New Insight into the Demography History, Evolution, and Phylogeography of Horseshoe Crabs with Special Emphasis on American Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Datasets Configuration
2.2. Study Area and Sample Collection in the Mexican Distribution Range
2.3. Laboratory Procedures
2.4. Analyses
2.4.1. Phylogeny and Phylogeography of Horseshoe Crabs
2.4.2. Divergence Time Estimation
2.4.3. Phylogeography and Demographic History in America
3. Results
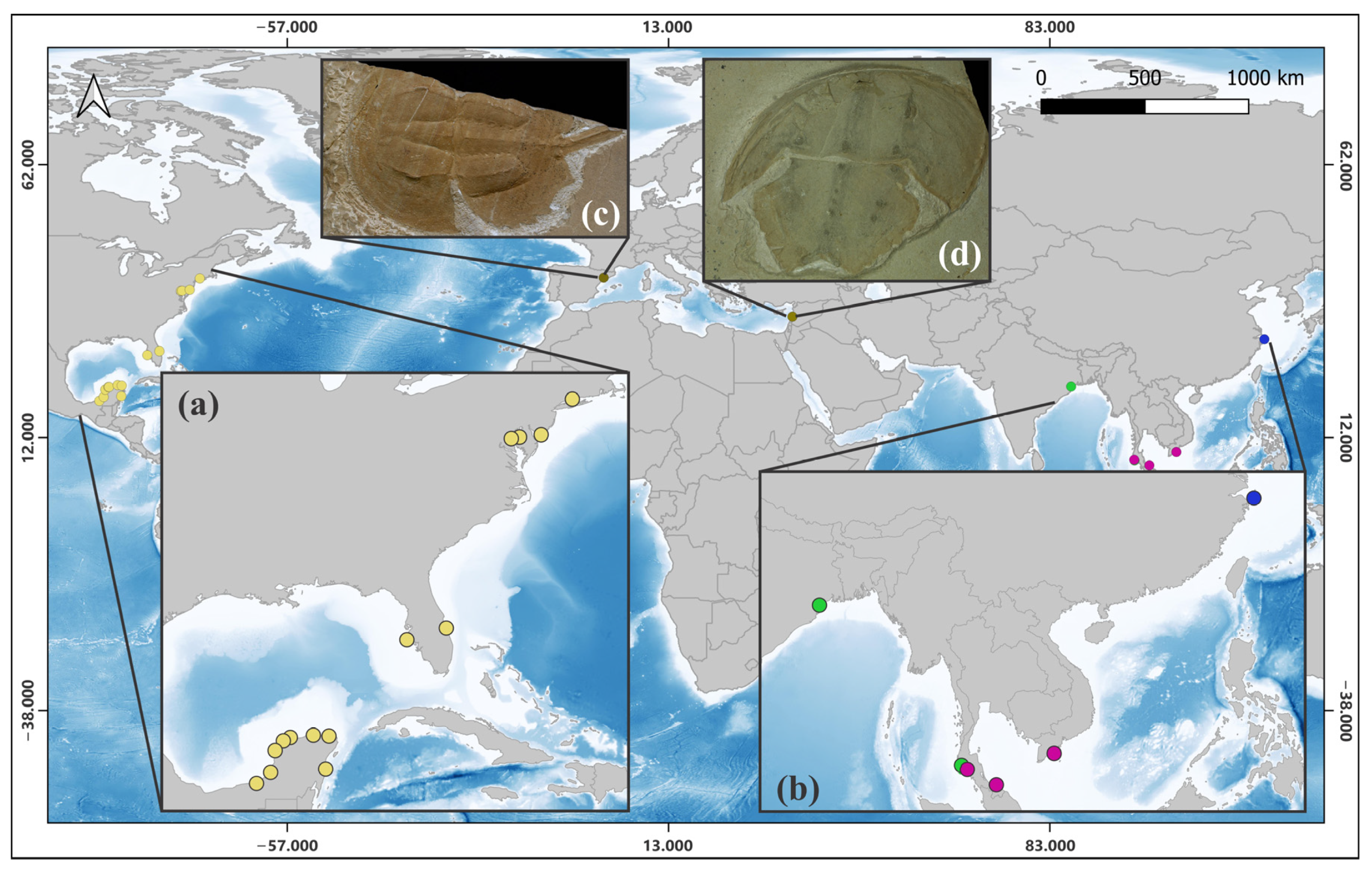
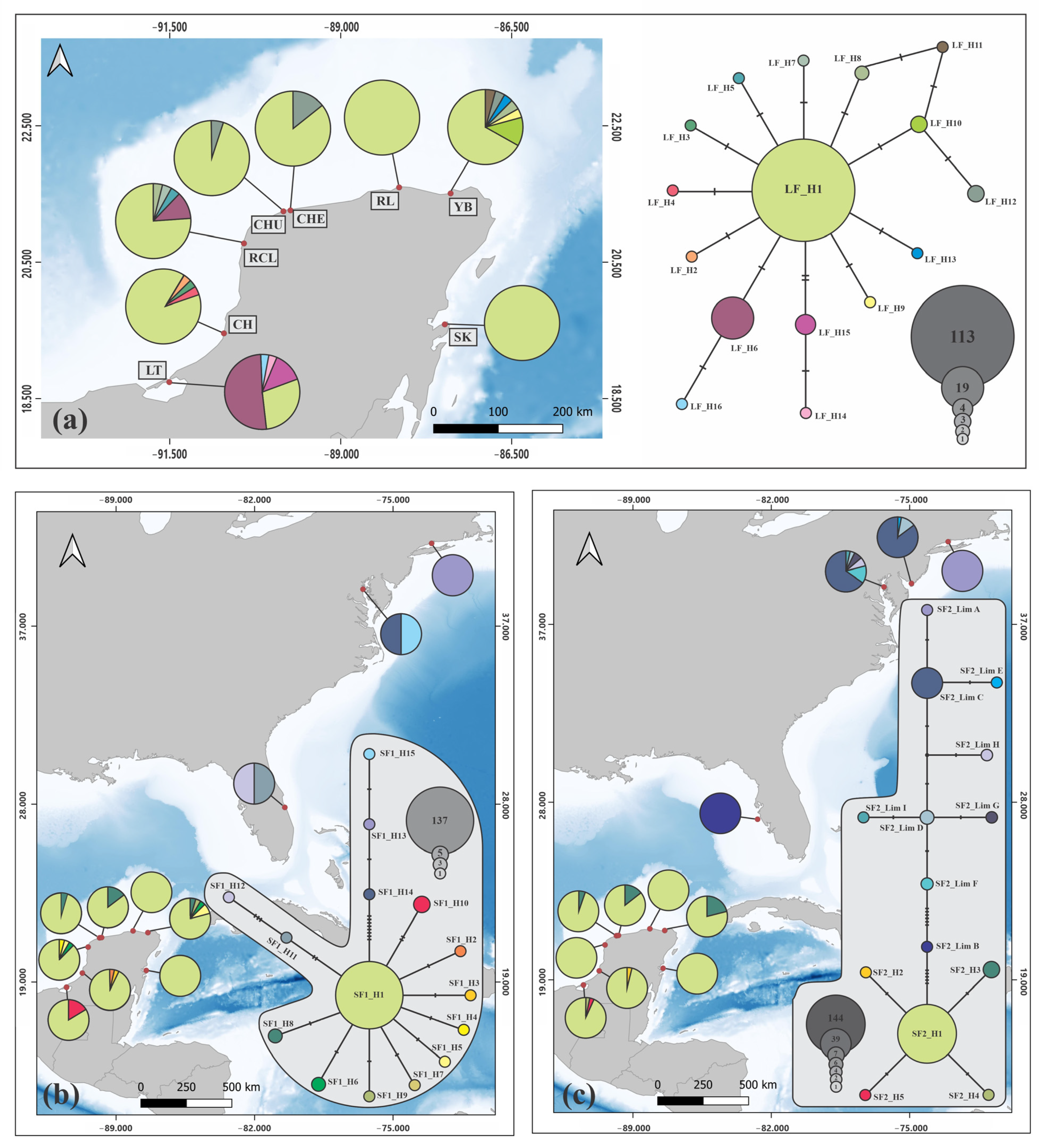
3.1. Phylogeny and Phylogeography of Horseshoe Crabs
3.2. Divergence Time Estimation
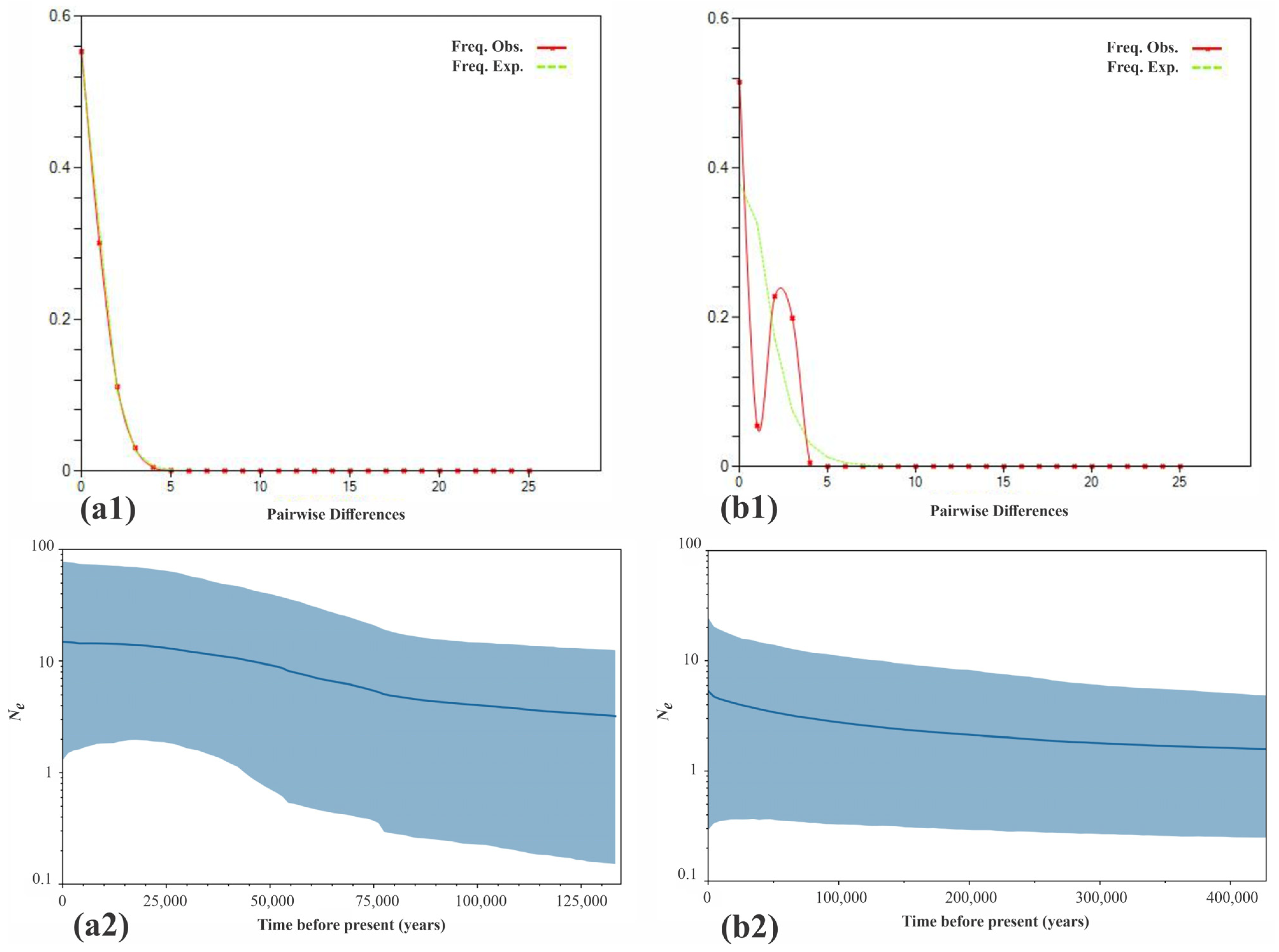
3.3. Phylogeography and Demographic History in America
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Roy, P.; Orr, P.J.; Botting, J.P.; Muir, L.A.; Vinther, J.; Lefebvre, B.; el Hariri, K.; Briggs, D.E.G. Ordovician faunas of Burgess shale type. Nature 2010, 465, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Eldredge, N.I. Differential evolutionary rates. Paleobiology 1976, 2, 174–177. [Google Scholar] [CrossRef]
- Lamsdell, J.C. Evolutionary history of the dynamic horseshoe crab. Int. Wader Stud. 2019, 21, 1–15. [Google Scholar]
- Lamsdell, J.C. The phylogeny and systematics of Xiphosura. PeerJ 2020, 8, e10431. [Google Scholar] [CrossRef]
- Lamsdell, J.C.; Isotalo, P.A.; Rudkin, D.M.; Martin, M.J. A new species of the Ordovician horseshoe crab Lunataspis. Geol. Mag. 2023, 160, 167–171. [Google Scholar] [CrossRef]
- Lamsdell, J.C. Horseshoe crab phylogeny and independent colonizations of fresh water: Ecological invasion as a driver for morphological innovation. Palaeontology 2016, 59, 181–194. [Google Scholar] [CrossRef]
- Barnosky, A.D.; Matzke, N.; Tomiya, S.; Wogan, G.O.U.; Swartz, B.; Quental, T.B.; Marshall, C.; McGuire, J.L.; Lindsey, E.L.; Maguire, K.C.; et al. Has the Earth’s sixth mass extinction already arrived? Nature 2011, 471, 51–57. [Google Scholar] [CrossRef]
- Sekiguchi, K.; Shuster, C.N. Limits on the Global Distribution of Horseshoe Crabs (Limulacea): Lessons learned from two lifetimes of observations: Asia and America. In Biology and Conservation of Horseshoe Crabs; Tanacredi, J., Botton, M., Smith, D., Eds.; Springer: Boston, MA, USA, 2009; pp. 5–24. [Google Scholar] [CrossRef]
- Brockmann, H.J.; Black, T.; King, T.L. Florida horseshoe crabs: Population, genetics and the marine-life harvest. In Changing Global Perspectives on Horseshoe Crab Biology, Conservation and Management; Carmichael, R., Botton, M., Shin, P., Cheung, S., Eds.; Springer: Cham, Switzerland, 2015; pp. 99–127. [Google Scholar] [CrossRef]
- Zaldívar-Rae, J.; Sapién-Silva, R.E.; Rosales-Raya, M.; Brockmann, H.J. American horseshoe crabs, Limulus polyphemus, in Mexico: Open possibilities. In Biology and Conservation of Horseshoe Crabs; Tanacredi, J., Botton, M., Smith, D., Eds.; Springer: Boston, MA, USA, 2009; pp. 97–113. [Google Scholar] [CrossRef]
- John, B.A.; Nelson, B.R.; Sheikh, H.I.; Cheung, S.G.; Wardiatno, Y.; Dash, B.P.; Tsuchiya, K.; Iwasaki, Y.; Pati, S. A review on fisheries and conservation status of Asian horseshoe crabs. Biodivers. Conserv. 2018, 27, 3573–3598. [Google Scholar] [CrossRef]
- Zhu, G.; Yuan, X.; Fan, J. Insight into intraspecific niche divergence and conservatism in American horseshoe crabs (Limulus polyphemus). Glob. Ecol. Conserv. 2020, 22, e00896. [Google Scholar] [CrossRef]
- Bicknell, R.D.C.; Pates, S. Pictorial atlas of fossil and extant horseshoe crabs, with focus on Xiphosurida. Front. Earth Sci. 2020, 8, 98. [Google Scholar] [CrossRef]
- Bicknell, R.D.C.; Birch, S.A.; Charbonnier, S.; Sautereau, F.; Hitij, T.; Campione, N.E.; Brougham, T. On the appendicular anatomy of the xiphosurid Tachypleus syriacus and the evolution of fossil horseshoe crab appendages. Sci. Nat. 2019, 106, 38. [Google Scholar] [CrossRef] [PubMed]
- Noah, K.E.; Hao, J.; Li, L.; Sun, X.; Foley, B.; Yang, Q.; Xia, X. Major revisions in arthropod phylogeny through improved supermatrix, with support for two possible waves of land invasion Chelicerates. Evol. Bioinform. 2020, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, J.A.; Santibáñez-López, C.E.; Baker, C.M.; Benavides, L.R.; Cunha, T.J.; Gainett, G.; Ontano, A.Z.; Setton, E.V.W.; Arango, C.P.; Gavish-Regev, E.; et al. Comprehensive species sampling and sophisticated algorithmic approaches refute the monophyly of Arachnida. Mol. Biol. Evol. 2022, 39, msac021. [Google Scholar] [CrossRef] [PubMed]
- Obst, M.; Faurby, S.; Bussarawit, S.; Funch, P. Molecular phylogeny of extant horseshoe crabs (Xiphosura, Limulidae) indicates Paleogene diversification of Asian species. Mol. Phylogenet. Evol. 2012, 62, 21–26. [Google Scholar] [CrossRef]
- Fisher, D.C. The Xiphosurida: Archetypes of bradytely? In Living Fossils; Eldredge, N., Stanley, S.M., Eds.; Springer: New York, NY, USA, 1984; pp. 196–213. [Google Scholar] [CrossRef]
- Lamsdell, J.C.; McKenzie, S.C. Tachypleus syriacus (Woodward)—A sexually dimorphic Cretaceous crown limulid reveals underestimated horseshoe crab divergence times. Org. Divers. Evol. 2015, 15, 681–693. [Google Scholar] [CrossRef]
- Donoghue, P.C.J.; Benton, M.J. Rocks and clocks: Calibrating the tree of life using fossils and molecules. Trends Ecol. Evol. 2007, 22, 424–431. [Google Scholar] [CrossRef]
- Smith, D.R.; Brockmann, H.J.; Carmichael, R.H.; Hallerman, E.M.; Watson, W.; Zaldivar-Rae, J.A. Assessment of recovery potential for the American horseshoe crab (Limulus polyphemus): An application of the IUCN green status process. Aquat. Conserv. Mar. Freshw. Ecosyst. 2023, 33, 1175–1199. [Google Scholar] [CrossRef]
- Smith, D.R.; Botton, M.L.; Shin, P.K.S. Recovering the American horseshoe crab through a commitment to collaboration. Fisheries 2025, vuae021. [Google Scholar] [CrossRef]
- Botton, M.L.; Tankersley, R.A.; Loveland, R.E. Developmental ecology of the American horseshoe crab Limulus polyphemus. Curr. Zool. 2010, 56, 550–562. [Google Scholar] [CrossRef]
- Hamilton, K.L.; Burnett, L.E.; Burnett, K.G.; Kalisperis, R.E.; Fowler, A.E. Physiological impacts of time in holding ponds, biomedical bleeding, and recovery on the Atlantic horseshoe crab, Limulus polyphemus. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2020, 239, 110554. [Google Scholar] [CrossRef]
- Faurby, S.; King, T.L.; Obst, M.; Hallerman, E.M.; Pertoldi, C.; Funch, P. Population dynamics of American horseshoe crabs-historic climatic events and recent anthropogenic pressures. Mol. Ecol. 2010, 19, 3088–3100. [Google Scholar] [CrossRef] [PubMed]
- Pierce, J.C.; Tan, G.; Gaffney, P.M. Delaware Bay and Chesapeake Bay populations of the horseshoe crab Limulus polyphemus are genetically distinct. Estuaries 2000, 23, 690–698. [Google Scholar] [CrossRef]
- King, T.L.; Eackles, M.S.; Spidle, A.P.; Brockmann, H.J. Regional differentiation and sex-biased dispersal among populations of the horseshoe crab Limulus polyphemus. Trans. Am. Fish. Soc. 2005, 134, 441–465. [Google Scholar] [CrossRef]
- King, T.L.; Eackles, M.S.; Aunins, A.W.; Brockmann, H.J.; Hallerman, E.; Brown, B.L. Conservation genetics of the American horseshoe crab (Limulus polyphemus): Allelic diversity, zones of genetic discontinuity, and regional differentiation. In Changing Global Perspectives on Horseshoe Crab Biology, Conservation and Management; Carmichael, R., Botton, M., Shin, P., Cheung, S., Eds.; Springer: Cham, Switzerland; Heidelberg, Germany; New York, NY, USA; Dordrecht, The Netherlands; London, UK, 2015; pp. 65–96. [Google Scholar] [CrossRef]
- Saunders, N.C.; Kessler, L.G.; Avise, J.C. Genetic variation and geographic differentiation in mitochondrial DNA of the horseshoe crab, Limulus polyphemus. Genetics 1986, 112, 613–627. [Google Scholar] [CrossRef]
- Smith, D.R.; Beekey, M.A.; Brockmann, H.J.; King, T.L.; Millard, M.J.; Zaldívar-Rae, J.A. Limulus polyphemus. The IUCN Red List of Threatened Spceies 2016: e.T11987A80159830. Available online: https://www.iucnredlist.org/species/11987/80159830 (accessed on 11 November 2023).
- García-Enríquez, J.M.; Machkour-M’rabet, S.; Rosas-Correa, C.O.; Hénaut, Y.; Carrillo, L. Genetic study of the American horseshoe crab throughout its Mexican distribution. Conservation and management implications. Biodivers. Conserv. 2023, 32, 489–507. [Google Scholar] [CrossRef]
- Noreña-Silva, K.L.; Rodríguez-Canul, R.; Améndola-Pimenta, M.; Zamora-Briseño, J.A.; Islas-Villanueva, V.; Pérez-Vega, J.A.; Zaldívar-Rae, J. Microsatellite and mitochondrial DNA based evidence reveals a single horseshoe crab (Limulus polyphemus) population on the northern Yucatan Peninsula, Mexico. Estuar. Coast. Shelf Sci. 2023, 292, 108466. [Google Scholar] [CrossRef]
- Smith, D.R.; Brockmann, H.J.; Beekey, M.A.; King, T.L.; Millard, M.J.; Zaldívar-Rae, J. Conservation status of the American horseshoe crab, (Limulus polyphemus): A regional assessment. Rev. Fish Biol. Fish. 2017, 27, 135–175. [Google Scholar] [CrossRef]
- Sandoval-Gío, J.J.; Avilés-Ramírez, G.; Ortiz-León, H.J.; Zamora-Bustillos, R.; Rosas-Correa, C.O.; Castro-Pérez, J.M. Effects of the octopus fishery on the American Horseshoe crab population in the Ría Lagartos biosphere, México. Cienc. Mar. 2020, 46, 77–88. [Google Scholar] [CrossRef]
- Ožana, S.; Pyszko, P.; Dolný, A. Determination of suitable insect part for non-lethal DNA sampling: Case study of DNA quality and regeneration capability of dragonflies. Insect Conserv. Divers. 2020, 13, 319–327. [Google Scholar] [CrossRef]
- Aljanabi, S.M.; Martinez, I. Universal and rapid salt-extraction of high quality genomic DNA for PCR- based techniques. Nucleic Acids Res. 1997, 25, 4692–4693. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- Tudu, S.; Gupta, S.K.; Dash, B.P. COI gene-based mitochondrial DNA variation of horseshoe crab (Tachypleus gigas) reveals high genetic variation and occurrence of distinct populations in the Bay of Bengal, India, and its comparison with other populations. Mar. Ecol. 2022, 43, e12701. [Google Scholar] [CrossRef]
- Lanfear, R.; Frandsen, P.B.; Wright, A.M.; Senfeld, T.; Calcott, B. PartitionFinder 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol. Biol. Evol. 2016, 34, 772–773. [Google Scholar] [CrossRef]
- Lanfear, R.; Calcott, B.; Ho, S.Y.W.; Guindon, S. PartitionFinder: Combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol. Biol. Evol. 2012, 29, 1695–1701. [Google Scholar] [CrossRef]
- Huelsenbeck, J.P.; Ronquist, F. MrBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010; pp. 1–8. [Google Scholar] [CrossRef]
- Clement, M.; Posada, D.C.K.A.; Crandall, K.A. TCS: A computer program to estimate gene genealogies. Mol. Ecol. 2000, 9, 1657–1659. [Google Scholar] [CrossRef]
- Drummond, A.J.; Suchard, M.A.; Xie, D.; Rambaut, A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 2012, 29, 1969–1973. [Google Scholar] [CrossRef] [PubMed]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Wolfe, J.M.; Daley, A.C.; Legg, D.A.; Edgecombe, G.D. Fossil calibrations for the arthropod Tree of Life. Earth-Sci. Rev. 2016, 160, 43–110. [Google Scholar] [CrossRef]
- Ksepka, D.T.; Parham, J.F.; Allman, J.F.; Benton, M.J.; Carrano, M.T.; Cranston, K.A.; Donoghue, P.C.J.; Head, J.J.; Hermsen, E.J.; Irmis, R.B.; et al. The Fossil Calibration Database—A new Resource for Divergence Dating. Syst. Biol. 2015, 64, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Cohen, K.M.; Finney, S.C.; Gibbard, P.L.; Fan, J.X. The ICS international chronostratigraphic chart. Episodes 2013, 36, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A.; Suchard, M.A.; Xie, W.; Drummond, A.J. Tracer vol 1.6.0: MCMC Trace Analysis Tool. 2013. Available online: http://beast.bio.ed.ac.uk/Tracer (accessed on 18 October 2023).
- Rambaut, A.; Drummond, A.J. Tree Annotator v1.8.2. 2014. Available online: http://beast.bio.ed.ac.uk/TreeAnnotator (accessed on 18 October 2023).
- Rambaut, A. FigTree v1.4.2. 2014. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 18 October 2023).
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef]
- Tajima, F. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 1989, 123, 585–595. [Google Scholar] [CrossRef]
- Fu, Y.X. Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics 1997, 147, 915–925. [Google Scholar] [CrossRef]
- Harpending, H.C. Signature of ancient population growth in a low-resolution mitochondrial DNA mistmatch distribution. Hum. Biol. 1994, 66, 591–600. [Google Scholar]
- Loveland, R.E.; Botton, M.L. Sea level rise in Delaware Bay, USA: Adaptations of spawning horseshoe crabs (Limulus polyphemus) to the glacial past, and the rapidly changing shoreline of the bay. In Changing Global Perspectives on Horseshoe Crab Biology, Conservation and Management; Carmichael, R., Botton, M., Shin, P., Cheung, S., Eds.; Springer: Cham, Switzerland; Heidelberg, Germany; New York, NY, USA; Dordrecht, The Netherlands; London, UK, 2015; pp. 41–63. [Google Scholar] [CrossRef]
- Rahmstorf, S.; Feulner, G. Paleoclimatic ocean circulation and sea-level changes. In Ocean Circulation Patterns; Siedler, G., Griffies, S.M., Gould, J., Church, J.A., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 31–56. [Google Scholar] [CrossRef]
- Kjær, K.H.; Winther Pedersen, M.; De Sanctis, B.; De Cahsan, B.; Korneliussen, T.S.; Michelsen, C.S.; Sand, K.K.; Jelavić, S.; Ruter, A.H.; Schmidt, A.M.A.; et al. A 2-million-year-old ecosystem in Greenland uncovered by environmental DNA. Nature 2022, 612, 283–291. [Google Scholar] [CrossRef]
- Avise, J.C.; Nelson, W.S.; Sugita, H. A speciational history of “living fossils”: Molecular evolutionary patterns in horseshoe crabs. Evolution 1994, 48, 1986–2001. [Google Scholar] [CrossRef]
- Shingate, P.; Ravi, V.; Prasad, A.; Tai, B.; Venkatesh, B. Chromosome-level genome assembly of the coastal horseshoe crab (Tachypleus gigas). Mol. Ecol. Resour. 2020, 20, 1748–1760. [Google Scholar] [CrossRef]
- Zhou, Y.; Liang, Y.; Yan, Q.; Zhang, L.; Chen, D.; Ruan, L.; Kong, Y.; Shi, H.; Chen, M.; Chen, J. The draft genome of horseshoe crab Tachypleus tridentatus reveals its evolutionary scenario and well-developed innate immunity. BMC Genom. 2020, 21, 137. [Google Scholar] [CrossRef]
- Lucas, S.G.; Orchard, M.J. Triassic. In Reference Module in Earth System and Environmental Sciences; Elsevier: Berlin, Germany, 2013. [Google Scholar] [CrossRef]
- Stubblefield, A.G.; Hatcher, R.D., Jr.; Horton, J.W., Jr.; Daniels, D.L. Unzipping supercontinent Pangea: Geologic, potential field data, and buried structures, and a case for sequential Atlantic opening. Tectonophysics 2023, 852, 229842. [Google Scholar] [CrossRef]
- Khosla, A.S.H.U.; Lucas, S.G. Cretaceous period: Biotic diversity and biogeography—An introduction. In Cretaceous Period: Biotic Diversity and Biogeography; New Mexico Museum of Natural History and Science: Albuquerque, NM, USA, 2016; Volume 71, pp. 1–4. [Google Scholar]
- Hou, Z.; Li, S. Tethyan changes shaped aquatic diversification. Biol. Rev. 2018, 93, 874–896. [Google Scholar] [CrossRef] [PubMed]
- Limaye, R.; Kumaran, K. Biogeographical and paleoclimate appraisal of mangrove vegetation in South Asia and Southeast Asia in the post Himalayan uplift scenario. Chin. Sci. Bull. 2013, 58, 126–133. [Google Scholar] [CrossRef]
- Walls, E.A.; Berkson, J.; Smith, S.A. The horseshoe crab, Limulus polyphemus: 200 million years of existence, 100 years of study. Rev. Fish. Sci. 2002, 10, 39–73. [Google Scholar] [CrossRef]
- Niles, L.J.; Dey, A.D.; Maslo, B. Overexploitation of marine species and its consequences for terrestrial biodiversity along coasts. Coastal conservation. In Coastal Conservation; Maslo, B., Lockwood, J.L., Eds.; Cambridge University Press: Cambridge, UK, 2014; pp. 347–368. [Google Scholar] [CrossRef]
- Kraft, J.C. Geology. In The Delaware Estuary: Rediscovering a Forgotten Resource; Bryant, T.L., Pennock, J.R., Eds.; University of Delaware Sea Grant College Program: Newark, DE, USA, 1988; pp. 31–41. [Google Scholar]
- Blakeslee, A.M.H.; Haram, L.E.; Altman, I.; Kennedy, K.; Ruiz, G.M.; Miller, A.W. Founder effects and species introductions: A host versus parasite perspective. Evol. Appl. 2020, 13, 559–574. [Google Scholar] [CrossRef]
- Mondin, L.A.D.C.; Machado, C.B.; Resende, E.K.D.; Marques, D.K.; Galetti, P.M., Jr. Genetic pattern and demographic history of Salminus brasiliensis: Population expansion in the Pantanal Region during the Pleistocene. Front. Genet. 2018, 9, 1. [Google Scholar] [CrossRef]
- Yang, M.C.; Hsieh, H.L.; Huang, H.; Chen, C.A. Phylogeography, demographic history, and reserves network of horseshoe crab, Tachypleus tridentatus, in the South and East China seaboards. In Biology and Conservation of Horseshoe Crabs; Tanacredi, J., Botton, M., Smith, D., Eds.; Springer: Boston, MA, USA, 2009; pp. 163–181. [Google Scholar] [CrossRef]
- Aini, N.K.; Wardiatno, Y.; Effendi, H.; Mashar, A.; Madduppa, H. High genetic diversity and mixing of coastal horseshoe crabs (Tachypleus gigas) across major habitats in Sundaland, Indonesia. PeerJ 2021, 9, e11739. [Google Scholar] [CrossRef]
- Slatkin, M. Isolation by distance in equilibrium and non-equilibrium populations. Evolution 1993, 47, 264–279. [Google Scholar] [CrossRef]
- Pinsky, M.L.; Palumbi, S.R. Meta-analysis reveals lower genetic diversity in overfished populations. Mol. Ecol. 2014, 23, 29–39. [Google Scholar] [CrossRef]
- Frévol, S.A.; MacNulty, D.R.; Anderson, M.; Carmichael, L.E.; Cluff, H.D.; Mech, L.D.; Musiani, M. Geographic isolation reduces genetic diversity of a wide-ranging terrestrial vertebrate, Canis lupus. Ecosphere 2023, 14, e4536. [Google Scholar] [CrossRef]
- De, A.K.; Muthiyan, R.; Ponraj, P.; Muniswamy, K.; Sunder, J.; Kundu, A.; Karunakaran, D.; George, Z.; Kundu, M.S.; Ahmed, S.K.Z.; et al. Mitogenome analysis of Indian isolate of Rhipicephalus microplus clade A sensu (Burger et al., 2014): A first report from Maritime South-East Asia. Mitochondrion 2019, 49, 135–148. [Google Scholar] [CrossRef] [PubMed]


| Locality | Code | N | N Hap. | S | h | π | Fs | D |
|---|---|---|---|---|---|---|---|---|
| Short Fragment 1 (SF1) | ||||||||
| Laguna de Términos | LT | 31 | 2 | 2 | 0.280 (SD 0.090) | 0.0009 | 0.741 | 0.237 |
| Champotón | CH | 28 | 3 | 2 | 0.140 (SD 0.087) | 0.0002 | −2.352 | −1.510 |
| Ría Celestún | RCL | 25 | 4 | 3 | 0.230 (SD 0.110) | 0.0004 | −2.352 | −1.511 |
| Chuburná | CHU | 20 | 2 | 1 | 0.100 (SD 0.088) | 0.0002 | −1.648 | −1.164 |
| Chelem | CHE | 7 | 2 | 1 | 0.286 (SD 0.196) | 0.0004 | −1.101 | −1.006 |
| Ría Lagartos | RL | 6 | 1 | 0 | NA | NA | NA | NA |
| Yum Balam | YB | 24 | 5 | 4 | 0.377 (SD 0.122) | 0.0006 | −2.067 | −1.689 |
| Sian Ka’an | SK | 13 | 1 | 0 | NA | NA | NA | NA |
| All MX | 154 | 10 | 10 | 0.208 (SD 0.044) | 0.0004 | −3.285 * | −2.029 * | |
| Florida | FL | 2 | 2 | 3 | 1.000 (SD 0.500) | 0.0046 | NA | NA |
| Maryland | ML | 2 | 2 | 2 | 1.000 (SD 0.500) | 0.0031 | NA | NA |
| Connecticut | CN | 1 | 1 | 0 | NA | NA | NA | NA |
| All US | 5 | 5 | 15 | 1.000 (SD 0.126) | 0.0127 | 1.082 | 1.015 | |
| Short Fragment 2 (SF2) | ||||||||
| Laguna de Términos | LT | 31 | 3 | 2 | 0.127 (SD 0.080) | 0.0003 | −2.396 | −1.505 |
| Champotón | CH | 28 | 2 | 1 | 0.071 (SD 0.065) | 0.0001 | −1.747 | −1.151 |
| Ría Celestún | RCL | 25 | 1 | 0 | NA | NA | NA | NA |
| Chuburná | CHU | 20 | 2 | 1 | 0.100 (SD 0.088) | 0.0002 | −1.647 | −1.164 |
| Chelem | CHE | 7 | 2 | 1 | 0.286 (SD 0.196) | 0.0006 | −1.101 | −1.006 |
| Ría Lagartos | RL | 6 | 1 | 0 | NA | NA | NA | NA |
| Yum Balam | YB | 24 | 2 | 1 | 0.344 (SD 0.099) | 0.0007 | 0.623 | 0.480 |
| Sian Ka’an | SK | 13 | 1 | 0 | NA | NA | NA | NA |
| All MX | 154 | 5 | 4 | 0.124 (SD 0.036) | 0.0003 | −2.875* | −1.521 | |
| Chesapeake bay | CHB | 14 | 6 | 6 | 0.846 (SD 0.061) | 0.004 | 0.658 | 0.362 |
| Delaware bay | DWB | 41 | 3 | 3 | 0.262 (SD 0.083) | 0.001 | −0.519 | −0.659 |
| All US | 55 | 7 | 7 | 0.486 (SD 0.079) | 0.0023 | −0.540 | −0.688 | |
| Long Fragment (LF) | ||||||||
| Laguna de Términos | LT | 31 | 5 | 5 | 0.652 (SD 0.063) | 0.001 | −0.548 | −0.111 |
| Champotón | CH | 28 | 4 | 3 | 0.206 (SD 0.100) | 0.0001 | −2.754 * | −1.733 |
| Ría Celestún | RCL | 25 | 5 | 4 | 0.420 (SD 0.117) | 0.0004 | −2.039 | −1.529 |
| Chuburná | CHU | 20 | 2 | 2 | 0.100 (SD 0.088) | 0.0002 | −2.188 | −1.513 |
| Chelem | CHE | 7 | 2 | 2 | 0.286 (SD 0.196) | 0.0005 | −1.374 | −1.237 |
| Ría Lagartos | RL | 6 | 1 | 0 | NA | NA | NA | NA |
| Yum Balam | YB | 24 | 7 | 5 | 0.554 (SD 0.116) | 0.0006 | −1.526 | −1.267 |
| Sian Ka’an | SK | 13 | 1 | 0 | NA | NA | NA | NA |
| All MX | 154 | 16 | 15 | 0.447 (SD 0.048) | 0.0005 | −3.664 * | −1.999 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Enríquez, J.M.; Machkour-M’Rabet, S.; Hénaut, Y.; Calmé, S.; Lesher-Gordillo, J.M. New Insight into the Demography History, Evolution, and Phylogeography of Horseshoe Crabs with Special Emphasis on American Species. Diversity 2025, 17, 269. https://doi.org/10.3390/d17040269
García-Enríquez JM, Machkour-M’Rabet S, Hénaut Y, Calmé S, Lesher-Gordillo JM. New Insight into the Demography History, Evolution, and Phylogeography of Horseshoe Crabs with Special Emphasis on American Species. Diversity. 2025; 17(4):269. https://doi.org/10.3390/d17040269
Chicago/Turabian StyleGarcía-Enríquez, José Manuel, Salima Machkour-M’Rabet, Yann Hénaut, Sophie Calmé, and Julia Maria Lesher-Gordillo. 2025. "New Insight into the Demography History, Evolution, and Phylogeography of Horseshoe Crabs with Special Emphasis on American Species" Diversity 17, no. 4: 269. https://doi.org/10.3390/d17040269
APA StyleGarcía-Enríquez, J. M., Machkour-M’Rabet, S., Hénaut, Y., Calmé, S., & Lesher-Gordillo, J. M. (2025). New Insight into the Demography History, Evolution, and Phylogeography of Horseshoe Crabs with Special Emphasis on American Species. Diversity, 17(4), 269. https://doi.org/10.3390/d17040269









