Patterns of Insect Distribution in Fruit Trees of South Romania and Their Role as Bacterial Vectors
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area, Sampling, and Sample Preparation
2.2. Insect Identification Based on Morphological Features and DNA Barcoding
2.3. Identification of Insect Bacterial Communities by 16S rRNA Gene Illumina Sequencing
2.4. Sequence Analyses
2.5. Statistical Analysis
3. Results
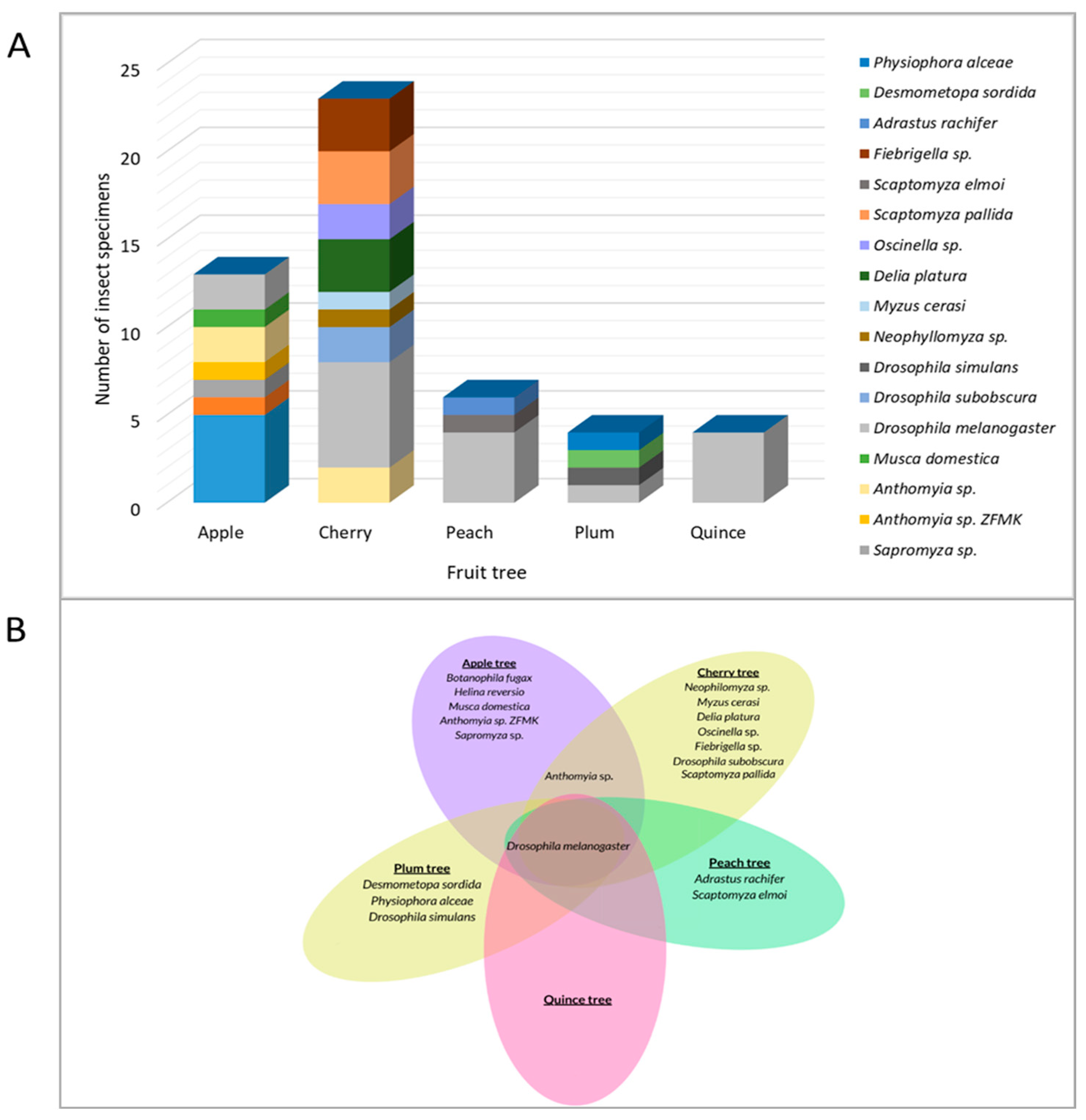
3.1. Identification and Distribution of Insect Species on Fruit Trees
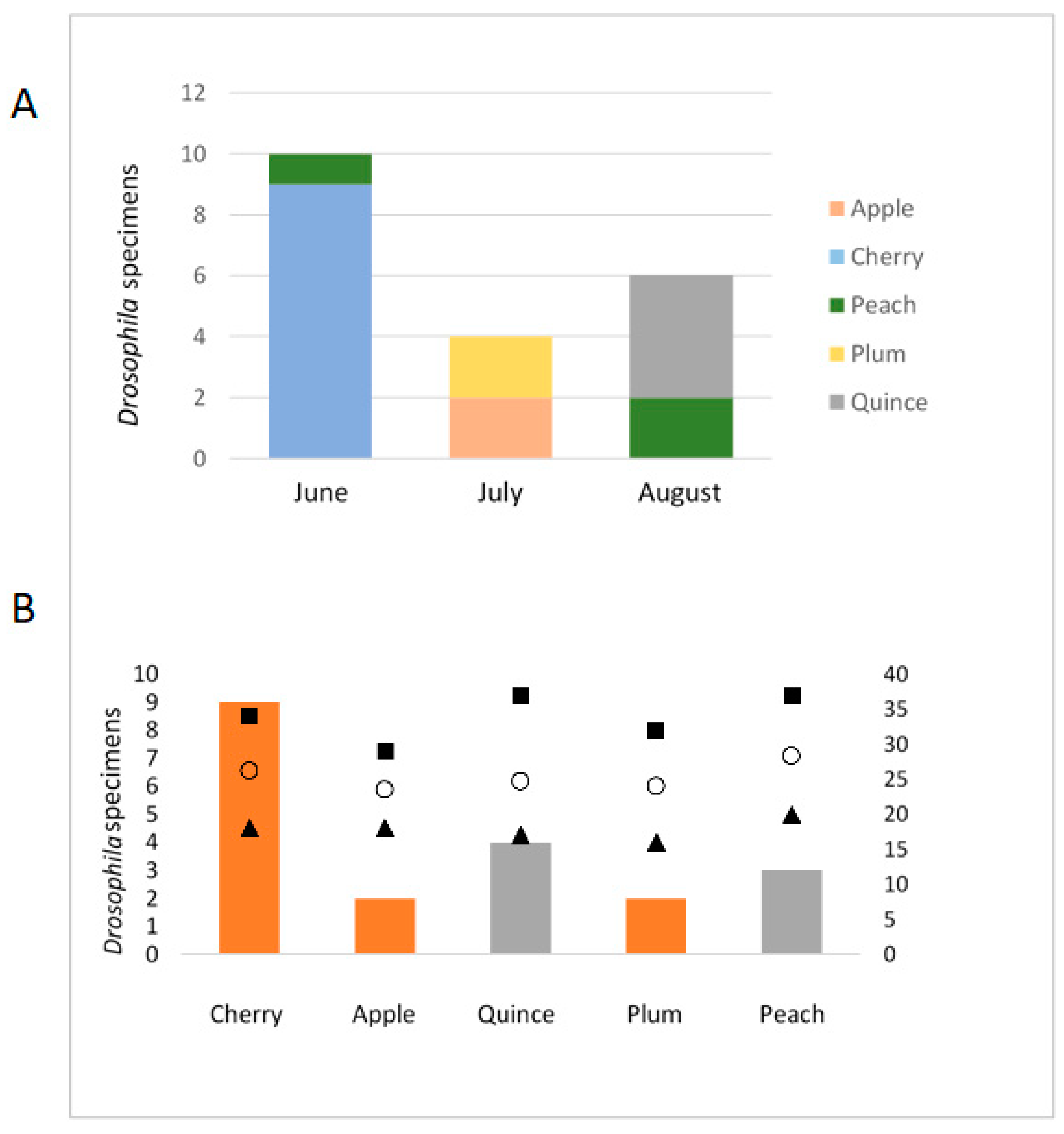
3.2. Influence of Temperature and Fruit Developmental Stage on Insect Presence
3.3. Bacterial Diversity of the Fruit Tree-Associated Insects
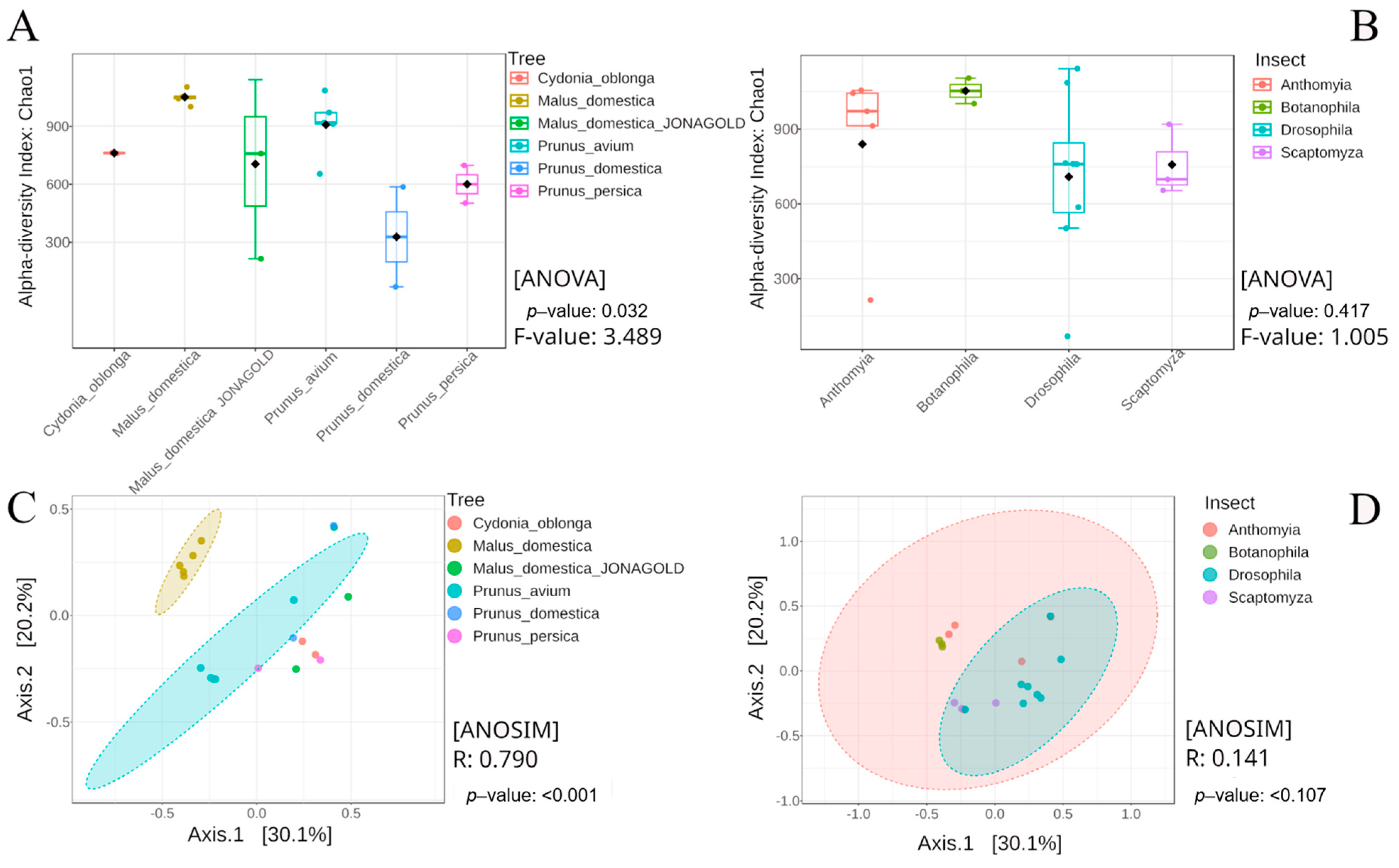
3.3.1. Alpha Diversity
3.3.2. Beta Diversity
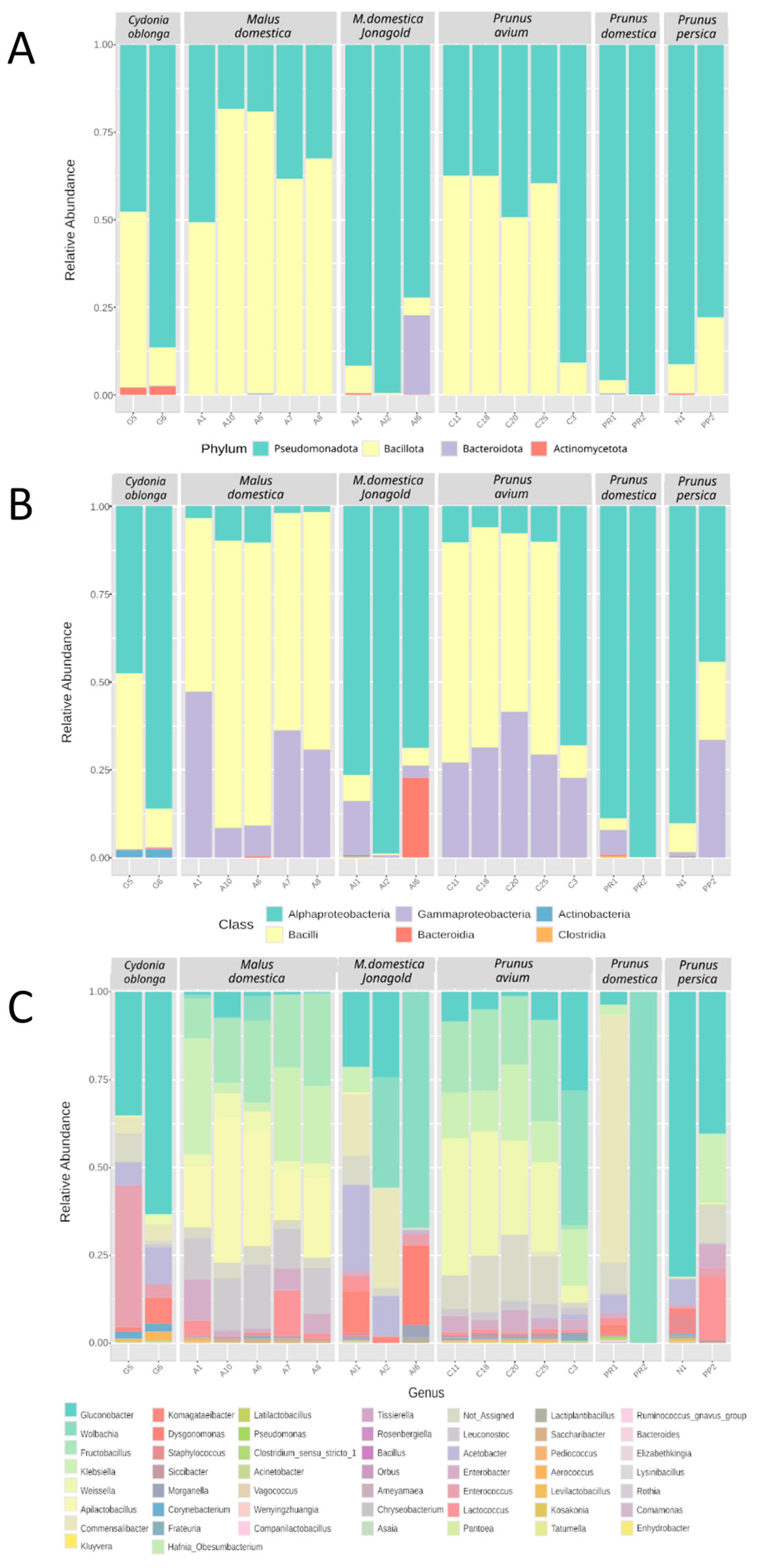
3.4. Taxonomic Profile of the Insect-Associated Bacterial Communities
4. Discussion
4.1. Fruit Tree Insects in South Romania Orchard
4.2. Insect–Bacteria Dynamics on Fruit Trees
4.3. Potential Pathogen Insect Carriers Between Fruit Trees
4.4. Potential Dynamics of Beneficial Bacteria
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holt, J.R.; Cavichiolli de Oliveira, N.; Medina, R.F.; Malacrinò, A.; Lindsey, A.R.I. Insect-microbe interactions and their influence on organisms and ecosystems. Ecol. Evol. 2024, 14, e11699. [Google Scholar] [CrossRef] [PubMed]
- Yasika, Y.; Shivakumar, M.S. A comprehensive account of functional role of insect gut microbiome in insect orders. J. Nat. Pestic. Res. 2025, 11, 100110. [Google Scholar] [CrossRef]
- Noman, A.; Aqeel, M.; Qasim, M.; Haider, I.; Lou, Y. Plant-insect-microbe interaction: A love triangle between enemies in ecosystem. Sci. Total Environ. 2020, 699, 134181. [Google Scholar] [CrossRef]
- Dascălu, I.; Iordănescu, O.A. Evolution of fruit growing sector in Romania. J. Hortic. For. Biotechnol. 2020, 24, 75–79. [Google Scholar]
- Marin, A.; Voicilă, D.N. The evolution of the dynamics of the fruit sector in Romania, in the period 2013–2022. Fruit Grow. Res. 2024, 40, 90–98. [Google Scholar] [CrossRef]
- Chandler, J.A.; Lang, J.M.; Bhatnagar, S.; Eisen, J.A.; Kopp, A. Bacterial communities of diverse Drosophila species: Ecological context of a host-microbe model system. PLoS Genet. 2011, 7, e1002272. [Google Scholar] [CrossRef]
- Reyes, J.A.; Lira-Noriega, A. Current and future global potential distribution of the fruit fly Drosophila suzukii (Diptera: Drosophilidae). Can. Entomol. 2020, 152, 587–599. [Google Scholar] [CrossRef]
- Kavallieratos, N.; Tomanović, Ž.; Stary, P.; Bogdanović, A. Parasitoids (Hymenoptera: Braconidae: Aphidiinae) Attacking Aphids Feeding on Prunoideae and Maloideae Crops in Southeast Europe: Aphidiine-Aphid-Plant Associations and Key. Zootaxa 2008, 1793, 47–64. [Google Scholar] [CrossRef]
- Kök, Ş.; Kasap, İ. Seasonal population fluctuation and life history in different temperatures of Myzus cerasi (Hemiptera: Aphididae) on cherry trees: A field and laboratory study. J. Econ. Entomol. 2024, 117, 865–875. [Google Scholar] [CrossRef]
- Borbély, C.; György, Z.; Szathmáry, E.; Markó, V. Apricot aphid, Myzus mumecola (Matsumura), a new and important pest of apricot in Hungary. J. Plant Dis. Prot. 2021, 128, 781–787. [Google Scholar] [CrossRef]
- Bevacqua, D.; Grechi, I.; Génard, M.; Lescourret, F. The consequences of aphid infestation on fruit production become evident in a multi-year perspective: Insights from a virtual experiment. Ecol. Model. 2016, 338, 11–16. [Google Scholar] [CrossRef]
- Knapp, L.; Mazzi, D.; Finger, R. The economic impact of Drosophila suzukii: Perceived costs and revenue losses of Swiss cherry, plum and grape growers. Pest Manag. Sci. 2021, 77, 978–1000. [Google Scholar] [CrossRef] [PubMed]
- Stewart, T.; Wang, X.; Molinar, A.; Daane, K. Factors Limiting Peach as a Potential Host for Drosophila suzukii (Diptera: Drosophilidae). J. Econ. Entomol. 2014, 107, 1771–1779. [Google Scholar] [CrossRef]
- Poyet, M.; Le Roux, V.; Gibert, P.; Meirland, A.; Prévost, G.; Eslin, P.; Chabrerie, O. The Wide Potential Trophic Niche of the Asiatic Fruit Fly Drosophila suzukii: The Key of Its Invasion Success in Temperate Europe? PLoS ONE 2015, 10, e0142785. [Google Scholar] [CrossRef]
- Markow, T.A.; O’Grady, P. Drosophila: A Guide to Species Identification and Use; Academic Press: Cambridge, MA, USA, 2005. [Google Scholar]
- Funmilola, A.S.; Babatunde, O.O.; Victor, O.; Segun, A.; Folake, O.I.; Ganiyu, O. Involvement of Cholinergic and Redox Impairments in Insecticidal Properties of Essential Oils from Fertility Tree and Horseradish Tree Leaves in Fruit Fly (Drosophila melanogaster). J. Oleo Sci. 2020, 69, 941–950. [Google Scholar] [CrossRef]
- Agrawal, A.; Tamrakar, V. Pattern of Distribution of Drosophila Species: A Global Scenario. Shodh. Darpan. 2016, 2, 1–6. [Google Scholar]
- Narciso, R.; Sario, S.; Mendes, R.J.; Santos, C. Drosophila suzukii displays a sex-dependent immune response to Microbacterium thalassium and Providencia sp. infection. Biol. Control 2023, 181, 105204. [Google Scholar] [CrossRef]
- Yee, W.L.; Rose, A.C.; Milnes, J.M.; Feder, J.L. Differential water deprivation tolerances of adult Rhagoletis indifferens and Rhagoletis pomonella (Diptera: Tephritidae) as a possible factor affecting their distributional abundances in Washington State, USA. Envrion. Entomol. 2024, 53, 1078–1092. [Google Scholar] [CrossRef]
- McCabe, L.M.; Boyle, N.K.; Pitts-Singer, T.L. Osmia lignaria (Hymenoptera: Megachilidae) increase pollination of Washington sweet cherry and pear crops. Environ. Entomol. 2024, 53, 698–705. [Google Scholar] [CrossRef]
- Henneberg, B.; Meiners, T.; Mody, K.; Obermaier, E. Morphological and olfactory tree traits influence the susceptibility and suitability of the apple species Malus domestica and M. sylvestris to the florivorous weevil Anthonomus pomorum (Coleoptera: Curculionidae). PeerJ 2022, 10, e13566. [Google Scholar] [CrossRef]
- Contreras-Miranda, J.A.; Piovesan, B.; Ueno, B.; Bernardi, D.; Botton, M.; Nava, D.E. Use of Preservatives in Vegetable Protein-Based Food Attractants for Monitoring Anastrepha fraterculus (Diptera: Tephritidae) in Peach Orchards. Neotrop. Entomol. 2021, 50, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Junnila, A.; Müller, G.C.; Schlein, Y. Attraction of Phlebotomus papatasi to common fruit in the field. J. Vector. Ecol. 2011, 36 (Suppl. S1), S206–S211. [Google Scholar] [CrossRef] [PubMed]
- Ling, J.; Li, Y.; Zheng, X.L.; Lu, W.; Wang, X.Y. Electroantennographic and behavioral responses of Bactrocera dorsalis (Diptera: Tephritidae) adults to the volatiles of plum fruits. J. Econ. Entomol. 2024, 117, 2400–2412. [Google Scholar] [CrossRef]
- Rakauskas, R.; Havelka, J.; Zaremba, A. Mitochondrial COI and morphological specificity of the mealy aphids (Hyalopterus ssp.) collected from different hosts in Europe (Hemiptera, Aphididae). Zookeys 2013, 319, 255–267. [Google Scholar] [CrossRef]
- Ciceoi, R.; Dobrin, I.; Ivan, E.; Dicianu, E.; Stanica, F. Emerging pests of Ziziphus jujuba crop in Romania. Sci. Papers. Ser. B Hortic. 2017, LXI, 143–153. [Google Scholar]
- Chireceanu, C.; Iamandei, M.; Stanica, F.; Chiriloaie, A. The Presence of The Mediterranean Fruit Fly Ceratitis capitata (Wied.), (Diptera: Tephritidae) in Romania. Rom. J. Plant Prot. 2013, 6, 92–97. [Google Scholar]
- Toma, O.-M.; Mitrea, I. Discovery and Impact of the Spotted Wing Drosophila (Drosophila suzukii) (Matsumura 1931) (Diptera: Drosophilidae) in a Mixed Raspberry and Blackberry Plantation in Perișor, Dolj, Romania. Ann. Univ. Craiova Biol. Hortic. Food Prod. Process. Technol. Environ. Eng. 2023, 28. [Google Scholar] [CrossRef]
- Chireceanu, C.; Chiriloaie, A.; Teodoru, A. First Record of the Spotted Wing Drosophila Drosophila suzukii (Diptera: Drosophilidae) in Romania. Rom. J. Plant Prot. 2015, 8, 86–95. [Google Scholar]
- Teodoru, A.; Chiriloaie, A.; Chireceanu, C. The hawthorn fruit fly, Anomoia purmunda Harris—A less known species in Romania. Rom. J Plant Prot. 2015, 8, 47–53. [Google Scholar]
- Olenici, N.; Duduman, M.-L.; Popa, I.; Isaia, G.; Paraschiv, M. Geographical Distribution of Three Forest Invasive Beetle Species in Romania. Insects 2022, 13, 621. [Google Scholar] [CrossRef]
- Laroche, M.; Raoult, D.; Parola, P. Insects and the Transmission of Bacterial Agents. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Heck, M. Insect Transmission of Plant Pathogens: A Systems Biology Perspective. mSystems 2018, 3, 00168-17. [Google Scholar] [CrossRef] [PubMed]
- Kirschbaum, D.S.; Funes, C.F.; Buonocore-Biancheri, M.J.; Suárez, L.; Ovruski, S.M. The Biology and Ecology of Drosophila suzukii (Diptera: Drosophilidae). In Drosophila suzukii Management; Garcia, F.R.M., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 41–91. [Google Scholar]
- Toledo-Hernández, E.; Torres-Quíntero, M.; Mancilla-Dorantes, I.; Sotelo Leyva, C.; Delgado-Núñez, E.; Velázquez, V.M.; Dunstand-Guzmán, E.; Peña-Chora, G. Entomopathogenic Bacteria Species and Toxins Targeting Aphids (Hemiptera: Aphididae): A Review. Plants 2025, 14, 943. [Google Scholar] [CrossRef]
- Moran, N.A. Microbe Profile: Buchnera aphidicola: Ancient aphid accomplice and endosymbiont exemplar. Microbiology 2021, 167, 001127. [Google Scholar] [CrossRef]
- Pedroncelli, A.; Puopolo, G. This tree is on fire: A review on the ecology of Erwinia amylovora, the causal agent of fire blight disease. J. Plant Pathol. 2024, 106, 823–837. [Google Scholar] [CrossRef]
- Lange, C.; Turrero Garcia, M.; Decimo, I.; Bifari, F.; Eelen, G.; Quaegebeur, A.; Boon, R.; Zhao, H.; Boeckx, B.; Chang, J.; et al. Relief of hypoxia by angiogenesis promotes neural stem cell differentiation by targeting glycolysis. Embo J. 2016, 35, 924–941. [Google Scholar] [CrossRef]
- Tafesh-Edwards, G.; Eleftherianos, I. The role of Drosophila microbiota in gut homeostasis and immunity. Gut Microbes 2023, 15, 2208503. [Google Scholar] [CrossRef]
- Broderick, N.A.; Lemaitre, B. Gut-associated microbes of Drosophila melanogaster. Gut Microbes 2012, 3, 307–321. [Google Scholar] [CrossRef]
- Zhu, Y.-X.; Yang, R.; Wang, X.-Y.; Wen, T.; Gong, M.-H.; Shen, Y.; Xu, J.-Y.; Zhao, D.-S.; Du, Y.-Z. Gut microbiota composition in the sympatric and diet-sharing Drosophila simulans and Dicranocephalus wallichii bowringi shaped largely by community assembly processes rather than regional species pool. iMeta 2022, 1, e57. [Google Scholar] [CrossRef]
- Zhu, Y.-X.; Zhang, Y.-Y.; Wang, X.-Y.; Yin, Y.; Du, Y.-Z. Wolbachia modify host cell metabolite profiles in response to short-term temperature stress. Environ. Microbiol. Rep. 2024, 16, e70013. [Google Scholar] [CrossRef]
- Gopikrishnan, V.; Radhakrishnan, M.; Krupakar, P.; Manigundan, K.; Abirami, B.; Reshma, S. Chapter 36—Endosymbiotic interactions of actinobacteria with the insects. In Microbial Symbionts; Dharumadurai, D., Ed.; Academic Press: Cambridge, MA, USA, 2023; pp. 645–658. [Google Scholar]
- Shin, S.C.; Kim, S.H.; You, H.; Kim, B.; Kim, A.C.; Lee, K.A.; Yoon, J.H.; Ryu, J.H.; Lee, W.J. Drosophila microbiome modulates host developmental and metabolic homeostasis via insulin signaling. Science 2011, 334, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Bharathi, T.; Sathiyanandam, V.K.; David, P.M.M. Attractiveness of some food baits to the melon fruit fly, Bactrocera cucurbitae (Coquillett) (Diptera: Tephritidae). Int. J. Trop. Insect Sci. 2004, 24, 125–134. [Google Scholar] [CrossRef]
- Gregor, F.; Rozkošný, R.; Bartak, M.; Vaňhara, J. The Muscidae (Diptera) of Central Europe. Folia Fac. Sci. Nat. Univ. Masaryk. Brun. Biol. 2002, 107, 1–280. [Google Scholar]
- P, G.; Popescu, I.E. Guide to Coleoptera of Romania, Vol. I. (Ghidul Coleopterelor din România, Volumul I); Ed. PIM Iaşi: Iaşi, Romania, 2012; ISBN 978-606-13-0960-3. [Google Scholar]
- Copoiu, D.S.; Purcarea, C. First report on the Presence Of Botanophila fugax (Meigen, 1826) (Diptera: Anthomyiidae) in Romania. Rom. J. Biol.-Zool. 2023, 68, 11–18. [Google Scholar] [CrossRef]
- Takahashi, S.; Tomita, J.; Nishioka, K.; Hisada, T.; Nishijima, M. Development of a prokaryotic universal primer for simultaneous analysis of Bacteria and Archaea using next-generation sequencing. PLoS ONE 2014, 9, e105592. [Google Scholar] [CrossRef]
- Johnson, M.; Zaretskaya, I.; Raytselis, Y.; Merezhuk, Y.; McGinnis, S.; Madden, T.L. NCBI BLAST: A better web interface. Nucleic Acids Res. 2008, 36, W5–W9. [Google Scholar] [CrossRef]
- Hebert, P.D.; Cywinska, A.; Ball, S.L.; deWaard, J.R. Biological identifications through DNA barcodes. Proc. Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 3. [Google Scholar] [CrossRef]
- Lu, Y.; Zhou, G.; Ewald, J.; Pang, Z.; Shiri, T.; Xia, J. MicrobiomeAnalyst 2.0: Comprehensive statistical, functional and integrative analysis of microbiome data. Nucleic Acids Res. 2023, 51, W310–W318. [Google Scholar] [CrossRef]
- Chen, H.; Boutros, P.C. VennDiagram: A package for the generation of highly-customizable Venn and Euler diagrams in R. BMC Bioinform. 2011, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 14 February 2025).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer International Publishing: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Mishra, P.; Singh, U.; Pandey, C.M.; Mishra, P.; Pandey, G. Application of student’s t-test, analysis of variance, and covariance. Ann. Card. Anaesth. 2019, 22, 407–411. [Google Scholar] [CrossRef]
- GBIF.org. GBIF Home Page. 2025. Available online: https://www.gbif.org (accessed on 21 February 2025).
- Cunningham, J.; Carlsson, M.; Villa, T.; Dekker, T.; Clarke, A. Do Fruit Ripening Volatiles Enable Resource Specialism in Polyphagous Fruit Flies? J. Chem. Ecol. 2016, 42, 1–10. [Google Scholar] [CrossRef]
- Bandzo, K.; Popovska, M.; Bandzo, S. Influence of the time of first fruit color change and the duration of fruit ripening of cherry varieties on the infestation by R. cerasi. Агрoзнање 2012, 13. [Google Scholar] [CrossRef]
- Vizitiu, D.; Buciumeanu, E.-C.; Guţă, I.; Sumedrea, D. Insect Species Diversity in Stefanesti Viticultural Centre from Romania. Sci. Papers. Ser. B Horticulture. 2019, 63, 109–114. [Google Scholar]
- Iancu, L.; Carter, D.O.; Junkins, E.N.; Purcarea, C. Using bacterial and necrophagous insect dynamics for post-mortem interval estimation during cold season: Novel case study in Romania. Forensic Sci. Int. 2015, 254, 106–117. [Google Scholar] [CrossRef]
- Rossmann, S.; Dees, M.W.; Perminow, J.; Meadow, R.; Brurberg, M.B. Soft Rot Enterobacteriaceae Are Carried by a Large Range of Insect Species in Potato Fields. Appl. Environ. Microbiol. 2018, 84, e00281-18. [Google Scholar] [CrossRef]
- Silva-López, J.; Godoy, P.; Jara, L.; Godoy-Herrera, R. Interaction and integration among behaviors of adult Drosophila in nature. PLoS ONE 2023, 18, e0278427. [Google Scholar] [CrossRef]
- Oakeshott, J.G.; Vacek, D.C.; Anderson, P.R. Effects of microbial floras on the distributions of five domestic Drosophila species across fruit resources. Oecologia 1989, 78, 533–541. [Google Scholar] [CrossRef]
- Ort, B.S.; Bantay, R.M.; Pantoja, N.A.; O’Grady, P.M. Fungal diversity associated with Hawaiian Drosophila host plants. PLoS ONE 2012, 7, e40550. [Google Scholar] [CrossRef] [PubMed]
- BEGON, M. Population densities in Drosophila obscura Fallén and D. subobscura Collin. Ecol. Entomol. 1978, 3, 1–12. [Google Scholar] [CrossRef]
- Marino, A.; Leonardi, M.; Berrilli, E.; Garzia, M.; Zambonelli, A.; Cerretti, P.; Iotti, M. Identification of Dipteran species inhabiting Tuber aestivum (the summer truffle) ascomata. Environ. Monit. Assess. 2024, 196, 1239. [Google Scholar] [CrossRef]
- O’Connor, T.K.; Humphrey, P.T.; Lapoint, R.T.; Whiteman, N.K.; O’Grady, P.M. Microbial interactions and the ecology and evolution of Hawaiian Drosophilidae. Front. Microbiol. 2014, 5, 616. [Google Scholar] [CrossRef]
- Coolen, S.; Magda, R.D.; Welte, C.U. The secret life of insect-associated microbes and how they shape insect-plant interactions. FEMS Microbiol. Ecol. 2022, 98, fiac083. [Google Scholar] [CrossRef]
- Ren, C.; Webster, P.; Finkel, S.E.; Tower, J. Increased internal and external bacterial load during Drosophila aging without life-span trade-off. Cell Metab. 2007, 6, 144–152. [Google Scholar] [CrossRef]
- Xin, X.F.; Kvitko, B.; He, S.Y. Pseudomonas syringae: What it takes to be a pathogen. Nat. Rev. Microbiol. 2018, 16, 316–328. [Google Scholar] [CrossRef]
- Yang, P.; Zhao, L.; Gao, Y.G.; Xia, Y. Detection, Diagnosis, and Preventive Management of the Bacterial Plant Pathogen Pseudomonas syringae. Plants 2023, 12, 1765. [Google Scholar] [CrossRef]
- Zierke, L.; Mourad, R.; Kohler, T.P.; Müsken, M.; Hammerschmidt, S. Influence of the polysaccharide capsule on virulence and fitness of Klebsiella pneumoniae. Front. Microbiol. 2025, 16, 1450984. [Google Scholar] [CrossRef]
- Cox, C.R.; Gilmore, M.S. Native microbial colonization of Drosophila melanogaster and its use as a model of Enterococcus faecalis pathogenesis. Infect Immun. 2007, 75, 1565–1576. [Google Scholar] [CrossRef]
- Moran, N.A.; Russell, J.A.; Koga, R.; Fukatsu, T. Evolutionary relationships of three new species of Enterobacteriaceae living as symbionts of aphids and other insects. Appl. Environ. Microbiol. 2005, 71, 3302–3310. [Google Scholar] [CrossRef] [PubMed]
- Montllor, C.; Maxmen, A.; Purcell, A. Facultative bacterial endosymbionts benefit pea aphids Acyrthosiphon pisum under heat stress. Ecol. Entomol. 2002, 27, 189–195. [Google Scholar] [CrossRef]
- Cadavid Sanchez, I.; Amat, E.; Gómez-P, L.-M. Enterobacteria Isolated from Synanthropic Flies (Diptera, Calyptratae) In Medellín, Colombia. Caldasia 2015, 37, 319. [Google Scholar] [CrossRef]
- Bertelloni, F.; Bresciani, F.; Cagnoli, G.; Scotti, B.; Lazzerini, L.; Marcucci, M.; Colombani, G.; Bilei, S.; Bossù, T.; De Marchis, M.L.; et al. House Flies (Musca domestica) from Swine and Poultry Farms Carrying Antimicrobial Resistant Enterobacteriaceae and Salmonella. Vet. Sci. 2023, 10, 118. [Google Scholar] [CrossRef]
- Stefani, S.; Goglio, A. Methicillin-resistant Staphylococcus aureus: Related infections and antibiotic resistance. Int. J. Infect Dis. 2010, 14 (Suppl. S4), S19–S22. [Google Scholar] [CrossRef]
- Panayidou, S.; Ioannidou, E.; Apidianakis, Y. Human pathogenic bacteria, fungi, and viruses in Drosophila: Disease modeling, lessons, and shortcomings. Virulence 2014, 5, 253–269. [Google Scholar] [CrossRef]
- Khan, A.S.; Dancer, S.J.; Humphreys, H. Priorities in the prevention and control of multidrug-resistant Enterobacteriaceae in hospitals. J. Hosp. Infect. 2012, 82, 85–93. [Google Scholar] [CrossRef]
- Leal, J.; Gregson, D.B.; Ross, T.; Church, D.L.; Laupland, K.B. Epidemiology of Clostridium species bacteremia in Calgary, Canada, 2000–2006. J. Infect. 2008, 57, 198–203. [Google Scholar] [CrossRef]
- Senneby, E.; Petersson, A.C.; Rasmussen, M. Clinical and microbiological features of bacteraemia with Aerococcus urinae. Clin. Microbiol. Infect. 2012, 18, 546–550. [Google Scholar] [CrossRef]
- Howard, A.; O’Donoghue, M.; Feeney, A.; Sleator, R.D. Acinetobacter baumannii: An emerging opportunistic pathogen. Virulence 2012, 3, 243–250. [Google Scholar] [CrossRef]
- Wong, D.W.; Nielsen, T.B.; Bonomo, R.A.; Pantapalangkoor, P.; Luna, B.M.; Spellberg, B. Clinical and Pathophysiological Overview of Acinetobacter Infections: A Century of Challenges. Clin. Microbiol. Rev. 2016, 30, 409–447. [Google Scholar] [CrossRef] [PubMed]
- Bottone, E.J. Bacillus cereus, a volatile human pathogen. Clin. Microbiol. Rev. 2010, 23, 382–398. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Yu, P.; Wang, J.; Li, C.; Guo, H.; Liu, C.; Kong, L.; Yu, L.; Wu, S.; Lei, T.; et al. A Study on Prevalence and Characterization of Bacillus cereus in Ready-to-Eat Foods in China. Front. Microbiol. 2019, 10, 3043. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, N.; Hess, C.; Hess, M.; Alispahic, M. Sequencing of five poultry strains elucidates phylogenetic relationships and divergence in virulence genes in Morganella morganii. BMC Genom. 2020, 21, 579. [Google Scholar] [CrossRef]
- Salas, B.; Conway, H.; Schuenzel, E.; Breaux, K.; Vitek, C.; Vacek, D. Morganella morganii (Enterobacteriales: Enterobacteriaceae) Is a Lethal Pathogen of Mexican Fruit Fly (Diptera: Tephritidae) Larvae. Fla. Entomol. 2017, 100, 743–751. [Google Scholar] [CrossRef]
- Rakauskas, R.; Havelka, J.; Zaremba, A.; Bernotienė, R. Mitochondrial COI and morphological evidence for host specificity of the black cherry aphids Myzus cerasi (Fabricius, 1775) collected from different cherry tree species in Europe (Hemiptera, Aphididae). Zookeys 2014, 388, 1–16. [Google Scholar] [CrossRef]
- Isac, M.; Preda, S.; Marcu, M. Aphid species—Vectors of plum pox virus. Acta Virol. 1998, 42, 233–234. [Google Scholar]
- Krams, R.; Gudra, D.; Popovs, S.; Willow, J.; Krama, T.; Munkevics, M.; Megnis, K.; Jõers, P.; Fridmanis, D.; Contreras Garduño, J.; et al. Dominance of Fructose-Associated Fructobacillus in the Gut Microbiome of Bumblebees (Bombus terrestris) Inhabiting Natural Forest Meadows. Insects 2022, 13, 98. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, H.; Lu, S.; Lai, C.; Liu, H.; Zhu, H. The metabolic flux regulation of Klebsiella pneumoniae based on quorum sensing system. Sci. Rep. 2016, 6, 38725. [Google Scholar] [CrossRef]
- Kersters, K.; Lisdiyanti, P.; Komagata, K.; Swings, J. The Family Acetobacteraceae: The Genera Acetobacter, Acidomonas, Asaia, Gluconacetobacter, Gluconobacter, and Kozakia. In The Prokaryotes: Volume 5: Proteobacteria: Alpha and Beta Subclasses; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.-H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2006; pp. 163–200. [Google Scholar]
- Jojima, Y.; Mihara, Y.; Suzuki, S.; Yokozeki, K.; Yamanaka, S.; Fudou, R. Saccharibacter floricola gen. nov., sp. nov., a novel osmophilic acetic acid bacterium isolated from pollen. Int. J. Syst. Evol. Microbiol. 2004, 54, 2263–2267. [Google Scholar] [CrossRef]
- Crotti, E.; Rizzi, A.; Chouaia, B.; Ricci, I.; Favia, G.; Alma, A.; Sacchi, L.; Bourtzis, K.; Mandrioli, M.; Cherif, A.; et al. Acetic acid bacteria, newly emerging symbionts of insects. Appl. Environ. Microbiol. 2010, 76, 6963–6970. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, A.R.; Tennessen, J.M.; Gelaw, M.A.; Jones, M.W.; Parish, A.J.; Newton, I.L.; Nemkov, T.; D’Alessandro, A.; Rai, M.; Stark, N. The intracellular symbiont Wolbachia alters Drosophila development and metabolism to buffer against nutritional stress. bioRxiv 2024. [Google Scholar] [CrossRef]
- Pagel, L.; Bultman, T.; Górzyńska, K.; Lembicz, M.; Leuchtmann, A.; Sangliana, A.; Richards, N. Botanophila flies, vectors of Epichloë fungal spores, are infected by Wolbachia. Mycology 2019, 10, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, P.; Butenko, A.; Linke, D.; Ghanavi, H.R.; Meier, J.I.; Wahlberg, N.; Matos-Maraví, P. Pervasive horizontal transmission of Wolbachia in natural populations of closely related and widespread tropical skipper butterflies. BMC Microbiol. 2025, 25, 5. [Google Scholar] [CrossRef]
- Montenegro, D.; Cortés-Cortés, G.; Balbuena-Alonso, M.G.; Warner, C.; Camps, M. Wolbachia-based emerging strategies for control of vector-transmitted disease. Acta Trop. 2024, 260, 107410. [Google Scholar] [CrossRef]
- Grève, P.; Moumen, B.; Bouchon, D. Three feminizing Wolbachia strains in a single host species: Comparative genomics paves the way for identifying sex reversal factors. Front. Microbiol. 2024, 15, 1416057. [Google Scholar] [CrossRef]
- Yuen, G.J.; Ausubel, F.M. Enterococcus infection biology: Lessons from invertebrate host models. J. Microbiol. 2014, 52, 200–210. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Copoiu, D.S.; Lavin, P.; Itcus, C.; Purcarea, C. Patterns of Insect Distribution in Fruit Trees of South Romania and Their Role as Bacterial Vectors. Diversity 2025, 17, 295. https://doi.org/10.3390/d17040295
Copoiu DS, Lavin P, Itcus C, Purcarea C. Patterns of Insect Distribution in Fruit Trees of South Romania and Their Role as Bacterial Vectors. Diversity. 2025; 17(4):295. https://doi.org/10.3390/d17040295
Chicago/Turabian StyleCopoiu, Dana S., Paris Lavin, Corina Itcus, and Cristina Purcarea. 2025. "Patterns of Insect Distribution in Fruit Trees of South Romania and Their Role as Bacterial Vectors" Diversity 17, no. 4: 295. https://doi.org/10.3390/d17040295
APA StyleCopoiu, D. S., Lavin, P., Itcus, C., & Purcarea, C. (2025). Patterns of Insect Distribution in Fruit Trees of South Romania and Their Role as Bacterial Vectors. Diversity, 17(4), 295. https://doi.org/10.3390/d17040295






