Zooplankton of Bahía de Los Ángeles (Gulf of California) in the Context of Other Coastal Regions of the Northeast Pacific
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Zooplankton Sampling and Identification
2.3. Temperature Data
2.4. Data Analysis
3. Results
3.1. Surface Sea Temperature
3.2. Major Zooplankton Taxa
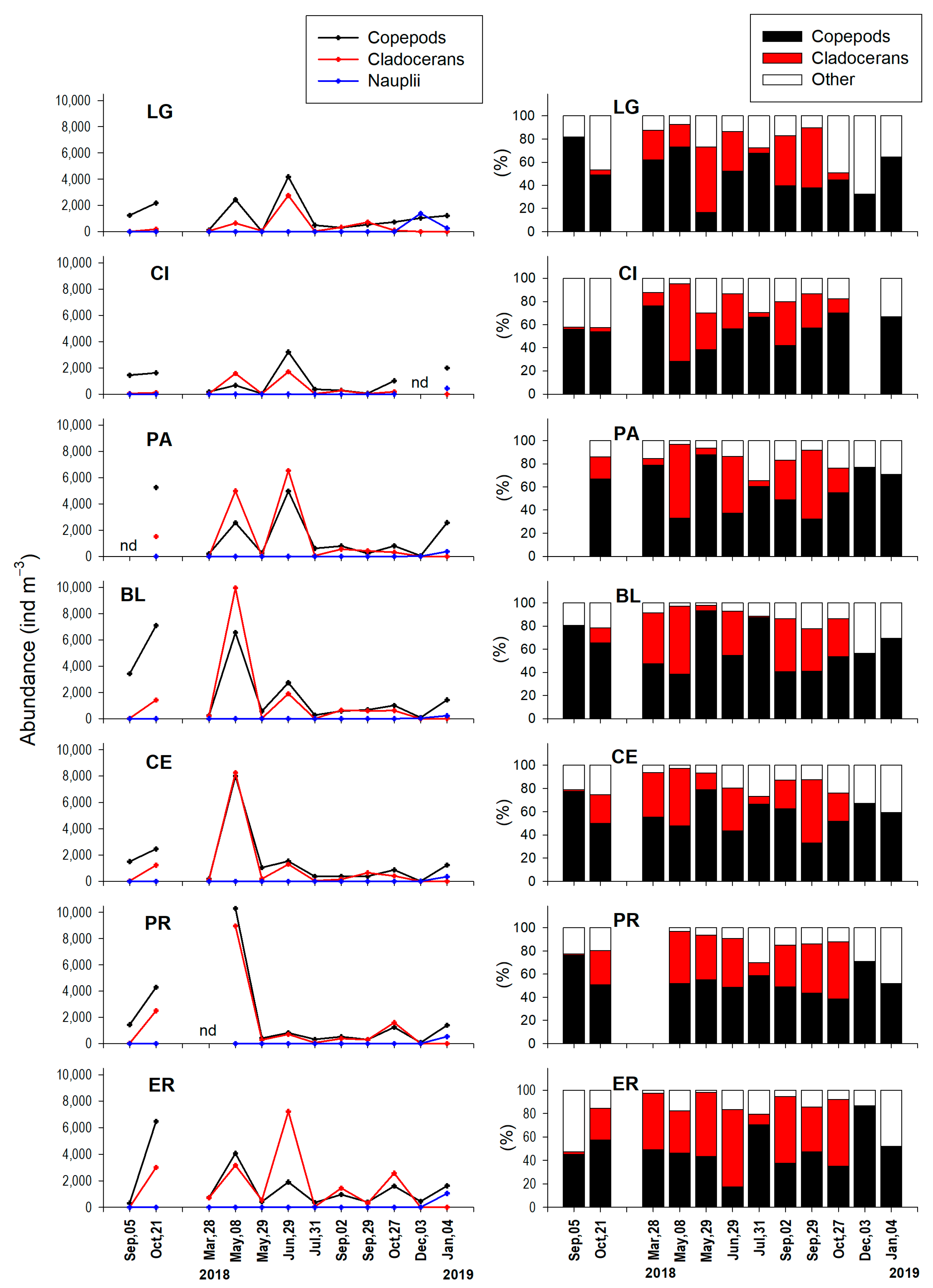
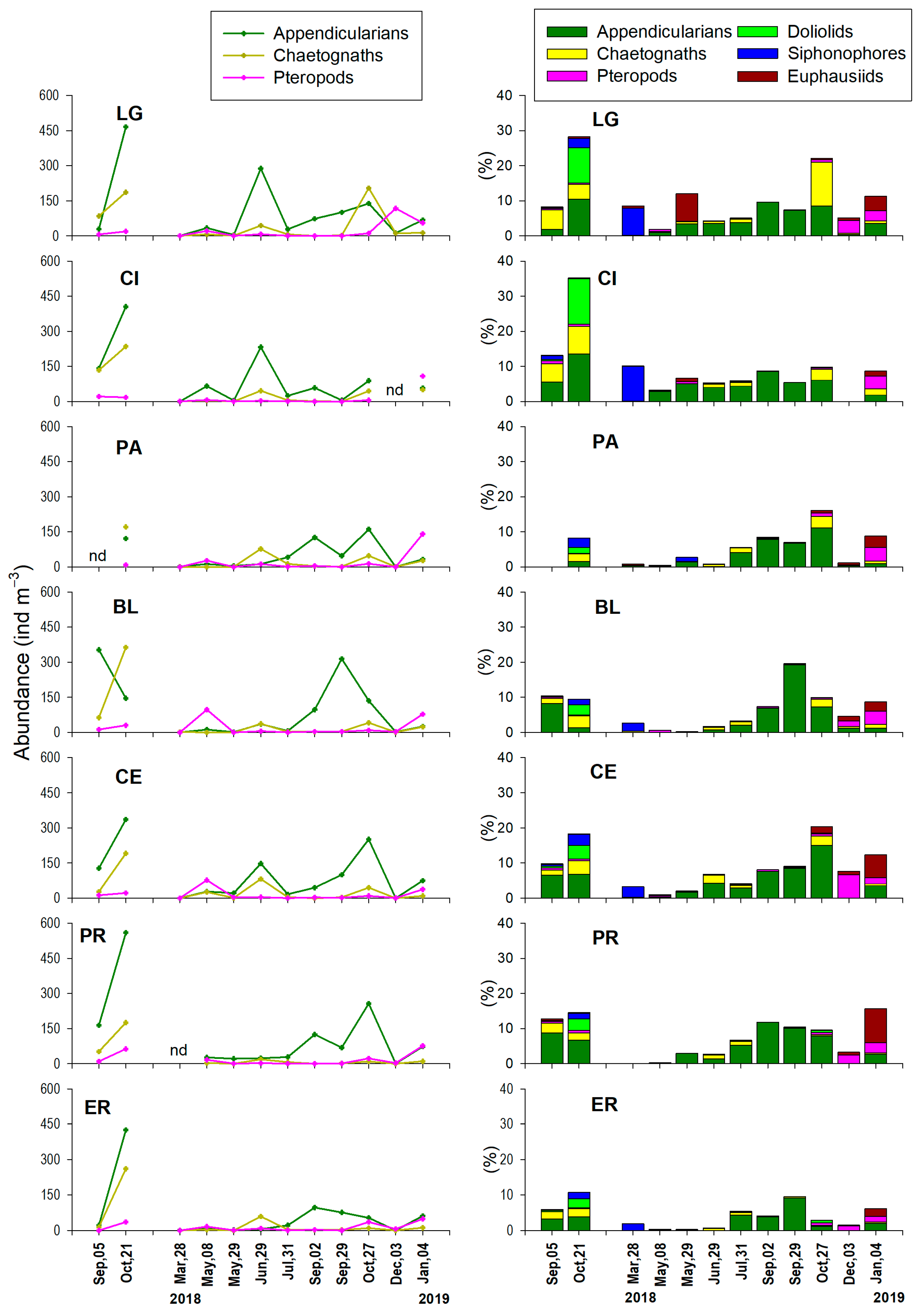
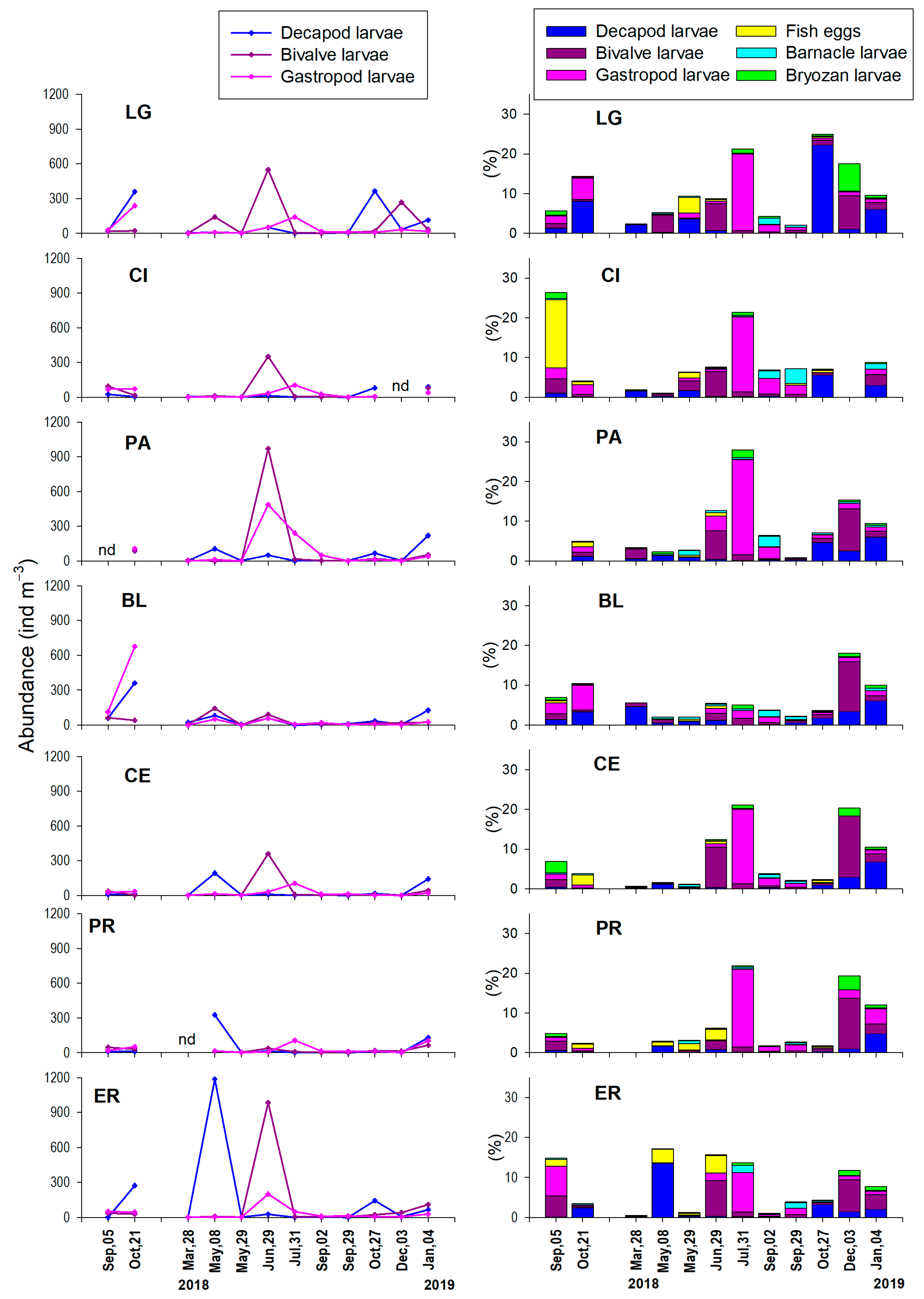
3.3. Monthly Variability of Zooplankton
3.3.1. Holozooplankton
3.3.2. Meroloplankton
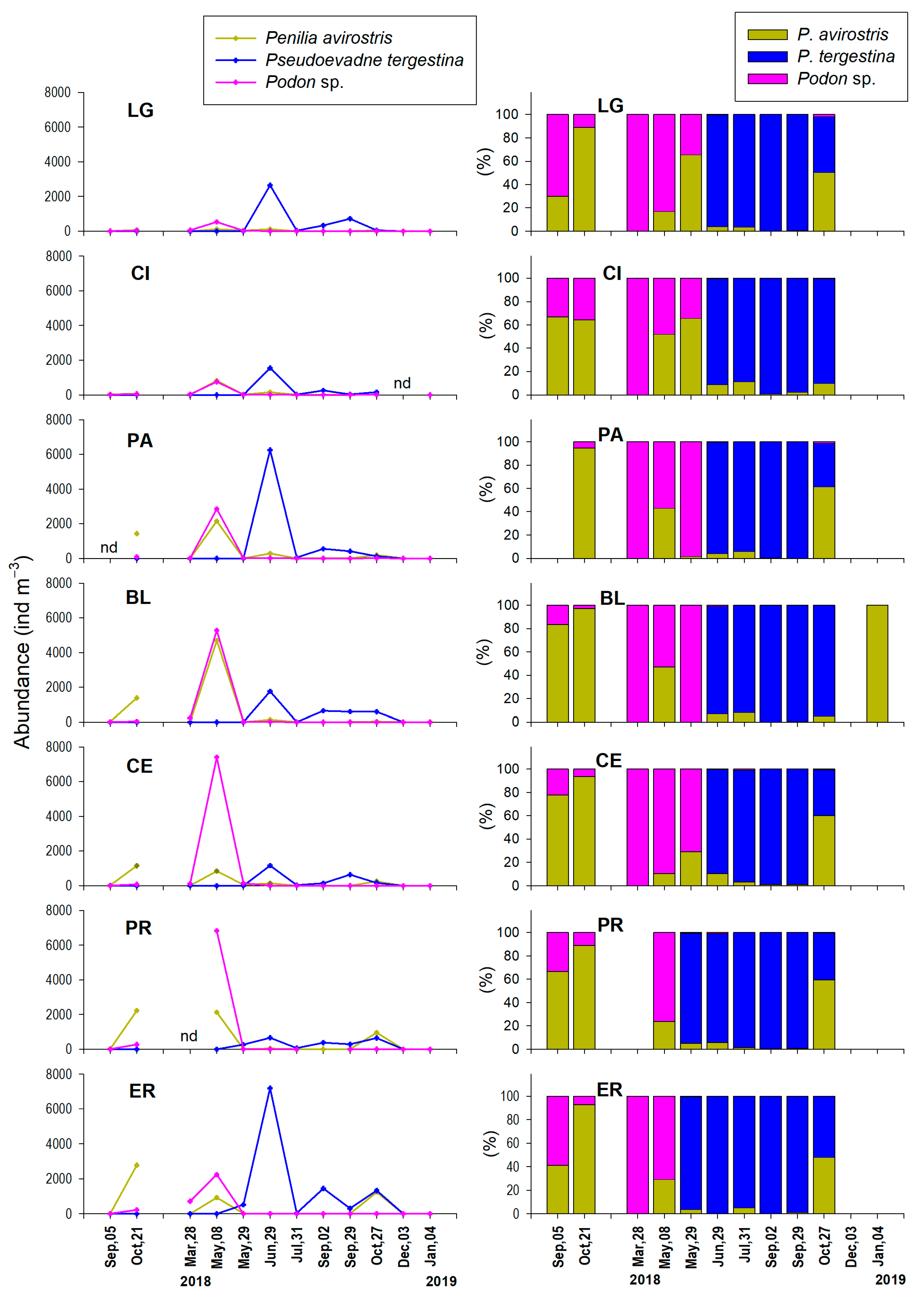
3.3.3. Cladoceran Species
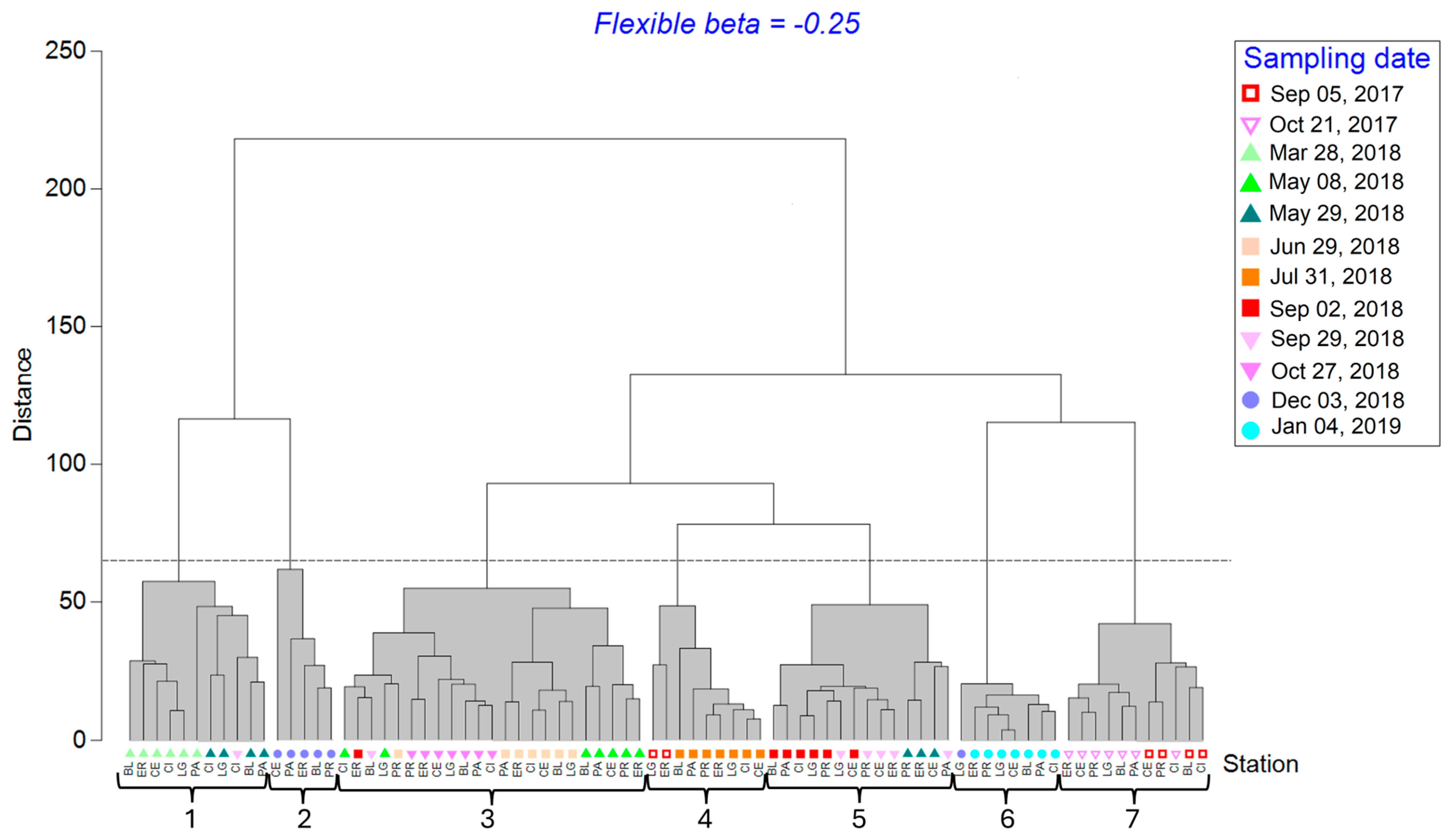
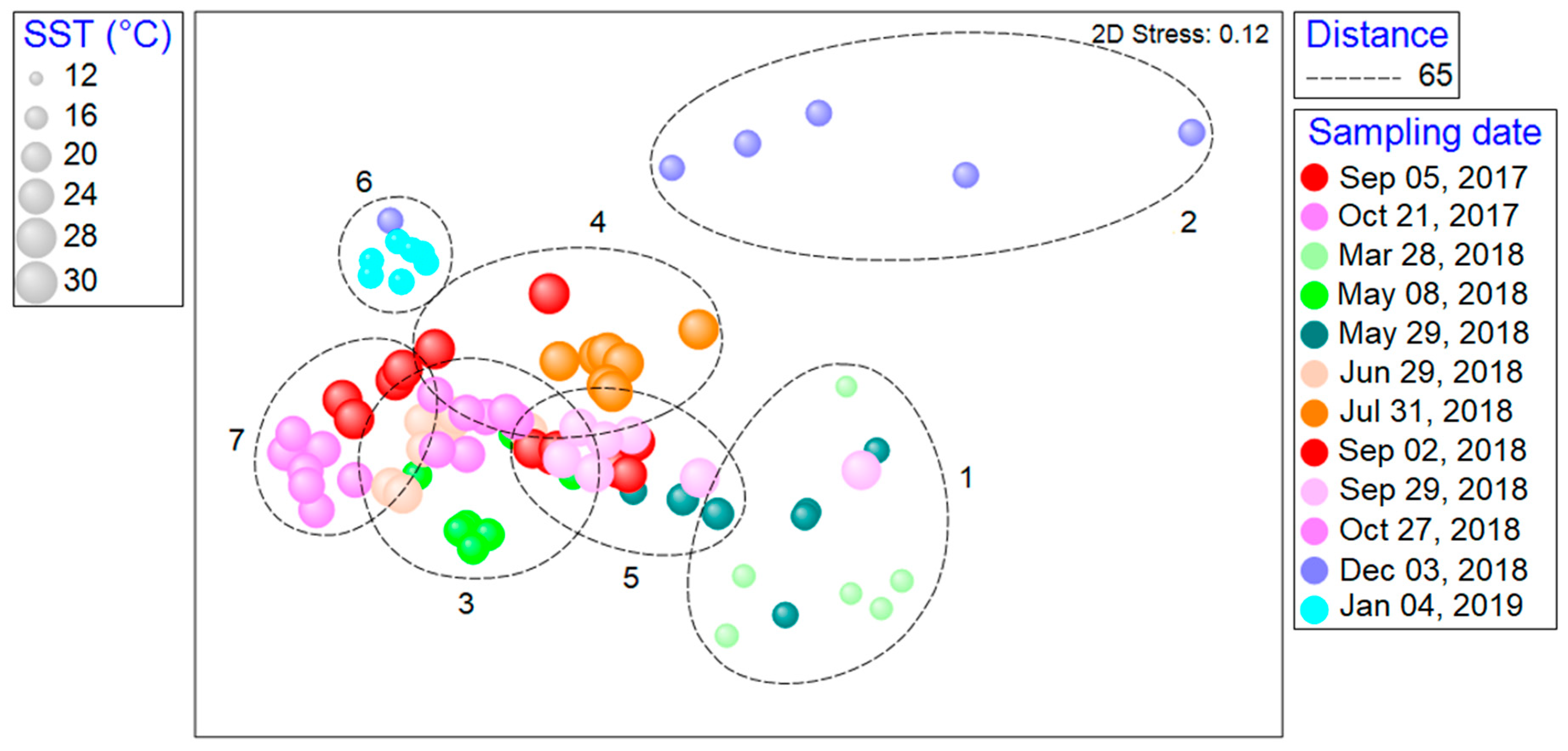
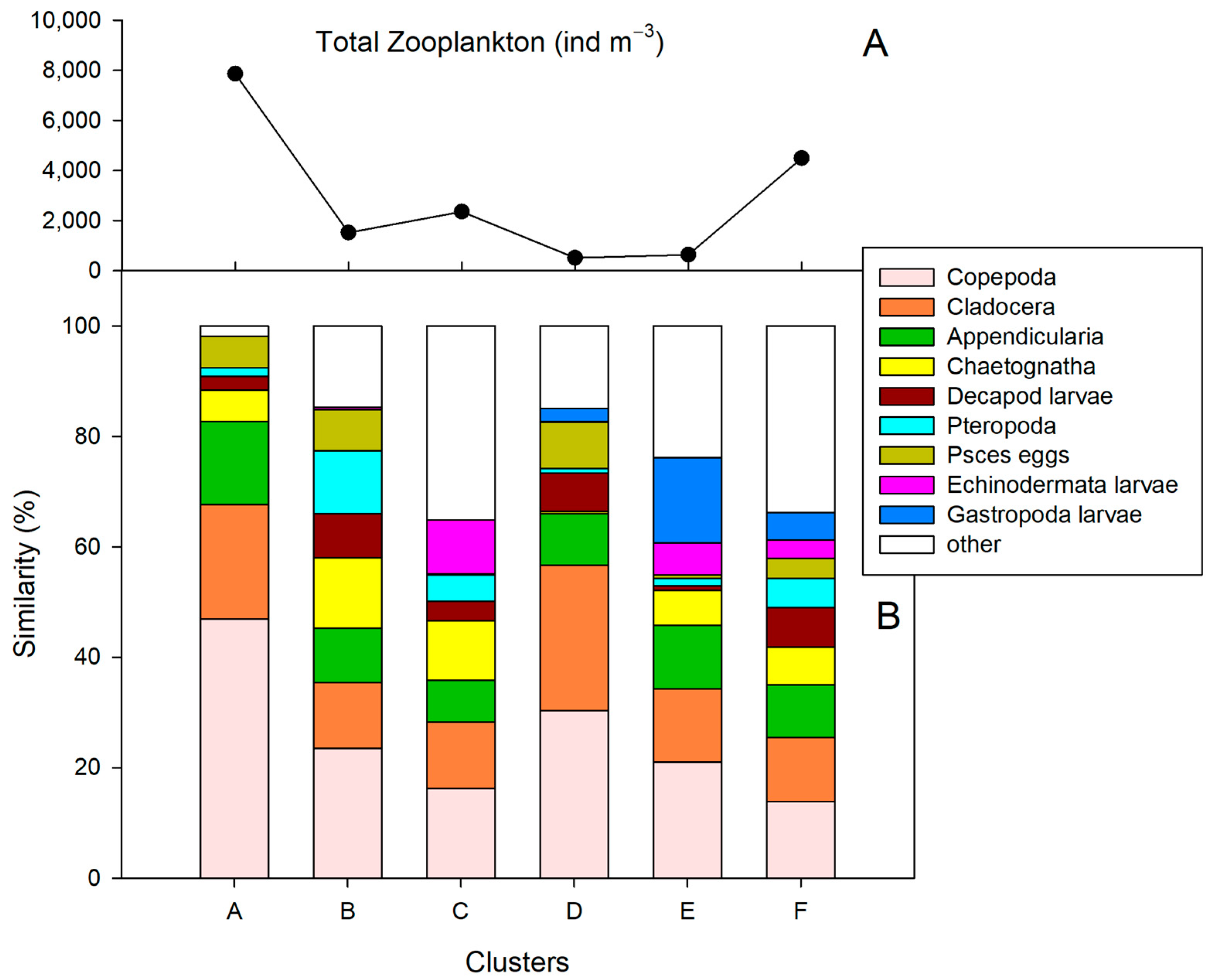
3.4. Space–Time Similarity of Zooplankton Communities
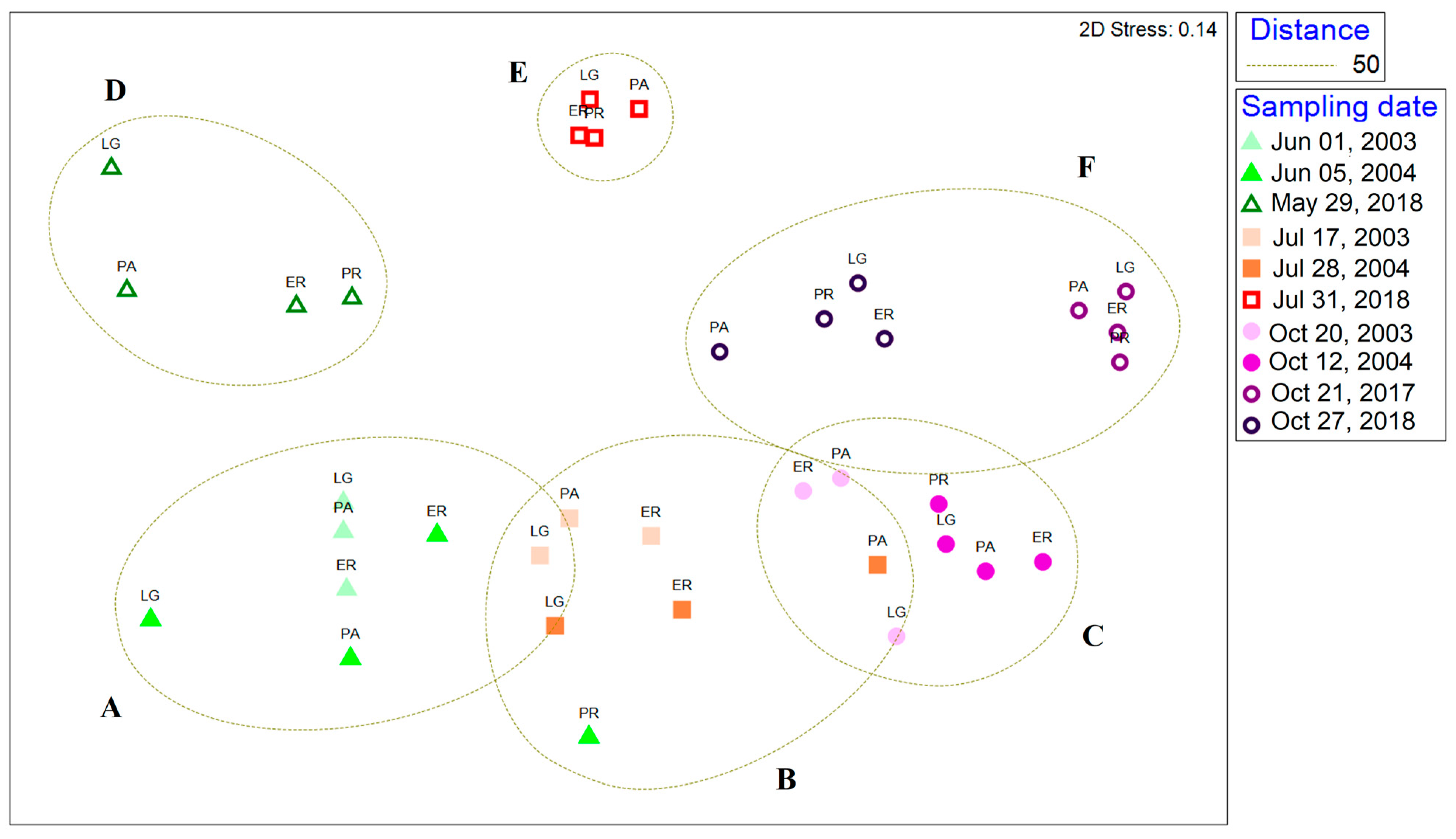
3.5. Comparison with Zooplankton from 2003 to 2004
4. Discussion
4.1. Structure of Zooplankton in the Context of Other Adjacent Coastal Regions
| Region | Date | Mesh | Abundance (ind m−3) | Holoplankton (%) | Meroplank. | Source | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| (µm) | Range | Mean | DMA | Range | Mean | DG | DG | |||
| GULF OF CALIFORNIA | ||||||||||
| Bahía de Los Ángeles, BC | 2003–2004 | 200 | 908–12,703 | 4626 | Spring | 78–99 | 94 | Copepods | Echinoderms. | [11] |
| 2017–2019 | 200 | 493–10,830 | 3114 | May | 80–98 | 91 | Coppods, cladocerans | Bivalves, decapods | This study | |
| La Paz Bay, BCS | 1975–1976 | 219 | 1.020–19,000 1 | 6.018 | May | 75–99 | 91 | Copepods | Gastropods | [45] |
| 1990–1992 | 300 | 163–3393 | 948 | Spring | 82–97 | 91 | Copepods | Decapods | [46] | |
| 2003–2004 | 333 | 276–1651 | 793 | June | 69–84 | 75 | Copepods, cladocerans | Decapods | [47] | |
| Ensenada Aripez (La Paz) | 1975–1976 | 219 | 950–4050 1 | 2583 | August | 78–96 | 90 | Copepods | Decapods | [45] |
| Las Guasimas Lagoon, Sonora | 2010 | 300 | 57–1000 | Spring | 96 | Copepods | Decapods, gastropods | [48] | ||
| Navachiste Lagoon, Sinaloa | 2002–2003 | 505 | Spring | 67 | Copepods | Decapods | [49] | |||
| El Verde Estuary, Sinaloa | 1977–1978 | 450 | 1–189 2 | 37 | August–September | 0–50 | 10 | Copepods | Decapods | [42] |
| Urías Estuary, Sinaloa | 1976–1977 | 450 | 429–1007 | 651 | November | 44–92 | 75 | Ctenohores | Decapods | [43] |
| El Rey Estuary, Nayarit | 1972–1973 | 200 | 525–13,667 1 | 2977 | September | Copepods | Cirripedians | [44] | ||
| El Pozo Estuary, Nayarit | 1972–1973 | 200 | 783–28,343 1 | 5808 | August | Copepods | Cirripedian, | [44] | ||
| WESTERN COAST OF BAJA CALIFORNIA AND USA | ||||||||||
| Neah Bay, Washington | 1961 | 110, 215 | 174–19,869 3 | 5682 | June | 99–100 | 99 | Copepods | Echinoderms, cirripedians | [50] |
| Newport, Oregon | 1970 | 240 | 105–28,374 4 | 10,127 | June | 96 | Copepods | Bivalves | [51] | |
| Tomales Bay, California | 1978–1979 | 240 | 32–965 5 | 256 | February | 74–99 | 95 | Copepods | Decapods | [52] |
| Santa Monica Bay, California | 1976–1977 | 253 | 121–32,631 4 | 6040 | April | 98–100 | 99 | Copepods | Cirripedians | [53] |
| Todos Santos Bay, BC | 1982–1983 | 505 | 40–367 | 194 | Summer | 80–95 | 90 | Copepods | Decapods | [54] |
| 1986–1987 | 300 | 1030–10,840 | 4208 | Summer | 77–98 | 90 | Copepods | Bryozoans | [55] | |
| Vizcaino Bay, BC | 1997–2013 | 505 | 131–394 | 260 | Spring–Summer | 95–99 | 97 | Copepods | Decapods | [56] |
| Gulf of Ulloa, BCS | 1998–2013 | 505 | 154–787 | 408 | Summer | 73–99 | 91 | Copepods | Decapods | [56] |
| Magdalena Bay, BCS | 1997–2001 | 333 | 673–36,645 | 14,229 | Summer | 68–98 | 87 | Copepods | Decapods | [57] |
| EASTERN TROPICAL PACIFIC | ||||||||||
| El Salado Estuary, Jalisco | 2001 | 505 | 2–52 | 34 | Winter | 49 | Chaetognaths | Decapods | [58] | |
| Chamela Bay, Jalisco | 2001–2002 | 150 | 173–3631 4 | 1512 | April | 20–96 | 85 | Copepods | Mollusks | [59] |
| Navidad Bay, Jalisco | 2010–2011 | 250 | 40–3600 1 | 985 | November | 85–97 | 91 | Copepods | Decapods | [60] |
| Barra de Navidad Lagoon, Jalisco | 2009–2010 | 505 | 37–441 | 143 | Winter | 6–33 | 20 | Copepods | Decapods | [61] |
| Manzanillo Bay, Colima | 2001–2002 | 150 | 155–6777 4 | 2878 | April | 62–98 | 91 | Copepods | Mollusks | [59] |
| Nuxco Lagoon, Guerrero | 1974 | ? | 10,000–50,000 6 | 23,333 | May | Copepods | Cirripedians | [62] | ||
| Coyuca Lagoon, Guerrero | 1999 | 250 | 39–282 | 109 | July | 77–100 | 92 | Copepods | Decapods | [63] |
| Chautengo Lagoon, Guerrero | 1974 | ? | 500–10,000 6 | 3833 | May | Copepods | Cirripedians | [62] | ||
| Chacahua Lagoon, Oaxaca | 1996–1997 | 500 | 9–1054 | 327 | June | 4–19 | 12 | Medusae | Decapods, fishes | [64] |
| La Pastoría Lagoon, Oaxaca | 1996–1997 | 500 | 32–402 | 258 | December | 7–66 | 30 | Copepods, mysids | Decapods | [64] |
| San Agustín Bay, Oaxaca | 1990–1991 | 250 | 17–246 | 103 | May | 83–95 | 81 | Copepods | Decapods | [65] |
| Santa Cruz Bay, Oaxaca | 1990–1991 | 250 | 66–1685 | 856 | May | 76–97 | 73 | Copepods | Echinoderms, decapods | [65] |
| Chahué Bay, Oaxaca | 1990–1991 | 250 | 128–750 | 542 | May | 44–94 | 64 | Copepods | Gastropods | [65] |
| Tangolunda Bay, Oaxaca | 1990–1991 | 250 | 90–1126 | 471 | May | 72–94 | 82 | Copepods | Decapods | [65] |
| Campon Lagoon, Chiapas | 1997 | 250 | 2228–2645 | 2436 | May | 95–97 | 96 | Copepods | Cirripedians, gastropods | [66] |
| Chantuto Lagoon, Chiapas | 1997 | 250 | 549–3233 | 1891 | July | 93–99 | 96 | Copepods | Cirripedians | [66] |
| Teculapan Lagoon, Chiapas | 1997 | 250 | 40–362 | 201 | July | 64–80 | 72 | Copepods | Cirripedians | [66] |
| Cerritos Lagoon, Chiapas | 1997 | 250 | 757–825 | 791 | July | 89–99 | 94 | Copepods | Decapods | [66] |
| Panzacola Lagoon, Chiapas | 1997 | 250 | 235–448 | 341 | May | 6–100 | 53 | Copepods | Cirripedians | [66] |
| Jiquilisco Bay, El Salvador | 2009 | 125 | 408–1807 1 | 1125 | Summer | 13–97 | 63 | Copepods | Cirripedians, decapods | [67] |
| 2013–2014 | 150 | 1028 | January | 89 | Copepods | Decapods, gastropods | [68] | |||
| Culebra Bay, Costa Rica | 2000 | 500 | 269–3625 1 | 1532 | February–March | Copepods | Decapods | [69] | ||
| Gulf of Dulce, Costa Rica | 1997–1998 | 153 | 3456–22,030 4 | 9865 | April | 95–99 | 98 | Copepods | Gastropods | [70] |
| Gulf of Nicoya, Costa Rica | 1997 | 280 | 1457–9142 | 4404 | April | 74 | Copepods | Crustaceans | [71] | |
| Gulf of Montijo, Panama | 2009–2010 | 243 | 300–4010 1 | 1318 | May | 84 | Copepods | Decapods | [72] | |
| Jaramijo Bay, Ecuador | 2008 | 335 | 1923–6435 5 | 4179 | April | 92–95 | 93 | Copepods, cladocerans | Fish eggs | [73] |
| Salado Estuary, Ecuador | 2016–2017 | 300 | 27–1330 | 380 | October | 6–74 | 39 | Copepods | Cirripedians | [74] |
4.2. Abundance and Seasonality of Cladocerans
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BLA | Bahía de Los Ángeles |
| GC | Gulf of California |
| ETP | Eastern Tropical Pacific |
| OSTIA | Sea Surface Temperature and Sea Ice Analysis |
| SST | Sea Surface Temperature |
| ER | Station El Rincon |
| PR | Station Punta Roja |
| CE | Station Central |
| BL | Station Bahía de Los Ángeles city |
| PA | Station Punta Arena |
| LG | Station La Gringa |
| CI | Station Cerraja Island |
References
- McLean, J.H. Marine mollusks from Los Angeles Bay, Gulf of California. Trans. San Diego Soc. Nat. Hist. 1961, 12, 449–476. [Google Scholar]
- Townsend, C.H. Voyage of the ‘Albatross’ to the Gulf of California in 1911. Bull. Am. Mus. Nat. Hist. 1916, 35, 399–476. [Google Scholar]
- Brinton, E.; Fleminger, A.H.; Siegel-Causey, D. The temperate and tropical planktonic biotas of the Gulf of California. Cal. Coop. Ocean. Fish. 1986, 27, 228–266. [Google Scholar]
- Lindsay, G.E.; Engstrand, I.H.W. History of scientific exploration in the Sea of Cortes. In Island Biogeography of the Sea of Cortes; Case, T.J., Cody, M.L., Ezcurra, E., Eds.; Oxford University Press: New York, NY, USA, 2002; pp. 3–13. [Google Scholar]
- Danemann, G.D.; Ezcurra, E.; Velarde, E. Conservación ecológica. In Bahía de Los Ángeles: Recursos Naturales y Comunidad; Danemann, G.D., Ezcurra, E., Eds.; Pronatura Noroeste, Secretaría de Medio Ambiente y Recursos Naturales, Instituto Nacional de Ecología, San Diego Natural History Museum: México, D.F., Mexico, 2008; pp. 695–729. [Google Scholar]
- Barnard, J.L.; Grady, J.R. A biological survey of Bahia de Los Angeles, Gulf of California. Mexico I. General account. Trans. San Diego Soc. Nat. Hist. 1968, 15, 51–66. [Google Scholar]
- Coan, E.V. A biological survey of Bahia de Los Angeles, Gulf of California, Mexico. III. Benthic Mollusca. Trans. San Diego Soc. Nat. Hist. 1968, 15, 107–132. [Google Scholar]
- Reish, D.J. A biological survey of Bahia de Los Angeles, Gulf of California, Mexico. II. Benthic polychaetous annelids. Trans. San Diego Soc. Nat. Hist. 1968, 15, 67–106. [Google Scholar]
- Barnard, J.L. A biological survey of Bahia de Los Angeles, Gulf of California, Mexico. IV. Benthic Amphipoda (Crustacea). Trans. San Diego Soc. Nat. Hist. 1969, 15, 175–228. [Google Scholar]
- Nelson, J.D.; Eckert, S.A. Foraging ecology of whale sharks (Rhincodon typus) within Bahía de Los Angeles, Baja California Norte, México. Fish. Res. 2007, 84, 47–64. [Google Scholar] [CrossRef]
- Lavaniegos, B.E.; Heckel, G.; Ladrón de Guevara, P. Seasonal variability of copepods and cladocerans in Bahía de Los Ángeles (Gulf of California) and importance of Acartia clausi as food for whale sharks. Cienc. Mar. 2012, 38, 11–30. [Google Scholar] [CrossRef]
- Hernández-Nava, M.F.; Álvarez-Borrego, S. Zooplankton in a whale shark (Rhincodon typus) feeding area of Bahía de Los Angeles (Gulf of California). Hidrobiológica 2013, 23, 198–208. [Google Scholar]
- Villagómez-Vélez, S.I.; Carreón-Palau, L.; Mejía-Zepeda, R.; González-Armas, R.; Aguíñiga-García, S.; Vázquez-Haikin, A.; Galván-Magaña, F. Fatty acid composition of whale shark (Rhincodon typus), and zooplankton in two aggregation sites in the Gulf of California. Reg. Stud. Mar. Sci. 2024, 76, 103577. [Google Scholar] [CrossRef]
- Amador-Buenrostro, A.; Serrano-Guzmán, S.J.; Argote-Espinoza, M.L. Numerical model of the circulation induced by the wind at Bahia de Los Angeles, BC., Mexico. Cienc. Mar. 1991, 17, 39–57. [Google Scholar] [CrossRef]
- Cavazos, T. Clima. In Bahía de Los Ángeles: Recursos Naturales y Comunidad; Danemann, G.D., Ezcurra, E., Eds.; Pronatura Noroeste, Secretaría de Medio Ambiente y Recursos Naturales, Instituto Nacional de Ecología, San Diego Natural History Museum: México, D.F., Mexico, 2008; pp. 67–90. [Google Scholar]
- Álvarez-Borrego, S. Oceanografía. In Bahía de Los Ángeles: Recursos Naturales y Comunidad; Danemann, G.D., Ezcurra, E., Eds.; Pronatura Noroeste, Secretaría de Medio Ambiente y Recursos Naturales, Instituto Nacional de Ecología, San Diego Natural History Museum: México, D.F., Mexico, 2008; pp. 45–65. [Google Scholar]
- Marinone, S.G.; Lavín, M.F. Residual flow and mixing in the large islands regions of the central Gulf of California. In Nonlinear Processes in Geophysical Fluid Dynamics; Velasco-Fuentes, O.U., Sheinbaum, J., Ochoa-de la Torre, J.L., Eds.; Kluwer Academic Publishers: Amsterdam, The Netherlands, 2003; pp. 213–236. [Google Scholar]
- Delgadillo-Hinojosa, F.; Gaxiola-Castro, G.; Segovia-Zavala, J.A.; Muñoz-Barbosa, A.; Orozco-Borbón, M.V. The effect of vertical mixing on primary production in a bay of the Gulf of California. Estuar. Coast. Shelf Sci. 1997, 45, 135–148. [Google Scholar] [CrossRef]
- Byrne, J.V.; Emery, K.O. Sediments of the Gulf of California. Bull. Geol. Soc. Am. 1960, 71, 983–1010. [Google Scholar] [CrossRef]
- Millán-Núñez, E. Red tide in Bahia de Los Angeles. Cienc. Mar. 1988, 14, 51–55. [Google Scholar] [CrossRef]
- Gilmartin, M.; Revelante, N. The phytoplankton characteristics of the barrier island lagoons of the Gulf of California. Estuar. Coast. Mar. Sci. 1978, 7, 29–47. [Google Scholar] [CrossRef]
- Canino-Herrera, S.R.; Gaxiola-Castro, G.; Segovia-Zavala, J.A. Effect of physical processes on the variation of chlorophyll, seston and primary productivity in the northern inlet of Bahia de Los Angeles (Summer 1986). Cienc. Mar. 1990, 16, 67–85. [Google Scholar] [CrossRef]
- Muñoz-Barbosa, A.; Gaxiola-Castro, G.; Segovia-Zavala, J.A. Temporal variability of primary productmty, chlorophyll and seston in Bahia de Los Angeles, Gulf of California. Cienc. Mar. 1991, 17, 47–68. [Google Scholar] [CrossRef]
- Parker, R.H. Zoogeography and ecology of some macroinvertebrates, particularly mollusks, in the Gulf of California and the continental slope off Mexico. Vidensk. Meddr. Dan. Naturn. Foren. 1964, 126, 1–178. [Google Scholar]
- Halfar, J.; Strasser, M.; Riegl, B.; Godinez-Orta, L. Oceanography, sedimentology and acoustic mapping of a bryomol carbonate factory in the northern Gulf of California, Mexico. In Cool-Water Carbonates: Depositional Systems and Palaeoenvironmental Controls; Geological Society, London, Special Publications: Bath, UK, 2006; No. 255; pp. 197–215. [Google Scholar]
- Yamaji, I. Illustrations of The Marine Plankton of Japan; Hoikusha Publ. Co.: Osaka, Japan, 1979; 537p. [Google Scholar]
- Todd, C.D.; Laverack, M.S. Coastal Marine Zooplankton: A Practical Manual for Students; Cambridge University Press: Cambridge, UK, 1991; pp. 1–106. [Google Scholar]
- Young, C.M.; Sewell, M.A.; Rice, M.E. (Eds.) Atlas of Marine Invertebrate Larvae; Academic Press: San Diego, CA, USA, 2002; 626p. [Google Scholar]
- Onbè, T. Ctenopoda and Onychopoda (=Cladocera). In South Atlantic Zooplankton; Boltovskoy, D., Ed.; Backhuys Publishers: Leiden, The Netherlands, 1999; pp. 797–814. [Google Scholar]
- Donlon, C.J.; Martin, M.; Stark, J.; Roberts-Jones, J.; Fiedler, E.; Wimmer, W. The operational sea surface temperature and sea ice analysis (OSTIA) system. Remote Sens. Environ. 2012, 116, 140–158. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N.; Somerfield, P.J. Change in Marine Communities: An Approach to Statistical Analysis, 3rd ed.; PRIMER-E: Plymouth, UK, 2014; 262p. [Google Scholar]
- SEMARNAT (Secretaría de Medio ambiente y Recursos Naturales). Decreto Por el que se Declara Área Natural Protegida, Con la Categoría de Reserva de la Biosfera, la Zona Marina Conocida Como Bahía de los Ángeles, Canales de Ballenas y de Salsipuedes; Diario Oficial de la Federación: Mexico, D.F., Mexico, 2007; 14p. [Google Scholar]
- Donath-Hernández, F.E. Cumaceos de Bahía de Los Ángeles, Baja California, Mexico (Crustacea, Peracarida). Cienc. Mar. 1993, 19, 461–471. [Google Scholar] [CrossRef]
- Leija-Tristán, A. Tamaño y densidad de Neaxius vivesi (Thalassinoidea: Axiidae), en Bahía de Los Ángeles, Baja California, México. Rev. Biol. Trop. 1994, 42, 719–721. [Google Scholar]
- Del Moral-Flores, L.F.; González-Acosta, A.F.; Espinosa-Pérez, H.; Ruiz-Campos, G.; Castro-Aguirre, J.L. Lista anotada de la ictiofauna de las islas del golfo de California, con comentarios sobre sus afinidades zoogeográficas. Rev. Mex. Biodivers. 2013, 84, 184–214. [Google Scholar] [CrossRef]
- Glockner Fagetti, A.; Calderon-Aguilera, L.; Herrero-Perezrul, M.D. Density decrease in an exploited population of brown sea cucumber Isostichopus fuscus in a Biosphere Reserve from the Baja California peninsula, Mexico. Ocean Coast Manag. 2016, 121, 49–59. [Google Scholar] [CrossRef]
- Herrero-Pérezrul, M.D.; Reyes-Bonilla, G.; Granja-Fernández, R. Effects of environmental factors on the abundances of the basket stars Astrocaneum spinosum and Astrodictyum panamense (Ophiuroidea: Gorgonocephalidae) in the northern Gulf of California, Mexico. Mar. Biol. Res. 2017, 13, 210–219. [Google Scholar]
- Alvarado-Rodríguez, J.F.; Nava, H.; Cabral-Tena, R.A.; Norzagaray-López, C.O.; Calderón-Aguilera, L.E. Contribución de los heterótrofos a la calcificación secundaria en arrecifes marginales del Pacífico mexicano. Hidrobiológica 2023, 33, 169–178. [Google Scholar]
- Viesca-Lobatón, C.; Balart, E.F.; González-Cabello, A.; Mascareñas-Osorio, I.; Aburto-Oropeza, O.; Reyes-Bonilla, H.; Torreblanca, E. Peces arrecifales. In Bahía de los Ángeles: Recursos Naturales y Comunidad; Danemann, G.D., Ezcurra, E., Eds.; Pronatura Noroeste, Secretaría de Medio Ambiente y Recursos Naturales, Instituto Nacional de Ecología, San Diego Natural History Museum: Mexico, D.F., Mexico, 2008; pp. 385–427. [Google Scholar]
- De la Lanza-Espino, G.; García-Calderón, J.L.; Tovilla-Hernández, C.; Arredondo-Figueroa, J.L. Ambientes y Pesquerías en el Litoral Pacífico Mexicano; Instituto Nacional de Estadística, Geografía e Informática: Aguascalientes, México, 1993; 97p. [Google Scholar]
- Brusca, R.C.; Cudney-Bueno, R.; Moreno-Báez, M. Gulf of California Esteros and Estuaries: Analysis, State of Knowledge and Conservation Priority Recommendations. Final Report to the David and Lucile Packard Foundation by the Arizona-Sonora Desert Museum. 2006. Available online: https://www.rickbrusca.com/http___www.rickbrusca.com_index.html/Papers_files/Packard%20Final%20Report%20SMALL.pdf (accessed on 8 April 2020).
- Sánchez-Osuna, L. Variaciones Estacionales del Zooplancton en el Estero El Verde, Sinaloa, México, con Especial Referencia a los Copepoda Calanoidea y Cladocera. Bachelor’s Thesis, National Polytechnic Institute, Mexico City, Mexico, 1980. [Google Scholar]
- Álvarez-León, R. Hidrología y zooplancton de tres esteros adyacentes a Mazatlán, Sinaloa, México. An. Cent. Cienc. Mar Limnol. UNAM 1980, 7, 177–194. [Google Scholar]
- Cortés-Guzmán, A.S.; Martínez-Guerrero, A. Identificación y cuantificación de larvas pedivelígeras de Crassostrea corteziensis Hertlein y balánidos, en el plancton de dos estuarios de San Blas, Nayarit, Pacífico de México. An. Cent. Cienc. Mar Limnol. UNAM 1979, 6, 37–51. [Google Scholar]
- Signoret, M.; Santoyo, H. Aspectos ecológicos del plancton de la Bahía de La Paz, Baja California sur. An. Inst. Cienc. Mar Limnol UNAM 1980, 7, 217–248. [Google Scholar]
- Lavaniegos, B.E.; González-Navarro, E. Grupos principales del zooplancton durante El Niño 1992–93 en el Canal de San Lorenzo, Golfo de California. Rev. Biol. Trop. 1999, 47 (Suppl. S1), 129–140. [Google Scholar]
- Hernández-Trujillo, S.; Nava-Torales, A.; Esqueda-Escárcega, G.M. El cambio estacional del mesozooplancton en la Bahía de La Paz, Baja California Sur, México (2003–2004). In Proceedings of the XX Congreso Nacional de Ciencia y Tecnología del Mar, Cabo San Lucas, Mexico, 1–4 October 2013; Secretaría de Educación Media Superior: Toluca de Lerdo, Mexico, 2013. [Google Scholar]
- Álvarez-Tello, F.J.; López-Martínez, J.; Funes-Rodríguez, R.; Lluch-Cota, D.B.; Rodríguez-Romero, J.; Flores-Coto, C. Composición, estructura y diversidad del mesozooplancton en Las Guásimas, Sonora, un sitio Ramsar en el Golfo de California, durante 2010. Hidrobiologica 2015, 25, 401–410. [Google Scholar]
- De Silva-Dávila, R.; Palomares-García, R.; Zavala-Norzagaray, A.; Escobedo-Urías, D.C. Ciclo anual de los grupos dominantes del zooplancton en Navachiste, Sinaloa. In Contributions to the Study of East Pacific Crustaceans; Hendrickx, M.E., Ed.; Instituto de Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México: Mexico City, Mexico, 2006; Volume 4, pp. 26–39. [Google Scholar]
- Peterson, W.K.; Anderson, G.C. Net zooplankton data from the northeast Pacific Ocean: Columbia River efluent area, 1961, 1962. Department of Oceanography, University of Wahington. Tech. Rep. 1966, 160, 1–225. [Google Scholar]
- Peterson, W.T.; Miller, C.B. Year to year variations in the planktology of the Oregon upwelling zone. Fish. Bull. 1975, 73, 642–653. [Google Scholar]
- Johnston, D.J. The Zooplankton Communities of Tomales Bay, California. Master’s Thesis, University of the Pacific, Stockton, CA, USA, 1980. [Google Scholar]
- Soule, D.F.; Oguri, M. Marine studies of San Pedro Bay, California. Part 13, The Marine ecology of Marina del Rey Harbor, California; Allan Hancock Foundation Technical Series No. 2; Allan Hancock Foundation (Harbors Environmental Projects) and the Office of Sea Grant Programs (University of Southern California): Los Angeles, CA, USA, 1977; pp. 1–424. [Google Scholar]
- Castro-Longoria, E.; Hammann, M.G. Zooplankton biomass and community structure in Todos Santos Bay, B.C., Mexico, during the 1982-83 El Niño event. Cienc. Mar. 1989, 15, 1–20. [Google Scholar] [CrossRef]
- Jiménez-Pérez, L.C. Temporal variation of zooplankton in Todos Santos Bay, Baja California, Mexico. Cienc. Mar. 1989, 15, 81–96. [Google Scholar] [CrossRef]
- Lavaniegos, B.E.; Molina-González, O.; Murcia-Riaño, M. Zooplankton functional groups from the California Current and climate variability during 1997–2013. Oceánides 2015, 30, 45–62. [Google Scholar] [CrossRef]
- Hernández-Trujillo, S.; Esqueda-Escárcega, G.; Palomares-García, R. Variabilidad de la abundancia de zooplancton en Bahía Magdalena, Baja California Sur, México (1997–2001). Lat. Am. J. Aquat. Res. 2010, 38, 438–446. [Google Scholar] [CrossRef]
- Navarro-Rodríguez, M.C.; Flores-Vargas, R.; González-Guevara, L.F. Variación estacional de los principales grupos zooplanctónicos del área natural protegida estero El Salado, Jalisco, México. Rev. Bio Cienc. 2015, 3, 103–115. [Google Scholar]
- Briseño-Avena, C. Biomasa y Composición del Zooplancton en la Bahía de Chamela, Jalisco y la Bahía de Manzanillo, Colima Durante un Ciclo Anual (2001–2002). Bachelor’s Thesis, University of Guadalajara, Jalisco, Mexico, 2004. [Google Scholar]
- Franco-Gordo, C.; Ambriz-Arreola, I.; Kozak, E.R.; Gómez-Gutiérrez, J.; Plascencia-Palomera, V.; Godínez-Domínguez, E.; Hinojosa-Larios, A. Seasonal succession of zooplankton taxonomic group assemblages in surface waters of Bahia de Navidad, Mexico (November 2010–December 2011). Hidrobiologica 2015, 25, 335–345. [Google Scholar]
- Flores-Vargas, R.; Navarro-Rodríguez, M.C.; González-Guevara, L.F.; Saucedo-Lozano, M. Variación estacional de los principales grupos zooplanctónicos y parámetros físicos del área natural protegida Laguna Barra de Navidad, Jalisco. Acta Pesq. 2017, 3, 34–50. [Google Scholar]
- Martínez-Guerrero, A. Distribución y variación estacional del zooplancton en cinco lagunas costeras del estado de Guerrero. México. An. Cent. Cienc. Mar Limnol UNAM 1978, 5, 201–214. [Google Scholar]
- Álvarez-Silva, C.; Torres-Alvarado, M.R. Composición y abundancia del zooplancton de la Laguna de Coyuca, Guerrero, México. Hidrobiologica 2013, 23, 241–249. [Google Scholar]
- Pantaleón-López, B.; Aceves, G.; Castellanos, I.A. Distribución y abundancia del zooplancton del complejo lagunar Chacahua-La Pastoría, Oaxaca, México. Rev. Mex. Biodivers. 2005, 76, 63–70. [Google Scholar]
- Sosa-Rosas, L. Composición y Variación del Zooplancton Presente en Algunas Localidades del Desarrollo Turístico Bahías de Huatulco, Oaxaca, Durante 1990–1991. Master’s Thesis, National Autonomous University of Mexico, Mexico City, Mexico, 1995. [Google Scholar]
- Álvarez-Silva, C.; Miranda-Arce, G.; De Lara-Isassi, G.; Gómez-Aguirre, S. Zooplancton de los sistemas estuarinos de Chantuto y Panzacola, Chiapas, en época de secas y de lluvias. Hidrobiologica 2006, 16, 175–182. [Google Scholar]
- Cuellar, T.C. Composición y abundancia del zooplancton. In El Ecosistema de Manglar de la Bahía de Jiquilisco; Rivera, C.G., Cuellar, T.C., Eds.; University of El Salvador: San Salvador, El Salvador, 2010; pp. 83–100. [Google Scholar]
- Galdámez-Castaneda, K.M. Composición y Abundancia del Zooplancton en el Área Los Remos, Bahía de Jiquilisco, El Salvador. Bachelor’s Thesis, University of El Salvador, San Salvador, El Salvador, 2014. [Google Scholar]
- Bednarski, M.; Morales-Ramírez, A. Composition, abundance and distribution of macrozooplankton in Culebra Bay, Gulf of Papagayo, Pacific coast of Costa Rica and its value as bioindicator of pollution. Rev. Biol. Trop. 2004, 52, 105–119. [Google Scholar]
- Quesada-Alpízar, M.A. Caracterización, Composición, Abundancia y Biomasa del Zooplancton en el Golfo Dulce Durante el Período 1997–1998. Master’s Thesis, University of Costa Rica, San Jose, Costa Rica, 2001. [Google Scholar]
- Brugnoli-Olivera, E.; Díaz-Ferguson, E.; Delfino-Machin, M.; Morales-Ramírez, A.; Dominici-Arosemena, A. Composition of the zooplankton community, with emphasis in copepods, in Punta Morales, Golfo de Nicoya, Costa Rica. Rev. Biol. Trop. 2004, 52, 897–902. [Google Scholar]
- Grimaldo, M.; Chang, J.; Muñoz, E.; Averza, A. Abundancia estacional del zooplancton en el Golfo de Montijo, Pacifico panameño. Tecnociencia 2013, 15, 33–42. [Google Scholar]
- Gualancañay, E.; Tapia, M.E.; Naranjo, C.; Cruz, M.; Villamar, F. Caracterización biológica de la Bahía de Jaramijó en la costa ecuatoriana, 2008. Acta Oceanogr. Pac. 2010, 16, 33–52. [Google Scholar]
- Castro-Madrid, R.A. Distribución y Abundancia del Zooplancton en un Área del Estero Salado, Entre Puente El Velero y Puente Zig-Zag, de la Ciudad de Guayaquil. Bachelor’s Thesis, University of Guayaquil, Guayaquil, Ecuador, 2017. [Google Scholar]
- Kimmerer, W.J. Distribution patterns of zooplankton in Tomales Bay, California. Estuaries 1993, 16, 264–272. [Google Scholar] [CrossRef]
- Álvarez-del Castillo, M.; Hendrickx, M.E.; Rodríguez, S. Crustáceos decápodos de la Laguna Barra de Navidad, Jalisco, México. Proc. San Diego Soc. Nat. Hist. 1992, 27, 1–9. [Google Scholar]
- González, G.; Rodríguez, E.A. El sistema lagunar: Cambios naturales, antropogénicos y su impacto en el ecosistema estuarino. In Chacahua: Reflejos de un Parque. Comisión Nacional de Áreas Naturales Protegidas; Alfaro, M., Sánchez, G., Eds.; Comisión Nacional de Áreas Protegidas, Programa de las Naciones Unidas para el Desarrollo, Secretaría de Medio ambiente y Recursos Naturales; Editorial Plaza y Valdés: México, D.F., Mexico, 2002; pp. 39–58. [Google Scholar]
- Ambler, J.W.; Cloern, J.E.; Hutchinson, A. Seasonal cycles of zooplankton from San Francisco Bay. Hydrobiologia 1985, 129, 177–197. [Google Scholar] [CrossRef]
- López-Serrano, A.; Serrano-Guzmán, S.J. Composición por grupos y abundancia del mesozooplancton en la Laguna Inferior (Sistema Lagunar Huave, Oaxaca, México), en mayo y septiembre-octubre de 2007. Cienc. Y Mar 2013, 19, 3–14. [Google Scholar]
- Valencia, B.; Giraldo, A.; Rivera-Gómez, M.; Izquierdo, V.; Cuellar-Chacón, A. Effects of seasonal upwelling on hydrography and mesozooplankton communities in a Pacific tropical cove off Colombia. Rev. Biol. Trop. 2019, 67, 945–962. [Google Scholar] [CrossRef]
- Naranjo, C.; Tapia, M. Variabilidad estacional del plancton en la Bahía de Manta en la costa ecuatoriana, durante el 2011. Acta Oceanogr. Pacif. 2013, 18, 65–74. [Google Scholar]
- Naranjo, C.; Tapia, M. Productividad planctónica en la Bahía de Pedernales, Manabí-Ecuador durante el 2013. Acta Oceanogr. Pacif. 2014, 19, 31–42. [Google Scholar]
- Egloff, D.A.; Fofonoff, P.W.; Onbé, T. Reproductive biology of marine cladocerans. Adv. Mar. Biol. 1997, 31, 79–167. [Google Scholar]
- Mejillón-del Pezo, Y.E. Estudio Sobre la Composición, Abundancia y Variación Espacio-Temporal del Orden Cladocera, Presentes en la Bahía de Santa Elena-La Libertad, Durante Octubre 2004 a Octubre 2005. Bachelor’s Thesis, Statal University of Santa Elena, La Libertad Canton, Ecuador, 2008. [Google Scholar]
- Jang, M.C.; Shin, K.; Jang, P.G.; Lee, W.J.; Choi, K.H. Mesozooplankton community in a seasonally hypoxic and highly eutrophic bay. Mar. Freshw. Res. 2015, 66, 719–729. [Google Scholar] [CrossRef]
- Hwang, O.M.; Shin, K.; Baek, S.H.; Lee, W.J.; Kim, S.; Jang, M.C. Annual variations in community structure of mesozooplankton by short-term sampling in Jangmok Harbor of Jinhae Bay. Ocean Polar Res. 2011, 33, 235–253. [Google Scholar] [CrossRef]
- Onbé, T. Seasonal fluctuations in the abundance of populations of marine cladocerans and their resting eggs in the inland Sea of Japan. Mar. Biol. 1985, 87, 83–88. [Google Scholar] [CrossRef]
- Tang, K.; Chen, Q.C.; Wong, C.K. Distribution and biology of marine cladocerans in the coastal waters of southern China. Hydrobiologia 1995, 307, 99–107. [Google Scholar] [CrossRef]
- Piontkovski, S.A.; Fonda-Umani, S.; De Olazabal, A.; Gubanova, A.D. Penilia avirostris: Regional and global patterns of seasonal cycles. Int. J. Ocean. Oceanogr. 2012, 6, 9–25. [Google Scholar]
- Goswami, S.C.; Devassy, V.P. Seasonal fluctuations in the occurrence of Cladocera in the Mandovi-Zuari estuarine waters of Goa. Indian J. Mar. Sci. 1991, 20, 138–142. [Google Scholar]
- Ezhilarasan, P.; Kanuri, V.V.; Sivasankar, R.; Kumar, P.S.; Murthy, M.R.; Rao, V.R.; Ramu, K. Surface mesozooplankton assemblages in a tropical coastal upwelling ecosystem: Southeastern Arabian Sea. Cont. Shelf Res. 2018, 168, 28–38. [Google Scholar] [CrossRef]
- Atienza, D.; Saiz, E.; Skovgaard, A.; Trepat, I.; Calbet, A. Life history and population dynamics of the marine cladoceran Penilia avirostris (Branchiopoda: Cladocera) in the Catalan Sea (NW Mediterranean). J. Plankton Res. 2008, 30, 345–357. [Google Scholar] [CrossRef]
- Calbet, A.; Garrido, S.; Saiz, E.; Alcaraz, M.; Duarte, C.M. Annual zooplankton succession in coastal NW Mediterranean waters: The importance of the smaller size fractions. J. Plankton Res. 2001, 23, 319–331. [Google Scholar] [CrossRef]
- Pestoric, B.; Lučić, D.; Joksimović, D. Cladocerans spatial and temporal distribution in the coastal south Adriatic waters (Montenegro). Stud. Mar. 2011, 25, 101–120. [Google Scholar]
- Gülsahin, N.; Tarkan, A.N. Seasonal changes in distribution and abundance of the cladoceran species in relation to environmental factors in Gökova Bay (Mugla, Aegean Sea, Turkey). Fresen. Environ. Bull. 2012, 21, 1853–1863. [Google Scholar]
- Terbıyık-Kurt, T.; Polat, S. Seasonal distribution of coastal mesozooplankton community in relation to the environmental factors in Iskenderun Bay (Northeast Levantine, Mediterranean Sea). J. Mar. Biol. Assoc. UK 2013, 93, 1163–1174. [Google Scholar] [CrossRef]
- Isinibilir, M. Annual crustacean zooplankton succession (Copepoda and Cladocera) in the upper layer of Ahirkapi coastal waters (northeastern Sea of Marmara). Crustaceana 2009, 82, 669–678. [Google Scholar] [CrossRef]
- Johns, D.G.; Edwards, M.; Greve, W.; Sjohn, A.W.G. Increasing prevalence of the marine cladoceran Penilia avirostris (Dana, 1852) in the North Sea. Helgol. Mar. Res. 2005, 59, 214–218. [Google Scholar] [CrossRef]
- D’Elbée, J.; Lalanne, Y.; Castège, I.; Bru, N.; D’Amico, F. Response of planktonic cladocerans (Class: Branchiopoda) to short-term changes in environmental variables in the surface waters of the Bay of Biscay. Deep Sea Res. II 2014, 106, 87–98. [Google Scholar] [CrossRef]
- Primo, A.L.; Azeiteiro, U.M.; Marques, S.C.; Martinho, F.; Pardal, M.A. Changes in zooplankton diversity and distribution pattern under varying precipitation regimes in a southern temperate estuary. Estuar. Coast. Shelf Sci. 2009, 82, 341–347. [Google Scholar] [CrossRef]
- Oghenekaro, E.U.; Chigbu, P. Population dynamics and life history of marine Cladocera in the Maryland coastal bays, U.S.A. J. Coast. Res. 2019, 35, 1225–1236. [Google Scholar] [CrossRef]
- Ambler, J.W.; Kumar, A.; Moisan, T.A.; Aulenbach, D.L.; Day, M.C.; Dix, S.A.; Winsor, M.A. Seasonal and spatial patterns of Penilia avirostris and three tunicate species in the southern Mid-Atlantic Bight. Cont. Shelf Res. 2013, 69, 141–154. [Google Scholar] [CrossRef]
- Rose, K.; Roff, J.C.; Hopcroft, R.R. Production of Penilia avirostris in Kingston Harbour, Jamaica. J. Plankton Res. 2004, 26, 605–615. [Google Scholar] [CrossRef]
- Sterza, J.M.; Fernandes, L.L. Distribution and abundance of Cladocera (Branchiopoda) in the Paraíba do Sul river estuary, Rio de Janeiro, Brazil. Braz. J. Oceanogr. 2006, 54, 193–204. [Google Scholar] [CrossRef]
- Marazzo, A.; Valentin, J.L. Spatial and temporal variations of Penilia avirostris and Evadne tergestina (Crustacea, Branchiopoda) in a tropical bay, Brazil. Hydrobiologia 2001, 445, 133–139. [Google Scholar] [CrossRef]
- Miyashita, L.K.; Pompeu, M.; Gaeta, S.A.; Lopes, R.M. Seasonal contrasts in abundance and reproductive parameters of Penilia avirostris (Cladocera, Ctenopoda) in a coastal subtropical area. Mar. Biol. 2010, 157, 2511–2519. [Google Scholar] [CrossRef]
- Della Posta, P.; Leme, M.H.A. Variação temporal de cladóceros marinhos em uma região estuarina de Ubatuba, SP, Brasil. Bol. Inst. Pesca 2015, 41, 327–334. [Google Scholar]
- Viñas, M.D.; Ramírez, F.C.; Santos, B.A.; Marrari, M. Spatial and temporal distribution patterns of Cladocera in the Argentine Sea. Hydrobiologia 2007, 594, 59–68. [Google Scholar] [CrossRef]
- Fryer, G. Morphology and the classification of the so-called Cladocera. Hydrobiologia 1987, 145, 19–28. [Google Scholar] [CrossRef]



| Taxa/Group | Habitat | Frequence (Num. Samples) |
|---|---|---|
| Phylum Foraminifera | HP | 34 |
| Phylum Cnidaria | ||
| Subphylum Medusozoa | ||
| Hydromedusa | HP | 46 |
| Siphonophora | HP | 32 |
| Larvae | MP | 10 |
| Phylum Ctenophora | HP | 5 |
| Phylum Chaetognatha | HP | 68 |
| Phylum Annelida | ||
| Polychaeta (pelagic) | HP | 72 |
| Polychaeta (larvae) | MP | 23 |
| Sipuncula (pelagosphera larva) | MP | 18 |
| Phylum Nemertea (pilidium larva) | MP | 19 |
| Phylum Mollusca | ||
| Pteropoda | HP | 67 |
| Pterotracheoidea 1 | HP | 25 |
| Gastropoda (larvae) | MP | 75 |
| Bivalvia (larvae) | MP | 80 |
| Phylum Platyhelminthes | B | 24 |
| Phylum Brachiopoda (larvae) | MP | 1 |
| Phylum Phoronida (actinotroch larvae) | MP | 24 |
| Phylum Bryozoa (cyphonaute larvae) | MP | 60 |
| Phylum Nematoda | B | 4 |
| Phylum Arthropoda | ||
| Subphylum Crustacea | ||
| Cladocera | HP | 69 |
| Ostracoda | HP | 5 |
| Cirripedia (larvae) | MP | 78 |
| Copepoda (adults and copepodites) | HP | 81 |
| Copepoda (nauplii) | HP | 13 |
| Isopoda | B | 24 |
| Cumacea | B | 1 |
| Amphipoda | HP | 20 |
| Mysida 2 | HP | 5 |
| Euphausiacea | HP | 41 |
| Decapoda (mainly larvae) | HP + MP | 75 |
| Stomatopoda (larvae) | MP | 19 |
| Phylum Echinodermata (larvae) | MP | 41 |
| Phylum Hemichordata (tornaria larvae) | MP | 3 |
| Phylum Chordata | ||
| Subphylum Cephalochordata | B | 2 |
| Subphylum Tunicata | ||
| Appendicularia | HP | 79 |
| Doliolida | HP | 28 |
| Salpida | HP | 17 |
| Subphylum Vertebrata | ||
| Fishes (eggs) | MP | 65 |
| Fishes (larvae) | MP | 47 |
| Invertebrates (larvae) 3 | MP | 37 |
| Season/Taxa | 2003 | 2004 | 2017 | 2018 | H | p |
|---|---|---|---|---|---|---|
| Spring | 1 June (n = 3) | 5 June (n = 3) | -- | 29 May (n = 4) | ||
| Fonaminifera | 0 | 0 | 2 | 6.5 | 0.039 | |
| Chaetognatha | 1 | 25 | <1 | 7.4 | 0.025 | |
| Polychaeta | 0 | 0 | <1 | 9.6 | 0.008 | |
| Copepoda | 5995 | 8796 | 328 | 7.1 | 0.029 | |
| Gastropod larvae | 0 | 0 | 1 | 6.4 | 0.041 | |
| Cirripedia larvae | 0 | 0 | 3 | 9.4 | 0.009 | |
| Penilia avirostris | 0 | 0 | 17 | 8.6 | 0.014 | |
| Podon sp. | 0 | 0 | 9 | 9.4 | 0.009 | |
| Summer | 17 July (n = 3) | 28 July (n = 3) | -- | 31 July (n = 4) | ||
| Polichaeta | 0 | 0 | 2 | 8.3 | 0.016 | |
| Pteropoda | 28 | 64 | 1 | 8.2 | 0.016 | |
| Copepoda | 3781 | 421 | 425 | 6.0 | 0.050 | |
| Euphausiacea | 3 | 0 | 0 | 6.5 | 0.039 | |
| Appendicularia | 183 | 12 | 28 | 7.4 | 0.025 | |
| Polychaeta larvae | <1 | 1 | 0 | 7.5 | 0.024 | |
| Gastropoda larvae | 0 | 0 | 123 | 8.3 | 0.016 | |
| Cirripedia larvae | 1 | 0 | 3 | 6.8 | 0.033 | |
| Decapoda larvae | 8 | 11 | 1 | 6.6 | 0.038 | |
| Echinodermata larvae | 0 | <1 | 5 | 7.2 | 0.028 | |
| Fish larvae | 8 | 14 | <1 | 7.0 | 0.029 | |
| Fish eggs | 15 | 5 | 1 | 7.0 | 0.030 | |
| Autumn | 20 October (n = 3) | 12 October (n = 4) | 21 October (n = 4) | 27 October (n = 4) | ||
| Hydromedusa | 6 | 10 | 25 | 3 | 7.8 | 0.050 |
| Siphonophora | 3 | 14 | 175 | 1 | 13.2 | 0.004 |
| Polychaeta | 0 | 0 | 10 | 3 | 15.2 | 0.007 |
| Pterotracheoidea | 0 | 0 | 4 | 2 | 10.5 | 0.015 |
| Copepoda | 526 | 1619 | 4775 | 1022 | 11.7 | 0.008 |
| Appendicularia | 24 | 25 | 445 | 150 | 10.7 | 0.014 |
| Ddoliolida | 23 | 90 | 274 | 9 | 10.2 | 0.017 |
| Polychaeta larvae | 5 | 16 | 8 | 1 | 8.6 | 0.035 |
| Gastropoda larvae | 0 | 0 | 78 | 10 | 13.4 | 0.004 |
| Cirripedia larvae | 2 | 0 | 10 | 7 | 10.4 | 0.016 |
| Echinodermata larvae | 150 | 94 | 19 | 6 | 8.4 | 0.038 |
| Fish eggs | <1 | 0 | 51 | 5 | 12.1 | 0.007 |
| Pseudoevadne tergestina | 13 | 54 | 0 | 379 | 10.6 | 0.014 |
| Podon sp. | 0 | 0 | 151 | 3 | 13.4 | 0.004 |
| Penilia avirostris | Pseudoevadne tergestina | |||||
|---|---|---|---|---|---|---|
| Region | Mesh (µm) | Abundance (ind m−3) | Date | Abundance (ind m−3) | Date | Source |
| EASTERN PACIFIC | ||||||
| Santa Monica Bay, California | 253 | 460 2 | September 1976 | [53] | ||
| Todos Santos Bay, BC | 505 | 81 | July 1983 | [54] | ||
| Bahia de Los Ángeles, BC | 200 | 51 | October 2003 | 538 | June 2003 | [11] |
| 930 | October 2004 | 372 | July 2004 | [11] | ||
| 1315 | October 2017 | This study | ||||
| 1664 | May 2018 | 3027 | June 2018 | This study | ||
| La Paz Bay, BCS | 219 | 1558 3 | May 1975 | 235 3 | March 1976 | [45] |
| Urías Estuary, Sinaloa 1 | 450 | 152 | November 1976 | [43] | ||
| San Agustín Bay, Oaxaca 1 | 250 | 21 4 | May 1991 | 7 4 | May 1991 | [65] |
| Santa Cruz Bay, Oaxaca 1 | 250 | 17 4 | May 1991 | 13 4 | May 1991 | [65] |
| Chahué Bay, Oaxaca 1 | 250 | 6 4 | May 1991 | 79 4 | May 1991 | [65] |
| Tangolunda Bay, Oaxaca 1 | 250 | 77 4 | May 1991 | 62 4 | May 1991 | [65] |
| Campon Lagoon, Chiapas | 250 | 11 | May 1997 | [66] | ||
| Santa Elena Bay, Ecuador | 335 | 17,547 5 | August 2005 | 488 5 | March 2005 | [84] |
| WESTERN PACIFIC | ||||||
| Masan Bay, South Korea | 200 | 2300 | July–August 2004–2005 | 1170 | July–August 2004–2005 | [85] |
| Jinhae Bay, South Korea | 200 | 21,354 3 | June 2009 | 39,906 3 | August 2009 | [86] |
| Seto Inland Sea, Japan | 100 | 7200 3 | July 1975 | 4600 3 | August 1975 | [87] |
| Zhujiang River Estuary, China | 125 | 35 6 | August 1980 | 4 6 | June 1981 | [88] |
| Shanwei coast, China | 125 | 500 6 | September 1982 | 7 6 | September 1982 | [88] |
| Tolo Harbor, Hong Kong | 125 | 4500 6 | March 1991 | 1100 | May 1990 | [88] |
| INDIAN OCEAN | ||||||
| Gulf of Oman | 150 | 50,000 7 | August 2004–2008 | [89] | ||
| Mandovi Estuary, India | 330 | 505 3 | May 1980 | 368 3 | May 1980 | [90] |
| Estuario Zuari, India | 330 | 4 3 | October 1980 | 280 3 | December 1979 | [90] |
| Kochi coast, India | 200 | 44 | August 2015 | 754 | August 2015 | [91] |
| MEDITERRANEAN | ||||||
| Port Olimpic, Balearic Sea | 53 | 2500 3 | July-August 2003 | [92] | ||
| Blanes Bay, Balearic Sea | 53 | 5650 6 | August 1995 | [93] | ||
| Gulf of Trieste, Mediterranean | 150 | 8770 7 | July 2004–2008 | [89] | ||
| Montenegro coast, Adriatic Sea | 125 | 29,900 | September 2009 | 751 | August 2009 | [94] |
| Gulf of Gokova, Aegean Sea | 200 | 2316 | September 2007 | 94 | July 2007 | [95] |
| Gulf of Alexandretta, Levantine Sea | 200 | 1725 | April 2008 | 24 | July 2008 | [96] |
| Istanbul coast, Sea of Marmara | 157 | 3317 3 | October 2005 | 2219 3 | April 2006 | [97] |
| Gulf of Sevastopol, Black Sea | 150 | 15,000 | August 2004–2008 | [89] | ||
| ATLANTIC OCEAN | ||||||
| Heligoland Bight, North Sea | 500 | 3176 | July 2004 | [98] | ||
| Bayona coast, Cantabrian Sea | 200 | 600 | September 2001–2008 | 600 | September 2001–2008 | [99] |
| Vigo coast, Cantabrian Sea | 150 | 8 7 | November 2004–2006 | [89] | ||
| Mondego Estuary, Portugal | 335 | 2297 | November 2006 | [100] | ||
| Maryland coast, USA | 200 | 38 | July 2012 | [101] | ||
| Virginia coast, USA | 80 | 313 | July 2005–2007 | [102] | ||
| Kingston Harbour, Jamaica | 200 | 22,500 6 | July 1993 | 2000 6 | June 1992 | [103] |
| Paraiba do Sul River Estuary, Brazil | 70 | 17,394 3 | March 2003 | [104] | ||
| Guanabara Bay, Brazil | 200 | 975 | March 1985 | 1204 | November 1985 | [105] |
| Ubatuba Bay, Brazil | 200 | 5881 3 | June 2008 | [106] | ||
| Rio Escuro Estuary, Brazil | 150 | 905 | July 2004 | 350 | July 2004 | [107] |
| 1088 | March 2005 | [107] | ||||
| Mar del Plata, Argentina | 67 | 3200 3 | March 2000 | 25 3 | January 2001 | [108] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lavaniegos, B.E.; Ortuño-Manzanares, G.; Cadena-Ramírez, J.L. Zooplankton of Bahía de Los Ángeles (Gulf of California) in the Context of Other Coastal Regions of the Northeast Pacific. Diversity 2025, 17, 316. https://doi.org/10.3390/d17050316
Lavaniegos BE, Ortuño-Manzanares G, Cadena-Ramírez JL. Zooplankton of Bahía de Los Ángeles (Gulf of California) in the Context of Other Coastal Regions of the Northeast Pacific. Diversity. 2025; 17(5):316. https://doi.org/10.3390/d17050316
Chicago/Turabian StyleLavaniegos, Bertha E., Guillermo Ortuño-Manzanares, and José Luis Cadena-Ramírez. 2025. "Zooplankton of Bahía de Los Ángeles (Gulf of California) in the Context of Other Coastal Regions of the Northeast Pacific" Diversity 17, no. 5: 316. https://doi.org/10.3390/d17050316
APA StyleLavaniegos, B. E., Ortuño-Manzanares, G., & Cadena-Ramírez, J. L. (2025). Zooplankton of Bahía de Los Ángeles (Gulf of California) in the Context of Other Coastal Regions of the Northeast Pacific. Diversity, 17(5), 316. https://doi.org/10.3390/d17050316






