Changes in the Species Composition and Structure of Large-Diameter Trees Along a Narrow Latitudinal Gradient in Subtropical China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Sampling Design and Plant Census
2.3. Statistical Analysis
2.3.1. Species Composition Analysis
2.3.2. Calculation of Species Diversity Indices
2.3.3. Species Rank Curve
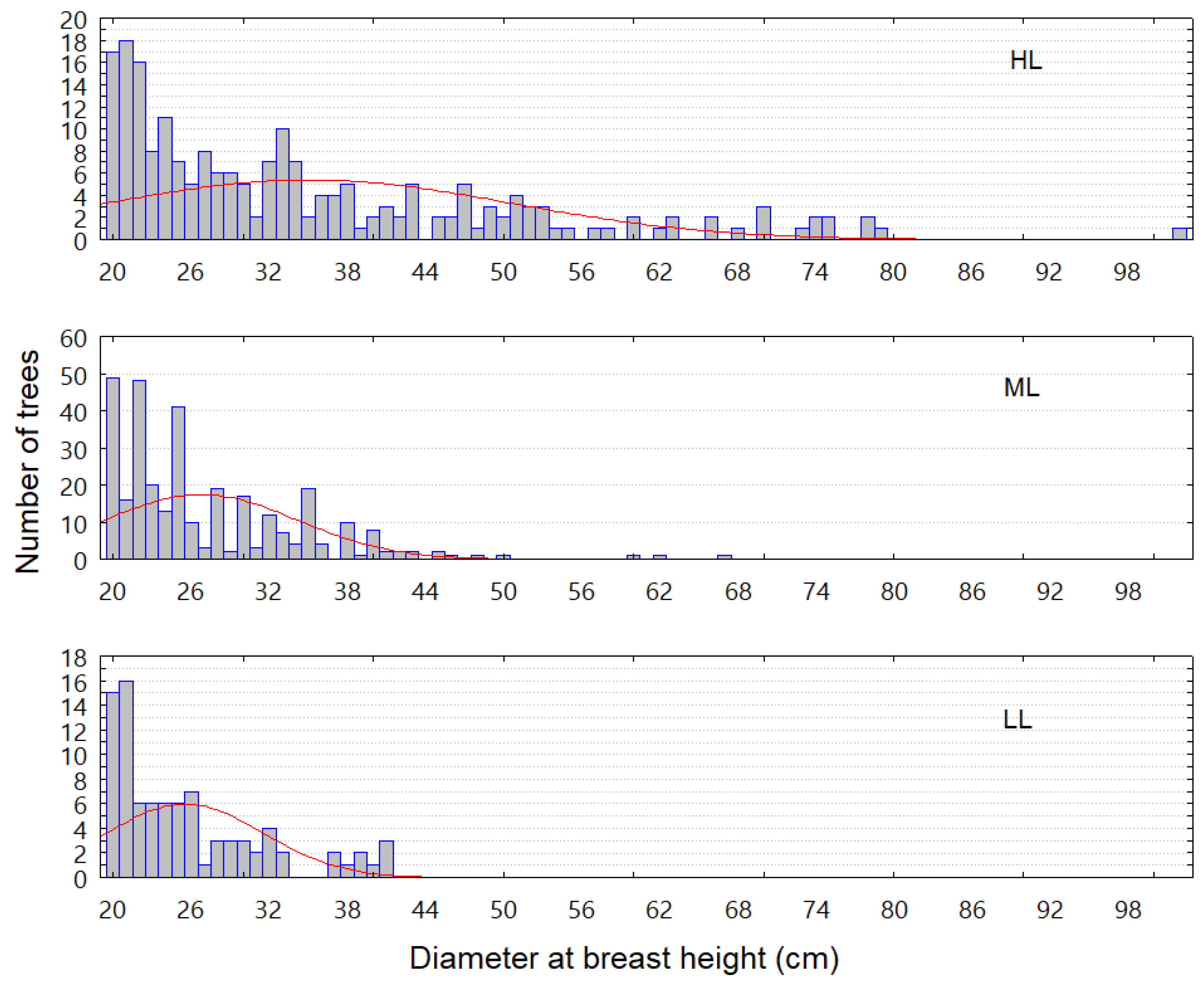
2.3.4. Diameter Structure Analysis
2.3.5. Principal Coordinates Analysis
2.3.6. Multi-Response Permutation Procedure
2.3.7. Indicator Species Analysis
2.3.8. Statistical Analyses
3. Results
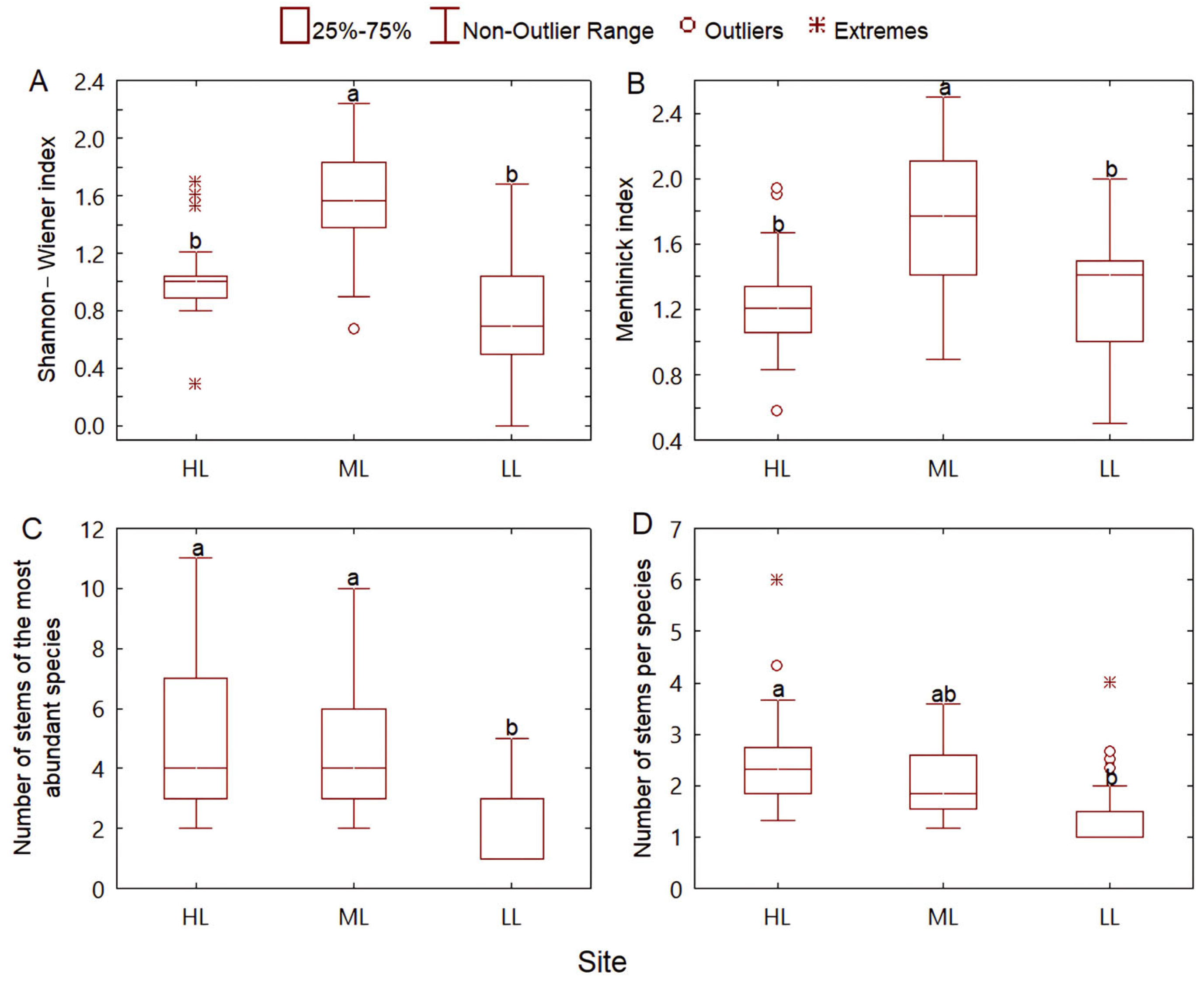
3.1. Species Composition and Diversity
3.2. Community Structure
3.3. Indicator Species
4. Discussion
4.1. Changes in the Species Composition of LDTs at Small-Scale Latitudes
4.2. Changes in the Community Structure and Indicator Species at Small-Scale Latitudes
4.3. Analysis of Suitable LDT Species in Guangdong
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ali, A.; Sanaei, A.; Li, M.; Nalivan, O.A.; Pour, M.J.; Valipour, A.; Karami, J.; Aminpour, M.; Kaboli, H.; Askari, Y. Big-trees—Energy mechanism underlies forest diversity and aboveground biomass. For. Ecol. Manag. 2020, 461, 117968. [Google Scholar] [CrossRef]
- Chiang, C.; Olsen, J.E.; Basler, D.; Bankestad, D.; Hoch, G. Latitude and Weather Influences on Sun Light Quality and the Relationship to Tree Growth. Forests 2019, 10, 610. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, Z.; Fang, J. The species-energy hypothesis as a mechanism for species richness patterns. Biodivers. Sci. 2009, 17, 613–624. (In Chinese) [Google Scholar]
- Willig, M.R.; Presley, S.J. Latitudinal Gradients of Biodiversity: Patterns, Processes, and Prospects. In Encyclopedia of Biodiversity, 3rd ed.; Academic Press: Cambridge, MA, USA, 2024; Volume 6, pp. 754–772. [Google Scholar]
- de Aledo, J.G.; Paneghel, M.; Cayuela, L.; Matas-Granados, L.; Ben Saadi, C.; Salinas, N.; La Torre-Cuadros, M.D.L.A.; Garcia-Villacorta, R.; Macia, M.J. Floristic diversity, composition and dominance across Amazonian forest types respond differently to latitude. Palabras Clave J. Biogeogr. 2023, 50, 685–698. [Google Scholar] [CrossRef]
- Hawkins, B.A.; Diniz, J.A.F. ‘Latitude’ and geographic patterns in species richness. Ecography 2004, 27, 268–272. [Google Scholar] [CrossRef]
- Pianka, R.E. Latitudinal gradients in species diversity. Trends Ecol. Evol. 1989, 8, 223. [Google Scholar] [CrossRef]
- Wu, C.; Jiang, B.; Yuan, W.; Shen, A.; Yang, S.; Yao, S.; Liu, J. On the Management of Large-Diameter Trees in China’s Forests. Forests 2020, 11, 111. [Google Scholar] [CrossRef]
- Macchioni, N.; Sozzi, L.; Fidanza, G.B. The Relationship between Carving Work and Timber Features: A Database for the Italian Wooden Statuary. Forests 2022, 13, 517. [Google Scholar] [CrossRef]
- Lutz, J.A.; Furniss, T.J.; Johnson, D.J.; Davies, S.J.; Allen, D.; Alonso, A.; Anderson-Teixeira, K.J.; Andrade, A.; Baltzer, J.; Becker, K.M.L.; et al. Global importance of large-diameter trees. Glob. Ecol. Biogeogr. 2018, 27, 849–864. [Google Scholar] [CrossRef]
- Hu, Y.; Su, Z.; Li, W.; Li, J.; Ke, X. Influence of Tree Species Composition and Community Structure on Carbon Density in a Subtropical Forest. PLoS ONE 2015, 10, e0136984. [Google Scholar] [CrossRef]
- Keeton, W.S.; Franklin, J.F. Do remnant old-growth trees accelerate rates of succession in mature Douglas-fir forests? Ecol. Monogr. 2005, 75, 103–118. [Google Scholar] [CrossRef]
- Sist, P.; Mazzei, L.; Blanc, L.; Rutishauser, E. Large trees as key elements of carbon storage and dynamics after selective logging in the Eastern Amazon. For. Ecol. Manag. 2014, 318, 103–109. [Google Scholar] [CrossRef]
- He, Z.; Yu, Q.; Hu, X.; Zhao, Q. Analysis on problems and countermeasures in cultivation of llarge-size timber forests in southern China under backaround of carbon neutrality. Guangxi For. Sci. 2024, 53, 116–123. (In Chinese) [Google Scholar]
- Cheng, K.; Yang, H.; Tao, S.; Su, Y.; Guan, H.; Ren, Y.; Hu, T.; Li, W.; Xu, G.; Chen, M.; et al. Carbon storage through China’s planted forest expansion. Nat. Commun. 2024, 15, 4106. [Google Scholar] [CrossRef]
- Yang, Y.; Jing, L.; Li, Q.; Liang, C.; Dong, Q.; Zhao, S.; Chen, Y.; She, D.; Zhang, X.; Wang, L.; et al. Big-sized trees and higher species diversity improve water holding capacities of forests in northeast China. Sci. Total Environ. 2023, 880, 163263. [Google Scholar] [CrossRef]
- Qiu, T.; Aravena, M.-C.; Andrus, R.; Ascoli, D.; Bergeron, Y.; Berretti, R.; Bogdziewicz, M.; Boivin, T.; Bonal, R.; Caignard, T.; et al. Is there tree senescence? The fecundity evidence. Proc. Natl. Acad. Sci. USA 2021, 118, e2106130118. [Google Scholar] [CrossRef]
- Cannon, C.H.; Piovesan, G.; Munne-Bosch, S. Old and ancient trees are life history lottery winners and vital evolutionary resources for long-term adaptive capacity. Nat. Plants 2022, 8, 136–145. [Google Scholar] [CrossRef]
- Ngo, D.T.; Le, A.V.; Le, H.T.; Stas, S.M.; Le, T.C.; Tran, H.D.; Pham, T.; Le, T.T.; Spracklen, B.D.; Langan, C.; et al. The potential for REDD plus to reduce forest degradation in Vietnam. Environ. Res. Lett. 2020, 15, 074025. [Google Scholar] [CrossRef]
- Huang, F.; Zhang, C.; Zeng, Y.; Yan, Y.; Li, M.; Su, Z.; Jia, X. Research Hotspots and Trends of Large-Diameter Trees Based on Bibliometric Data. Sustainability 2024, 16, 4826. [Google Scholar] [CrossRef]
- Yan, S.; Wang, R.; Deng, H.; Zheng, H.; Hu, D.; Wei, R. Study on impact factors of large-diameter wood formation of Cunninghamia lanceolatain Nanling Mountains. J. South China Agric. Univ. 2021, 42, 80–89. (In Chinese) [Google Scholar]
- Lindenmayer, D.B.; Laurance, W.F. The ecology, distribution, conservation and management of large old trees. Biol. Rev. 2017, 92, 1434–1458. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D. Research on the Regional System of Ecological Geography in China; The Commercial Press: Beijing, China, 2008. [Google Scholar]
- Li, L.; Wen, Z.; Wei, S.; Lian, J.; Ye, W. Functional Diversity and Its Influencing Factors in a Subtropical Forest Community in China. Forests 2022, 13, 966. [Google Scholar] [CrossRef]
- Song, X.; Shu, Z.; Dai, W.; Chen, W.; Fang, B.; Qin, X.; Miao, S. Species Diversity and Structure of Dominant Species Populations of Cunninghamia lanceolata Plantation at Chebaling in Northern Guangdong. For. Environ. Sci. 2017, 33, 1–8. (In Chinese) [Google Scholar]
- Yu, P.; Chen, H.; Zhong, P.; Xiong, L.; Chen, Y.; Gan, X. Distribution of Orchidaceae Resources in Guangdong Conghua Chenhedong Provincial Nature Reserve. For. Environ. Sci. 2024, 40, 135–141. (In Chinese) [Google Scholar]
- Feng, M.; Xie, H.; Deng, N.; Li, J.; Luo, Z.; Lu, D.; Lin, N. Niche Characteristics of Dominant Tree Species of Subtropical Evergreen Broad-leaved Secondary Forest in Dongguan City, Guangdong Province, China. J. Trop. Subtrop. Bot. 2024, 32, 747–756. (In Chinese) [Google Scholar]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future Koppen-Geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef]
- Hubbell, S.P.; Foster, R.B. Commonness and rarity in a neotropical forest: Implications for tropical tree conservation. In Conservation Biology: The Science of Scarcity and Diversity; Soule, M.E., Ed.; Sinauer Associates: Soule, Argentina, 1986; pp. 205–231. [Google Scholar]
- Jain, M.; Flynn, D.F.B.; Prager, C.M.; Hart, G.M.; DeVan, C.M.; Ahrestani, F.S.; Palmer, M.I.; Bunker, D.E.; Knops, J.M.H.; Jouseau, C.F.; et al. The importance of rare species: A trait-based assessment of rare species contributions to functional diversity and possible ecosystem function in tall-grass prairies. Ecol. Evol. 2014, 4, 104–112. [Google Scholar] [CrossRef]
- Tong, Y.; Qu, L.; Fu, Q.; Chen, Y.; Xiang, X.; Zhu, W.; Qi, G.; Dai, L. Species diversity of forest plant communities on the southern slope of the DabieMountains and its relationship with altitude factors. Acta Ecol. Sin. 2024, 44, 5307–5317. (In Chinese) [Google Scholar]
- Su, Y.; Zhang, Y.; Jia, X.; Xue, Y. Application of several diversity indexes in forest community analysis. Ecol. Sci. 2017, 36, 132–138. (In Chinese) [Google Scholar]
- Ou, Y.; Wang, C.; Su, Z. The dynamics of different growth form ground vegetation following a natural disturbance. Acta Ecol. Sin. 2015, 35, 4500–4507. (In Chinese) [Google Scholar]
- Chavez, V.; Macdonald, S.E. The influence of canopy patch mosaics on understory plant community composition in boreal mixedwood forest. For. Ecol. Manag. 2010, 259, 1067–1075. [Google Scholar] [CrossRef]
- Leitão, R.P.; Zuanon, J.; Villéger, S.; Williams, S.E.; Baraloto, C.; Fortunel, C.; Mendonça, F.P.; Mouillot, D. Rare species contribute disproportionately to the functional structure of species assemblages. Proc. R. Soc. B-Biol. Sci. 2016, 283, 20160084. [Google Scholar] [CrossRef]
- Scholl, J.P.; Urbina-Casanova, R.; Iler, A.M. The importance of negative density dependence for rare species persistence. Biol. Conserv. 2022, 274, 109729. [Google Scholar] [CrossRef]
- LaManna, J.A.; Mangan, S.A.; Alonso, A.; Bourg, N.A.; Brockelman, W.Y.; Bunyavejchewin, S.; Chang, L.-W.; Chiang, J.-M.; Chuyong, G.B.; Clay, K.; et al. Plant diversity increases with the strength of negative density dependence at the global scale. Science 2017, 356, 1389–1392. [Google Scholar] [CrossRef]
- Chisholm, R.A.; Fung, T. Comment on “Plant diversity increases with the strength of negative density dependence at the global scale”. Science 2018, 360, eaar4685. [Google Scholar] [CrossRef]
- Xiao, L.; Yu, S.; Li, M.; Wang, Y. Community Compensatory Trend Prevails from Tropical to Temperate Forest. PLoS ONE 2012, 7, e38621. [Google Scholar] [CrossRef][Green Version]
- Yu, D.; Hao, Z.; Ji, L.; Li, Y.; Xiong, Z.; Ye, J. Dissimilarity of plant communities with changes in altitudes on the northern slope of Changbai Mountain. Chin. J. Ecol. 2003, 22, 1–5. (In Chinese) [Google Scholar]
- He, S.; Zhong, Y.; Sun, Y.; Su, Z.; Jia, X.; Hu, Y.; Zhou, Q. Topography-associated thermal gradient predicts warming effects on woody plant structural diversity in a subtropical forest. Sci. Rep. 2017, 7, 40387. [Google Scholar] [CrossRef]
- De Frenne, P.; Brunet, J.; Shevtsova, A.; Kolb, A.; Graae, B.J.; Chabrerie, O.; Cousins, S.A.; Decocq, G.; De Schrijver, A.; Diekmann, M.; et al. Temperature effects on forest herbs assessed by warming and transplant experiments along a latitudinal gradient. Glob. Change Biol. 2011, 17, 3240–3253. [Google Scholar] [CrossRef]
- Jin, Y.-S.; Hu, Y.-K.; Wang, J.; Liu, D.-D.; Lin, Y.-H.; Liu, G.; Zhang, Y.-H.; Zhou, Z.-Q. Diversity of Understory Communities in Boreal Forests: Influences of Forest Type, Latitude, and Spatial Scale. Forests 2019, 10, 1003. [Google Scholar] [CrossRef]
- Fu, X.-Y.; Cheng, Z.-C.; Ni, H.-W.; Zhang, R.-T. Latitude variations of soil bacterial community diversity and composition in three typical forests of temperate, northeastern of China. Front. Earth Sci. 2023, 10, 1096931. [Google Scholar] [CrossRef]
- Liu, W.; Zang, R.; Ding, Y. Community features of two types of typical tropical monsoon forests in BawanglingNature Reserve, Hainan Island. Acta Ecol. Sin. 2009, 29, 3465–3476. (In Chinese) [Google Scholar]
- Wu, Z.; Wang, Z.; Luan, F.; Shu, Z.; Li, B. Community Composition and Floral Characteristics of the Chebaling 20 hm2 Forest Dynamic Plot in A Mid-subtropical Evergreen Broad-leaved Forest. For. Environ. Sci. 2021, 37, 86–91. (In Chinese) [Google Scholar]
- Chen, X.; Zhang, C.; Li, B. A syudy on the spermatophyta in the national Chebaling nature reserve of Guangdong. Guihaia 1994, 14, 321–333. (In Chinese) [Google Scholar]
- Huang, L.; Guo, Y.; Zhang, S.; Xiong, L. Flora of Vascular Plants in Chenhedong Nature Reserve from Conghua of Guangdong. Subtrop. Plant Sci. 2019, 48, 169–175. (In Chinese) [Google Scholar]
- Gu, W.; Liang, Y.; Xu, D.; Deng, Z.; Chen, J.; Lu, M.; Tang, J.; Zhang, Z. As-sessment of Ecological Service Function of Forests along Yinpingshan Forest Park in Dongguan City. For. Environ. Sci. 2022, 38, 111–118. (In Chinese) [Google Scholar]
- Branco, M.; Brockerhoff, E.G.; Castagneyrol, B.; Orazio, C.; Jactel, H. Host range expansion of native insects to exotic trees increases with area of introduction and the presence of congeneric native trees. J. Appl. Ecol. 2015, 52, 69–77. [Google Scholar] [CrossRef]
- Karvemo, S.; Schroeder, M.; Ranius, T. Beetle diversity in dead wood is lower in non-native than native tree species, especially those more distantly related to native species. J. Appl. Ecol. 2023, 60, 170–180. [Google Scholar] [CrossRef]
- Qiu, L.; Lu, D.; Li, Y.; Wu, X.; Liu, T.; Chang, H. Quantitative analysis of geographical distributions in all genera of Fagaceae in China. Acta Bot. Boreali-Occident. Sin. 2019, 39, 343–348. (In Chinese) [Google Scholar]
- Qiu, L.; Wu, X.; Liu, T. Spatial Diversities and Differences of All Genera in Fagaceae of China. Acta Bo-Tanica Boreali-Occident. Sin. 2016, 36, 2103–2108. (In Chinese) [Google Scholar]
- Feng, Y.; Schmid, B.; Loreau, M.; Forrester, D.I.; Fei, S.; Zhu, J.; Tang, Z.; Zhu, J.; Hong, P.; Ji, C.; et al. Multispecies forest plantations outyield monocultures across a broad range of conditions. Science 2022, 376, 865. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Qian, H. Drivers of the differentiation between broad-leaved trees and shrubs in the shift from evergreen to deciduous leaf habit in forests of eastern Asian subtropics. Plant Divers. 2023, 45, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Jump, A.S.; Mátyás, C.; Peñuelas, J. The altitude-for-latitude disparity in the range retractions of woody species. Trends Ecol. Evol. 2009, 24, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Thomas, F.M.; Blank, R.; Hartmann, G. Abiotic and biotic factors and their interactions as causes of oak decline in Central Europe. Forest Pathol. 2002, 32, 277–307. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, L.; Li, Y.; Zhu, W.; Chen, Y. Maxent Modelling Predicts a Shift in Suitable Habitats of a Subtropical Evergreen Tree (Cyclobalanopsis glauca (Thunberg) Oersted) under Climate Change Scenarios in China. Forests 2022, 13, 126. [Google Scholar] [CrossRef]
- Thurm, E.A.; Hernandez, L.; Baltensweiler, A.; Ayan, S.; Rasztovits, E.; Bielak, K.; Zlatanov, T.M.; Hladnik, D.; Balic, B.; Freudenschuss, A.; et al. Alternative tree species under climate warming in managed European forests. For. Ecol. Manag. 2018, 430, 485–497. [Google Scholar] [CrossRef]
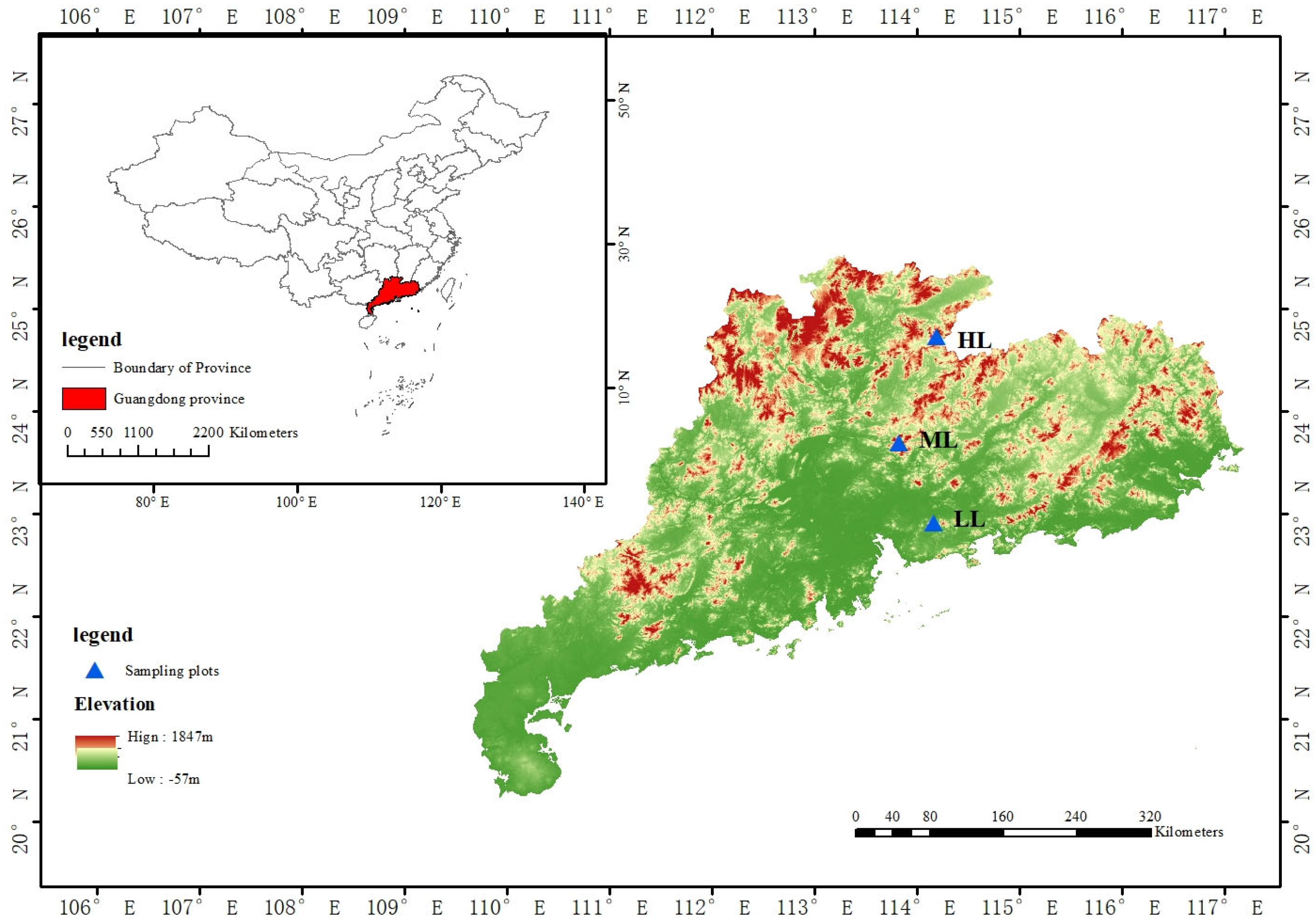


| Site | Time Since Established | Geographic Location | Plot Elevation |
|---|---|---|---|
| Chebaling National Nature Reserve (HL) | 1988-05-09 | 24°40′–24°46′ N 114°09′–114°16′ E | 435 m |
| Chenhedong Nature Reserve (ML) | 1999-12-01 | 23°32′–23°50′ N 113°45′–113°54′ E | 560 m |
| Yinpingshan Nature Reserve (LL) | 2000-12-01 | 22°52′–22°56′ N 114°10′–114°15′ E | 360 m |
| Family | Species | Abundance | RF | RA | RP | IV | |||
|---|---|---|---|---|---|---|---|---|---|
| Total | HL | ML | LL | ||||||
| Fagaceae | Castanopsis eyrei | 96 | 82 | 14 | 0 | 9.51 | 15.51 | 30.74 | 18.59 |
| Fagaceae | Castanopsis fabri | 50 | 0 | 50 | 0 | 5.57 | 8.08 | 7.96 | 7.2 |
| Fagaceae | Castanopsis fissa | 56 | 56 | 0 | 0 | 4.59 | 9.05 | 6.66 | 6.77 |
| Pentaphylacaceae | Ternstroemia gymnanthera | 31 | 29 | 2 | 0 | 5.57 | 5.01 | 7.54 | 6.04 |
| Fagaceae | Lithocarpus hancei | 46 | 0 | 46 | 0 | 4.92 | 7.43 | 5.01 | 5.79 |
| Fagaceae | Cyclobalanopsis jenseniana | 37 | 0 | 37 | 0 | 3.61 | 5.98 | 5.36 | 4.98 |
| Theaceae | Schima superba | 25 | 16 | 6 | 3 | 4.92 | 4.04 | 5.27 | 4.74 |
| Lauraceae | Cinnamomum porrectum | 25 | 2 | 8 | 15 | 5.57 | 4.04 | 2.88 | 4.16 |
| Lauraceae | Cinnamomum austro-sinensis | 26 | 0 | 26 | 0 | 4.26 | 4.2 | 3.42 | 3.96 |
| Altingiaceae | Altingia chinensis | 23 | 0 | 23 | 0 | 2.62 | 3.72 | 2.79 | 3.04 |
| Magnoliaceae | Michelia maudiae | 16 | 0 | 16 | 0 | 2.95 | 2.58 | 1.54 | 2.36 |
| Pinaceae | Pinus massoniana | 13 | 0 | 0 | 13 | 2.62 | 2.1 | 1.73 | 2.15 |
| Fagaceae | Castanopsis fordii | 12 | 0 | 12 | 0 | 2.95 | 1.94 | 1.18 | 2.02 |
| Lauraceae | Machilus chinensis | 12 | 1 | 9 | 2 | 2.62 | 1.94 | 1.33 | 1.96 |
| Elaeocarpaceae | Elaeocarpus decipiens | 11 | 0 | 11 | 0 | 2.62 | 1.78 | 1.35 | 1.92 |
| Lauraceae | Machilus chekiangensis | 11 | 0 | 0 | 11 | 2.3 | 1.78 | 1.37 | 1.82 |
| Araliaceae | Schefflera octophylla | 10 | 0 | 0 | 10 | 2.3 | 1.62 | 1.1 | 1.67 |
| Magnoliaceae | Manglietia pachyphylla | 9 | 0 | 9 | 0 | 0.98 | 1.45 | 1.15 | 1.2 |
| Rutaceae | Acronychia pedunculata | 6 | 0 | 0 | 6 | 1.97 | 0.97 | 0.47 | 1.14 |
| Juglandaceae | Engelhardtia roxburghiana | 8 | 0 | 0 | 8 | 0.98 | 1.29 | 0.99 | 1.09 |
| Pentaphylacaceae | Pentaphylax euryoides | 7 | 0 | 5 | 2 | 1.31 | 1.13 | 0.55 | 1 |
| Magnoliaceae | Michelia foveolata | 6 | 0 | 6 | 0 | 1.31 | 0.97 | 0.64 | 0.97 |
| Ericaceae | Rhododendron cavaleriei | 6 | 6 | 0 | 0 | 1.31 | 0.97 | 0.6 | 0.96 |
| - | total | 542 | 192 | 280 | 70 | - | - | - | - |
| Family | Species | Abundance | RF | RA | RP | IV | |||
|---|---|---|---|---|---|---|---|---|---|
| Total | HL | ML | LL | ||||||
| Moraceae | Artocarpus hypargyreus | 5 | 0 | 0 | 5 | 0.98 | 0.81 | 0.5 | 0.76 |
| Fagaceae | Lithocarpus glaber | 5 | 1 | 0 | 4 | 1.64 | 0.81 | 0.49 | 0.98 |
| Sabiaceae | Meliosma squamulata | 5 | 0 | 5 | 0 | 1.31 | 0.81 | 0.44 | 0.85 |
| Fagaceae | Castanopsis fargesii | 4 | 3 | 1 | 0 | 1.31 | 0.65 | 0.3 | 0.75 |
| Fagaceae | Quercus myrsinifolia | 4 | 0 | 0 | 4 | 1.31 | 0.65 | 0.32 | 0.76 |
| Juglandaceae | Engelhardtia fenzelii | 4 | 1 | 3 | 0 | 0.98 | 0.65 | 0.35 | 0.66 |
| Myricaceae | Myrica rubra | 4 | 2 | 2 | 0 | 1.31 | 0.65 | 0.55 | 0.84 |
| Lauraceae | Neolitsea zeylanica | 4 | 4 | 0 | 0 | 0.98 | 0.65 | 0.47 | 0.7 |
| Fagaceae | Quercus championii | 3 | 0 | 3 | 0 | 0.66 | 0.48 | 0.41 | 0.52 |
| Rubiaceae | Gardenia jasminoides | 3 | 0 | 0 | 3 | 0.98 | 0.48 | 0.58 | 0.68 |
| Lauraceae | Litsea elongata | 3 | 0 | 3 | 0 | 0.98 | 0.48 | 0.35 | 0.61 |
| Lauraceae | Neolitsea levinei | 3 | 0 | 3 | 0 | 0.66 | 0.48 | 0.35 | 0.5 |
| Proteaceae | Helicia reticulata | 2 | 0 | 2 | 0 | 0.33 | 0.32 | 0.18 | 0.28 |
| Rosaceae | Photinia prunifolia | 2 | 0 | 2 | 0 | 0.66 | 0.32 | 0.23 | 0.4 |
| Euphorbiaceae | Sapium discolor | 2 | 0 | 2 | 0 | 0.66 | 0.32 | 0.13 | 0.37 |
| Theaceae | Pyrenaria spectabilis | 2 | 0 | 2 | 0 | 0.66 | 0.32 | 0.17 | 0.38 |
| Fabaceae | Adenathera pavonina | 1 | 0 | 0 | 1 | 0.33 | 0.16 | 0.08 | 0.19 |
| Theaceae | Adinandra millettii | 1 | 0 | 1 | 0 | 0.33 | 0.16 | 0.08 | 0.19 |
| Fagaceae | Castanopsis chinensis | 1 | 1 | 0 | 0 | 0.33 | 0.16 | 0.19 | 0.23 |
| Fagaceae | Castanopsis hystrix | 1 | 0 | 1 | 0 | 0.33 | 0.16 | 0.1 | 0.2 |
| Daphniphyllaceae | Daphniphyllum oldhamii | 1 | 1 | 0 | 0 | 0.33 | 0.16 | 0.19 | 0.23 |
| Araliaceae | Dendropanax proteus | 1 | 0 | 1 | 0 | 0.33 | 0.16 | 0.37 | 0.28 |
| Ebenaceae | Diospyros morrisiana | 1 | 1 | 0 | 0 | 0.33 | 0.16 | 0.09 | 0.19 |
| Elaeocarpaceae | Elaeocarpus chinensis | 1 | 0 | 1 | 0 | 0.33 | 0.16 | 0.08 | 0.19 |
| Elaeocarpaceae | Elaeocarpus japonicus | 1 | 0 | 1 | 0 | 0.33 | 0.16 | 0.13 | 0.21 |
| Guttiferae | Garcinia oblongifolia | 1 | 0 | 0 | 1 | 0.33 | 0.16 | 0.07 | 0.19 |
| Fagaceae | Lithocarpus polystachyus | 1 | 0 | 1 | 0 | 0.33 | 0.16 | 0.06 | 0.18 |
| Lauraceae | Machilus pauhoi | 1 | 0 | 1 | 0 | 0.33 | 0.16 | 0.1 | 0.2 |
| Lauraceae | Machilus thunbergii | 1 | 0 | 1 | 0 | 0.33 | 0.16 | 0.14 | 0.21 |
| Magnoliaceae | Michelia skinnerana | 1 | 0 | 1 | 0 | 0.33 | 0.16 | 0.1 | 0.2 |
| Fabaceae | Ormosia fordiana | 1 | 1 | 0 | 0 | 0.33 | 0.16 | 0.18 | 0.22 |
| Fabaceae | Ormosia semicastrata | 1 | 0 | 1 | 0 | 0.33 | 0.16 | 0.06 | 0.18 |
| Oleaceae | Osmanthus marginatus | 1 | 0 | 1 | 0 | 0.33 | 0.16 | 0.06 | 0.18 |
| Fagaceae | Quercus myrsinaefolia | 1 | 1 | 0 | 0 | 0.33 | 0.16 | 0.13 | 0.21 |
| Rosaceae | Rhaphiolepis indica | 1 | 1 | 0 | 0 | 0.33 | 0.16 | 0.07 | 0.19 |
| Sapotaceae | Sinosideroxylon pedunculatum | 1 | 0 | 0 | 1 | 0.33 | 0.16 | 0.11 | 0.2 |
| Elaeocarpaceae | Sloanea sinensis | 1 | 0 | 1 | 0 | 0.33 | 0.16 | 0.06 | 0.18 |
| Ericaceae | Vaccinium bracteatum | 1 | 1 | 0 | 0 | 0.33 | 0.16 | 0.06 | 0.18 |
| - | total | 77 | 18 | 40 | 19 | - | - | - | - |
| Sites | T | A | p |
|---|---|---|---|
| Overall comparison | −36.21 | 0.18 | 10−7 |
| Pairwise comparison | |||
| HL vs. ML | −26.15 | 0.17 | 10−7 |
| HL vs. LL | −24.07 | 0.16 | 10−7 |
| ML vs. LL | −19.50 | 0.10 | 10−7 |
| Latitude | Indicator Species | Generic Areal-Type | Indicator Value | p |
|---|---|---|---|---|
| HL | Castanopsis eyrei | Temperate | 78.6 | 0.001 |
| HL | Castanopsis fissa | Temperate | 56 | 0.001 |
| HL | Ternstroemia gymnanthera | Pantropic | 59.9 | 0.001 |
| HL | Schima superba | Tropical Asia | 25.6 | 0.009 |
| HL | Rhododendron cavaleriei | Temperate | 16 | 0.039 |
| ML | Altingia chinensis | Tropical Asia | 32 | 0.001 |
| ML | Castanopsis fabri | Temperate | 68 | 0.001 |
| ML | Castanopsis fordii | Temperate | 36 | 0.001 |
| ML | Cinnamomum austro-sinensis | Tropical Asia and Tropical Australasia | 52 | 0.001 |
| ML | Cyclobalanopsis jenseniana | Tropical Asia | 44 | 0.001 |
| ML | Elaeocarpus decipiens | Pantropic | 32 | 0.001 |
| ML | Lithocarpus hancei | Temperate | 60 | 0.001 |
| ML | Michelia maudiae | Tropical Asia | 36 | 0.001 |
| ML | Meliosma squamulata | Temperate | 16 | 0.031 |
| ML | Machilus chinensis | Tropical Asia | 18 | 0.035 |
| ML | Michelia foveolata | Tropical Asia | 16 | 0.039 |
| LL | Machilus chekiangensis | Tropical Asia | 28 | 0.001 |
| LL | Pinus massoniana Lamb. | Temperate | 32 | 0.001 |
| LL | Schefflera octophylla | Pantropic | 28 | 0.002 |
| LL | Acronychia pedunculata | Tropical Asia and Tropical Australasia | 24 | 0.004 |
| LL | Quercus myrsinifolia | Tropical Asia | 16 | 0.026 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Huang, F.; Jia, X. Changes in the Species Composition and Structure of Large-Diameter Trees Along a Narrow Latitudinal Gradient in Subtropical China. Diversity 2025, 17, 309. https://doi.org/10.3390/d17050309
Li M, Huang F, Jia X. Changes in the Species Composition and Structure of Large-Diameter Trees Along a Narrow Latitudinal Gradient in Subtropical China. Diversity. 2025; 17(5):309. https://doi.org/10.3390/d17050309
Chicago/Turabian StyleLi, Mengxian, Fei Huang, and Xiaorong Jia. 2025. "Changes in the Species Composition and Structure of Large-Diameter Trees Along a Narrow Latitudinal Gradient in Subtropical China" Diversity 17, no. 5: 309. https://doi.org/10.3390/d17050309
APA StyleLi, M., Huang, F., & Jia, X. (2025). Changes in the Species Composition and Structure of Large-Diameter Trees Along a Narrow Latitudinal Gradient in Subtropical China. Diversity, 17(5), 309. https://doi.org/10.3390/d17050309






