Aerobic Composting of Auricularia auricula (L.) Residues: Investigating Nutrient Dynamics and Microbial Interactions with Different Substrate Compositions
Abstract
:1. Introduction
- (1)
- Supplementation of A. auricula residues with weed biomass would optimize the initial C:N ratio and porosity, thereby accelerating both organic matter mineralization and humification rates.
- (2)
- Co-composting would foster synergistic interactions between lignin-degrading fungi (e.g., Basidiomycota) and cellulose-degrading bacteria (e.g., Pseudomonas), enhancing functional diversity and metabolic efficiency.
- (3)
- This study uniquely emphasizes microbial diversity as a key factor in composting performance, hypothesizing that herbaceous materials may significantly stimulate microbial activity by augmenting carbon availability and structural heterogeneity, thereby advancing current knowledge of microbial succession in lignocellulosic waste composting.
2. Materials and Methods
2.1. Composting Feedstock and Experimental Design
2.2. Measurement of Experimental Parameters During Composting
2.2.1. Basic Physiochemical Properties
2.2.2. Enzyme Activities
2.2.3. Humic Substance Characterization
2.2.4. High-Throughput Sequencing
2.3. Data Processing and Statistical Analysis
3. Results and Discussion
3.1. Compost Safety Index
3.2. Basic Physical and Chemical Indexes in Composting Process
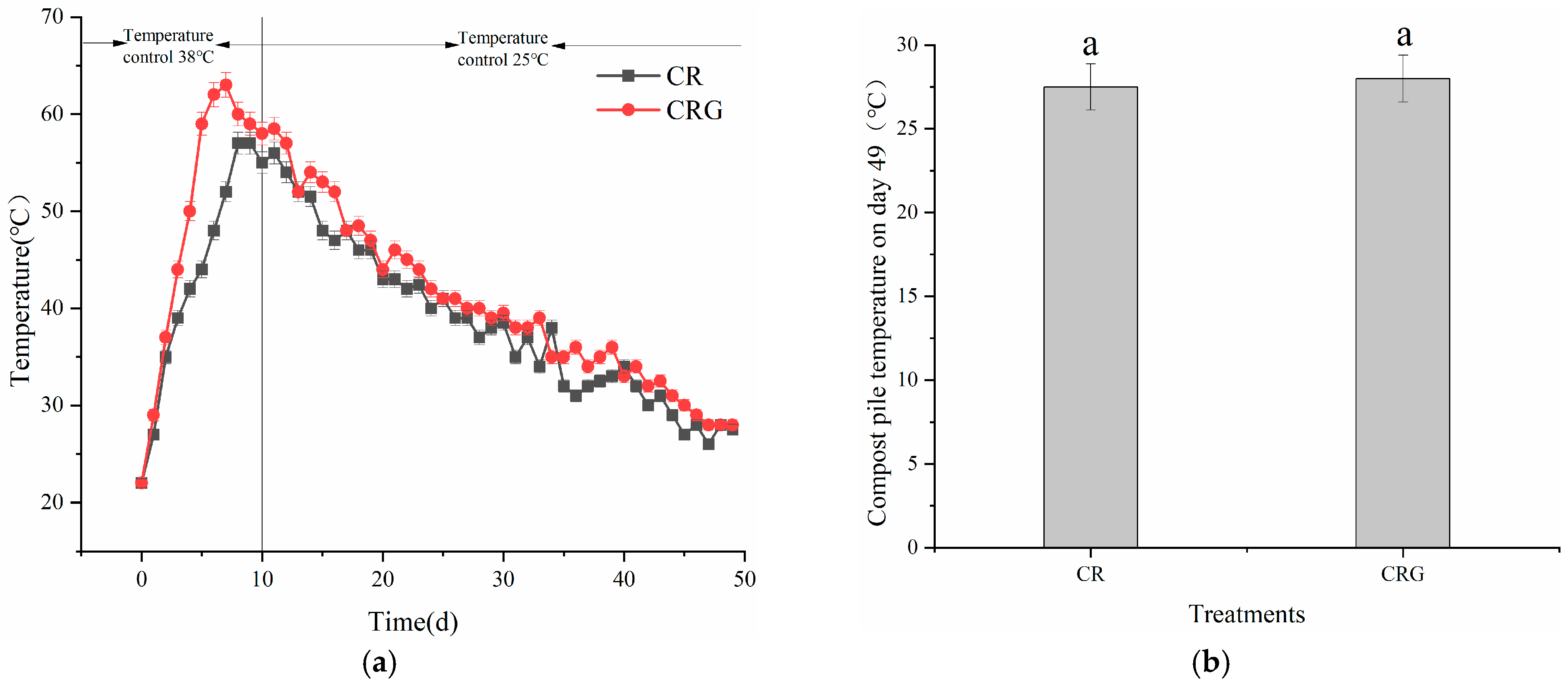
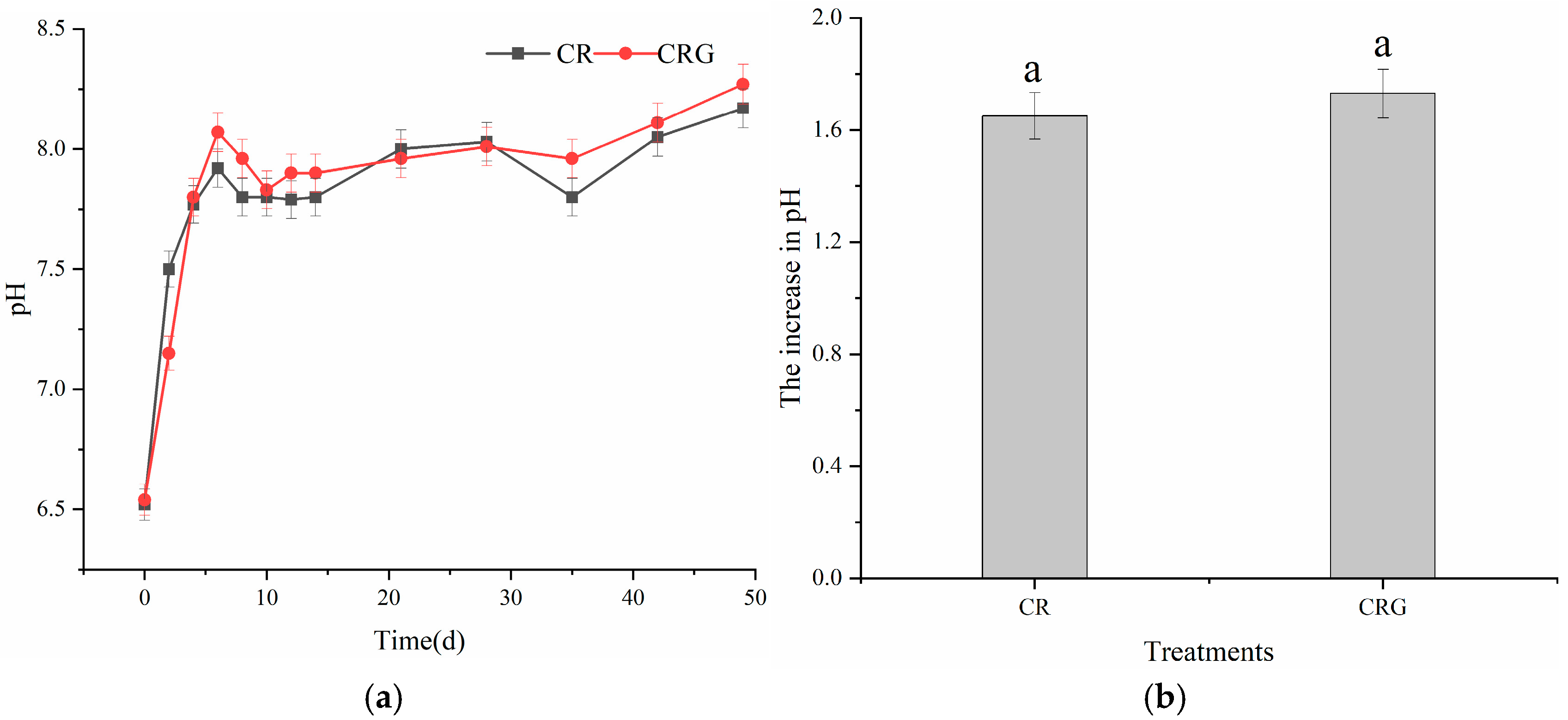
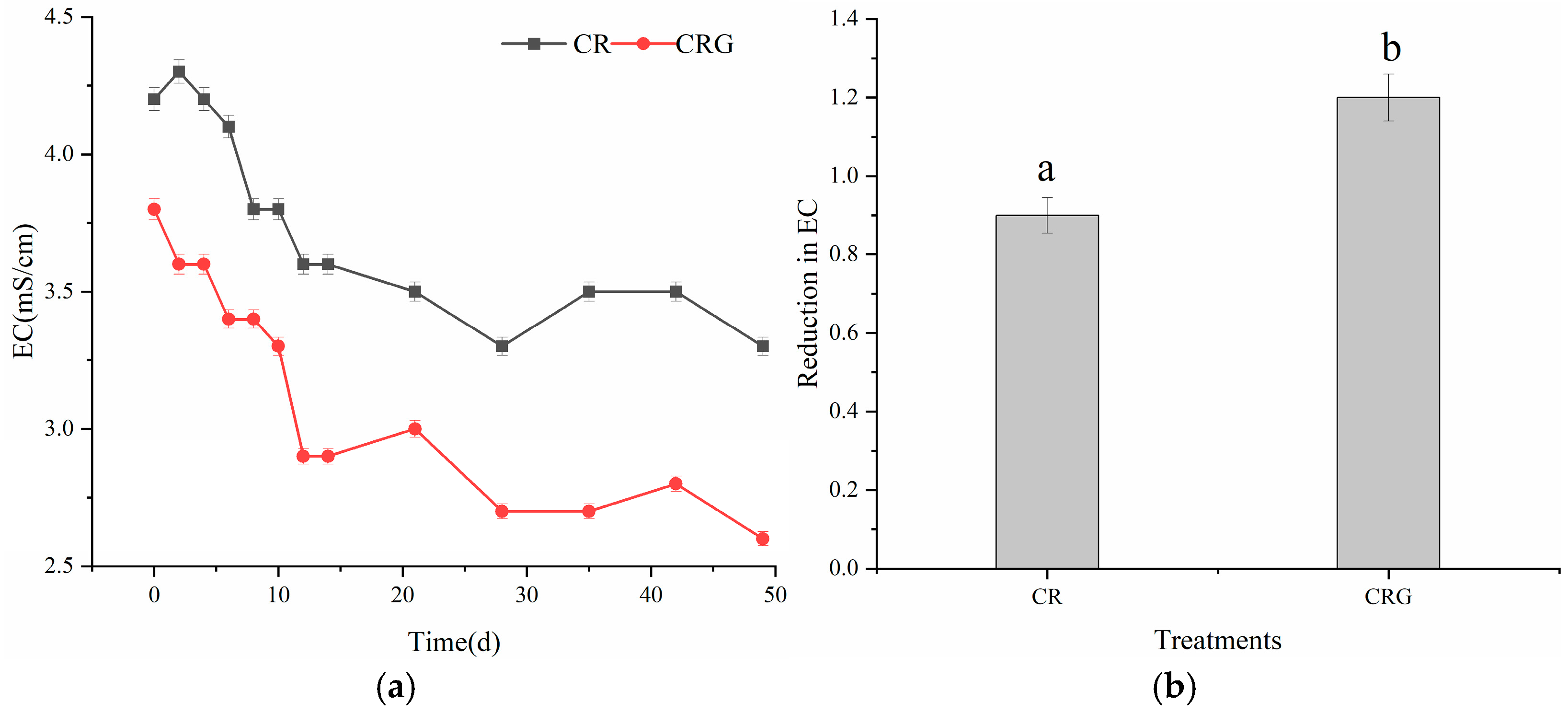
3.2.1. Temperature, pH, and EC Values
3.2.2. Total Organic Carbon (TOC)
3.2.3. Total Nitrogen (TN)
3.2.4. Total Phosphorus (TP)
3.2.5. Total Potassium (TK)
3.3. Changes in Enzyme Activity During the Composting Process
3.3.1. Urease Activity
3.3.2. Cellulase Activity
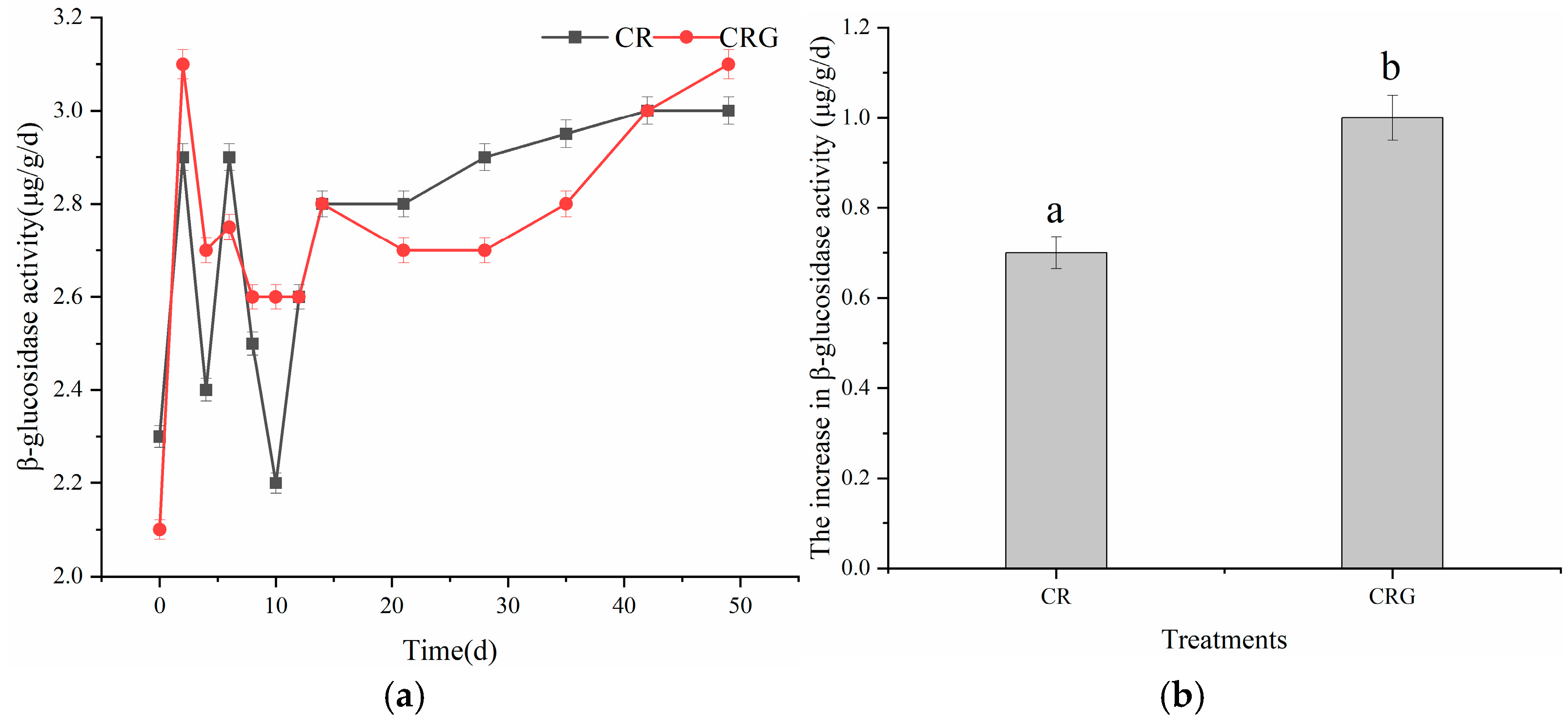
3.3.3. β-Glucosidase Activity
3.4. Changes in Humic Substance Components During the Composting Process
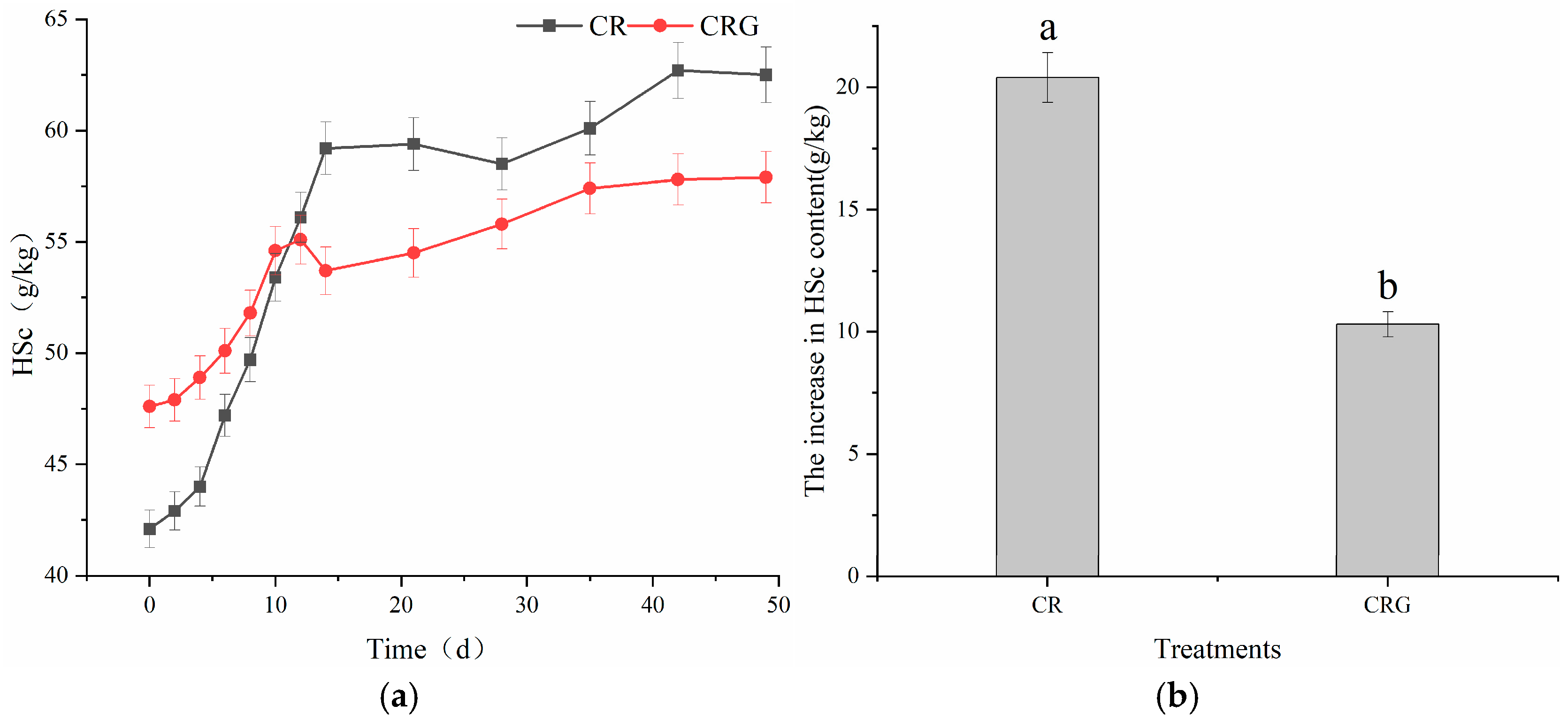
3.4.1. Humic Substance Carbon (HSc)
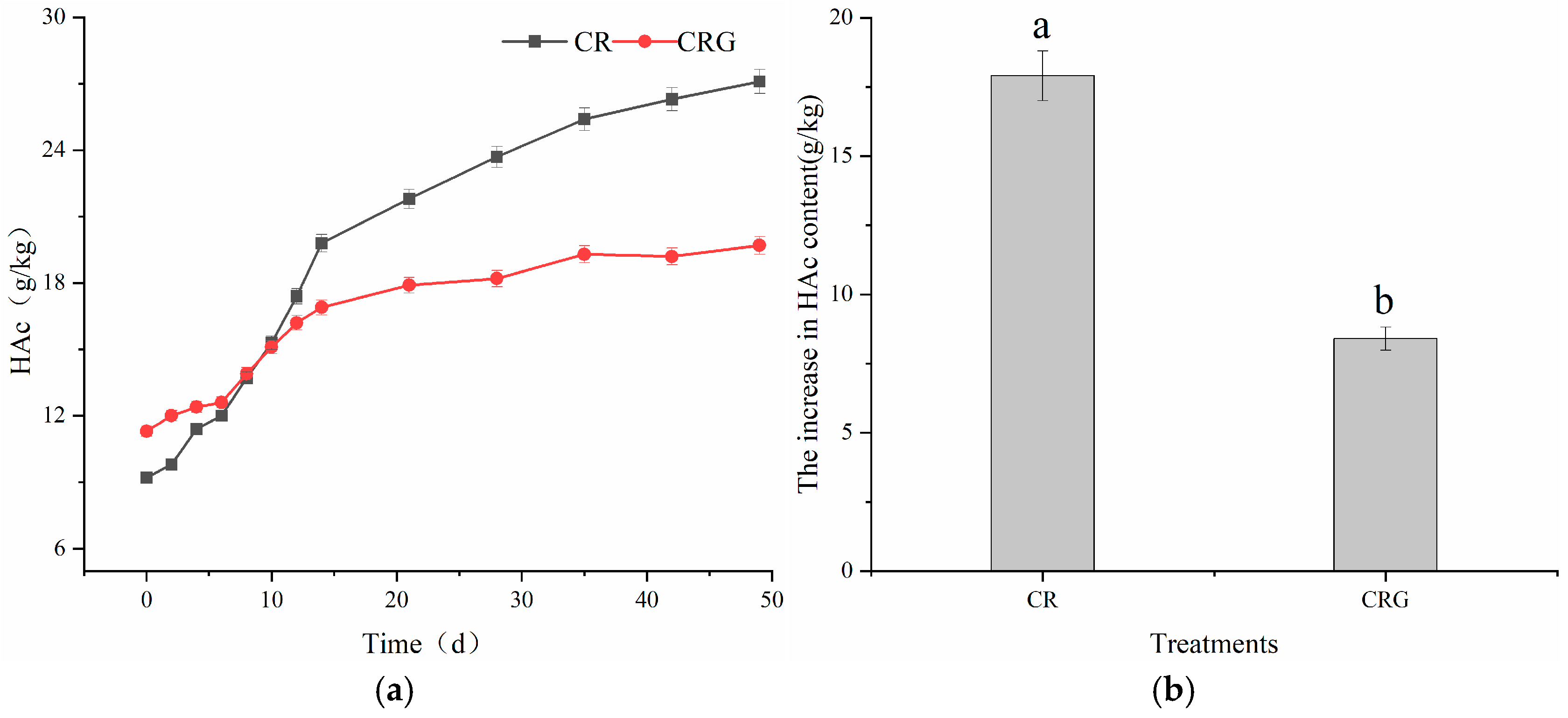
3.4.2. Humic Acid Carbon (HAc)
3.4.3. Fulvic Acid Carbon (FAc)
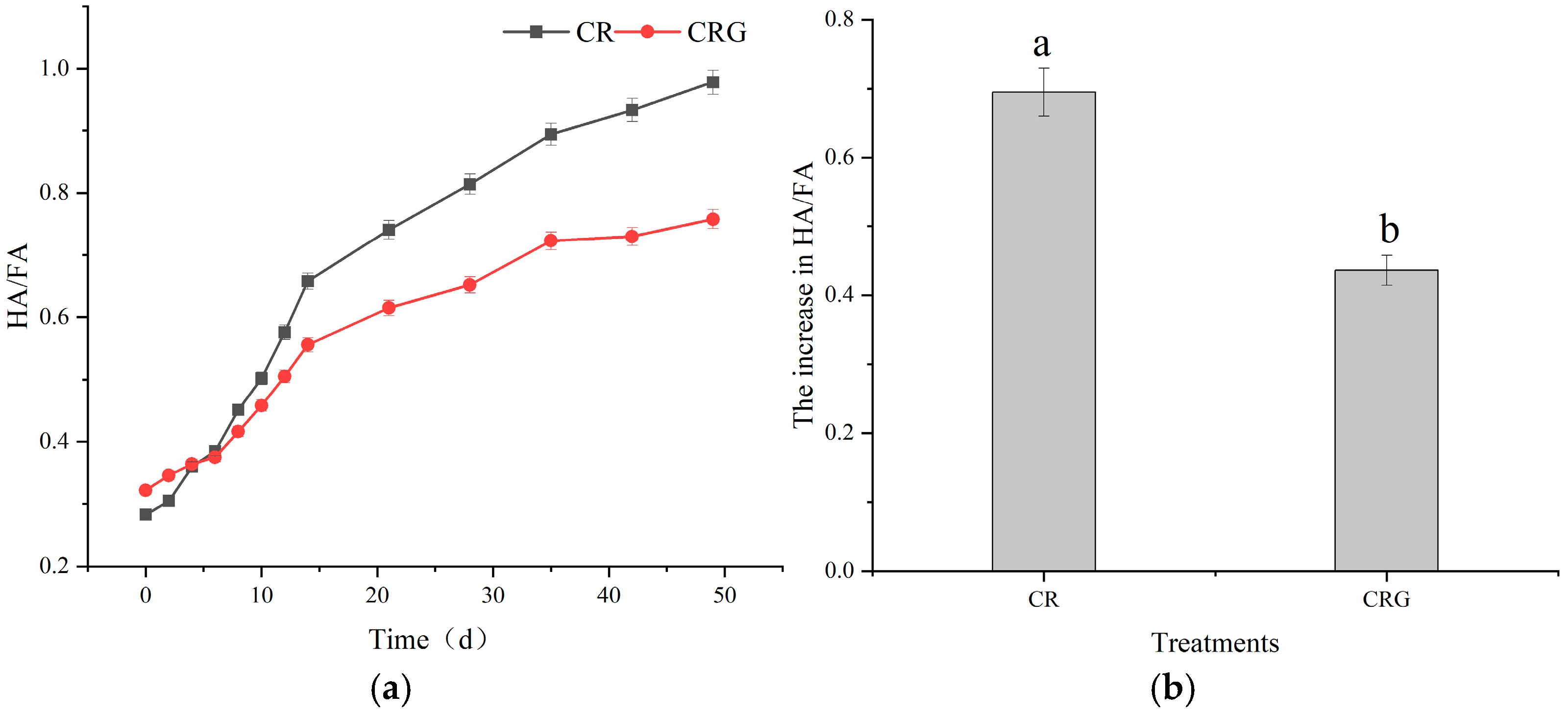
3.4.4. Humification Index (HA/FA)
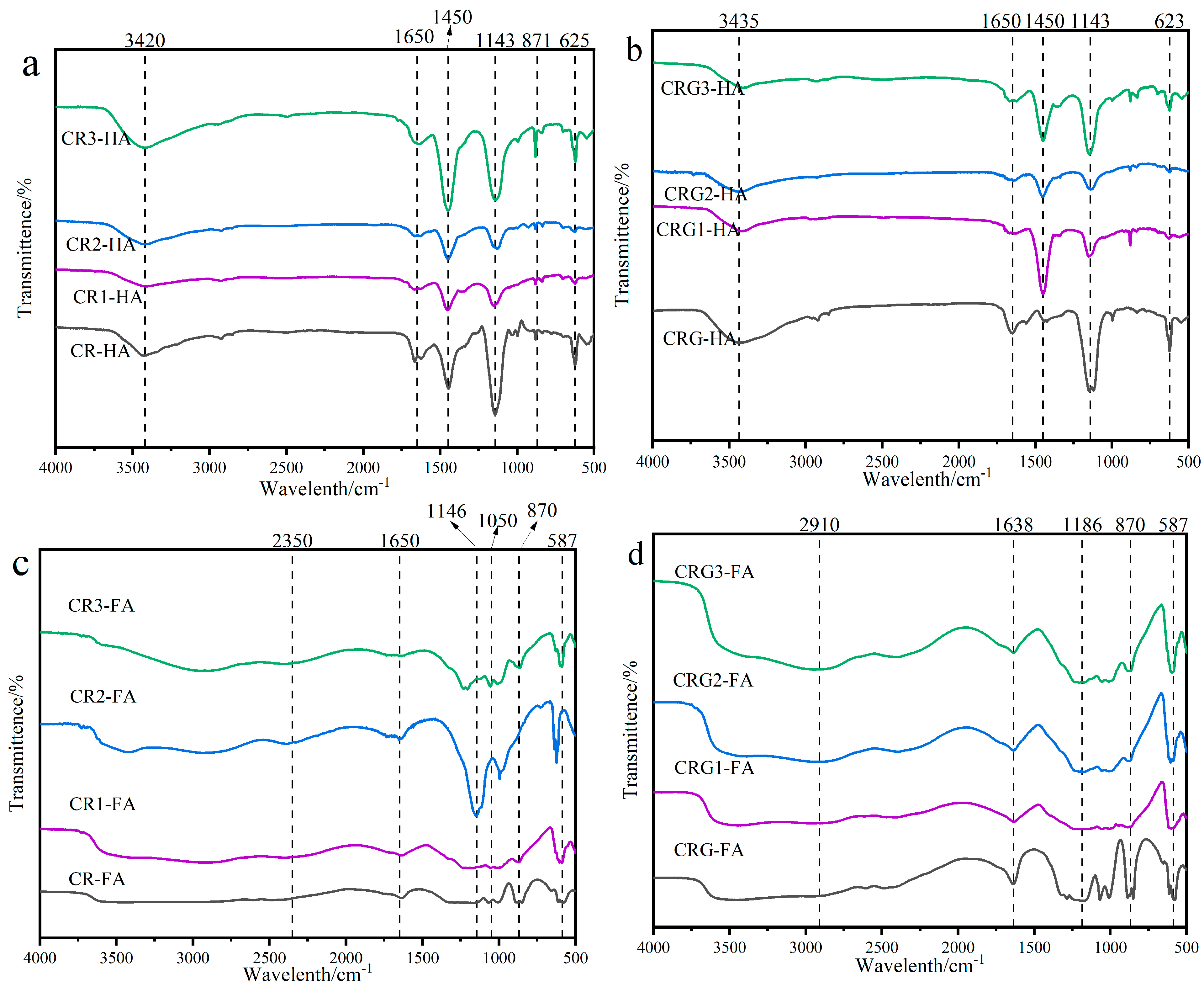
3.5. HA and FA Infrared Spectral Changes
3.5.1. Humic Acid (HA)
3.5.2. Fulvic Acid (FA)
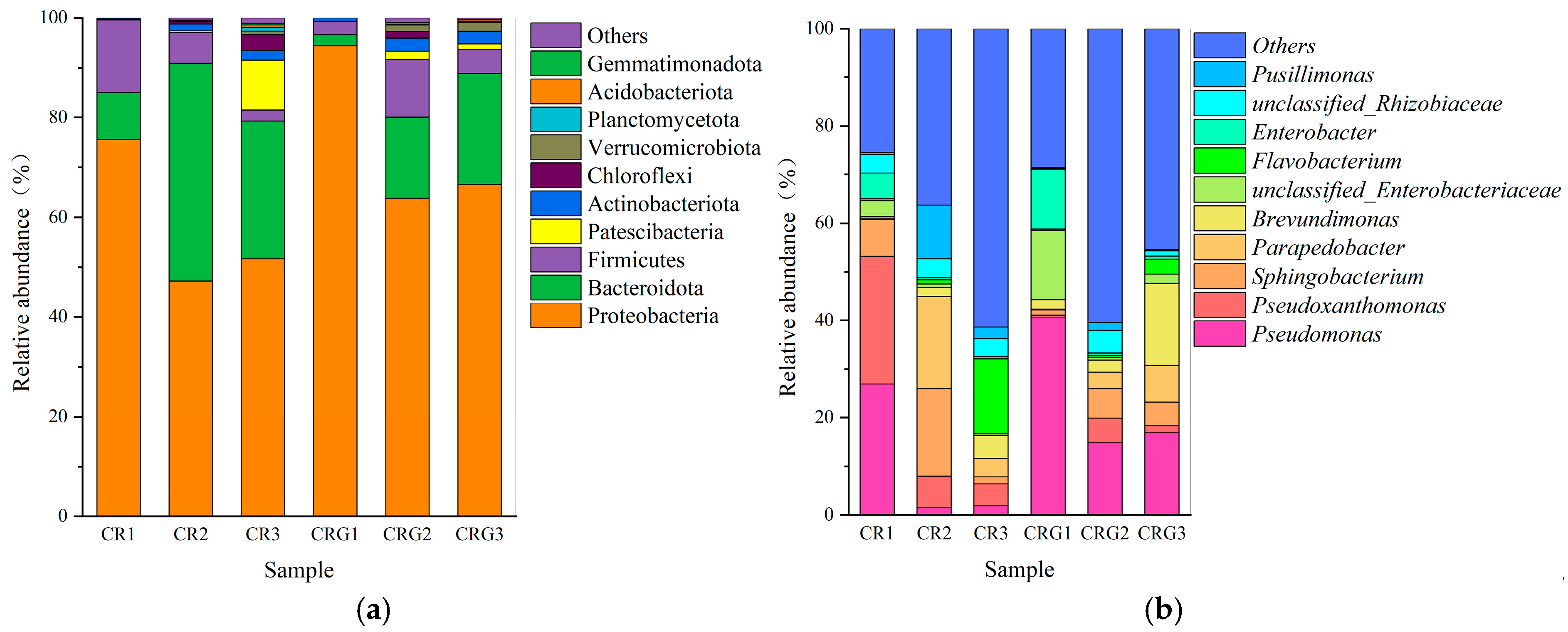
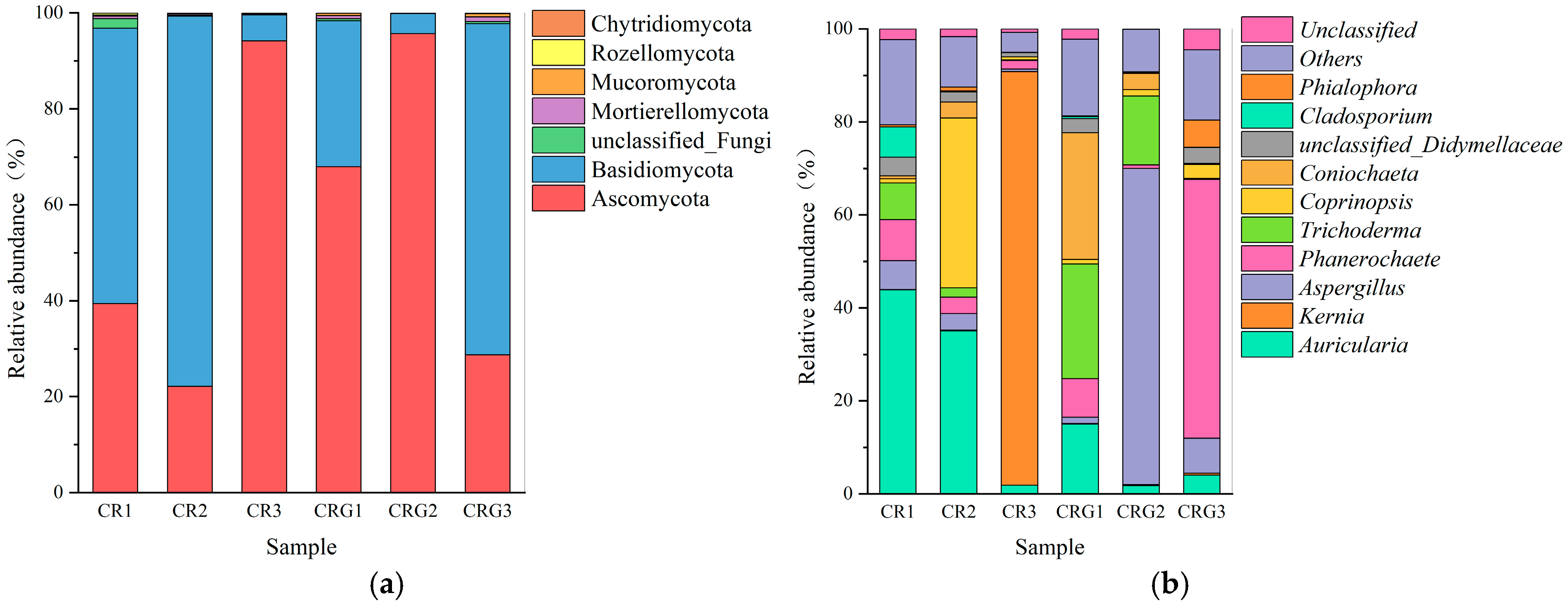
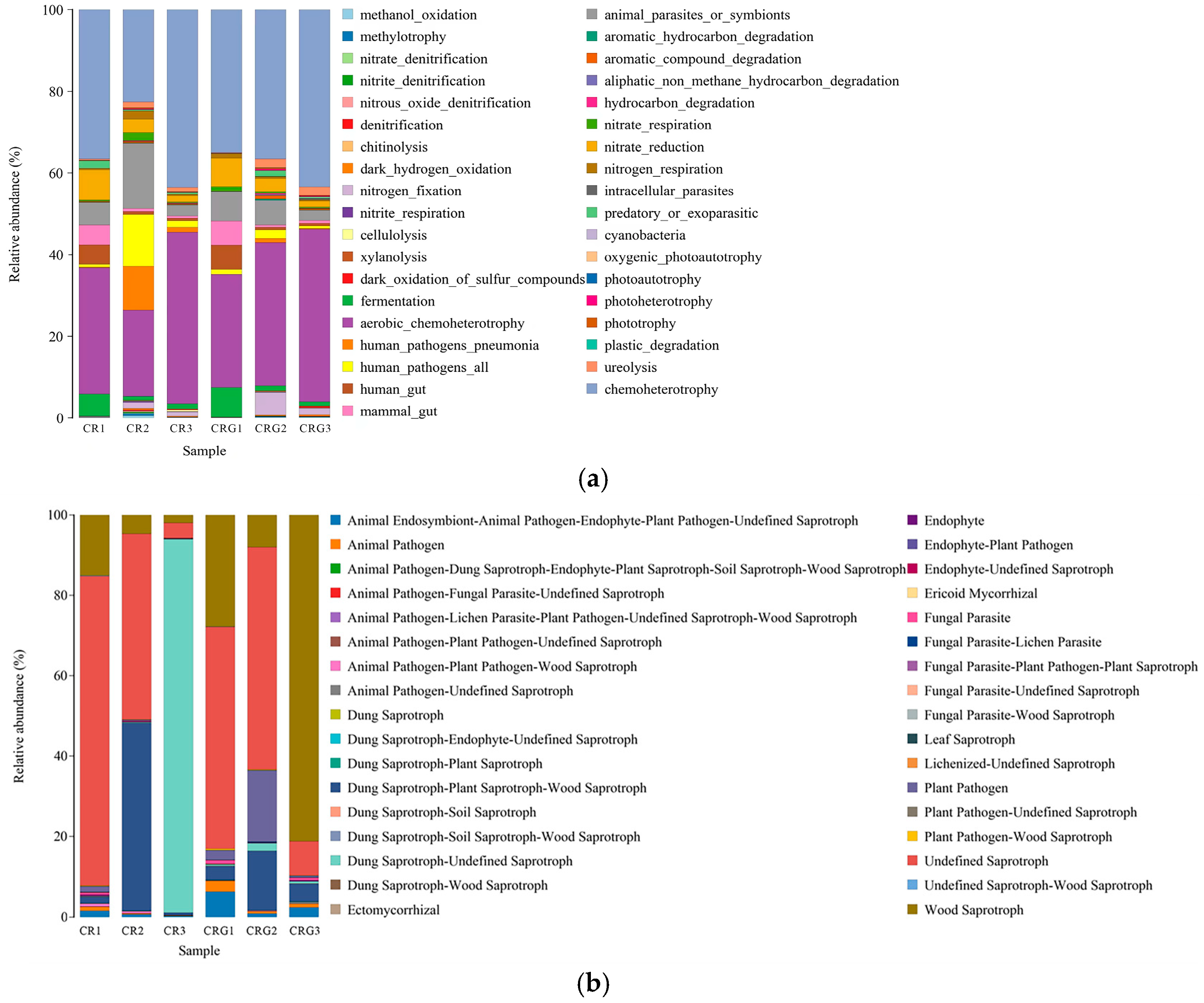
3.6. Microbial Community Dynamics and Functional Implications
3.6.1. Microbial Dynamics
3.6.2. Functional Implications
3.7. Composting Correlation Between Microbial Community Structure and Nutrient Indicators in Compost
3.8. Partial Least Squares Path Model Analysis of Compost Maturity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Malpotra, M.; Garg, M.; Singh, N.; Neha, S.; Susmita, D.S.; Rajni, C.; Bhupesh, S. An overview of bioactive components and phytopharmaceutical potentials of Hygrophila auriculata–A herbaceous medicinal plant. Phytomed. Plus 2025, 5, 100737. [Google Scholar] [CrossRef]
- Tian, B.; Pan, Y.; Wang, J.; Cai, M.; Ye, B.; Yang, K.; Sun, P. Insoluble Dietary Fibers from By-Products of Edible Fungi Industry: Basic Structure, Physicochemical Properties, and Their Effects on Energy Intake. Front. Nutr. 2022, 9, 851228. [Google Scholar] [CrossRef]
- Walia, A.; Mehta, P.; Guleria, S.; Chauhan, A.; Shirkot, C.K. Molecular Cloning and Sequencing of Alkalophilic Cellulosimicrobium cellulans CKMX1 Xylanase Gene Isolated from Mushroom Compost and Characterization of the Gene Product. Braz. Arch. Biol. Technol. 2015, 58, 913–922. [Google Scholar] [CrossRef]
- Oluwabamiro, K.S.; Adenipekun, C.O.; Ijimbili, S.B.; Oyetunji, O.J. Comparative Effect of Spent Mushroom Compost and NPK Fertilizer on the Growth of Sorghum bicolor L. Moench and Zea mays L. Using Fodder Hydroponics Techniques. Biotechnol. J. Int. 2023, 27, 92–102. [Google Scholar] [CrossRef]
- Feng, X.; Pan, J.S.; Du, Y.L.; Yang, D.X.; Ling, L.Z.; Yang, X.X.; Ma, J.; Zhang, M.; Jin, Y.H.; Geng, C.Y. Investigation of edible fungus bran resources and nutritional value evaluation in Jilin Province. Feed Ind. 2024, 45, 115–119. [Google Scholar]
- Wang, C.; Wang, Y.; Yan, S.; Li, Y.; Zhang, P.; Ren, P.; Wang, M.; Kuang, S. Biochar-amended composting of lincomycin fermentation dregs promoted microbial metabolism and reduced antibiotic resistance genes. Bioresour. Technol. 2022, 367, 128253. [Google Scholar] [CrossRef] [PubMed]
- Rashid, A.; Singh, A.; Laishram, N.; Pandey, R.K. Nutrient Status and Yield of French marigold as Influenced by Application of Spent Mushroom Compost, Biofertilizer and MKP Foliar Application. Int. J. Plant Soil Sci. 2022, 34, 1077–1083. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, Y.Y.; Sun, H.R.; Xu, Z. Analysis of humus formation and factors for driving the humification process during composting of different agricultural wastes. Front. Environ. Sci. 2022, 10, 954158. [Google Scholar] [CrossRef]
- Ran, X.L.; Li, S.; Uppuluri, T.S.; Deng, Y.; Su, Y.; Huang, G.Q.; Dong, R.J.; Müller, J.; Wei, Q.; Guo, J.B.; et al. Phosphorus transformation and bioavailability in livestock manure through aerobic composting and anaerobic digestion. Chem. Eng. J. 2025, 505, 159285. [Google Scholar] [CrossRef]
- Dang, Q.; Zhao, X.; Yang, T.; Gong, T.; He, X.; Tan, W.; Xi, B. Coordination of bacterial biomarkers with the dominant microbes enhances triclosan biodegradation in soil amended with food waste compost and cow dung compost. Sci. Total Environ. 2022, 824, 153837. [Google Scholar] [CrossRef]
- Piadeh, F.; Offie, I.; Behzadian, K.; Rizzuto, J.P.; Bywater, A.; Córdoba-Pachón, J.R.; Walker, M. A critical review for the impact of anaerobic digestion on sustainable development goals. J. Environ. Manag. 2024, 349, 15. [Google Scholar] [CrossRef]
- Hu, L.; Li, T.; Jing, Y. Thermal Pyrolysis Behavior and Decomposition Mechanism of Lignin Revealed by Stochastic Cluster Dynamics Simulations. J. Phys. Chem. C 2024, 128, 3832–3838. [Google Scholar] [CrossRef]
- Bhartiya, D.K.; Nath, G.; Singh, K. Vermicomposting: A technology for vermiremediation of heavy metals from sewage sludge and animal dung. J. Int. J. Recycl. Org. Waste Agric. 2024, 13, 1–6. [Google Scholar]
- Yang, Z. Enhancing the Quality of Tomato Straw Waste Composting: The Role of Earthworm Stocking Density in Composting–Vermicomposting Integrated Systems. Sustainability 2024, 17, 175. [Google Scholar] [CrossRef]
- Huang, W.Z. Resource Utilization of Agricultural Waste: From Biomass Energy to Organic Fertilizer. J. Energy Biosci. 2024, 15, 221–232. [Google Scholar] [CrossRef]
- Pan, X.R.; Shang-Guan, P.K.; Li, S.H.; Zhang, C.H.; Lou, J.M.; Guo, L.; Liu, L.; Lu, Y. The influence of carbon dioxide on fermentation products, microbial community, and functional gene in food waste fermentation with uncontrol pH. J. Environ. Res 2025, 267, 120645. [Google Scholar] [CrossRef]
- Liao, H.P.; Zhao, Q.; Cui, P.; Chen, Z.; Yu, Z.; Geisen, S.; Friman, V.; Zhou, S.G. Efficient reduction of antibiotic residues and associated resistance genes in tylosin antibiotic fermentation waste using hyperthermophilic composting. Environ. Int. 2019, 133, 105203. [Google Scholar] [CrossRef]
- Rastogi, M.; Nandal, M.; Nain, L. Tailoring the compost matrix with hybrid Bacillus commune to improve the biodegradation of municipal solid waste. Int. J. Environ. Sci. Technol. 2024, 21, 1447–1466. [Google Scholar] [CrossRef]
- Wang, K.; Ma, X.C.; Yin, X.Q.; Wu, C.D.; Wang, Z.; Wu, Y.Q.; Zhao, Y.; Tian, Y. Difference and interplay of microbial communities, metabolic functions, trophic modes and influence factors between sludge and bulking agent in a composting matrix. Bioresour. Technol. 2021, 336, 125085. [Google Scholar] [CrossRef]
- Li, T.; Yan, Z.X.; Sun, Y.; Hu, X.; Peng, C.L.; Zhao, S.J.; Xu, D.B.; Liu, D.Y.; Shen, Q.R. Inorganic and organic additives differently regulate compost microbiomes in response to heavy metals immobilization. Chem. Eng. J. 2024, 502, 158087. [Google Scholar] [CrossRef]
- He, X.Q.; Peng, Z.H.; Zhu, Y.X.; Chen, Y.F.; Huang, Y.P.; Xiong, J.P.; Fang, C.; Du, S.; Long, W.; Ling, Z. Wheat straw biochar as an additive in swine manure Composting: An in-depth analysis of mixed material particle characteristics and interface interactions. Waste Manag. 2024, 176, 41–51. [Google Scholar] [CrossRef]
- Fan, T.Y.; Zhang, X.M.; Wan, Y.; Deng, R.L.; Zhu, H.H.; Wang, X.H.; Wang, S.; Wang, X.M. Effect of Different Livestock Manure Ratios on the Decomposition Process of Aerobic Composting of Wheat Straw. Agronomy 2023, 13, 2916. [Google Scholar] [CrossRef]
- Zhou, S.F. The Inhibitory Effect of Xiaofeipeng Composting and Its Inhibitory Composition on Barnyard Grass. Ph.D. Thesis, Hunan University, Hunan, China, 2021; pp. 1–122. [Google Scholar]
- Ma, J.W.; Yang, W.Y.; Li, S.; Yang, Z.Y.; Qiao, C.; Liu, D.; Wang, M. Comprehensive effects of tea branch biochar on antibiotic resistance profiles and C/N/S cycling in the compost microbiota of animal manure. Sci. Total Environ. 2024, 957, 177457. [Google Scholar] [CrossRef] [PubMed]
- Baidoo, A.E.; Manka’abusi, D.; Apuri, L.; Marschner, B.; Frimpong, A.K. Biochar addition influences C and N dynamics during biochar co-composting and the nutrient content of the biochar co-compost. Sci. Rep. 2024, 14, 23781. [Google Scholar]
- Gurmessa, B.; Cocco, S.; Ashworth, A.J.; Udawatta, R.P.; Cardelli, V.; Serrani, D.; Ilari, A.; Pedretti, E.F.; Fornasier, F.; Corti, G. Enzyme activities and microbial nutrient limitations in response to digestate and compost additions in organic matter poor soils in the Marches, Italy. Soil Tillage Res. 2024, 242, 106–136. [Google Scholar] [CrossRef]
- Mahongnao, S.; Sharma, P.; Singh, D.; Ahamad, A.; Kumar, P.; Kumar, P.; Nanda, S. Formation and characterization of leaf waste into organic compost. Environ. Sci. Pollut. Res. 2023, 30, 75823. [Google Scholar] [CrossRef]
- Xi, B.D.; Zhao, X.Y.; He, X.S.; Huang, C.H.; Tan, W.B.; Gao, R.T.; Zhang, H.; Li, D. Successions and diversity of humic-reducing microorganisms and their association with physical-chemical parameters during composting. Bioresour. Technol. 2016, 219, 204–211. [Google Scholar] [CrossRef]
- Duan, H.Y.; Ji, M.H.; Chen, A.; Zhang, B.G.; Shi, J.P.; Liu, L.; Li, X.; Sun, J.S. Evaluating the impact of rice husk on successions of bacterial and fungal communities during cow manure composting. Environ. Technol. Innov. 2021, 24, 102084. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, G.Z.; Li, L.B.; Su, F.K.; Yang, J. Optimal Color Development Conditions and Spectrophotometric Wavelength for Determination of Total Phosphorus in Soil by Mo-Sb Anti Colorimetric Method. Soil Fertil. Sci. China 1992, 2, 46–48. [Google Scholar]
- Tao, S.H.; Gong, H.R.; Chen, Z.W.; Chen, Y.Z.; Miao, X.X.; Wang, J.M. Determination of Total Potassium in Plants by Microwave Digestion-Flame Photometry. Hubei Agric. Sci. 2019, 58, 142–145. [Google Scholar]
- Guan, Y.S. Soil Enzymes and Their Research Methods; Agriculture Press: Beijing, China, 1986. [Google Scholar]
- Kuwatsuka, S.; Watanable, A.; Itoh, K.; Arail, S. Comparision of Two Methods of Preparation of Humic and Fulvic Acids, IHSS Method and NAGOYA Method. Soil Sci. Plant Nutr. 1992, 38, 23–30. [Google Scholar] [CrossRef]
- Wang, G.Y.; Kong, Y.L.; Yang, Y.; Ma, R.N.; Shen, Y.J.; Li, G.X.; Yuan, J. Superphosphate, Biochar, and a Microbial Inoculum Regulate Phytotoxicity and Humification During Chicken Manure Composting. Sci. Total Environ. 2022, 824, 153958. [Google Scholar] [CrossRef] [PubMed]
- White, J.R.; Nagarajan, N.; Pop, M. Statistical methods for detecting differentially abundant features in clinical metagenomic samples. PLoS Comput. Biol. 2009, 5, 30–52. [Google Scholar] [CrossRef] [PubMed]
- Nevase, D.; Rathod, J.; Phatake, Y. Waste to Compost: Application of Indigenous Microbes for Conversion of Vegetable Waste into Compost. Biol. Bull. 2024, 51, S66–S74. [Google Scholar] [CrossRef]
- Correa-Bustos, A.; Berti, F.; Salas-Sanjuán, M.d.C.; Segura-Pérez, M. Characterization of Mixtures of Rugulopteryx okamurae Compost and Plant Residues to Determine the Most Effective Composition as a Substrate and Source of Nutrients. Horticulturae 2024, 10, 567. [Google Scholar] [CrossRef]
- Parzych, A.E. Urban Leaf Litters as a Potential Compost Component. J. Ecol. Eng. 2022, 23, 250–260. [Google Scholar] [CrossRef]
- Lalremruati, M.; Devi, A.S. Changes in physico-chemical properties during composting of three common household organic solid wastes amended with garden soil. Bioresour. Technol. Rep. 2021, 15, 100727. [Google Scholar] [CrossRef]
- Goyal, S.; Dhull, S.K.; Kapoor, K.K. Chemical and biological changes during composting of different organic wastes and assessment of compost maturity. Bioresour. Technol. 2005, 96, 1584–1591. [Google Scholar] [CrossRef]
- Sülük, K.; Tosun, İ.; Ekinci, K. Co-composting of two-phase olive-mill pomace and poultry manure with tomato harvest stalks. J. Environ. Technol. 2017, 38, 923–932. [Google Scholar] [CrossRef]
- Wang, X.H.; He, X.L.; Liang, J. Succession of Microbial Community during the Co-Composting of Food Waste Digestate and Garden Waste. Int. J. Environ. Res. Public Health 2022, 19, 9945. [Google Scholar] [CrossRef]
- Duan, Y.M.; Awasthi, M.K.; Wu, H.H.; Yang, J.F.; Li, Z.L.; Ni, X.H.; Zhang, J.T.; Zhang, Z.Q.; Li, H.K. Biochar regulates bacterial-fungal diversity and associated enzymatic activity during sheep manure composting. Bioresour. Technol. 2022, 346, 126647. [Google Scholar] [CrossRef] [PubMed]
- Li, J.N.; Zhang, H.Y.; Zheng, L. Influence of Organic Amendments Based on Garden Waste for Microbial Community Growth in Coastal Saline Soil. Sustainability 2023, 15, 5038. [Google Scholar] [CrossRef]
- Fu, Y.; Yu, Y.; Yang, S.; Yang, G.; Huang, H.; Yang, Y.; Du, M. Effects of Fertilization on Soil Physicochemical Properties and Enzyme Activities of Zanthoxylum planispinum var. Dingtanensis Plantation. Forests 2025, 16, 418. [Google Scholar] [CrossRef]
- Ming, L.; Dou, S.; Zhou, J.; Wang, H.; Yang, D. Biochar Regulates the Humification of Kitchen Waste and the Effects of the Humic Acid Structure of Products on Black Soil. Agronomy 2024, 14, 2503. [Google Scholar] [CrossRef]
- Wu, F.L.; Geng, G.; Wu, X.M.; Ye, M.F.; Lin, D.Y. Effects of Different Carbon-to-Nitrogen Ratios on the Composting of Spirulina Algal Sludge and Pig Manure Residue. J. Fujian Agric. Sci. Technol. 2022, 53, 9–14. [Google Scholar]
- Tiquia, S.M.; Tam, N.F. Fate of nitrogen during composting of chicken litter. Environ. Pollut. 2000, 110, 535–541. [Google Scholar] [CrossRef]
- Nie, L.; Wan, W. Nutrient-cycling functional gene diversity mirrors phosphorus transformation during chicken manure composting. Bioresour. Technol 2023, 10, 129504. [Google Scholar] [CrossRef]
- Alises, M.O.; Palomo, E.S.; González-Viñas, M.Á. Wine Waste Compost Addition to a Vineyard of Chelva Grape of Central Spain: Effect on Wine Volatile Compounds. Horticulturae 2025, 11, 219. [Google Scholar] [CrossRef]
- Kang, J.; Song, G.; Wang, X.; Qiu, W.; Pei, F.; Ling, H.; Ping, W.; Ge, J. Aerobic composting with sauerkraut fermentation wastewater: Increasing the stability and complexity of bacterial community and changing bacterial community assembly processes. Bioresour. Technol. 2023, 376, 128883. [Google Scholar] [CrossRef]
- Kazemi, K.; Zhang, B.; Lye, L.M. Assessment of Microbial Communities and Their Relationship with Enzymatic Activity during Composting. World J. Eng. Technol. 2017, 5, 93–102. [Google Scholar] [CrossRef]
- Finore, I.; Feola, A.; Russo, L.; Cattaneo, A.; Di Donato, P.; Nicolaus, B.; Poli, A.; Romano, I. Thermophilic bacteria and their thermozymes in composting processes: A review. Chem. Biol. Technol. Agric. 2023, 10, 7. [Google Scholar] [CrossRef]
- Dai, Q.P.; Xiang, D.; Chang, Z.F.; Guo, H.B.; Lu, Z.Y.; Zheng, D.; Zhang, L.Y.; Yu, Y.J. Effect of agricultural and forestry wastes on organic matter conversion andenzymatic activity in livestock manure composting. J. Environ. Eng. Technol. 2024, 14, 1541–1549. [Google Scholar]
- Hubbe, M.A.; Nazhad, M.; Sanchez, C. Composting as a way to convert cellulosic biomass and organic waste into high-value soil amendments: A review. Bioresources 2010, 5, 2808–2854. [Google Scholar] [CrossRef]
- Pastorelli, R.; Casagli, A.; Rocchi, F.; Tampio, E.; Laaksonen, I.; Becagli, C.; Lagomarsino, A. Effects of Anaerobic Digestates and Biochar Amendments on Soil Health, Greenhouse Gas Emissions, and Microbial Communities: A Mesocosm Study. Appl. Sci. 2024, 14, 1917. [Google Scholar] [CrossRef]
- Zang, X.Y.; Liu, M.T.; Wang, H.W.; Fan, Y.H.; Zhang, H.C.; Liu, J.W.; Xing, E.L.; Xu, X.H.; Li, H.T. The distribution of active beta-glucosidase producing microbial communities in composting. Can. J. Microbiol. 2017, 12, 998–1008. [Google Scholar] [CrossRef]
- Sathya, T.A.; Alarjani, K.M.; Elshikh, M.S.; Flanetraj, S.R.; Ponnuswamy, V. Co-Composting of Green Leaves and Kitchen Waste: Characterization of Organic Amendments, Microbial Activity and Analysis of Defence Enzymes in Plants. Biomass Convers. Biorefin. 2024, 4, 1–12. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Zhao, Z.H.; Zhou, K.Y.; Zhan, X.M.; Wang, Y.; Liu, N.; Han, X.R.; Li, X. Soil Organic Carbon and Humus Characteristics: Response and Evolution to Long-Term Direct/Carbonized Straw Return to Field. Agronomy 2024, 14, 2400. [Google Scholar] [CrossRef]
- Wang, Z.H.; Yang, X.P.; Mao, J.; Wang, Z.F.; Xie, Y.Q.; Zhou, L.Y. Effect of exogenous bacterial agents on microbial communities and decay effect during cotton straw composting. J. Ecol. Rural Environ. 2024, 40, 1376–1384. [Google Scholar]
- Yang, X.Y.; Yan, R.X.; Yang, C.Z.; Zhang, H.W.; Lyu, H.Y.; Li, S.Q.; Liu, T.R.; Li, R.H.; Yao, Y.Q.; Li, W.T.; et al. Soil accelerates the humification involved in co-composting of wheat straw and cattle manure by promoting humus formation. Chem. Eng. J. 2024, 479, 147583. [Google Scholar] [CrossRef]
- Ying, J.; Zhang, X.; Wu, W.; Nan, Q.; Wang, G.; Dong, D. The effects of long-term rice straw and biochar return on soil humus composition and structure in paddy soil. Plant Soil Environ. 2024, 70, 12. [Google Scholar] [CrossRef]
- Gong, X.Q.; Zou, L.; Wang, L.; Zhang, B.; Jiang, J.X. Biochar improves compost humification, maturity and mitigates nitrogen loss during the vermicomposting of cattle manure-maize straw. J. Environ. Manag. 2023, 325, 116432. [Google Scholar] [CrossRef] [PubMed]
- Scotti, R.; Agostino, D.N.; Pane, C.; Zaccardelli, M. Humic acids and compost tea from compost for sustainable agriculture management. Acta Hortic. 2016, 1146, 115–120. [Google Scholar] [CrossRef]
- Maffia, A.; Marra, F.; Canino, F.; Battaglia, S.; Mallamaci, C.; Oliva, M.; Muscolo, A. Humic Substances from Waste-Based Fertilizers for Improved Soil Fertility. Agronomy 2024, 14, 2657. [Google Scholar] [CrossRef]
- Xu, Y.; Bi, Z.T.; Zhang, Y.C.; Wu, H.; Zhou, L.; Zhang, H.Q. Impact of wine grape pomace on humification performance and microbial dynamics during pig manure composting. Bioresour. Technol. 2022, 358, 127380. [Google Scholar] [CrossRef]
- Tao, Z.D.; Liu, X.C.; Sun, L.L.; He, X.X.; Wu, Z.S. Effects of two types of nitrogen sources on humification processes and phosphorus dynamics during the aerobic composting of spent mushroom substrate. Bioresour. Technol. 2022, 317, 115453. [Google Scholar]
- Chang, H.Y.; Xi, B.D.; Sun, X.J.; Chen, S.X.; Wang, Y.; Tan, Z.H.; Zhang, J. Effect of electric field on the structural characteristics of fumenic acid in composite sludge compost. Res. Environ. Sci. 2024, 254, 123835. [Google Scholar]
- Yang, Y.J.; Du, W.; Ren, X.N.; Cui, Z.Y.; Zhou, W.; Lv, J.L. Effect of bean dregs amendment on the organic matter degradation, humification, maturity and stability of pig manure composting. Sci. Total Environ. 2020, 708, 134623. [Google Scholar] [CrossRef]
- Wang, G.M.; Huang, S.S.; Wang, L.G.; Zheng, G.R. Dynamic change of decay degree index and infrared spectrum during slag aerobic composting. J. Eco. Environ. 2019, 28, 2416–2424. [Google Scholar]
- Wang, D.M.; Mao, Y.L.; Mai, L.W.; Yu, Z.; Lin, J.C.; Li, Q.F.; Yuan, J.; Li, G.X. Insight into humification of mushroom residues under addition of Rich-N sources: Comparing key molecular evolution processes using EEM-PARAFAC and 2D-FTIR-COS analysis. J. Environ. Manag. 2023, 329, 117079. [Google Scholar] [CrossRef]
- Zhou, X.L.; Li, J.B.; Zhang, J.; Deng, F.; Chen, Y.H.; Zhou, P.; Li, D. Bioaugmentation Mechanism on Humic Acid Formation during Composting of Food Wast. Sci. Total Environ. 2022, 830, 154783. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.Y.; Lv, T.T.; Meng, G.F.; Zhang, J. Study on the formation rule and structural change of humic acid in chicken manure composting. J. Qilu Univ. Technol. 2024, 38, 67–75. [Google Scholar]
- Bai, L. Study on the humization process and regulation mechanism of aerobic composting. D. Jiangnan Univ. 2020, 1–105. [Google Scholar]
- Dong, L.C.; Wang, X.X.; Ma, M.T.; Wang, Y.X. Effect of lignite addition on the spectroscopic change characteristics of water-soluble organic matter during fermentation of organic fertilizer in sheep manure. Spectrosc. Spectr. Anal. 2019, 39, 3579–3584. [Google Scholar]
- Biyada, S.; Merzouki, M.; Dėmčėnko, T.; Vasiliauskienė, D.; Urbonavičius, J.; Marčiulaitienė, E.; Vasarevičius, S.; Benlemlih, M. Evolution of Microbial Composition and Enzymatic Activities during the Composting of Textile Waste. Appl. Sci. 2020, 10, 3758. [Google Scholar] [CrossRef]
- Sun, X.J.; Tan, Z.H.; Zhang, M.X.; Wang, Y.B.; Ou, C.Q.Y. Effect of different carbon sources on the electron transfer capacity of humus during the sludge composting process. J. Guilin Univ. Technol. 2024, 44, 304–312. [Google Scholar]
- Cui, H.; Ou, Y.; Wang, L.X.; Yan, B.X.; Li, Y.X.; Bao, M.W. Dissolved organic carbon, a critical factor to increase the bioavailability of phosphorus during biochar-amended aerobic composting. J. Environ. Sci. 2022, 113, 356–364. [Google Scholar] [CrossRef]
- Qiu, Z.P.; Li, M.X.; Song, L.Y.; Wang, C.; Yang, S.; Yan, Z.Y.; Wang, Y.Q. Study on nitrogen-retaining microbial agent to reduce nitrogen loss during chicken manure composting and nitrogen transformation mechanism. J. Clean. Prod. 2021, 285, 124813. [Google Scholar] [CrossRef]
- Zhong, X.Z.; Li, X.X.; Zeng, Y.; Wang, S.P.; Sun, Z.Y.; Tang, Y.Q. Dynamic change of bacterial community during dairy manure composting process revealed by high-throughput sequencing and advanced bioinformatics tools. Bioresour. Technol. 2020, 306, 123091. [Google Scholar] [CrossRef]
- Zhang, W.H.; Men, M.Q.; Xu, B.S.; Xu, X.H.; Cheng, L.J.; Meng, Q.X.; Deng, L.Y.; Jiang, X.; Wu, X.T.; Sheng, S.Y. Dynamic characterization of fungal community composition in novel static composting of cattle manure and rice straw. J. Agric. Environ. Sci. 2018, 37, 2029–2036. [Google Scholar]
- Shi, M.Z.; Wei, Z.M.; Wang, L.Q.; Wu, J.Q.; Zhang, D.Y.; Wei, D.; Tang, Y.; Zhao, Y. Response of humic acid formation to elevated nitrate during chicken manure composting. J. Bioresour. Technol. 2018, 258, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fan, B.; Zhao, L.; Zhao, C.; Yang, F. Biochar promotes compost humification by regulating bacterial and fungal communities. Front. Microbiol. 2024, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Ozer, H.; Ozdemir, N.; Ates, A.; Koklu, R.; Erdem, O.S.; Ozdemir, S. Circular Utilization of Coffee Grounds as a Bio-Nutrient through Microbial Transformation for Leafy Vegetables. Life 2024, 14, 1299. [Google Scholar] [CrossRef]
- Guo, H.N.; Liu, H.T.; Wu, S. Immobilization pathways of heavy metals in composting: Interactions of microbial community and functional gene under varying C/N ratios and bulking agents. J. Hazard. Mater. 2022, 426, 128103. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.H.; Lu, T.J.; Feng, W.; Jin, Q.C.; Wu, Z.G.; Yang, Y. Development of the degradation bacteria in household food waste and analysis of the microbial community in aerobic composting. Biotechnol. Appl. Biochem. 2023, 70, 622–633. [Google Scholar] [CrossRef]
- Qu, H.; Cao, J.; Wang, P.; Li, R.; Qi, Z.; Fu, J.; Chen, Y.; Chen, M. Volatile organic compounds and dominant bacterial community during aerobic composting of vegetable waste and cow manure co-complexing. BioResources 2022, 17, 1338–1353. [Google Scholar] [CrossRef]
- Gałązka, A.; Jankiewicz, U.; Orzechowski, S. The Role of Ligninolytic Enzymes in Sustainable Agriculture: Applications and Challenges. Agronomy 2025, 15, 451. [Google Scholar] [CrossRef]
- Kakumyan, P.; Yang, L.; Liu, S.J.; Yu, C.X.; Li, Z.P.; Chen, M.J.; Popluechai, S.; Zhao, Y. Comparison of the Bacterial and Fungal Communities and Metabolic Functions of Cottonseed Hull Waste Compost Associated with High and Low Yields of Straw Mushroom Volvariella volvacea. Microorganisms 2025, 13, 437. [Google Scholar] [CrossRef]
- Xu, J.L.; Wang, X.Y.; Feng, J.Y.; Zhang, W.Q.; Sun, J.T. Study of the Relationship Between Nitrogen, Phosphorus Content, and Microbial Community Changes in Deer Manure Compost with Different Conditioners. Fermentation 2025, 11, 66. [Google Scholar] [CrossRef]
- Zhao, L.; Huang, Y.H.; Ran, X.; Xu, Y.W.; Chen, Y.Y.; Wu, C.S.; Tang, J. Nitrogen Transformation Mechanisms and Compost Quality Assessment in Sustainable Mesophilic Aerobic Composting of Agricultural Waste. Sustainability 2025, 17, 575. [Google Scholar] [CrossRef]
- Biyada, S.; Tauraitė, D.; Urbonavičius, J.; Merzouki, M. Accelerated Co-Composting of Textile Waste Using the New Strains and Microbial Consortium: Evaluation of Maturity, Stability and Microbial Activity. Appl. Sci. 2024, 14, 11976. [Google Scholar] [CrossRef]
- Bang, D.; Chung, W.; Chang, S. Influence of Effective Microbial Additives Inoculation on Indigenous Bacterial Community Dynamics and Co-Occurrence Patterns During the Composting of Mixed Food Waste and Livestock Manure. Agronomy 2024, 14, 2973. [Google Scholar] [CrossRef]
- Jia, P.H.; Huang, Y.M.; Chen, M.L.; Qi, X.P.; Hou, H.Y. Comprehensive evaluation of spent mushroom substrate-chicken manure co-composting by garden waste improvement: Physicochemical properties, humification process, and the spectral characteristics of dissolved organic matter. Environ. Sci. Pollut. Res. 2023, 30, 8987–8997. [Google Scholar] [CrossRef]
- Gu, Z.; He, L.; Liu, T.; Xing, M.Y.; Feng, L.Y.; Luo, G.L. Exploration of Bacterial Community Structure Profiling and Functional Characteristics in the Vermicomposting of Wasted Sludge and Kitchen Waste. Water 2024, 16, 3107. [Google Scholar] [CrossRef]
- Zhang, Z.C.; Yang, H.K.; Wang, B.; Chen, C.; Zou, X.S.; Cheng, T.; Li, J. Aerobic co-composting of mature compost with cattle manure: Organic matter conversion and microbial community characterization. Bioresour. Technol. 2023, 382, 129187. [Google Scholar] [CrossRef]
- Wang, W.W.; Zhang, F.; Zhao, Q.; Liu, C.J.; Jim, C.Y.; Johnson, C.V.; Tan, L.M. Determining the main contributing factors to nutrient concentration in rivers in arid northwest China using partial least squares structural equation modeling. J. Environ. Manag. 2023, 343, 118249. [Google Scholar] [CrossRef]
- Sarstedt, M.; Ringle, C.M.; Smith, D.; Reams, R.; HairJr, F.J. Partial least squares structural equation modeling (PLS-SEM): A useful tool for family business researchers. J. Fam. Bus. Strateg. 2014, 5, 105–115. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, X.J.; Yao, Q.; Zhu, H.H. Both Soil Bacteria and Soil Chemical Property Affected the Micropredator Myxobacterial Community: Evidence from Natural Forest Soil and Greenhouse Rhizosphere Soil. Microorganisms 2023, 8, 1387. [Google Scholar] [CrossRef]
- Wang, X.; Wang, M.; Zhang, J.; Kong, Z.; Shen, Q. Contributions of the biochemical factors and bacterial community to the humification process of in situ large-scale aerobic composting. Bioresour. Technol. 2020, 323, 124599. [Google Scholar] [CrossRef]

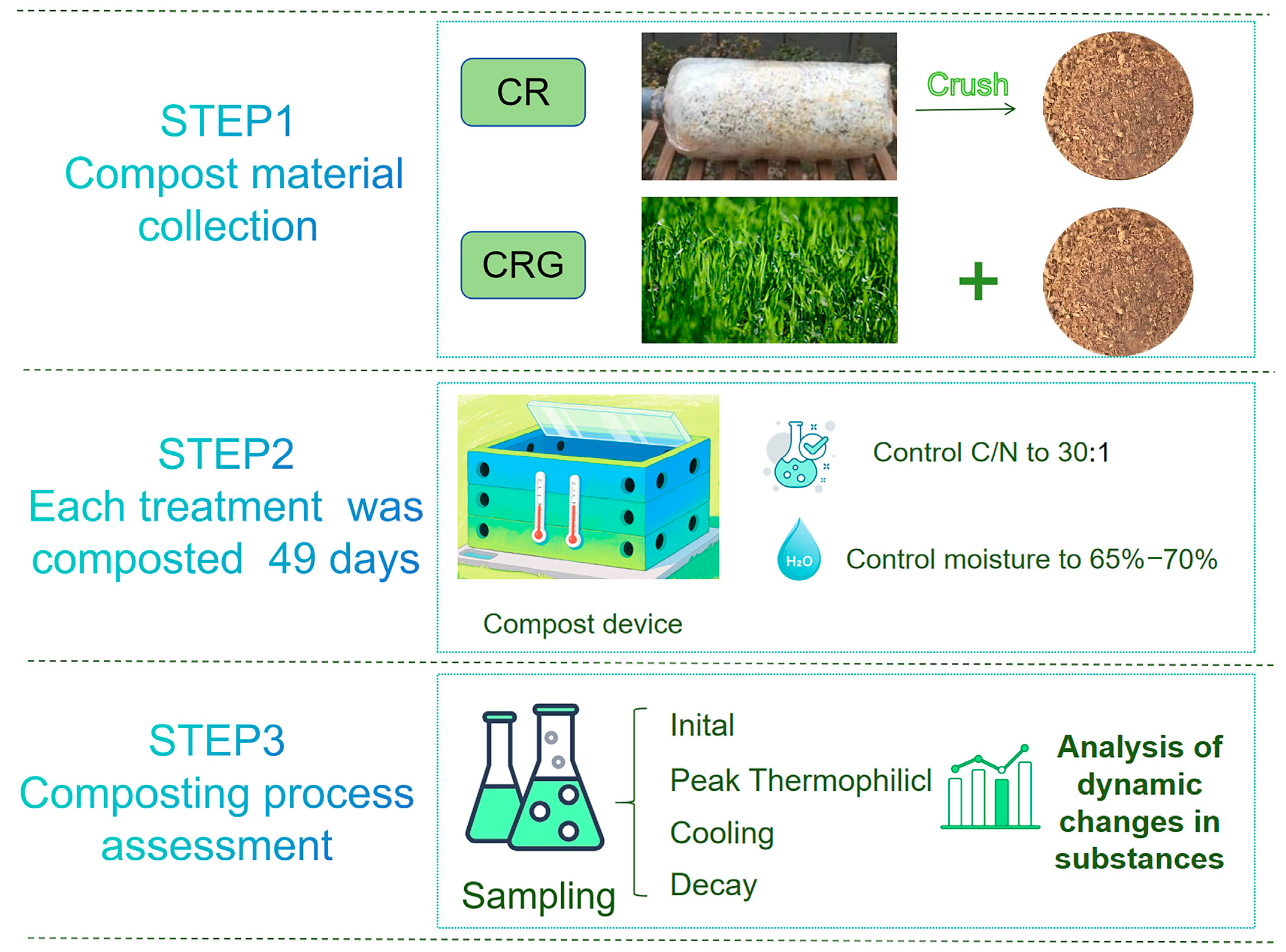
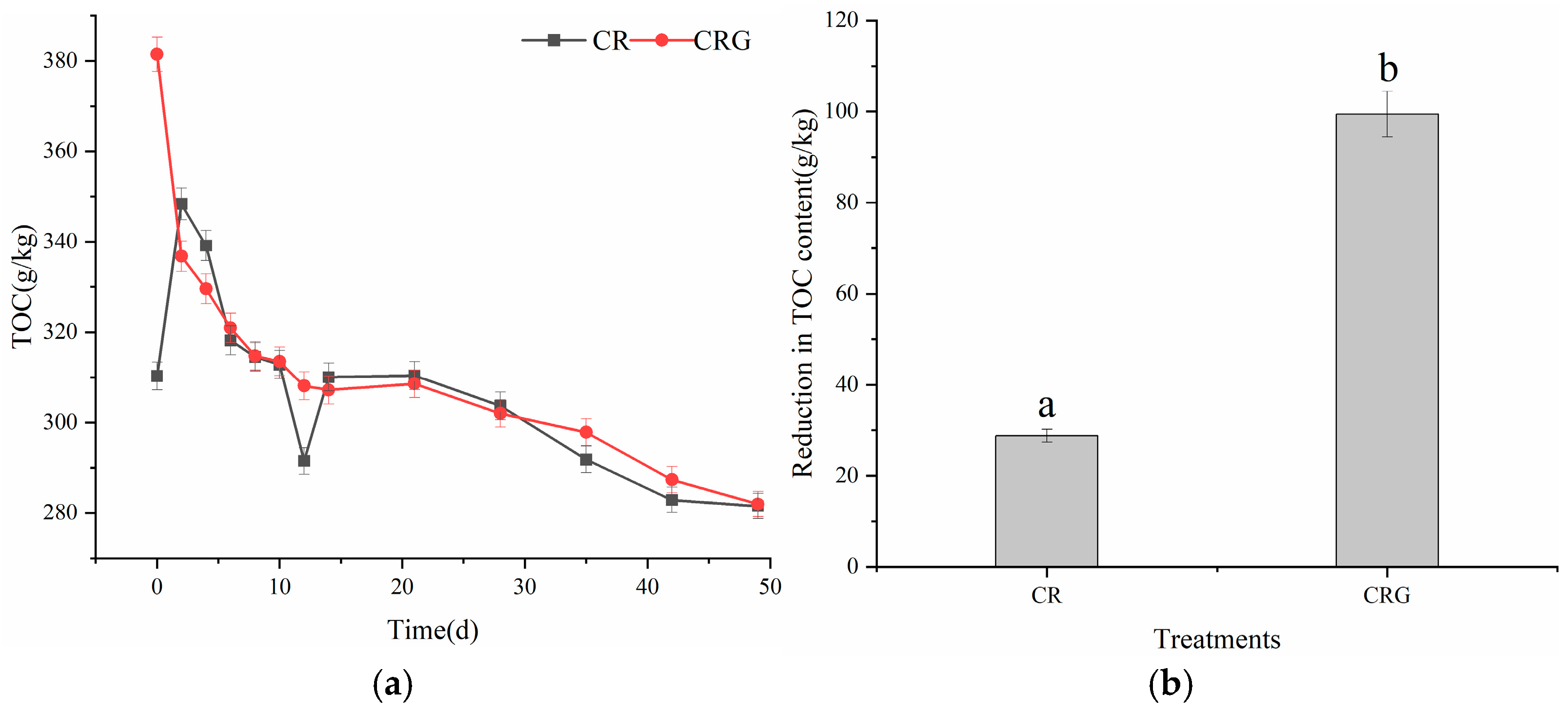
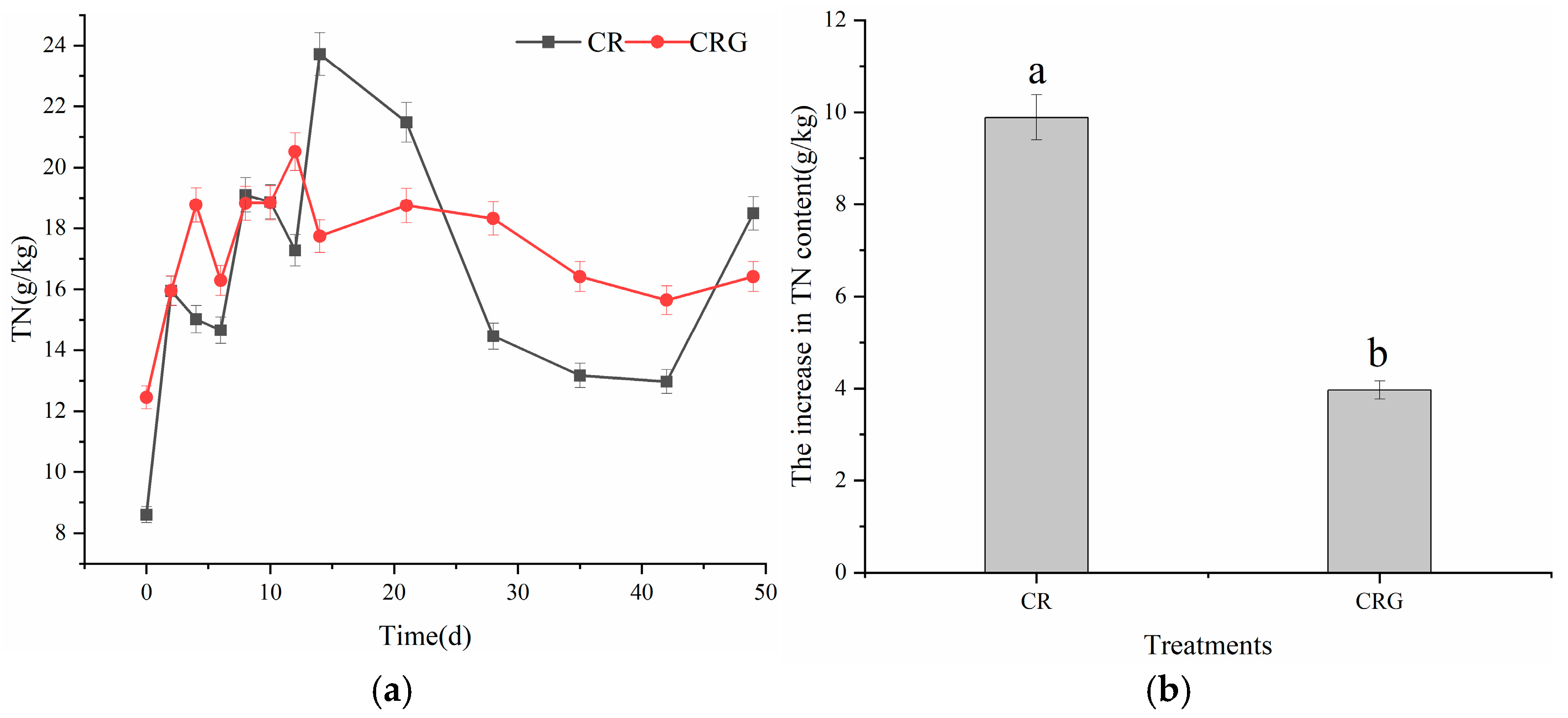
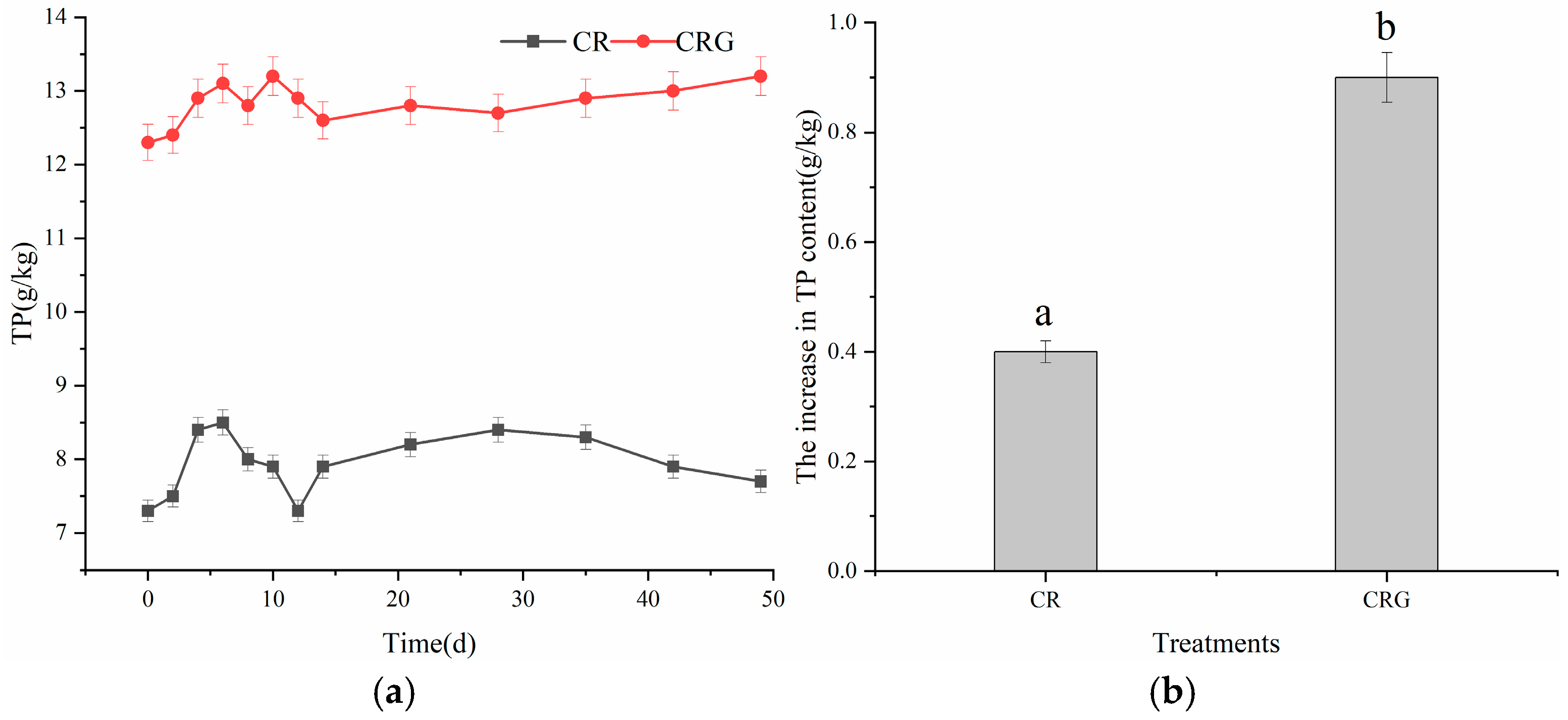


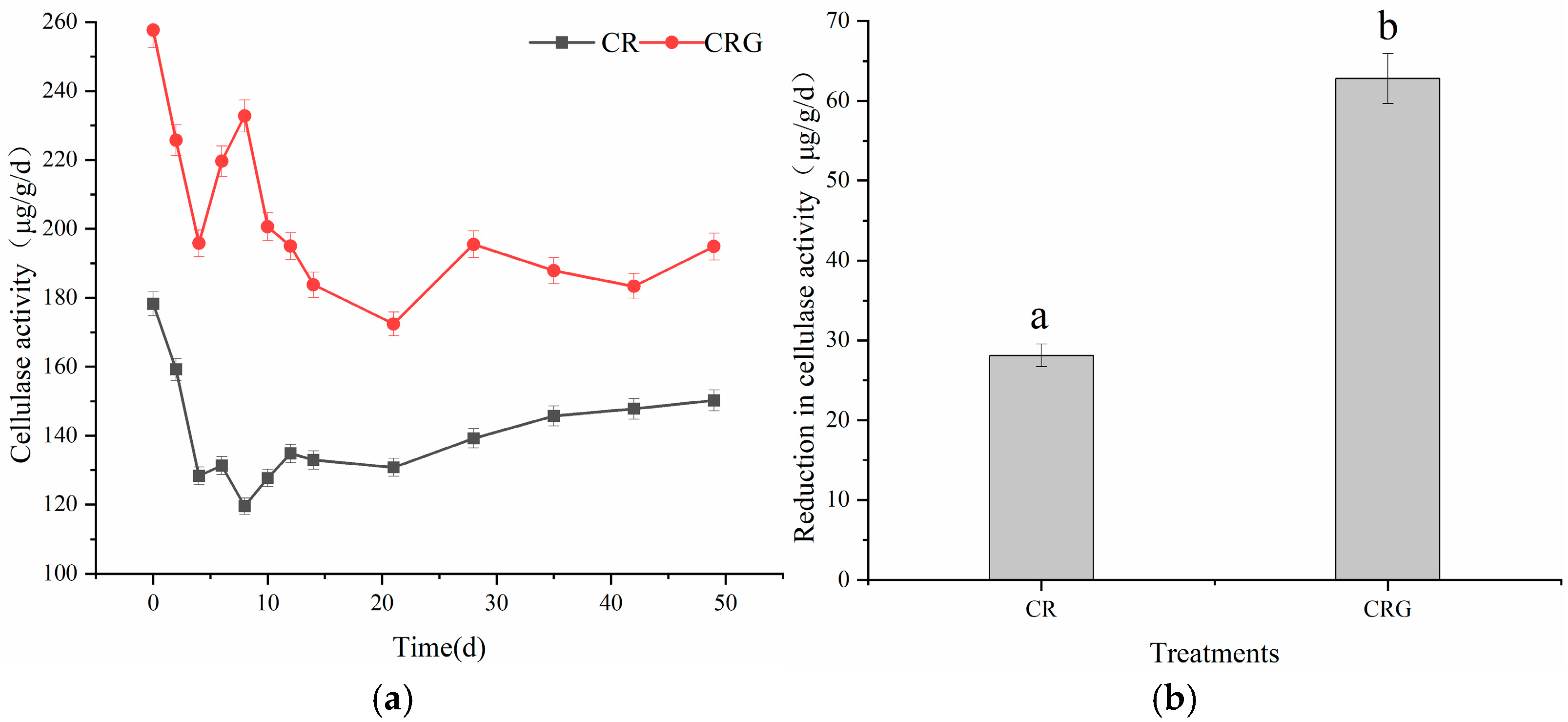



| Treatment | TOC (g/kg) | TN (g/kg) | TP (g/kg) | TK (g/kg) | Urea (g/kg) | Moisture Content (%) | C/N | |||
| CR | 310.29 ± 5.29 a | 8.60 ± 0.20 a | 7.33 ± 0.21 a | 25.52 ± 0.34 a | 3.74 ± 0.04 | 65–70% | 30:1 | |||
| CRG | 381.43 ± 6.43 b | 12.45 ± 0.15 b | 12.31 ± 0.23 b | 15.74 ± 0.23 b | / | 65–70% | 30:1 | |||
| Basic physical and chemical properties of test materials (dry base) | ||||||||||
| Treatment | pH | EC (mS/cm) | Lignin (g/kg) | Cellulose (g/kg) | Hemicellulose (g/kg) | |||||
| CR | 6.52 ± 0.03 a | 4.22 ± 0.12 a | 55.56 ± 5.52 a | 100.29 ± 10.01 a | 65.18 ± 14.82 a | |||||
| CRG | 6.54 ± 0.02 a | 3.80 ± 0.13 a | 40.22 ± 12.08 b | 137.50 ± 12.59 b | 90.24 ± 9.72 b | |||||
| Sample Collection | CR | CRG |
|---|---|---|
| Initial Stage | 0 d (7.2) | 0 d (7.2) |
| Peak Thermophilic Stage | 2, 4, 6, 8 d (7.4, 7.6, 7.8, 7.10) | 2, 4, 6, 9 d (7.4, 7.6, 7.8, 7.11) |
| Cooling Stage | 10, 12, 14, 21, 28, 35, 42 d (7.12, 7.14, 7.16, 7.23, 7.30, 8.6, 8.13) | 10, 12, 14, 21, 28, 35, 42 d (7.12, 7.14, 7.16, 7.23, 7.30, 8.6, 8.13) |
| Decay Stage | 49 d (8.20) | 49 d (8.20) |
| Samples | GI (%) | Pb (mg/kg) | Cd (mg/kg) | Cr (mg/kg) | As (mg/kg) | Hg (mg/kg) |
|---|---|---|---|---|---|---|
| CR | 132 ± 5.2 a | 13.2 ± 0.7 a | 0.7 ± 0.1 a | 34.2 ± 1.2 a | 1.8 ± 0.2 a | 0.2 ± 0.05 a |
| CRG | 118 ± 4.9 b | 17.2 ± 0.6 b | 1.3 ± 0.2 b | 44.2 ± 3.3 b | 3.3 ± 0.2 b | 0.6 ± 0.1 b |
| Sample ID | ACE Index | Chao1 Index | Simpson Index | Shannon Index | Coverage |
|---|---|---|---|---|---|
| CR1 | 657.81 ± 65.78 Aa | 661.00 ± 66.10 Aa | 0.92 ± 0.09 Aa | 5.57 ± 0.56 Aa | 0.9999 |
| CR2 | 797.53 ± 79.75 Ba | 798.50 ± 79.85 Ba | 0.96 ± 0.10 Aa | 6.36 ± 0.64 Ba | 0.9999 |
| CR3 | 951.90 ± 95.19 Ca | 954.00 ± 95.40 Ca | 0.97 ± 0.10 Aa | 7.45 ± 0.75 Ca | 0.9996 |
| CRG1 | 524.89 ± 52.49 Ab | 524.60 ± 52.46 Ab | 0.97 ± 0.10 Aa | 6.06 ± 0.61 Ab | 0.9999 |
| CRG2 | 886.15 ± 88.62 Bb | 887.46 ± 88.75 Bb | 0.99 ± 0.10 Aa | 7.71 ± 0.77 Bb | 0.9997 |
| CRG3 | 1092.06 ± 109.21 Cb | 1093.21 ± 109.32 Cb | 0.97 ± 0.10 Aa | 7.51 ± 0.75 Ba | 0.9997 |
| Sample ID | ACE Index | Chao1 Index | Simpson Index | Shannon Index | Coverage |
|---|---|---|---|---|---|
| CR1 | 314.91 ± 31.49 Aa | 320.33 ± 32.03 Aa | 0.89 ± 0.09 Aa | 4.63 ± 0.46 Aa | 0.9996 |
| CR2 | 276.75 ± 27.68 Ba | 276.25 ± 27.63 Ba | 0.87 ± 0.09 Aa | 3.92 ± 0.39 Ba | 0.9998 |
| CR3 | 187.95 ± 18.80 Ca | 188.50 ± 18.85 Ca | 0.28 ± 0.03 Ba | 1.36 ± 0.14 Ca | 0.9999 |
| CRG1 | 273.58 ± 27.36 Ab | 276.71 ± 27.67 Aa | 0.88 ± 0.09 Aa | 4.27 ± 0.43 Aa | 0.9997 |
| CRG2 | 183.87 ± 18.39 Bb | 189.83 ± 18.98 Bb | 0.68 ± 0.07 Ba | 2.73 ± 0.27 Ba | 0.9998 |
| CRG3 | 233.87 ± 23.39 Ba | 235.00 ± 23.50 Ba | 0.74 ± 0.07 Bb | 3.72 ± 0.37 Ba | 0.9992 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Tian, Y.; Wu, P.; Zheng, J.; Xing, Y.; Qu, Y.; Guo, X.; Zhang, X. Aerobic Composting of Auricularia auricula (L.) Residues: Investigating Nutrient Dynamics and Microbial Interactions with Different Substrate Compositions. Diversity 2025, 17, 279. https://doi.org/10.3390/d17040279
Liu Q, Tian Y, Wu P, Zheng J, Xing Y, Qu Y, Guo X, Zhang X. Aerobic Composting of Auricularia auricula (L.) Residues: Investigating Nutrient Dynamics and Microbial Interactions with Different Substrate Compositions. Diversity. 2025; 17(4):279. https://doi.org/10.3390/d17040279
Chicago/Turabian StyleLiu, Qian, Yuxin Tian, Pengbing Wu, Junyan Zheng, Yuhe Xing, Ying Qu, Xingchi Guo, and Xu Zhang. 2025. "Aerobic Composting of Auricularia auricula (L.) Residues: Investigating Nutrient Dynamics and Microbial Interactions with Different Substrate Compositions" Diversity 17, no. 4: 279. https://doi.org/10.3390/d17040279
APA StyleLiu, Q., Tian, Y., Wu, P., Zheng, J., Xing, Y., Qu, Y., Guo, X., & Zhang, X. (2025). Aerobic Composting of Auricularia auricula (L.) Residues: Investigating Nutrient Dynamics and Microbial Interactions with Different Substrate Compositions. Diversity, 17(4), 279. https://doi.org/10.3390/d17040279






