Abstract
Coral reefs are threatened by multiple stressors that have driven a decline in the cover of reef-building coral species, resulting in a loss of reef structure and function. Restoration reef science provides useful conservation tools to preserve and restore the key species and ecological functions of these ecosystems. However, gaps remain in restoration at large scales. This study provides a guide of how to invest and apply innovative solutions and immediate action strategies from the tourism-hotel sector in alliance with academia and key stakeholders, through the development and implementation of a multi-species restoration program at two sites in the Mexican Caribbean: Manchoncitos Reef, Riviera Maya and La Francesita Reef, Cozumel. We have identified effective propagation and outplanting techniques for key critically endangered species, as well as genotypes resistant to temperature stress and Stony Coral Tissue Loss Disease (SCTLD), based on pre-restoration nursery trials. We include a comparative analysis over time (2020–2022) showing increased coral cover, structural complexity and fish biomass. Baseline assessment of the study areas will allow adaptation of repopulation techniques not only for hard corals, but also to advance in the comprehensive restoration of the ecosystem, incorporating new elements to the reef, such as fish, crab or sea urchin post larvae. These organisms could accelerate herbivory functions and in turn could improve the natural processes of the coral reefs. Our results improve the understanding of the use of restoration as a tool for climate change adaptation led by the private sector.
1. Introduction
For decades, coral reefs have faced complex and additive interactions with risk drivers, which have generated important modifications in their structure and functions, such that they have undergone relatively rapid changes among ecological states of equilibrium [1]. Their conservation has created major challenges for communities, researchers, governments, and those involved in coral reef work.
Caribbean reefs have experienced significant losses of key species, such as Acroporids, since the 1970s [2,3,4], and, more recently, the loss of coral communities and populations due to bleaching [5,6,7] increased the intensity of storm and hurricane frequency [8,9], phase shifts [10,11] and the prevalence of emerging diseases [12,13,14], such as the case of Stony Coral Tissue Loss Disease (SCTLD), first reported in Florida in 2014 [15].
In the Mexican Caribbean, SCTLD has been observed since 2018, and to date has caused the mortality of over 80% of the most susceptible coral populations, mainly of the Meandrinadae family and the Faviinae subfamily, affecting important reef-building species [16]. In the Puerto Morelos region, between 2020 and 2021, reefs were also exposed to extreme events, such as storms and hurricanes, causing physical and physiological damage to coral species (Tropical Storm Cristobal, June 2020; Hurricane Gamma Category 1, Hurricane Delta Category 2, and Hurricane Zeta Category 1, all three in October 2020; and Hurricane Grace Category 1, August 2021). Hurricanes Delta and Grace caused coral bleaching after their impact (personal observations).
Considering that disturbances on Caribbean coral reefs will increase in the foreseeable future, it is necessary to improve conservation strategies at the local level (i.e., planning urban development, reducing sedimentation, water pollution, and overfishing, establishing no-take protected areas, etc.) and effectively implement active restoration actions in the coming decades [17,18]. With these interventions, widespread coral reef degradation under increasingly adverse conditions might be mitigated.
Currently, most coral reef restoration programs in the Caribbean focus on tissue production, generally using fast-growing species such as Acropora palmata and Acropora cervicornis [19,20,21]. Although significant efforts have been made in Asia, Australia and the Caribbean, few programs consider the sexual reproduction of other mass-growing type species [20,21,22,23,24,25]. In most cases the projects are characterized by the implementation and monitoring over short periods of time (1–2 years), with no baseline data, and gaps in knowledge, either of the genotypic identity stress tolerance of the corals being used, or the water quality information in the restoration site [20,21,26,27]. In most programs, the metrics used to determine effectiveness do not generally contemplate ecological aspects such as the recovery of ecosystem functions and structural complexity [20,21,28].
Furthermore, there is a need to implement coral reef restoration projects that include policy, governance, and economic investment, as well as socio-ecological models that support financial strategies [29]. Scientific commitment is also necessary to develop novel research that addresses the challenges of climate change, as well as interventions beyond the reef, considering environmental education and the formation of community leaders [18,22,26,30,31,32,33].
The tourism industry is commencing to adopt responsible tourism practices through regenerative leadership [29,34,35]. However, gaps remain between sustainability initiatives and comprehensive coral reef conservation and restoration. Iberostar Group is strategically scaling up these efforts, using solid scientific investment, by hiring researchers within its staff and establishing alliances with academia in each of the destinations where they operate in the Caribbean region [21,30,31,34,35]. Here we present the results of ecological assessments of a coral reef restoration program in Mexico, from 2020 to 2022, considering changes in coral cover and the Reef Functional Index (RFI). The latter is used as a measure of coral community functioning [30]. We also show an outline of the program, integrating academia and government authorities in activities to catalyze coral biodiversity for resilience, including the selection of appropriate reefs for restoration, the selection of coral species to maximize functional diversity, the use of heat stress resistant coral material, water quality monitoring, and the assessment of ecological changes over time as a result of the restoration strategy.
2. Materials and Methods
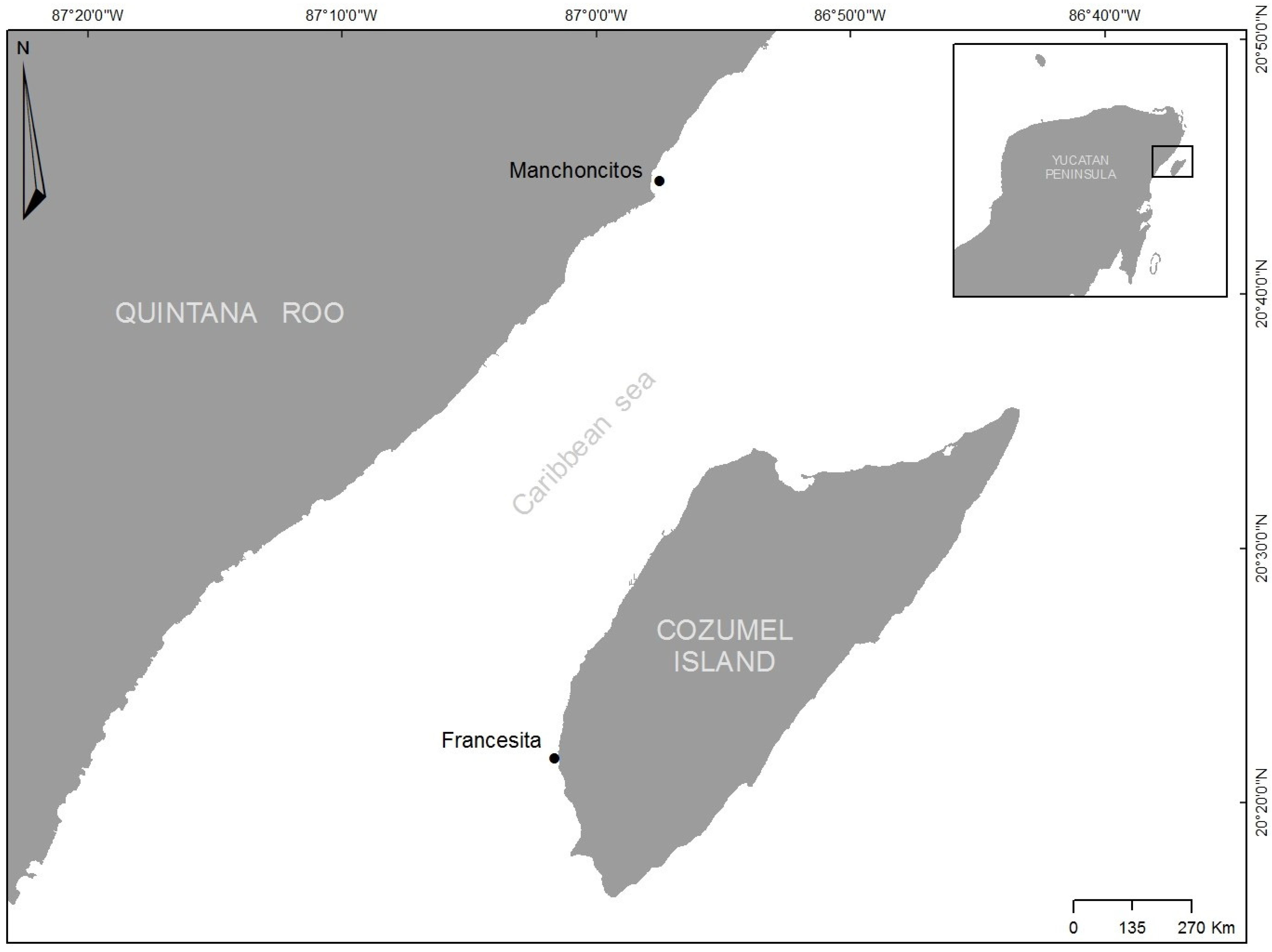
The study was conducted at Manchoncitos reef in Riviera Maya (20°45′34″ N 86°57′00″ W) and Francesita reef on Cozumel Island (20°21′47″ N 87°01′36″ W), which are part of the Mesoamerican Reef System (MAR), the second largest barrier reef in the world (Figure 1). There are well-studied reefs for these two areas [36,37,38,39,40,41]. However, there is an absence of historical data in both peer reviewed and non-peer reviewed literature for these two reefs. Therefore, to our knowledge, the data provided in this manuscript constitute the first scientific report of baseline records for these reefs.
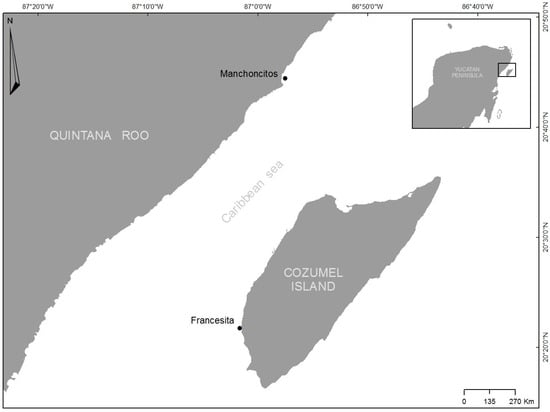
Figure 1.
Location of Manchoncitos reef and Francesita reefs in the Mexican Caribbean.
Manchoncitos is an area with reef patches between 5 and 13 m deep. It has an approximate length of 500 m and an area of 0.15 km2. It presents elevated bottom formations between one and seven meters high, mainly due to the presence of colonies of Orbicella spp. that reach between six and seven meters in diameter. This species dominates along with colonies of Montastraea cavernosa. There are still colonies of Diploria labyrinthiformis, Colpophyllia spp. and Pseudodiploria spp. that survived SCTLD and other disturbances that have occurred over the period of the study. Few isolated colonies of A.cervicornis and A. palmata are present, where the eroded skeletons of the latter remain. Most of the year, mobile invertebrates of commercial and ecological importance are present, such as lobsters (Panulirus argus) and sea cucumbers (Holothuria ssp.), along with vertebrates such as green turtles (Chelonia mydas). In 2022, two juvenile specimens of top predators (sharks Ginglymostoma cirratum and Carcharias taurus) were recorded.
Francesita is a smaller fringe reef formation located on the west side of Cozumel Island. This reef is approximately 200 m long, and 7–10 m deep, with an estimated area of 0.0048 km2. The reef is influenced by strong currents most of the year, as is the case for most reefs located in the Cozumel Reefs National Park. The reef is surrounded by sand, followed by an area of approximately 0.06 km2 of seagrass (Thalassia testudinum and Syringodium filiforme). It is common to observe hosting rays (Hypanus americanus, Urobatis jamaicensis), queen conch (Lobatus gigas) and sea cucumbers (Holothuria spp.). The reef is dominated by species such as Porites spp. and Agaricia spp. and skeletons of the colonies that died recently due to SCTLD (M. cavernosa, D. labyrinthiformis, Pseudodiploria spp., Orbicella annularis, Orbicella faveolata, Orbicella franksi and Eusmilia fastigiata). In addition, some areas have been colonized by eroding sponges and macroalgae. During the study, one G. cirratum and three large adults >40 cm of Sphyraena barracuda specimens were recorded. This site is a popular snorkeling spot, where c.a. 600 people visit per day.
In the middle of 2020, two coral nurseries were installed on each reef. Each one of them had 20 structures of three different types with a capacity of 25 to 30 fragments each. Types of structures included coral fragments attached to hexagonal steel rod structures, called “spiders”, 30 cm high from the bottom, painted with fiberglass resin and coated with coarse beach sand to provide a rough substrate for the corals. In addition, corals were attached to steel rod dome-shaped structures (100 cm). Other structures used in the nurseries, made with a mix of concrete and sand, were used to form a platform (2 × 2 m) for the structures on which the coral fragments were mounted. (Supplementary Figure S1).
Two outplantings were performed in May and July 2021 on each of the reefs, and a third one in August 2021, just after hurricane Grace (this was done with opportunity coral fragments that were detached by the hurricane), and two more in May and July 2022. (Supplementary Table S1).
All criteria and steps used in the design and implementation of the restoration program are described in detail in the planning and design guide for coral reef restoration programs [42,43,44].
2.1. Ecological Monitoring
The ecological assessment was carried out through annual monitoring of both reefs at the outplanted sites between 2020 and 2022. Based on the Atlantic and Gulf Reef Rapid Reef Assessment protocol (AGRRA) Version 5.4 [45], six permanent transects of 10 m were randomly located at the sites to carry out the assessments. For the benthos survey, the point intercept methodology was used with measurements collected every 10 cm along each of the transects, recording the category corresponding to the substrate observed just below each point. The benthic community was grouped into six categories: coral (scleractinian coral), CCA (crustose coralline algae), cyanobacteria, other invertebrates, macroalgae and abiotic substrate.
To measure fish abundance, six transects were performed (30 m long × 2 m wide) in the same habitat as the permanent transects. The number of individuals corresponding to the reef fish species of commercial and ecological importance covered by the AGRRA protocol were recorded, as well as their sizes in the class size ranges proposed in the protocol [45]. This was complemented by a survey of coral and fish species richness at each of the reefs.
In addition, data for photomosaic analysis were collected from both reefs at the same time for 2020 and 2021 in order to have complementary databases and high-resolution spatial mapping available. Data for 2022 are still under analysis. Photos were taken with two parallel GOPRO Hero 8 cameras separated by approximately 1 m. Images were taken at a 0.5 s interval, making several tracks in an area of approximately 250 m2 [46]. For each reef survey, photos were imported into Agisoft Metashape (v. 1.7) to construct photomosaics using a standardized processing pipeline. This entailed first aligning the photos, then manually inspecting and correcting alignment errors and finally optimizing the camera distortion modeling to achieve a scene model with the greatest degree of accuracy possible. Vector files based on the photomosaics were uploaded into QGIS. AGRRA codes [45] were used to identify and categorize all points distributed across the photomosaics into living and non-living benthic categories. All live corals (LC) were identified to the lowest level possible (species and genus). Coral health status was noted when there was visual evidence of disease, bleaching, paleness, or predation at the colony level. Epibenthic sponges and gorgonians were characterized in other invertebrates (OINV), while aggressive sponges (Cliona spp., Chondrilla caribensis, Palythoa caribaeorum, among others) and Millepora spp. were characterized in aggressive invertebrates (AINV). Abiotic factors (ABIO) were divided into the following descriptive codes: sand, rock, rubble, mud, and hole. All unidentifiable points, due to image quality (blurriness/distortion/artifacts) or the presence of something blocking clear sight to the benthic floor (i.e., fish, sea fan, scale bars, etc.), were marked as unknown (UNK). For instances in which points fell within holes in the photomosaic itself (e.g., due to a lack of imagery overlay), the points were marked as having no data (N/A). Point count data files were exported from QGIS and compiled in RStudio (version 4.0.5) before summary statistics were acquired using Excel.
Constant environmental monitoring has also been carried out on both outplanted sites over the years. Temperature and light measurements were recorded by hanging HOBO data loggers. One was placed at each site at a depth of 10 m. Each logger was programmed to record every 20 min, with logger replacement every two months. During this time, cleaning and removal of algae that could cover the sensors was carried out. In addition, annual water quality analyses have been performed for the following parameters: dissolved O2, pH, temperature, nitrates, nitrites, salinity, enterococci by defined chromogenic substrate, total phosphorus digestion, total phosphorus and total phosphates (from total P), during the months of October. These were analyzed by ABC Intertek Laboratories, a qualified and certified external company that provides water quality analysis services in the State of Quintana Roo, Mexico.
2.2. Ecological Indicators Assessment over Time
To determine the effects related to active restoration and outplanting efforts, ecological indicators were estimated. Three main variables considered as condition indicators were calculated: (1) coral cover, obtained directly from the benthos percentage cover data, (2) Reef Functional Index (RFI), calculated considering the values and equation presented by González-Barrios and Álvarez-Filip [47], which focuses on quantifying the relative contribution of abundance (live coral cover) of the species present in a coral reef system by integrating mean calcification rate, rugosity, and the height of each species—a value close to one represents dominance of species with the highest calcification rates and the highest values of structural complexity—and (3) total fish biomass, obtained using the abundance and size class data, considering the length–weight relationship equation W = aLb described by Bonsack and Harper [48]. Constants (a and b) for length–weight relationships for each species were obtained from Froese and Pauly [49]; a logarithmic transformation was performed to improve the visualization of the data.
Data was not normally distributed. Therefore, Friedman tests were performed to compare the values of the three indicators between years for each reef, followed by Conover’s post hoc tests for pairwise comparisons. All analyses were performed at a significance of α = 0.05 and carried out with the statistical program R, using customized scripts.
2.3. Genetic Intervention Strategies
For genetic characterization, 1 cm2 tissue samples were collected from twenty-four colonies of A. palmata and three colonies of A. cervicornis from Cozumel. Samples were placed in vials with 95% ethanol, stored, and sent to Eurofins BioDiagnosis laboratory (Madison, Wisconsin, USA) for DNA extraction and Single Nucleotide Polymorphisms (SNPs) analysis. This process and genetic information were analyzed through the Galaxy web-based portal described by Kitchen et al. [50]. A cluster plot was generated from a multilocus genotype assay searching for associations between genotypes using Galaxy. These multi-locus genotypes were then compared against each other and a database of acroporid reference genotypes to identify genets [51].
As an approach to integrate assisted translocation into the restoration program, 13 of the 24 genotyped A. palmate colonies which had been maintained and adapted to temperatures 1 °C above typically reported on the continent during 23 months in Francesita’s nursery, were translocated to the Manchoncitos outplanting site.
Finally, to have a comprehensive restoration program, as part of the efforts led by the Government of the State of Quintana Roo in compliance with the SEMA-Zone 4 project under parametric insurance, and with the collaboration of INAPESCA and UNAM (Spanish acronyms), 43 individuals of Caribbean King Crab (Maguimithrax spinosissimus), were released at the same outplanted site in Manchoncitos.
3. Results
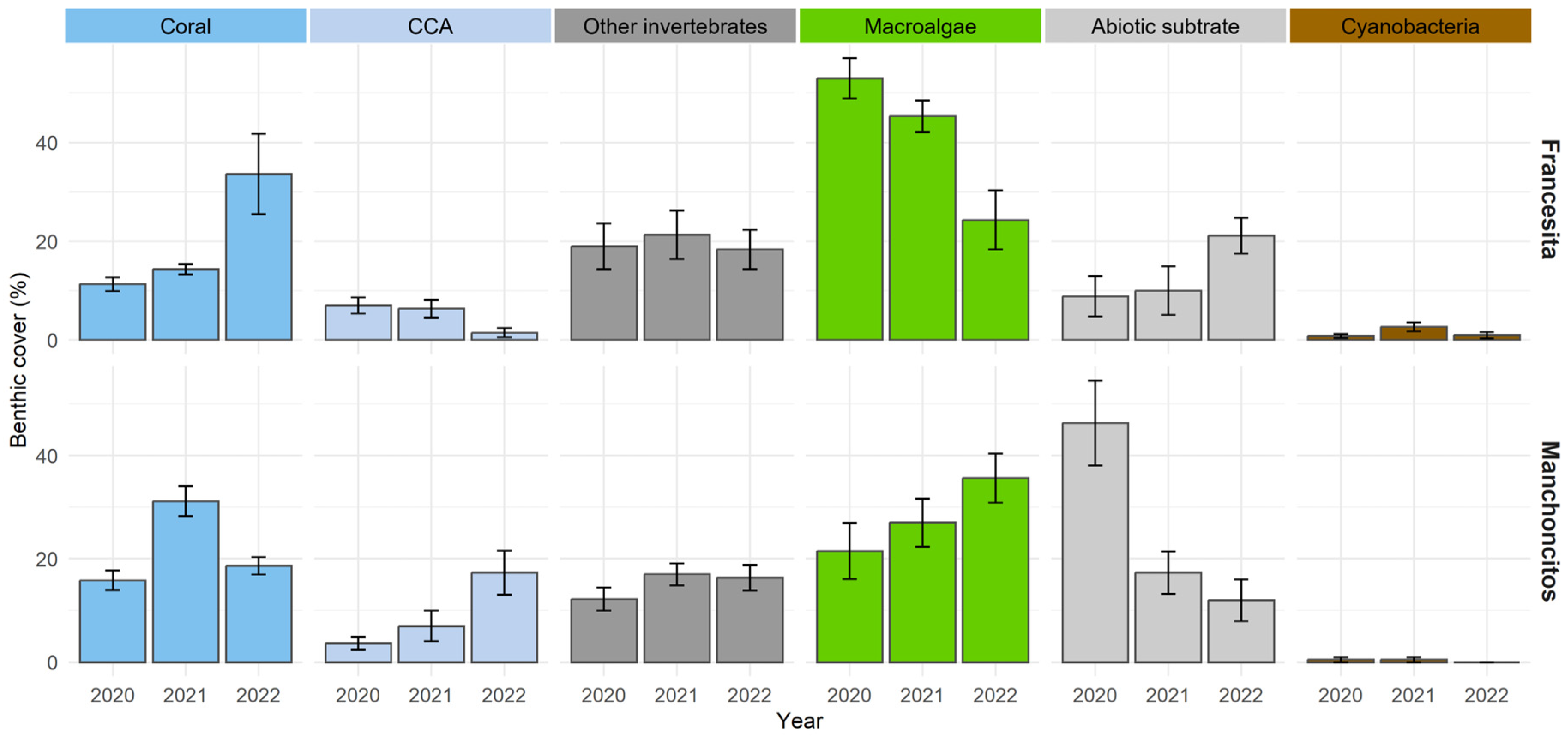
The results obtained from the monitoring analysis using the AGRRA methodology (Figure 2), at the outplanting sites of both reefs over time, showed an increasing trend in the scleractinian coral cover, along with a decrease in macroalgae and abiotic substrate. In Francesita reef, for 2020, macroalgae cover (53 ± 10.01%) was predominant, followed by other invertebrates (19 ± 11.40%), corals (11.33 ± 3.44%) and abiotic substrate (8.83 ± 10.06%). In 2021, there was a decrease in macroalgae cover (45.33 ± 7.71%) and an increase in coral (14.33 ± 2.58%) and abiotic substrate cover (10 ± 12.11%). Finally, in 2022 there was a substantial increase in coral cover (33.66 ± 20.02%), which contrasts with the decrease in the percentage of macroalgae cover (24.33 ± 14.73%). In the case of Manchoncitos, in 2020 abiotic substrate cover predominated (46.33 ± 0.19%), followed by macroalgae (21.50 ± 13.27%), other invertebrates (12.16 ± 5.45), and coral cover (15.83 ± 4.57%), with minor contributions of CCA (3.66 ± 3.07%) and cyanobacteria (0.5 ± 1.22%). In 2021, there was an increase in coral cover (31.16 ± 7.16%) and a considerable decrease in abiotic substrate (17.33 ± 10.07%). In 2022, the percentage coral cover (30.16 ± 14.49%) and the other benthic categories remained the same as the previous year, except for cyanobacteria with no observations in 2022.
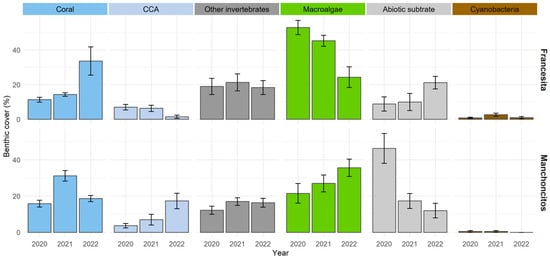
Figure 2.
Changes in benthic community structure at Francesita reef and Manchoncitos reef over time. Bar lines represent the standard deviation (±SD).
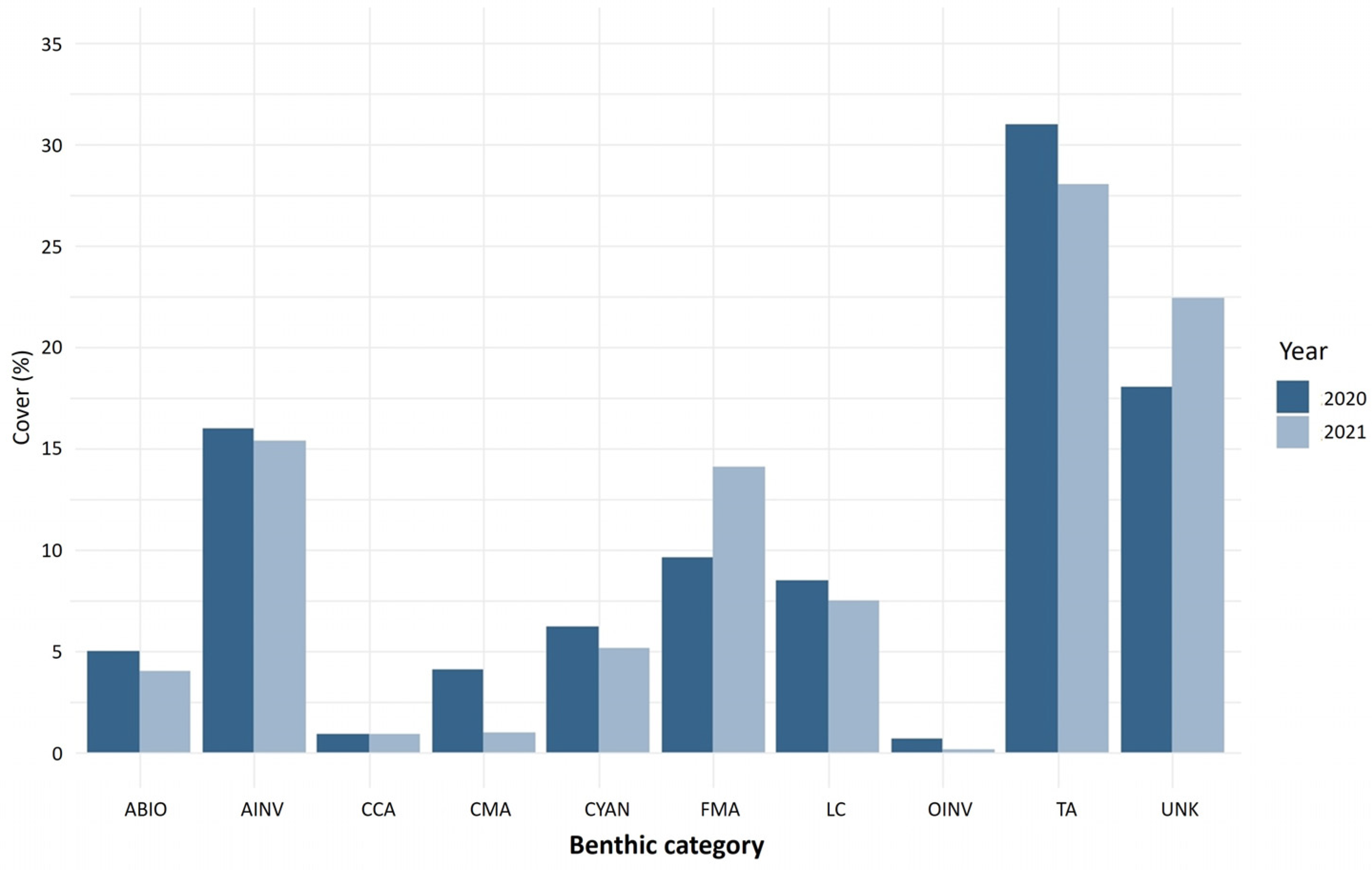
Based on the results obtained from the analysis of photomosaics, turf algae was the most abundant benthic component in Manchoncitos reef in both 2020 and 2021. Invertebrates and fleshy macroalgae were the next most abundant benthic organisms. The live coral cover was less than 10% for both years (Figure 3). The coral composition was dominated by some of the main Caribbean reef building species, including O. annularis, O. faveolata and M. cavernosa—totaling 65.0% in 2020 and 70.9% in 2021 of the species observed. This reef was affected by SCTLD, mainly colonies of O. faveolata in 2020 and 2021, and Siderastrea siderea in 2021.
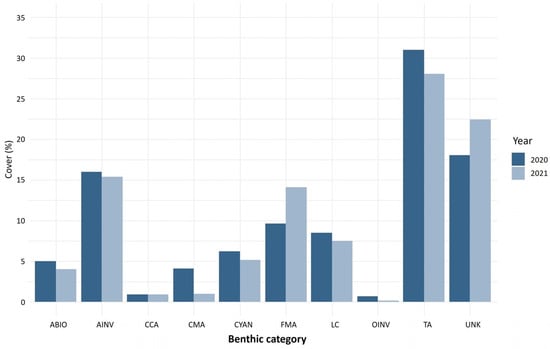
Figure 3.
Benthic reef cover (%) for Manchoncitos reef (Mexico) for 2020 (black bars) and 2021 (white bars). Benthic category codes are LC: live coral, FMA: fleshy macroalgae, TA: turf algae, CMA: calcareous macroalgae, CCA: crustose coralline algae, AINV: aggressive invertebrate, OINV: other invertebrate, CYAN: cyanobacteria, ABIO: abiotic substrate, UNK: unknown/unidentifiable substrates.
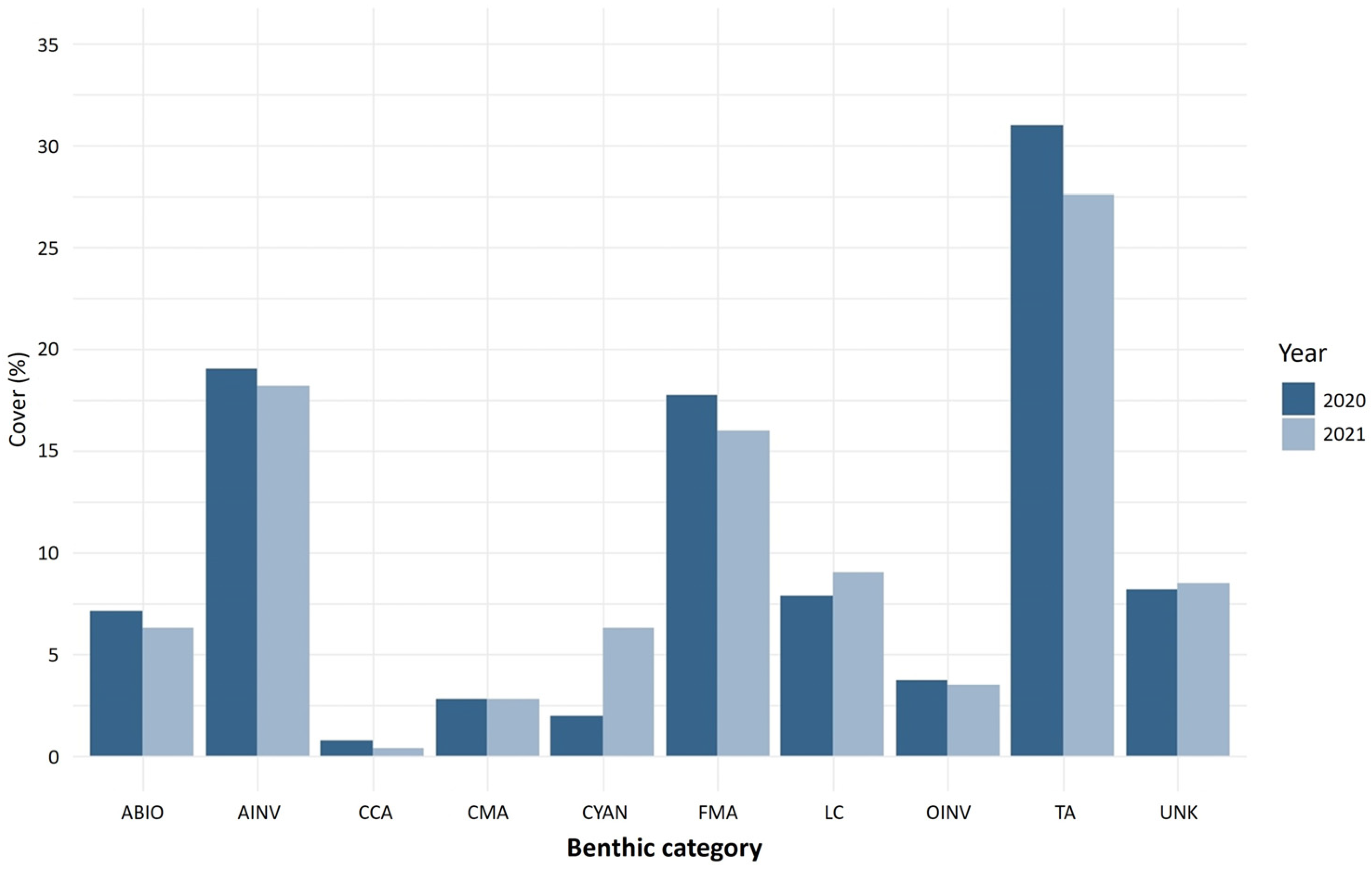
The main benthic components of Francesita reef were turf algae, invertebrates and fleshy macroalgae (Figure 4). Live coral cover was 7.8% in 2020 and 9.2% in 2021. Brooders are the primary coral species in Francesita reef (>90% in 2020 and >85% in 2021), with A. agaricites being the most abundant, followed by Porites porites and Porites furcata. Reef building species (e.g., Orbicella genus) cover less than 5% of all coral cover in both 2020 and 2021. The coverage of the most abundant species was similar between years. However, P. furcata shifted from 2.9% in 2020 to 15.1% in 2021. The species affected by SCTLD in Francesita were A. agaricites in 2020 and S. siderea in 2021.
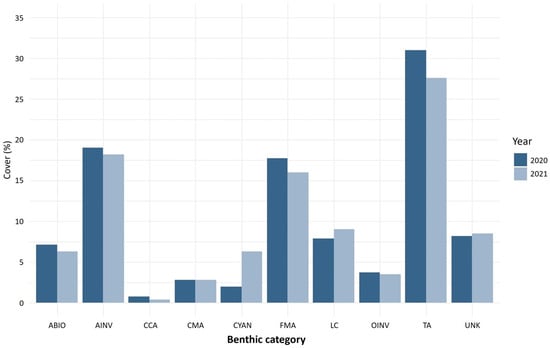
Figure 4.
Benthic reef cover (%) for Francesita reef (Mexico) for 2020 (black bars) and 2021 (white bars). Benthic category codes are LC: live coral, FMA: fleshy macroalgae, TA: turf algae, CMA: calcareous macroalgae, CCA: crustose coralline algae, AINV: aggressive invertebrate, OINV: other invertebrate, CYAN: cyanobacteria, ABIO: abiotic substrate, UNK: unknown/unidentifiable substrates.
At Manchoncitos Reef 35 coral species and 54 fish species were registered, while at Francesita reef 26 coral species and 69 fish species were observed (Supplementary Table S2). At both reefs, the Haemulidae family presented the highest abundance.
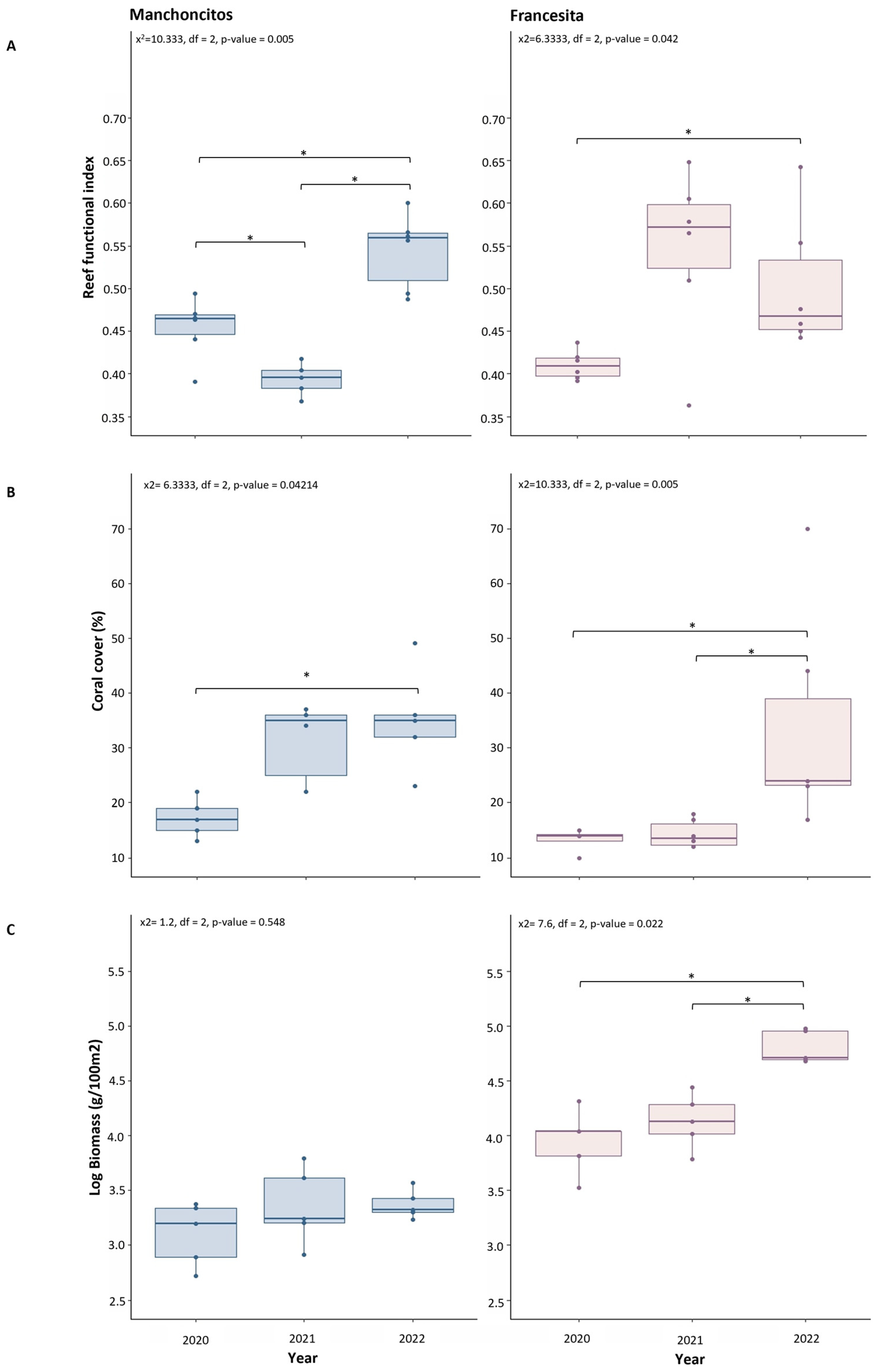
A Friedman test showed significant differences between years for the RFI (Figure 5A), with an increase of ~0.10 at Manchoncitos Reef (x2 = 10.333, df = 2, p-value = 0.0057) and an increase of ~0.09 at Francesita reef from 2020 to 2022 (x2 = 6.3333, df = 2, p-value = 0.04214). Post hoc pairwise comparisons revealed significant differences in RFI at Francesita reef between 2020 and 2022 (Conover test, p-value = 0.036), and 2022 compared to 2020 and 2021 (Conover test, p-value = 0.0017, 4.5 × 10−5) at Manchoncitos reef. The RFI increased for both sites between 2020 and 2022, which could be related to the transplantation efforts.
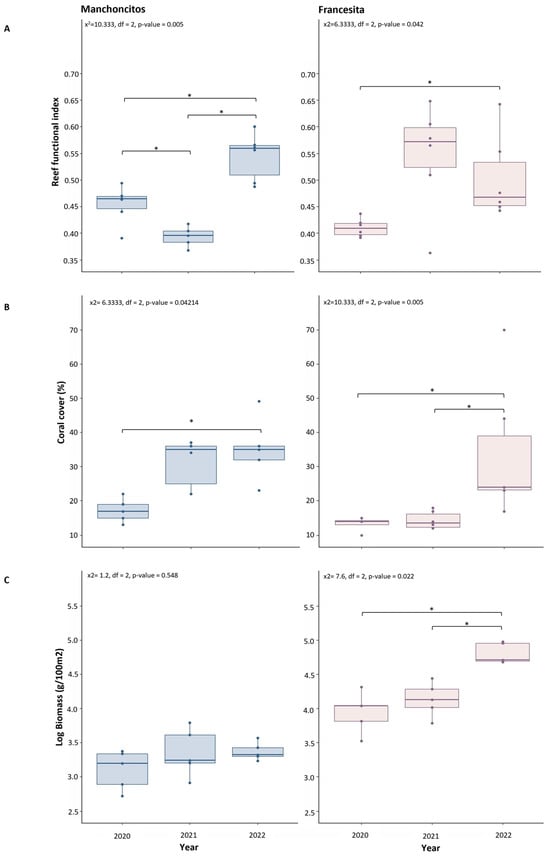
Figure 5.
Ecological benefits due to outplanting. Considering date as a descriptive variable. Reef Functional Index (RFI) (A); Coral cover (B); Total fish biomass (C). Significantly different values of the three indicators between years for each reef, except biomass in Manchoncitos, according to Friedman test (p < 0.05) and Conover’s test post-hoc denoted by different asterisks: * (p-value < 0.05).
Live coral cover was significantly different over time at the two reefs. Manchoncitos reef showed an increase of ~15% (x2 = 6.3333, df = 2, p-value = 0.04214) in hard coral cover over time, between 2020 and 2022 (Conover test, p-value = 0.036), while at Francesita reef there was a ~22% increase in hard coral cover (x2 = 10.333, df = 2, p-value = 0.005704) (Figure 5B), presenting a significant gain in 2022 compared to 2020 and 2021 (Conover test, p-value = 4.5 × 10−5, 0.0017).
No significant differences were observed between years for the total fish biomass at Manchoncitos reef (x2 = 1.2, df = 2, p-value = 0.5488). However, in Francesita fish biomass changed over time (x2 = 7.6, df = 2, p-value = 0.02237) (Figure 5C), in 2020/2022 and 2021/2022 (Bonferroni test, p-value = 0.005, 0.011).
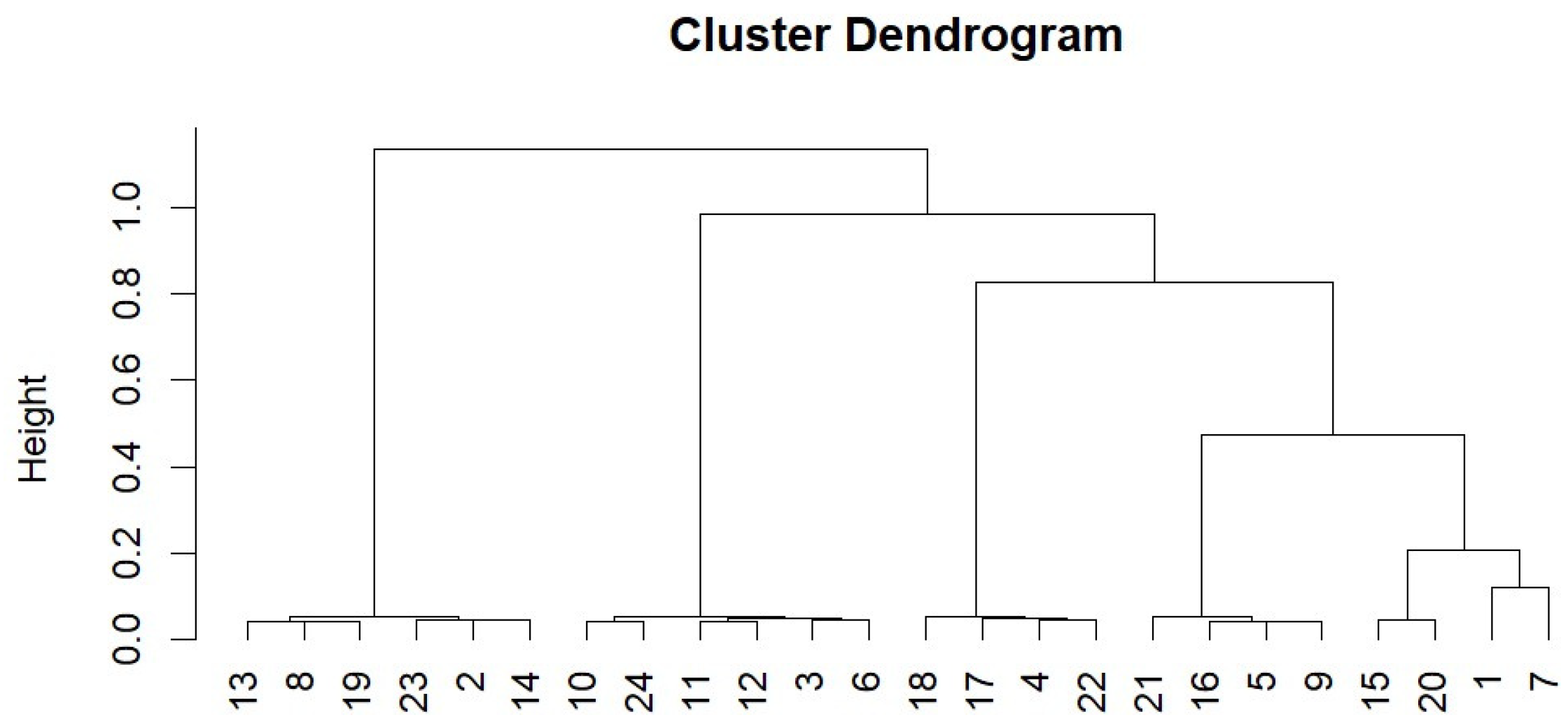
A coral multilocus genotype plot showed that the main Cozumel nursery contained five genotypes in the 24 ramets studied (Figure 6).
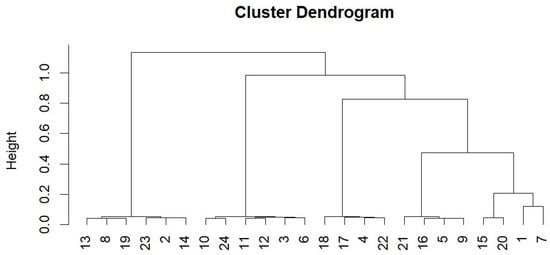
Figure 6.
Clustering dendrogram of A. ramets in the Cozumel nursery.
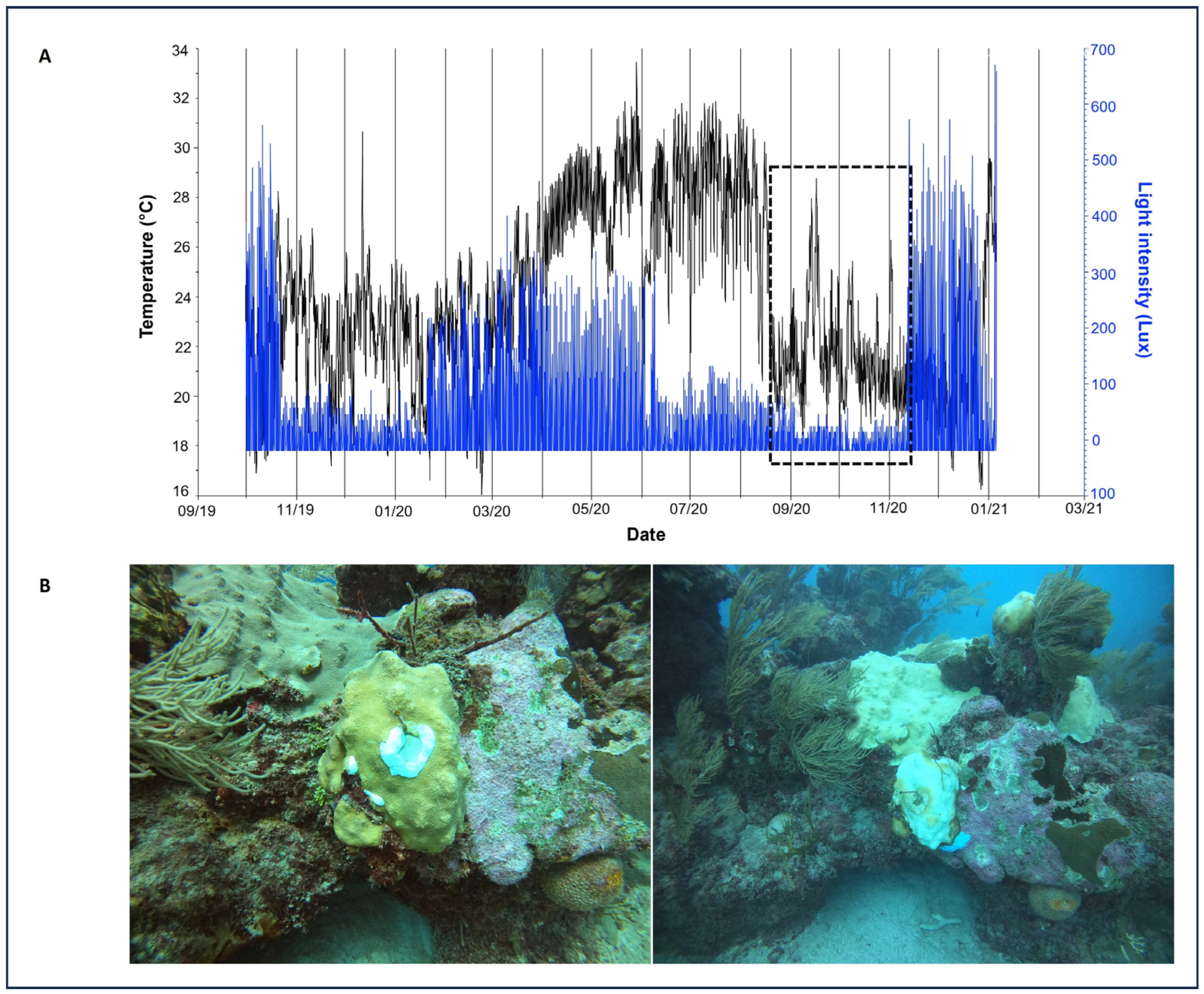
Temperature and light at both reefs during 2020–2022 presented similar data, as did mean dissolved oxygen (DO), pH, salinity, nitrites, nitrates, phosphates, and enterococci (Supplementary Table S3). However, in 2020, after Tropical Storm Cristobal and Hurricanes Gamma, Delta, and Zeta in the second half of the year there was a strong decrease in water temperature, which was more evident at Manchoncitos reef (Figure 7A). This might be related to the strong mass bleaching event observed, mainly affecting O. faveolata colonies (Figure 7B).
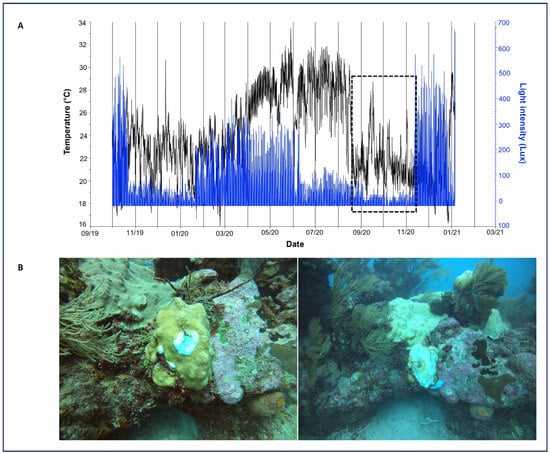
Figure 7.
Temperature (°C) and luminosity (Lux) trend at Manchoncitos Reef in 2020. (A) Black line represents temperature (°C) and blue line represents light intensity (Lux) over time. The black dashed box highlights a period of decrease inluminosity during hurricanes Gamma, Delta, and Zeta. (B) O. faveolata colonies before Hurricane Delta (left) and bleached O. faveolata colonies after Hurricane Delta (right).
4. Discussion
This study suggests that active restoration actions appear to be a suitable approach to maximize diversity at different scales (reef, species and genetic level) and positively influence the maintenance of reef ecological functionality. Our results suggest a direct relationship to the significant increase in the percentage of live coral cover obtained through restoration actions, and the maintenance of genetic variation and coral species richness, from selecting and growing coral colonies in nurseries [33,52]. The significant increases in live coral tissue, the constant amount of fish biomass recorded, and the maintenance of bottom structural complexity over the years at both sampling sites (Manchoncitos and Francesita reefs), are greater than what has been reported in other reef areas with similar characteristics throughout the Mexican Caribbean. In the region, slight increases have been reported ca 3.8% to 8.9% of coral cover during the first decade of the 2000s [53,54]. In addition, in recent years, prolonged heat waves and the outbreak of new and severe diseases, such as hard coral tissue loss disease (SCTLD), have limited the increase in coral cover [55,56]. However, the significant increase in coral cover recorded at both intervention sites, with values higher than those observed in the region, suggests that active restoration actions, such as those carried out in this study, seem to positively influence the maintenance and recovery of ecological processes and functions within the locally treated reefs.
Generally, the tourism sector has played a purely financial role in restoration projects, as it has long-term economic stability compared to sectors that depend on external funding cycles for the implementation of restoration programs [29]. In the case of Mexico, collaborative work with different stakeholders has allowed the incorporation of innovative techniques for restoration programs [31], such as molecular biology, analysis of spectral signatures, implementation of digital underwater photogrammetry, among others. Molecular biology is becoming a useful tool for understanding coral reefs, generating information that helps to improve coral adaptation to climate change [36]. Additionally, as highlighted by Blanco-Pimentel et al. (2022) [35], the tourism sector can play a pivotal role in coral reef restoration beyond financial contributions. Their study emphasizes the importance of integrating tourism operators into long-term restoration efforts by fostering collaboration between hotels, local communities, and scientific institutions. In this context, our study aligns with these recommendations by implementing a multi-stakeholder restoration program that directly involves the tourism sector in monitoring and conservation actions.
A key example of this commitment is the Wave of Change innovation center, through which the hospitality industry is actively engaging in marine conservation by hiring specialized scientists to design, develop, and implement various strategies, experiments, and innovative solutions. This initiative not only generates new approaches to ecosystem restoration but also ensures that findings and results are disseminated through open-access scientific publications, presentations at national and international forums, and the publication of applied science protocols and nature-based solutions [21,30,31,34,35,51,57,58,59,60,61,62]. Furthermore, the industry’s efforts extend beyond coral reef conservation and restoration programs to include coastal dune stabilization, mangrove rehabilitation, and seagrass restoration, enhancing ecosystem connectivity across all destinations where the company operates.
In addition to its direct involvement in marine ecosystem restoration, the hospitality industry has made significant strides in sustainable operations. Since 2020, all of its more than 120 hotels have eliminated single-use plastics, setting a precedent for large-scale corporate sustainability. In 2023, the company achieved its goal of sourcing 100% responsible seafood in Mexico, demonstrating a strong commitment to ocean health beyond reef restoration. Moreover, it has launched two Circular Economy and Coastal Health roadmaps and a guide of Decarbonizing Hotel Food Systems, aimed at reducing its operational carbon footprint by 2030, reinforcing the role of the private sector in advancing climate resilience and sustainability https://waveofchange.com/ (accessed on 3 February 2025).
As part of this holistic approach to ocean conservation, scientific research plays a crucial role in ensuring the effectiveness of restoration efforts. One key aspect is understanding the genetic diversity of coral reef species, which varies considerably between species and even among individuals of the same species. This knowledge allows for the identification of more resilient genetic variants that can be strategically applied in restoration programs [37,38,39,58,59,60,61,62]. To this end, our research has been conducted in collaboration with research centers and universities in Mexico, leveraging scientific innovation to identify and utilize coral genotypes that are better adapted to changing environmental conditions, further enhancing the long-term success of reef restoration initiatives.
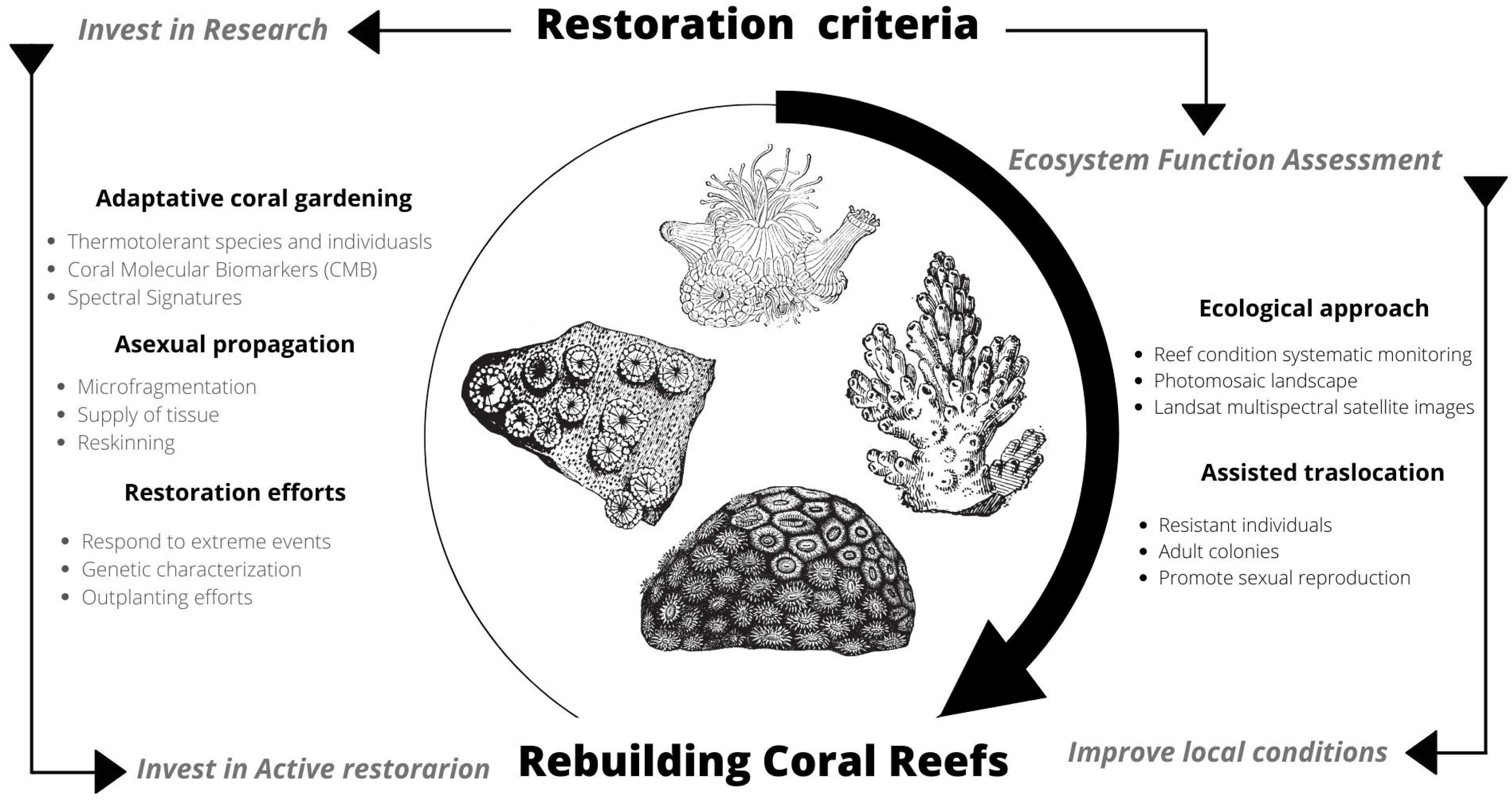
Challenges remain in achieving results towards comprehensive ecological restoration. Hence the importance of continuing to consolidate multidisciplinary collaborations and catalyze solutions for future reef restoration, incorporating research, passive and active restoration actions, local support, and long-term monitoring (Figure 8). If humanity were to succeed in reducing greenhouse gas emissions, it is still necessary to continue investing and working on these types of strategies and solutions that can be replicated and implemented in different locations where coral reef restoration is needed [30,41,42].
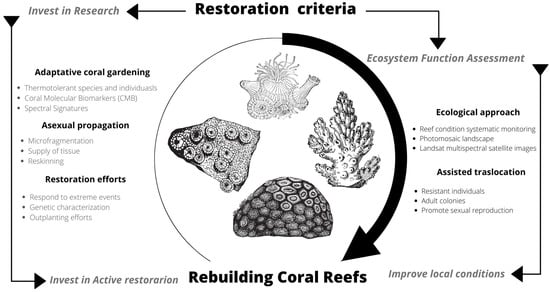
Figure 8.
Outline for rebuilding coral reefs through the organizational capacity of the private sector to invest in research, active restoration, improve local considerations and long-term engagement.
Based on the results obtained, both reefs are degraded. Francesita reef has low coral cover (%) and species richness, and Manchoncitos reef has high algal cover (turf and fleshy) and cyanobacteria. Despite the degraded conditions, a remarkable result is the increase in coral cover from 2020 to 2022 observed in both reefs. This increase is associated with a decrease in macroalgae cover at Francesita reef and the low cover of abiotic substrate at Manchoncitos reef, at least in the areas of active intervention. Furthermore, despite the impact of four hurricanes in these areas in 2020, coral cover increased or was maintained and could be considered as a positive indicator of active restoration efforts.
Both photomosaics and AGRRA analyses for Francesita indicated that there was an increase in coral cover in 2021. However, for Manchoncitos the photomosaic analysis indicated a decrease in coral cover, while AGRRA analysis showed an increase. The contrast of these results could be related to the sampling method used. The use of visual or in situ coral cover estimation methods, such as the AGRRA protocol or the Point Intercept Transect (PIT), have been previously considered to overestimate the percentages of live coral cover [43,63,64] compared to underwater photogrammetry.
Our results of coral cover obtained with digital photogrammetry also contrast with those reported by visual surveys for different reefs in the Caribbean region Reyes-Bonilla et al. (2014) [65,66,67].
Notwithstanding the discrepancies between visual methods and photogrammetry, both methods can complement each other to obtain a more complete assessment of coral cover condition and changes over time.
In the case of Francesita, a reef with a smaller area (0.0048 km2) and considering that the ideal sampling area using UDP is c.a. 0.00038 km2 [44], the restoration efforts carried out in the intervened area (0.001 km2) suggest a positive impact on the changes observed over the whole reef. However, at Manchoncitos (0.15 km2), with an intervened area of 0.0015 km2, active restoration efforts should be increased to see results in all the reefs. It is possible that these changes will be reflected in the long term in ecological succession processes, where demographic monitoring of populations is evaluated to determine changes in population growth rates or sexual recruitment rates [36]. The results indicate that the approach and actions proposed here may accelerate the ecological succession processes needed to scale up restoration [27,45,46,47,48].
To date, these results also show the ecological benefits of outplanted colonies, mainly manifested in an increase in coral cover and greater structural complexity reflected in the RFI. This was also reported by Calle-Triviño et al. [21], in an area of the Arrecifes del Sureste Marine Sanctuary in the Dominican Republic.
Periodic active restoration actions can influence the decrease in the cover of opportunistic species [49], as well as in the processes of herbivory and corallivory. Furthermore, including massive corals is also favorable because they have shown higher outplanted survival, influencing the repair and maintenance of ecological services and functions [50].
In this study, the observed increase in fish biomass at Francesita Reef carries significant functional implications for the ecosystem. One of the most critical roles of increased fish biomass is its contribution to nutrient cycling. Reef-associated fish facilitate the redistribution of nutrients through excretion, enhancing primary production and supporting coral health [68,69,70].
Beyond macroalgae regulation, an increase in fish biomass influences the composition of the trophic structure, promoting a more balanced ecosystem [71,72]. Moreover, a well-structured fish community enhances ecosystem resilience, buffering against environmental stressors such as coral bleaching and disease outbreaks [73,74].
From a socio-economic perspective, increased fish biomass has direct implications for food security and local fisheries. Healthy fish populations ensure sustainable seafood resources for coastal communities, providing both economic benefits and nutritional support [75,76]. Additionally, well-managed reef ecosystems attract ecotourism, further reinforcing conservation efforts and local economic stability [77,78].
Overall, the positive shift in fish biomass at Francesita Reef highlights the importance of effective fisheries management and conservation strategies in maintaining ecological balance and ensuring the long-term sustainability of reef-associated livelihoods [79,80].
The ecology and structural functionality of Caribbean coral reefs have undergone severe ecological changes due to the abrupt mortality of massive and large corals as a consequence of bleaching events and the presence of SCTLD. Álvarez-Filip et al. [16] described a 30% reduction in the ability of coral communities in the Mexican Caribbean region to produce calcium carbonate. If this scenario of coral cover loss continues, in the absence of natural recovery processes, the structural complexity will be modulated only by destructive forces, representing a high risk for coastal protection. This is particularly relevant considering the unprecedented long heat wave observed in 2023 in the Caribbean. Because of this, hard coral cover needs to be increased as a step towards increasing reef resilience. This must be done not only with active calcification processes by building corals [51] but also by forming a protective surface layer on a reef framework [52] using different restoration techniques. In this regard, preliminary results on coral assessment at Manchoncitos reef in 2023, have shown a 97.5% survival rate of the translocated A. palmata colonies that were adapted to higher temperatures, which needs to be further explored.
Obtaining the greatest possible quantity and quality of data from the area will allow us to adapt outplanting techniques, incorporate new elements to the system, such as the introduction of fish post larvae [22], crabs or urchins that could accelerate herbivory functions and in turn improve the natural processes of the coral reefs, allowing them to return to a more favorable state.
Due to the current high numbers of Coral Bleaching Heat Stress Degree Heating Week (DHW) in the Caribbean Basin [53], we are working on the development of probiotics to increase the adaptive capacity of corals and reduce the percentage of coral losses due to environmental stress and disease.
It is important that we do whatever possible to preserve as many genetically unique colonies as possible to allow for the greatest chance of successful future propagation and restoration. We are trying to solve this problem by creating a treatment for SCTLD that can eradicate the pathogens from infected colonies and fragments. The creation of this treatment could allow our community to preserve the living tissue of genetic individuals at a large scale.
It will be fundamental to continue building relationships between government, academia, tourism, and local communities to increase restoration efforts and expand watershed-based approaches to make rehabilitation and restoration processes more efficient, as well as to promote nature-based solutions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d17040268/s1, Figure S1. Coral Reef Restoration Program in Mexico, all tables have captions. Table S1. Outplanted colonies in Manchoncitos reef and Francesita reef over time. Table S2. Coral and fish species observed in the Manchoncitos Reef and Francesita Reef. Table S3. Water Quality in Francesita reef and Manchoncitos reef.
Author Contributions
Conceptualization, J.C.-T.; Methodology, J.C.-T., D.R.-C., L.A.N.-T., M.B.-P., C.C.-U. and R.R.-N.; Formal analysis, J.C.-T., D.R.-C., L.A.N.-T. and R.R.-N.; Investigation, J.C.-T. and C.C.-U.; Resources, J.C.-T., M.B.-P., J.E.A.-G. and C.C.-U.; Data curation, J.C.-T., D.R.-C., L.A.N.-T., M.B.-P., C.C.-U. and R.R.-N.; Writing–original draft, J.C.-T., D.R.-C., L.A.N.-T., N.C.-G., R.C.H.-L., M.B.-P., J.E.A.-G., C.C.-U. and R.R.-N.; Writing–review & editing, J.C.-T., D.R.-C., N.C.-G., R.C.H.-L., J.E.A.-G. and R.R.-N.; Funding acquisition, J.C.-T., M.B.-P. and C.C.-U. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
We would like to thank the Iberostar Group, Dressel Divers for aquatic and logistical support, Centro de Investigación y de Estudios Avanzados del Instituto Politécnico Nacional (National Polytechnical Institute Research and Advanced Studies Center, CINVESTAV), students from the National Autonomous University of Mexico (UNAM-ENES, Merida), The National Commission of Natural Protected Areas (CONANP), Mexican Caribbean Biosphere Reserve (MCRB) and Cozumel Reefs National Park (CRNP), Perry Institute for photomosaic analysis, Megan Morikawa for all the support provided in the creation and development of the program, Iliana Baums and Sheila Kitchen for SNPs analysis. Handling of species and development of research activities were possible thanks to the Iberostar-Cinvestav alliance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nyström, M.; Folke, C.; Moberg, F. Coral reef disturbance and resilience in a human-dominated environment. Trends Ecol. Evol. 2000, 15, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Davis, G.E. A century of natural change in coral distribution at the Dry Tortugas: A comparison of reef maps from 1881 and 1976. Bull. Mar. Sci. 1982, 32, 608–623. [Google Scholar]
- Miller, M.W.; Bourque, A.S.; Bohnsack, J.A. An analysis of the loss of acroporid corals at Looe Key, Florida, USA: 1983–2000. Coral Reefs 2002, 21, 179–182. [Google Scholar] [CrossRef]
- Aronson, R.; Bruckner, A.; Moore, J.; Precht, B.; Weil, E. Acropora cervicornis. In The IUCN Red List of Threatened Species; International Union for the Conservation of Nature and Natural Resources: Gland, Switzerland, 2008; e.T133381A3716457. [Google Scholar] [CrossRef]
- Eakin, C.M.; Morgan, J.A.; Heron, S.F.; Smith, T.B.; Liu, G.; Alvarez-Filip, L.; Baca, B.; Bartels, E.; Bastidas, C.; Bouchon, C.; et al. Caribbean corals in crisis: Record thermal stress, bleaching, and mortality in 2005. PLoS ONE 2010, 5, e13969. [Google Scholar] [CrossRef] [PubMed]
- Levas, S.; Schoepf, V.; Warner, M.E.; Aschaffenburg, M.; Baumann, J.; Grottoli, A.G. Long-term recovery of Caribbean corals from bleaching. J. Exp. Mar. Biol. Ecol. 2018, 506, 124–134. [Google Scholar] [CrossRef]
- Bellwood, D.R.; Pratchett, M.S.; Morrison, T.H.; Gurney, G.G.; Hughes, T.P.; Álvarez-Romero, J.G.; Day, J.C.; Grantham, R.; Grech, A.; Hoey, A.S.; et al. Coral reef conservation in the Anthropocene: Confronting spatial mismatches and prioritizing functions. Biol. Conserv. 2019, 236, 604–615. [Google Scholar] [CrossRef]
- Przeslawski, R.; Ahyong, S.; Byrne, M.; Wörheide, G.; Hutchings, P. Beyond corals and fish: The effects of climate change on noncoral benthic invertebrates of tropical reefs. Glob. Change Biol. 2008, 14, 2773–2795. [Google Scholar] [CrossRef]
- Jokiel, P.L.; Jury, C.P.; Kuffner, I.B. Coral calcification and ocean acidification. In Coral Reefs at the Crossroads. Coral Reefs of the World; Hubbard, D., Rogers, C.S., Lipps, J.H., Stanley, G.D., Jr., Eds.; Springer: Dordrecht, The Netherlands, 2016; pp. 7–45. [Google Scholar]
- Aronson, R.B.; Macintyre, I.G.; Precht, E.F.; Murdoch, T.J.T.; Wapnick, C.M. The expanding scale of species turnover events on coral reefs in Belize. Ecol. Monogr. 2002, 72, 233–249. [Google Scholar]
- Arias-González, J.E.; Fung, T.; Seymour, R.M.; Garza-Pérez, J.R.; Acosta-González, G.; Bozec, Y.-M.; Johnson, C.R. A coral-algal phase shift in Mesoamerica not driven by changes in herbivorous fish abundance. PLoS ONE 2017, 12, e0174855. [Google Scholar] [CrossRef] [PubMed]
- Antonius, A.; Ballesteros, E. Epizoism: A new threat to coral health in Caribbean reefs. Rev. Biol. Trop. 1998, 46, 5. [Google Scholar]
- Harvell, C.D.; Kim, K.; Burkholder, J.M.; Colwell, R.R.; Epstein, P.R.; Grimes, D.J.; Hofmann, E.E.; Lipp, E.K.; Osterhaus, A.D.M.E.; Overstreet, R.M.; et al. Emerging marine diseases–climate links and anthropogenic factors. Science 1999, 285, 1505–1510. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.P.; Anderson, K.D.; Connolly, S.R.; Heron, S.F.; Kerry, J.T.; Lough, J.M.; Baird, A.H.; Baum, J.K.; Berumen, M.L.; Bridge, T.C.; et al. Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 2018, 359, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Precht, W.F.; Gintert, B.E.; Robbart, M.L.; Fura, R.; Van Woesik, R. Unprecedented disease-related coral mortality in Southeastern Florida. Sci. Rep. 2016, 6, 31374. [Google Scholar] [CrossRef]
- Álvarez-Filip, L.; González-Barrios, F.J.; Pérez-Cervantes, E.; Molina-Hernández, A.; Estrada Saldívar, N. Stony coral tissue loss disease decimated Caribbean coral populations and reshaped reef functionality. Commun. Biol. 2022, 5, 440. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, E.V.; Perry, C.T.; Halloran, P.R.; Iglesias-Prieto, R.; Schönberg, C.H.; Wisshak, M.; Form, A.U.; Carricart-Ganivet, J.P.; Fine, M.; Eakin, C.M.; et al. Avoiding coral reef functional collapse requires local and global action. Curr. Biol. 2013, 23, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Setter, R.O.; Franklin, E.C.; Mora, C. Co-occurring anthropogenic stressors reduce the timeframe of environmental viability for the world’s coral reefs. PLoS Biol. 2022, 20, e3001821. [Google Scholar] [CrossRef] [PubMed]
- Young, C.; Schopmeyer, S.; Lirman, D. A review of reef restoration and coral propagation using the threatened genus Acropora in the Caribbean and western Atlantic. Bull. Mar. Sci. 2012, 88, 1075–1098. [Google Scholar] [CrossRef]
- Boström-Einarsson, L.; Babcock, R.C.; Bayraktarov, E.; Ceccarelli, D.; Cook, N.; Ferse, S.C.A.; Hancock, B.; Harrison, P.; Hein, M.; Shaver, E.; et al. Coral restoration–A systematic review of current methods, successes, failures and future directions. PLoS ONE 2020, 15, e0226631. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bayraktarov, E.; Banaszak, A.T.; Montoya, P.M.; Kleypas, J.; Arias-González, J.E.; Blanco, M.; Calle-Triviño, J.; Charuvi, N.; Cortés-Useche, C.; Galván, V.; et al. Coral reef restoration efforts in Latin American countries and territories. PLoS ONE 2020, 15, e0228477. [Google Scholar] [CrossRef] [PubMed]
- Calle-Triviño, J.; Cortés-Useche, C.; Sellares-Blasco, R.I.; Arias-González, J.E. Assisted fertilization of threatened Staghorn Coral to complement the restoration of nurseries in Southeastern Dominican Republic. Reg. Stud. Mar. Sci. 2018, 18, 129–134. [Google Scholar] [CrossRef]
- Sellares-Blasco, R.I.; Villalpando, M.F.; Guendulain-García, S.D.; Croquer, A. Assisted coral reproduction in the Dominican Republic: A successful story to replicate in the Caribbean. Front. Mar. Sci. 2021, 8, 669505. [Google Scholar] [CrossRef]
- Toh, T.C.; Guest, J.; Chou, L. Coral larval rearing in Singapore: Observations on spawning timing, larval development and settlement of two common scleractinian coral species. In Contributions to Marine Science; National University of Singapore: Singapore, 2012; pp. 81–87. [Google Scholar]
- Gilmour, J.; Speed, C.W.; Babcock, R. Coral reproduction in Western Australia. PeerJ 2016, 4, e2010. [Google Scholar] [CrossRef]
- Calle-Triviño, J.; Rivera-Madrid, R.; León-Pech, M.G.; Cortés-Useche, C.; Sellares-Blasco, R.I.; Aguilar-Espinosa, M.; Arias-González, J.E. Assessing and genotyping threatened staghorn coral Acropora cervicornis nurseries during restoration in southeast Dominican Republic. PeerJ 2020, 8, e8863. [Google Scholar] [CrossRef] [PubMed]
- Rivas, N.; Hesley, D.; Kaufman, M.; Unsworth, J.; D’Alessandro, M.; Lirman, D. Developing best practices for the restoration of massive corals and the mitigation of predation impacts: Influences of physical protection, colony size, and genotype on outplant mortality. Coral Reefs 2021, 40, 1227–1241. [Google Scholar] [CrossRef]
- Calle-Triviño, J.; Muñiz-Castillo, A.I.; Cortés-Useche, C.; Morikawa, M.; Sellares-Blasco, R.; Arias-González, J.E. Approach to the Functional Importance of Acropora cervicornis in Outplanting Sites in the Dominican Republic. Front. Mar. Sci. 2021, 8, 668325. [Google Scholar] [CrossRef]
- Suggett, D.J.; Edwards, M.; Cotton, D.; Hein, M.; Camp, E.F. An integrative framework for sustainable coral reef restoration. One Earth 2023, 6, 666–681. [Google Scholar] [CrossRef]
- Cortés-Useche, C.; Hernández-Delgado, E.A.; Calle-Triviño, J.; Sellares Blasco, R.; Galván, V.; Arias-González, J.E. Conservation actions and ecological context: Optimizing coral reef local management in the Dominican Republic. PeerJ 2021, 9, e10925. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Useche, C.; Reyes-Gamboa, W.; Cabrera-Pérez, J.L.; Calle-Triviño, J.; Cerón-Flores, A.; Raigoza-Figueras, R.; Yathiraj, R.; Arias-González, J.E. Capture, Culture and Release of Postlarvae Fishes: Proof-of-Concept as a Tool Approach to Support Reef Management. Front. Mar. Sci. 2021, 8, 718526. [Google Scholar] [CrossRef]
- Kleypas, J.; Allemand, D.; Anthony, K.; Baker, A.C.; Beck, M.W.; Hale, L.Z.; Hilmi, N.; Hoegh-Guldberg, O.; Hughes, T.; Kaufman, L.; et al. Designing a blueprint for coral reef survival. Biol. Conserv. 2021, 257, 109107. [Google Scholar] [CrossRef]
- Quigley, K.M.; Hein, M.; Suggett, D.J. Translating the 10 golden rules of reforestation for coral reef restoration. Conserv. Biol. 2022, 36, e13890. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Roach, S.; Duarte, C.; Hauser, C.A.; Aranda, M. Beyond reef restoration: Next-generation techniques for coral gardening, landscaping, and outreach. Front. Mar. Sci. 2020, 7, 672. [Google Scholar] [CrossRef]
- Blanco-Pimentel, M.; Evensen, N.R.; Cortés-Useche, C.; Calle-Triviño, J.; Barshis, D.J.; Galván, V.; Harms, E.; Morikawa, M.K. All-inclusive coral reef restoration: How the tourism sector can boost restoration efforts in the caribbean. Front. Mar. Sci. 2022, 9, 931302. [Google Scholar] [CrossRef]
- Contreras-Silva, A.I.; Tilstra, A.; Migani, V.; Thiel, A.; Pérez-Cervantes, E.; Estrada-Saldívar, N.; Elias-Ilosvay, X.; Mott, C.; Alvarez-Filip, L.; Wild, C. A meta-analysis to assess long-term spatiotemporal changes of benthic coral and macroalgae cover in the Mexican Caribbean. Sci. Rep. 2020, 10, 8897. [Google Scholar] [CrossRef]
- Caballero-Aragón, H.; Perera-Valderrama, S.; Cerdeira-Estrada, S.; Martell-Dubois, R.; la Cruz, L.R.; Álvarez-Filip, L.; Pérez-Cervantes, E.; Estrada-Saldivar, N.; Ressl, R. Dataset of coral reefs monitoring, Puerto Morelos, Mexico. Data Brief 2022, 42, 108253. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Rendis, A.; Acosta-González, G.; Arias-González, J.E. A spatio-temporal long-term assessment on the ecological response of reef communities in a Caribbean marine protected area. Aquat. Conserv. Mar. Freshw. Ecosyst. 2020, 30, 273–289. [Google Scholar] [CrossRef]
- Medina-Valmaseda, A.E.; Rodríguez-Martínez, R.E.; Alvarez-Filip, L.; Jordan-Dahlgren, E.; Blanchon, P. The role of geomorphic zonation in long-term changes in coral-community structure on a Caribbean fringing reef. PeerJ 2020, 8, e10103. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, R.E.; Ruíz-Rentería, F.; van Tussenbroek, B.; Barba-Santos, G.; Escalante-Mancera, E.; Jordán-Garza, G.; Jordán-Dahlgren, E. Environmental state and tendencies of the Puerto Morelos CARICOMP site, Mexico. Rev. Biol. Trop. 2010, 58 (Suppl. S3), 23–43. [Google Scholar] [PubMed]
- Hernández-Landa, R.C.; Barrera-Falcon, E.; Rioja-Nieto, R. Size-frequency distribution of coral assemblages in insular shallow reefs of the Mexican Caribbean using underwater photogrammetry. PeerJ 2020, 8, e8957. [Google Scholar] [CrossRef] [PubMed]
- Calle-Triviño, J.; Niño-Torres, L.A.; Rojas-Cano, D.; Flores-Quintal, K.; Ruiz-Flores, A.; Cortés-Martínez, E.; Soto-Alonso, M. Manual Planificación y Diseño de Programas de Restauración de Arrecifes Coralinos. Wave of Change 2022. Available online: https://coralmar.org/wp-content/uploads/2024/03/Manual-planificacion-y-diseno.pdf (accessed on 15 May 2023).
- Johnson, M.E.; Lustic, C.; Bartels, E.; Baums, I.B.; Guillam, D.S.; Larson, L.; Lirman, D.; Miller, M.W.; Nedimyer, K.; Schopmeyer, S. Caribbean Acropora Restoration Guide: Best Practices for Propagation and Population Ehancement; TNC: Arlington VA, USA, 2011. [Google Scholar]
- Goergen, E.A.; Schopmeyer, S.; Moulding, A.L.; Moura, A.; Kramer, P.; Viehman, T.S. Coral Reef Restoration Monitoring Guide: Methods to Evaluate Restoration Success from Local to Ecosystem Scales; NOAA Technical Memorandum NOSNCCOS 279; NOAA NOS NCCOS: Silver Spring, MD, USA, 2020; 145p. [Google Scholar] [CrossRef]
- Lang, J.C.; Marks, W.; Kramer, P.; Richards-Kramer, P.A.; Ginsburg, R.N. AGRRA Protocols Version 5.4. 2010. Available online: https://www.researchgate.net/publication/265148106_Agrra_protocols_version_54 (accessed on 15 May 2023).
- Neufeld, A.M.; Fundakowski, G. Coral Restoration Foundation Photomosaic Manual; Coral Restoration Foundation: Key Largo, FL, USA, 2019; 30p. [Google Scholar]
- González-Barrios, F.J.; Álvarez-Filip, L. A framework for measuring coral species-specific contribution to reef functioning in the Caribbean. Ecol. Indic. 2018, 95, 877–886. [Google Scholar] [CrossRef]
- Bonsack, J.A.; Harper, D.E. Length-Weight Relationships of Selected Marine Reef Fishes from the Southeastern United States and the Caribbean; NOAA. NOAA Tech Memo NMFS-SEFC 215; NOAA NOS NCCOS: Silver Spring, MD, USA, 1988. [Google Scholar]
- Froese, R.; Pauly, D. FishBase. 2019. Available online: http://www.fishbase.org (accessed on 15 May 2023).
- Kitchen, S.A.; Von Kuster, G.; Kuntz, K.L.; Reich, H.G.; Miller, W.; Griffin, S.; Fogarty, N.D. STAGdb: A 30K SNP genotyping array and science gateway for Acropora corals and their dinoflagellate symbionts. Sci. Rep. 2020, 10, 12488. [Google Scholar] [CrossRef]
- Blanco-Pimentel, M.; Kenkel, D.C.; Kitchen, S.A.; Calle-Triviño, J.; Baums, I.B.; Cortés-Useche, C.; Morikawa, M.K. Overcoming barriers to reef restoration: Field-based method for approximate genotyping of Acropora cervicornis. Restor. Ecol. 2023, 32, 14073. [Google Scholar] [CrossRef]
- Baums, I.B.; Baker, A.C.; Davies, S.W.; Grottoli, A.G.; Kenkel, C.D.; Kitchen, S.A.; Kuffner, I.B.; LaJeunesse, T.C.; Matz, M.V.; Miller, M.W.; et al. Considerations for maximizing the adaptive potential of restored coral populations in the western Atlantic. Ecol. Appl. 2019, 29, e01978. [Google Scholar] [CrossRef] [PubMed]
- Rioja-Nieto, R.; Álvarez-Filip, L. Coral reef systems of the Mexican Caribbean: Status, recent trends and conservation. Mar. Pollut. Bull. 2019, 140, 616–625. [Google Scholar] [CrossRef]
- Díaz-López, A.M.; Hernández-Arana, H.A.; Vega-Zepeda, A.; Ruiz-Zárate, M.A.; Victoria-Salazar, I. Changes in the community structure of stony corals in the southern Mexican Caribbean. Mar. Environ. Res. 2023, 191, 106154. [Google Scholar] [CrossRef]
- Randall, C.J.; Van Woesik, R. Some coral diseases track climate oscillations in the Caribbean. Sci. Rep. 2017, 7, 5719. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Saldívar, N.; Jordán-Dalhgren, E.; Rodríguez-Martínez, R.E.; Perry, C.; Álvarez-Filip, L. Functional consequences of the long-term decline of reef-building corals in the Caribbean: Evidence of across-reef functional convergence. R. Soc. Open Sci. 2019, 6, 190298. [Google Scholar] [CrossRef]
- Croquer, A.; Zambrano, S.; King, S.; Reyes, A.; Sellares-Blanco, R.; Trinidad, A.V.; Villalpando, M.; Rodriguez-Jerez, Y.; Vargas, E.; Cortes-Useche, C.; et al. Stony Coral Tissue Loss Disease and Other Diseases Affect Adults and Recruits of Major Reef Builders at Different Spatial Scales in the Dominican Republic. Gulf Caribb. Res. 2022, 33, GCFI1–GCFI13. [Google Scholar] [CrossRef]
- Blanco-Pimentel, M.; Calle-Triviño, J.; Morikawa, M. Grafting Method for Estimating Genotypic Diversity in Acropora cervicornis. 2023. Available online: https://dx.doi.org/10.17504/protocols.io.bp2l6xxnklqe/v1 (accessed on 18 August 2023).
- Pelose, G.; Blanco-Pimentel, M.; Calle-Triviño, J.; Leon, A.; Galván, V.; Schopmeyer, S.; Foster, D.; Harms, E.; Burdett, C.; Morikawa, M. Novel Ex-Situ Stony Coral Tissue Loss Disease Treatment Protocol. 2024. Available online: https://dx.doi.org/10.17504/protocols.io.dm6gpzknplzp/v1 (accessed on 13 December 2024).
- Colín-García, N.A.; Ocaña-Mendoza, C.; Chiappa-Carrara, X.; Rioja-Nieto, R.; Arena Ortíz, M.L.; Calle-Triviño, J.; Alvarado-Recillas, N.; Campos, J. Thermal stress response in the Montastraea cavernosa coral-symbiont complex in the Mexican Caribbean, through the expression of the HSP70 gene. Bull. Mar. Sci. 2024, 100, 779–791. [Google Scholar] [CrossRef]
- O’Donnell, S.E.; Ruggeri, M.; Blanco-Pimentel, M.; Morikawa, M.K.; Harms, E.; Calle-Triviño, J.; Flanagan, B.A.; Carlson, H.K.; Kenkel, C.D.; Million, W.C. Species-specific patterns of population genetic structure differ on a microgeographic scale. Coral Reefs 2025, 44, 353–358. [Google Scholar] [CrossRef]
- Colín Garcia, N.A.; Ocaña-Mendoza, C.; Chiappa-Carrara, X.; Rioja-Nieto, R.; Calle-Triviño, J.; Pérez-Ángel, D.A. Characterization and Expression of Hsp70 Gene in Corals: A Comparative Responses of Coral Hosts and Symbiodinium to Thermal Stress in Three Coral Species. 2025. Available online: https://ssrn.com/abstract=5119646 (accessed on 3 February 2025).
- Barrera-Falcon, E.; Rioja-Nieto, R.; Hernández-Landa, R.C.; Torres-Irineo, E. Comparison of Standard Caribbean Coral Reef Monitoring Protocols and Underwater Digital Photogrammetry to Characterize Hard Coral Species Composition, Abundance and Cover. Front. Mar. Sci. 2021, 8, 722569. [Google Scholar] [CrossRef]
- Urbina-Barreto, I.; Garnier, R.; Elise, S.; Pinel, R.; Dumas, P.; Mahamadaly, V.; Facon, M.; Bureau, S.; Peignon, C.; Quod, J.-P.; et al. Which method for which purpose? A comparison of line intercept transect and underwater photogrammetry methods for coral reef surveys. Front. Mar. Sci. 2021, 8, 577. [Google Scholar] [CrossRef]
- Reyes-Bonilla, H.; Millet, E.; Alvarez-Filip, L. Community structure of scleractinian corals outside protected areas in Cozumel Island, Mexico. Atoll Res. Bull. 2014, 601, 1–16. [Google Scholar] [CrossRef][Green Version]
- Barranco, L.; Carriquiry, J.; Rodriguez Zaragoza, F.; Cupul-Magaña, A.; Villaescusa, J.; Calderon-Aguilera, L. Spatiotemporal variations of live coral cover in the northern Mesoamerican reef system, Yucatan peninsula, Mexico. Sci. Mar. 2016, 80, 143–150. [Google Scholar] [CrossRef]
- McField, M.; Kramer, P.; Alvarez-Filip, L.; Drysdale, I.; Flores, M.; Petersen, A.; Soto, M. 2018 Mesoamerican Reef Report Card (Washington, DC). ResearchGate 2018, 13. [Google Scholar] [CrossRef]
- Allgeier, J.E.; Burkepile, D.E.; Layman, C.A. Animal pee in the sea: Consumer-mediated nutrient dynamics in the world’s changing oceans. Glob. Change Biol. 2017, 23, 2166–2178. [Google Scholar] [CrossRef] [PubMed]
- Brandl, S.J.; Tornabene, L.; Goatley, C.R.H.; Casey, J.M.; Morais, R.A.; Côté, I.M.; Baldwin, C.C.; Parravicini, V.; Schiettekatte, N.M.D.; Bellwood, D.R. Demographic dynamics of the smallest marine vertebrates fuel coral-reef ecosystem functioning. Science 2019, 364, 1189–1192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, Y.; Huang, H.; Li, X.B.; Zheng, W.; Wang, D.R. Biogeochemical Dynamics of Coral Reef Systems. In Coral Reefs of the Western Pacific Ocean in a Changing Anthropocene; Zhang, J., Yeemin, T., Morrison, R.J., Hong, G.H., Eds.; Coral Reefs of the World; Springer: Cham, Switzerland, 2022; Volume 14. [Google Scholar] [CrossRef]
- Sandin, S.A.; Smith, J.E.; DeMartini, E.E.; Dinsdale, E.A.; Donner, S.D.; Friedlander, A.M.; Konotchick, T.; Malay, M.; Maragos, J.E.; Obura, D.; et al. Baselines and Degradation of Coral Reefs in the Northern Line Islands. PLoS ONE 2008, 3, e1548. [Google Scholar] [CrossRef]
- Bozec, Y.M.; O’Farrell, S.; Bruggemann, J.H.; Luckhurst, B.E.; Mumby, P.J. Tradeoffs between fisheries harvest and the resilience of coral reefs. Proc. Natl. Acad. Sci. USA 2016, 113, 4536–4541. [Google Scholar]
- McClanahan, T.R.; Graham, N.A.J.; MacNeil, M.A.; Muthiga, N.A.; Cinner, J.E.; Bruggemann, J.H.; Wilson, S.K. Critical Thresholds and Tangible Targets for Ecosystem-Based Management o (PDF) Environmental Variability Indicates a Climate-Adaptive Center Under Threat in Northern Mozambique Coral Reefs. 2011. Available online: https://www.researchgate.net/publication/317755982_Environmental_variability_indicates_a_climate-adaptive_center_under_threat_in_northern_Mozambique_coral_reefs (accessed on 3 February 2025).
- Graham, N.A.J.; Jennings, S.; MacNeil, M.A.; Mouillot, D.; Wilson, S.K. Predicting climate-driven regime shifts versus rebound potential in coral reefs. Nature 2015, 518, 94–97. [Google Scholar] [CrossRef]
- Cinner, J.E.; Huchery, C.; MacNeil, M.A.; Graham, N.A.; McClanahan, T.R.; Maina, J.; Maire, E.; Kittinger, J.N.; Hicks, C.C.; Mora, C.; et al. Bright spots among the world’s coral reefs. Nature 2016, 535, 416–419. [Google Scholar] [CrossRef]
- Pauly, D.; Zeller, D. Catch reconstructions reveal that global marine fisheries catches are higher than reported and declining. Nat. Commun. 2016, 7, 10244. [Google Scholar] [CrossRef] [PubMed]
- Bruggemann, J.H.; Rodier, M.; Guillaume, M.M.; Andréfouët, S.; Arfi, R.; Cinner, J.E.; Pichon, M.; Ramahatratra, F.; Rasoamanendrika, F.; Zinke, J.; et al. Wicked social-ecological problems forcing unprecedented change on the latitudinal margins of coral reefs: The case of Southwest Madagascar. Ecol. Soc. 2012, 17, 47. [Google Scholar] [CrossRef]
- Cesar, H.; Burke, L.; Pet-Soede, L. The Economics of Worldwide Coral Reef Degradation. International System for Agricultural Science and Technology. 2003. Available online: http://www.icran.org/pdf/cesardegradationreport.pdf (accessed on 3 February 2025).
- Hoegh-Guldberg, O.; Mumby, P.J.; Hooten, A.J.; Steneck, R.S.; Greenfield, P.; Gomez, E.; Harvell, C.D.; Sale, P.F.; Edwards, A.J.; Caldeira, K.; et al. Coral Reefs Under Rapid Climate Change and Ocean Acidification. Science 2007, 318, 1737–1742. [Google Scholar] [CrossRef]
- McClanahan, T.R.; Muthiga, N.A.; Kamukuru, A.T.; Machano, H.; Kiambo, R.W. The effects of marine parks and fishing on coral reefs of northern Tanzania. Biol. Conserv. 1999, 89, 161–182. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).