Variations in Impacts of Climate Change on Giant Lobelia Species in East Africa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Model Species
2.2. Distribution and Environmental Data
2.3. Environmental Variables
2.4. Species Distribution Modeling and Evaluation
2.5. Conservation Status in the Changing Climatic Scenarios
3. Results
3.1. The Distribution Pattern of Giant Lobelia
3.2. Model Performance of Giant Lobelia
3.3. Contribution of Bioclimatic Variables
3.4. The Current Suitable Habitat
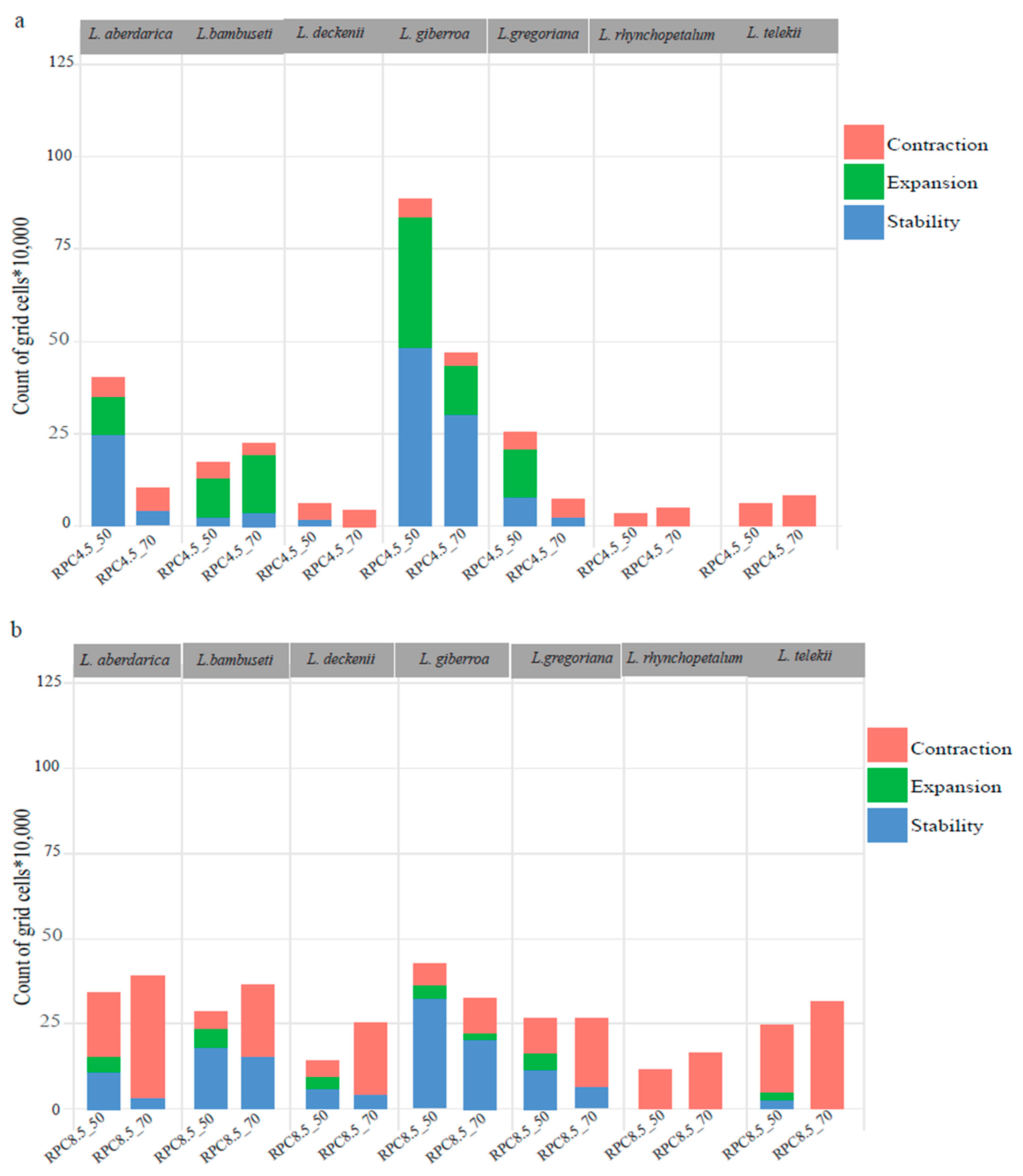
3.5. The Dynamics of Future Distribution
4. Discussion
4.1. Models Performance
4.2. Giant Lobelia Distribution Change and Environmental Variables
4.3. Extinction Risks of Giant Lobelia Species
4.4. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lloyd, A.H.; Bunn, A.G. Responses of the circumpolar boreal forest to 20th century climate variability. Environ. Res. Lett. 2007, 2, 045013. [Google Scholar] [CrossRef]
- Sinervo, B.; Méndez-De-La-Cruz, F.; Miles, D.B.; Heulin, B.; Bastiaans, E.; Villagrán-Santa Cruz, M.; Lara-Resendiz, R.; Martínez-Méndez, N.; Calderón-Espinosa, M.L.; Meza-Lázaro, R.N.; et al. Erosion of lizard diversity by climate change and altered thermal niches. Science 2010, 328, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.C.; Hill, J.K.; Ohlemüller, R.; Roy, D.B.; Thomas, C.D. Rapid range shifts of species associated with high levels of climate warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species distribution models: Ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Abdelaal, M.; Fois, M.; Fenu, G.; Bacchetta, G. Using MaxEnt modeling to predict the potential distribution of the endemic plant Rosa arabica Crép. in Egypt. Ecol. Inform. 2019, 50, 68–75. [Google Scholar] [CrossRef]
- Li, R.; Xu, M.; Wong, M.H.G.; Qiu, S.; Sheng, Q.; Li, X.; Song, Z. Climate change-induced decline in bamboo habitats and species diversity: Implications for giant panda conservation. Divers. Distrib. 2015, 21, 379–391. [Google Scholar] [CrossRef]
- Booth, T.H.; Nix, H.A.; Busby, J.R.; Hutchinson, M.F. BIOCLIM: The first species distribution modelling package, its early applications and relevance to most current MAXENT studies. Divers. Distrib. 2014, 20, 1–9. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W.; Zimmermann, N.E. Habitat Suitability and Distribution Models: With Applications in R; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar]
- Grabherr, G.; Gottfried, M.; Pauli, H. Climate change impacts in alpine environments. Geogr. Compass 2010, 4, 1133–1153. [Google Scholar] [CrossRef]
- Hussein, M.A. Climate Change Impacts on East Africa. Econ. Soc. Political Elem. Clim. Change 2010, 1, 589–601. [Google Scholar]
- Fort, M. Impact of Climate Change on Mountain Environment Dynamics. An Introduction. J. Alp. Res.|Rev. Géographie Alp. 2015, 103-2. [Google Scholar] [CrossRef]
- Testolin, R.; Attorre, F.; Borchardt, P.; Brand, R.F.; Bruelheide, H.; Chytrý, M.; De Sanctis, M.; Dolezal, J.; Finckh, M.; Haider, S.; et al. Global Patterns and Drivers of Alpine Plant Species Richness. Glob. Ecol. Biogeogr. 2021, 30, 1218–1231. [Google Scholar] [CrossRef]
- Thapliyal, N.; Bhojak, P.; Sekar, K.C.; Bisht, K.; Mehta, P.; Arya, D.; Joshi, S. Potential Drivers of Plant Diversity and Composition in High-Altitude Alpine Regions of Himalaya. Community Ecol. 2024, 26, 1–19. [Google Scholar] [CrossRef]
- Ssali, F.; Mugerwa, B.; Van Heist, M.; Sheil, D.; Kirunda, B.; Musicante, M.; Seimon, A.; Halloy, S. Plant Diversity and Composition Vary with Elevation on Two Equatorial High Mountains in Uganda: Baselines for Assessing the Influence of Climate Change. Alp. Bot. 2023, 133, 49–61. [Google Scholar] [CrossRef]
- Foden, W.B.; Young, B.E.; Akçakaya, H.R.; Garcia, R.A.; Hoffmann, A.A.; Stein, B.A.; Thomas, C.D.; Wheatley, C.J.; Bickford, D.; Carr, J.A. Climate change vulnerability assessment of species. Wiley Interdiscip. Rev. Clim. Change 2019, 10, e551. [Google Scholar] [CrossRef]
- Lenoir, J.; Svenning, J.C. Climate-related range shifts—A global multidimensional synthesis and new research directions. Ecography 2015, 38, 15–28. [Google Scholar] [CrossRef]
- Salariato, D.L.; Delfini, C.F.; Zuloaga, F.O. Climate change impact assessments on the Andean genus Menonvillea (Brassicaceae) reveal uneven vulnerability among major phylogenetic and biogeographic groups. Flora 2024, 315, 152511. [Google Scholar] [CrossRef]
- Antonelli, A. Have giant lobelias evolved several times independently? Life form shifts and historical biogeography of the cosmopolitan and highly diverse subfamily Lobelioideae (Campanulaceae). BMC Biol. 2009, 7, 1–21. [Google Scholar] [CrossRef]
- Zhao, S.-Y.; Chen, L.-Y.; Muchuku, J.K.; Hu, G.-W.; Wang, Q.-F. Genetic adaptation of giant lobelias (Lobelia aberdarica and Lobelia telekii) to different altitudes in East African Mountains. Front. Plant Sci. 2016, 7, 488. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, B.; Linder, H.P. Species Richness, Endemism and Species Composition in the Tropical Afroalpine Flora. Alp. Bot. 2014, 124, 165–177. [Google Scholar] [CrossRef]
- Knox, E.B.; Jeffrey, D.P. Chloroplast DNA Evidence on the Origin and Radiation of the Giant Lobelias in Eastern Africa. Syst. Bot. 1998, 32, 109–149. [Google Scholar] [CrossRef]
- Coe, M. Biogeographic Affinities of the High Mountains of Tropical Africa. In Quaternary and Environmental Research on East African Mountains; CRC Press: Boca Raton, FL, USA, 2021; pp. 257–278. [Google Scholar]
- Manes, S.; Costello, M.J.; Beckett, H.; Debnath, A.; Devenish-Nelson, E.; Grey, K.-A.; Jenkins, R.; Khan, T.M.; Kiessling, W.; Krause, C.; et al. Endemism Increases Species’ Climate Change Risk in Areas of Global Biodiversity Importance. Biol. Conserv. 2021, 257, 109070. [Google Scholar] [CrossRef]
- Vale, M.M.; Arias, P.A.; Ortega, G.; Cardoso, M.; Oliveira, B.F.; Loyola, R.; Scarano, F.R. Climate Change and Biodiversity in the Atlantic Forest: Best Climatic Models, Predicted Changes and Impacts, and Adaptation Options. In The Atlantic Forest: History, Biodiversity, Threats and Opportunities of the Mega-Diverse Forest; Springer: Cham, Switzerland, 2021; pp. 253–267. [Google Scholar]
- Nzei, J.M.; Ngarega, B.K.; Mwanzia, V.M.; Musili, P.M.; Wang, Q.F.; Chen, J.M. The Past, Current, and Future Distribution Modeling of Four Water Lilies (Nymphaea) in Africa Indicates Varying Suitable Habitats and Distribution in Climate Change. Aquat. Bot. 2021, 173, 103416. [Google Scholar] [CrossRef]
- Mkala, E.M.; Mutinda, E.S.; Wanga, V.O.; Oulo, M.A.; Oluoch, W.A.; Waswa, E.N.; Odago, W.; Nanjala, C.; Mwachala, G.; Hu, G.W.; et al. Modeling Impacts of Climate Change on the Potential Distribution of Three Endemic Aloe Species Critically Endangered in East Africa. Ecol. Inform. 2022, 71, 101765. [Google Scholar] [CrossRef]
- Mkala, E.M.; Jost, M.; Wanke, S.; Ngarega, B.K.; Hughes, A.; Mutinda, E.S.; Waswa, E.N.; Mwanzia, V.M.; Oulo, M.A.; Wanga, V.O.; et al. How Vulnerable Are Holoparasitic Plants with Obligate Hosts to Negative Climate Change Impacts? Ecol. Inform. 2022, 69, 101636. [Google Scholar] [CrossRef]
- Gómez-Undiano, I.; Francis, M.; Wilfred, L.M.; Grace, M.D.; Roger, D.; Regan, E.; Kenneth, W. Predicting Potential Global and Future Distributions of the African Armyworm (Spodoptera Exempta) Using Species Distribution Models. Sci. Rep. 2022, 12, 16234. [Google Scholar]
- Muchuku, J.K.; Gichira, A.W.; Zhao, S.-Y.; Chen, J.-M.; Chen, L.-Y.; Wang, Q.-F. Distribution pattern and habitat preference for Lobelia species (Campanulaceae) in five countries of East Africa. PhytoKeys 2020, 159, 45. [Google Scholar] [CrossRef]
- Perkins, T.D. Haeitat and Population Ecology of Lobelia kalmii on Presoue Isle, Pa Correlated with Changes in Lake Erie Water Levels. J. Pennsyljzania Acad. Sci. 1992, 4, 123. [Google Scholar]
- Beck, E. Ecophysiology of Niche Occupation by Two Giant Rosette Plants, Lobelia gibberoa Hemsl and Solanecio gigas (Vatke) C. Jeffrey, in an Afromontane Forest Valley. Ann. Bot. 2001, 88, 267–278. [Google Scholar]
- Buytaert, W.; Cuesta-Camacho, F.; Tobón, C. Potential impacts of climate change on the environmental services of humid tropical alpine regions. Glob. Ecol. Biogeogr. 2011, 20, 19–33. [Google Scholar] [CrossRef]
- Nagy, L.; Grabherr, G. The Biology of Alpine Habitats; Oxford University Press: Cary, NC, USA, 2009. [Google Scholar]
- Barbet, M.M.; Jiguet, F.; Albert, C.H. Thuiller W Selecting pseudo absences for species distribution models: How, where and how many? Methods Ecol. Evol. 2012, 3, 327–338. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Zomer, R.J.; Xu, J.; Trabucco, A. Version 3 of the global aridity index and potential evapotranspiration database. Sci. Data 2022, 9, 409. [Google Scholar] [CrossRef]
- Mehra, N.; Swain, J.B. Assessment of land use land cover change and its effects using artificial neural network-based cellular automation. J. Eng. Appl. Sci. 2024, 71, 70. [Google Scholar] [CrossRef]
- Naimi, B. usdm: Uncertainty Analysis for Species Distribution Models. –R Package Ver. 1.1-15. R Package; CRAN: Vienna, Austria, 2015. [Google Scholar]
- Hijmans, R.; Van Etten, J.; Cheng, J.; Mattiuzzi, M.; Sumner, M.; Greenberg, J.; Lamigueiro, O.; Bevan, A.; Racine, E.; Shortridge, A. Package ‘Raster’. R Package; CRAN: Vienna, Austria, 2015; 734, 473. [Google Scholar] [CrossRef]
- Yamazaki, D.; Ikeshima, D.; Tawatari, R.; Yamaguchi, T.; O’Loughlin, F.; Neal, J.C.; Sampson, C.C.; Kanae, S.; Bates, P.D. A high-accuracy map of global terrain elevations. Geophys. Res. Lett. 2017, 44, 5844–5853. [Google Scholar] [CrossRef]
- The IUCN Red List of Threatened Species. Version 2025-1. Available online: https://www.iucnredlist.org (accessed on 2 February 2025).
- Ackerley, D.; Dietmar, D. Atmosphere-Only Gcm (Access1. 0) Simulations with Prescribed Land Surface Temperatures. Geosci. Model Dev. 2016, 9, 2077–2098. [Google Scholar] [CrossRef]
- Naimi, B.; Miguel, B.A. Sdm: A Reproducible and Extensible R Platform for Species Distribution Modelling. Ecography 2016, 39, 368–375. [Google Scholar] [CrossRef]
- Thuiller, W.; Guéguen, M.; Renaud, J.; Karger, D.N.; Zimmermann, N.E. Uncertainty in ensembles of global biodiversity scenarios. Nat. Commun. 2019, 10, 1446. [Google Scholar] [CrossRef]
- Peterson, A.T.; Soberón, J.; Pearson, R.G.; Anderson, R.P.; Martínez-Meyer, E.; Nakamura, M.; Araújo, M.B. Ecological Niches and Geographic Distributions. In Ecological Niches and Geographic Distributions; Princeton University Press: Princeton, NJ, USA, 2011. [Google Scholar]
- Beaumont, L.J.; Graham, E.; Duursma, D.E.; Wilson, P.D.; Cabrelli, A.; Baumgartner, J.B.; Hallgren, W.; Esperón-Rodríguez, M.; Nipperess, D.A.; Warren, D.L.; et al. Which Species Distribution Models Are More (or Less) Likely to Project Broad-Scale, Climate-Induced Shifts in Species Ranges? Ecol. Model. 2016, 342, 135–146. [Google Scholar] [CrossRef]
- Lei, Y.; Yiming, Y. Stochastic Proximal Auc Maximization. J. Mach. Learn. Res. 2021, 22, 1–45. [Google Scholar]
- Smeraldo, S.; Bosso, L.; Salinas-Ramos, V.B.; Ancillotto, L.; Sánchez-Cordero, V.; Gazaryan, S.; Russo, D. Generalists yet Different: Distributional Responses to Climate Change May Vary in Opportunistic Bat Species Sharing Similar Ecological Traits. Mammal Rev. 2021, 51, 571–584. [Google Scholar] [CrossRef]
- Hughes, T.P.; James, T.K.; Álvarez-Noriega, M.; Álvarez-Romero, G.J.; Kristen, D.A.; Andrew, H.B.; Russell, C.B.; Maria, B.; David, R.B.; Ray, B. Global Warming and Recurrent Mass Bleaching of Corals. Nature 2017, 543, 373–377. [Google Scholar] [CrossRef]
- Kaky, E.; Gilbert, F. Assessment of the extinction risks of medicinal plants in Egypt under climate change by integrating species distribution models and IUCN Red List criteria. J. Arid Environ. 2019, 170, 103988. [Google Scholar]
- Mahmoodi, S.; Heydari, M.; Ahmadi, K.; Khwarahm, N.R.; Karami, O.; Almasieh, K.; Naderi, B.; Bernard, P.; Mosavi, A. The Current and Future Potential Geographical Distribution of Nepeta Crispa Willd., an Endemic, Rare and Threatened Aromatic Plant of Iran: Implications for Ecological Conservation and Restoration. Ecol. Indic. 2022, 137, 108752. [Google Scholar] [CrossRef]
- Qazi, A.W.; Saqib, Z.; Zaman-ul-Haq, M. Trends in Species Distribution Modelling in Context of Rare and Endemic Plants: A Systematic Review. Ecol. Process. 2022, 11, 1–11. [Google Scholar] [CrossRef]
- Guillera-Arroita, G.; Lahoz-Monfort, J.J.; Elith, J.; Gordon, A.; Kujala, H.; Lentini, P.E.; McCarthy, M.A.; Tingley, R.; Wintle, B.A. Is My Species Distribution Model Fit for Purpose? Matching Data and Models to Applications. Glob. Ecol. Biogeogr. 2015, 24, 276–292. [Google Scholar] [CrossRef]
- Wiens, J.A.; Stralberg, D.; Jongsomjit, D.; Howell, C.A.; Snyder, M.A. Niches, Models, and Climate Change: Assessing the Assumptions and Uncertainties. Proc. Natl. Acad. Sci. USA 2009, 106, 19729–19736. [Google Scholar] [CrossRef]
- Wu, Y.M.; Shen, X.L.; Tong, L.; Lei, F.W.; Mu, X.Y.; Zhang, Z.X. Impact of Past and Future Climate Change on the Potential Distribution of an Endangered Montane Shrub Lonicera Oblata and Its Conservation Implications. Forests 2021, 12, 125. [Google Scholar] [CrossRef]
- Varol, T.; Ugur, C.; Mehmet, C.; Halil, B.O.; Hakan, S. Impacts of Climate Change Scenarios on European Ash Tree (Fraxinus excelsior L.) in Turkey. For. Ecol. Manag. 2021, 491, 119199. [Google Scholar] [CrossRef]
- Hedberg, O. Features of afroalpine plant ecology. In Sv. Växtgeografiska Sällsk; Almqvist & Wiksells: Stockholm, Sweden, 1964. [Google Scholar]
- Smith, A.P.; Young, T.P. Tropical alpine plant ecology. Annu. Rev. Ecol. Syst. 1987, 18, 137–158. [Google Scholar] [CrossRef]
- Fetene, M.; Gashaw, M.; Nauke, P.; Beck, E. Microclimate and ecophysiological significance of the tree-like life-form of Lobelia rhynchopetalum in a tropical alpine environment. Oecologia 1998, 113, 332–340. [Google Scholar] [CrossRef]
- Jump, A.S.; Mátyás, C.; Peñuelas, J. The altitude-for-latitude disparity in the range retractions of woody species. Trends Ecol. Evol. 2009, 24, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Agudelo-Hz, W.J.; Urbina-Cardona, N.; Armenteras-Pascual, D. Critical shifts on spatial traits and the risk of extinction of Andean anurans: An assessment of the combined effects of climate and land-use change in Colombia. Perspect. Ecol. Conserv. 2019, 17, 206–219. [Google Scholar] [CrossRef]
- Díaz, S.M.; Settele, J.; Brondízio, E.; Ngo, H.; Guèze, M.; Agard, J.; Arneth, A.; Balvanera, P.; Brauman, K.; Butchart, S. The Global Assessment Report on Biodiversity and Ecosystem Services: Summary for Policy Makers; CONICET: Buenos Aires, Argentina, 2019.
- Kidane, Y.O.; Steinbauer, M.J.; Beierkuhnlein, C. Dead end for endemic plant species? A biodiversity hotspot under pressure. Glob. Ecol. Conserv. 2019, 19, e00670. [Google Scholar] [CrossRef]
- Årevall, J.; Early, R.; Estrada, A.; Wennergren, U.; Eklöf, A.C. Conditions for successful range shifts under climate change: The role of species dispersal and landscape configuration. Divers. Distrib. 2018, 24, 1598–1611. [Google Scholar] [CrossRef]


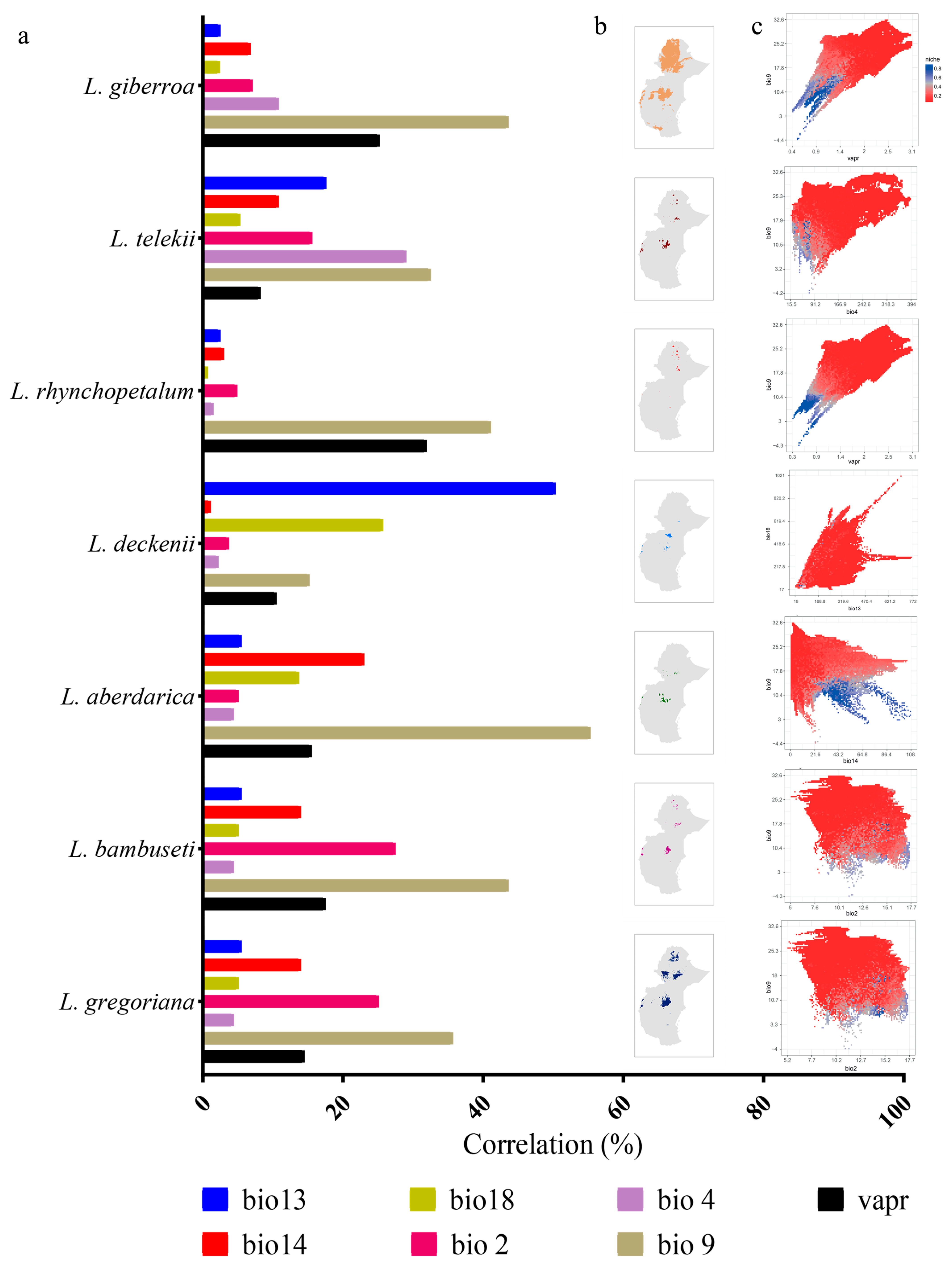
| Code | Description | VIF |
|---|---|---|
| bio13 | Precipitation of Wettest Month (mm) | 3.90 |
| bio14 | Precipitation of Driest Month (mm) | 2.88 |
| bio15 | Precipitation Seasonality (Coefficient of Variation) (mm) | 2.45 |
| bio18 | Precipitation of Warmest Quarter (mm) | 2.52 |
| bio19 | Precipitation of Coldest Quarter (mm) | 3.63 |
| bio2 | Mean Diurnal Range (°C) | 3.26 |
| bio3 | Isothermality | 7.39 |
| bio4 | Temperature Seasonality (°C) | 5.77 |
| bio9 | Mean temperature of the driest quarter (°C) | 6.30 |
| lc | Land cover | 1.35 |
| srad | Solar radiation | 2.58 |
| vapr | Water vapor | 8.80 |
| Method | AUC | COR | TSS | Deviance |
|---|---|---|---|---|
| glm | 0.98 | 0.9 | 0.95 | 0.15 |
| brt | 0.99 | 0.89 | 0.96 | 0.15 |
| maxlike | 0.92 | 0.47 | 0.86 | 2.59 |
| rf | 1 | 0.94 | 0.98 | 0.05 |
| rpart | 0.97 | 0.9 | 0.93 | 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salah, R.; Ezzat, M.; Mkala, E.M.; Dakhil, M.A.; Wan, T.; Wan, J.-N. Variations in Impacts of Climate Change on Giant Lobelia Species in East Africa. Diversity 2025, 17, 274. https://doi.org/10.3390/d17040274
Salah R, Ezzat M, Mkala EM, Dakhil MA, Wan T, Wan J-N. Variations in Impacts of Climate Change on Giant Lobelia Species in East Africa. Diversity. 2025; 17(4):274. https://doi.org/10.3390/d17040274
Chicago/Turabian StyleSalah, Radwa, Mohamed Ezzat, Elijah Mbandi Mkala, Mohammed A. Dakhil, Tao Wan, and Jun-Nan Wan. 2025. "Variations in Impacts of Climate Change on Giant Lobelia Species in East Africa" Diversity 17, no. 4: 274. https://doi.org/10.3390/d17040274
APA StyleSalah, R., Ezzat, M., Mkala, E. M., Dakhil, M. A., Wan, T., & Wan, J.-N. (2025). Variations in Impacts of Climate Change on Giant Lobelia Species in East Africa. Diversity, 17(4), 274. https://doi.org/10.3390/d17040274







