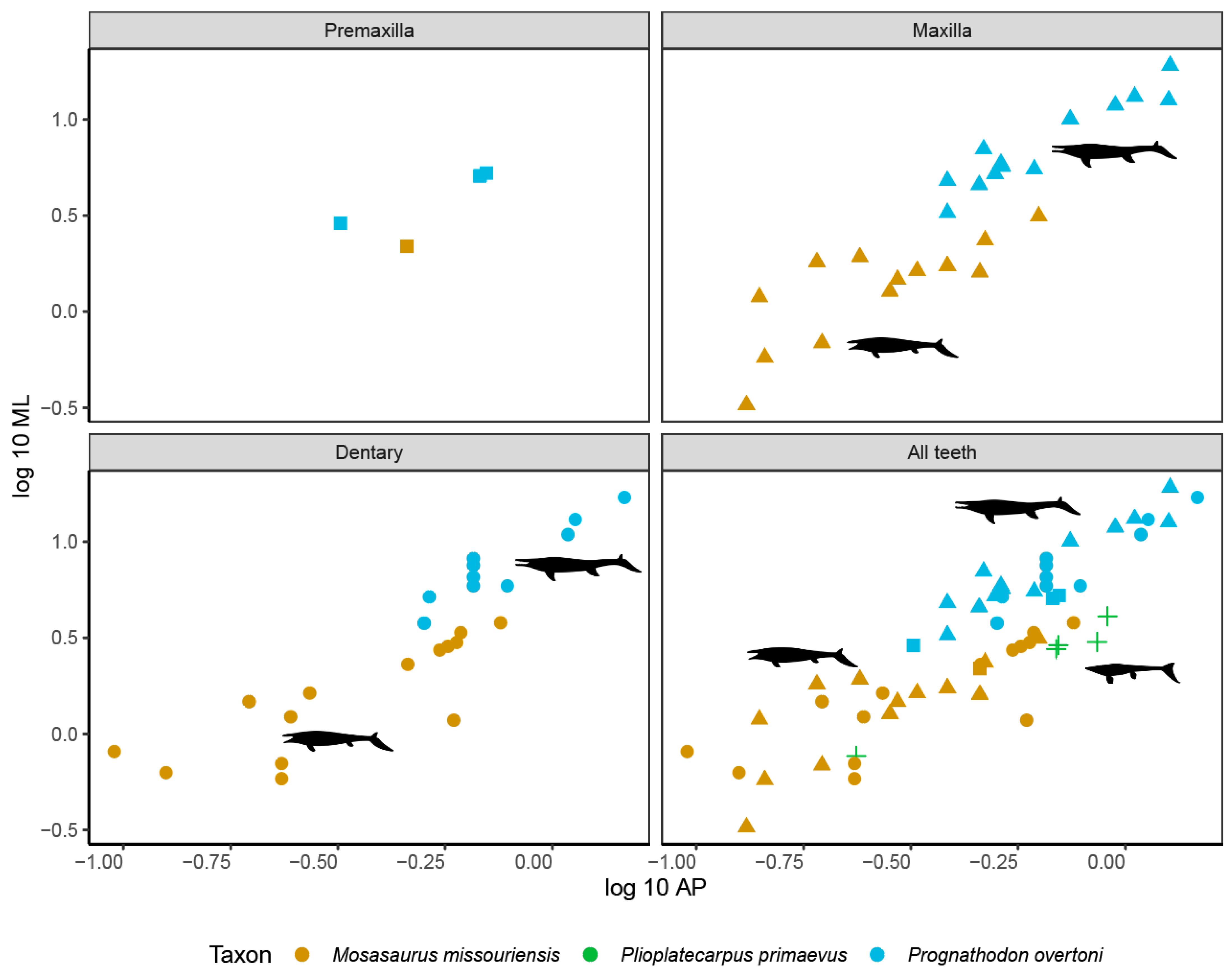
3.3. Tooth Bending Strength
4. Discussion
4.1. EDX-Based Sr/Ca and Ba/Ca Differences
4.2. Inferred Diet and Niche Partitioning
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eberth, D.A. Review and comparison of Belly River Group and Edmonton Group stratigraphy and stratigraphic architecture in the southern Alberta Plains. In Conference of the Canadian Society of Petroleum Geology, Calgary, Alberta, Abstracts 117; 2002. Available online: https://www.researchgate.net/publication/237349569_Review_and_Comparison_of_Belly_River_Group_and_Edmonton_Group_Stratigraphy_and_Stratigraphic_Architecture_in_the_Southern_Alberta_Plains (accessed on 18 October 2024).
- Zubalich, R.; Capozzi, R.; Fanti, F.; Catuneanu, O. Evolution of the Western Interior Seaway in west-central Alberta (late Campanian, Canada): Implications for hydrocarbon exploration. Mar. Pet. Geol. 2020, 124, 104779. [Google Scholar] [CrossRef]
- Wall, J.H.; Sweet, A.R.; Hills, L.V. Paleoecology of the Bearpaw and contiguous Upper Cretaceous formations in the C.P.O.G. Strathmore well, southern Alberta. Bull. Can. Pet. Geol. 1971, 19, 691–702. [Google Scholar] [CrossRef]
- Mychaluk, K.A. Update on Ammolite Production from Southern Alberta, Canada. Gems Gemol. 2009, 45, 192–196. [Google Scholar] [CrossRef]
- Tanke, D. Personal, historical and technical perspectives on the growing role of light and heavy industry on ver-tebrate palaeontology in Alberta, Canada. In Eighteenth Annual Symposium, Abstracts; Alberta Palaeontological Society: Calgary, AB, Canada, 2014; pp. 49–50. [Google Scholar]
- Russell, D.A. Systematics and Morphology of American Mosasaurs (Reptilia, Sauria); Yale University Press: New Haven, CT, USA, 1967. [Google Scholar]
- Polcyn, M.J.; Jacobs, L.L.; Araújo, R.; Schulp, A.S.; Mateus, O. Physical drivers of mosasaur evolution. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2014, 400, 17–27. [Google Scholar] [CrossRef]
- Nicholls, E.L.; Russell, A.P. Paleobiogeography of the Cretaceous Western Interior Seaway of North America: The vertebrate evidence. Palaeogeogr. Palaeoclim. Palaeoecol. 1990, 79, 149–169. [Google Scholar] [CrossRef]
- Konishi, T.; Brinkman, D.; Massare, J.A.; Caldwell, M.W. New exceptional specimens of Prognathodon overtoni (Squamata, Mosasauridae) from the upper Campanian of Alberta, Canada, and the systematics and ecology of the genus. J. Vertebr. Paléontol. 2011, 31, 1026–1046. [Google Scholar] [CrossRef]
- Konishi, T.; Newbrey, M.G.; Caldwell, M.W. A small, exquisitely preserved specimen of Mosasaurus missouriensis (Squamata, Mosasauridae) from the upper Campanian of the Bearpaw Formation, western Canada, and the first stomach contents for the genus. J. Vertebr. Paléontol. 2014, 34, 802–819. [Google Scholar] [CrossRef]
- Holwerda, F.M.; Beatty, B.L.; Schulp, A.S. Dental macro-and microwear in Carinodens belgicus, a small mosasaur from the type Maastrichtian. Neth. J. Geosci. 2013, 92, 267–274. [Google Scholar] [CrossRef]
- Holwerda, F.M.; Bestwick, J.; Purnell, M.A.; Jagt, J.W.M.; Schulp, A.S. Three-dimensional dental microwear in type-Maastrichtian mosasaur teeth (Reptilia, Squamata). Sci. Rep. 2023, 13, 18720. [Google Scholar] [CrossRef]
- Teaford, M.F.; Walker, A. Quantitative differences in dental microwear between primate species with different diets and a comment on the presumed diet of Sivapithecus. Am. J. Phys. Anthr. 1984, 64, 191–200. [Google Scholar] [CrossRef]
- Sillen, A. Biogenic and diagenetic Sr/Ca in Plio-Pleistocene fossils of the Omo Shungura Formation. Paleobiology 1986, 12, 311–323. [Google Scholar] [CrossRef]
- Sillen, A. Elemental and isotopic analyses of mammalian fauna from Southern Africa and their implications for paleodietary research. Am. J. Phys. Anthr. 1988, 76, 49–60. [Google Scholar] [CrossRef]
- Terrill, D.F.; Jarochowska, E.; Henderson, C.M.; Shirley, B.; Bremer, O. Sr/Ca and Ba/Ca ratios support trophic partitioning within a Silurian conodont community from Gotland, Sweden. Paleobiology 2022, 48, 601–621. [Google Scholar] [CrossRef]
- Mihlbachler, M.C.; Beatty, B.L.; Caldera-Siu, A.; Chan, D.; Lee, R. Error rates and observer bias in dental microwear analysis using light microscopy. Palaeontol. Electron. 2012, 15, 1–22. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; Version 3.6.3; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: http://www.r-project.org/ (accessed on 18 October 2024).
- Shirley, B.; Jarochowska, E. Chemical characterisation is rough: The impact of topography and measurement parameters on energy-dispersive X-ray spectroscopy in biominerals. Facies 2022, 68, 7. [Google Scholar] [CrossRef]
- Farlow, J.O.; Brinkman, D.L.; Abler, W.L.; Currie, P.J. Size, shape, and serration density of theropod dinosaur lateral teeth. Mod. Geol. 1991, 16, 161–198. [Google Scholar]
- Peek, S.; Clementz, M.T. Sr/Ca and Ba/Ca variations in environmental and biological sources: A survey of marine and terrestrial systems. Geochim. Cosmochim. Acta 2012, 95, 36–52. [Google Scholar] [CrossRef]
- Kiernan, C.R. Stratigraphic distribution and habitat segregation of mosasaurs in the Upper Cretaceous of western and central Alabama, with an historical review of alabama mosasaur discoveries. J. Vertebr. Paléontol. 2002, 22, 91–103. [Google Scholar] [CrossRef]
- Everhart, M.J. Plesiosaurs as the food of mosasaurs; new data on the stomach contents of a Tylosaurus proriger (Squamata; Mosasauridae) from the Niobrara Formation of western Kansas. Mosasaur 2004, 7, 41–46. [Google Scholar]
- McHenry, C.R.; Cook, A.G.; Wroe, S. Bottom-Feeding Plesiosaurs. Science 2005, 310, 75. [Google Scholar] [CrossRef]
- Sørensen, A.M.; Surlyk, F.; Lindgren, J. Food resources and habitat selection of a diverse vertebrate fauna from the upper lower Campanian of the Kristianstad Basin, southern Sweden. Cretac. Res. 2013, 42, 85–92. [Google Scholar] [CrossRef]
- Cicimurri, D.J.; Everhart, M.J. An Elasmosaur with Stomach Contents and Gastroliths from the Pierre Shale (Late Cretaceous) of Kansas. Trans. Kans. Acad. Sci. 2001, 104, 129–143. [Google Scholar] [CrossRef]
- White, J.M.; Barron, A.; McCurry, M.R.; Denham, T. Investigating gut contents of the leptocleidian plesiosaur Umoonasaurus demoscyllus using micro-CT imaging. Alcheringa Australas. J. Palaeontol. 2023, 47, 206–210. [Google Scholar] [CrossRef]
- Cuny, G.; Suteethorn, V.; Kamha, S.; Buffetaut, E. Hybodont sharks from the lower Cretaceous Khok Kruat Formation of Thailand, and hybodont diversity during the Early Cretaceous. Geol. Soc. Lond. Spéc. Publ. 2008, 295, 93–107. [Google Scholar] [CrossRef]
- Vullo, R. Direct evidence of hybodont shark predation on Late Jurassic ammonites. Naturwissenschaften 2011, 98, 545–549. [Google Scholar] [CrossRef]
- Wright, J.K.; Bassett-Butt, L.; Collinson, M. Fatally bitten ammonites from the Lower Calcareous Grit Formation (Upper Jurassic) of NE Yorkshire, UK. Proc. Yorks. Geol. Soc. 2014, 60, 1–8. [Google Scholar] [CrossRef]
- Cumbaa, S.L.; Underwood, C.J.; Schröder-Adams, C.J.; Arratia, G. Paleoenvironments and paleoecology of the vertebrate fauna from a Late Cretaceous marine bonebed, Canada. Mesoz. Fishes 2013, 5, 509–524. [Google Scholar]
- Massare, J.A. Tooth morphology and prey preference of Mesozoic marine reptiles. J. Vertebr. Paléontol. 1987, 7, 121–137. [Google Scholar] [CrossRef]
- Young, M.T.; Brusatte, S.L.; Beatty, B.L.; De Andrade, M.B.; Desojo, J.B. Tooth-On-Tooth Interlocking Occlusion Suggests Macrophagy in the Mesozoic Marine Crocodylomorph Dakosaurus. Anat. Rec. 2012, 295, 1147–1158. [Google Scholar] [CrossRef]
- Bestwick, J.; Unwin, D.M.; Henderson, D.M.; Purnell, M.A. Dental microwear texture analysis along reptile tooth rows: Complex variation with non-dietary variables. R. Soc. Open Sci. 2021, 8, 201754. [Google Scholar] [CrossRef]
- Holmes, R.B. Evaluation of the photosensory characteristics of the lateral and pineal eyes of Plioplatecarpus (Squamata, Mosasauridae) based on an exceptionally preserved specimen from the Bearpaw Shale (Campanian, Upper Cretaceous) of southern Alberta. J. Vertebr. Paléontol. 2024, 43, e2335174. [Google Scholar] [CrossRef]
- Polcyn, M.J.; Bardet, N.; Albright, L.B.; Titus, A. A new lower Turonian mosasaurid from the Western Interior Seaway and the antiquity of the unique basicranial circulation pattern in Plioplatecarpinae. Cretac. Res. 2023, 151, 105621. [Google Scholar] [CrossRef]
- Kauffman, E.G. Mosasaur Predation on Upper Cretaceous Nautiloids and Ammonites from the United States Pacific Coast. Palaios 2004, 19, 96–100. [Google Scholar] [CrossRef]
- Klompmaker, A.A.; Waljaard, N.A.; Fraaije, R.H. Ventral bite marks in Mesozoic ammonoids. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2009, 280, 245–257. [Google Scholar] [CrossRef]
- Tsujita, C.J.; Westermann, G.E. Were limpets or mosasaurs responsible for the perforations in the ammonite Placenticeras? Palaeogeogr. Palaeoclimatol. Palaeoecol. 2001, 169, 245–270. [Google Scholar] [CrossRef]
- Schulp, A.S.; Vonhof, H.B.; van der Lubbe, J.H.J.L.; Janssen, R.; van Baal, R.R. On diving and diet: Resource partitioning in type-Maastrichtian mosasaurs. Neth. J. Geosci. 2013, 92, 165–170. [Google Scholar] [CrossRef]
- Cortés, D.; Larsson, H.C. Top of the food chains: An ecological network of the marine Paja Formation biota from the Early Cretaceous of Colombia reveals the highest trophic levels ever estimated. Zool. J. Linn. Soc. 2024, 202, zlad092. [Google Scholar] [CrossRef]



| Taxon | Mosasaurus | Prognathodon | Tylosaurus | Plioplatecarpus | Elasmosaur | Sawfish | Shark |
|---|---|---|---|---|---|---|---|
| Type | Skull and teeth | Skull and teeth | Teeth | Teeth | Teeth | Teeth | Teeth |
| Locality | Lethbridge | Lethbridge | Manyberries | Lethbridge | DPP | Iddlesleigh | Iddlesleigh |
| Formation | Bearpaw | Bearpaw | Bearpaw | Bearpaw | Bearpaw | Bearpaw | Bearpaw |
| Microwear | 10 | 5 | 2 | 3 | - | - | - |
| EDX | 16 | 16 | 9 | 7 | 5 | 4 | 15 |
| Bending Strength | 30 | 28 | - | 5 | - | - | - |
| Taxon | Mosasaurus | Prognathodon | Tylosaurus | Plioplatecarpus |
|---|---|---|---|---|
| Sr/Ca range | 0.2–0.73 | 0.11–0.57 | 0.31–0.77 | 0.17–0.71 |
| Ba/Ca range | 0.06–0.33 | 0.03–0.33 | 0.13–0.21 | 0.03–0.11 |
| Dominant microwear | Fine scratches | Pits | Gouges | Fine scratches |
| Inferred prey | Large range of vertebrate prey items Large range of invertebrate prey items | Harder vertebrate prey items (turtles and large fish) Harder invertebrate prey items (shellfish and ammonites) | Fish and larger vertebrates | Softer prey items (squid and fish) Harder prey items (large fish and ammonites) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holwerda, F.M.; Mitchell, M.T.; van de Kerk, M.; Schulp, A.S. Mosasaur Feeding Ecology from the Campanian Bearpaw Formation, Alberta, Canada: A Preliminary Multi-Proxy Approach. Diversity 2025, 17, 205. https://doi.org/10.3390/d17030205
Holwerda FM, Mitchell MT, van de Kerk M, Schulp AS. Mosasaur Feeding Ecology from the Campanian Bearpaw Formation, Alberta, Canada: A Preliminary Multi-Proxy Approach. Diversity. 2025; 17(3):205. https://doi.org/10.3390/d17030205
Chicago/Turabian StyleHolwerda, Femke M., Mark T. Mitchell, Madelon van de Kerk, and Anne S. Schulp. 2025. "Mosasaur Feeding Ecology from the Campanian Bearpaw Formation, Alberta, Canada: A Preliminary Multi-Proxy Approach" Diversity 17, no. 3: 205. https://doi.org/10.3390/d17030205
APA StyleHolwerda, F. M., Mitchell, M. T., van de Kerk, M., & Schulp, A. S. (2025). Mosasaur Feeding Ecology from the Campanian Bearpaw Formation, Alberta, Canada: A Preliminary Multi-Proxy Approach. Diversity, 17(3), 205. https://doi.org/10.3390/d17030205







