The Evolution of Mosasaurid Foraging Behavior Through the Lens of Stable Carbon Isotopes
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Selection
2.2. Sample Pretreatment
2.3. Sample Enamel Removal
2.4. Sample Analysis
2.5. Compiled Data
2.6. Taxonomic Identifications
2.7. Institutional Abbreviations
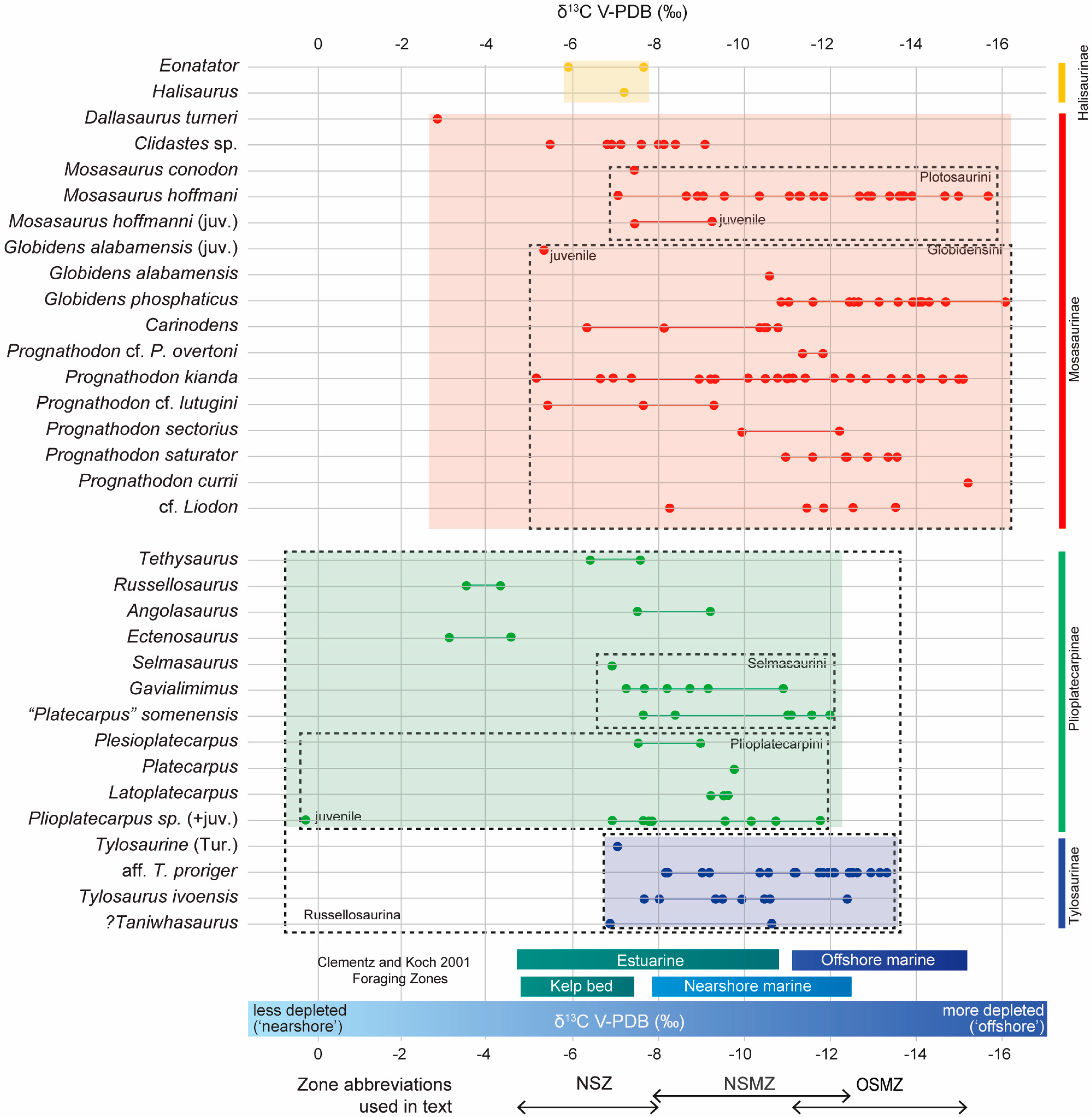
3. Results
3.1. δ13C by Taxon
3.1.1. Halisaurinae
3.1.2. Early-Diverging Plioplatecarpinae
3.1.3. Plioplatecarpinae: Plioplatecarpini
3.1.4. Plioplatecarpinae: Selmasaurini
3.1.5. Tylosaurinae
3.1.6. Early-Diverging Mosasaurinae
3.1.7. Mosasaurinae: Plotosaurini
3.1.8. Mosasaurinae: Globidensini
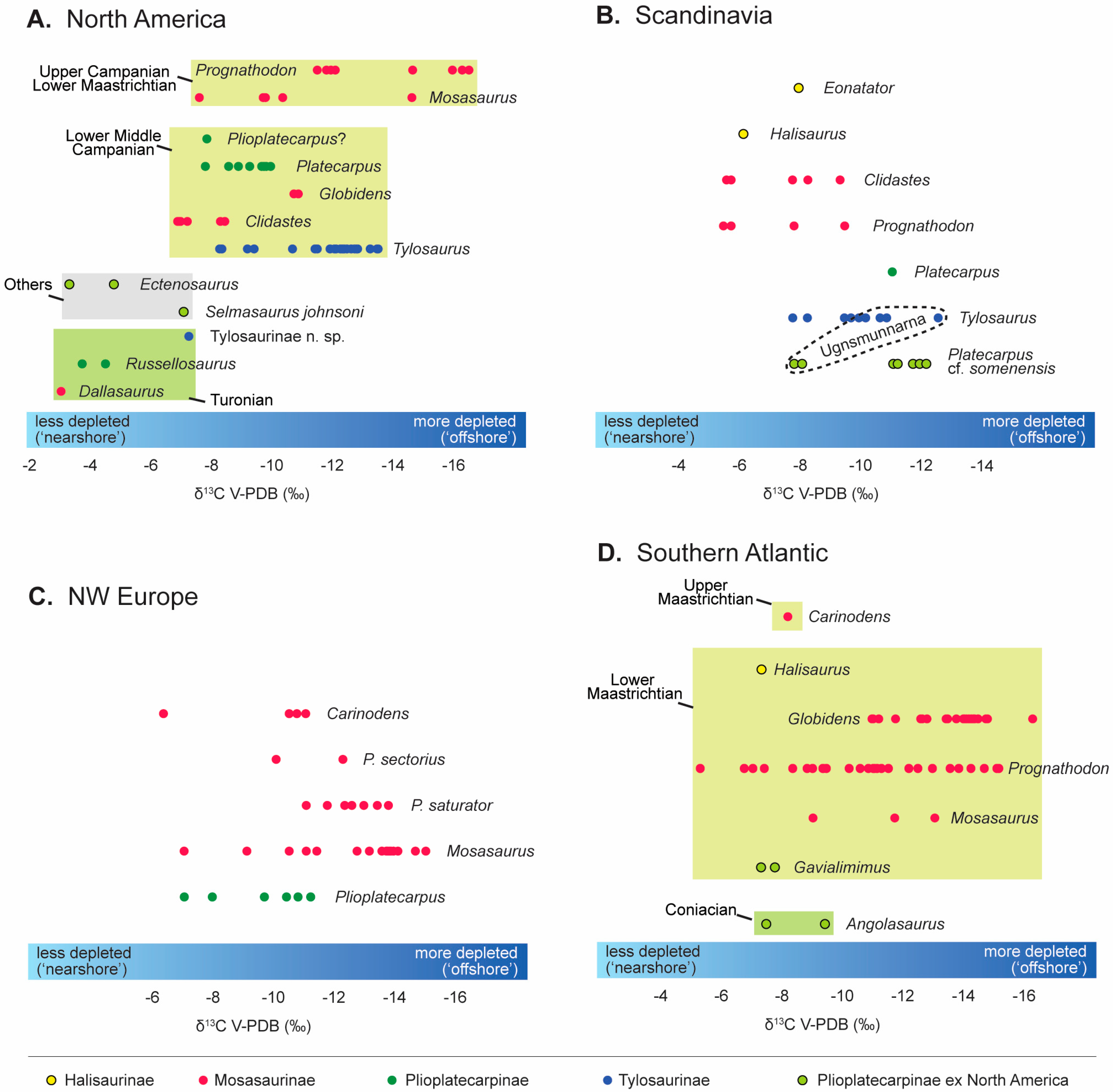
3.2. δ13C Through Time
3.2.1. Early Radiations
3.2.2. Coniacian–Santonian
3.2.3. Campanian
3.2.4. Maastrichtian
4. Discussion
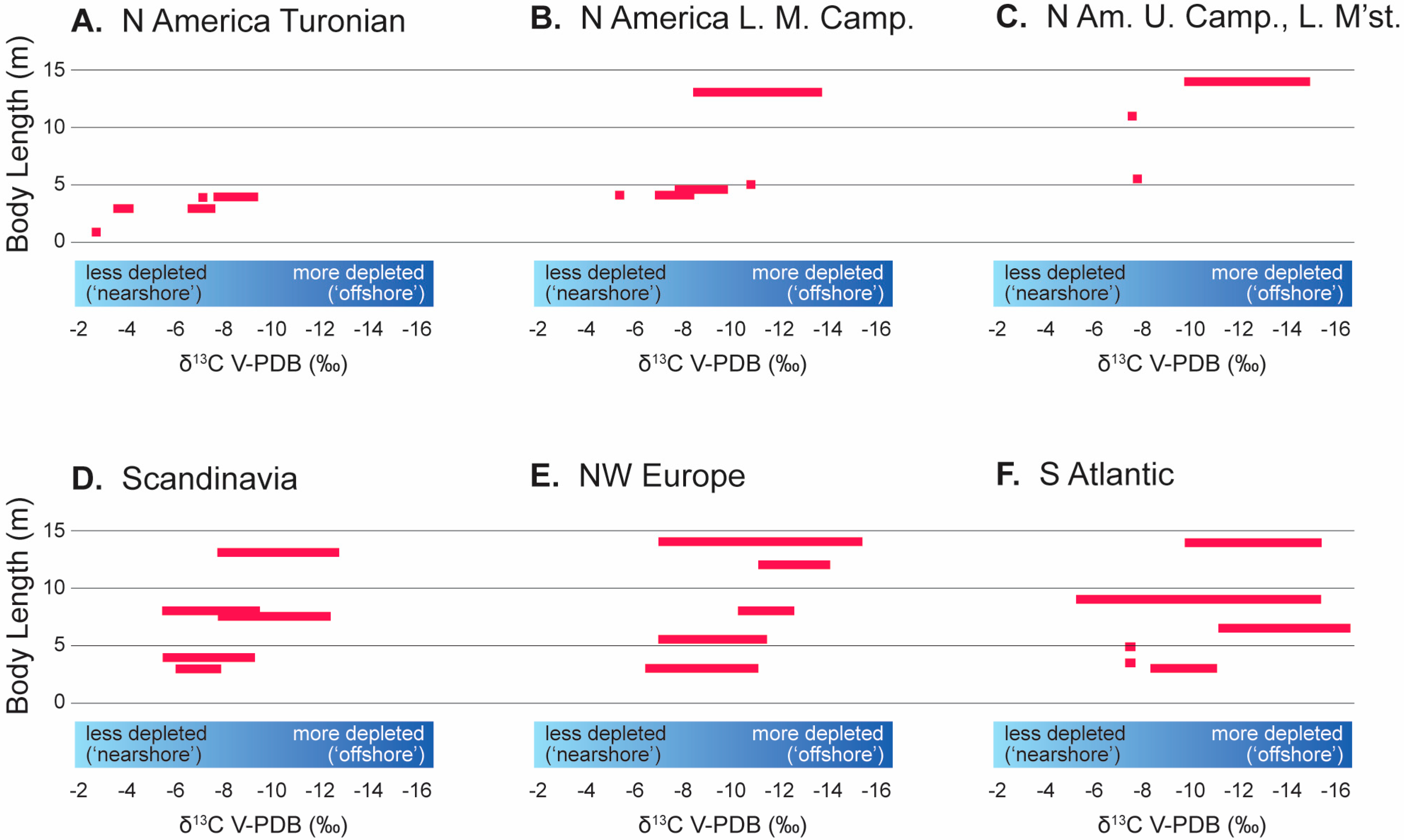
4.1. Trophic Level and Foraging Habit
4.2. Physiological Factors
4.3. Other Factors
4.4. Evolution of Foraging Behavior in Mosasaurs
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Specimen # (Sample #) | Taxon | Region | δ13C | δ18O | wt.% Carb * | Data Src. |
|---|---|---|---|---|---|---|
| Asen1 (1) | Eonatator sp. | Scandinavia | −7.7 | −3.7 | 7.8 | * |
| Asen1 (2) | Eonatator sp. | Scandinavia | −5.9 | −2.8 | 7.1 | * |
| Asen2 (1) | Clidastes cf. C. propython | Scandinavia | −9.1 | −3.3 | 8.2 | * |
| Asen3 (1) | Clidastes cf. C. propython | Scandinavia | −5.4 | −3.1 | 6.4 | * |
| Asen3 (2) | Clidastes cf. C. propython | Scandinavia | −5.4 | −2.1 | 4.2 | * |
| Asen3 (3) | Clidastes cf. C. propython | Scandinavia | −7.5 | −2.3 | 4.5 | * |
| Asen4 (1) | Clidastes cf. C. propython | Scandinavia | −8.0 | - | 7.8 | * |
| SMU76391 (1) | Clidastes sp. | North Texas | −7.1 | −3.0 | 5.4 | [36] |
| SMU76391 (2) | Clidastes sp. | North Texas | −8.1 | −3.5 | 4.9 | [36] |
| SMU76404 (1) | Clidastes sp. | North Texas | −8.3 | −3.1 | 3.2 | [36] |
| SMU76281 (1) | Clidastes sp. | North Texas | −6.8 | −3.1 | 5.1 | [36] |
| SMU72184 (1) | Clidastes cf. C. propython | North Texas | −6.7 | −4.3 | 4.6 | * |
| SMU62504 (1) | Clidastes sp. | North Texas | −6.7 | −3.9 | 4.4 | * |
| TMM43209-1 (1) | Dallasaurus turneri | North Texas | −2.8 | −4.4 | 4.8 | * |
| MGUAN PA149 (1) | Globidens phosphaticus | West Africa | −13.8 | −2.5 | 4.2 | [36] |
| MGUAN PA149 (2) | Globidens phosphaticus | West Africa | −14.6 | −2.3 | 4.4 | [36] |
| MGUAN PA149 (3) | Globidens phosphaticus | West Africa | −14.0 | 4.5 | [36] | |
| MGUAN PA149 (4) | Globidens phosphaticus | West Africa | −14.0 | 4.8 | [36] | |
| MGUAN PA149 (5) | Globidens phosphaticus | West Africa | −13.9 | 3.2 | [36] | |
| MGUAN PA149 (6) | Globidens phosphaticus | West Africa | −14.1 | 5.0 | [36] | |
| MGUAN PA149 (7) | Globidens phosphaticus | West Africa | −14.0 | 5.9 | [36] | |
| MGUAN PA149 (8) | Globidens phosphaticus | West Africa | −14.1 | 6.0 | [36] | |
| MGUAN PA149 (9) | Globidens phosphaticus | West Africa | −14.2 | 4.0 | [36] | |
| MGUAN PA149 (10) | Globidens phosphaticus | West Africa | −14.1 | 5.8 | [36] | |
| MGUAN PA149 (11) | Globidens phosphaticus | West Africa | −14.0 | 3.0 | [36] | |
| MGUAN PA149 (12) | Globidens phosphaticus | West Africa | −14.0 | 4.4 | [36] | |
| MGUAN PA149 (13) | Globidens phosphaticus | West Africa | −13.6 | 5.0 | [36] | |
| MGUAN PA149 (14) | Globidens phosphaticus | West Africa | −14.1 | 5.7 | [36] | |
| MGUAN PA149 (15) | Globidens phosphaticus | West Africa | −14.1 | 2.8 | [36] | |
| MGUAN PA149 (16) | Globidens phosphaticus | West Africa | −13.9 | 4.4 | [36] | |
| MGUAN PA149 (17) | Globidens phosphaticus | West Africa | −14.1 | 4.0 | [36] | |
| MGUAN PA149 (18) | Globidens phosphaticus | West Africa | −14.2 | 3.9 | [36] | |
| MGUAN PA149 (19) | Globidens phosphaticus | West Africa | −13.9 | 3.3 | [36] | |
| MGUAN PA149 (20) | Globidens phosphaticus | West Africa | −13.9 | 5.0 | [36] | |
| SMU76241 (1) | Globidens alabamaensis (juv.) | North Texas | −5.2 | −2.6 | 4.1 | [36] |
| SMU76241 (2) | Globidens alabamaensis (juv.) | North Texas | −5.3 | −2.5 | 3.4 | [36] |
| SMU76241 (3) | Globidens alabamaensis (juv.) | North Texas | −5.4 | −2.6 | 3.7 | [36] |
| SMU76280 (1) | Globidens alabamaensis (adult) | North Texas | −10.5 | −3.4 | 3.6 | [36] |
| SMU76280 (2) | Globidens alabamaensis (adult) | North Texas | −10.5 | −2.2 | 4.3 | [36] |
| SMU76348 (1) | Mosasaurus cf. M. conodon | North Texas | −7.4 | −1.3 | 5.0 | * |
| SMU76242 (1) | Mosasaurus hoffmanni | North Texas | −9.5 | −5.3 | 1.0 | [36] |
| SMU62079 (1) | Prognathodon sp. | North Texas | −10.1 | −3.2 | 2.9 | * |
| SMU76393 (1) | Prognathodon kianda | South Atlantic | −14.5 | −2.1 | 4.3 | [36] |
| SMU76393 (2) | Prognathodon kianda | South Atlantic | −14.9 | −2.1 | 4.2 | [36] |
| SMU76393 (3) | Prognathodon kianda | South Atlantic | −15.0 | −2.0 | 5.0 | [36] |
| HUJ OR 100 (1) | Prognathodon currii | Israel | −15.1 | −2.4 | 3.5 | * |
| SMU76504 (1) | Prognathodon cf. P. overtoni | North Texas | −11.3 | −4.7 | 7.5 | * |
| SMU76504 (2) | Prognathodon cf. P. overtoni | North Texas | −11.7 | −4.5 | 7.5 | * |
| Asen5 (1) | ?Prognathodon cf. P. lutugini | Scandinavia | −5.4 | −2.9 | 9.2 | * |
| Asen5 (2) | ?Prognathodon cf. P. lutugini | Scandinavia | −5.4 | −2.9 | 8.6 | * |
| Asen5 (3) | ?Prognathodon cf. P. lutugini | Scandinavia | −7.6 | −2.6 | 7.9 | * |
| Asen5 (4) | ?Prognathodon cf. P. lutugini | Scandinavia | −9.3 | −2.4 | 7.6 | * |
| SMU76503 (1) | ?Prognathodon sp. | North Texas | −11.6 | −2.3 | 5.4 | * |
| SMU76503 (2) | ?Prognathodon sp. | North Texas | −14.4 | −2.2 | 8.1 | * |
| SMU76503 (3) | ?Prognathodon sp. | North Texas | −11.8 | −3.4 | 9.1 | * |
| SMU76503 (4) | ?Prognathodon sp. | North Texas | −16.0 | −2.6 | 8.4 | * |
| SMU76503 (5) | ?Prognathodon sp. | North Texas | −16.2 | −2.7 | 6.6 | * |
| SMU76503 (6) | ?Prognathodon sp. | North Texas | −15.7 | −2.6 | 8.4 | * |
| SMU76334 (1) | Tethysaurusnopscai | North Africa | −7.5 | −4.8 | 3.0 | * |
| SMU76334 (2) | Tethysaurusnopscai | North Africa | −6.4 | −3.9 | 2.0 | * |
| MGUAN PA1 (1) | Angolasaurus bocagei | West Africa | −7.4 | −0.6 | 3.7 | * |
| MGUAN PA65 (1) | Angolasaurus bocagei | West Africa | −9.2 | −2.8 | 4.5 | * |
| SMU76350 (1) | Ectenosaurus sp. | West Texas | −3.1 | −4.4 | 4.0 | * |
| SMU76350 (2) | Ectenosaurus sp. | West Texas | −4.6 | −3.0 | 4.0 | * |
| FHSM VP16582 (1) | Latoplatecarpus sp. | Western Kansas | −7.6 | −5.3 | 4.7 | * |
| SMU76501 (1) | Plesioplatecarpus sp. | Western Kansas | −8.8 | −5.8 | 4.8 | * |
| Asen6 (1) | Platecarpus sp. | Scandinavia | −10.9 | 7.4 | * | |
| Asen7 (1) | “Platecarpus” cf. P. somenensis | Scandinavia | −12.0 | −4.6 | 8.2 | * |
| Asen7 (2) | “Platecarpus” cf. P. somenensis | Scandinavia | −11.1 | −4.3 | 8.3 | * |
| Asen7 (3) | “Platecarpus” cf. P. somenensis | Scandinavia | −11.0 | −2.9 | 8.4 | * |
| Asen7 (4) | “Platecarpus” cf. P. somenensis | Scandinavia | −11.6 | −4.1 | 7.8 | * |
| Ugn0 (1) | “Platecarpus” cf. P. somenensis | Scandinavia | −7.8 | −4.2 | 8.4 | * |
| Ugn0 (2) | “Platecarpus” cf. P. somenensis | Scandinavia | −7.6 | −4.1 | 8.4 | * |
| SMU61617 (1) | Latoplatecarpus sp. | North Texas | −9.6 | −4.7 | 6.1 | * |
| SMU61617 (2) | Latoplatecarpus sp. | North Texas | −9.6 | −4.4 | 4.8 | * |
| SMU61617 (3) | Latoplatecarpus sp. | North Texas | −9.6 | −3.2 | 5.5 | * |
| SMU61617 (4) | Latoplatecarpus sp. | North Texas | −9.1 | −2.8 | 5.4 | * |
| SMU61617 (5) | Latoplatecarpus sp. | North Texas | −9.1 | −4.1 | 5.0 | * |
| SMU61617 (6) | Latoplatecarpus sp. | North Texas | −9.5 | −5.1 | 4.9 | * |
| SMU61617 (7) | Latoplatecarpus sp. | North Texas | −9.1 | −3.9 | 4.5 | * |
| SMU61617 (8) | Latoplatecarpus sp. | North Texas | −9.5 | −3.6 | 4.9 | * |
| SMU76381 (1) | Indet. sp. | North Texas | −8.4 | −4.2 | 3.7 | * |
| SMU76497 (1) | Plesioplatecarpus sp. | Texas | −7.5 | −3.4 | 4.6 | * |
| SMU76393 (1) | Plioplatecarpus sp. | Western Arkansas | −7.7 | −2.8 | 5.4 | * |
| SMU76498 (1) | ?Plioplatecarpus sp. | North Texas | −7.6 | −5.9 | 4.1 | * |
| SMU73056 (1) | Russellosaurus coheni | North Texas | −4.3 | −5.8 | 4.5 | * |
| SMU73056 (2) | Russellosaurus coheni | North Texas | −3.5 | −4.5 | 5.2 | * |
| FHSM VP 13910 (1) | Selmasaurus johnsoni | Western Kansas | −6.9 | −5.9 | 4.1 | * |
| Asen8 (1) | Tylosaurus ivoensis | Scandinavia | −9.3 | −3.6 | 6.1 | * |
| Asen8 (2) | Tylosaurus ivoensis | Scandinavia | −7.6 | −3.7 | 8.9 | * |
| Kris1 (1) | Tylosaurus ivoensis | Scandinavia | −9.5 | −2.5 | 5.9 | * |
| Kris2 (1) | Tylosaurus ivoensis | Scandinavia | −8.0 | −2.6 | 6.3 | * |
| Ugn1 (1) | Tylosaurus ivoensis | Scandinavia | −12.4 | −5.8 | 5.8 | * |
| Ugn1 (2) | Tylosaurus ivoensis | Scandinavia | −10.4 | −3.5 | 5.8 | * |
| Ugn1 (3) | Tylosaurus ivoensis | Scandinavia | −9.5 | −3.3 | 5.7 | * |
| Ugn1 (4) | Tylosaurus ivoensis | Scandinavia | −9.9 | −3.3 | 5.7 | * |
| Ugn1 (5) | Tylosaurus ivoensis | Scandinavia | −10.6 | −3.8 | 4.4 | * |
| SMU76502 (1) | Tylosaurus sp. | North Texas | −8.2 | −2.8 | 2.9 | * |
| SMU61667 (1) | Tylosaurus sp. | North Texas | −8.1 | −3.7 | 6.9 | * |
| SMU61667 (2) | Tylosaurus sp. | North Texas | −9.0 | −3.5 | 4.9 | * |
| SMU61667 (3) | Tylosaurus sp. | North Texas | −10.5 | −3.7 | 5.3 | * |
| SMU61667 (4) | Tylosaurus sp. | North Texas | −11.2 | −3.3 | 5.8 | * |
| SMU61667 (5) | Tylosaurus sp. | North Texas | −11.7 | −3.4 | 5.7 | * |
| SMU61667 (6) | Tylosaurus sp. | North Texas | −11.9 | −4.5 | 6.5 | * |
| SMU76500 (1) | Tylosaurus sp. | North Texas | −13.3 | −6.7 | 3.8 | * |
| SMU61113 (1) | Tylosaurus sp. | North Texas | −11.8 | −3.6 | 5.1 | * |
| SMU61113 (2) | Tylosaurus sp. | North Texas | −12.4 | −3.7 | 6.2 | * |
| SMU61113 (3) | Tylosaurus sp. | North Texas | −13.2 | −4.2 | 5.7 | * |
| SMU61113 (4) | Tylosaurus sp. | North Texas | −12.5 | −4.2 | 6.3 | * |
| SMU61113 (5) | Tylosaurus sp. | North Texas | −12.1 | −3.9 | 5.5 | * |
| SMU61113 (6) | Tylosaurus sp. | North Texas | −12.1 | −4.9 | 6.3 | * |
| SMU61113 (7) | Tylosaurus sp. | North Texas | −12.1 | −3.5 | 4.4 | * |
| SMU61113 (8) | Tylosaurus sp. | North Texas | −12.6 | −3.1 | 5.1 | * |
| SMU61113 (9) | Tylosaurus sp. | North Texas | −12.1 | −2.9 | 4.9 | * |
| SMU76392 (1) | Tylosaurus sp. | North Texas | −13.0 | −5.8 | 5.4 | * |
| SMU76392 (2) | Tylosaurus sp. | North Texas | −12.6 | −4.5 | 4.1 | * |
| SMU76392 (3) | Tylosaurus sp. | North Texas | −12.5 | −6.1 | 5.6 | * |
| SMU76392 (4) | Tylosaurus sp. | North Texas | −12.5 | −4.8 | 4.3 | * |
| SMU76392 (5) | Tylosaurus sp. | North Texas | −12.2 | −4.2 | 4.5 | * |
| LO7786 (1) | Tylosaurus sp. | North Texas | −7.0 | −4.8 | 6.7 | * |
| SMU75374 (1) | Tylosaurus sp. | North Texas | −11.7 | −6.2 | 6.3 | * |
| SMU75374 (2) | Tylosaurus sp. | North Texas | −11.2 | −6.3 | 5.9 | * |
| SMU75374 (3) | Tylosaurus sp. | North Texas | −11.8 | −6.9 | 6.8 | * |
| SMU75374 (4) | Tylosaurus sp. | North Texas | −12.0 | −6.2 | 5.3 | * |
| SMU76382 (1) | Tylosaurus sp. | North Texas | −9.2 | −4.2 | 2. | * |
| SMU75586 (1) | Tylosaurus sp. | North Texas | −10.3 | 8.2 | * | |
| NHMM 1980.6 (1) | Carinodensbelgicus | Northern Europe | −10.8 | −4.0 | [37] | |
| NHMM 1980.7 (1) | Carinodensbelgicus | Northern Europe | −10.5 | −2.3 | [37] | |
| NHMM 1980.7 (2) | Carinodensbelgicus | Northern Europe | –10.5 | −2.3 | [37] | |
| NHMM 7354 (1) | Carinodensbelgicus | Northern Europe | –10.3 | −3.6 | [37] | |
| NHMM 1984.88.1A (1) | Plioplatecarpusmarshi | Northern Europe | −10.7 | −4.5 | [37] | |
| NHMM 1984.88.1A (2) | Plioplatecarpusmarshi | Northern Europe | –11.1 | −4.5 | [37] | |
| NHMM 1984.88.1B | Plioplatecarpusmarshi | Northern Europe | –10.3 | [37] | ||
| NHMM 1995 031 (1) | Prognathodon sectorius | Northern Europe | −9.9 | −3.4 | [37] | |
| NHMM LV 150 (1) | Prognathodon sectorius | Northern Europe | −12.2 | −3.0 | [37] | |
| NHMM 1998 141 (1) | Prognathodonsaturator | Northern Europe | −12.8 | [37] | ||
| NHMM 1998 141 (2) | Prognathodonsaturator | Northern Europe | –13.3 | −3.4 | [37] | |
| NHMM 1998 141 (3) | Prognathodonsaturator | Northern Europe | –13.6 | −3.0 | [37] | |
| NHMM 1998 141 (4) | Prognathodonsaturator | Northern Europe | –12.3 | −2.8 | [37] | |
| NHMM 1998 141 (5) | Prognathodonsaturator | Northern Europe | –12.3 | −3.1 | [37] | |
| NHMM 1317.02 (1) | Mosasaurushoffmanni | Northern Europe | −9.3 | −3.2 | [37] | |
| NHMM MK 591 (1) | Mosasaurushoffmanni | Northern Europe | −7.1 | [37] | ||
| NHMM 4560 (1) | Mosasaurushoffmanni | Northern Europe | −11.3 | −3.8 | [37] | |
| NHMM 1446 (1) | Mosasaurushoffmanni | Northern Europe | −13.7 | [37] | ||
| NHMM 1446 (2) | Mosasaurushoffmanni | Northern Europe | –13.0 | −4.4 | [37] | |
| NHMM 1446 (3) | Mosasaurushoffmanni | Northern Europe | –12.6 | −4.0 | [37] | |
| NHMM 1446 (4) | Mosasaurushoffmanni | Northern Europe | –13.8 | −6.2 | [37] | |
| NHMM 1446 (5) | Mosasaurushoffmanni | Northern Europe | –14.0 | −4.4 | [37] | |
| NHMM 1446 (6) | Mosasaurushoffmanni | Northern Europe | –13.4 | −5.3 | [37] | |
| NHMM 1446 (7) | Mosasaurushoffmanni | Northern Europe | –13.6 | −4.0 | [37] | |
| NHMM 1446 (8) | Mosasaurushoffmanni | Northern Europe | –14.9 | −3.9 | [37] | |
| NHMM 1446 (9) | Mosasaurushoffmanni | Northern Europe | –14.5 | −3.9 | [37] | |
| NHMM 1446 (10) | Mosasaurushoffmanni | Northern Europe | –13.7 | −3.8 | [37] | |
| NHMM 1446 (11) | Mosasaurushoffmanni | Northern Europe | –13.6 | −6.2 | [37] | |
| MGUAN PA 171 (1) | Carinodens sp. | West Africa | −8.1 | −1.5 | [39] | |
| MGUAN PA 04 (1) | Globidens phosphaticus | West Africa | −14.6 | −2.1 | [39] | |
| MGUAN PA 05 (1) | Globidens phosphaticus | West Africa | −13.2 | −1.7 | [39] | |
| MGUAN PA 29 (1) | Globidens phosphaticus | West Africa | −14.3 | −1.2 | [39] | |
| MGUAN PA 30 (1) | Globidens phosphaticus | West Africa | −10.8 | −1.3 | [39] | |
| MGUAN PA 301 (1) | Globidens phosphaticus | West Africa | −12.6 | −1.1 | [39] | |
| MGUAN PA 307 (1) | Globidens phosphaticus | West Africa | −11.6 | −1.7 | [39] | |
| MGUAN PA 31 (1) | Globidens phosphaticus | West Africa | −12.4 | −1.1 | [39] | |
| MGUAN PA 313 (1) | Globidens phosphaticus | West Africa | −12.5 | −1.5 | [39] | |
| MGUAN PA 33 (1) | Globidens phosphaticus | West Africa | −11.0 | −2.4 | [39] | |
| MGUAN PA 500 (1) | Globidens phosphaticus | West Africa | −16.1 | −2.1 | [39] | |
| MGUAN PA 61 (1) | Globidens phosphaticus | West Africa | −14.3 | −1.6 | [39] | |
| MGUAN PA 314 (1) | Halisaurus sp. | West Africa | −7.2 | −2.2 | [39] | |
| MGUAN PA 309 (1) | Mosasaurus sp. | West Africa | −11.6 | −1.4 | [39] | |
| MGUAN PA 309 (2) | Mosasaurus sp. | West Africa | −8.9 | −1.4 | [39] | |
| MGUAN PA 44 (1) | Mosasaurus sp. | West Africa | −11.6 | −2.6 | [39] | |
| MGUAN PA 46 (1) | Mosasaurus sp. | West Africa | −12.9 | −1.9 | [39] | |
| MGUAN PA 177 (1) | Gavialimimus sp. | West Africa | −7.2 | −2.1 | [39] | |
| MGUAN PA 312 (1) | Gavialimimus sp. | West Africa | −7.2 | −2.1 | [39] | |
| MGUAN PA 312 (2) | Gavialimimus sp. | West Africa | −7.6 | −2.4 | [39] | |
| MGUAN PA 525 (1) | Gavialimimus sp. | West Africa | −9.2 | −2.6 | [39] | |
| MGUAN PA 525 (2) | Gavialimimus sp. | West Africa | −9.2 | −3.3 | [39] | |
| MGUAN PA 321 (1) | Gavialimimus sp. | West Africa | −8.2 | −2.3 | [39] | |
| MGUAN PA 38 (1) | Gavialimimus sp. | West Africa | −10.9 | −1.5 | [39] | |
| MGUAN PA 55 (1) | Gavialimimus sp. | West Africa | −8.7 | −3.6 | [39] | |
| MGUAN PA528 (1) | Prognathodon kianda | West Africa | −8.9 | −1.4 | [39] | |
| MGUAN PA 526 (1) | Prognathodon kianda | West Africa | −9.3 | −1.9 | [39] | |
| MGUAN PA 527 (1) | Prognathodon kianda | West Africa | −9.2 | −2.3 | [39] | |
| MGUAN PA 28 (2) | Prognathodon kianda | West Africa | −11.1 | −1.6 | [39] | |
| MGUAN PA 28 (1) | Prognathodon kianda | West Africa | −11.4 | −2.3 | [39] | |
| MGUAN PA 304 (1) | Prognathodon kianda | West Africa | −7.3 | −1.5 | [39] | |
| MGUAN PA 304 (2) | Prognathodon kianda | West Africa | −6.9 | −1.6 | [39] | |
| MGUAN PA 306 (1) | Prognathodon kianda | West Africa | −12.1 | −2.4 | [39] | |
| MGUAN PA 315 (1) | Prognathodon kianda | West Africa | −10.1 | −1.8 | [39] | |
| MGUAN PA 318 (1) | Prognathodon kianda | West Africa | −12.4 | −2.4 | [39] | |
| MGUAN PA 35 (1) | Prognathodon kianda | West Africa | −10.7 | −1.5 | [39] | |
| MGUAN PA 40 (1) | Prognathodon kianda | West Africa | −11.0 | −1.7 | [39] | |
| MGUAN PA 41 (1) | Prognathodon kianda | West Africa | −5.2 | −1.9 | [39] | |
| MGUAN PA 45 (1) | Prognathodon kianda | West Africa | −10.4 | −3.3 | [39] | |
| MGUAN PA 47 (1) | Prognathodon kianda | West Africa | −13.7 | −2.4 | [39] | |
| MGUAN PA 48 (1) | Prognathodon kianda | West Africa | −10.9 | −1.6 | [39] | |
| MGUAN PA 50 (1) | Prognathodon kianda | West Africa | −14.1 | −3.7 | [39] | |
| MGUAN PA 50 (2) | Prognathodon kianda | West Africa | −13.4 | −1.9 | [39] | |
| MGUAN PA 53 (1) | Prognathodon kianda | West Africa | −12.8 | −3.5 | [39] | |
| MGUAN PA 56 (1) | Prognathodon kianda | West Africa | −6.6 | −2.0 | [39] | |
| NHMD 157504 (1) | Carinodensminalmamar | Scandinavia | −6.3 | −3.1 | [40] | |
| NHMD 227349 (1) | Mosasaurus sp. | Scandinavia | −11.1 | −2.5 | [40] | |
| OESM 8783 (1) | Mosasaurus sp. | Scandinavia | −8.6 | −2.6 | [40] | |
| NHMM 1984089-1 (1) | Mosasaurushoffmanni | Northern Europe | −10.3 | −2.7 | [40] | |
| NHMM 1984089-1 (2) | Mosasaurushoffmanni | Northern Europe | −9.0 | −3.4 | [40] | |
| NHMD 226499 (1) | Mosasaurus cf. M. hoffmanni | Scandinavia | −6.9 | −2.8 | [40] | |
| NHMD 226499 (2) | Mosasaurus cf. M. hoffmanni | Scandinavia | −11.0 | −2.7 | [40] | |
| NHMD 227350 (1) | Plioplatecarpus sp. | Scandinavia | 0.2 | −3.4 | [40] | |
| NHMD 189763 (1) | Plioplatecarpus sp. | Scandinavia | −9.5 | −2.8 | [40] | |
| NHMM 1997289 (1) | Plioplatecarpusmarshi | Northern Europe | −7.0 | −2.4 | [40] | |
| NHMM 1997289 (2) | Plioplatecarpusmarshi | Northern Europe | −7.9 | −4.0 | [40] | |
| NHMM 1998141-11 (1) | Prognathodonsaturator | Northern Europe | −10.9 | −2.7 | [40] | |
| NHMM 1998141-7 (1) | Prognathodonsaturator | Northern Europe | −11.6 | −2.5 | [40] | |
| MLP 15-I-24-41a: ARG-1 (1) | Mosasauridae indet. | Antarctica | −8.2 | −4.0 | [41] | |
| MLP 15-I-24-44: ARG-3 (1) | Globidensini indet. | Antarctica | −11.2 | −4.6 | [41] | |
| MLP 15-I-24-48: ARG-4 (1) | ?Mosasaurus indet. | Antarctica | −11.8 | −4.4 | [41] | |
| MLP 15-I-24-33a: ARG-5 (1) | cf. Liodon indet. | Antarctica | −13.5 | −3.9 | [41] | |
| MLP 15-I-24-33a: ARG-5 (2) | cf. Liodon indet. | Antarctica | −11.4 | −3.6 | [41] | |
| MLP 15-I-24-29: ARG-8 (1) | ?Taniwhasaurus indet. | Antarctica | −10.6 | −4.8 | [41] | |
| MLP 15-I-24-55a: ARG-10 (1) | ?Taniwhasaurus indet. | Antarctica | −6.8 | −4.8 | [41] | |
| MLP 15-I-24-25: ARG-13 (1) | cf. Liodon indet. | Antarctica | −11.8 | −3.5 | [41] | |
| MLP 15-I-24-33b: ARG-14 (1) | cf. Liodon indet. | Antarctica | −12.5 | −2.6 | [41] | |
| MLP 13-XI-19-38: ARG-18 (1) | ?Mosasaurinae indet. | Argentina | −11.1 | −5.1 | [41] | |
| MLP 13-XI-19-38: ARG-19 | ?Mosasaurinae indet. | Argentina | −11.1 | −4.2 | [41] |
References
- Polcyn, M.J.; Jacobs, L.L.; Araújo, R.; Schulp, A.S.; Mateus, O. Physical Drivers of Mosasaur Evolution. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2014, 400, 17–27. [Google Scholar] [CrossRef]
- Augusta, B.G.; Zaher, H.; Polcyn, M.J.; Fiorillo, A.R.; Jacobs, L.L. A Review of Non-Mosasaurid (Dolichosaur and Aigialosaur) Mosasaurians and Their Relationships to Snakes. In The Origin and Early Evolutionary History of Snakes; Gower, D.J., Zaher, H., Eds.; Systematics Association Special Volume Series; Cambridge University Press: Cambridge, UK, 2022; pp. 157–179. ISBN 978-1-108-93889-1. [Google Scholar]
- Lindgren, J.; Polcyn, M.J.; Young, B.A. Landlubbers to Leviathans: Evolution of Swimming in Mosasaurine Mosasaurs. Paleobiology 2011, 37, 445–469. [Google Scholar] [CrossRef]
- Dollo, L. Le Hainosaure et Les Nouveaux Vertébrés Fossiles Du Musée de Bruxelles. Rev. Des. Quest. Sci. 1887, 21, 504–539. [Google Scholar]
- Williston, S. Some Additional Characters of the Mosasaurs. Kans. Univ. Q. 1899, 8, 39–41. [Google Scholar]
- Sternberg, C.H. Explorations of the Permian of Texas and the Chalk of Kansas, 1918. Trans. Kans. Acad. Sci. 1919, 30, 119–120. [Google Scholar] [CrossRef]
- Camp, C.L. California Mosasaurs. Mem. Univ. Calif. 1942, 13, 1. [Google Scholar]
- Massare, J.A. Tooth Morphology and Prey Preference of Mesozoic Marine Reptiles. J. Vertebr. Paleontol. 1987, 7, 121–137. [Google Scholar] [CrossRef]
- Martin, J.E.; Bjork, P.P. Gastric Residues Associated with a Mosasaur from the Late Cretaceous (Campanian) Pierre Shale in South Dakota. In Papers in Vertebrate Paleontology in Honor of Morton Green; Martin, J.E., Ostrander, G.E., Eds.; Dakoterra; South Dakota School of Mines & Technology: Rapid City, SD, USA, 1987. [Google Scholar]
- Everhart, M. Plesiosaurs as the Food of Mosasaurs; New Data on the Stomach Contents of a Tylosaurus proriger (Squamata; Mosasauridae) from the Niobrara Formation of Western Kansas. Mosasaur 2004, 7, 41–46. [Google Scholar]
- Schulp, A.S. Feeding the Mechanical Mosasaur: What Did Carinodens Eat? Neth. J. Geosci. 2005, 84, 345–357. [Google Scholar] [CrossRef]
- Martin, J.E.; Fox, J.E. Stomach Contents of Globidens, a Shell-Crushing Mosasaur (Squamata), from the Late Cretaceous Pierre Shale Group, Big Bend Area of the Missouri River, Central South Dakota. In The Geology and Paleontology of the Late Cretaceous Marine Deposits of the Dakotas; Martin, J.E., Paris, D., Eds.; Geological Society of America Special Papers; Geological Society of America: Boulder, CO, USA, 2007; Volume 427, pp. 167–176. [Google Scholar]
- Einarsson, E.; Lindgren, J.; Kear, B.P.; Siverson, M. Mosasaur Bite Marks on a Plesiosaur Propodial from the Campanian (Late Cretaceous) of Southern Sweden. GFF 2010, 132, 123–128. [Google Scholar] [CrossRef]
- Poynter, J.M. Using Dental Microwear Analysis to Predict Feeding Types in Mesozoic Marine Reptiles. Ph.D. Thesis, Northwest Missouri State University, Maryville, MO, USA, 2011. [Google Scholar]
- Konishi, T.; Brinkman, D.; Massare, J.A.; Caldwell, M.W. New Exceptional Specimens of Prognathodon overtoni (Squamata, Mosasauridae) from the Upper Campanian of Alberta, Canada, and the Systematics and Ecology of the Genus. J. Vertebr. Paleontol. 2011, 31, 1026–1046. [Google Scholar] [CrossRef]
- Konishi, T.; Newbrey, M.G.; Caldwell, M.W. A Small, Exquisitely Preserved Specimen of Mosasaurus missouriensis (Squamata, Mosasauridae) from the Upper Campanian of the Bearpaw Formation, Western Canada, and the First Stomach Contents for the Genus. J. Vertebr. Paleontol. 2014, 34, 802–819. [Google Scholar] [CrossRef]
- Bardet, N.; Houssaye, A.; Vincent, P.; Pereda Suberbiola, X.; Amaghzaz, M.; Jourani, E.; Meslouh, S. Mosasaurids (Squamata) from the Maastrichtian Phosphates of Morocco: Biodiversity, Palaeobiogeography and Palaeoecology Based on Tooth Morphoguilds. Gondwana Res. 2015, 27, 1068–1078. [Google Scholar] [CrossRef]
- Longrich, N.R.; Jalil, N.-E.; Khaldoune, F.; Yazami, O.K.; Pereda-Suberbiola, X.; Bardet, N. Thalassotitan atrox, a Giant Predatory Mosasaurid (Squamata) from the Upper Maastrichtian Phosphates of Morocco. Cretac. Res. 2022, 140, 105315. [Google Scholar] [CrossRef]
- Holwerda, F.M.; Bestwick, J.; Purnell, M.A.; Jagt, J.W.M.; Schulp, A.S. Three-Dimensional Dental Microwear in Type-Maastrichtian Mosasaur Teeth (Reptilia, Squamata). Sci. Rep. 2023, 13, 18720. [Google Scholar] [CrossRef]
- Polcyn, M.J.; Schulp, A.S.; Goncalves, A.O. Remarkably Well-Preserved in-Situ Gut-Content in a Specimen of Prognathodon kianda (Squamata: Mosasauridae) Reveals Multispecies Intrafamilial Predation, Cannibalism, and a New Mosasaurine Taxon. In Windows into Sauropsid and Synapsid Evolution; Lee, Y.-N., Ed.; Dinosaur Science Center Press: Hwaseong City, Republic of Korea, 2023; pp. 66–98. [Google Scholar]
- Russell, D.A. Systematics and Morphology of American Mosasaurs; Yale University Press: New Haven, CT, USA, 1967; ISBN 978-1-933789-45-3. [Google Scholar]
- Holmes, R.; Caldwell, M.W.; Cumbaa, S.L. A New Specimen of Plioplatecarpus (Mosasauridae) from the Lower Maastrichtian of Alberta: Comments on Allometry, Functional Morphology, and Paleoecology. Can. J. Earth Sci. 1999, 36, 363–369. [Google Scholar] [CrossRef]
- Kiernan, C.R. Stratigraphic Distribution and Habitat Segregation of Mosasaurs in the Upper Cretaceous of Western and Central Alabama, with an Historical Review of Alabama Mosasaur Discoveries. J. Vertebr. Paleontol. 2002, 22, 91–103. [Google Scholar] [CrossRef]
- Nicholls, E.L.; Meckert, D. Marine Reptiles from the Nanaimo Group (Upper Cretaceous) of Vancouver Island. Can. J. Earth Sci. 2002, 39, 1591–1603. [Google Scholar] [CrossRef]
- Jacobs, L.L.; Polcyn, M.J.; Taylor, L.H.; Ferguson, K. Sea-Surface Temperatures and Palaeoenvironments of Dolichosaurs and Early Mosasaurs. Geol. Mijnb. 2005, 84, 269–281. [Google Scholar] [CrossRef]
- Ifrim, C.; Stinnesbeck, W.; Frey, E. Upper Cretaceous (Cenomanian-Turonian and Turonian-Coniacian) Open Marine Plattenkalk Deposits in NE Mexico. Neues Jahrb. Für Geol. Und Paläontologie Abh. 2007, 245, 71–81. [Google Scholar] [CrossRef]
- Patrick, D.; Martin, J.; Parris, D.; Grandstaff, D. Paleoenvironmental Interpretations of Rare Earth Element Signatures in Mosasaurs (Reptilia) from the Upper Cretaceous Pierre Shale, Central South Dakota, USA. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2004, 212, 277–294. [Google Scholar] [CrossRef]
- Kocsis, L.; Ősi, A.; Vennemann, T.; Trueman, C.N.; Palmer, M.R. Geochemical Study of Vertebrate Fossils from the Upper Cretaceous (Santonian) Csehbánya Formation (Hungary): Evidence for a Freshwater Habitat of Mosasaurs and Pycnodont Fish. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2009, 280, 532–542. [Google Scholar] [CrossRef]
- Harrell, T.L.; Pérez-Huerta, A. Habitat Preference of Mosasaurs Indicated by Rare Earth Element (REE) Content of Fossils from the Upper Cretaceous Marine Deposits of Alabama, New Jersey, and South Dakota (USA). Neth. J. Geosci. 2015, 94, 145–154. [Google Scholar] [CrossRef]
- Reisdorf, A.G.; Bux, R.; Wyler, D.; Benecke, M.; Klug, C.; Maisch, M.W.; Fornaro, P.; Wetzel, A. Float, Explode or Sink: Postmortem Fate of Lung-Breathing Marine Vertebrates. Palaeobiodivers. Palaeoenviron. 2012, 92, 67–81. [Google Scholar] [CrossRef]
- DeNiro, M.J.; Epstein, S. Influence of Diet on the Distribution of Carbon Isotopes in Animals. Geochim. Cosmochim. Acta 1978, 42, 495–506. [Google Scholar] [CrossRef]
- Clementz, M.T.; Koch, P.L. Differentiating Aquatic Mammal Habitat and Foraging Ecology with Stable Isotopes in Tooth Enamel. Oecologia 2001, 129, 461–472. [Google Scholar] [CrossRef]
- Fry, B.; Wainright, S.C. Diatom Sources of 13C-Rich Carbon in Marine Food Webs. Mar. Ecol. Prog. Ser. 1991, 76, 149–157. [Google Scholar] [CrossRef]
- Rau, G.H.; Takahashi, T.; Des Marais, D.J.; Repeta, D.J.; Martin, J.H. The Relationship between δ13C of Organic Matter and [CO2(Aq)] in Ocean Surface Water: Data from a JGOFS Site in the Northeast Atlantic Ocean and a Model. Geochim. Cosmochim. Acta 1992, 56, 1413–1419. [Google Scholar] [CrossRef]
- Hemminga, M.; Mateo, M. Stable Carbon Isotopes in Seagrasses:Variability in Ratios and Use in Ecological Studies. Mar. Ecol. Prog. Ser. 1996, 140, 285–298. [Google Scholar] [CrossRef]
- Robbins, J.; Ferguson, K.M.; Polcyn, M.J.; Jacobs, L.L. Application of Stable Carbon Isotope Analysis to Mosasaur Ecology. In Proceedings of the Second Mosasaur Meeting; Everhart, M.J., Ed.; Sternberg Museum of Natural History, Fort Hays State University: Hays, KS, USA, 2008; Volume Special Volume; pp. 123–130. ISBN 978-0-615-23109-9. [Google Scholar]
- Schulp, A.S.; Vonhof, H.B.; Van Der Lubbe, J.H.J.L.; Janssen, R.; Van Baal, R.R. On Diving and Diet: Resource Partitioning in Type-Maastrichtian Mosasaurs. Neth. J. Geosci. 2013, 92, 165–170. [Google Scholar] [CrossRef]
- Schulp, A.S.; Janssen, R.; Van Baal, R.R.; Jagt, J.W.M.; Mulder, E.W.A.; Vonhof, H.B. Stable Isotopes, Niche Partitioning and the Paucity of Elasmosaur Remains in the Maastrichtian Type Area. Neth. J. Geosci. 2017, 96, 29–33. [Google Scholar] [CrossRef]
- Strganac, C.; Jacobs, L.L.; Polcyn, M.J.; Mateus, O.; Myers, T.S.; Salminen, J.; May, S.R.; Araújo, R.; Ferguson, K.M.; Gonçalves, A.O.; et al. Geological Setting and Paleoecology of the Upper Cretaceous Bench 19 Marine Vertebrate Bonebed at Bentiaba, Angola. Neth. J. Geosci. 2015, 94, 121–136. [Google Scholar] [CrossRef]
- Giltaij, T.J.; Jeroen, V.D.L.; Lindow, B.; Schulp, A.S.; Jagt, J.W.M. Carbon Isotope Trends in North-West European Mosasaurs (Squamata; Late Cretaceous). Bull. Geol. Soc. Den. 2021, 69, 59–70. [Google Scholar] [CrossRef]
- Leuzinger, L.; Kocsis, L.; Luz, Z.; Vennemann, T.; Ulyanov, A.; Fernández, M. Latest Maastrichtian Middle- and High-Latitude Mosasaurs and Fish Isotopic Composition: Carbon Source, Thermoregulation Strategy, and Thermal Latitudinal Gradient. Paleobiology 2023, 49, 353–373. [Google Scholar] [CrossRef]
- Strong, C.R.C.; Caldwell, M.W.; Konishi, T.; Palci, A. A New Species of Longirostrine Plioplatecarpine Mosasaur (Squamata: Mosasauridae) from the Late Cretaceous of Morocco, with a Re-Evaluation of the Problematic Taxon ‘Platecarpus’ ptychodon. J. Syst. Palaeontol. 2020, 18, 1769–1804. [Google Scholar] [CrossRef]
- Polcyn, M.J.; Bardet, N.; Albright, L.B.; Titus, A. A New Lower Turonian Mosasaurid from the Western Interior Seaway and the Antiquity of the Unique Basicranial Circulation Pattern in Plioplatecarpinae. Cretac. Res. 2023, 151, 105621. [Google Scholar] [CrossRef]
- Zietlow, A.R.; Boyd, C.A.; Van Vranken, N.E. Jormungandr walhallaensis: A New Mosasaurine (Squamata: Mosasauroidea) from the Pierre Shale Formation (Pembina Member: Middle Campanian) of North Dakota. Bull. Am. Mus. Nat. Hist. 2023, 464, 1–82. [Google Scholar] [CrossRef]
- Longrich, N.R.; Polcyn, M.J.; Jalil, N.-E.; Pereda-Suberbiola, X.; Bardet, N. A Bizarre New Plioplatecarpine Mosasaurid from the Maastrichtian of Morocco. Cretac. Res. 2024, 160, 105870. [Google Scholar] [CrossRef]
- Polcyn, M.J.; Bell, G.L., Jr.; Shimada, K.; Everhart, M.J. The Oldest North American Mosasaurs (Squamata: Mosasauridae) from the Turonian (Upper Cretaceous) of Kansas and Texas with Comments on the Radiations of Major Mosasaur Clades. In Proceedings of the Second Mosasaur Meeting; Everhart, M.J., Ed.; Sternberg Museum of Natural History, Fort Hays State University: Hays, KS, USA, 2008; Volume Special Volume; pp. 137–155. ISBN 978-0-615-23109-9. [Google Scholar]
- Bell, G.L. A Phylogenetic Revision of North American and Adriatic Mosasauroidea. In Ancient Marine Reptiles; Elsevier: Amsterdam, The Netherlands, 1997; pp. 293–332. ISBN 978-0-12-155210-7. [Google Scholar]
- Páramo-Fonseca, M.E. Yaguarasaurus columbianus (Reptilia, Mosasauridae), a Primitive Mosasaur from the Turonian (Upper Cretaceous) of Colombia. Hist. Biol. 2000, 14, 121–131. [Google Scholar] [CrossRef]
- Bardet, N.; Suberbiola, X.P.; Jalil, N.-E. A New Mosasauroid (Squamata) from the Late Cretaceous (Turonian) of Morocco. Comptes Rendus Palevol 2003, 2, 607–616. [Google Scholar] [CrossRef]
- Polcyn, M.J.; Bell, G.L. Russellosaurus coheni n. gen., n. sp., a 92 Million-Year-Old Mosasaur from Texas (USA), and the Definition of the Parafamily Russellosaurina. Neth. J. Geosci. 2005, 84, 321–333. [Google Scholar] [CrossRef]
- Polcyn, M.J.; Everhart, M.J. Description and Phylogenetic Analysis of a New Species of Selmasaurus (Mosasauridae: Plioplatecarpinae) from the Niobrara Chalk of Western Kansas. In Proceedings of the Second Mosasaur Meeting; Everhart, M.J., Ed.; Sternberg Museum of Natural History, Fort Hays State University: Hays, KS, USA, 2008; Volume Special Volume; pp. 13–28. ISBN 978-0-615-23109-9. [Google Scholar]
- Konishi, T.; Caldwell, M.W. Two New Plioplatecarpine (Squamata, Mosasauridae) Genera from the Upper Cretaceous of North America, and a Global Phylogenetic Analysis of Plioplatecarpines. J. Vertebr. Paleontol. 2011, 31, 754–783. [Google Scholar] [CrossRef]
- Leblanc, A.R.H.; Caldwell, M.W.; Bardet, N. A New Mosasaurine from the Maastrichtian (Upper Cretaceous) Phosphates of Morocco and Its Implications for Mosasaurine Systematics. J. Vertebr. Paleontol. 2012, 32, 82–104. [Google Scholar] [CrossRef]
- Palci, A.; Caldwell, M.W.; Papazzoni, C.A. A New Genus and Subfamily of Mosasaurs from the Upper Cretaceous of Northern Italy. J. Vertebr. Paleontol. 2013, 33, 599–612. [Google Scholar] [CrossRef]
- Willman, A.J.; Konishi, T.; Caldwell, M.W. A New Species of Ectenosaurus (Mosasauridae: Plioplatecarpinae) from Western Kansas, USA, Reveals a Novel Suite of Osteological Characters for the Genus1. Can. J. Earth Sci. 2021, 58, 741–755. [Google Scholar] [CrossRef]
- Kiernan, C.R.; Ebersole, J.A. Two New Plioplatecarpine Mosasaurs (Mosasauridae; Plioplatecarpinae) of the Genus Ectenosaurus from the Upper Cretaceous of North America. PaleoBios 2023, 40, 1–28. [Google Scholar] [CrossRef]
- Bell, G.L.; Barnes, K.R.; Polcyn, M.J. Late Cretaceous Mosasauroids (Reptilia, Squamata) of the Big Bend Region in Texas, USA. Earth Environ. Sci. Trans. R. Soc. Edinb. 2012, 103, 571–581. [Google Scholar] [CrossRef]
- Bell, G.L.; Polcyn, M.J. Dallasaurus turneri, a New Primitive Mosasauroid from the Middle Turonian of Texas and Comments on the Phylogeny of Mosasauridae (Squamata). Neth. J. Geosci. 2005, 84, 177–194. [Google Scholar] [CrossRef]
- Lively, J.R. Taxonomy and Historical Inertia: Clidastes (Squamata: Mosasauridae) as a Case Study of Problematic Paleobiological Taxonomy. Alcheringa Australas. J. Palaeontol. 2018, 42, 516–527. [Google Scholar] [CrossRef]
- Everhart, M.J. Revisions to the Biostratigraphy of the Mosasauridae (Squamata) in the Smoky Hill Chalk Member of the Niobrara Chalk (Late Cretaceous) of Kansas. Trans. Kans. Acad. Sci. 2001, 104, 59–78. [Google Scholar] [CrossRef]
- Holwerda, F.M.; Mitchell, M.T.; Van De Kerk, M.; Schulp, A.S. Mosasaur Feeding Ecology from the Campanian Bearpaw Formation, Alberta, Canada: A Preliminary Multi-Proxy Approach. Diversity 2025, 17, 205. [Google Scholar] [CrossRef]
- Russell, D.A. A New Species of Globidens from South Dakota, and a Review of Globidentine Mosasaurs; Fieldiana, Geology; Field Museum of Natural History: Chicago, IL, USA, 1975; Volume 33. [Google Scholar]
- Schulp, A.S.; Polcyn, M.J.; Mateus, O.; Jacobs, L.L.; Morais, M.L. A New Species of Prognathodon (Squamata, Mosasauridae) from the Maastrichtian of Angola, and the Affinities of the Mosasaur Genus Liodon. In Proceedings of the Second Mosasaur Meeting; Fort Hays State University Fort Hays: Hays, KS, USA, 2008; Volume 3, pp. 1–12. [Google Scholar]
- DeBraga, M.; Carroll, R.L. The Origin of Mosasaurs as a Model of Macroevolutionary Patterns and Processes. In Evolutionary Biology; Hecht, M.K., MacIntyre, R.J., Clegg, M.T., Eds.; Springer US: Boston, MA, USA, 1993; pp. 245–322. ISBN 978-1-4613-6248-7. [Google Scholar]
- Polcyn, M.J.; Lindgren, J.; Bardet, N.; Cornelissen, D.; Verding, L.; Schulp, A.S. Description of New Specimens of Halisaurus arambourgi Bardet and Pereda Suberbiola, 2005 and the Relationships of Halisaurinae. Bull. De La Société Géologique De Fr. 2012, 183, 123–136. [Google Scholar] [CrossRef]
- Polcyn, M.J.; Augusta, B.G.; Zaher, H. Reassessing the Morphological Foundations of the Pythonomorph Hypothesis. In The Origin and Early Evolutionary History of Snakes; Gower, D.J., Zaher, H., Eds.; Systematics Association; Cambridge University Press: Cambridge, UK, 2022; Volume Special Volume Series, pp. 125–156. ISBN 978-1-108-93889-1. [Google Scholar]
- Koch, P.L.; Tuross, N.; Fogel, M.L. The Effects of Sample Treatment and Diagenesis on the Isotopic Integrity of Carbonate in Biogenic Hydroxylapatite. J. Archaeol. Sci. 1997, 24, 417–429. [Google Scholar] [CrossRef]
- Passey, B.H.; Cerling, T.E.; Levin, N.E. Temperature Dependence of Oxygen Isotope Acid Fractionation for Modern and Fossil Tooth Enamels. Rapid Comm Mass Spectrom. 2007, 21, 2853–2859. [Google Scholar] [CrossRef]
- Kusaka, S.; Nakano, T. Carbon and Oxygen Isotope Ratios and Their Temperature Dependence in Carbonate and Tooth Enamel Using a GasBench II Preparation Device: Letter to the Editor. Rapid Commun. Mass Spectrom. 2014, 28, 563–567. [Google Scholar] [CrossRef]
- Swart, P.K.; Burns, S.J.; Leder, J.J. Fractionation of the Stable Isotopes of Oxygen and Carbon in Carbon Dioxide during the Reaction of Calcite with Phosphoric Acid as a Function of Temperature and Technique. Chem. Geol. Isot. Geosci. Sect. 1991, 86, 89–96. [Google Scholar] [CrossRef]
- Chesson, L.A.; Kenyhercz, M.W.; Regan, L.A.; Berg, G.E. Addressing Data Comparability in the Creation of Combined Data Sets of Bioapatite Carbon and Oxygen Isotopic Compositions. Archaeometry 2019, 61, 1193–1206. [Google Scholar] [CrossRef]
- Christiansen, P.; Bonde, N. A New Species of Gigantic Mosasaur from the Late Cretaceous of Israel. J. Vertebr. Paleontol. 2002, 22, 629–644. [Google Scholar] [CrossRef]
- Polcyn, M.J.; Jacobs, L.L.; Schulp, A.S.; Mateus, O. The North African Mosasaur Globidens phosphaticus from the Maastrichtian of Angola. Hist. Biol. 2010, 22, 175–185. [Google Scholar] [CrossRef]
- Mateus, O.; Polcyn, M.J.; Jacobs, L.L.; Araújo, R.; Schulp, A.S.; Marinheiro, J.; Pereira, B.; Vineyard, D. Cretaceous Amniotes from Angola: Dinosaurs, Pterosaurs, Mosasaurs, Plesiosaurs, and Turtles. In Proceedings of the V International Conference on Dinosaur Paleontology and their Environment, Salas de los Infantes, Burgos, 26 July 2012; pp. 71–105. [Google Scholar]
- Lindgren, J. Dental and Vertebral Morphology of the Enigmatic Mosasaur Dollosaurus (Reptilia, Mosasauridae) from the Lower Campanian (Upper Cretaceous) of Southern Sweden. Bull. Geol. Soc. Den. 2005, 52, 17–25. [Google Scholar] [CrossRef]
- Fernandez, M.; Martin, J.E. Description and Phylogenetic Relationships of Taniwhasaurus antarcticus (Mosasauridae, Tylosaurinae) from the Upper Campanian (Cretaceous) of Antarctica. Cretac. Res. 2009, 30, 717–726. [Google Scholar] [CrossRef]
- Fischer, V.; Bennion, R.F.; Foffa, D.; MacLaren, J.A.; McCurry, M.R.; Melstrom, K.M.; Bardet, N. Ecological Signal in the Size and Shape of Marine Amniote Teeth. Proc. R. Soc. B Biol. Sci. 2022, 289, 20221214. [Google Scholar] [CrossRef] [PubMed]
- Jagt, J.W.; Mulder, E.W.; Dortangs, R.W.; Kuypers, M.; Peeters, H.; Verding, L. Recent Additions to the Late Maastrichtian Mosasaur Faunas of Liège-Limburg (The Netherlands, Belgium). Sargetia (Acta Musei Devensis Ser. Sci. Naturae) 2002, 19, 13–26. [Google Scholar]
- Dortangs, R.W.; Schulp, A.S.; Mulder, E.W.A.; Jagt, J.W.M.; Peeters, H.H.G.; De Graaf, D.T. A Large New Mosasaur from the Upper Cretaceous of The Netherlands. Neth. J. Geosci. 2002, 81, 1–8. [Google Scholar] [CrossRef]
- Clementz, M.T. Sea Cows, Seagrasses, and Stable Isotopes: Biogeochemical Evaluation of the Ecology and Evolution of the Sirenia and Desmostylia; University of California: Santa Cruz, CA, USA, 2002. [Google Scholar]
- Clementz, M.T.; Fox-Dobbs, K.; Wheatley, P.V.; Koch, P.L.; Doak, D.F. Revisiting Old Bones: Coupled Carbon Isotope Analysis of Bioapatite and Collagen as an Ecological and Palaeoecological Tool. Geol. J. 2009, 44, 605–620. [Google Scholar] [CrossRef]
- Lee-Thorp, J.A.; Sealy, J.C.; Van Der Merwe, N.J. Stable Carbon Isotope Ratio Differences between Bone Collagen and Bone Apatite, and Their Relationship to Diet. J. Archaeol. Sci. 1989, 16, 585–599. [Google Scholar] [CrossRef]
- Tieszen, L.L.; Fagre, T. Effect of Diet Quality and Composition on the Isotopic Composition of Respiratory CO2, Bone Collagen, Bioapatite, and Soft Tissues. In Prehistoric Human Bone; Lambert, J.B., Grupe, G., Eds.; Springer: Berlin/Heidelberg, Germany, 1993; pp. 121–155. ISBN 978-3-662-02896-4. [Google Scholar]
- Toperoff, A.K. Examination of Diet of Harbor Porpoise (Phocoena phocoena) from Central California Using Stomach Content and Stable Isotope Analysis from Multiple Tissues; San Jose State University: San Jose, CA, USA, 2002. [Google Scholar]
- Walker, J.L.; Macko, S.A. Dietary Studies of Marine Mammals Using Stable Carbon and Nitrogen Isotopic Ratios of Teeth. Mar. Mammal Sci. 1999, 15, 314–334. [Google Scholar] [CrossRef]
- Mendes, S.; Newton, J.; Reid, R.J.; Frantzis, A.; Pierce, G.J. Stable Isotope Profiles in Sperm Whale Teeth: Variations between Areas and Sexes. J. Mar. Biol. Assoc. UK 2007, 87, 621–627. [Google Scholar] [CrossRef]
- Rey-Iglesia, A.; Wilson, T.; Routledge, J.; Skovrind, M.; Garde, E.; Heide-Jørgensen, M.P.; Szpak, P.; Lorenzen, E.D. Combining δ13C and δ15N from Bone and Dentine in Marine Mammal Palaeoecological Research: Insights from Toothed Whales. Isot. Environ. Health Stud. 2023, 59, 66–77. [Google Scholar] [CrossRef]
- Benner, R.; Biddanda, B.; Black, B.; McCarthy, M. Abundance, Size Distribution, and Stable Carbon and Nitrogen Isotopic Compositions of Marine Organic Matter Isolated by Tangential-Flow Ultrafiltration. Mar. Chem. 1997, 57, 243–263. [Google Scholar] [CrossRef]
- Boeuf, B.J.L.; Crocker, D.E.; Grayson, J.; Gedamke, J.; Webb, P.M.; Blackwell, S.B.; Costa, D.P. Respiration and Heart Rate at the Surface Between Dives in Northern Elephant Seals. J. Exp. Biol. 2000, 203, 3265–3274. [Google Scholar] [CrossRef]
- Truchot, J.-P. Comparative Aspects of Extracellular Acid-Base Balance; Zoophysiology; Springer: Berlin/Heidelberg, Germany, 1987; Volume 20, ISBN 978-3-642-83132-4. [Google Scholar]
- Randall, D.J.; Burggren, W.W.; French, K.; Eckert, R. Eckert Animal Physiology: Mechanisms and Adaptations, 5th ed.; W.H. Freeman and Co: New York, NY, USA, 2002; ISBN 978-0-7167-3863-3. [Google Scholar]
- Lutz, P.L.; Storey, K.B. Adaptations to Variations in Oxygen Tension by Vertebrates and Invertebrates. In Comprehensive Physiology; Prakash, Y.S., Ed.; Wiley: Hoboken, NJ, USA, 1997; pp. 1479–1522. ISBN 978-0-470-65071-4. [Google Scholar]
- Ackerman, R.; White, F. Cyclic Carbon Dioxide Exchange in the Turtle Pseudemys scripta. Physiol. Zool. 1979, 52, 378–389. [Google Scholar] [CrossRef]
- Lutcavage, M.E.; Lutz, P.L. Diving Physiology. In The Biology of Sea Turtles; Lutz, P.L., Musick, J.A., Eds.; CRC Press: Boca Raton, FL, USA, 1997; Volume 1, p. 410. ISBN 978-0-203-73708-8. [Google Scholar]
- McConnaughey, T.A.; Burdett, J.; Whelan, J.F.; Paull, C.K. Carbon Isotopes in Biological Carbonates: Respiration and Photosynthesis. Geochim. Et Cosmochim. Acta 1997, 61, 611–622. [Google Scholar] [CrossRef]
- Biasatti, D.M. Stable Carbon Isotopic Profiles of Sea Turtle Humeri: Implications for Ecology and Physiology. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2004, 206, 203–216. [Google Scholar] [CrossRef]
- Roe, L.J.; Thewissen, J.G.M.; Quade, J.; O’Neil, J.R.; Bajpai, S.; Sahni, A.; Hussain, S.T. Isotopic Approaches to Understanding the Terrestrial-to-Marine Transition of the Earliest Cetaceans. In The Emergence of Whales; Thewissen, J.G.M., Ed.; Springer: Boston, MA, USA, 1998; pp. 399–422. ISBN 978-1-4899-0161-3. [Google Scholar]
- Lindgren, J. Stratigraphical Distribution of Campanian and Maastrichtian Mosasaurs in Sweden—Evidence of an Intercontinental Marine Extinction Event? GFF 2004, 126, 221–229. [Google Scholar] [CrossRef]
- Lindgren, J.; Siverson, M. The First Record of the Mosasaur Clidastes from Europe and Its Palaeogeographical Implications. Acta Palaeontologica Polonica 2004, 49, 219. [Google Scholar]
- Jagt, J.W.M. Stratigraphic Ranges of Mosasaurs in Belgium and the Netherlands (Late Cretaceous) and Cephalopod-Based Correlations with North America. Neth. J. Geosci. 2005, 84, 283–301. [Google Scholar] [CrossRef]
- Mulder, E.W.A.; Formanoy, P.; Gallagher, W.B.; Jagt, J.W.M.; Schulp, A.S. The first North American record of Carinodens belgicus (Squamata, Mosasauridae) and correlation with the youngest in situ examples from the Maastrichtian type area: Palaeoecological implications. Neth. J. Geosci 2013, 92, 145–152. [Google Scholar] [CrossRef]
- Wendler, I. A Critical Evaluation of Carbon Isotope Stratigraphy and Biostratigraphic Implications for Late Cretaceous Global Correlation. Earth Sci. Rev. 2013, 126, 116–146. [Google Scholar] [CrossRef]
- Cavin, L.; Tong, H.; Boudad, L.; Meister, C.; Piuz, A.; Tabouelle, J.; Aarab, M.; Amiot, R.; Buffetaut, E.; Dyke, G.; et al. Vertebrate Assemblages from the Early Late Cretaceous of Southeastern Morocco: An Overview. J. Afr. Earth Sci. 2010, 57, 391–412. [Google Scholar] [CrossRef]
- Grigoriev, D. Redescription of Prognathodon lutugini (Squamata, Mosasauridae). Proc. Zool. Inst. RAS 2013, 317, 246–261. [Google Scholar] [CrossRef]
- Gilmore, C.W. A New Mosasauroid Reptile from the Cretaceous of Alabama. Proc. United States Natl. Mus. 1912, 41, 479–484. [Google Scholar] [CrossRef]
- Martin, J.E. A New Species of the Durophagous Mosasaur Globidens (Squamata: Mosasauridae) from the Late Cretaceous Pierre Shale Group of Central South Dakota, USA. In The Geology and Paleontology of the Late Cretaceous Marine Deposits of the Dakotas; Special Paper; Geological Society of America: Boulder, CO, USA, 2007; pp. 177–198. ISBN 978-0-8137-2427-0. [Google Scholar]
- Dollo, L. Nouvelle Note Sur Les Vertébrés Fossiles Récemment Offerts Au Musée de Bruxelles Par M. Alfred Lemonnier. Bull. Société Belg. Géologie Paléontologie D’hydrologie 1889, 3, 214–215. [Google Scholar]
- Schulp, A.S.; Polcyn, M.J.; Mateus, O.; Jacobs, L.L. Two Rare Mosasaurs from the Maastrichtian of Angola and the Netherlands. Neth. J. Geosci. 2013, 92, 3–10. [Google Scholar] [CrossRef]
- Woodward, A.S. III.—Note on Tooth of an Extinct Alligator (Bottosaurus belgicus, sp. nov.) from the Lower Danian of Ciply, Belgium. Geol. Mag. 1891, 8, 114–115. [Google Scholar] [CrossRef]
- Thurmond, J. New Name for the Mosasaur Compressidens Dollo, 1924. J. Paleontol. 1969, 43, 1298. [Google Scholar]
- Bardet, N.; Suberbiola, X.P.; Iarochène, M.; Amalik, M.; Bouya, B. Durophagous Mosasauridae (Squamata) from the Upper Cretaceous Phosphates of Morocco, with Description of a New Species of Globidens. Neth. J. Geosci. 2005, 84, 167–175. [Google Scholar] [CrossRef]
- Lingham-Soliar, T. Mosasaurs from the Upper Cretaceous of Niger. Palaeontology 1991, 34, 653–670. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polcyn, M.J.; Robbins, J.A.; Schulp, A.S.; Lindgren, J.; Jacobs, L.L. The Evolution of Mosasaurid Foraging Behavior Through the Lens of Stable Carbon Isotopes. Diversity 2025, 17, 291. https://doi.org/10.3390/d17040291
Polcyn MJ, Robbins JA, Schulp AS, Lindgren J, Jacobs LL. The Evolution of Mosasaurid Foraging Behavior Through the Lens of Stable Carbon Isotopes. Diversity. 2025; 17(4):291. https://doi.org/10.3390/d17040291
Chicago/Turabian StylePolcyn, Michael J., John A. Robbins, Anne S. Schulp, Johan Lindgren, and Louis L. Jacobs. 2025. "The Evolution of Mosasaurid Foraging Behavior Through the Lens of Stable Carbon Isotopes" Diversity 17, no. 4: 291. https://doi.org/10.3390/d17040291
APA StylePolcyn, M. J., Robbins, J. A., Schulp, A. S., Lindgren, J., & Jacobs, L. L. (2025). The Evolution of Mosasaurid Foraging Behavior Through the Lens of Stable Carbon Isotopes. Diversity, 17(4), 291. https://doi.org/10.3390/d17040291






