Habitat and Predator Influences on the Spatial Ecology of Nine-Banded Armadillos
Abstract
1. Introduction
2. Materials and Methods
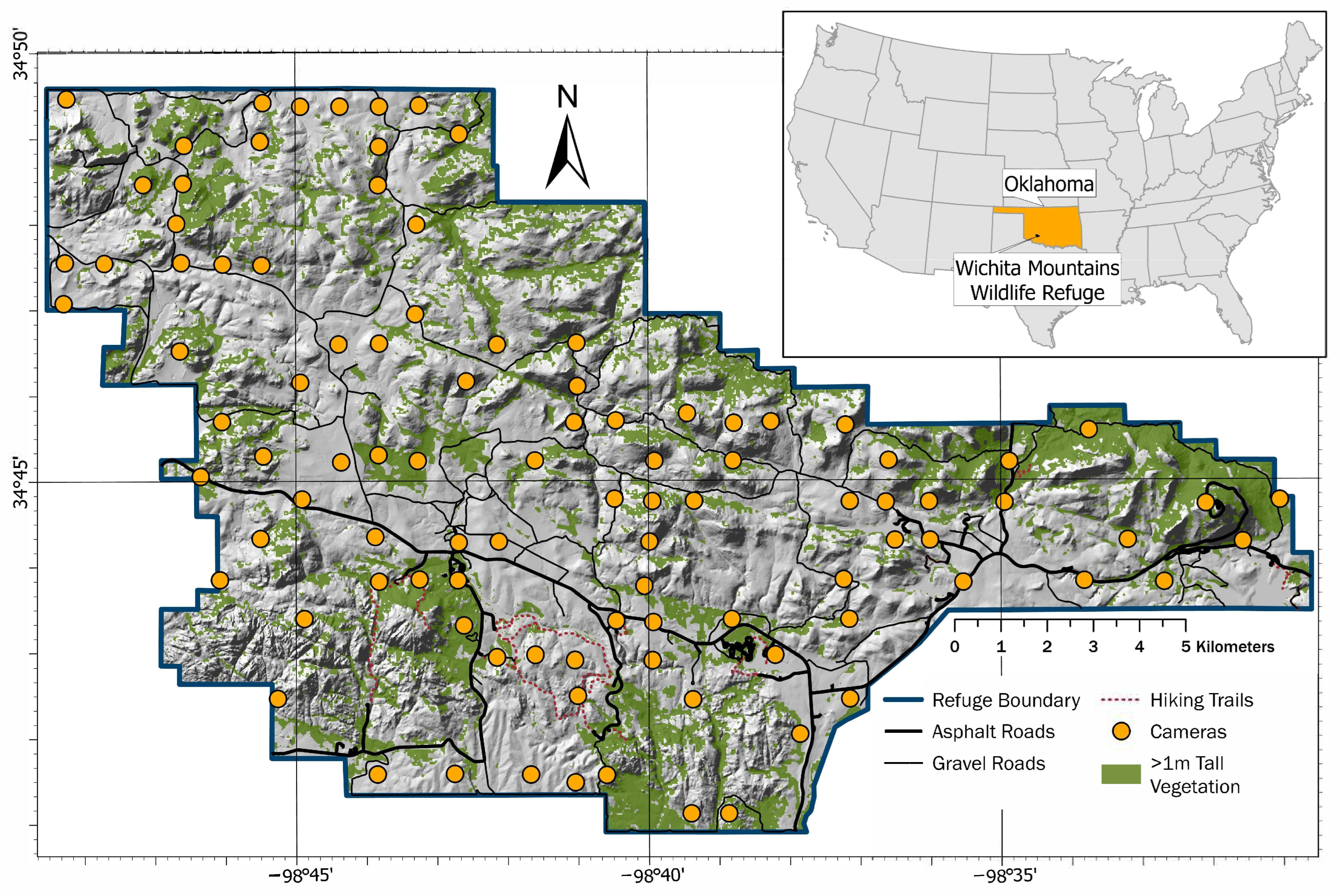
2.1. Study Area
2.2. Field Surveys and Image Processing
2.3. Detection and Occupancy Covariates
2.4. Occupancy Analyses
2.5. Diel Activity Analyses
3. Results
3.1. Data Collection
3.2. Environmental Characteristics and Occupancy Patterns
3.3. Diel Activity Patterns
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crooks, K.R.; Soulé, M.E. Mesopredator Release and Avifaunal Extinctions in a Fragmented System. Nature 1999, 400, 563–566. [Google Scholar] [CrossRef]
- Ritchie, E.G.; Johnson, C.N. Predator Interactions, Mesopredator Release and Biodiversity Conservation. Ecol. Lett. 2009, 12, 982–998. [Google Scholar] [CrossRef] [PubMed]
- Vance-Chalcraft, H.D.; Rosenheim, J.A.; Vonesh, J.R.; Osenberg, C.W.; Sih, A. The Influence of Intraguild Predation on Prey Suppression and Prey Release: A Meta-Analysis. Ecology 2007, 88, 2689–2696. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.E.; Feit, A.; Gru, J.; Letnic, M. Mesopredator Suppression by an Apex Predator Alleviates the Risk of Predation Perceived by Small Prey. Proc. R. Soc. B 2015, 282, 20142870. [Google Scholar] [CrossRef]
- Palomares, F.; Caro, T.M. Interspecific Killing among Mammalian Carnivores. Am. Nat. 1999, 153, 492–508. [Google Scholar] [CrossRef]
- Lourenço, R.; Penteriani, V.; Rabaça, J.E.; Korpimäki, E. Lethal Interactions among Vertebrate Top Predators: A Review of Concepts, Assumptions and Terminology. Biol. Rev. 2014, 89, 270–283. [Google Scholar] [CrossRef]
- Newman, C.; Buesching, C.D.; Wolff, J.O. The Function of Facial Masks in “Midguild” Carnivores. Oikos 2005, 108, 623–633. [Google Scholar] [CrossRef]
- Stankowich, T.; Campbell, L.A. Living in the Danger Zone: Exposure to Predators and the Evolution of Spines and Body Armor in Mammals. Evolution 2016, 70, 1501–1511. [Google Scholar] [CrossRef]
- Walker, H.; Caro, T.; Bell, D.; Ferguson, A.; Stankowich, T. Predation Risk Drives Aposematic Signal Conformity. Evolution 2023, 77, 2492–2503. [Google Scholar] [CrossRef]
- Fay, C.; Young, J.K.; Stankowich, T. Aposematic Learning in a Mammalian Predator–Prey System. Anim. Behav. 2024, 212, 39–48. [Google Scholar] [CrossRef]
- Peplinski, J.; Malone, M.A.; Fowler, K.J.; Potratz, E.J.; Pergams, A.G.; Charmoy, K.L.; Rasheed, K.; Avdieiev, S.S.; Whelan, C.J.; Brown, J.S. Ecology of Fear: Spines, Armor and Noxious Chemicals Deter Predators in Cancer and in Nature. Front. Ecol. Evol. 2021, 9, 682504. [Google Scholar] [CrossRef]
- Hunter, J.S. Familiarity Breeds Contempt: Effects of Striped Skunk Color, Shape, and Abundance on Wild Carnivore Behavior. Behav. Ecol. 2009, 20, 1315–1322. [Google Scholar] [CrossRef]
- Prange, S.; Gehrt, S.D. Response of Skunks to a Simulated Increase in Coyote Activity. J. Mammal. 2007, 88, 1040–1049. [Google Scholar] [CrossRef]
- Wang, Y.; Allen, M.L.; Wilmers, C.C. Mesopredator Spatial and Temporal Responses to Large Predators and Human Development in the Santa Cruz Mountains of California. Biol. Conserv. 2015, 190, 23–33. [Google Scholar] [CrossRef]
- McBee, K.; Baker, R.J. Dasypus novemcinctus . Mamm. Species 1982, 162, 1–9. [Google Scholar] [CrossRef]
- Ober, H.K.; Degroote, L.W.; McDonough, C.M.; Mizell, R.F.; Mankin, R.W. Identification of an Attractant for the Nine-Banded Armadillo, Dasypus novemcinctus. Wildl. Soc. Bull. 2011, 35, 421–429. [Google Scholar] [CrossRef]
- Sawyer, C.F.; Brinkman, D.C.; Walker, V.D.; Covington, T.D.; Stienstraw, E.A. The Zoogeomorphic Characteristics of Burrows and Burrowing by Nine-Banded Armadillos (Dasypus novemcinctus). Geomorphology 2012, 157–158, 122–130. [Google Scholar] [CrossRef]
- DeGregorio, B.A.; Veon, J.T.; Massey, A. Wildlife Associates of Nine-Banded Armadillo (Dasypus novemcinctus) Burrows in Arkansas. Ecol. Evol. 2022, 12, e8858. [Google Scholar] [CrossRef]
- Taulman, J.F.; Robbins, L.W. Recent Range Expansion and Distributional Limits of the Nine-Banded Armadillo (Dasypus novemcinctus) in the United States. J. Biogeogr. 1996, 23, 635–648. [Google Scholar] [CrossRef]
- Taulman, J.F.; Robbins, L.W. Range Expansion and Distributional Limits of the Nine-Banded Armadillo in the United States: An Update of Taulman & Robbins (1996). J. Biogeogr. 2014, 41, 1626–1630. [Google Scholar] [CrossRef]
- Davidson, A.D.; Detling, J.K.; Brown, J.H. Ecological Roles and Conservation Challenges of Social, Burrowing, Herbivorous Mammals in the World’s Grasslands. Front. Ecol. Environ. 2012, 10, 477–486. [Google Scholar] [CrossRef]
- Lamb, B.D.; Anderson, C.D.; McDonough, C.M.; Lockhart, J.M.; Butler, Z.P. A Comparison of Vertebrate Associates of Gopher Tortoise and Nine-Banded Armadillo Burrows in South Georgia. Chelonian Conserv. Biol. 2023, 22, 184–196. [Google Scholar] [CrossRef]
- Feng, X.; Papeş, M. Ecological Niche Modelling Confirms Potential North-East Range Expansion of the Nine-Banded Armadillo (Dasypus novemcinctus) in the USA. J. Biogeogr. 2015, 42, 803–807. [Google Scholar] [CrossRef]
- Gompper, M.E. Top Carnivores in the Suburbs? Ecological and Conservation Issues Raised by Colonization of North-eastern North America by Coyotes. Bioscience 2002, 52, 185. [Google Scholar] [CrossRef]
- Best, T.L.; Hoditschek, B.; Thomas, H.H. Foods of Coyotes (Canis latrans) in Oklahoma. Southwest. Nat. 1981, 26, 67–69. [Google Scholar] [CrossRef]
- Cherry, M.J.; Turner, K.L.; Howze, M.B.; Cohen, B.S.; Conner, L.M.; Warren, R.J. Coyote Diets in a Longleaf Pine Ecosystem. Wildl. Biol. 2016, 22, 64–70. [Google Scholar] [CrossRef]
- Watine, L.N.; Giuliano, W.M. Factors Determining Coyote (Canis latrans) Diets. Open J. Ecol. 2017, 7, 650–666. [Google Scholar] [CrossRef]
- McDonough, C.M.; Loughry, W.J. Patterns of Mortality in a Population of Nine-Banded Armadillos, Dasypus novemcinctus. Am. Midl. Nat. 1997, 138, 299–305. [Google Scholar] [CrossRef]
- Rooney, B.; Kays, R.; Cove, M.V.; Jensen, A.; Goldstein, B.R.; Pate, C.; Castiblanco, P.; Abell, M.E.; Adley, J.; Agenbroad, B.; et al. Snapshot USA 2019–2023: The First Five Years of Data from a Coordinated Camera Trap Survey of the United States. Glob. Ecol. Biogeogr. 2025, 34, e13941. [Google Scholar] [CrossRef]
- MacKenzie, D.I.; Nichols, J.D.; Lachman, G.B.; Droege, S.; Royle, J.A.; Langtimm, C.A. Estimating Site Occupancy Rates When Detection Probabilities Are Less than One. Ecology 2002, 83, 2248–2255. [Google Scholar] [CrossRef]
- Richmond, O.M.W.; Hines, J.E.; Beissinger, S.R. Two-Species Occupancy Models: A New Parameterization Applied to Co-Occurrence of Secretive Rails. Ecol. Appl. 2010, 20, 2036–2046. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, D.I.; Nichols, J.D.; Royle, J.A.; Pollock, K.H.; Bailey, L.L.; Hines, J.E. Occupancy Estimation and Modeling: Inferring Patterns and Dynamics of Species Occurrence, 2nd ed.; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Pasa, J.B.; Arrais, R.C.; Massara, R.L.; Pereira, G.; de Azevedo, F.C.C. Factors Influencing the Habitat Use by Ocelots in One of the Last Large Atlantic Forest Remnants in Southeastern Brazil. Ecol. Evol. 2021, 11, 4631–4643. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.R.V.; Bailey, L.L.; Smart, A.H. Patch Utilization and Flower Visitations by Wild Bees in a Honey Bee-Dominated, Grassland Landscape. Ecol. Evol. 2021, 11, 14888–14904. [Google Scholar] [CrossRef] [PubMed]
- Broadway, M.S.; Todaro, H.M.; Koeck, M.M.; Dotterweich, C.N.; Cain, S.A.; Buehler, L.; Chitwood, M.C.; Lonsinger, R.C. Interspecific Effects of Invasive Wild Pigs (Sus scrofa) on Native Nine-banded Armadillos (Dasypus novemcinctus). J. Mammal. 2025, in press.
- Costa, R.T.; Fornitano, L.; de Cassia Bianchi, R. Nine-banded Armadillos Temporally Avoid Sites Visited by Domestic Dogs and Native Carnivores. Wildl. Res. 2024, 51, WR23047. [Google Scholar] [CrossRef]
- DeGregorio, B.A.; Gale, C.; Lassiter, E.V.; Massey, A.; Roberts, C.P.; T. Veon, J. Nine-Banded Armadillo (Dasypus novemcinctus) Activity Patterns Are Influenced by Human Activity. Ecol. Evol. 2021, 11, 15874–15881. [Google Scholar] [CrossRef]
- Saldo, E.A.; Jensen, A.J.; Muthersbaugh, M.S.; Butfiloski, J.W.; Cantrell, J.; Kilgo, J.C.; Ruth, C.; Yarrow, G.K.; Jachowski, D.S. Spatiotemporal Overlap with Invasive Wild Pigs (Sus scrofa) Varies by Species and Season in a Temperate Ecosystem. Ecosphere 2023, 14, e4500. [Google Scholar] [CrossRef]
- Wichita Mountains Wildlife Refuge [WMWR]. Wichita Mountains Wildlife Refuge Comprehensive Conservation Plan and Environmental Assessment; U.S. Fish and Wildlife Service: Idahome, OK, USA, 2013. [Google Scholar]
- National Oceanic and Atmospheric Administration [NOAA]. Climate Data Online. 2023. Available online: https://water.weather.gov/precip/download.php (accessed on 13 November 2023).
- Murley, B.P. Feral Swine Space Use and Effects on Ecological Communities in the Wichita Mountains Wildlife Refuge. Master’s Thesis, Oklahoma State University, Stillwater, OK, USA, 2024. [Google Scholar]
- Loughry, W.J.; McDonough, C.M. Spatial Patterns in a Population of Nine-Banded Armadillos (Dasypus novemcinctus). Am. Midl. Nat. 1998, 140, 161–169. [Google Scholar] [CrossRef]
- Gese, E.M.; Rongstad, O.J.; Mytton, W.R. Home Range and Habitat Use of Coyotes in Southeastern Colorado. J. Wildl. Manage. 1988, 52, 640–646. [Google Scholar] [CrossRef]
- Nelson, J.L.; Cypher, B.L.; Bjurlin, C.D.; Creel, S. Effects of Habitat on Competition between Kit Foxes and Coyotes. J. Wildl. Manage. 2007, 71, 1467–1475. [Google Scholar] [CrossRef]
- Rota, C.T.; Fletcher, R.J.; Dorazio, R.M.; Betts, M.G. Occupancy Estimation and the Closure Assumption. J. Appl. Ecol. 2009, 46, 1173–1181. [Google Scholar] [CrossRef]
- Gould, M.J.; Gould, W.R.; Cain, J.W.; Roemer, G.W. Validating the Performance of Occupancy Models for Estimating Habitat Use and Predicting the Distribution of Highly-Mobile Species: A Case Study Using the American Black Bear. Biol. Conserv. 2019, 234, 28–36. [Google Scholar] [CrossRef]
- Cove, M.V.; Kays, R.; Bontrager, H.; Bresnan, C.; Lasky, M.; Frerichs, T.; Klann, R.; Lee, T.E.; Crockett, S.C.; Crupi, A.P.; et al. Snapshot USA 2019: A Coordinated National Camera Trap Survey of the United States. Ecology 2021, 102, e03353. [Google Scholar] [CrossRef]
- Werdel, T.J.; Piper, C.W.; Ricketts, A.M.; Peek, M.S.; Ahlers, A.A. Scale-Specific Landscape Effects Impose Range-Limiting Constraints on the Distribution of a Prairie-Obligate Carnivore. Landsc. Ecol. 2022, 37, 2065–2079. [Google Scholar] [CrossRef]
- Greenberg, S.; Godin, T.; Whittington, J. Design Patterns for Wildlife-Related Camera Trap Image Analysis. Ecol. Evol. 2019, 9, 13706–13730. [Google Scholar] [CrossRef]
- Lonsinger, R.C.; Dart, M.M.; Larsen, R.T.; Knight, R.N. Efficacy of Machine Learning Image Classification for Automated Occupancy-Based Monitoring. Remote Sens. Ecol. Conserv. 2024, 10, 56–71. [Google Scholar] [CrossRef]
- Iannarilli, F.; Erb, J.; Arnold, T.W.; Fieberg, J.R. Evaluating Species-Specific Responses to Camera-Trap Survey Designs. Wildl. Biol. 2021, 2021, 1–12. [Google Scholar] [CrossRef]
- Goldstein, B.R.; Jensen, A.J.; Kays, R.; Cove, M.V.; McShea, W.J.; Rooney, B.; Kierepka, E.M.; Pacifici, K. Guidelines for Estimating Occupancy from Autocorrelated Camera Trap Detections. Methods Ecol. Evol. 2024, 15, 1177–1191. [Google Scholar] [CrossRef]
- Johansen, K. Temperature Regulation in the Nine-Banded Armadillo (Dasypus novemcinctus mexicanus). Physiol. Zool. 1961, 34, 126–144. [Google Scholar] [CrossRef]
- McNab, B.K. Energetics and the Limits to a Temperate Distribution in Armadillos. J. Mammal. 1980, 61, 606–627. [Google Scholar] [CrossRef]
- McDonough, C.M.; Loughry, W.J. Influences on Activity Patterns in a Population of Nine-Banded Armadillos. J. Mammal. 1997, 78, 932–941. [Google Scholar] [CrossRef]
- Dart, M.M.; Perkins, L.B.; Jenks, J.A.; Hatfield, G.; Lonsinger, R.C. The Effect of Scent Lures on Detection Is Not Equitable among Sympatric Species. Wildl. Res. 2023, 50, 190–200. [Google Scholar] [CrossRef]
- Lesmeister, D.B.; Nielsen, C.K.; Schauber, E.M.; Hellgren, E.C. Spatial and Temporal Structure of a Mesocarnivore Guild in Midwestern North America. Wildl. Monogr. 2015, 191, 1–61. [Google Scholar] [CrossRef]
- Madsen, A.E.; Corral, L.; Fontaine, J.J. Weather and Exposure Period Affect Coyote Detection at Camera Traps. Wildl. Soc. Bull. 2020, 44, 342–350. [Google Scholar] [CrossRef]
- Oikolab. Weather and Climate Data for Analysts. 2023. Available online: https://oikolab.com (accessed on 13 November 2023).
- Sollmann, R. Mt or Not Mt: Temporal Variation in Detection Probability in Spatial Capture-Recapture and Occupancy Models. Peer Community J. 2024, 4, e1. [Google Scholar] [CrossRef]
- Bender, L.C.; Rosas-Rosas, O.C.; Weisenberger, M.E. Seasonal Occupancy of Sympatric Larger Carnivores in the Southern San Andres Mountains, South-Central New Mexico, USA. Mammal Res. 2017, 62, 323–329. [Google Scholar] [CrossRef]
- LANDFIRE. LANDFIRE 2.2.0. U.S. Department of the Interior, Geological Survey, and U.S. Department of Agriculture. 2023. Available online: http://www.landfire.gov (accessed on 13 November 2023).
- Golightly, R.T.; Ohmart, R.D. Water Economy of Two Desert Canids: Coyote and Kit Fox. J. Mammal. 1984, 65, 51–58. [Google Scholar] [CrossRef]
- Rodrigues, T.F.; Chiarello, A.G. Native Forests within and Outside Protected Areas Are Key for Nine-Banded Armadillo (Dasypus novemcinctus) Occupancy in Agricultural Landscapes. Agric. Ecosyst. Environ. 2018, 266, 133–141. [Google Scholar] [CrossRef]
- Lombardi, J.V.; Comer, C.E.; Scognamillo, D.G.; Conway, W.C. Coyote, Fox, and Bobcat Response to Anthropogenic and Natural Landscape Features in a Small Urban Area. Urban Ecosyst. 2017, 20, 1239–1248. [Google Scholar] [CrossRef]
- Atwood, T.C.; Fry, T.L.; Leland, B.R. Partitioning of Anthropogenic Watering Sites by Desert Carnivores. J. Wildl. Manage. 2011, 75, 1609–1615. [Google Scholar] [CrossRef]
- DeGregorio, B.A.; McElroy, M.R.; Johansson, E.P. Occupancy and Activity Patterns of Nine-Banded Armadillos (Dasypus novemcinctus) in a Suburban Environment. Diversity 2023, 15, 907. [Google Scholar] [CrossRef]
- Zimmerman, J.W. Burrow Characteristics of the Nine-Banded Armadillo, Dasypus novemcinctus. Southwest. Nat. 1990, 35, 226–227. [Google Scholar] [CrossRef]
- Koehler, G.M.; Hornocker, M.G. Seasonal Resource Use among Mountain Lions, Bobcats, and Coyotes. J. Mammal. 1991, 72, 391–396. [Google Scholar] [CrossRef]
- Cherry, M.J.; Howell, P.E.; Seagraves, C.D.; Warren, R.J.; Conner, L.M. Effects of Land Cover on Coyote Abundance. Wildl. Res. 2016, 43, 662–670. [Google Scholar] [CrossRef]
- Lonsinger, R.C.; Gese, E.M.; Bailey, L.L.; Waits, L.P. The Roles of Habitat and Intraguild Predation by Coyotes on the Spatial Dynamics of Kit Foxes. Ecosphere 2017, 8, e01749. [Google Scholar] [CrossRef]
- Jorge, M.H.; Garrison, E.P.; Conner, L.M.; Cherry, M.J. Fire and Land Cover Drive Predator Abundances in a Pyric Landscape. For. Ecol. Manage. 2020, 461, 117939. [Google Scholar] [CrossRef]
- Stevenson, E.R.; Lashley, M.A.; Chitwood, M.C.; Garabedian, J.E.; Swingen, M.B.; Deperno, C.S.; Moorman, C.E. Resource Selection by Coyotes (Canis latrans) in a Longleaf Pine (Pinus palustris) Ecosystem: Effects of Anthropogenic Fires and Landscape Features. Can. J. Zool. 2019, 97, 165–171. [Google Scholar] [CrossRef]
- Burton, A.C.; Beirne, C.; Gaynor, K.M.; Sun, C.; Granados, A.; Allen, M.L.; Alston, J.M.; Alvarenga, G.C.; Álvarez Calderón, F.S.; Amir, Z.; et al. Mammal Responses to Global Changes in Human Activity Vary by Trophic Group and Landscape. Nat. Ecol. Evol. 2024, 8, 924–935. [Google Scholar] [CrossRef]
- Trovati, R.G. Differentiation and Characterization of Burrows of Two Species of Armadillos in the Brazilian Cerrado. Rev. Chil. Hist. Nat. 2015, 88, 19. [Google Scholar] [CrossRef]
- Haywood, C.J.; Nielsen, C.K.; Jiménez, F.A. Potential Distribution of Colonizing Nine-Banded Armadillos at Their Northern Range Edge. Diversity 2021, 13, 266. [Google Scholar] [CrossRef]
- Hinton, J.W.; Van Manen, F.T.; Chamberlain, M.J. Space Use and Habitat Selection by Resident and Transient Coyotes (Canis latrans). PLoS ONE 2015, 10, e0132203. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, J.V.; Mackenzie, D.I.; Tewes, M.E.; Perotto-Baldivieso, H.L.; Mata, J.M.; Campbell, T.A. Co-Occurrence of Bobcats, Coyotes, and Ocelots in Texas. Ecol. Evol. 2020, 10, 4903–4917. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A Review of Methods to Deal with It and a Simulation Study Evaluating Their Performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Multimodel Inference: Understanding AIC and BIC in Model Selection. Sociol. Methods Res. 2004, 33, 261–304. [Google Scholar] [CrossRef]
- Doherty, P.F.; White, G.C.; Burnham, K.P. Comparison of Model Building and Selection Strategies. J. Ornithol. 2012, 152, 317–323. [Google Scholar] [CrossRef]
- Arnold, T.W. Uninformative Parameters and Model Selection Using Akaike’s Information Criterion. J. Wildl. Manage. 2010, 74, 1175–1178. [Google Scholar] [CrossRef]
- Sutherland, C.; Hare, D.; Johnson, P.J.; Linden, D.W.; Montgomery, R.A.; Droge, E. Practical Advice on Variable Selection and Reporting Using Akaike Information Criterion. Proc. R. Soc. B Biol. Sci. 2023, 290. [Google Scholar] [CrossRef]
- Fiske, I.; Chandler, R. Overview of Unmarked: An R Package for the Analysis of Data From Unmarked Animals. 2015. Available online: https://cran.r-project.org/web/packages/unmarked/index.html (accessed on 1 October 2024).
- Steenweg, R.; Whittington, J.; Hebblewhite, M.; Forshner, A.; Johnston, B.; Petersen, D.; Shepherd, B.; Lukacs, P.M. Camera-Based Occupancy Monitoring at Large Scales: Power to Detect Trends in Grizzly Bears across the Canadian Rockies. Biol. Conserv. 2016, 201, 192–200. [Google Scholar] [CrossRef]
- Lonsinger, R.C.; Knight, R.N.; Waits, L.P. Detection Criteria and Post-Field Sample Processing Influence Results and Cost Efficiency of Occupancy-Based Monitoring. Ecol. Appl. 2021, 31, e02404. [Google Scholar] [CrossRef]
- White, G.C.; Burnham, K.P. Program MARK: Survival Estimation from Populations of Marked Animals. Bird Study 1999, 46, S120–S139. [Google Scholar] [CrossRef]
- Lashley, M.A.; Cove, M.V.; Chitwood, M.C.; Penido, G.; Gardner, B.; DePerno, C.S.; Moorman, C.E. Estimating Wildlife Activity Curves: Comparison of Methods and Sample Size. Sci. Rep. 2018, 8, 4173. [Google Scholar] [CrossRef] [PubMed]
- Ridout, M.S.; Linkie, M. Estimating Overlap of Daily Activity Patterns from Camera Trap Data. J. Agric. Biol. Environ. Stat. 2009, 14, 322–337. [Google Scholar] [CrossRef]
- Rowcliffe, J.M.; Kays, R.; Kranstauber, B.; Carbone, C.; Jansen, P.A. Quantifying Levels of Animal Activity Using Camera Trap Data. Methods Ecol. Evol. 2014, 5, 1170–1179. [Google Scholar] [CrossRef]
- Niedballa, J.; Sollmann, R.; Courtiol, A.; Wilting, A. camtrapR: An R Package for Efficient Camera Trap Data Management. Methods Ecol. Evol. 2016, 7, 1457–1462. [Google Scholar] [CrossRef]
- Meredith, M.; Ridout, M.S. Overview of the Overlap Package. Central R Archive Network Online. 2014. Available online: https://kar.kent.ac.uk/id/eprint/41474 (accessed on 5 December 2024).
- Agostinelli, C.; Lund, U. R Package ‘Circular’. Circular Statistics (Version 0.4-7). 2013. Available online: https://cran.r-project.org/web/packages/circular/index.html (accessed on 5 December 2024).
- Stankowich, T. Armed and Dangerous: Predicting the Presence and Function of Defensive Weaponry in Mammals. Adapt. Behav. 2012, 20, 32–43. [Google Scholar] [CrossRef]
- Stankowich, T.; Romero, A.N. The Correlated Evolution of Antipredator Defences and Brain Size in Mammals. Proc. R. Soc. B Biol. Sci. 2017, 284, 20161857. [Google Scholar] [CrossRef]
- Haswell, P.M.; Jones, K.A.; Kusak, J.; Hayward, M.W. Fear, Foraging and Olfaction: How Mesopredators Avoid Costly Interactions with Apex Predators. Oecologia 2018, 187, 573–583. [Google Scholar] [CrossRef]
- Litvaitis, J.A.; Shaw, J.H. Coyote Movements, Habitat Use, and Food Habits in Southwestern Oklahoma. J. Wildl. Manage. 1980, 44, 62–68. [Google Scholar] [CrossRef]
- McNab, B.K. The Influence of Food Habits on the Energetics of Eutherian Mammals. Ecol. Monogr. 1986, 56, 1–19. [Google Scholar] [CrossRef]
- Sulc, R.M.; Tracy, B.F. Integrated Crop-Livestock Systems in the U.S. Corn Belt. Agron. J. 2007, 99, 335–345. [Google Scholar] [CrossRef]
- Wehr, N.H.; Litton, C.M.; Lincoln, N.K.; Hess, S.C. Relationships between Soil Macroinvertebrates and Nonnative Feral Pigs (Sus scrofa) in Hawaiian Tropical Montane Wet Forests. Biol. Invasions 2020, 22, 577–586. [Google Scholar] [CrossRef]
- Engle, D.M.; Fuhlendorf, S.D.; Roper, A.; Leslie, D.M. Invertebrate Community Response to a Shifting Mosaic of Habitat. Rangel. Ecol. Manag. 2008, 61, 55–62. [Google Scholar] [CrossRef]
- Litt, A.R.; Steidl, R.J. Interactive Effects of Fire and Nonnative Plants on Small Mammals in Grasslands. Wildl. Monogr. 2011, 176, 1–31. [Google Scholar] [CrossRef]
- Butler, A.; Davis, C.A.; Fuhlendorf, S.D.; Wilder, S.M. Effects of Fire on Ground-Dwelling Arthropods in a Shrub-Dominated Grassland. Ecol. Evol. 2021, 11, 427–442. [Google Scholar] [CrossRef]
- Royle, J.A.; Nichols, J.D. Estimating Abundance from Repeated Presence–Absence Data or Point Counts. Ecology 2003, 84, 777–790. [Google Scholar] [CrossRef]
- Staller, E.L.; Palmer, W.E.; Carroll, J.P.; Thornton, R.P.; Sisson, D.C. Identifying Predators at Northern Bobwhite Nests. J. Wildl. Manage. 2005, 69, 124–132. [Google Scholar] [CrossRef]
- Sikes, R.S.; Animal Care and Use Committee of the American Society of Mammalogists. 2016 Guidelines of the American Society of Mammalogists for the Use of Wild Mammals in Research and Education. J. Mammal. 2016, 97, 663–688. [Google Scholar] [CrossRef]
- Lonsinger, R.C.; Murley, B.P. Nine-Banded Armadillo and Coyote Detection Data and Site-Specific Data from the Wichita Mountains Wildlife Refuge During Summer 2023; U.S. Geological Survey: Reston, VA, USA, 2025. [Google Scholar] [CrossRef]

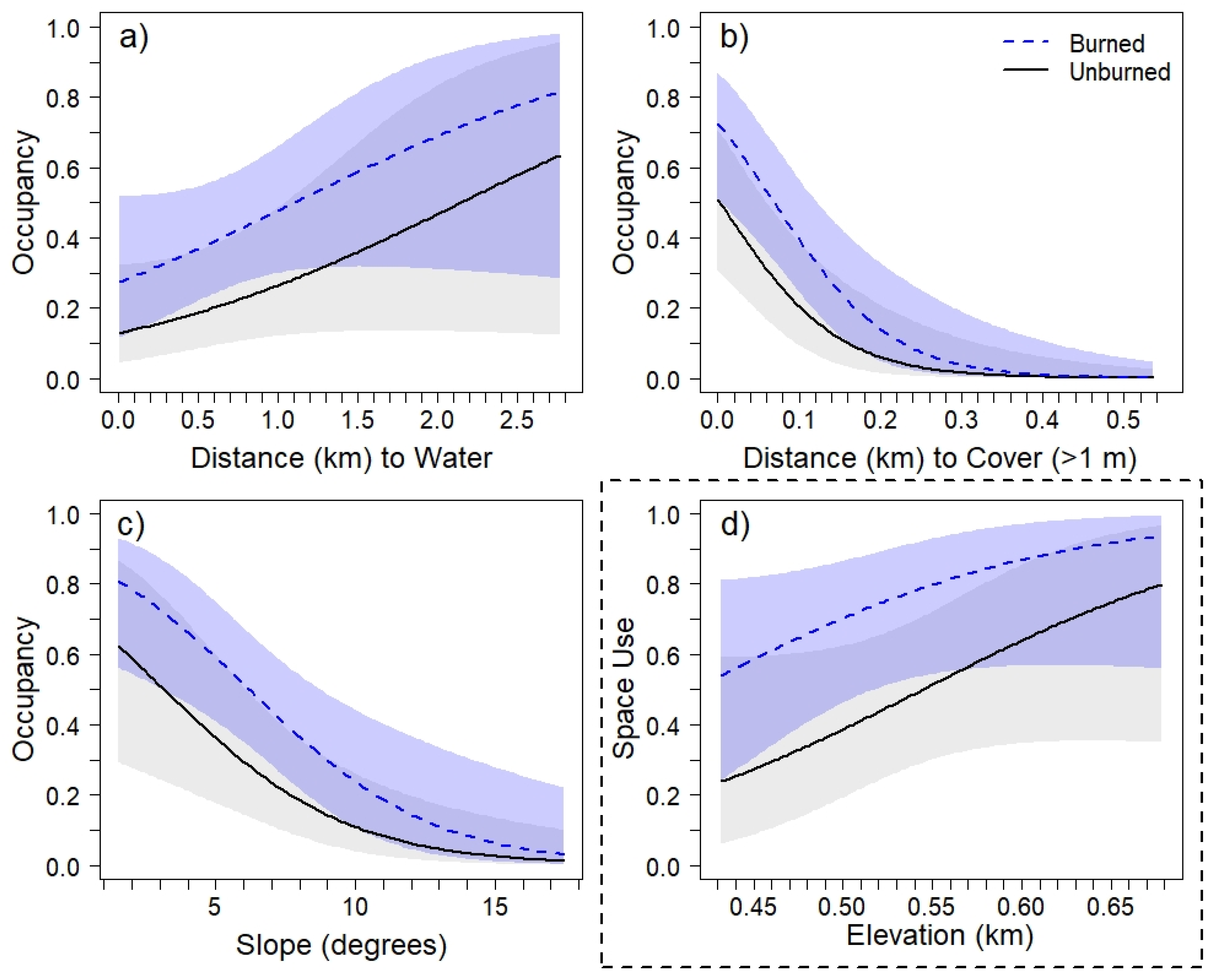
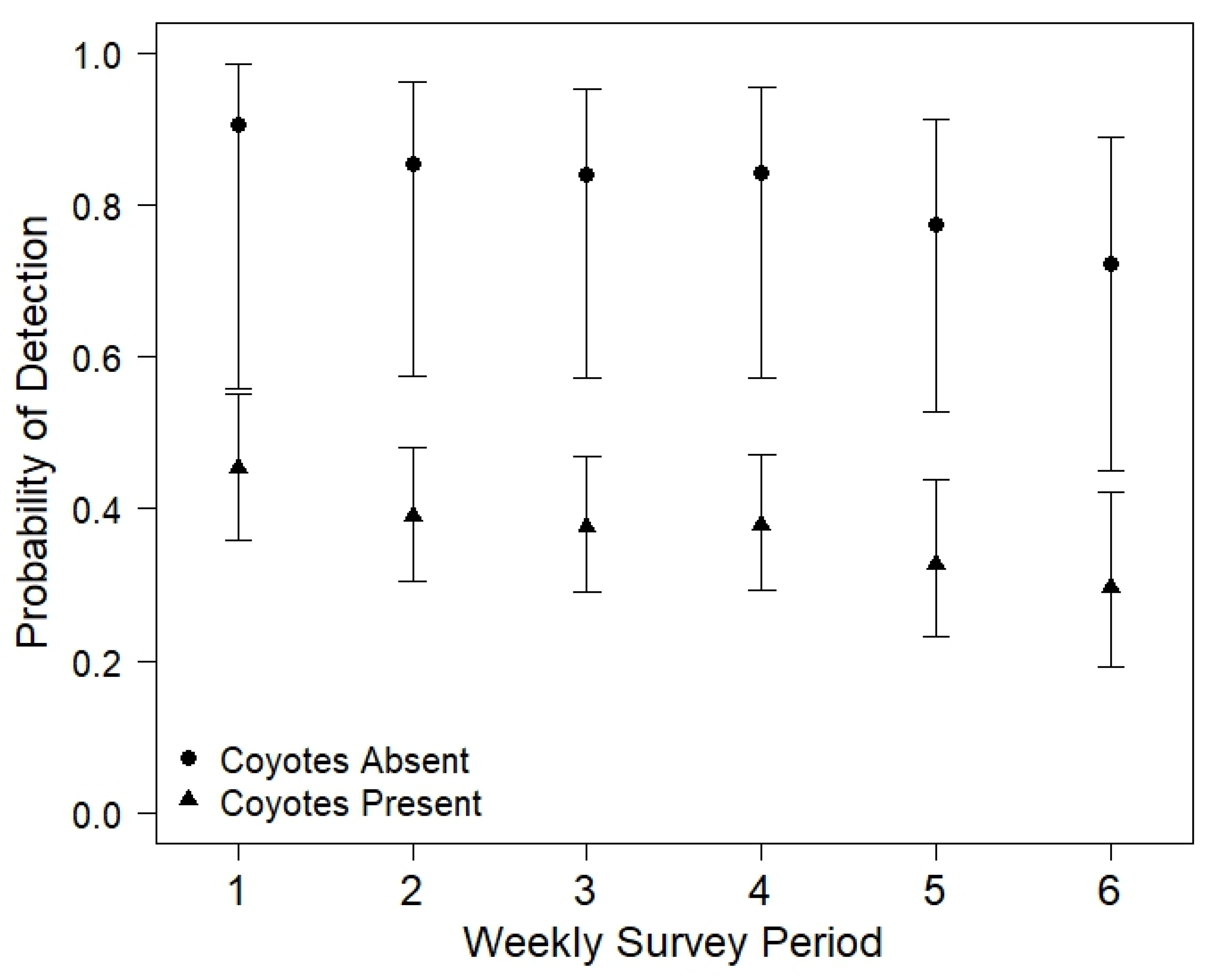

| Armadillo | Parameter | β | SE | LCL | UCL | Σwi | Coyote | Parameter | β | SE | LCL | UCL | Σwi |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p | Intercept | 2.00 | 0.99 | 0.57 | 3.43 | - | p | Intercept | −3.55 | 1.32 | −5.45 | −1.65 | - |
| Effort | - | - | - | - | 0.45 | Effort | 0.34 | 0.19 | 0.06 | 0.61 | 0.77 | ||
| Temp | −0.10 | 0.05 | −0.17 | −0.03 | 0.61 | Temp | - | - | - | - | 0.44 | ||
| Prec | - | - | - | - | 0.33 | Prec | - | - | - | - | 0.27 | ||
| ψ | Intercept | 1.74 | 0.89 | 0.46 | 3.03 | - | ψ | Intercept | −5.61 | 3.40 | −10.50 | −0.71 | - |
| RxB | 0.93 | 0.56 | 0.13 | 1.74 | 0.64 | RxB | 1.32 | 0.66 | 0.37 | 2.27 | 0.66 | ||
| DistW | 0.09 | 0.06 | 0.01 | 0.17 | 0.57 | DistW | - | - | - | - | 0.43 | ||
| DistRd | - | - | - | - | 0.34 | DensRd | - | - | - | - | 0.25 | ||
| Slope | −0.31 | 0.10 | −0.45 | −0.17 | 0.99 | Elevation | 0.10 | 0.06 | 0.01 | 0.19 | 0.55 | ||
| DistCov | −1.40 | 0.39 | −1.95 | −0.84 | 1.00 | %Forest | - | - | - | - | 0.40 |
| Model Structure | K | AICc | ΔAICc | wi | Dev. |
|---|---|---|---|---|---|
| ψA, ψBA = ψBa, pA = rA, pB, rBA = rBa | 14 | 828.924 | 0.000 | 0.49 | 795.92 |
| ψA, ψBA = ψBa, pA = rA, pB, rBA, rBa | 16 | 830.592 | 1.668 | 0.21 | 791.96 |
| ψA, ψBA = ψBa, pA = rA, pB = rBA = rBa | 12 | 830.715 | 1.791 | 0.20 | 803.09 |
| ψA, ψBA = ψBa, pA, rA, pB, rBA = rBa | 16 | 833.510 | 4.586 | 0.05 | 794.88 |
| ψA, ψBA = ψBa, pA, rA, pB, rBA, rBa | 18 | 834.761 | 5.837 | 0.03 | 790.21 |
| ψA, ψBA = ψBa, pA, rA, pB = rBA = rBa | 14 | 835.497 | 6.573 | 0.02 | 802.50 |
| ψA, ψBA, ψBa, pA = rA, pB, rBA = rBa | 19 | 837.946 | 9.022 | 0.01 | 790.33 |
| ψA, ψBA, ψBa, pA = rA, pB, rBA, rBa | 21 | 841.269 | 12.345 | 0.00 | 787.27 |
| ψA, ψBA, ψBa, pA, rA, pB, rBA = rBa | 21 | 841.433 | 12.509 | 0.00 | 787.43 |
| ψA, ψBA, ψBa, pA = rA, pB = rBA = rBa | 17 | 841.835 | 12.911 | 0.00 | 800.28 |
| ψA, ψBA, ψBa, pA, rA, pB, rBA, rBa | 23 | 844.143 | 15.219 | 0.00 | 783.42 |
| ψA, ψBA, ψBa, pA, rA, pB = rBA = rBa | 19 | 847.020 | 18.096 | 0.00 | 799.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lonsinger, R.C.; Murley, B.P.; McDonald, D.T.; Fallon, C.E.; White, K.M. Habitat and Predator Influences on the Spatial Ecology of Nine-Banded Armadillos. Diversity 2025, 17, 290. https://doi.org/10.3390/d17040290
Lonsinger RC, Murley BP, McDonald DT, Fallon CE, White KM. Habitat and Predator Influences on the Spatial Ecology of Nine-Banded Armadillos. Diversity. 2025; 17(4):290. https://doi.org/10.3390/d17040290
Chicago/Turabian StyleLonsinger, Robert C., Ben P. Murley, Daniel T. McDonald, Christine E. Fallon, and Kara M. White. 2025. "Habitat and Predator Influences on the Spatial Ecology of Nine-Banded Armadillos" Diversity 17, no. 4: 290. https://doi.org/10.3390/d17040290
APA StyleLonsinger, R. C., Murley, B. P., McDonald, D. T., Fallon, C. E., & White, K. M. (2025). Habitat and Predator Influences on the Spatial Ecology of Nine-Banded Armadillos. Diversity, 17(4), 290. https://doi.org/10.3390/d17040290







