Australian Tropical Marine Micromolluscs: An Overwhelming Bias
Abstract
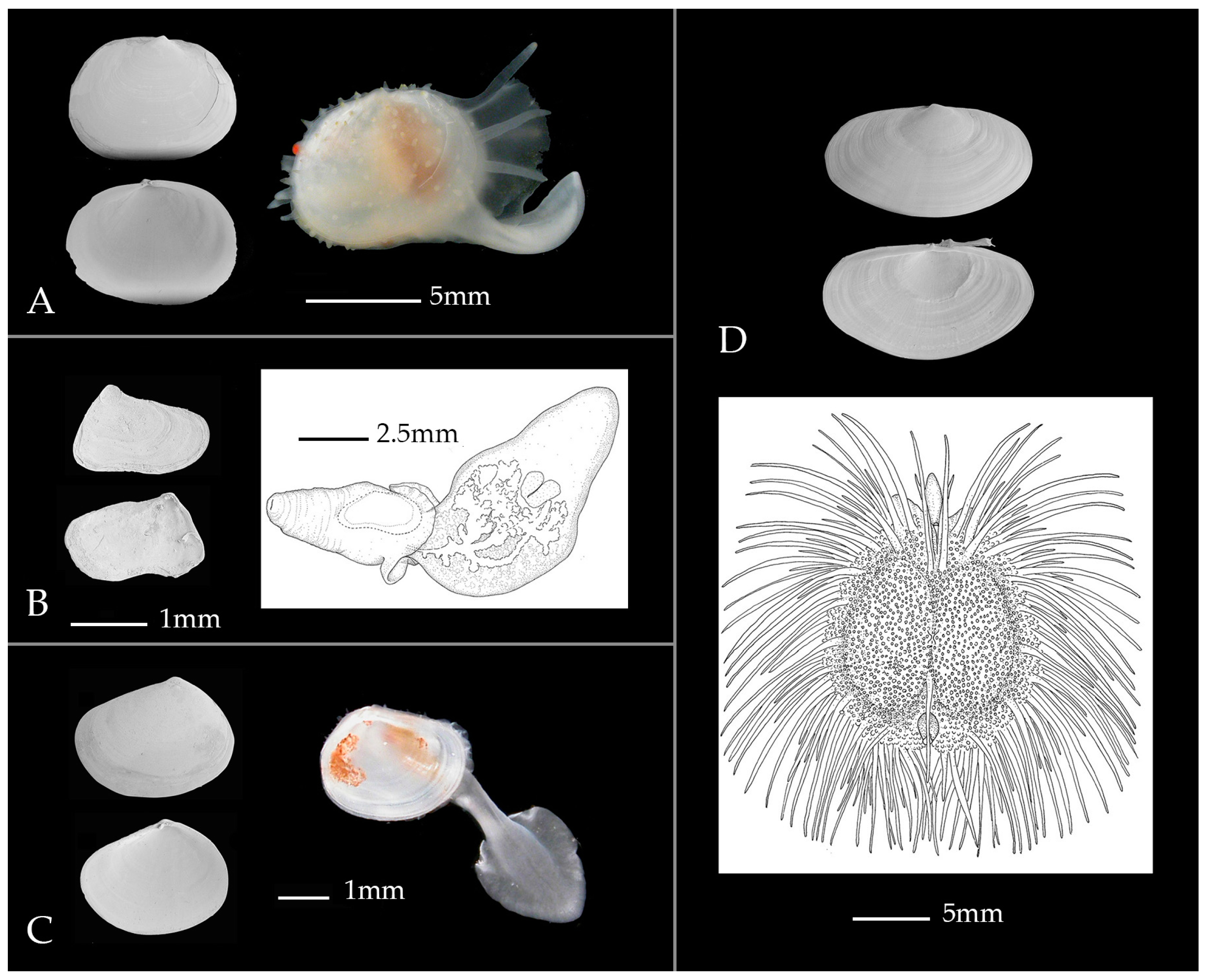
:1. What Is the Problem?
2. Collection Activity in Australia, a Telling Measure
3. The Dreaded Taxonomic “Cul-De-Sac”
4. Connecting Parallel Taxonomies—Species Names, Morphospecies, and DNA Taxonomy
5. Why Would Anyone Care?
6. How Do We Move forward?
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mora, C.; Tittensor, D.P.; Adl, S.; Simpson, A.G.; Worm, B. How many species are there on Earth and in the ocean? PLoS Biol. 2011, 9, e1001127. [Google Scholar] [CrossRef] [PubMed]
- Appeltans, W.; Ahyong, S.T.; Anderson, G.; Angel, M.V.; Artois, T.; Bailly, N.; Bamber, R.; Barber, A.; Bartsch, I.; Berta, A.; et al. The magnitude of global marine species diversity. Curr. Biol. 2012, 22, 2189–2202. [Google Scholar] [CrossRef] [PubMed]
- Ponder, W.; Hutchings, P.; Chapman, R. Overview of the Conservation of Australian Marine Invertebrates. Report for Environment Australia 2002. Available online: http://malsocaus.org/marine_invert/ (accessed on 10 June 2016).
- Valentine, J.W.; Jablonski, D. A twofold role for global energy gradients in marine biodiversity trends. J. Biogeogr. 2015, 42, 997–1005. [Google Scholar] [CrossRef]
- Hausmann, I.; Nützel, A. Diversity and palaeoecology of a highly diverse Late Triassic marine biota from the Cassian Formation at the Stuores Wiesen (North Italy, Dolomites). Lethaia 2015, 48, 235–255. [Google Scholar] [CrossRef]
- Nützel, A.; Kaim, A. Diversity, palaeoecology and systematics of a marine fossil assemblage from the Late Triassic Cassian Formation at Settsass Scharte, N Italy. Paläontologische Z. 2014, 88, 405–431. [Google Scholar] [CrossRef]
- Geiger, D.L.; Marshall, B.A.; Ponder, W.F.; Sasaki, T.; Warén, A. Techniques for collecting, handling, preparing, storing and examining small molluscan specimens. Molluscan Res. 2007, 27, 1–50. [Google Scholar]
- Bouchet, P.; Lozouet, P.; Maestrati, P.; Heros, V. Assessing the magnitude of species richness in tropical marine environments: Exceptionally high numbers of molluscs at a New Caledonia site. Biol. J. Linn. Soc. 2002, 75, 421–436. [Google Scholar] [CrossRef]
- Sasaki, T. Micromolluscs in Japan: Taxonomic composition, habitats, and future topics. Zoosymposia 2008, 1, 147–232. [Google Scholar] [CrossRef]
- Australian Faunal Directory (AFD). Available online: http://www.environment.gov.au/biodiversity/abrs/online-resources/fauna/ (accessed on 2 April 2016).
- Bouchet, P. From specimens to data, and from seashells to molluscs: The Panglao Marine Biodiversity Project. Vita Malacol. 2009, 8, 1–8. [Google Scholar]
- Haas, F.; Häuser, C.L. Taxonomists: An endangered species. In Success Stories in Implementation of the Programmes of Work on Dry and Sub-Humid Lands and the Global Taxonomy Initiative; Secretariat of the Convention on Biological Diversity: Montreal, Canada, 2005; volume 87. [Google Scholar]
- Costello, M.J.; May, R.M.; Stork, N.E. Can We Name Earth’s Species Before They Go Extinct? Science 2013, 339, 413–416. [Google Scholar] [CrossRef] [PubMed]
- Atlas of Living Australia (ALA). Available online: http://www.ala.org.au (accessed on 26 April 2016).
- Laseron, C. New South Wales Marginellidae. Rec. Aust. Mus. 1948, 22, 35–48. [Google Scholar] [CrossRef]
- Laseron, C.F. Review of the Rissoidae of New South Wales. Rec. Aust. Mus. 1950, 22, 257–287. [Google Scholar] [CrossRef]
- Laseron, C. The New South Wales Pyramidellidae and the genus Mathilda. Rec. Aust. Mus. 1951, 22, 298–334. [Google Scholar] [CrossRef]
- Laseron, C. Revision of the New South Wales Cerithiopsidae. Aust. Zool. 1951, 11, 351–368. [Google Scholar]
- Laseron, C. Minute bivalves from New South Wales. Rec. Aust. Mus. 1953, 23, 33–54. [Google Scholar] [CrossRef]
- Laseron, C. Revision of the New South Wales Triphoridae. Rec. Aust. Mus. 1954, 23, 139–158. [Google Scholar] [CrossRef]
- Laseron, C. Revision of the Liotiidae of New South Wales. Aust. Zool. 1954, 12, 1–25. [Google Scholar]
- Laseron, C. Revision of the New South Wales eulimoid shells. Aust. Zool. 1955, 12, 83–107. [Google Scholar]
- Laseron, C. The Family Cerithiopsidae (Mollusca) from the Solanderian and Dampierian Zoogeographical Provinces. Aust. J. Mar. Freshw. Res. 1956, 7, 151–182. [Google Scholar] [CrossRef]
- Laseron, C. The families Rissoinidae and Rissoidae (Mollusca) from the Solanderian and Damperian zoogeographical provinces. Aust. J. Mar. Freshw. Res. 1956, 7, 384–484. [Google Scholar] [CrossRef]
- Laseron, C. A Revision of the New South Wales Leptonidae. Mollusca: Pelecypoda. Rec. Aust. Mus. 1956, 24, 7–21. [Google Scholar] [CrossRef]
- Laseron, C. A new classification of the Australian Marginellidae (Mollusca), with a review of species from the Solanderian and Dampierian Zoogeographical Provinces. Aust. J. Mar. Freshw. Res. 1957, 8, 274–311. [Google Scholar] [CrossRef]
- Laseron, C. The Family Triphoridae (Mollusca) from Northern Australia, also Triphoridae from Christmas Island (Indian Ocean). Aust. J. Mar. Freshw. Res. 1958, 9, 569–658. [Google Scholar]
- Laseron, C. Liotiidae and allied molluscs from the Dampierian Zoogeographical Province. Rec. Aust. Mus. 1958, 24, 165–182. [Google Scholar] [CrossRef]
- Laseron, C. The family Pyramidellidae (Mollusca) from northern Australia. Aust. J. Mar. Freshw. Res. 1959, 10, 177–267. [Google Scholar] [CrossRef]
- Ponder, W.F.; Yoo, E.K. A revision of the Australian and tropical Indo-Pacific Tertiary and Recent species of Pisinna (=Estea) (Mollusca: Gastropoda: Rissoidae). Rec. Aust. Mus. 1976, 30, 150–247. [Google Scholar] [CrossRef]
- Ponder, W.F.; Yoo, E.K. A revision of the Australian species of the Rissoellidae (Mollusca: Gastropoda). Rec. Aust. Mus. 1977, 31, 133–185. [Google Scholar] [CrossRef]
- Ponder, W.F.; Yoo, E.K. A Revision of the Eatoniellidae of Australia (Mollusca, Gastropoda, Littorinacea). Rec. Aust. Mus. 1978, 31, 606–658. [Google Scholar] [CrossRef]
- Ponder, W.F. Review of the genera of the Barleeidae (Mollusca: Gastropoda: Rissoacea). Rec. Aust. Mus. 1983, 35, 231–281. [Google Scholar] [CrossRef]
- Ponder, W.F. A review of the genera of the Rissoidae (Mollusca: Mesogastropoda: Rissoacea). Rec. Aust. Mus. Suppl. 1985, 4, 1–221. [Google Scholar] [CrossRef]
- Ponder, W.F. The truncatelloidean (= Rissoacean) radiation—A preliminary phylogeny. Malacol. Rev. Suppl. 1988, 4, 129–166. [Google Scholar]
- Ponder, W.F. The anatomy and relationships of the Orbitestellidae (Gastropoda: Heterobranchia). J. Molluscan Stud. 1990, 56, 515–532. [Google Scholar] [CrossRef]
- Ponder, W.F. The anatomy and relationships of a marine valvatoidean (Gastropoda: Heterobranchia). J. Molluscan Stud. 1990, 56, 533–555. [Google Scholar] [CrossRef]
- Ponder, W.F. The anatomy of Diala, with an assessment of its taxonomic position (Mollusca: Cerithioidea). In Proceedings of the Third International Marine Biological Workshop: The Marine Flora and Fauna of Albany, Western Australia, Perth, Australia, 11–18 January 1991; Wells, F., Ed.; Australian Marine Sciences, Western Australian Branch: Perth, Australia, 1991; Volume 2, pp. 499–519. [Google Scholar]
- Ponder, W.F. The anatomy and relationships of Finella and Scaliola (Caenogastropoda: Cerithioidea: Scalioidae). In The Malacofauna of Hong Kong and Southern China, 3rd ed.; Morton, B., Ed.; Hong Kong University Press: Hong Kong, China, 1994; pp. 215–241. [Google Scholar]
- Hanken, J.; Wake, D.B. Miniaturization of body size: Organismal consequences and evolutionary significance. Annu. Rev. Ecol. Syst. 1993, 24, 501–519. [Google Scholar] [CrossRef]
- Lützen, J.; Nielsen, C. Galeommatid bivalves from Phuket, Thailand. Zool. J. Linn. Soc. 2005, 144, 261–308. [Google Scholar] [CrossRef]
- Todt, C. Aplacophoran Mollusks—Still Obscure and Difficult? Am. Malacol. Bull. 2013, 31, 181–187. [Google Scholar] [CrossRef]
- Middelfart, P. A revision of the Australian Condylocardiinae (Bivalvia: Carditoidea: Condylocardiidae). Molluscan Res. 2002, 22, 23–85. [Google Scholar] [CrossRef]
- Middelfart, P.U. Revision of the Australian Cuninae sensu lato (Bivalvia: Carditoidea: Condylocardiidae). Zootaxa 2002, 112, 1–124. [Google Scholar]
- Le Renard, J.; Bouchet, P. New species and genera of the family Pickworthiidae (Mollusca, Caenogastropoda). Zoosystema 2003, 25, 569–591. [Google Scholar]
- Criscione, F.; Ponder, W.F. A phylogenetic analysis of rissooidean and cingulopsoidean families (Gastropoda: Caenogastropoda). Mol. Phylogenet. Evol. 2012, 66, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Warén, A. A generic revision of the family Eulimidae (Gastropoda, Prosobranchia). J. Molluscan Stud. Suppl. 1984, 13, 1–96. [Google Scholar] [CrossRef]
- Marshall, B.A. Cerithiopsidae (Mollusca: Gastropoda) of New Zealand, and a provisional classification of the family. N. Z. J. Zool. 1978, 5, 47–120. [Google Scholar] [CrossRef]
- Nützel, A. Über die Stammesgeschichte der Ptenoglossa (Gastropoda). Berl. Geowiss. Abh. E 1997, 26, 1–229. [Google Scholar]
- Marshall, B.A. A revision of the Recent Triphoridae of southern Australia (Mollusca: Gastropoda). Rec. Aust. Mus. Suppl. 1983, 2, 1–119. [Google Scholar] [CrossRef]
- Criscione, F.; Ponder, W.F.; Kohler, F.; Takano, T.; Kano, Y. A molecular phylogeny of Rissoidae (Caenogastropoda: Rissooidea) allows testing the diagnostic utility of morphological traits. Zool. J. Linn. Soc. 2016, 1–18. [Google Scholar] [CrossRef]
- Golding, R.E. Molecular phylogeny and systematics of Australian and East Timorese Stenothyridae (Caenogastropoda: Truncatelloidea). Molluscan Res. 2014, 34, 102–126. [Google Scholar] [CrossRef]
- Iredale, T.; Laseron, C.F. The systematic status of Ctiloceras and some comparative genera. Proc. R. Zool. Soc. N.S.W. 1957, 1955–1956, 97–109. [Google Scholar]
- Bandel, K. Phylogeny of the Caecidae (Caenogastropoda). Mitteilungen Geol.-Paläontologischen Inst. Univ. Hambg. 1996, 79, 53–115. [Google Scholar]
- Pizzini, M.; Raines, B.K.; Vannozzi, A. The Family Caecidae in the South-West Pacific (Gastropoda: Rissooidea). Bollettino malacologico 2013, 49 (Suppl. 10), 1–78. [Google Scholar]
- Marshall, B.A. Skeneidae, Vitrinellidae and Orbitestellidae (Mollusca: Gastropoda) associated with biogenic substrata from bathyal depths off New Zealand and New South Wales. J. Nat. Hist. 1988, 22, 949–1004. [Google Scholar] [CrossRef]
- Warén, A. New and little known Mollusca from Iceland and Scandinavia. Sarsia 1993, 78, 159–201. [Google Scholar] [CrossRef]
- Grindel, J.; Nützel, A. Evolution and Classification of Mesozoic mathildoid gastropods. Acta Paletontol. Pol. 2013, 58, 803–826. [Google Scholar] [CrossRef]
- Warén, A. Murchisonellidae: Who are they, where are they and what are they doing? (Gastropoda, lowermost Heterobranchia). Vita Malacol. 2013, 11, 1–14. [Google Scholar]
- Brenzinger, B.; Wilson, N.G.; Schrödl, M. Microanatomy of shelled Koloonella cf. minutissima (Laseron, 1951) (Gastropoda: ‘lower’ Heterobranchia: Murchisonellidae) does not contradict a sister-group relationship with enigmatic Rhodopemorpha slugs. J. Molluscan Stud. 2014, 80, 518–540. [Google Scholar]
- Haszprunar, G.; Heß, M. A new Rhodope from the Roscoff area (Bretagne), with a review of Rhodope species (Gastropoda: Nudibranchia?). Spixiana 2005, 28, 193. [Google Scholar]
- Brenzinger, B.; Wilson, N.G.; Schrödl, M. 3D microanatomy of a gastropod ‘worm’, Rhodope rousei n. sp. (Heterobranchia) from Southern Australia. J. Molluscan Stud. 2011, 77, 375–387. [Google Scholar] [CrossRef]
- Brenzinger, B.; Haszprunar, G.; Schrödl, M. At the limits of a successful body plan–3D microanatomy, histology and evolution of Helminthope. (Mollusca: Heterobranchia: Rhodopemorpha), the most worm-like gastropod. Front. Zool. 2013, 10, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Peñas, A.; Rolán, E. Deep Water Pyramidelloidea of the Tropical South Pacific: Turbonilla and Related Genera; Gofas, S., Ed.; Mémoires du Muséum National d’Histoire Naturelle: Paris, France, 2010; Volume 26, pp. 1–436, In Tropical Deep Sea Benthos; 200. [Google Scholar]
- Schander, C.; van Aartsen, J.J.; Corgan, J.X. Families and genera of the Pyramidelloidea (Mollusca: Gastropoda). Boll. Malacol. 1999, 34, 145–166. [Google Scholar]
- Schander, C.; Hori, S.; Lundberg, J. Anatomy, phylogeny and biology of Odostomella and Herviera, with the description of a new species of Odostomella. Ophelia 1999, 51, 39–76. [Google Scholar] [CrossRef]
- Kano, Y.; Brenzinger, B.; Nutzel, A.; Wilson, N.G.; Schrodl, M. Ringiculid bubble snails recovered as the sister group to sea slugs (Nudipleura). Sci. Rep. 2016, 6, 30908. [Google Scholar]
- Geiger, D. Monograph of the Little Slit-Shells; Volume 1: Introduction, Scissurellidae; Volume 2: Anatomidae, Larocheidae, Depressizonidae, Sutilizonidae, Temnocinclidae; Santa Barbara Museum of Natural History: Santa Barbara, CA, USA, 2012; p. 1291. [Google Scholar]
- World Register of Marine Species (WoRMS). Available online: http://www.marinespecies.org/index.php (accessed on 20 April 2016).
- Coan, E.V. The tropical eastern Pacific species of the Condylocardiidae (Bivalvia). Nautilus 2003, 117, 47–61. [Google Scholar]
- Hedley, C. The Mollusca of Mast Head Reef, Capricorn Group, Queensland. Proc. Linn. Soc. N.S.W. 1906, 31, 453–479. [Google Scholar] [CrossRef]
- Albano, P.G.; Sabelli, B.; Bouchet, P. The challenge of small and rare species in marine biodiversity surveys: Microgastropod diversity in a complex tropical coastal environment. Biodivers. Conserv. 2011, 20, 3223–3237. [Google Scholar] [CrossRef]
- Moorea Biocode Project. Available online: http://mooreabiocode.org/ (accessed on 10 April 2016).
- Golding, R.E.; Jones, A.S. Micro-CT as a novel technique for 3D reconstruction of molluscan anatomy. Molluscan Res. 2007, 27, 123–128. [Google Scholar]
- Willan, R.C.; Bryce, C.; Slack-Smith, S.M. Kimberley marine biota. Historical data: Molluscs. Rec. West. Aust. Mus. Suppl. 2015, 84, 287–343. [Google Scholar] [CrossRef]
- Wilson, N.G.; Kirkendale, L.A. Putting the ‘Indo’ back into the Indo-Pacific: Resolving marine phylogeographic gaps. Invertebr. Syst. 2016, 30, 86–94. [Google Scholar] [CrossRef]
- Olabarria, C.; Chapman, M.G. Comparison of patterns of spatial variation of microgastropods between two contrasting intertidal habitats. Mar. Ecol. Prog. Ser. 2001, 220, 201–211. [Google Scholar] [CrossRef]
- Beesley, P.L. Mollusca: The southern synthesis. In Fauna of Australia; Beesley, P.L., Ross, G.J.B., Wells, A., Eds.; Australian Government Publishing Service (with CSIRO): Melbourne, Australia, 1998; Volume 5, Part A xvi 563p, Part B viii 565–1234. [Google Scholar]
- Leray, M.; Meyer, C.P.; Mills, S.C. Metabarcoding dietary analysis of coral dwelling predatory fish demonstrates the minor contribution of coral mutualists to their highly partitioned, generalist diet. PeerJ 2015, 3, e1047. [Google Scholar] [CrossRef] [PubMed]
- Berry, O.; Bulman, C.; Bunce, M.; Coghlan, M.; Murray, D.C.; Ward, R.D. Comparison of morphological and DNA metabarcoding analyses of diets in exploited marine fishes. Mar. Ecol. Prog. Ser. 2015, 540, 167–181. [Google Scholar] [CrossRef]
- Schrödl, M.; Jörger, K.M.; Klussmann-Kolb, A.; Wilson, N.G. Bye bye “Opisthobranchia”! A review on the contribution of mesopsammic sea slugs to euthyneuran systematics. Thalassas 2011, 27, 101–112. [Google Scholar]
- Valentich-Scott, P.; Foighil, D.Ó.; Li, J. Where’s Waldo? A new commensal species, Waldo arthuri (Mollusca, Bivalvia, Galeommatidae), from the Northeastern Pacific Ocean. Zookeys 2013, 316, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Foighil, D.Ó.; Middelfart, P. The evolutionary ecology of biotic association in a megadiverse bivalve superfamily: Sponsorship required for permanent residency in sediment. PLoS ONE 2012, 7, e42121. [Google Scholar] [CrossRef] [PubMed]
- Heard, S.B.; Hauser, D.L. Key evolutionary innovations and their ecological mechanisms. Hist. Biol. 1995, 10, 151–173. [Google Scholar] [CrossRef]
- Hunter, J.P. Key innovations and the ecology of macroevolution. Trends Ecol. Evol. 1998, 13, 31–36. [Google Scholar] [CrossRef]
- Leasi, F.; Andrade, S.C.; Norenburg, J.L. At least some meiofaunal species are not everywhere. Indication of geographic, ecological and geological barriers affecting the dispersion of species of Ototyphlonemertes (Nemertea, Hoplonemertea). Mol. Ecol. 2016, 25, 1381–1397. [Google Scholar] [CrossRef] [PubMed]
- Jörger, K.M.; Norenburg, J.L.; Wilson, N.G.; Schrödl, M. Barcoding against a paradox? Combined molecular species delineations reveal multiple cryptic lineages in elusive meiofaunal sea slugs. BMC Evol. Biol. 2012, 12, 245. [Google Scholar]
- Halt, M.N.; Kupriyanova, E.K.; Cooper, S.J.; Rouse, G.W. Naming species with no morphological indicators: Species status of Galeolaria caespitosa (Annelida: Serpulidae) inferred from nuclear and mitochondrial gene sequences and morphology. Invertebr. Syst. 2009, 23, 205–222. [Google Scholar] [CrossRef]
- Cook, L.G.; Edwards, R.D.; Crisp, M.D.; Hardy, N.B. Need morphology always be required for new species descriptions? Invertebr. Syst. 2010, 24, 322–326. [Google Scholar] [CrossRef]
- Jörger, K.M.; Schrödl, M. How to describe a cryptic species? Practical challenges of molecular taxonomy. Front. Zool. 2013, 10. [Google Scholar] [CrossRef] [PubMed]





| Higher Level Systematics | Family (Unless Stated Otherwise) | Key Literature |
|---|---|---|
| Class Aplacophora | [42] * | |
| Class Bivalvia | Neoleptonidae | [19] NSW focus * |
| Class Bivalvia | Condylocardiidae | [43,44] Australian focus |
| Class Bivalvia | Cyamiidae | [19] NSW focus * |
| Class Bivalvia | Superfamily Galeommatoidea | * |
| Class Gastropoda, Caenogastropoda | Superfamily Cerithioidea, Pickworthiidae | [45] * |
| Class Gastropoda, Caenogastropoda | Superfamily Cerithioidea, Scaliolidae | [39] * |
| Class Gastropoda, Caenogastropoda | Superfamily Cerithioidea, Dialidae | [38] * |
| Class Gastropoda, Caenogastropoda | Superfamily Cingulopsoidea | [31,46] Australian Eatoniellidae * |
| Class Gastropoda, Caenogastropoda | Superfamily Eulimoidea, Eulimidae | [47] * |
| Class Gastropoda, Caenogastropoda | Superfamily Triphoroidea, Cerithiopsidae | [48,49] NZ |
| Class Gastropoda, Caenogastropoda | Superfamily Triphoroidea, Triphoridae | [50] Southern Australia |
| Class Gastropoda, Caenogastropoda | Superfamily Rissoidea | [16,24,30,34,35,46,51] Rissoidae NSW, [33] Barleeidae |
| Class Gastropoda, Caenogastropoda | Superfamily Truncatelloidea | [52] Australian and East Timorese Stenothyridae, [30] Australian and tropical IP Tertiary and Recent species of Pisinna (=Estea), [53] Ctiloceras and some comparative genera, [54] Caecidae, [55] Caecidae, Southwest Pacific, [56] NZ and NSW, Tornidae |
| Class Gastropoda, Caenogastropoda | Superfamily Vanikoroidea, Vanikoridae | Very limited information * |
| Class Gastropoda, Heterobranchia | Cimidae | [57] Very limited information * |
| Class Gastropoda, Heterobranchia | Tofanellidae | [58] Very limited information * |
| Class Gastropoda, Heterobranchia | Murchisonellidae | [59,60] Very limited information * |
| Class Gastropoda, Heterobranchia | Rhodopidae | [61,62,63] Southern Australia |
| Class Gastropoda, Heterobranchia | Omalogyridae | [21] NSW, [28] Dampierian Zoogeographical Province |
| Class Gastropoda, Heterobranchia | Orbitestellidae | [21,36] NSW, [28] Dampierian Zoogeographical Province, [56] NZ and NSW |
| Class Gastropoda, Heterobranchia | Pyramidellidae | [17] NSW Pyramidellidae, Mathilda, [29] Northern Australia, [64] Tropical South Pacific, [65,66] Odostomella and Herviera |
| Class Gastropoda, Heterobranchia | Rissoellidae | [31] Australia |
| Class Gastropoda, Heterobranchia | Ringiculidae | [67] Very limited information |
| Class Gastropoda, Heterobranchia | Cornirostridae | [37] Very limited information * |
| Class Gastropoda, Vetigastropoda | Scissurellidae | [68] Anatomidae, Larocheidae, Depressizonidae, Sutilizonidae, Temnocinclidae |
| Class Gastropoda, Vetigastropoda | Superfamily Trochoidea, Skeneidae | [56] NZ and NSW |
| Class Gastropoda, Vetigastropoda | Superfamily Trochoidea, Liotiidae | [21] NSW |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Middelfart, P.U.; Kirkendale, L.A.; Wilson, N.G. Australian Tropical Marine Micromolluscs: An Overwhelming Bias. Diversity 2016, 8, 17. https://doi.org/10.3390/d8030017
Middelfart PU, Kirkendale LA, Wilson NG. Australian Tropical Marine Micromolluscs: An Overwhelming Bias. Diversity. 2016; 8(3):17. https://doi.org/10.3390/d8030017
Chicago/Turabian StyleMiddelfart, Peter U., Lisa A. Kirkendale, and Nerida G. Wilson. 2016. "Australian Tropical Marine Micromolluscs: An Overwhelming Bias" Diversity 8, no. 3: 17. https://doi.org/10.3390/d8030017
APA StyleMiddelfart, P. U., Kirkendale, L. A., & Wilson, N. G. (2016). Australian Tropical Marine Micromolluscs: An Overwhelming Bias. Diversity, 8(3), 17. https://doi.org/10.3390/d8030017







