DNA Barcoding of Red Algae from Bocas del Toro, Panamá, with a Description of Gracilaria bocatorensis sp. nov. and G. dreckmannii sp. nov. (Gracilariales, Gracilariaceae)
Abstract
1. Introduction
2. Materials and Methods
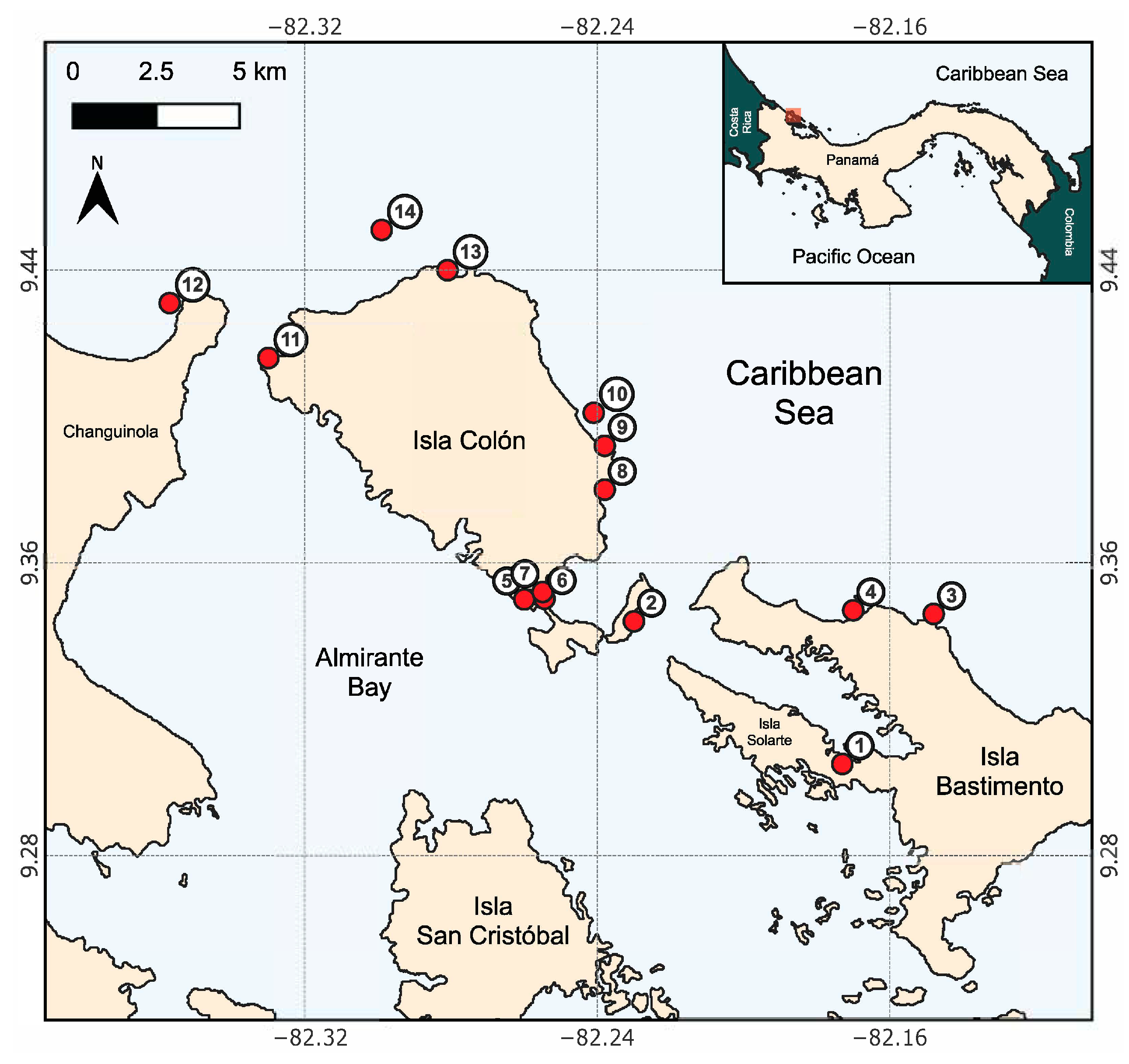
2.1. Sample Collection and Processing
2.2. DNA Extraction, Amplification, and Sequencing
2.3. Sequence Identification and Phylogenetic Analysis
2.4. Morphological Analysis
3. Results
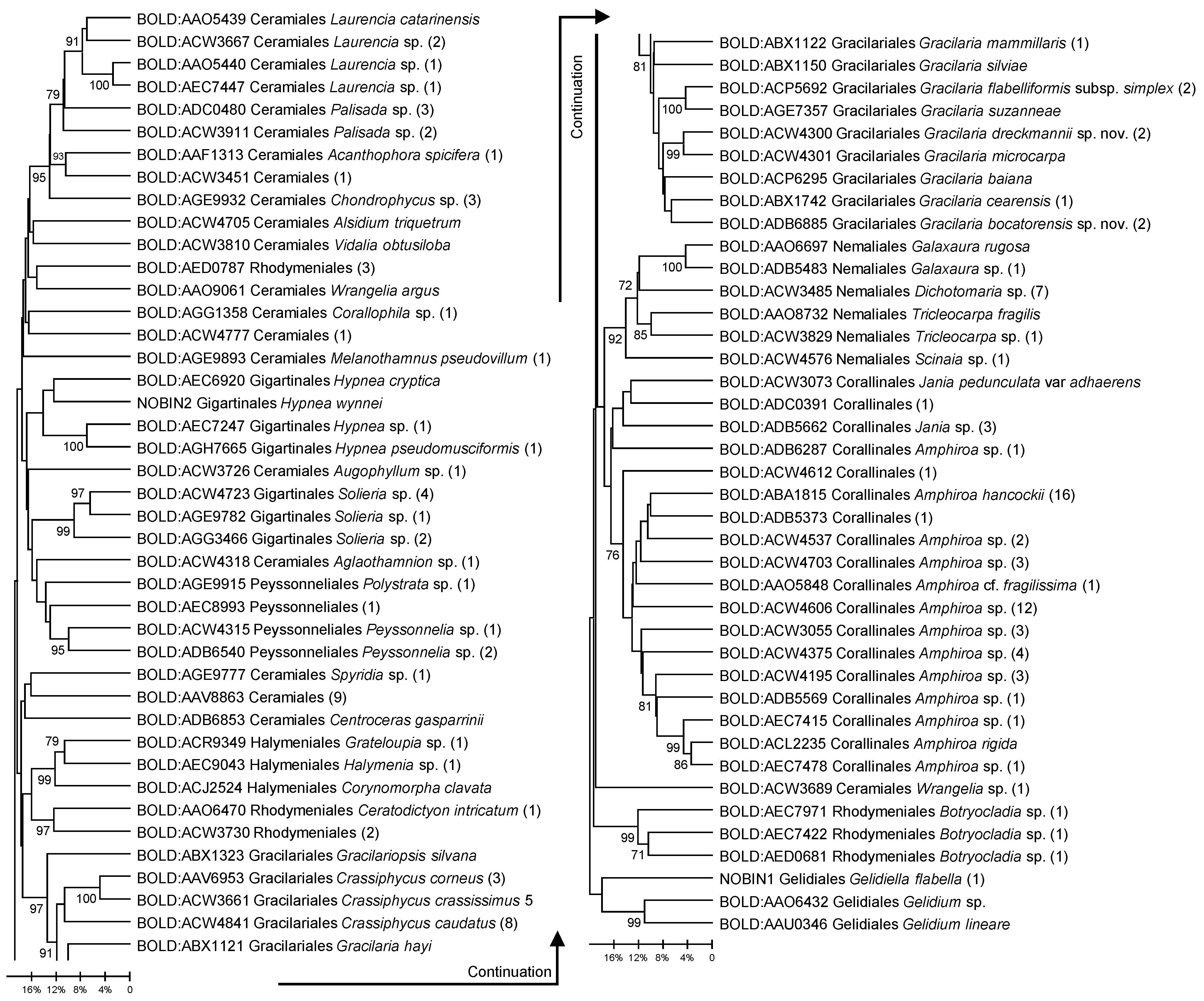
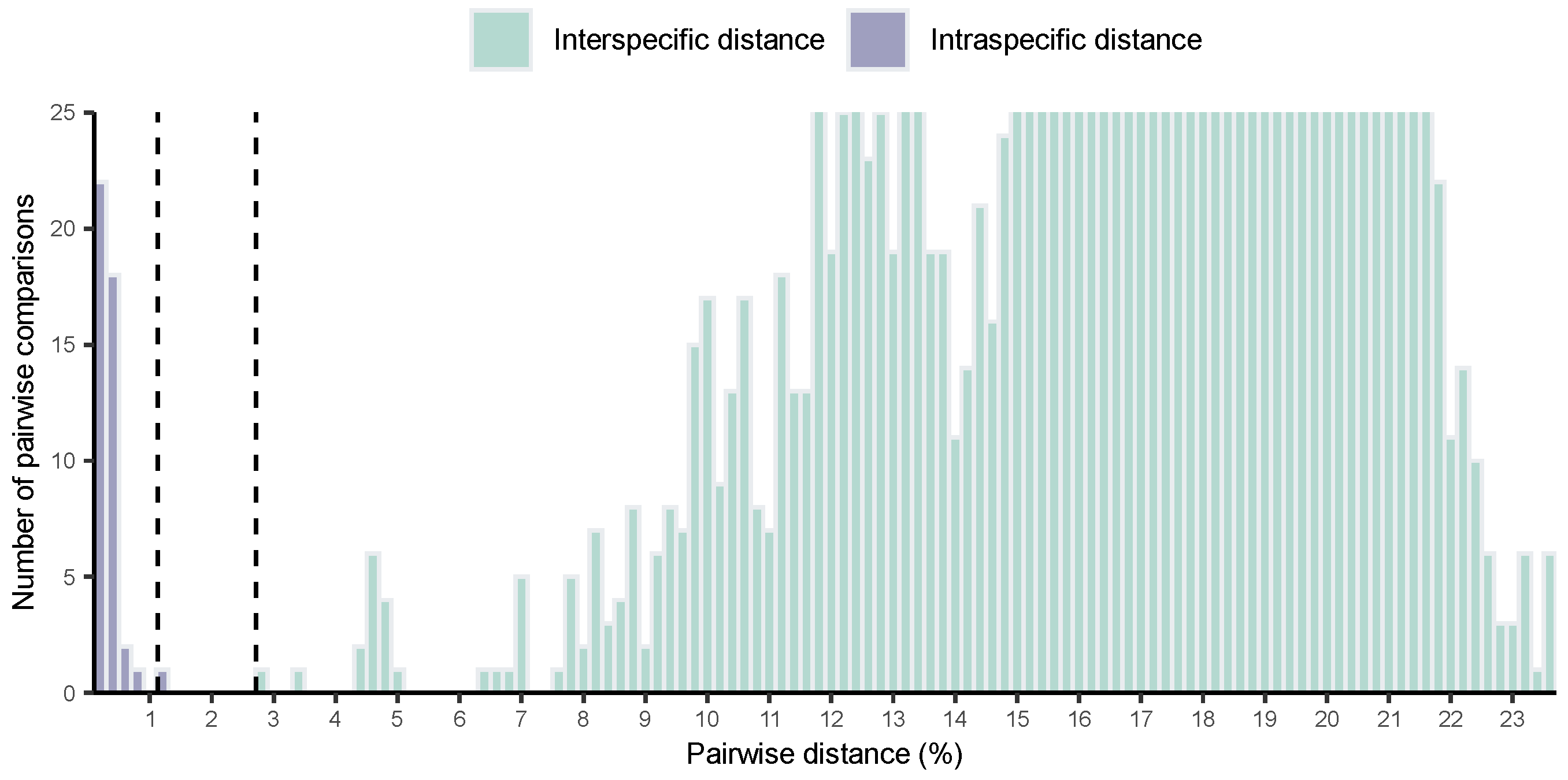
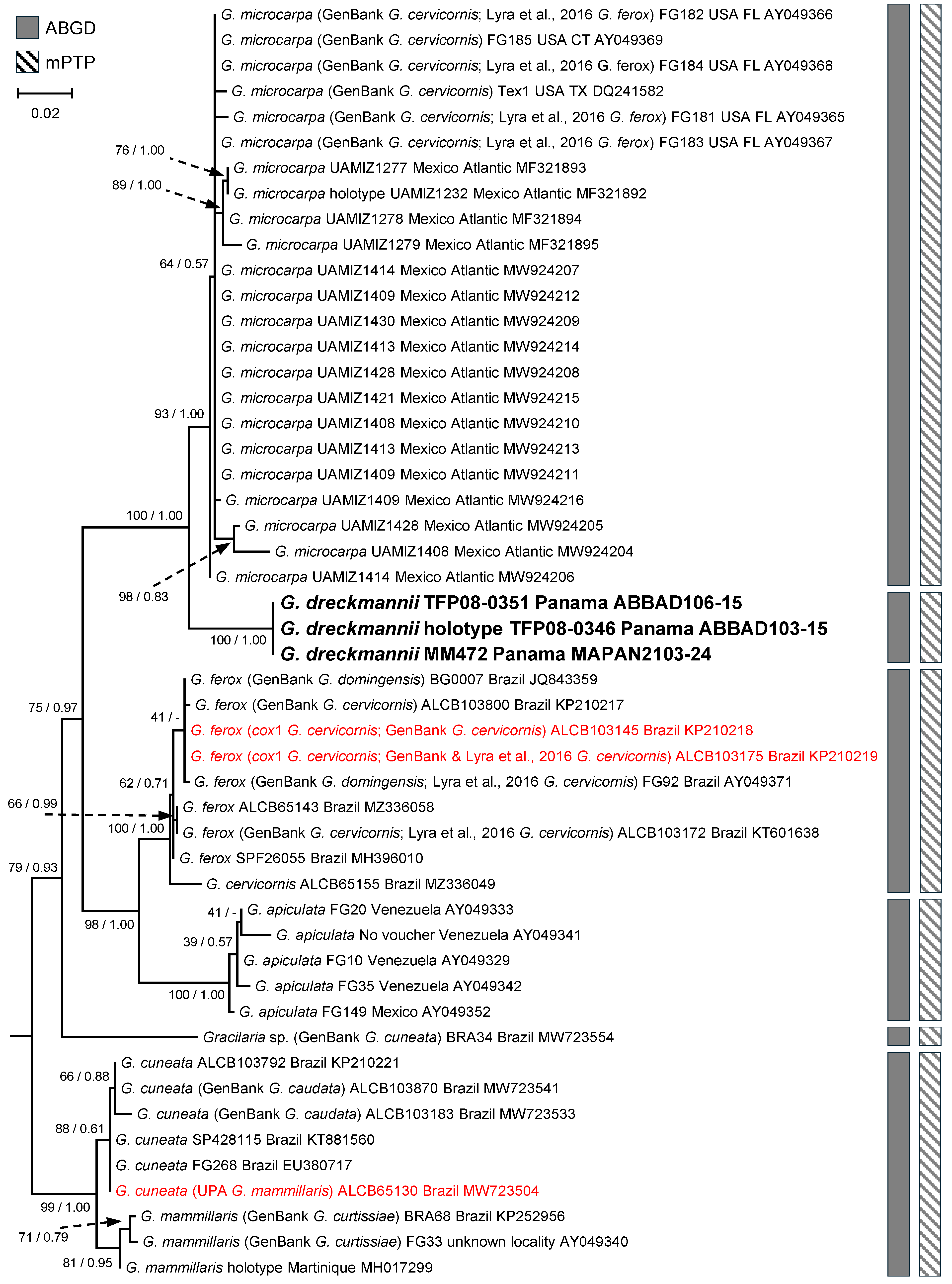
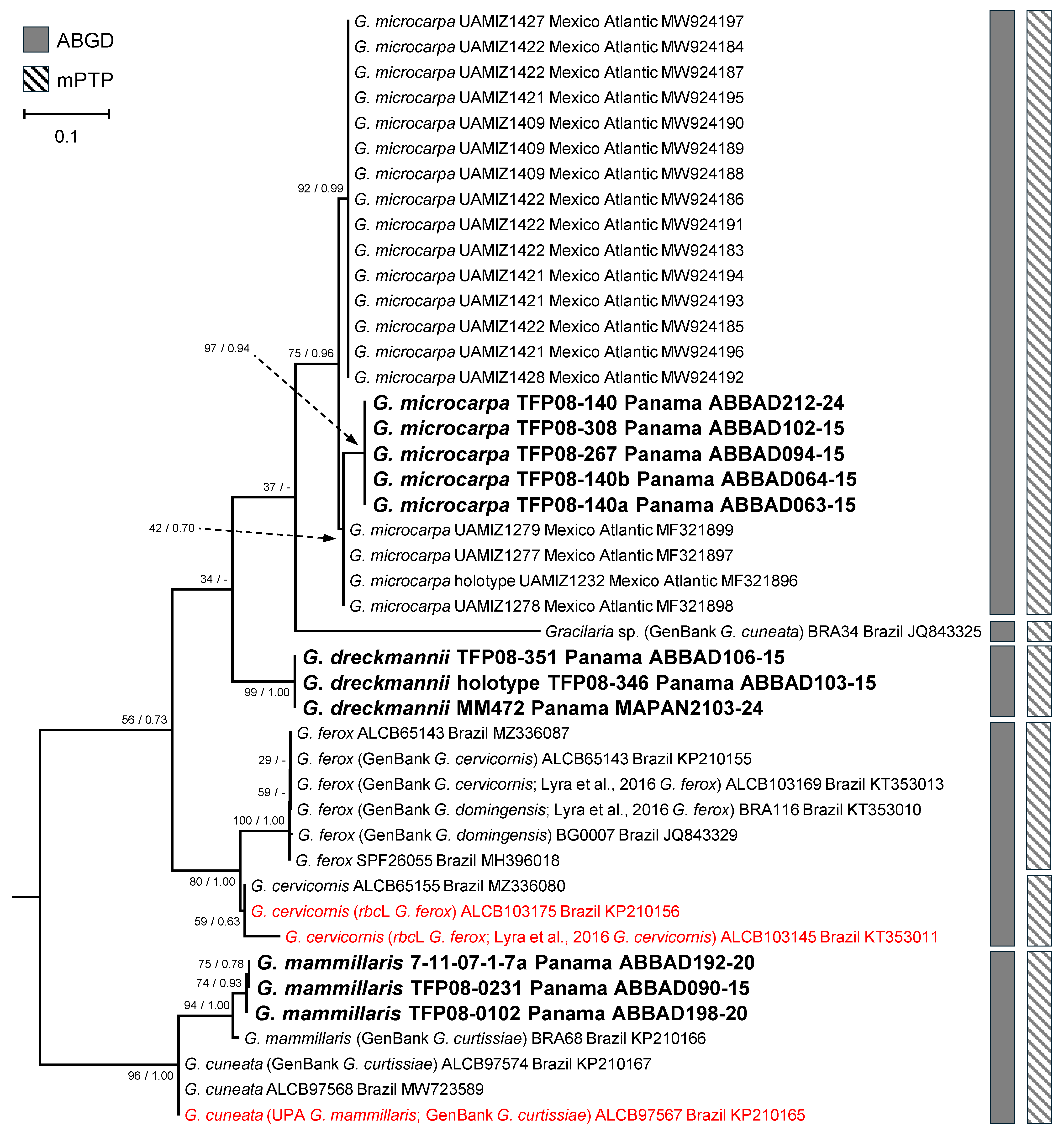
3.1. Barcode Analyses
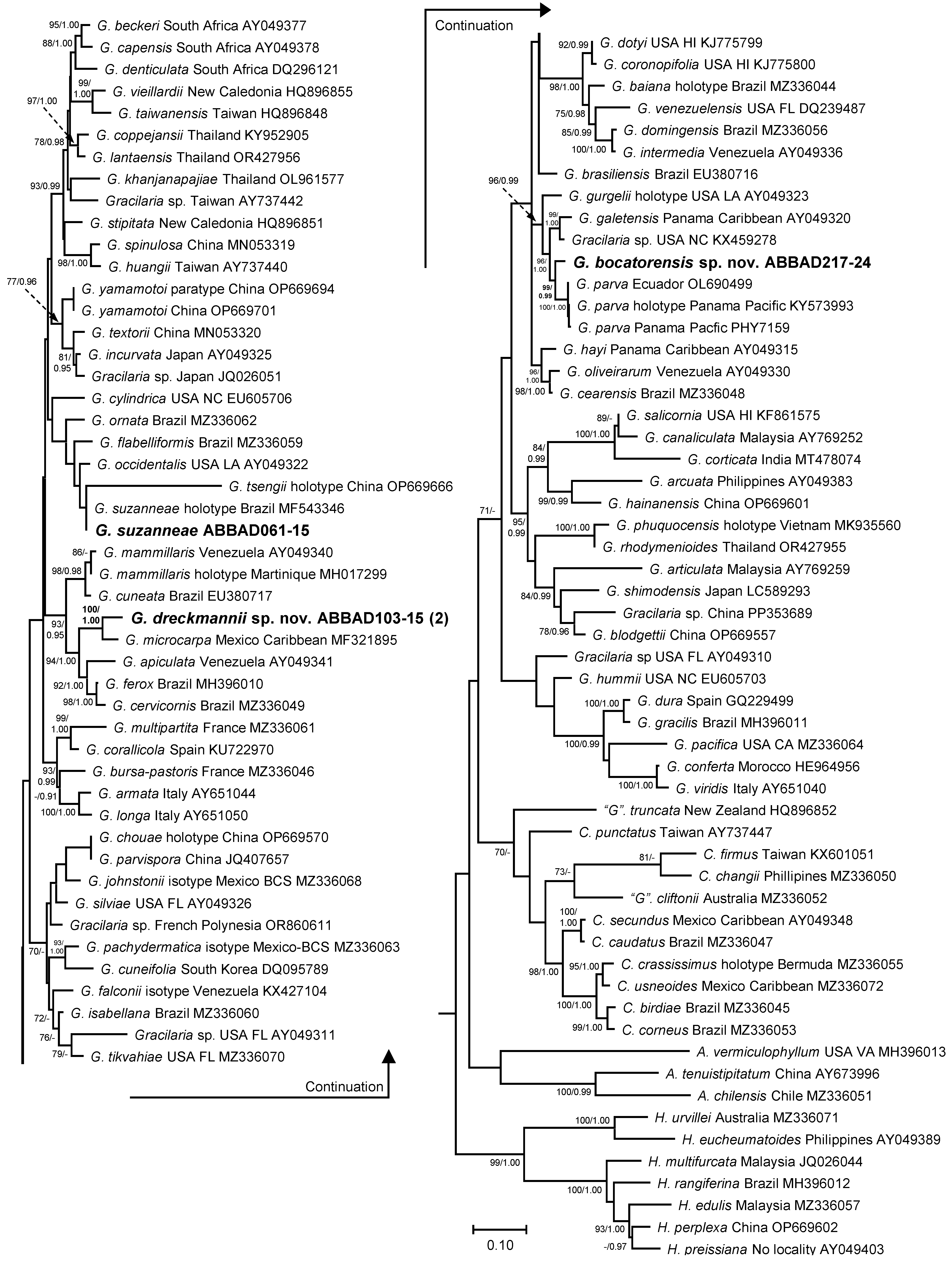
3.2. Gracilaria Specific Sequence Analyses and New Species Support
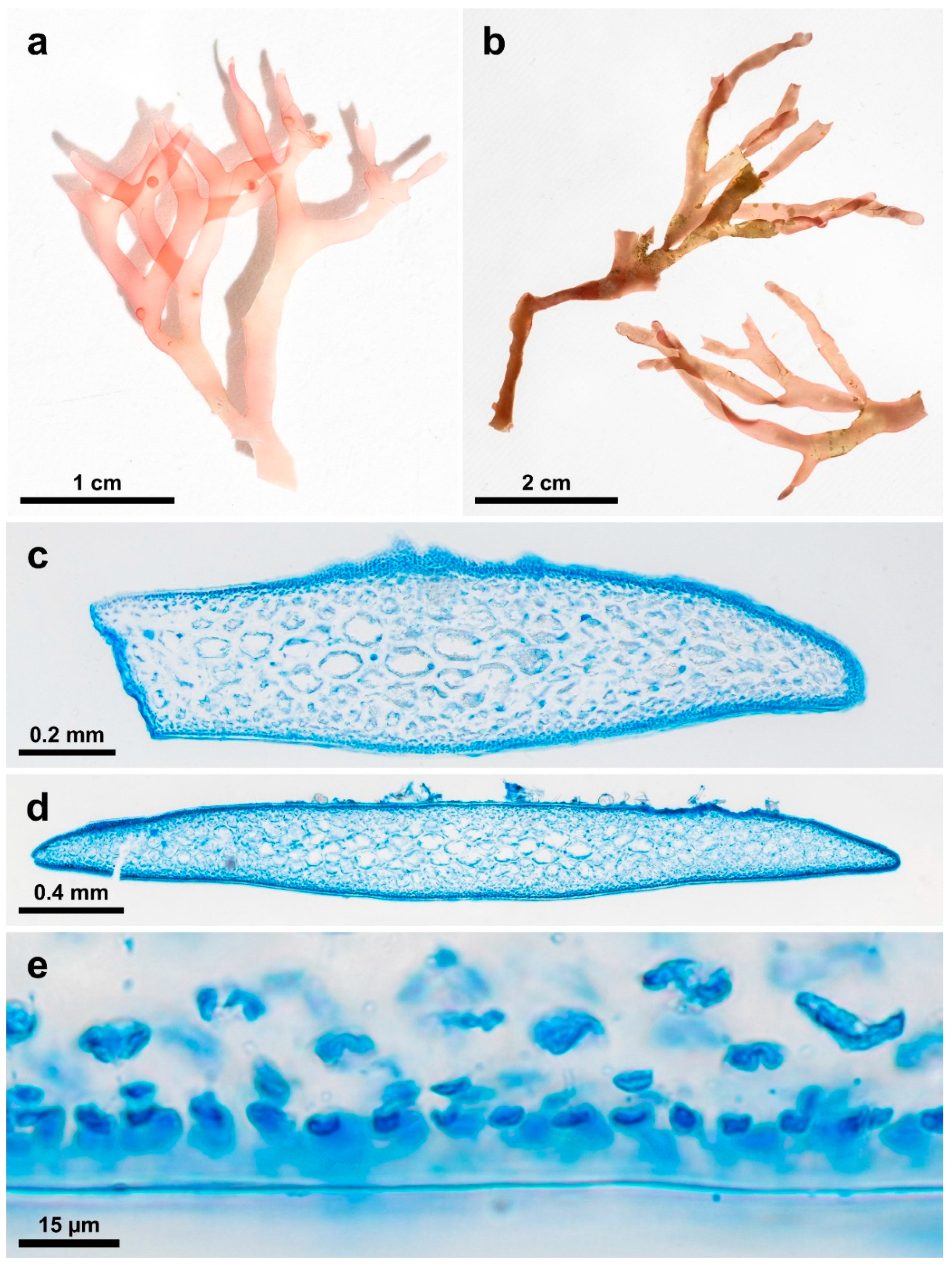
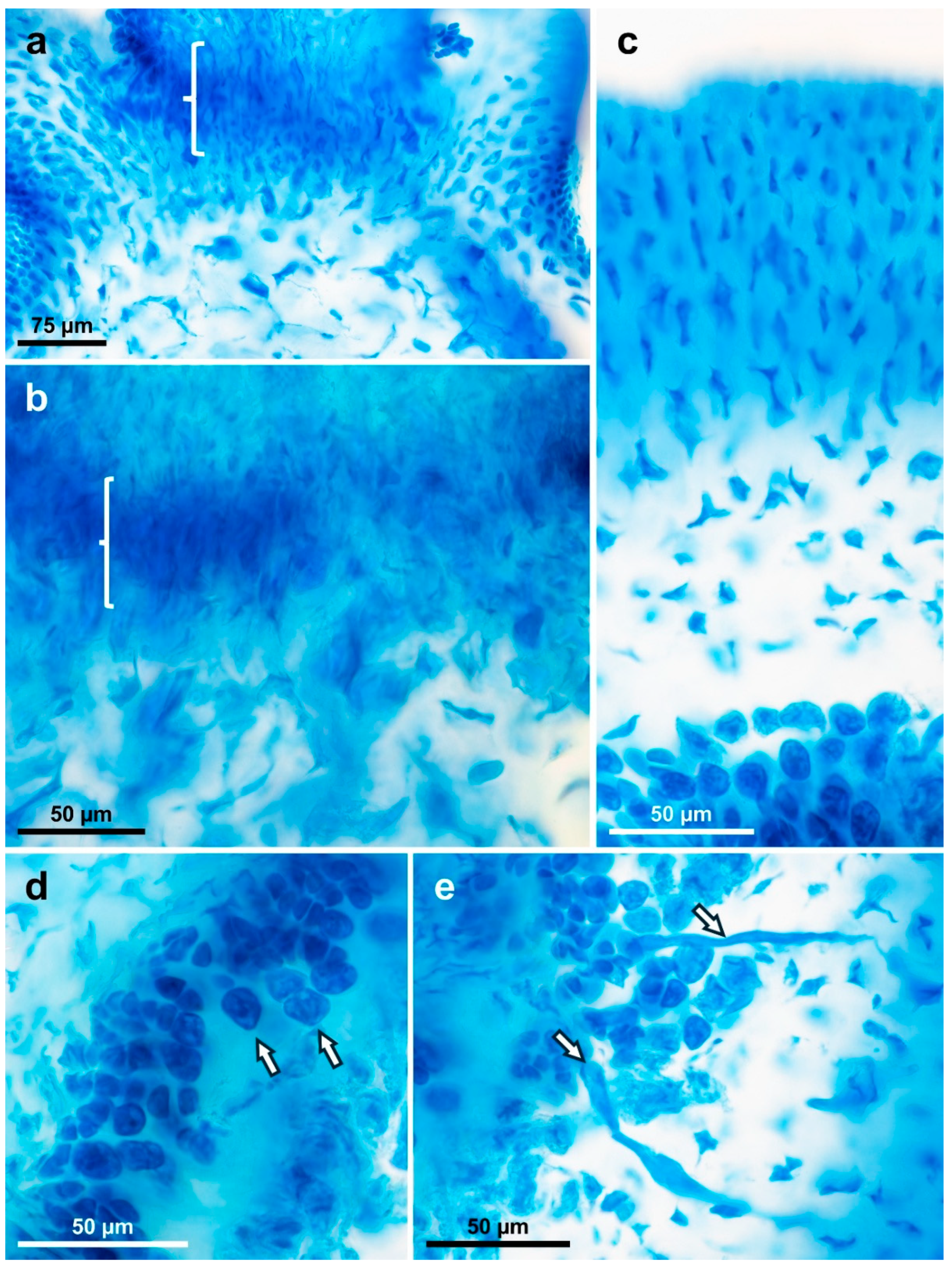
3.3. New Species Descriptions
3.4. New Gracilaria Species Morphological Comparisons
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guzmán, H.M.; Guevara, C.A. Arrecifes coralinos de Bocas del Toro, Panamá: I. Distribución, estructura y estado de conservación de Ios arrecifes continentales de la Laguna de Chiriquí y la Bahía Almirante. Rev. Biol. Trop. 1998, 46, 601–622. [Google Scholar]
- Guzmán, H.M.; Guevara, C.A. Arrecifes coralinos de Bocas del Toro, Panamá: II. Distribución, estructura y estado de conservación de los arrecifes de las Islas Bastimentos, Solarte, Carenero y Colón. Rev. Biol. Trop. 1998, 46, 889–912. [Google Scholar] [CrossRef]
- Guzmán, H.M.; Guevara, C.A. Arrecifes coralinos de Bocas del Toro, Panamá: III. Distribución, estructura, diversidad y estado de conservación de los arrecifes de las islas Pastores, Cristóbal, Popa y Cayo Agua. Rev. Biol. Trop. 1999, 47, 659–676. [Google Scholar] [CrossRef]
- Guzman, H.M.; Guevara, C.A. Arrecifes coralinos de Bocas del Toro, Panama: IV. Distribucion, estructura y estado de conservacion de los arrecifes continentales de Peninsula Valiente. Rev. Biol. Trop. 2001, 49, 53–67. [Google Scholar]
- Collin, R. Ecological monitoring and biodiversity surveys at the smithsonian tropical research institute s Bocas Del Toro research station. Caribb. J. Sci. 2005, 41, 367–373. [Google Scholar]
- Collin, R.; Adelson, A.E.; Altieri, A.H.; Clark, K.E.; Davis, K.; Giddings, S.N.; Kastner, S.; Mach, L.; Pawlak, G.; Sjögersten, S. Using forty years of research to view Bahía Almirante on the caribbean coast of Panama as an integrated social-ecological system. Estuar. Coast. Shelf Sci. 2024, 306, 108878. [Google Scholar] [CrossRef]
- Maslakova, S.; Ellison, C.I.; Hiebert, T.C.; Conable, F.; Heaphy, M.C.; Venera-Pontón, D.E.; Norenburg, J.L.; Schwartz, M.L.; Moss, N.D.; Boyle, M.J. Sampling multiple life stages significantly increases estimates of marine biodiversity. Biol. Lett. 2022, 18, 20210596. [Google Scholar] [CrossRef]
- Bolanos, D.M.; Quiroga, S.Y.; Litvaitis, M.K. Five new species of cotylean flatworms (Platyhelminthes: Polycladida) from the wider Caribbean. Zootaxa 2007, 1650, 1–23. [Google Scholar] [CrossRef]
- Calder, D.R.; Kirkendale, L. Hydroids (Cnidaria, Hydrozoa) from shallow-water environments along the Caribbean coast of Panama. Caribb. J. Sci. 2005, 41, 476–491. [Google Scholar]
- Collin, R.; Diaz, M.C.; Norenburg, J.L.; Rocha, R.d.; Sanchez, J.A.; Schulz, A.; Schwartz, M.L.; Valdes, A. Photographic identification guide to some common marine invertebrates of Bocas Del Toro, Panama. Caribb. J. Sci. 2005, 41, 638–707. [Google Scholar]
- Diaz, M.C. Common sponges from shallow marine habitats from Bocas del Toro region, Panama. Caribb. J. Sci. 2005, 41, 465–475. [Google Scholar]
- Miglietta, M.P.; Piraino, S.; Pruski, S.; Alpizar Gonzalez, M.; Castellanos-Iglesias, S.; Jerónimo-Aguilar, S.; W. Lawley, J.; Maggioni, D.; Martell, L.; Matsumoto, Y. An integrative identification guide to the Hydrozoa (Cnidaria) of Bocas del Toro, Panama. Neotrop. Biodivers. 2018, 4, 103–113. [Google Scholar] [CrossRef]
- Pardos, F.; Sánchez, N.; Herranz, M. Two sides of a coin: The phylum Kinorhyncha in Panama. I) Caribbean Panama. Zool. Anz. -A J. Comp. Zool. 2016, 265, 3–25. [Google Scholar] [CrossRef]
- Rocha, R.M.; Faria, S.B.; Moreno, T.R. Ascidians from Bocas del Toro, Panama. I. Biodiversity. Caribb. J. Sci. 2005, 41, 600–612. [Google Scholar]
- White, K.N. Caribbean Leucothoidae (Crustacea: Amphipoda) of Panama. Gulf Caribb. Res. 2011, 23, 23–35. [Google Scholar] [CrossRef]
- Allen, N.S.; Dauphin, G.; Villarreal, J.C.; Caswell-Levy, C.; Cox, E.R.; Gudiño, J.; Hernández-Rodríguez, E.; Magaña-Marcial, K.Y.; Mezăka, A.; Ramírez-Román, J.D. Bryophytes of mangroves of Bocas del Toro, Panama. Bryophyt. Divers. Evol. 2022, 45, 133–150. [Google Scholar] [CrossRef]
- Goodheart, J.A.; Ellingson, R.A.; Vital, X.G.; Galvão Filho, H.C.; McCarthy, J.B.; Medrano, S.M.; Bhave, V.J.; García-Méndez, K.; Jiménez, L.M.; López, G. Identification guide to the heterobranch sea slugs (Mollusca: Gastropoda) from Bocas del Toro, Panama. Mar. Biodivers. Rec. 2016, 9, 1–31. [Google Scholar] [CrossRef]
- De Grave, S.; Anker, A. An annotated checklist of marine caridean and stenopodidean shrimps (Malacostraca: Decapoda) of the Caribbean coast of Panama. Nauplius 2017, 25, e2017015. [Google Scholar] [CrossRef]
- Hay, M.E.; Norris, J.N. Seasonal reproduction and abundance of six sympatric species of Gracilaria Grev. (Gracilariaceae; Rhodophyta) on a Caribbean subtidal sand plain. In Proceedings of the Eleventh International Seaweed Symposium: Eleventh International Seaweed Symposium, Qingdao, China, 19–25 June 1983; Volume 116, pp. 63–72. [Google Scholar]
- Hay, M.E. The functional morphology of turf-forming seaweeds: Persistence in stressful marine habitats. Ecology 1981, 62, 739–750. [Google Scholar] [CrossRef]
- Sangil, C.; Guzman, H.M. Macroalgal communities on multi-stressed coral reefs in the Caribbean: Long-term changes, spatial variations, and relationships with environmental variables. J. Sea Res. 2016, 117, 7–19. [Google Scholar] [CrossRef]
- Sangil, C.; Guzman, H.M. Macroalgal community response to herbivores and sediment deposition: An indicator of coral reef degradation. J. Appl. Phycol. 2020, 32, 1405–1419. [Google Scholar] [CrossRef]
- Earle, S.A. A review of the marine plants of Panama. Bull. Biol. Soc. Wash. 1972, 2, 69–87. [Google Scholar]
- Taylor, W.R. Caribbean Marine Algae of the Allan Hancock Expedition, 1939; University of Southern California Press: Los Angeles, CA, USA, 1942. [Google Scholar]
- Taylor, W.R. Notes on algae from the tropical Atlantic Ocean. Am. J. Bot. 1929, 16, 621–630. [Google Scholar] [CrossRef]
- Wysor, B.; Kooistra, W. An annotated list of marine Chlorophyta from the Caribbean coast of the Republic of Panama. Nova Hedwig. 2003, 77, 487–523. [Google Scholar] [CrossRef]
- Wysor, B.; De Clerck, O. An updated and annotated list of marine brown algae (Phaeophyceae) of the Caribbean coast of the Republic of Panama. Bot. Mar. 2003, 46, 151–160. [Google Scholar] [CrossRef]
- Mamoozadeh, N.R.; Freshwater, D.W. Polysiphonia sensu lato (Ceramiales, Florideophyceae) species of Caribbean Panama including Polysiphonia lobophoralis sp. nov. and Polysiphonia nuda sp. nov. Bot. Mar. 2012, 55, 317–347. [Google Scholar] [CrossRef]
- Freshwater, D.W.; Tudor, K.; O’shaughnessy, K.; Wysor, B. DNA barcoding in the red algal order Gelidiales: Comparison of COI with rbcL and verification of the ”barcoding gap”. Cryptogam. Algol. 2010, 31, 435. [Google Scholar]
- Freshwater, D.W.; Shahnaz, L. Phylogenetic relationships of Pakistan Gelidium (Gelidiales, Rhodophyta) species with recognition of Gelidium pakistanicum stat. nov. Bot. Mar. 2019, 62, 141–147. [Google Scholar] [CrossRef]
- Gabrielson, P.W.; Hughey, J.R.; Diaz-Pulido, G. Genomics reveals abundant speciation in the coral reef building alga Porolithon onkodes (Corallinales, Rhodophyta). J. Phycol. 2018, 54, 429–434. [Google Scholar] [CrossRef]
- Hernandez-Kantun, J.J.; Gabrielson, P.; Hughey, J.R.; Pezzolesi, L.; Rindi, F.; Robinson, N.M.; Peña, V.; Riosmena-Rodriguez, R.; Gall, L.L.; Adey, W. Reassessment of branched Lithophyllum spp. (Corallinales, Rhodophyta) in the Caribbean Sea with global implications. Phycologia 2016, 55, 619–639. [Google Scholar] [CrossRef]
- Iha, C.; O’Shaughnessy, K.A.; Guimarães, S.M.; Oliveira, M.C.; Freshwater, D.W. Taxonomic reappraisal of Gelidium coarctatum (Gelidiales, Rhodophyta) and Gelidium lineare sp. nov. from the tropical western Atlantic. Phycologia 2016, 55, 555–563. [Google Scholar] [CrossRef]
- Krayesky, D.M.; Norris, J.N.; West, J.A.; Fredericq, S. The Caloglossa leprieurii complex (Delesseriaceae, Rhodophyta) in the Americas: The elucidation of overlooked species based on molecular and morphological evidence. Cryptogam. Algol. 2011, 32, 37–62. [Google Scholar] [CrossRef]
- Richards, J.L.; Sauvage, T.; Schmidt, W.E.; Fredericq, S.; Hughey, J.R.; Gabrielson, P.W. The coralline genera Sporolithon and Heydrichia (Sporolithales, Rhodophyta) clarified by sequencing type material of their generitypes and other species. J. Phycol. 2017, 53, 1044–1059. [Google Scholar] [CrossRef]
- Richards, J.L.; Schmidt, W.E.; Fredericq, S.; Sauvage, T.; Pena, V.; Le Gall, L.; Mateo-Cid, L.E.; Mendoza-Gonzalez, A.C.; Hughey, J.R.; Gabrielson, P.W. DNA sequencing of type material and newly collected specimens reveals two heterotypic synonyms for Harveylithon munitum (Metagoniolithoideae, Corallinales, Rhodophyta) and three new species. J. Phycol. 2021, 57, 1234–1253. [Google Scholar] [CrossRef]
- Rodriguez-Prieto, C.; Freshwater, D.W.; Hommersand, M.H. Morphology and phylogenetic systematics of Ptilocladiopsis horrida and proposal of the Ptilocladiopsidaceae fam. nov.(Gigartinales, Rhodophyta). Phycologia 2014, 53, 383–395. [Google Scholar] [CrossRef]
- Schmidt, W.E.; Gurgel, C.F.D.; Fredericq, S.L. Taxonomic transfer of the red algal genus Gloiosaccion to Chrysymenia (Rhodymeniaceae, Rhodymeniales), including the description of a new species, Chrysymenia pseudoventricosa, for the Gulf of Mexico. Phytotaxa 2016, 243, 54–70. [Google Scholar] [CrossRef]
- Won, B.Y.; Cho, T.O.; Fredericq, S. Morphological and molecular characterization of species of the genus Centroceras (Ceramiaceae, Ceramiales), including two new species. J. Phycol. 2009, 45, 227–250. [Google Scholar] [CrossRef]
- Averza Colamarco, A.A.; Almodóvar, L.R.; Martínez, A. Compendio bibliográfico de las algas del Caribe de Panamá: Las algas verdes. Tecnociencia 2002, 4, 141–160. [Google Scholar]
- Averza Colamarco, A.A. Registro de algas pardas del caribe de Panamá. Tecnociencia 2006, 8, 115–127. [Google Scholar]
- Collin, R.; Madrid, M. STRI—SYMBIOTA Panamabiota Portal. Project Algae of Panama. Available online: https://panamabiota.org/stri/projects/index.php?pid=18 (accessed on 23 January 2025).
- Hurtado, A. Different colour morphotypes of Kappaphycus alvarezii and Kappaphycus striatum used in commercial farming. Taxon. S. Asian Seaweeds II Monogr. Ser. 2013, 15, 83–92. [Google Scholar]
- Meneses, I. Morphological variation in three species of the genus Ceramium (Ceramiales, Rhodophyta) from Hawaii: Differences between reproductive phases and phenotypic plasticity. Bot. Mar. 1992, 35, 461–474. [Google Scholar] [CrossRef]
- Nauer, F.; Jesus, P.B.; Cassano, V.; Nunes, J.M.C.; Schnadelbach, A.S.; Oliveira, M.C. A taxonomic review of the genus Hypnea (Gigartinales, Rhodophyta) in Brazil based on DNA barcode and morphology. Braz. J. Bot. 2019, 42, 561–574. [Google Scholar] [CrossRef]
- Zanolla, M.; Carmona, R.; De la Rosa, J.; Salvador, N.; Sherwood, A.; Andreakis, N.; Altamirano, M. Morphological differentiation of cryptic lineages within the invasive genus Asparagopsis (Bonnemaisoniales, Rhodophyta). Phycologia 2014, 53, 233–242. [Google Scholar] [CrossRef]
- Kim, M.-S.; Yang, E.C.; Boo, S.M. Taxonomy and phylogeny of flattened species of Gracilaria (Gracilariceae, Rhodophyta) from Korea based on morphology and protein-coding plastid rbcL and psbA sequences. Phycologia 2006, 45, 520–528. [Google Scholar] [CrossRef]
- Saunders, G.W. Applying DNA barcoding to red macroalgae: A preliminary appraisal holds promise for future applications. Philos. Trans. R. Soc. B-Biol. Sci. 2005, 360, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, A.R.; Kurihara, A.; Conklin, K.Y.; Sauvage, T.; Presting, G.G. The Hawaiian Rhodophyta Biodiversity Survey (2006-2010): A summary of principal findings. BMC Plant Biol. 2010, 10, 1–29. [Google Scholar] [CrossRef]
- Bringloe, T.T.; Sjøtun, K.; Saunders, G.W. A DNA barcode survey of marine macroalgae from Bergen (Norway). Mar. Biol. Res. 2019, 15, 580–589. [Google Scholar] [CrossRef]
- Silva, M.Y.; Hughey, J.R. Complete mitochondrial genome of the holotype specimen of Wildemania schizophylla (Bangiales: Rhodophyta). Mitochondrial DNA Part A 2016, 27, 1001–1002. [Google Scholar] [CrossRef]
- Taylor, R.L.; Bailey, J.C.; Freshwater, D.W. Systematics of Cladophora spp. (Chlorophyta) from North Carolina, USA, based upon morphology and DNA sequence data with a description of Cladophora subtilissima sp nov. J. Phycol. 2017, 53, 541–556. [Google Scholar] [CrossRef]
- Hughey, J.R.; Maggs, C.A.; Mineur, F.; Jarvis, C.; Miller, K.A.; Shabaka, S.H.; Gabrielson, P.W. Genetic analysis of the Linnaean Ulva lactuca (Ulvales, Chlorophyta) holotype and related type specimens reveals name misapplications, unexpected origins, and new synonymies. J. Phycol. 2019, 55, 503–508. [Google Scholar] [CrossRef]
- Boo, G.H.; Hughey, J.R.; Miller, K.A.; Boo, S.M. Mitogenomes from type specimens, a genotyping tool for morphologically simple species: Ten genomes of agar-producing red algae. Sci. Rep. 2016, 6, 13. [Google Scholar] [CrossRef]
- Boo, G.H.; Hughey, J.R. Phylogenomics and multigene phylogenies decipher two new cryptic marine algae from California, Gelidium gabrielsonii and G. kathyanniae (Gelidiales, Rhodophyta). J. Phycol. 2019, 55, 160–172. [Google Scholar] [CrossRef]
- de Jesus, P.B.; de Mattos Lyra, G.; Zhang, H.; Fujii, M.T.; Nauer, F.; de Castro Nunes, J.M.; Davis, C.C.; Oliveira, M.C. Phylogenomics and taxon-rich phylogenies of new and historical specimens shed light on the systematics of Hypnea (Cystocloniaceae, Rhodophyta). Mol. Phylogenetics Evol. 2023, 183, 107752. [Google Scholar] [CrossRef] [PubMed]
- Gavio, B.; Fredericq, S. Botryocladia caraibica (Rhodymeniales, Rhodophyta), a new species from the Caribbean. Cryptogam. Algol. 2003, 24, 93–106. [Google Scholar]
- Gurgel, C.F.D.; Liao, L.M.; Fredericq, S.; Hommersand, M.H. Systematics of Gracilariopsis (Gracilariales, Rhodophyta) based on rbcL sequence analyses and morphological evidence. J. Phycol. 2003, 39, 154–171. [Google Scholar] [CrossRef]
- Gurgel, C.F.D.; Fredericq, S. Systematics of the Gracilariaceae (Gracilariales, Rhodophyta): A critical assessment based on rbcL sequence analyses. J. Phycol. 2004, 40, 138–159. [Google Scholar] [CrossRef]
- Kantun, J.J.H.; Riosmena-Rodriguez, R.; Adey, W.H.; Rindi, F. Analysis of the cox2-3 spacer region for population diversity and taxonomic implications in rhodolith-forming species (Rhodophyta: Corallinales). Phytotaxa 2014, 190, 331–354. [Google Scholar] [CrossRef][Green Version]
- Giorgi, A.; Monti, M.; Maggioni, D.; Gabrielson, P.W.; Steneck, R.S.; Kocot, K.; Olson, J.B. DNA sequencing reveals higher taxonomic diversity of coralline algae (Corallinales and Hapalidiales, Rhodophyta) in the tropical western North Atlantic that complicates ecological studies. Bot. Mar. 2024, 67, 561–586. [Google Scholar] [CrossRef]
- Rösler, A.; Perfectti, F.; Peña, V.; Braga, J.C. Phylogenetic relationships of corallinaceae (Corallinales, Rhodophyta): Taxonomic implications for reef-building corallines. J. Phycol. 2016, 52, 412–431. [Google Scholar] [CrossRef]
- Cramer, K.L.; Jackson, J.B.; Angioletti, C.V.; Leonard-Pingel, J.; Guilderson, T.P. Anthropogenic mortality on coral reefs in Caribbean Panama predates coral disease and bleaching. Ecol. Lett. 2012, 15, 561–567. [Google Scholar] [CrossRef]
- Cramer, K.L. History of human occupation and environmental change in western and central Caribbean Panama. Bull. Mar. Sci. 2013, 89, 955–982. [Google Scholar] [CrossRef]
- Seemann, J.; González, C.T.; Carballo-Bolaños, R.; Berry, K.; Heiss, G.A.; Struck, U.; Leinfelder, R.R. Assessing the ecological effects of human impacts on coral reefs in Bocas del Toro, Panama. Environ. Monit. Assess. 2014, 186, 1747–1763. [Google Scholar] [CrossRef]
- García Armuelles, L. Empresa Desarrollará Cultivo de Algas en La Comarca Ngäbe Buglé y Bocas del Toro. La Estrella De Panamá. 2024. Available online: https://www.laestrella.com.pa/economia/empresa-desarrollara-cultivo-de-algas-en-la-comarca-ngabe-bugle-y-bocas-del-toro-MG9954718 (accessed on 12 January 2025).
- Carrasquilla, E. Por la cual se autoriza provisionalmente a Algas Panameñas, S.A., para operar y desarrollar la maricultura (cultivo de algas), por el periodo de un (1) año, en un espejo de agua de mar con una superficie de 8441 ha + 5336.93 m2, ubicado en la comarca de Ngäbe Bugle distrito de Kusapin, Kankitú y Jirondai, corregimiento de Bahía Azul, Bisiria y Gwaribiara; y provincia de Bocas del Toro, distrito de Bocas del Toro, corregimiento de Punta Laurel, Cauchero y Tierra Oscura. GACETA 30185, jueves 26 de diciembre de 2024, Resolución N° ADM/ARAP 096. 2024. Available online: https://www.gacetaoficial.gob.pa/ (accessed on 12 January 2025).
- Schloder, C.; Canning-Clode, J.; Saltonstall, K.; Strong, E.E.; Ruiz, G.M.; Torchin, M.E. The Pacific bivalve Anomia peruviana in the Atlantic: A recent invasion across the Panama Canal? Aquat. Invasions 2013, 8, 443–448. [Google Scholar] [CrossRef]
- Saunders, G.W.; McDevit, D.C. Methods for DNA barcoding photosynthetic protists emphasizing the macroalgae and diatoms. In DNA Barcodes: Methods and Protocols; Kress, W.J., Erickson, D.L., Eds.; Humana Press: Totowa, NJ, USA, 2012; pp. 207–222. [Google Scholar]
- Sherwood, A.R.; Presting, G.G. Universal primers amplify a 23S rDNA plastid marker in eukaryotic algae and cyanobacteria. J. Phycol. 2007, 43, 605–608. [Google Scholar] [CrossRef]
- Freshwater, D.W.; Rueness, J. Phylogenetic-relationships of some european Gelidium (Gelidiales, Rhodophyta) species, based on rbcL nucleotide-sequence analysis. Phycologia 1994, 33, 187–194. [Google Scholar] [CrossRef]
- Freshwater, D.W.; Montgomery, F.; Greene, J.K.; Hamner, R.M.; Williams, M.; Whitfield, P.E. Distribution and identification of an invasive Gracilaria species that is hampering commercial fishing operations in southeastern North Carolina, USA. Biol. Invasions 2006, 8, 631–637. [Google Scholar] [CrossRef]
- Hommersand, M.H.; Freshwater, D.W. Gracilaria hummii sp nov (Gracilariales, Rhodophyta), a new name for the agarophyte “Gracilaria confervoides” harvested in north carolina during World War II. J. Phycol. 2009, 45, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Croce, M.E.; Freshwater, D.W. Intertidal Species of Gelidium from the Temperate Coast of Argentina. Diversity 2024, 16, 399. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Puillandre, N.; Lambert, A.; Brouillet, S.; Achaz, G. ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Mol. Ecol. 2012, 21, 1864–1877. [Google Scholar] [CrossRef] [PubMed]
- Kapli, P.; Lutteropp, S.; Zhang, J.; Kobert, K.; Pavlidis, P.; Stamatakis, A.; Flouri, T. Multi-rate Poisson tree processes for single-locus species delimitation under maximum likelihood and Markov chain Monte Carlo. Bioinformatics 2017, 33, 1630–1638. [Google Scholar] [CrossRef] [PubMed]
- Gurgel, C.F.D.; Fredericq, S.; Norris, J.N.; Guiry, M.D. Crassiphycus Guiry, Gurgel, J.N.Norris & Fredericq, gen. nov., a replacement name for Crassa Gurgel, J.N.Norris & Fredericq, nom. inval. (Gracilariaceae, Rhodophyta), with some additional nomenclatural notes. Not. Algarum 2018, 82, 1–4. [Google Scholar]
- Gurgel, C.F.D.; Norris, J.N.; Schmidt, W.E.; Le, H.N.; Fredericq, S. Systematics of the Gracilariales (Rhodophyta) including new subfamilies, tribes, subgenera, and two new genera, Agarophyton gen. nov. and Crassa gen. nov. Phytotaxa 2018, 374, 1–23. [Google Scholar] [CrossRef]
- Gurgel, C.F.D.; Soares, L.P.; Norris, J.N.; Fujii, M.T.; Schmidt, W.E.; Fredericq, S. Molecular systematics of Crassiphycus and Hydropuntia (Gracilariales, Rhodophyta) with the description of poorly known taxa in the Western Atlantic Ocean. Eur. J. Phycol. 2021, 56, 216–229. [Google Scholar] [CrossRef]
- Lyra, G.d.M.; Iha, C.; Grassa, C.J.; Cai, L.; Zhang, H.; Lane, C.; Blouin, N.; Oliveira, M.C.; Nunes, J.M.C.; Davis, C.C. Phylogenomics, divergence time estimation and trait evolution provide a new look into the Gracilariales (Rhodophyta). Mol. Phylogenet. Evol. 2021, 165, 107294. [Google Scholar] [CrossRef]
- Littler, D.S.; Littler, M.M. Caribbean Reef Plants; Offshore Graphics Incorporated: Washington, DC, USA, 2000. [Google Scholar]
- Thiers, B.M. Index Herbariorum. Available online: https://sweetgum.nybg.org/science/ih/ (accessed on 25 December 2024).
- Guiry, M.D.; Guiry, G.M. AlgaeBase. Listing the World’s Algae. Available online: https://www.algaebase.org/ (accessed on 22 January 2025).
- Lyra, G.d.M.; Gurgel, C.F.D.; Costa, E.d.S.; de Jesus, P.B.; Oliveira, M.C.; Oliveira, E.C.; Davis, C.C.; Nunes, J.M.d.C. Delimitating cryptic species in the Gracilaria domingensis complex (Gracilariaceae, Rhodophyta) using molecular and morphological data. J. Phycol. 2016, 52, 997–1017. [Google Scholar] [CrossRef]
- Dreckmann, K.M.; Nunez Resendiz, M.L.; Sentíes, A. Gracilaria microcarpa sp. nov. (Gracilariaceae, Rhodophyta) from the southwestern Gulf of Mexico. Bot. Mar. 2018, 61, 115–125. [Google Scholar] [CrossRef]
- Iha, C.; Grassa, C.J.; Lyra, G.d.M.; Davis, C.C.; Verbruggen, H.; Oliveira, M.C. Organellar genomics: A useful tool to study evolutionary relationships and molecular evolution in Gracilariaceae (Rhodophyta). J. Phycol. 2018, 54, 775–787. [Google Scholar] [CrossRef]
- Hardesty, D.M.; Freshwater, D.W. Studies of North Carolina marine algae XIV: Increased diversity of flattened offshore Gracilaria (Gracilariales, Rhodophyta) species revealed by DNA sequences of contemporary specimens and the G. mammillaris holotype. Bot. Mar. 2018, 61, 407–413. [Google Scholar] [CrossRef]
- Gurgel, C.F.D.; Fredericq, S.; Norris, J.N. Molecular systematics and taxonomy of flattened species of Gracilaria Greville (Gracilariaceae, Gracilariales, Rhodophyta) from the Western Atlantic. Taxon. Econ. Seaweeds Ref. Pac. Other Locat. 2004, 9, 159–199. [Google Scholar]
- Freshwater, D.W.; Williamson, B.; Gabrielson, P.W.; Brandt, M. Gracilaria parva sp. nov. (Gracilariales, Rhodophyta) a diminutive Species from the Tropical Eastern Pacific. Taxonomy 2022, 2, 48–56. [Google Scholar] [CrossRef]
- Børgesen, F. The marine algae of the Danish West Indies. Part3. Rhodophyceae. Dan. Bot. Ark. 1915, 3, 1–498. [Google Scholar]
- Lyra, G.d.M.; Nunes, J.M.d.C.; Pestana, E.M.d.S.; de Matos, J.C.G.; Caires, T.A.; de Jesus, P.B.; Costa, E.d.S.; Oliveira, M.C. Diversity of Gracilariaceae (Rhodophyta) in Brazil: Integrating morphological and molecular data. Phytotaxa 2021, 496, 1–53. [Google Scholar] [CrossRef]
- Soares, L.P.; Gurgel, C.F.D.; Fujii, M.T. Taxonomic reassessment of Gracilaria cearensis (Rhodophyta, Gracilariales), a poorly defined yet common flattened species based on morphological and molecular analysis including topotype collections. Phytotaxa 2015, 201, 241–255. [Google Scholar] [CrossRef]
- Gurgel, C.F.D.; Fredericq, S.; Norris, J.N. Gracilaria apiculata and G. flabelliformis (Gracilariaceae, Rhodophyta): Restoring old names for common tropical western Atlantic species, including the recognition of three new subspecies, and a replacement name for G. lacinulata. Cryptogam. Algol. 2004, 25, 367–396. [Google Scholar]
- Collin, R.; Venera-Pontón, D.E.; Macdonald, K.; Driskell, A.C.; Boyle, M.J. Knots, spoons, and cloches: DNA barcoding unusual larval forms helps document the diversity of Neotropical marine annelids. Invertebr. Biol. 2021, 140, e12311. [Google Scholar] [CrossRef]
- Venera-Pontón, D.E.; Driskell, A.C.; De Grave, S.; Felder, D.L.; Scioli, J.A.; Collin, R. Documenting decapod biodiversity in the Caribbean from DNA barcodes generated during field training in taxonomy. Biodivers. Data J. 2020, 8, e47333. [Google Scholar] [CrossRef]
- Neill, J.L.; Dillon, M.M.; Bilecki, C.; Bollinger, S.; Dansereau, S.C.; Flores, A.; Garcia, K.B.G.; Hernandez-Thorn, R.A.; Kim, M.; Martinez, P.V. New contributions to the heterobranch sea slug biodiversity of Bocas del Toro, Panama. Nautilus 2024, 138, 75–81. [Google Scholar]
- Freshwater, D.W.; Idol, J.N.; Parham, S.L.; Fernández-García, C.; León, N.; Gabrielson, P.W.; Wysor, B. Molecular assisted identification reveals hidden red algae diversity from the Burica Peninsula, Pacific Panama. Diversity 2017, 9, 19. [Google Scholar] [CrossRef]
- Lyra, G.d.M.; Gurgel, C.F.D.; Costa, E.d.S.; De Jesus, P.B.; Caires, T.A.; de Matos, J.C.G.; Oliveira, M.C.; Oliveira, E.C.; Nunes, J. A new tropical species of Gracilariaceae (Rhodophyta, Gracilariales): Gracilaria silviae sp. nov. Phytotaxa 2015, 222, 199–210. [Google Scholar] [CrossRef]
- Lyra, G.d.M.; Costa, E.d.S.; de Jesus, P.B.; de Matos, J.C.G.; Caires, T.A.; Oliveira, M.C.; Oliveira, E.C.; Xi, Z.; Nunes, J.M.d.C.; Davis, C.C. Phylogeny of Gracilariaceae (Rhodophyta): Evidence from plastid and mitochondrial nucleotide sequences. J. Phycol. 2015, 51, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Soares, L.P.; Gurgel, C.F.D.; Fujii, M.T. Gracilaria suzannae sp. nov. (Gracilariales, Rhodophyta), a new flattened species from northeast Brazil based on morphological and molecular evidence. Phycologia 2018, 57, 345–353. [Google Scholar] [CrossRef]
- Vilchis, M.I.; Dreckmann, K.M.; Hernández, O.E.; Palma Ortíz, C.A.; Núñez Resendiz, M.L.; Sentíes, A. Molecular assessment of the species of Gracilariaceae (Gracilariales, Rhodophyta) from the Yucatan Peninsula, Mexico, including two new records for the Mexican Atlantic. Bot. Sci. 2022, 100, 493–505. [Google Scholar] [CrossRef]
- Torrano-Silva, B.N.; Vieira, B.R.; Riosmena-Rodríguez, R.; Oliveira, M.C. Guidelines for DNA barcoding of coralline algae, focusing on Lithophylloideae (Corallinales) from Brazil. Bot. Mar. 2018, 61, 127–140. [Google Scholar] [CrossRef]
- Gabriel, D.; Schmidt, W.E.; Micael, J.; Moura, M.; Fredericq, S. DNA Barcode-Assisted Inventory of the Marine Macroalgae from the Azores, Including New Records. Phycology 2024, 4, 65–86. [Google Scholar] [CrossRef]
- Manghisi, A.; Miladi, R.; Minicante, S.A.; Genovese, G.; Le Gall, L.; Abdelkafi, S.; Saunders, G.W.; Morabito, M. DNA barcoding sheds light on novel records in the Tunisian red algal flora. Cryptogam. Algol. 2019, 40, 5–27. [Google Scholar] [CrossRef]
- Kogame, K.; Uwai, S.; Anderson, R.; Choi, H.-G.; Bolton, J. DNA barcoding of South African geniculate coralline red algae (Corallinales, Rhodophyta). S. Afr. Bot. 2017, 108, 337–341. [Google Scholar] [CrossRef]
- Castrellón, M.G.; Lu, C.; Domínguez, I.; Matos, R.; Anguizola, K.; Popescu, I. Spatiotemporal distribution of salinity in Gatun Lake and the Panama Canal pre-and post-expansion. J. Hydrol. Reg. Stud. 2025, 58, 102199. [Google Scholar] [CrossRef]
- Mamoozadeh, N.R.; Freshwater, D.W. Taxonomic notes on Caribbean Neosiphonia and Polysiphonia (Ceramiales, Florideophyceae): Five species from Florida, USA and Mexico. Bot. Mar. 2011, 54, 269–292. [Google Scholar] [CrossRef]
- Pérez González, C.M. Caracterización biológica y química de Kappaphycus alvarezii de Panamá. Ph.D. Thesis, Universidad de Las Palmas de Gran Canaria, Las Palmas, Spain, 2013. [Google Scholar]
- Batista De Vega, G.E. Cultivo ecosostenible de Kappaphycus alvarezii en Panama. Ph.D. Thesis, Universidad de Las Palmas de Gran Canaria, Las Palmas de Gran Canaria, Las Palmas, Spain, 2009. [Google Scholar]
- Sellers, A.J.; Saltonstall, K.; Davidson, T.M. The introduced alga Kappaphycus alvarezii (Doty ex PC Silva, 1996) in abandoned cultivation sites in Bocas del Toro, Panama. BioInvasions Rec. Int. J. Field Res. Biol. Invasions 2015, 4, 1–7. [Google Scholar]
- Andreakis, N.; Procaccini, G.; Maggs, C.; Kooistra, W.H. Phylogeography of the invasive seaweed Asparagopsis (Bonnemaisoniales, Rhodophyta) reveals cryptic diversity. Mol. Ecol. 2007, 16, 2285–2299. [Google Scholar] [CrossRef]
- Lin, S.M.; Fredericq, S.; Hommersand, M.H. Systematics of the Delesseriaceae (Ceramiales, Rhodophyta) based on large subunit rDNA and rbcL sequences, including the Phycodryoideae, subfam. nov. J. Phycol. 2001, 37, 881–899. [Google Scholar] [CrossRef]
- Krayesky, D.M.; Norris, J.N.; West, J.A.; Kamiya, M.; Viguerie, M.; Wysor, B.S.; Fredericq, S. Two new species of Caloglossa (Delesseriaceae, Rhodophyta) from the Americas, C. confusa and C. fluviatilis spp. nov. Phycologia 2012, 51, 513–530. [Google Scholar] [CrossRef][Green Version]
- Zuccarello, G.C.; West, J.A. Phylogeography of the Bostrychia calliptera–B. pinnata complex (Rhodomelaceae, Rhodophyta) and divergence rates based on nuclear, mitochondrial and plastid DNA markers. Phycologia 2002, 41, 49–60. [Google Scholar] [CrossRef]
- Zuccarello, G.C.; Muangmai, N.; Preuss, M.; Sanchez, L.B.; De Goër, S.L.; West, J.A. The Bostrychia tenella species complex: Morphospecies and genetic cryptic species with resurrection of B. binderi. Phycologia 2015, 54, 261–270. [Google Scholar] [CrossRef]
- Stuercke, B.; Freshwater, D.W. Two new species of Polysiphonia (Ceramiales, Florideophyceae) from the western Atlantic. Bot. Mar. 2010, 53, 301–311. [Google Scholar] [CrossRef]
- de Jesus, P.B.; Nauer, F.; Lyra, G.d.M.; Cassano, V.; Oliveira, M.C.; Nunes, J.M.d.C.; Schnadelbach, A.S. Species-delimitation and phylogenetic analyses of some cosmopolitan species of Hypnea (Rhodophyta) reveal synonyms and misapplied names to H. cervicornis, including a new species from Brazil. J. Phycol. 2016, 52, 774–792. [Google Scholar] [CrossRef]
- Gavio, B.; Hickerson, E.; Fredericq, S. Platoma chrysymenioides sp. nov. (Schizymeniaceae), and Sebdenia integra sp. nov. (Sebdeniaceae), two new red algal species from the northwestern Gulf of Mexico, with a phylogenetic assessment of the Cryptonemiales complex (Rhodophyta). Gulf Mex. Sci. 2005, 23, 5. [Google Scholar] [CrossRef]
- Zhang, M.; Li, H.; Xue, J.; Pang, T.; de Vega, G.B.; Xia, B.; Liu, J. A preliminary evaluation of the red seaweed Gracilariopsis silvana, isolated from Colon, Panama, as a potential new agar-producing cultivar. J. Appl. Phycol. 2021, 33, 4125–4136. [Google Scholar] [CrossRef]
- Mateo-Cid, L.E.; Mendoza-González, A.C.; Gavio, B.; Fredericq, S. Grateloupia huertana sp. nov.(Halymeniaceae, Rhodophyta), a peculiar new prostrate species from tropical Pacific Mexico. Phycologia 2005, 44, 4–16. [Google Scholar] [CrossRef]
- Richards, J.L.; Vieira-Pinto, T.; Schmidt, W.E.; Sauvage, T.; Gabrielson, P.W.; Oliveira, M.C.; Fredericq, S. Molecular and morphological diversity of Lithothamnion spp. (Hapalidiales, Rhodophyta) from deepwater rhodolith beds in the Northwestern Gulf of Mexico. Phytotaxa 2016, 278, 81–114. [Google Scholar] [CrossRef]
- Krayesky-Self, S.; Richards, J.L.; Rahmatian, M.; Fredericq, S. Aragonite infill in overgrown conceptacles of coralline Lithothamnion spp.(Hapalidiaceae, Hapalidiales, Rhodophyta): New insights in biomineralization and phylomineralogy. J. Phycol. 2016, 52, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Sauvage, T.; Schmidt, W.E.; Suda, S.; Fredericq, S. A metabarcoding framework for facilitated survey of endolithic phototrophs with tufA. BMC Ecol. 2016, 16, 1–21. [Google Scholar] [CrossRef]
- Nelson, W.A.; Sutherland, J.E.; Farr, T.J.; Hart, D.R.; Neill, K.F.; Kim, H.J.; Yoon, H.S. Multi-gene phylogenetic analyses of New Zealand coralline algae: Corallinapetra Novaezelandiae gen. et sp. nov. and recognition of the Hapalidiales ord. nov. J. Phycol. 2015, 51, 454–468. [Google Scholar] [CrossRef]
- Gabrielson, P.W.; Hughey, J.R.; Peña, V.; Richards, J.L.; Saunders, G.W.; Twist, B.; Farr, T.; Nelson, W.A. Asia Pacific Sporolithon (Corallinophycidae, Rhodophyta) species revised based on DNA sequencing of type specimens and including S. crypticum sp. nov., S. immotum sp. nov. and S. nodosum sp. nov. Phycologia 2023, 62, 593–607. [Google Scholar] [CrossRef]






| Taxon and BOLD BIN | Maximum NCBI BLAST % Identity and GenBank Accessions | GenBank Accessions |
|---|---|---|
| CORALLINALES | ||
| 2 Corallinales ACW4612 | 89.2% Corallinaceae HQ544204 | PV136126 |
| 2 Corallinales ADB5373 | 90.8% Titanoderma sp. OQ943746 | PV136176 |
| 2 Corallinales ADC0391 | 87.7% Cheilosporum cultratum (Harvey) Areschoug 1852 LC071762 | PV136224 |
| Amphiroa cf. fragilissima (Linnaeus) J.V.Lamouroux 1816 AAO5848 | 99.6% Amphiroa cf. fragilissima (Linnaeus) J.V.Lamouroux 1816 MG521326 | PV136085 |
| PV136212 | ||
| PV136230 | ||
| 2 Amphiroa hancockii W.R.Taylor 1942 ABA1815 | 96.2–96.6% Corallinales GQ917680 | PV136132 |
| PV136054 | ||
| PV136104 | ||
| PV136092 | ||
| PV136077 | ||
| PV136089 | ||
| PV136070 | ||
| PV136141 | ||
| PV136180 | ||
| PV136155 | ||
| PV136137 | ||
| PV136120 | ||
| Amphiroa rigida J.V.Lamouroux 1816 ACL2235 | 98.2% Amphiroa rigida J.V.Lamouroux 1816 MG521335 | PV136170 |
| 2 Amphiroa sp. AEC7415 | 95.2% Lithophyllum corallinae (P.Crouan & H.Crouan) Heydrich 1897 MG521354 | PV136164 |
| 2 Amphiroa sp. AEC7478 | 96.7% Lithophyllum corallinae (P.Crouan & H.Crouan) Heydrich 1897 MG521354 | PV136098 |
| 2 Amphiroa sp. ACW3055 | 91.1% Amphiroa beauvoisii J.V.Lamouroux 1816 LC071729 | PV136066 |
| PV136106 | ||
| PV136172 | ||
| 2 Amphiroa sp. ACW4195 | 91.9% Amphiroa sp. SA-2023 LC767501 | PV136067 |
| PV136096 | ||
| PV136078 | ||
| 2 Amphiroa sp. ACW4375 | 93.7–94.1% Amphiroa beauvoisii J.V.Lamouroux 1816 LC071729 | PV136133 |
| PV136109 | ||
| PV136185 | ||
| PV136118 | ||
| 2 Amphiroa sp. ACW4537 | 95.3% Corallinales GQ917303 | PV136163 |
| PV136149 | ||
| Amphiroa sp. ACW4606 | 98.2–98.3% Corallinales GQ917679 | PV136211 |
| PV136158 | ||
| PV136139 | ||
| PV136058 | ||
| PV136123 | ||
| PV136099 | ||
| PV136074 | ||
| PV136196 | ||
| PV136084 | ||
| PV136201 | ||
| PV136143 | ||
| 2 Amphiroa sp. ACW4703 | 92.8–93.1% Amphiroa foliacea J.V.Lamouroux 1824 OM460663 | PV136129 |
| PV136203 | ||
| Amphiroa sp. ADB5569 | 99.8% Corallinales GQ917676 | PV136081 |
| 2 Amphiroa sp. ADB6287 | 92.9% Corallinales GQ917652 | PV136151 |
| 1 Jania pedunculata var. adhaerens (J.V.Lamouroux) A.S.Harvey, Woelkerling & Reviers 2020 ACW3073 | 99.2% Jania pedunculata var. adhaerens (J.V.Lamouroux) A.S.Harvey, Woelkerling & Reviers 2020 OR192975 | PV136090 |
| 1,2 Jania sp. ADB5662 | 97–97.3% Jania rosea (Lamarck) Decaisne 1842 LC071777 | PV136204 |
| PV136193 | ||
| GRACILARIALES | ||
| 1 Gracilaria baiana Lyra, Gurgel, M.C.Oliveira & Nunes 2016 ACP6295 | 98.8% Gracilaria baiana Lyra, Gurgel, M.C.Oliveira & Nunes 2016 KP210196 | PV136114 |
| PV136202 | ||
| PV136117 | ||
| PV136227 | ||
| PV136194 | ||
| PV136207 | ||
| 1 Crassiphycus caudatus (J.Agardh) Gurgel, J.N.Norris & Fredericq 2018 ACW4841 | 97.6–99.5% Gracilaria caudata (J.Agardh) Gurgel, J.N.Norris & Fredericq 2018 KP210151 | PV136072 |
| PV136186 | ||
| PV136095 | ||
| PV136157 | ||
| PV136184 | ||
| PV136182 | ||
| PV136144 | ||
| 1,2 Gracilaria bocatorensis sp. nov. ADB6885 | 95.2% Gracilaria sp. KY656553 | PV136199 |
| 3 PV136446 | ||
| 4 PV136450 | ||
| PV136111 | ||
| Gracilaria cearensis (A.B.Joly & Pinheiro) A.B.Joly & Pinheiro 1966 ABX1742 | 99.8% Gracilaria cearensis (A.B.Joly & Pinheiro) A.B.Joly & Pinheiro 1966 KP210189 | PV136135 |
| 1 Crassiphycus corneus (J.Agardh) Gurgel, J.N.Norris & Fredericq 2018 AAV6953 | 99.5–99.7% Hydropuntia cornea (J.Agardh) M.J.Wynne 1989 KP636771 | PV136055 |
| PV136116 | ||
| PV136225 | ||
| Crassiphycus crassissimus (J.Agardh) Gurgel, J.N.Norris & Fredericq 2018 ACW3661 | 99.4–99.8% Crassiphycus crassissimus (J.Agardh) Gurgel, J.N.Norris & Fredericq 2018 MZ336084 | PV136190 |
| PV136206 | ||
| PV136173 | ||
| 1,2 Gracilaria dreckmannii sp. nov. ACW4300 | 95.6% Gracilaria microcarpa Dreckmann, Núñez-Resendiz & Sentíes 2018 MF321896 | PV136121 |
| 3 PV136444 | ||
| 4 PV136448 | ||
| PV136071 | ||
| 3 PV136443 | ||
| 4 PV136447 | ||
| 1 Gracilaria flabelliformis subsp. simplex Gurgel, Fredericq & J.N.Norris 2004 ACP5692 | 99.5–99.8% Gracilaria flabelliformis subsp. simplex Gurgel, Fredericq & J.N.Norris 2004 KP210178 | PV136119 |
| PV136102 | ||
| PV136112 | ||
| PV136076 | ||
| PV136226 | ||
| PV136087 | ||
| PV136195 | ||
| Gracilaria hayi Gurgel, Fredericq & J.N.Norris 2004 ABX1121 | 100% Gracilaria hayi Gurgel, Fredericq & J.N.Norris 2004 KP210179 | PV136103 |
| Gracilaria mammillaris (Montagne) M.Howe 1918 ABX1122 | 98.6–98.8% Gracilaria mammillaris (Montagne) M.Howe 1918 KP210166 | PV136115 |
| PV136083 | ||
| PV136189 | ||
| 1 Gracilaria microcarpa Dreckmann, Núñez-Resendiz & Sentíes 2018 ACW4301 | 99.1% Gracilaria microcarpa Dreckmann, Núñez-Resendiz & Sentíes 2018 MF321896 | PV136192 |
| PV136222 | ||
| PV136079 | ||
| PV136154 | ||
| PV136181 | ||
| Gracilaria silviae Lyra, Gurgel, M.C.Oliveira & J.M.C.Nunes 2015 ABX1150 | 99.8% Gracilaria silviae Lyra, Gurgel, M.C.Oliveira & J.M.C.Nunes 2015 KP210168 | PV136062 |
| 1 Gracilaria suzanneae L.P.Soares, Gurgel & M.T.Fujii 2018 AGE7357 | 97.4% Gracilaria occidentalis (Børgesen) M.Bodard 1965 MW924175 | PV136165 |
| 3 PV136445 | ||
| 4 PV136449 | ||
| 1 Gracilariopsis silvana Gurgel, Fredericq & J.N.Norris 2003 ABX1323 | 99.8% Gracilariopsis silvana Gurgel, Fredericq & J.N.Norris 2003 KP210202 | PV136053 |
| PV136205 | ||
| PV136231 | ||
| PV136191 | ||
| PV136215 | ||
| PV136145 | ||
| PV136093 | ||
| PV136105 | ||
| CERAMIALES | ||
| 2 Ceramiales AAV8863 | 85.7–85.8% Membranoptera alata (Hudson) Stackhouse 1809 JX111884 | PV136061 |
| PV136229 | ||
| PV136124 | ||
| PV136177 | ||
| PV136213 | ||
| PV136169 | ||
| 2 Ceramiales ACW3451 | 90.5% Acanthophora dendroides Harvey 1855 MT876665 | PV136187 |
| 2 Ceramiales ACW4777 | 86.7% Placophora monocarpa (Montagne) Papenfuss 1956 KU564367 | PV136136 |
| Acanthophora spicifera (Vahl) Børgesen 1910 AAF1313 | 100% Acanthophora spicifera (Vahl) Børgesen 1910 MH388705 | PV136069 |
| PV136064 | ||
| PV136128 | ||
| 2 Aglaothamnion sp. ACW4318 | 88.8% Callithamnion acutum Kylin 1925 PP735967 | PV136088 |
| Alsidium triquetrum (S.G.Gmelin) Trevisan 1845 ACW4705 | 99.7% Alsidium triquetrum (S.G.Gmelin) Trevisan 1845 MN165082 | PV136130 |
| PV136065 | ||
| PV136073 | ||
| PV136056 | ||
| PV136125 | ||
| 2 Augophyllum sp. ACW3726 | 95.2% Augophyllum wysorii Showe M.Lin, Fredericq & Hommersand 2004 OM460639 | PV136161 |
| Centroceras gasparrinii (Meneghini) Kützing 1849 ADB6853 | 98.2% Centroceras gasparrinii (Meneghini) Kützing 1849 KP222749 | PV136228 |
| 2 Chondrophycus sp. AGE9932 | 93.6–93.8% Chondrophycus sp. MZ855311 | PV136167 |
| PV136059 | ||
| 2 Corallophila sp. AGG1358 | 97.4% Corallophila sp. MW354762 | PV136214 |
| 2 Laurencia sp. AAO5440 | 96.6% Laurencia dendroidea J.Agardh 1852 MH388711 | PV136159 |
| 2 Laurencia sp. ACW3667 | 95% Laurencia australis M.Preuss, Diaz-Tapia, Verbruggen & Zuccarello 2023 OQ863292 | PV136188 |
| PV136091 | ||
| 2 Laurencia sp. AEC7447 | 96.3% Laurencia dendroidea J.Agardh 1852 MH388711 | PV136122 |
| 1 Laurencia catarinensis Cordeiro-Marino & Fujii 1985 AAO5439 | 99.8% Laurencia catarinensis Cordeiro-Marino & Fujii 1985 OQ863298 | PV136097 |
| Melanothamnus pseudovillum (Hollenberg) Díaz-Tapia & Maggs 2017 AGE9893 | 99.8% Melanothamnus pseudovillum (Hollenberg) Díaz-Tapia & Maggs 2017 HM573524 | PV136140 |
| 2 Palisada sp. ACW3911 | 91.4% Palisada corallopsis (Montagne) Sentíes, M. T. Fujii & Díaz-Larrea 2008 PP974343 | PV136221 |
| PV136152 | ||
| 2 Palisada sp. ADC0480 | 96.97–96.98% Palisada perforata (Bory) K.W.Nam 2007 MH388710 | PV136068 |
| PV136209 | ||
| 2 Spyridia sp. AGE9777 | 97.4% Spyridia americana Durant 1850 MW770747 | PV136219 |
| 1 Vidalia obtusiloba (Mertens ex C.Agardh) J.Agardh 1863 ACW3810 | 99.5% Vidalia obtusiloba (Mertens ex C.Agardh) J.Agardh 1863 MG188846 | PV136138 |
| Wrangelia argus (Montagne) Montagne 1856 AAO9061 | 100% Wrangelia argus (Montagne) Montagne 1856 OQ561846 | PV136086 |
| 2 Wrangelia sp. ACW3689 | 93.4% Wrangelia ryancraigii C.W.Schneider & G.W.Saunders 2024 OQ561822 | PV136110 |
| GIGARTINALES | ||
| 1 Hypnea cryptica P.B.Jesus & J.M.C.Nunes 2019 AEC6920 | 99.5% Hypnea cryptica P.B.Jesus & J.M.C.Nunes 2019 OR589246 | PV136217 |
| 1 Hypnea pseudomusciformis Nauer, Cassano & M.C.Oliveira 2015 AGH7665 | 99.35–99.57% Hypnea pseudomusciformis Nauer, Cassano & M.C.Oliveira 2015 MH482171 | PV136153 |
| PV136107 | ||
| PV136174 | ||
| 2 Hypnea sp. AEC7247 | 97.4% Hypnea musciformis (Wulfen) J.V.Lamouroux 1813 PP898380 | PV136179 |
| 1 Hypnea wynnei NOBIN2 | 100% Hypnea wynnei Nauer, Cassano & M.C.Oliveira 2016 MZ855294 | PV136162 |
| Solieria sp. ACW4723 | 98.5% Solieria sp. MG018946 | PV136220 |
| PV136210 | ||
| PV136156 | ||
| PV136200 | ||
| 2 Solieria sp. AGG3466 | 97.3% Solieria filiformis (Kützing) P.W.Gabrielson 1985 KJ202080 | PV136168 |
| PV136108 | ||
| 2 Solieria sp. AGE9782 | 93.4% Solieria sp. MG018946 | PV136075 |
| RHODYMENIALES | ||
| 2 Rhodymeniales ACW3730 | 90.6% Lomentaria sp. KU707864 | PV136094 |
| PV136147 | ||
| 2 Rhodymeniales AED0787 | 88.6–89% Neogastroclonium subarticulatum (Turner) L.Le Gall, Dalen & G.W.Saunders 2008 MN447949 | PV136178 |
| PV136100 | ||
| 2 Botryocladia sp. AEC7422 | 94% Botryocladia skottsbergii (Børgesen) Levring 1941 HQ423132 | PV136080 |
| Botryocladia sp. AEC7971 | 98.8% Botryocladia sp. KR011965 | PV136223 |
| 2 Botryocladia sp. AED0681 | 91% Botryocladia leptopoda (J.Agardh) Kylin 1931 MT876667 | PV136148 |
| Ceratodictyon intricatum (C.Agardh) R.E.Norris 1987 AAO6470 | 99.8–100% Ceratodictyon intricatum (C.Agardh) R.E.Norris 1987 OK641564 | PV136160 |
| PV136218 | ||
| NEMALIALES | ||
| Dichotomaria sp. ACW3485 | 99.3–99.7% Dichotomaria sp. KF752542 | PV136197 |
| PV136175 | ||
| PV136057 | ||
| PV136131 | ||
| 2 Galaxaura sp. ADB5483 | 96.2% Galaxaura rugosa (J.Ellis & Solander) J.V.Lamouroux 1816 MT472792 | PV136060 |
| Galaxaura rugosa (J.Ellis & Solander) J.V.Lamouroux 1816 AAO6697 | 99.4% Galaxaura rugosa (J.Ellis & Solander) J.V.Lamouroux 1816 MT472804 | PV136134 |
| 2 Scinaia sp. ACW4576 | 95.5% Scinaia hormoides Setchell 1914 HQ422966 | PV136082 |
| 2 Tricleocarpa sp. ACW3829 | 96.7% Tricleocarpa cylindrica (J.Ellis & Solander) Huisman & Borowitzka 1990 KY656534 | PV136198 |
| Tricleocarpa fragilis (Linnaeus) Huisman & R.A.Townsend 1993 AAO8732 | 99.4% Tricleocarpa fragilis (Linnaeus) Huisman & R.A.Townsend 1993 KU321670 | PV136150 |
| GELIDIALES | ||
| 1 Gelidiella flabella G.H.Boo & Le Gall 2016 NOBIN1 | 99.8% Gelidiella flabella G.H.Boo & Le Gall 2016 KT207971 | PV136063 |
| Gelidium lineare Iha & Freshwater 2016 AAU0346 | 98.9% Gelidium lineare Iha & Freshwater 2016 KT208015 | PV136183 |
| 2 Gelidium sp. AAO6432 | 97.9% Gelidium microdonticum W.R.Taylor 1969 KT208002 | PV136166 |
| HALYMENIALES | ||
| Corynomorpha clavata (Harvey) J.Agardh 1872 ACJ2524 | 98.9% Corynomorpha clavata (Harvey) J.Agardh 1872 MK919031 | PV136127 |
| Grateloupia sp. ACR9349 | 99.7% Grateloupia sp. MK919065 | PV136113 |
| 2 Halymenia sp. AEC9043 | 93.3% Halymenia durvillei Bory 1828 MK812802 | PV136101 |
| PEYSSONNELIALES | ||
| 2 Peyssonneliales AEC8993 | 93.2% Peyssonneliales sp. 1 OM902126 | PV136208 |
| 2 Peyssonnelia sp. ACW4315 | 92% Peyssonnelia sp. HQ545338 | PV136146 |
| 2 Peyssonnelia sp. ADB6540 | 91.9% Peyssonnelia sp. JX969736 | PV136142 |
| PV136171 | ||
| 2 Polystrata sp. AGE9915 | 93.4% Polystrata sp. OM902137 | PV136216 |
| Loci | Species | Mean Intra. (%) | Maximum Intra. (%) | Nearest Neighbor | Min. Dist. to NN (%) |
|---|---|---|---|---|---|
| rbcL | G. apiculata | 0.39 | 0.76 | G. cervicornis | 1.84 |
| G. cervicornis (G. ferox) | 0.36 | 0.89 | G. apiculata | 1.84 | |
| G. dreckmannii sp. nov. | 0 | 0 | G. microcarpa | 1.58 | |
| G. mammillaris (G. curtissiae and G. cuneata) | 0.65 | 1.12 | Gracilaria sp. SPF57166 | 2.80 | |
| G. microcarpa | 0.50 | 1.41 | G. dreckmannii sp. nov. | 1.58 | |
| Gracilaria sp. SPF57166 | 0 | 0 | G. mammillaris | 2.80 | |
| cox1 | G. cervicornis (G. ferox) | 1.99 | 3.29 | G. dreckmannii sp. nov. | 4.52 |
| G. dreckmannii sp. nov. | 0 | 0 | G. microcarpa | 4.43 | |
| G. mammillaris (G. curtissiae and G. cuneata) | 1.63 | 2.57 | G. cervicornis | 6.42 | |
| G. microcarpa | 1.01 | 1.47 | G. dreckmannii sp. nov. | 4.43 | |
| Gracilaria sp. SPF57166 | 0 | 0 | G. dreckmannii sp. nov. | 5.57 | |
| UPA | G. cervicornis (G. ferox) | 0.54 | 0.54 | Gracilaria sp. SPF57166 | 0.81 |
| G. mammillaris (G. curtissiae and G. cuneata) | 0.4 | 0.54 | Gracilaria sp. SPF57166 | 1.08 | |
| G. dreckmannii sp. nov. | 0 | 0 | Gracilaria sp. SPF57166 | 1.08 | |
| Gracilaria sp. SPF57166 | 0 | 0 | G. cervicornis | 0.81 |
| Characters | G. bocatorensis (This Study) | G. galetensis [91] | G. parva [92] | G. gurgelii [91] | G. oliveirarum [91] | G. smithsoniensis [91] | G. hayi [91] | G. occidentalis [91,93] |
|---|---|---|---|---|---|---|---|---|
| Thallus | Compressed to flattened | Flattened | Compressed to flattened | Flattened | Flattened | Compressed | Flattened | Flattened |
| Plant length (cm) | 2.5–7.7 | 8.0–20.0 | Up to 2.5 | 7.0–10.0 | 10.0–14.0 | 3.0–5.5 | 5.0–15.0 | Up to 25 |
| Axis width (mm) | 1.7–4.4 | 5.0–8.0 | 0.5–4.0 | 5.0–8.0 | 5.0–12.0 | 2.0–2.5 | 5.0–25.0 | 4.0 |
| Thallus thickness (µm) | 328–417 | 254–275 | - | 250–330 | 500–750 | ‘Thin”, up to 300 | ‘Thin’, up to 100 | ca. 800 at margins |
| Holdfast | Not observed | Small, discoid | Small, discoid | Small, tough, irregular shaped | Small, discoid | Small, discoid | Discoid | - |
| Branching | Subdichotomous to irregular | Dichotomous to subdichotomous | Dichotomous or cervicorn | Dichotomous to subdichotomous | Dichotomous to subdichotomous to irregular | Dichotomous | Dichotomous to subdichotomous | Dichotomous to subdichotomous |
| Orders of branching | Up to 5 | 2–3 | Up to 4 | 2–3 (3+) 1 | 3–5+ | Up to 5+ | Up to 5+ | Up to 5+ |
| Apices | Obtuse | Obtuse | Obtuse to truncate | Obtuse | Not described | Obtuse | Obtuse | - |
| Cortical cell layers | 1–3 | 1–3 | 2–3 | 1–2 | 1–2 | 1–2 | 1–2 | 1–2 |
| Cortical cell shape | Quadrate | Variable, mostly anticlinally elongated | Polyhedral | Isodiametric, sometimes anticlinally elongated | Variable | Variable, periclinally to anticlinally elongated | Periclinally compressed | Rounded polyhedral, often compressed |
| Cortical cell diameter (µm) | 2.7–10.0 | 3.75–12.5 | 4.0–10.0 | 3.75–11.8 | 3.0–9.0 | 3.7–7.5 | 2.5–10.0 | 5.0–16.2 |
| Medullary cell layers | 4–8 | 4–5 | 3–6 | 4–5 | 4–5 | 5–6 | 3–5 | 5–6 |
| Medullary cell shape | Globose | Rounded, isodiametric, most slightly compressed | Globose to longitudinally ovoid | Isodiametric–slightly compressed | Inner cells ovoid, outer globose to ovoid | Isodiametric | Globose to ovoid | Globose to ovoid |
| Medullary cell diameter (µm) | 56.0–102.0 | 56.0–154.0 | 70.0–160.0 | 100.0–185.0 | Inner cells 150.0–400.0 | 61.7–116.0 | 50.0–86.0 | 103.0–135.0 |
| Cortex-to-medulla transition | Abrupt | Gradual | Abrupt | Abrupt | Abrupt | Abrupt | Abrupt | Abrupt |
| Spermatia type | Not observed | Shallow textorii-type | Shallow textorii-type | - | - | Shallow textorii-type | Shallow textorii-type | - |
| Cystocarp shape | Hemispherical | Hemispherical to urceolate | Hemispherical | - | - | Hemispherical | Hemispherical | - |
| Cystocarp diameter (mm) | 0.7–0.8 | Up to 1.0 | Not described | - | - | 1.0–2.0 | 1.0–2.0 | - |
| Outer pericarp cell layers | 9–11 | 12–14 | 11–17 | - | - | 12–14 | 12–14 | - |
| Tubular nutritive cells | Few, attached to inner pericarp | Not described | Attached to inner and outer pericarp | - | - | Attached to lower outer pericarp | Prominent, attached to outer pericarp | - |
| Characters | G. dreckmannii (This Study) | G. cervicornis [87,88,94] | G. macrocarpa [88] | G. mammillaris [91] | G. cuneata [94,95] | G. ferox [87,88] | G. apiculate [96] |
|---|---|---|---|---|---|---|---|
| Thallus | Cylindrical | Flattened | Cylindrical | Flattened | Flattened | Flattened mostly | Flattened, occasionally cylindrical |
| Plant length (cm) | 2.4–4.8 | 6.0–35.0 | 8.0–22.0 | - | 3.0–12.0 | Up to 38.0 | 5.0–25.0 |
| Axis width (mm) | 1.9–3.0 | - | 0.43–0.62 | - | 10.0–20.0 | To 2.0 | 2–4 (flattened); 1+ (cylindrical |
| Thallus thickness (µm) | 660–1200 | - | 430–620 | 620–1000 | 600–1000 | - | Not described |
| Holdfast | Discoid | - | Small, discoid | - | - | Irregular, cushion-like | Small, discoid |
| Branching | Subdichotomous to irregular | Sparse, irregular | Dichotomous sometimes trichotomous | - | Mostly dichotomous | - | Mostly alternate but variable |
| Orders of branching | Up to 7 | Up to 2 | 4–5+ | - | - | - | Up to 3 |
| Apices | - | Acute | Acute | - | Obtuse to acute | - | Not described |
| Cortical cell layers | 1–2 | 2–3 | 2–3 | 1–2 | 1–2 | 2–7 | 1–2 |
| Cortical cell shape | Globose to elongate | - | Spherical | Isodiametric | Quadratic | Radially elongate | Radially elongate, elipsoid |
| Cortical cell diameter (µm) | 3–10 | 5–9 | 2–7 | 3–7 | 7–10 | - | 3–11 |
| Medullary cell layers | 7–11 | 4–6 | 8–9 | 3–4 | 2–5 | 2–5 | 6–12 |
| Medullary cell shape | Ovoid to globose | Ovoid to polyhedral | Isodiometric to irregular | Ovoid to globose | - | - | Rounded to compressed |
| Medullary cell diameter (µm) | 88–117 | 55–133 | 30–110 | 261–398 | 25–207 | 12–15 | 70–175 |
| Cortex-to-medulla transition | Abrupt | Gradual | Abrupt | Abrupt | Gradual | Gradual | Somewhat gradual |
| Spermatia type | Not observed | Shallow textorii-type | Not observed | - | Shallow textorii-type | Shallow textorii-type | Not observed |
| Cystocarp shape | Dome shaped constricted around the base | Subspherical and pedunculate | Hemispherical to subspherical and pedunculate | - | Hemispherical or subspherical and basally constricted | - | Variable, some urceolate and pedunculate |
| Cystocarp diameter (mm) | 0.5 | 0.75–0.80 | 0.28–0.30 | - | - | 0.50–1.00 | 1.5–2.0 |
| Outer pericarp cell layers | 9–11 | 6–10 | 12–14 | - | 10–14 | 8–12 | 9–13 |
| Tubular nutritive cells | Attached to outer pericarp | Attached to chamber floor | Attached to outer pericarp | - | Attached to chamber floor, rarely to outer pericarp | Absent | Abundant, attached to outer pericarp and chamber floor |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madrid Concepcion, M.E.; Collin, R.; Macdonald, K.S., III; Driskell, A.C.; Fredericq, S.; Wysor, B.; Freshwater, D.W. DNA Barcoding of Red Algae from Bocas del Toro, Panamá, with a Description of Gracilaria bocatorensis sp. nov. and G. dreckmannii sp. nov. (Gracilariales, Gracilariaceae). Diversity 2025, 17, 222. https://doi.org/10.3390/d17040222
Madrid Concepcion ME, Collin R, Macdonald KS III, Driskell AC, Fredericq S, Wysor B, Freshwater DW. DNA Barcoding of Red Algae from Bocas del Toro, Panamá, with a Description of Gracilaria bocatorensis sp. nov. and G. dreckmannii sp. nov. (Gracilariales, Gracilariaceae). Diversity. 2025; 17(4):222. https://doi.org/10.3390/d17040222
Chicago/Turabian StyleMadrid Concepcion, Maycol Ezequiel, Rachel Collin, Kenneth S. Macdonald, III, Amy C. Driskell, Suzanne Fredericq, Brian Wysor, and D. Wilson Freshwater. 2025. "DNA Barcoding of Red Algae from Bocas del Toro, Panamá, with a Description of Gracilaria bocatorensis sp. nov. and G. dreckmannii sp. nov. (Gracilariales, Gracilariaceae)" Diversity 17, no. 4: 222. https://doi.org/10.3390/d17040222
APA StyleMadrid Concepcion, M. E., Collin, R., Macdonald, K. S., III, Driskell, A. C., Fredericq, S., Wysor, B., & Freshwater, D. W. (2025). DNA Barcoding of Red Algae from Bocas del Toro, Panamá, with a Description of Gracilaria bocatorensis sp. nov. and G. dreckmannii sp. nov. (Gracilariales, Gracilariaceae). Diversity, 17(4), 222. https://doi.org/10.3390/d17040222







