Polychaetes Associated with Calcareous Red Algae Corallina officinalis in the Northern Adriatic Sea
Abstract
:1. Introduction
2. Materials and Methods
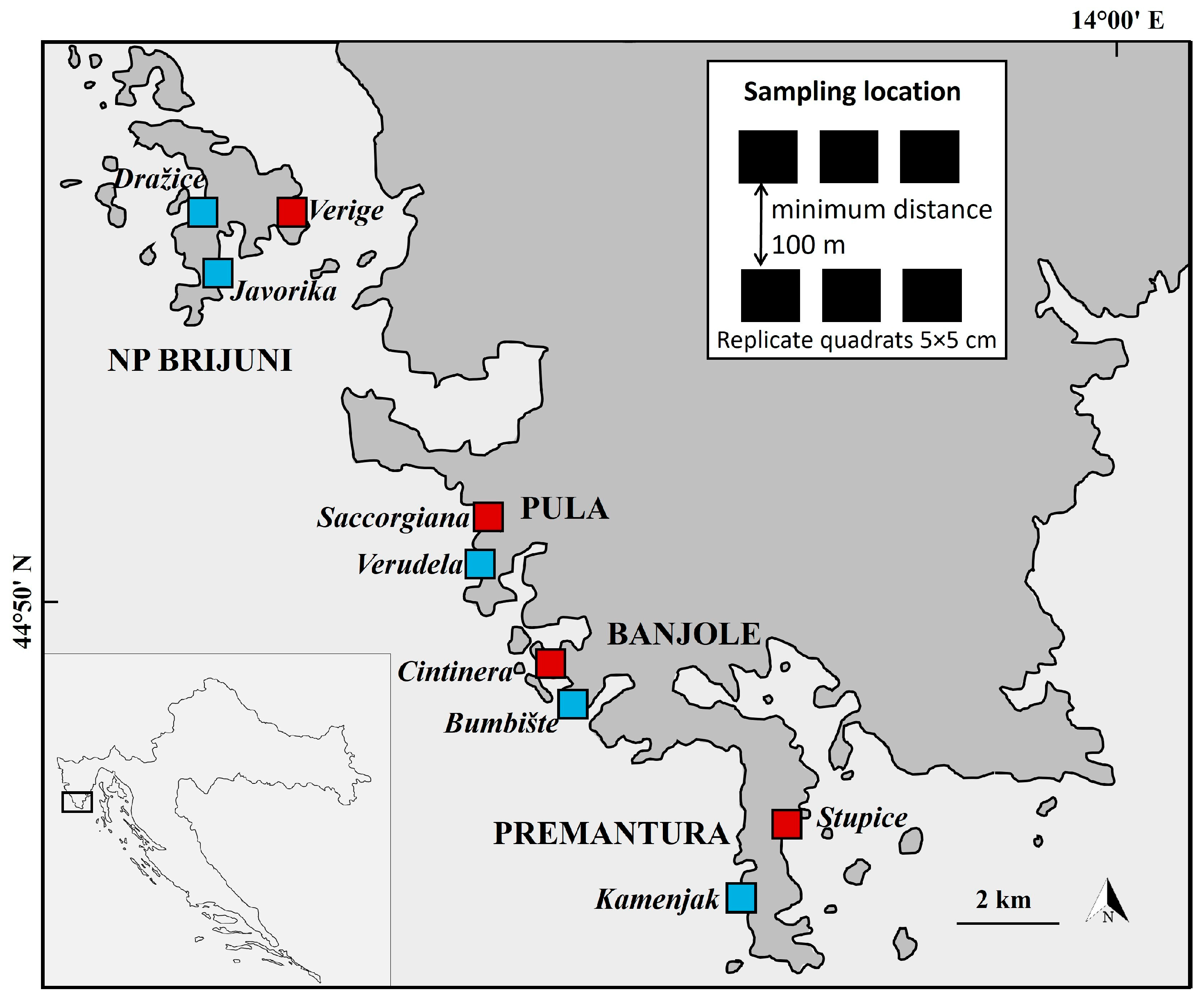
2.1. Study Area
2.2. Sampling Methods and Processing
2.3. Assessing Anthropogenic Pressure
2.4. Species Composition Analysis
3. Results
3.1. Categorization of Anthropogenic Pressure
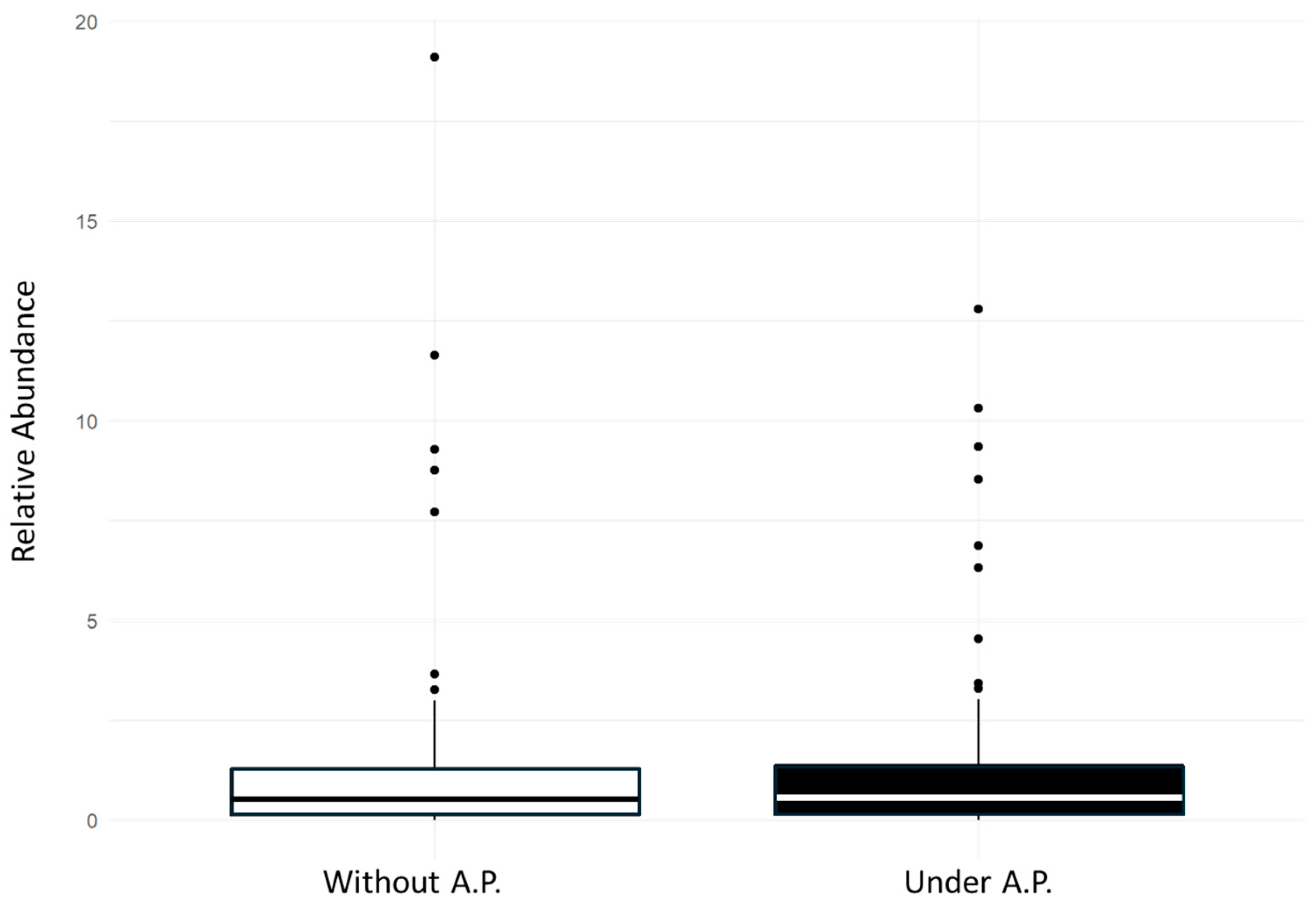
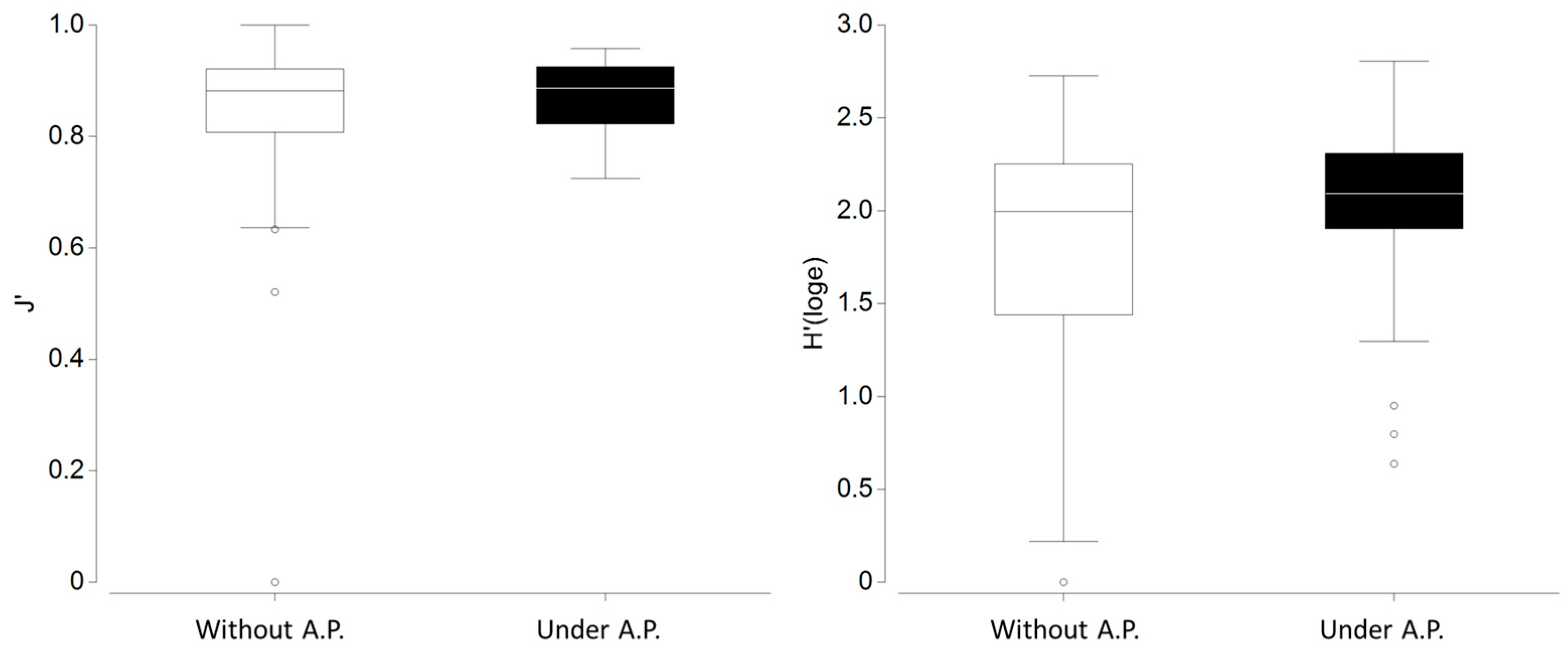
3.2. Polychaete Diversity Within Corallina officinalis Assemblages
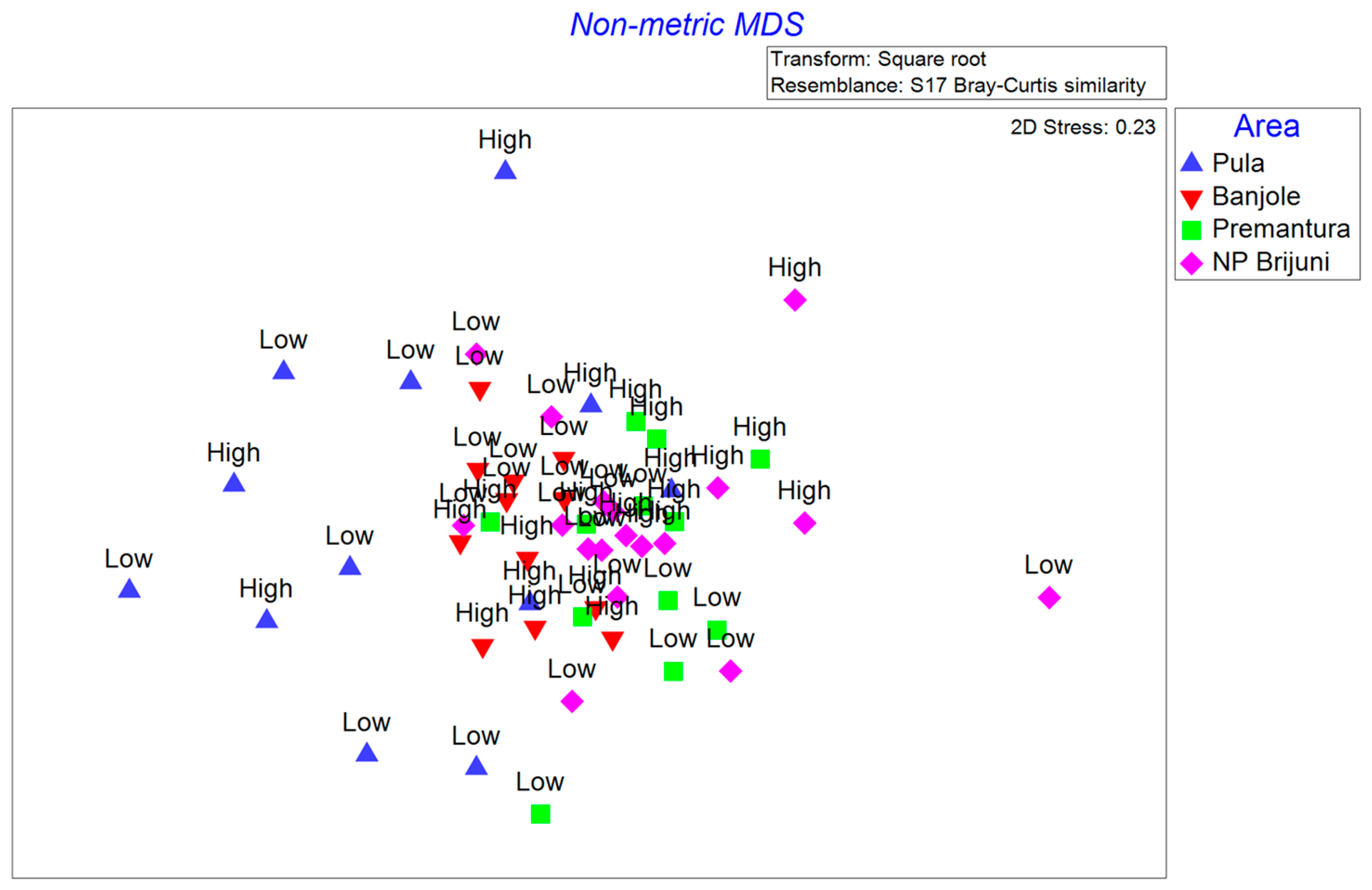
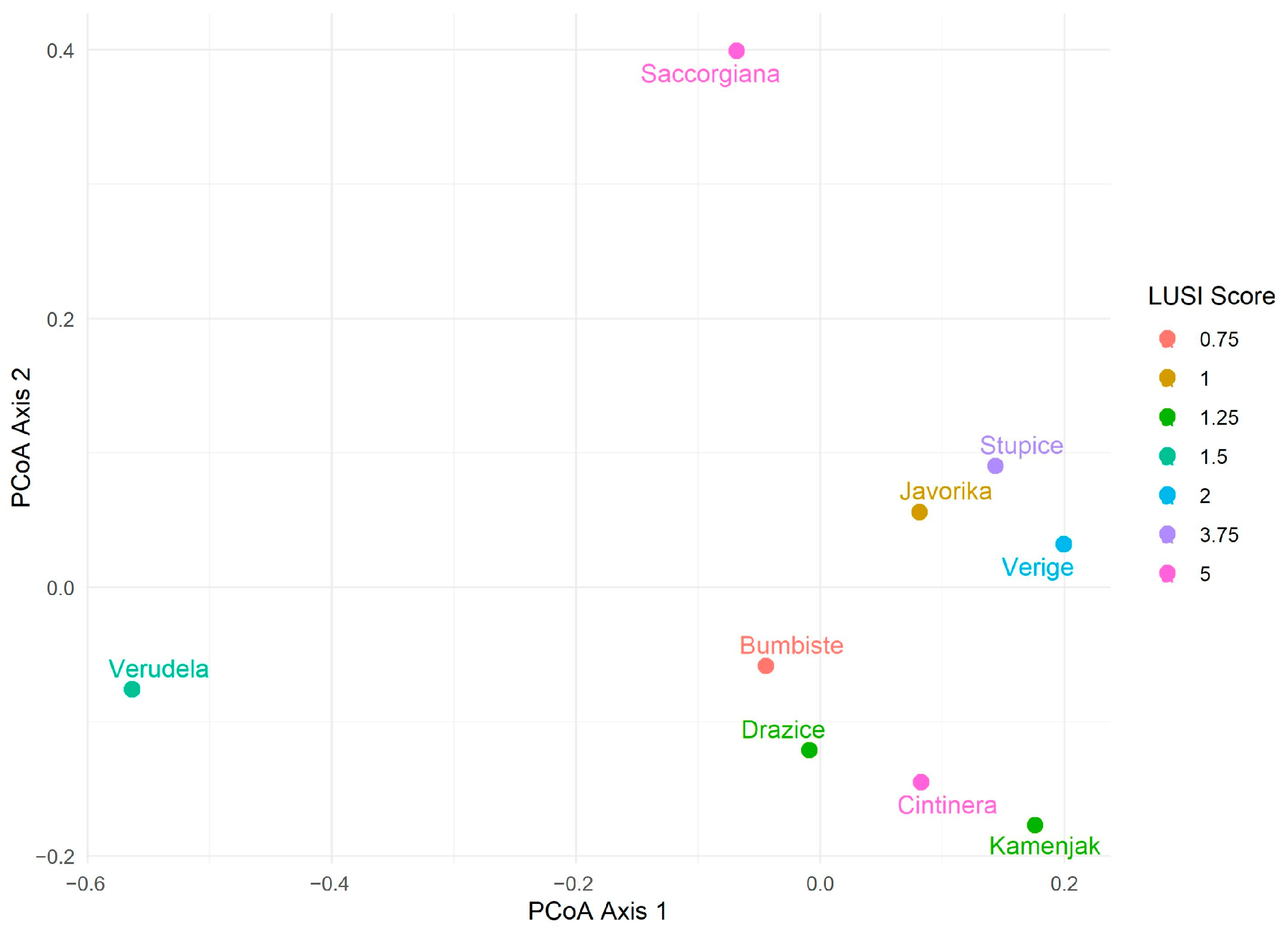
3.3. Polychaete Species Composition Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mikac, B. A Sea of Worms: Polychaete Checklist of the Adriatic Sea. Zootaxa 2015, 3943, 1–172. [Google Scholar] [CrossRef] [PubMed]
- Giangrande, A.; Licciano, M.; Pagliara, P. The Diversity of Diets in Syllidae (Annelida: Polychaeta). Cah. Biol. Mar. 2000, 41, 55–66. [Google Scholar]
- Jumars, P.A.; Dorgan, K.M.; Lindsay, S.M. Diet of Worms Emended: An Update of Polychaete Feeding Guilds. Annu. Rev. Mar. Sci. 2015, 7, 497–520. [Google Scholar] [CrossRef]
- Ockelmann, K.W.; Vahl, O. On the Biology of the Polychaete Glycera Alba, Especially Its Burrowing and Feeding. Ophelia 1970, 8, 275–294. [Google Scholar] [CrossRef]
- Pardo, E.V.; Amaral, A.C.Z. Feeding Behavior of Scolelepis sp. (Polychaeta: Spionidae). Braz. J. Oceanogr. 2004, 52, 74–79. [Google Scholar] [CrossRef]
- Stabili, L.; Licciano, M.; Giangrande, A.; Fanelli, G.; Cavallo, R.A. Sabella spallanzanii Filter-Feeding on Bacterial Community: Ecological Implications and Applications. Mar. Environ. Res. 2006, 61, 74–92. [Google Scholar] [CrossRef]
- Banta, G.T.; Holmer, M.; Jensen, M.H.; Kristensen, E. Effects of Two Polychaete Worms, Nereis Diversicolor and Arenicola Marina, on Aerobic and Anaerobic Decomposition in a Sandy Marine Sediment. Aquat. Microb. Ecol. 1999, 19, 189–204. [Google Scholar] [CrossRef]
- Volkenborn, N.; Hedtkamp, S.I.C.; van Beusekom, J.E.E.; Reise, K. Effects of Bioturbation and Bioirrigation by Lugworms (Arenicola marina) on Physical and Chemical Sediment Properties and Implications for Intertidal Habitat Succession. Estuar. Coast. Shelf Sci. 2007, 74, 331–343. [Google Scholar] [CrossRef]
- Andresen, M.; Kristensen, E. The Importance of Bacteria and Microalgae in the Diet of the Deposit-Feeding Polychaete Arenicola Marina. Ophelia 2002, 56, 179–196. [Google Scholar] [CrossRef]
- Golubkov, S.; Tiunov, A.; Golubkov, M. Food-Web Modification in the Eastern Gulf of Finland after Invasion of Marenzelleria Arctia (Spionidae, Polychaeta). NeoBiota 2021, 66, 75–94. [Google Scholar] [CrossRef]
- Giangrande, A.; Cavallo, A.; Licciano, M.; Mola, E.; Pierri, C.; Trianni, L. Utilization of the Filter Feeder Polychaete Sabella. Aquacult Int. 2005, 13, 129–136. [Google Scholar] [CrossRef]
- Martin, D.; Britayev, T.A. Symbiotic Polychaetes Revisited: An Update of the Known Species and Relationships (1998–2017). In Oceanography and Marine Biology; CRC Press: Boca Raton, FL, USA, 2018; ISBN 978-0-429-45445-5. [Google Scholar]
- Pulido Mantas, T.; Pola, L.; Cerrano, C.; Gambi, M.C.; Calcinai, B. Bioerosion Features of Boring Polydorid Polychaetes in the North Adriatic Sea. Hydrobiologia 2022, 849, 1969–1980. [Google Scholar] [CrossRef]
- Nogueira, M.; Magalhães, W.; Mariano-Neto, E.; Neves, E.; Johnsson, R. Taxonomical and Functional Analyses of Epifaunal Polychaetes Associated with Mussismilia spp.: The Effects of Coral Growth Morphology. PeerJ 2023, 11, e15144. [Google Scholar] [CrossRef]
- Chapman, N.D.; Moore, C.G.; Harries, D.B.; Lyndon, A.R. The Community Associated with Biogenic Reefs Formed by the Polychaete, Serpula Vermicularis. J. Mar. Biol. Assoc. UK 2012, 92, 679–685. [Google Scholar] [CrossRef]
- Giangrande, A.; Licciano, M.; Musco, L. Polychaetes as Environmental Indicators Revisited. Mar. Pollut. Bull. 2005, 50, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Mikac, B.; Licciano, M.; Jaklin, A.; Iveša, L.; Giangrande, A.; Musco, L. Diversity and Distribution Patterns of Hard Bottom Polychaete Assemblages in the North Adriatic Sea (Mediterranean). Diversity 2020, 12, 408. [Google Scholar] [CrossRef]
- Damianidis, P.; Chintiroglou, C.-C. Structure and Functions of Polychaetofauna Living in Mytilus galloprovincialis Assemblages in Thermaikos Gulf (North Aegean Sea). Oceanol. Acta 2000, 23, 323–337. [Google Scholar] [CrossRef]
- Cabana, D.; Sigala, K.; Nicolaidou, A.; Reizopoulou, S. Towards the Implementation of the Water Framework Directive in Mediterranean Transitional Waters: The Use of Macroinvertebrates as Biological Quality Elements. Adv. Oceanogr. Limnol. 2013, 4, 212–240. [Google Scholar] [CrossRef]
- Borja, A.; Franco, J.; Pérez, V. A Marine Biotic Index to Establish the Ecological Quality of Soft-Bottom Benthos Within European Estuarine and Coastal Environments. Mar. Pollut. Bull. 2000, 40, 1100–1114. [Google Scholar] [CrossRef]
- Muxika, I.; Borja, Á.; Bald, J. Using Historical Data, Expert Judgement and Multivariate Analysis in Assessing Reference Conditions and Benthic Ecological Status, According to the European Water Framework Directive. Mar. Pollut. Bull. 2007, 55, 16–29. [Google Scholar] [CrossRef]
- Simboura, N.; Zenetos, A. Benthic Indicators to Use in Ecological Quality Classification of Mediterranean Soft Bottom Marine Ecosystems, Including a New Biotic Index. Mediterr. Mar. Sci. 2002, 3, 77–111. [Google Scholar] [CrossRef]
- Rosenberg, R.; Blomqvist, M.; Nilsson, H.C.; Cederwall, H.; Dimming, A. Marine Quality Assessment by Use of Benthic Species-Abundance Distributions: A Proposed New Protocol within the European Union Water Framework Directive. Mar. Pollut. Bull. 2004, 49, 728–739. [Google Scholar] [CrossRef]
- Antoniadou, C.; Nicolaidou, A.; Chintiroglou, C. Polychaetes Associated with the Sciaphilic Alga Community in the Northern Aegean Sea: Spatial and Temporal Variability. Helgol. Mar. Res. 2004, 58, 168–182. [Google Scholar] [CrossRef]
- Dauvin, J.C.; Ruellet, T. Polychaete/Amphipod Ratio Revisited. Mar. Pollut. Bull. 2007, 55, 215–224. [Google Scholar] [CrossRef]
- Buršić, M.; Iveša, L.; Jaklin, A.; Pijevac, M.A. A Preliminary Study on the Diversity of Invertebrates Associated with Corallina Officinalis Linnaeus in Southern Istrian Peninsula. Acta Adriat. 2019, 60, 127–136. [Google Scholar] [CrossRef]
- Buršić, M.; Jaklin, A.; Arko Pijevac, M.; Bruvo Mađarić, B.; Neal, L.; Pustijanac, E.; Burić, P.; Iveša, N.; Paliaga, P.; Iveša, L. Seasonal Variations in Invertebrates Sheltered among Corallina Officinalis (Plantae, Rodophyta) Turfs along the Southern Istrian Coast (Croatia, Adriatic Sea). Diversity 2023, 15, 1099. [Google Scholar] [CrossRef]
- Abbiati, M.; Bianchi, C.N.; Castelli, A.; Giangrande, A.; Lardicci, C. Distribution of Polychaetes on Hard Substrates of the Midlittoral-Infralittoral Transition Zone, Western Mediterranean. In Systematics, Biology and Morphology of World Polychaeta; Brill: Leiden, The Netherlands, 1991; pp. 421–432. ISBN 978-90-04-62974-5. [Google Scholar]
- Langeneck, J.; Lezzi, M.; Del Pasqua, M.; Musco, L.; Gambi, M.C.; Castelli, A.; Giangrande, A. Non-Indigenous Polychaetes along the Coasts of Italy: A Critical Review. Mediterr. Mar. Sci. 2020, 21, 238–275. [Google Scholar] [CrossRef]
- Gravina, M.F.; Pierri, C.; Mercurio, M.; Nonnis Marzano, C.; Giangrande, A. Polychaete Diversity Related to Different Mesophotic Bioconstructions along the Southeastern Italian Coast. Diversity 2021, 13, 239. [Google Scholar] [CrossRef]
- Faulwetter, S.; Simboura, N.; Katsiaras, N.; Chatzigeorgiou, G.; Arvanitidis, C. Polychaetes of Greece: An Updated and Annotated Checklist. Biodivers. Data J. 2017, 5, e20997. [Google Scholar] [CrossRef]
- Mikac, B.; Giangrande, A.; Licciano, M. Sabellidae and Fabriciidae (Polychaeta) of the Adriatic Sea with Particular Retrospect to the Northern Adriatic and the Description of Two New Species. J. Mar. Biol. Assoc. UK 2013, 93, 1511–1524. [Google Scholar] [CrossRef]
- Dommasnes, A. On the Fauna of Corallina officinalis L. in Western Norway. Sarsia 1969, 38, 71–86. [Google Scholar] [CrossRef]
- Giangrande, A. Polychaete Zonation and Its Relation to Algal Distribution down a Vertical Cliff in the Western Mediterranean (Italy): A Structural Analysis. J. Exp. Mar. Biol. Ecol. 1988, 120, 263–276. [Google Scholar] [CrossRef]
- Somaschini, A. Policheti Della Biocenosi Ad Alghe Fotofile (Facies a Corallina elongata) Nel Lazio Settentrionale. Atti Della Soc. Toscana Sci. Nat. Mem. Ser. B 1988, 95, 83–94. [Google Scholar]
- Tena, J.; Capaccioni-Azzati, R.; Torres-Gavila, F.J.; García-Carrascosa, A.M. Polychaetes Associated with Different Facies of the Photophilic Algal Community in the Chafarinas Archipelago (SW Mediterranean). Bull. Mar. Sci. 2000, 67, 55–72. [Google Scholar]
- Serrano, A.; Martín, G.S.; López, E. Ecology of Syllidae (Annelida: Polychaeta) from Shallow Rocky Environments in the Cantabrian Sea (South Bay of Biscay). Sci. Mar. 2006, 70, 225–235. [Google Scholar] [CrossRef]
- Fraschetti, S.; Bianchi, C.N.; Terlizzi, A.; Fanelli, G.; Morri, C.; Boero, F. Spatial Variability and Human Disturbance in Shallow Subtidal Hard Substrate Assemblages: A Regional Approach. Mar. Ecol. Prog. Ser. 2001, 212, 1–12. [Google Scholar] [CrossRef]
- Bertolino, M.; Betti, F.; Bo, M.; Cattaneo-Vietti, R.; Pansini, M.; Romero, J.; Bavestrello, G. Changes and Stability of a Mediterranean Hard Bottom Benthic Community over 25 Years. J. Mar. Biol. Assoc. UK 2016, 96, 341–350. [Google Scholar] [CrossRef]
- Tillin, H.M.; Budd, G. Coralline Crust-Dominated Shallow Eulittoral Rockpools; Marine Biological Association of the United Kingdom: Plymouth, UK, 2016. [Google Scholar]
- Fauchald, K. The Polychaete Worms. In Definitions and Keys to the Orders, Families and Genera; Science Series; Natural History Museum of Los Angeles County: Los Angeles, CA, USA, 1977. [Google Scholar]
- San Martin, G. Fauna Ibérica, Volume 21—Annelida: Polychaeta II; Museo Nacional de Ciencias Naturales, Consejo Superior de Investigaciones Científicas: Madrid, Spain, 2003; ISBN 978-84-00-08294-9. [Google Scholar]
- Vieitez, J.M. Fauna Ibérica, Volume 25—Annelida: Polychaeta I; Museo Nacional de Ciencias Naturales, Consejo Superior de Investigaciones Científicas: Madrid, Spain, 2004; ISBN 978-84-00-07010-6. [Google Scholar]
- Parapar, J.; Alós, C.; Núñez, J.; Moreira, J.; López, E.; Aguirrezabalaga, F.; Besteiro, C.; Martínez, A. Fauna Ibérica, Volume 36—Annelida: Polychaeta III; Museo Nacional de Ciencias Naturales, Consejo Superior de Investigaciones Científicas: Madrid, Spain, 2012; ISBN 978-84-00-08294-9. [Google Scholar]
- Parapar, J.; Moreira, J.; Núñez, J.; Barnich, R.; del Carmen Brito, M.; Fiege, D.; Capaccioni-Azzati, R.; El-Haddad, M. Fauna Ibérica, Volume 41—Annelida: Polychaeta IV; Museo Nacional de Ciencias Naturales, Consejo Superior de Investigaciones Científicas: Madrid, Spain, 2015; ISBN 978-84-00-08294-9. [Google Scholar]
- Blake, J.A.; Göransson, P. Redescription of Tharyx Killariensis (Southern) from Ireland and Description of Two New Species of Tharyx from the Kattegat, Sweden (Polychaeta, Cirratulidae). Zootaxa 2015, 4039, 501–515. [Google Scholar] [CrossRef]
- Gravina, M.; Lezzi, M.; Bonifazi, A.; Giangrande, A. The Genus Nereis L., 1758 (Polychaeta, Nereididae): State of the Art for Identification of Mediterranean Species. Atti Della Soc. Toscana Sci. Nat. Mem. Ser. B 2015, 122, 147–164. [Google Scholar] [CrossRef]
- Surugiu, V.; Boltachova, N.A.; Lisitskaya, E.V. The Current Status of Eunereis Longissima (Johnston, 1840) (Polychaeta: Nereididae) in the Black Sea. Cah. Biol. Mar. 2018, 59, 61–69. [Google Scholar] [CrossRef]
- WoRMS Editorial Board WoRMS—World Register of Marine Species—IMIS. Available online: https://www.marinespecies.org/imis.php?dasid=1447&doiid=170 (accessed on 11 March 2025).
- Buršić, M.; Iveša, L.; Jaklin, A.; Arko Pijevac, M.; Bruvo Mađarić, B.; Neal, L.; Pustijanac, E.; Burić, P.; Iveša, N.; Paliaga, P. Changes in Composition of Mollusks within Corallina Officinalis Turfs in South Istria, Adriatic Sea, as a Response to Anthropogenic Impact. Diversity 2023, 15, 939. [Google Scholar] [CrossRef]
- Flo, E.; Garcés, E.; Camp, J. Land Uses Simplified Index (LUSI): Determining Land Pressures and Their Link With Coastal Eutrophication. Front. Mar. Sci. 2019, 6, 18. [Google Scholar] [CrossRef]
- Tischler, W. Grundzüge der Terrestrischen Tierökologie; Springer: Berlin/Heidelberg, Germany, 1949; ISBN 978-3-663-02549-8. [Google Scholar]
- Travizi, A. The Nematode Fauna of the Northern Adriatic Offshore Sediments: Community Structure and Biodiversity. Acta Adriat. 2010, 51, 169–180. [Google Scholar]
- Năstase, A.; Honț, Ș.; Iani, M.; Paraschiv, M.; Cernișencu, I.; Năvodaru, I. Ecological Status of Fish Fauna from Razim Lake and the Adjacent Area, the Danube Delta Biosphere Reserve, Romania. Acta Ichthyol. Piscat. 2022, 52, 43–52. [Google Scholar] [CrossRef]
- Clarke, K.R.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation, 2nd ed.; PRIMER-E, Ltd., Plymouth Marine Laboratory: Plymouth, UK, 2001. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. In R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2023. [Google Scholar]
- Clarke, K.R.; Gorley, R.N. PRIMER v6: User Manual/Tutorial; PRIMER-E, Ltd., Plymouth Marine Laboratory: Plymouth, UK, 2006. [Google Scholar]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods—ScienceOpen; PRIMER-E: Plymouth, UK, 2008. [Google Scholar]
- Zavodnik, D. Prispevek k Poznavanju Naselja Cystoseira Barbata (Good. & Wood.) C. Ag. v Severnem Jadranu. Biol. Vest 1965, 13, 87–101. [Google Scholar]
- Zavodnik, D. Dinamika Litoralnega Fitala Na Zahodnoistrski Obali. Razpr. Slov. Akad. Znan. Umet. 1967, 10, 5–71. [Google Scholar]
- Amoureux, L.; Katzmann, W. Note Faunistique et Écologique Sur Une Collection d’Annélides Polychètes de Substrats Rocheux Circalittoraux de La Région de Rovinj (Yougoslavie). Zool Anz 1971, 186, 114–122. [Google Scholar]
- Katzmann, W. Polychaeten (Errantier, Sedentarier) aus nordadriatischen Cystoseira-Beständen und deren Epiphyten. Oecologia 1971, 8, 31–51. [Google Scholar] [CrossRef]
- Amoureux, L. Annélides Polychètes de l’îlot Banjole (Prés de Rovinj, Haute–Adriatique). Cah. Biol. Mar. 1975, 16, 231–244. [Google Scholar]
- Mikac, B.; Musco, L. Faunal and Biogeographic Analysis of Syllidae (Polychaeta) from Rovinj (Croatia, Northern Adriatic Sea). Sci. Mar. 2010, 74, 353–370. [Google Scholar] [CrossRef]
- Pitacco, V.; Mavrič, B.; Orlando-Bonaca, M.; Lipej, L. Rocky Macrozoobenthos Mediolittoral Community in the Gulf of Trieste (North Adriatic) along a Gradient of Hydromorphological Modifications. Acta Adriat. 2013, 54, 67–86. [Google Scholar]
- Pitacco, V.; Orlando-Bonaca, M.; Mavrič, B.; Lipej, L. Macrofauna Associated with a Bank of Cladocora Caespitosa (Anthozoa, Scleractinia) in the Gulf of Trieste (Northern Adriatic). Ann. Anal. Istrske Mediter. Štud. Ser. Hist. Nat. 2014, 24, 1–14. [Google Scholar]
- Pitacco, V.; Chatzigeorgiou, G.; Mikac, B.; Lipej, L. Ecological Patterns of Polychaete Assemblages Associated with the Mediterranean Stony Coral Cladocora Caespitosa (Linnaeus, 1767): A Comparison of Sites in Two Biogeographic Zones (Adriatic and Aegean Sea). Mediterr. Mar. Sci. 2021, 22, 532–551. [Google Scholar] [CrossRef]
- Fraschetti, S.; Giangrande, A.; Terlizzi, A.; Miglietta, M.; Della Tommasa, L.; Boero, F. Spatio-Temporal Variation of Hydroids and Polychaetes Associated with Cystoseira Amentacea (Fucales: Phaeophyceae). Mar. Biol. 2002, 140, 949–957. [Google Scholar] [CrossRef]
- Cantone, G. Distribution of Benthic Polychaetous Annelids in the Adriatic Sea with Zoogeographic Consideration. Biogeogr.-J. Integr. Biogeogr. 2003, 24, 169–193. [Google Scholar] [CrossRef]
- Giangrande, A.; Delos, A.L.; Fraschetti, S.; Musco, L.; Licciano, M.; Terlizzi, A. Polychaete Assemblages along a Rocky Shore on the South Adriatic Coast (Mediterranean Sea): Patterns of Spatial Distribution. Mar. Biol. 2003, 143, 1109–1116. [Google Scholar] [CrossRef]
- Çinar, M.E.; Gonlugur-Demirci, G. Polychaete Assemblages on Shallow-Water Benthic Habitats along the Sinop Peninsula (Black Sea, Turkey). Cah. Biol. Mar. 2005, 46, 253. [Google Scholar]
- Gambi, M.C.; Musco, L.; Giangrande, A.; Badalamenti, F.; Micheli, F.; Kroeker, K.J. Distribution and Functional Traits of Polychaetes in a CO2 Vent System: Winners and Losers among Closely Related Species. Mar. Ecol. Prog. Ser. 2016, 550, 121–134. [Google Scholar] [CrossRef]
- Benedetti-Cecchi, L.; Bulleri, F.; Cinelli, F. The Interplay of Physical and Biological Factors in Maintaining Mid-Shore and Low-Shore Assemblages on Rocky Coasts in the North-West Mediterranean. Oecologia 2000, 123, 406–417. [Google Scholar] [CrossRef]
- Martins, R.; Magalhães, L.; Peter, A.; San Martín, G.; Rodrigues, A.M.; Quintino, V. Diversity, Distribution and Ecology of the Family Syllidae (Annelida) in the Portuguese Coast (Western Iberian Peninsula). Helgol. Mar. Res. 2013, 67, 775–788. [Google Scholar] [CrossRef]
- Çinar, M.E. Ecology of Syllidae (Annelida: Polychaeta) from Northern Cyprus (Eastern Mediterranean Sea). Bull. Mar. Sci. 2003, 72, 795–811. [Google Scholar]
- Musco, L. Ecology and Diversity of Mediterranean Hard-Bottom Syllidae (Annelida): A Community-Level Approach. Mar. Ecol. Prog. Ser. 2012, 461, 107–119. [Google Scholar] [CrossRef]
- Smith, V.H. Eutrophication of Freshwater and Coastal Marine Ecosystems a Global Problem. Env. Sci. Pollut. Res. 2003, 10, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Krause-Jensen, D.; Middelboe, A.L.; Carstensen, J.; Dahl, K. Spatial Patterns of Macroalgal Abundance in Relation to Eutrophication. Mar. Biol. 2007, 152, 25–36. [Google Scholar] [CrossRef]
- Collén, J.; Pinto, E.; Pedersén, M.; Colepicolo, P. Induction of Oxidative Stress in the Red Macroalga Gracilaria Tenuistipitata by Pollutant Metals. Arch. Env. Contam. Toxicol. 2003, 45, 337–342. [Google Scholar] [CrossRef]
- Dafforn, K.A.; Kelaher, B.P.; Simpson, S.L.; Coleman, M.A.; Hutchings, P.A.; Clark, G.F.; Knott, N.A.; Doblin, M.A.; Johnston, E.L. Polychaete Richness and Abundance Enhanced in Anthropogenically Modified Estuaries Despite High Concentrations of Toxic Contaminants. PLoS ONE 2013, 8, e77018. [Google Scholar] [CrossRef]
- Žunec, A.; Cvitković, I.; Despalatović, M.; Žuljević, A.; Nejašmić, J.; Lučić, P. New Records of Hard-Bottom Polychaete Species in the Central Adriatic Sea. Acta Adriat. 2024, 65, 51–59. [Google Scholar] [CrossRef]
- Travizi, A.; Jaklin, A.; Mikac, B.; Nerlović, V.; Balković, I. A Baseline Study of Macrofauna from the North Adriatic Seaports Raša, Rijeka, Bakar and Omišalj. In Proceedings of the 13th Croatian Biological Congress with International Participation, Croatian Biological Society, Poreč, Croatia, 19–23 September 2018; pp. 242–243. [Google Scholar]
- Spagnolo, A.; Auriemma, R.; Bacci, T.; Balković, I.; Bertasi, F.; Bolognini, L.; Cabrini, M.; Cilenti, L.; Cuicchi, C.; Cvitković, I.; et al. Non-Indigenous Macrozoobenthic Species on Hard Substrata of Selected Harbours in the Adriatic Sea. Mar. Pollut. Bull. 2019, 147, 150–158. [Google Scholar] [CrossRef]
- Çinar, M.E. Re-Description of Timarete Punctata (Polychaeta: Cirratulidae) and Its Occurrence in the Mediterranean Sea. Sci. Mar. 2007, 71, 755–764. [Google Scholar] [CrossRef]
- Sala-Mirete, A.; López, E.; Sánchez-Fernández, O.; Marcos, C.; Pérez-Ruzafa, A. First Records of Non-Indigenous Timarete Caribous (Grube, 1859) (Polychaeta; Cirratulidae) in the Western Mediterranean, and Its Ecology in the Mar Menor (Murcia, SE Spain). BioInvasions Rec. 2023, 12, 167–185. [Google Scholar] [CrossRef]
- Lezzi, M.; (ARPAE, Regional Agency for Environmental Prevention and Energy of Emilia-Romagna, Oceanographic Unit Daphne—Cesenatico (FC)). Personal Comunication, 2025.



| Family | Species | Relative Abundance (%) | Frequency of Occurrence (%) | ||||
|---|---|---|---|---|---|---|---|
| Without A.P. | Under A.P. | All Locations | Without A.P. | Under A.P. | All Locations | ||
| Cirratulidae | Tharyx killariensis | 0.26 | 0.14 | 0.20 | 6.67 | 4.17 | 5.56 |
| Timarete sp. | 0.53 | 0.55 | 0.54 | 10.00 | 16.67 | 12.96 | |
| Cirratulidae indet. | 1.58 | 0.83 | 1.22 | 3.33 | 8.33 | 5.56 | |
| Dorvilleidae | Dorvillea rubrovittata | 0.13 | 0.00 | 0.07 | 3.33 | 0.00 | 1.85 |
| Eunicidae | Lysidice collaris | 0.00 | 0.14 | 0.07 | 0.00 | 4.17 | 1.85 |
| Lysidice ninetta | 0.26 | 0.14 | 0.20 | 3.33 | 4.17 | 3.70 | |
| Lysidice unicornis | 0.66 | 0.69 | 0.68 | 13.33 | 20.83 | 16.67 | |
| Lumbrinereidae | Scoletoma funchalensis | 0.00 | 0.28 | 0.14 | 0.00 | 8.33 | 3.70 |
| Scoletoma laurentiana | 0.13 | 0.00 | 0.07 | 3.33 | 0.00 | 1.85 | |
| Maldanidae | Nicomachinae indet. | 0.13 | 0.00 | 0.07 | 3.33 | 0.00 | 1.85 |
| Nereididae | Eunereis longissima | 0.13 | 0.00 | 0.07 | 3.33 | 0.00 | 1.85 |
| Neanthes sp. | 0.00 | 0.28 | 0.14 | 0.00 | 8.33 | 3.70 | |
| Nereis pelagica | 0.40 | 0.97 | 0.68 | 10.00 | 20.83 | 14.81 | |
| Nereis cf. pelagica | 9.37 | 6.38 | 7.91 | 63.33 | 70.83 | 66.67 | |
| Nereis perivisceralis | 0.53 | 0.14 | 0.34 | 13.33 | 4.17 | 9.26 | |
| Nereis rava | 1.85 | 1.11 | 1.49 | 26.67 | 16.67 | 22.22 | |
| Nereis sp. | 0.13 | 1.39 | 0.74 | 3.33 | 12.50 | 7.41 | |
| Perinereis cultrifera | 0.00 | 0.83 | 0.41 | 0.00 | 20.83 | 9.26 | |
| Perinereis macropus | 0.00 | 0.28 | 0.14 | 0.00 | 4.17 | 1.85 | |
| Platynereis dumerilii | 8.84 | 3.05 | 6.02 | 60.00 | 37.50 | 50.00 | |
| Platynereis nadie | 0.13 | 0.00 | 0.07 | 3.33 | 0.00 | 1.85 | |
| Nereididae indet. | 1.58 | 2.08 | 1.83 | 33.33 | 37.50 | 35.19 | |
| Oenoidae | Arabella iricolor | 1.06 | 1.39 | 1.22 | 20.00 | 29.17 | 24.07 |
| Ophellidae | Ophellidae indet. juv. | 0.00 | 0.14 | 0.07 | 0.00 | 4.17 | 1.85 |
| Orbinidae | Nainereis cf. quadricuspida | 0.00 | 0.28 | 0.14 | 0.00 | 8.33 | 3.70 |
| Phyllodocidae | Eteone foliosa | 0.13 | 0.00 | 0.07 | 3.33 | 0.00 | 1.85 |
| Phyllodocidae indet. | 0.13 | 0.00 | 0.07 | 3.33 | 0.00 | 1.85 | |
| Polynoidae | Harmothoe imbricata | 0.13 | 0.00 | 0.07 | 3.33 | 0.00 | 1.85 |
| Lepidonotus clava | 0.13 | 0.28 | 0.20 | 3.33 | 8.33 | 5.56 | |
| Lepidonotus sp. | 0.53 | 0.97 | 0.74 | 13.33 | 16.67 | 14.81 | |
| Polynoidae indet. | 0.13 | 0.00 | 0.07 | 3.33 | 0.00 | 1.85 | |
| Sabellidae | Sabellidae indet. | 1.19 | 0.83 | 1.01 | 23.33 | 8.33 | 16.67 |
| Syllidae/Syllinae | Branchiosyllis exilis | 0.13 | 0.00 | 0.07 | 3.33 | 0.00 | 1.85 |
| Haplosyllis spongicola | 0.00 | 0.42 | 0.20 | 0.00 | 8.33 | 3.70 | |
| Syllis alternata | 0.79 | 1.25 | 1.01 | 16.67 | 12.50 | 14.81 | |
| Syllis armillaris | 1.32 | 0.97 | 1.15 | 16.67 | 8.33 | 12.96 | |
| Syllis beneliahua | 1.06 | 1.53 | 1.28 | 20.00 | 25.00 | 22.22 | |
| Syllis columbretensis | 1.19 | 0.28 | 0.74 | 20.00 | 8.33 | 14.81 | |
| Syllis corallicola | 0.00 | 0.14 | 0.07 | 0.00 | 4.17 | 1.85 | |
| Syllis gerlachi | 1.98 | 3.47 | 2.70 | 33.33 | 37.50 | 35.19 | |
| Syllis gerundensis | 2.51 | 10.40 | 6.36 | 43.33 | 75.00 | 57.41 | |
| Syllis garciai | 0.13 | 0.28 | 0.20 | 3.33 | 4.17 | 3.70 | |
| Syllis gracilis | 1.58 | 1.39 | 1.49 | 36.67 | 16.67 | 27.78 | |
| Syllis krohnii | 0.66 | 0.55 | 0.61 | 16.67 | 12.50 | 14.81 | |
| Syllis rosea | 19.26 | 8.60 | 14.06 | 80.00 | 75.00 | 77.78 | |
| Syllis prolifera | 11.74 | 9.43 | 10.62 | 53.33 | 54.17 | 53.70 | |
| Syllis pulvinata | 0.00 | 0.28 | 0.14 | 0.00 | 8.33 | 3.70 | |
| Syllis sp. | 0.53 | 3.33 | 1.89 | 13.33 | 20.83 | 16.67 | |
| Syllis variegata | 1.72 | 1.80 | 1.76 | 26.67 | 41.67 | 33.33 | |
| Trypanosyllis coeliaca | 0.13 | 0.00 | 0.07 | 3.33 | 0.00 | 1.85 | |
| Syllidae/Exogoninae | Brania arminii | 1.45 | 0.00 | 0.74 | 23.33 | 0.00 | 12.96 |
| Brania pusilla | 0.79 | 1.80 | 1.28 | 10.00 | 41.67 | 24.07 | |
| Exogone (Exogone) dispar | 3.30 | 3.05 | 3.18 | 43.33 | 50.00 | 46.30 | |
| Exogone (Exogone) verugera | 0.40 | 0.28 | 0.34 | 10.00 | 8.33 | 9.26 | |
| Salvatoria clavata | 1.06 | 0.55 | 0.81 | 20.00 | 12.50 | 16.67 | |
| Salvatoria limbata | 2.37 | 1.80 | 2.10 | 23.33 | 29.17 | 25.93 | |
| Salvatoria yraidae | 1.06 | 0.55 | 0.81 | 20.00 | 12.50 | 16.67 | |
| Sphaerosyllis austriaca | 3.69 | 6.93 | 5.27 | 46.67 | 54.17 | 50.00 | |
| Sphaerosyllis pirifera | 7.78 | 12.90 | 10.28 | 53.33 | 58.33 | 55.56 | |
| Syllidae/Eusyllinae | Paraehlersia ferrugina | 3.03 | 4.58 | 3.79 | 43.33 | 45.83 | 44.44 |
| Pyonosyllis dionisi | 0.00 | 0.28 | 0.14 | 0.00 | 4.17 | 1.85 | |
| Syllidae/Autolitinae | Autolitinae indet. | 0.26 | 0.00 | 0.14 | 6.67 | 0.00 | 3.70 |
| Relative Abundance | Without A.P. | Under A.P. | ||
|---|---|---|---|---|
| Number of Species | % | Number of Species | % | |
| eudominant (>10%) | 2 | 3.70 | 2 | 3.70 |
| dominant (5–10%) | 3 | 5.56 | 4 | 7.41 |
| subdominant (2–5%) | 5 | 9.26 | 5 | 9.26 |
| recedent (1–2%) | 11 | 20.37 | 9 | 16.67 |
| subrecedent (<1%) | 33 | 61.11 | 34 | 62.96 |
| TOTAL | 54 | 100.00 | 54 | 100.00 |
| Frequency of Occurrence | Without A.P. | Under A.P. | ||
|---|---|---|---|---|
| Number of Species | % | Number of Species | % | |
| very frequent (75–100%) | 1 | 1.85 | 0 | 0.00 |
| frequent (50–75%) | 4 | 7.41 | 6 | 11.11 |
| widespread (25–50%) | 8 | 14.81 | 8 | 14.81 |
| rare (0–25%) | 41 | 75.93 | 40 | 74.07 |
| TOTAL | 54 | 100.00 | 54 | 100.00 |
| Species | Contribution | SD | Ratio | Mean High | Mean Low |
|---|---|---|---|---|---|
| Sphaerosyllis pirifera | 0.0628 | 0.0520 | 1.2075 | 23.25 | 11.80 |
| Syllis rosea | 0.0615 | 0.0565 | 1.0891 | 15.50 | 29.20 |
| Syllis prolifera | 0.0541 | 0.0382 | 1.4172 | 17.00 | 17.80 |
| Syllis gerundensis | 0.0488 | 0.0409 | 1.1948 | 18.75 | 3.80 |
| Platynereis dumerilii | 0.0361 | 0.0320 | 1.1257 | 5.50 | 13.40 |
| Sphaerosyllis austriaca | 0.0320 | 0.0464 | 0.6890 | 12.50 | 5.60 |
| Nereis cf. pelagica | 0.0292 | 0.0218 | 1.3390 | 11.50 | 14.20 |
| Paraehlersia ferrugina | 0.0217 | 0.0223 | 0.9754 | 8.25 | 4.60 |
| Syllis sp. | 0.0198 | 0.0193 | 1.0273 | 6.00 | 0.80 |
| Syllis gerlachi | 0.0176 | 0.0188 | 0.9345 | 6.25 | 3.00 |
| Exogone dispar | 0.0130 | 0.0099 | 1.3134 | 5.50 | 5.00 |
| Salvatoria limbata | 0.0109 | 0.0086 | 1.2663 | 3.25 | 3.60 |
| Brania pusilla | 0.0107 | 0.0095 | 1.1254 | 3.25 | 1.20 |
| Syllis gracilis | 0.0092 | 0.0061 | 1.4943 | 2.50 | 2.40 |
| Syllis armillaris | 0.0085 | 0.0097 | 0.8784 | 1.75 | 2.00 |
| Syllis variegata | 0.0080 | 0.0062 | 1.2922 | 3.25 | 2.60 |
| Nereis rava | 0.0074 | 0.0050 | 1.4831 | 2.00 | 2.80 |
| Brania arminii | 0.0072 | 0.0053 | 1.3599 | 0.00 | 2.20 |
| Arabella iricolor | 0.0072 | 0.0084 | 0.8601 | 2.50 | 1.60 |
| Nereis sp. | 0.0071 | 0.0120 | 0.5892 | 2.50 | 0.20 |
| Syllis alternata | 0.0065 | 0.0051 | 1.2800 | 2.25 | 1.20 |
| Syllis beneliahua | 0.0063 | 0.0088 | 0.7153 | 2.75 | 1.60 |
| Lepidonotus sp. | 0.0061 | 0.0073 | 0.8355 | 1.75 | 0.80 |
| Salvatoria clavata | 0.0054 | 0.0049 | 1.1065 | 1.00 | 1.60 |
| Syllis columbretensis | 0.0054 | 0.0053 | 1.0188 | 0.50 | 1.80 |
| Nereis pelagica | 0.0050 | 0.0039 | 1.2872 | 1.75 | 0.60 |
| Perinereis cultrifera | 0.0046 | 0.0042 | 1.0917 | 1.50 | 0.00 |
| Lysidice unicornis | 0.0038 | 0.0028 | 1.3515 | 1.25 | 1.00 |
| Timarete sp. | 0.0038 | 0.0040 | 0.9448 | 1.00 | 0.80 |
| Salvatoria yraidae | 0.0036 | 0.0030 | 1.2065 | 1.00 | 1.60 |
| Syllis krohnii | 0.0033 | 0.0035 | 0.9650 | 1.00 | 1.00 |
| Nereis perivisceralis | 0.0024 | 0.0023 | 1.0331 | 0.25 | 0.80 |
| Exogone verugera | 0.0024 | 0.0022 | 1.0839 | 0.50 | 0.60 |
| Haplosyllis spongicola | 0.0023 | 0.0025 | 0.9121 | 0.75 | 0.00 |
| Syllis garciai | 0.0020 | 0.0027 | 0.7428 | 0.50 | 0.20 |
| Neanthes sp. | 0.0019 | 0.0020 | 0.9279 | 0.50 | 0.00 |
| Tharyx killariensis | 0.0019 | 0.0025 | 0.7426 | 0.25 | 0.40 |
| Pyonosyllis dionisi | 0.0019 | 0.0034 | 0.5446 | 0.50 | 0.00 |
| Lysidice ninetta | 0.0017 | 0.0025 | 0.6811 | 0.25 | 0.40 |
| Lepidonotus clava | 0.0016 | 0.0018 | 0.9166 | 0.50 | 0.20 |
| Nainereis cf. quadricuspida | 0.0016 | 0.0029 | 0.5491 | 0.50 | 0.00 |
| Syllis pulvinata | 0.0015 | 0.0016 | 0.9385 | 0.50 | 0.00 |
| Perinereis macropus | 0.0014 | 0.0025 | 0.5524 | 0.50 | 0.00 |
| Scoletoma funchalensis | 0.0014 | 0.0025 | 0.5524 | 0.50 | 0.00 |
| Branchiosyllis exilis | 0.0009 | 0.0018 | 0.4801 | 0.00 | 0.20 |
| Eunereis longissima | 0.0009 | 0.0018 | 0.4801 | 0.00 | 0.20 |
| Platynereis nadie | 0.0009 | 0.0018 | 0.4801 | 0.00 | 0.20 |
| Syllis corallicola | 0.0007 | 0.0012 | 0.5524 | 0.25 | 0.00 |
| Lysidice collaris | 0.0007 | 0.0012 | 0.5524 | 0.25 | 0.00 |
| Dorvillea rubrovittata | 0.0006 | 0.0013 | 0.4833 | 0.00 | 0.20 |
| Harmothoe imbricata | 0.0006 | 0.0013 | 0.4833 | 0.00 | 0.20 |
| Scoletoma laurentiana | 0.0006 | 0.0012 | 0.4839 | 0.00 | 0.20 |
| Trypanosyllis coeliaca | 0.0005 | 0.0010 | 0.4849 | 0.00 | 0.20 |
| Eteone foliosa | 0.0005 | 0.0010 | 0.4849 | 0.00 | 0.20 |
| Source | Df | SS | MS | Pseudo-F | P(perm) |
|---|---|---|---|---|---|
| Pressure = Pr | 1 | 4093.2 | 4093.2 | 1.7515 | 0.028 |
| Area = Ar | 3 | 22,863 | 7620.9 | 3.2611 | 0.0001 |
| Pr × Ar | 3 | 9322.5 | 3107.5 | 1.3297 | 0.0601 |
| Res | 46 | 107,500 | 2336.9 | ||
| Total | 53 | 143,320 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pitacco, V.; Buršić, M.; Žunec, A.; Burić, P.; Iveša, N.; Kovačić, I.; Pustijanac, E.; Iveša, L.; Vojvoda Zeljko, T.; Mavrič, B. Polychaetes Associated with Calcareous Red Algae Corallina officinalis in the Northern Adriatic Sea. Diversity 2025, 17, 302. https://doi.org/10.3390/d17050302
Pitacco V, Buršić M, Žunec A, Burić P, Iveša N, Kovačić I, Pustijanac E, Iveša L, Vojvoda Zeljko T, Mavrič B. Polychaetes Associated with Calcareous Red Algae Corallina officinalis in the Northern Adriatic Sea. Diversity. 2025; 17(5):302. https://doi.org/10.3390/d17050302
Chicago/Turabian StylePitacco, Valentina, Moira Buršić, Ante Žunec, Petra Burić, Neven Iveša, Ines Kovačić, Emina Pustijanac, Ljiljana Iveša, Tanja Vojvoda Zeljko, and Borut Mavrič. 2025. "Polychaetes Associated with Calcareous Red Algae Corallina officinalis in the Northern Adriatic Sea" Diversity 17, no. 5: 302. https://doi.org/10.3390/d17050302
APA StylePitacco, V., Buršić, M., Žunec, A., Burić, P., Iveša, N., Kovačić, I., Pustijanac, E., Iveša, L., Vojvoda Zeljko, T., & Mavrič, B. (2025). Polychaetes Associated with Calcareous Red Algae Corallina officinalis in the Northern Adriatic Sea. Diversity, 17(5), 302. https://doi.org/10.3390/d17050302








