Improving Triethylamine-Sensing Performance of WO3 Nanoplates through In Situ Heterojunction Construction
Abstract
:1. Introduction
2. Experimental Section
2.1. Material Synthesis
2.2. Preparation of WO3 Nanoplates
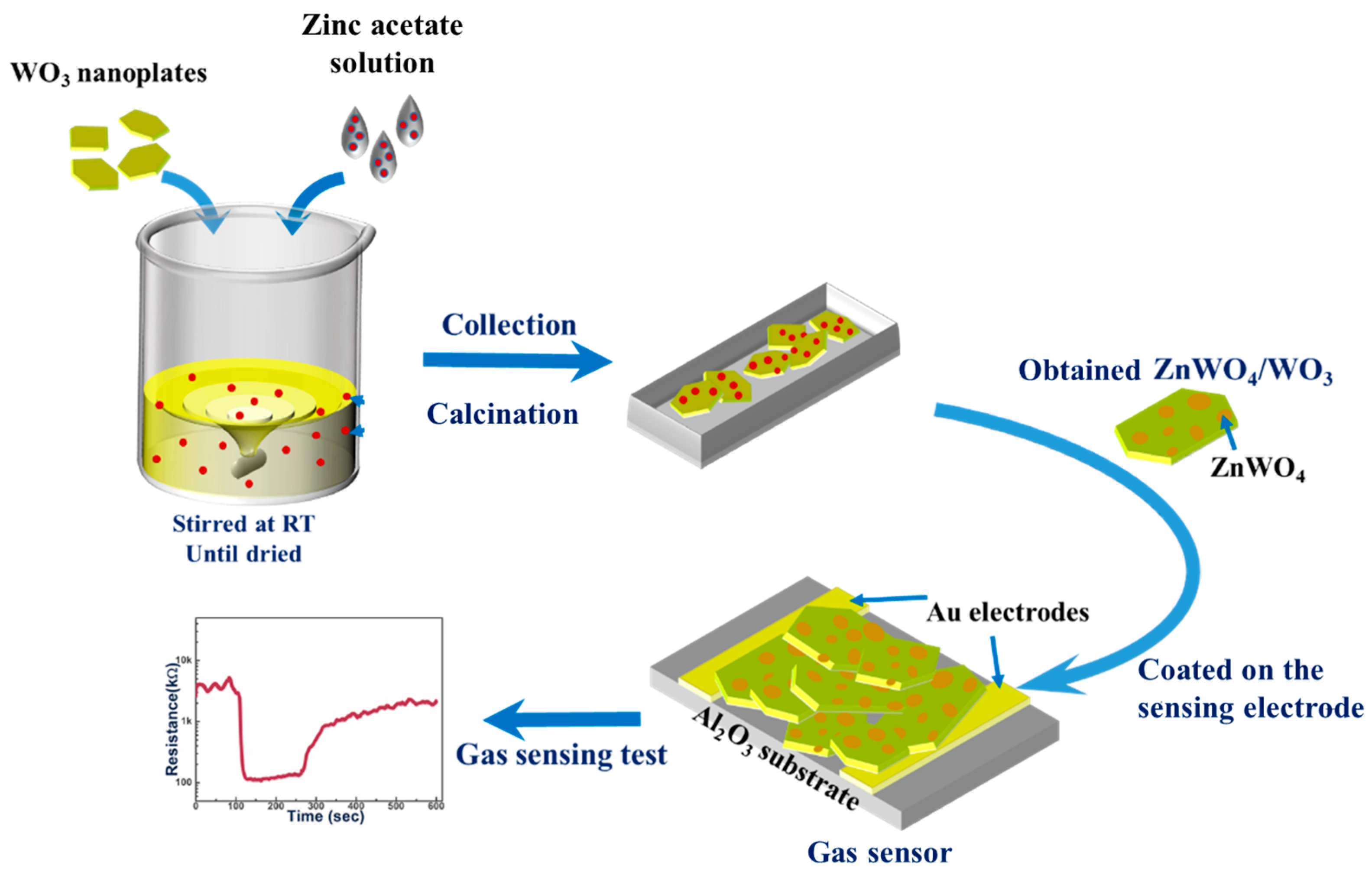
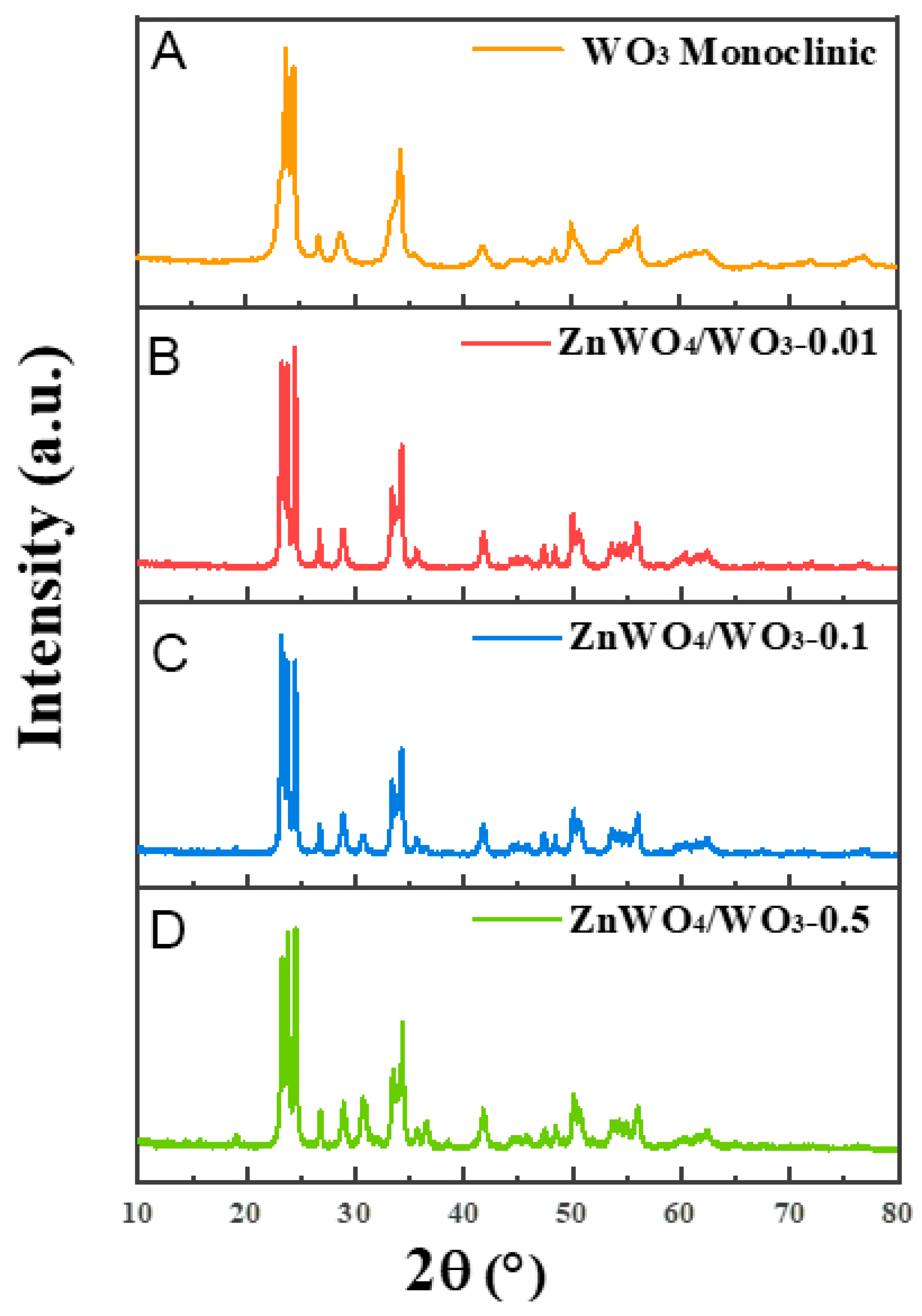
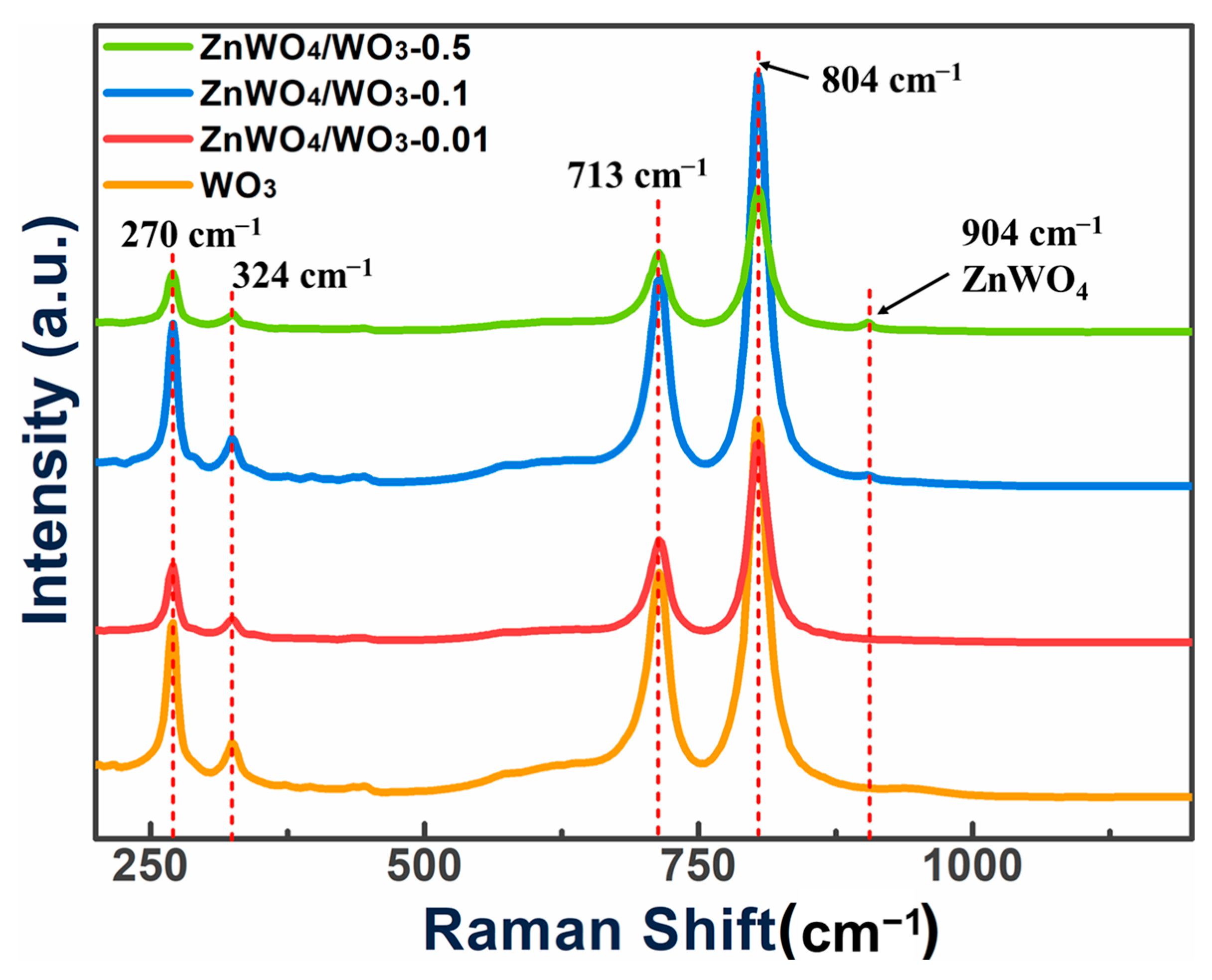
2.3. Preparation of Nanoplates with ZnWO4/WO3 Surface Heterojunction
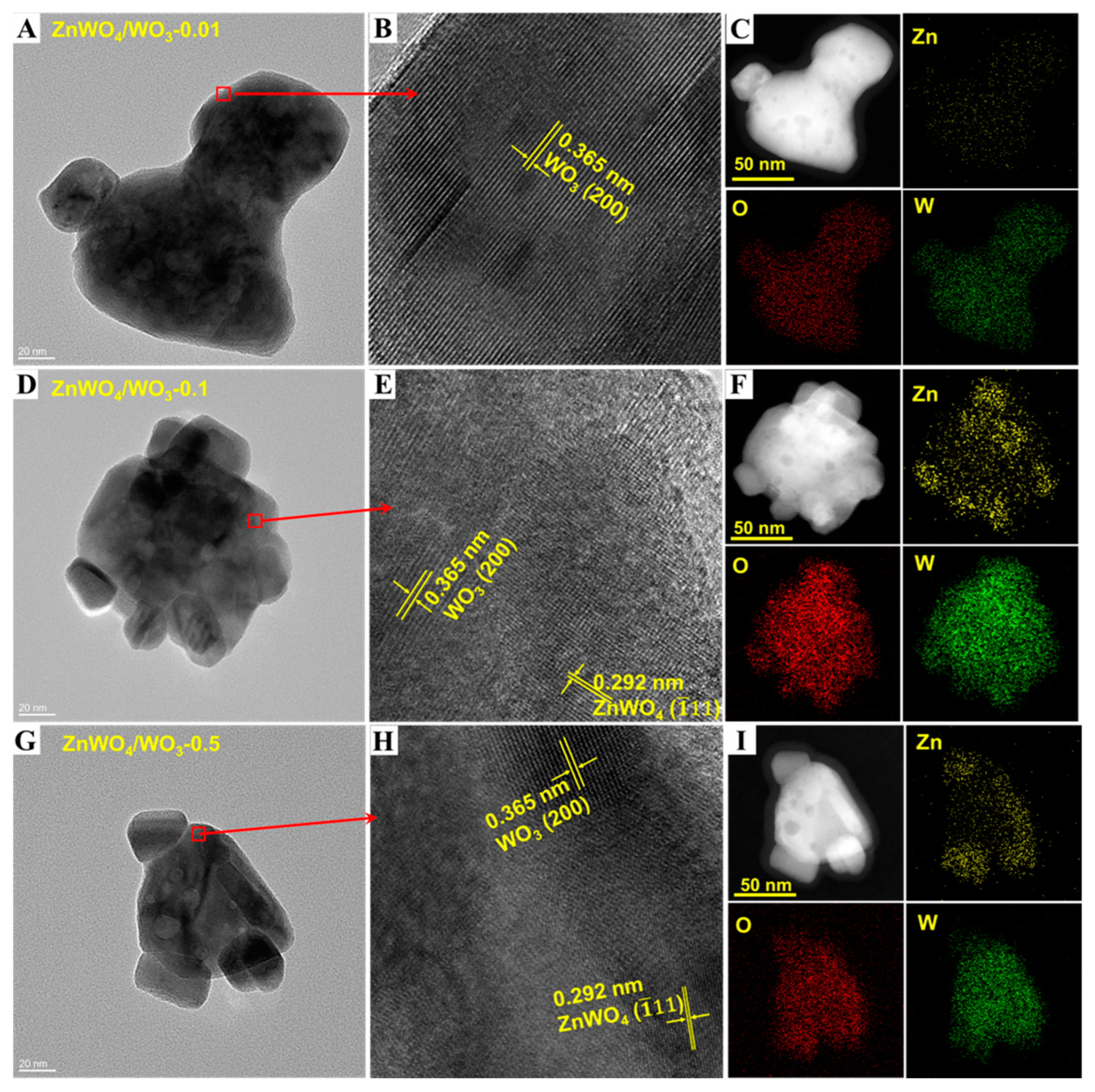
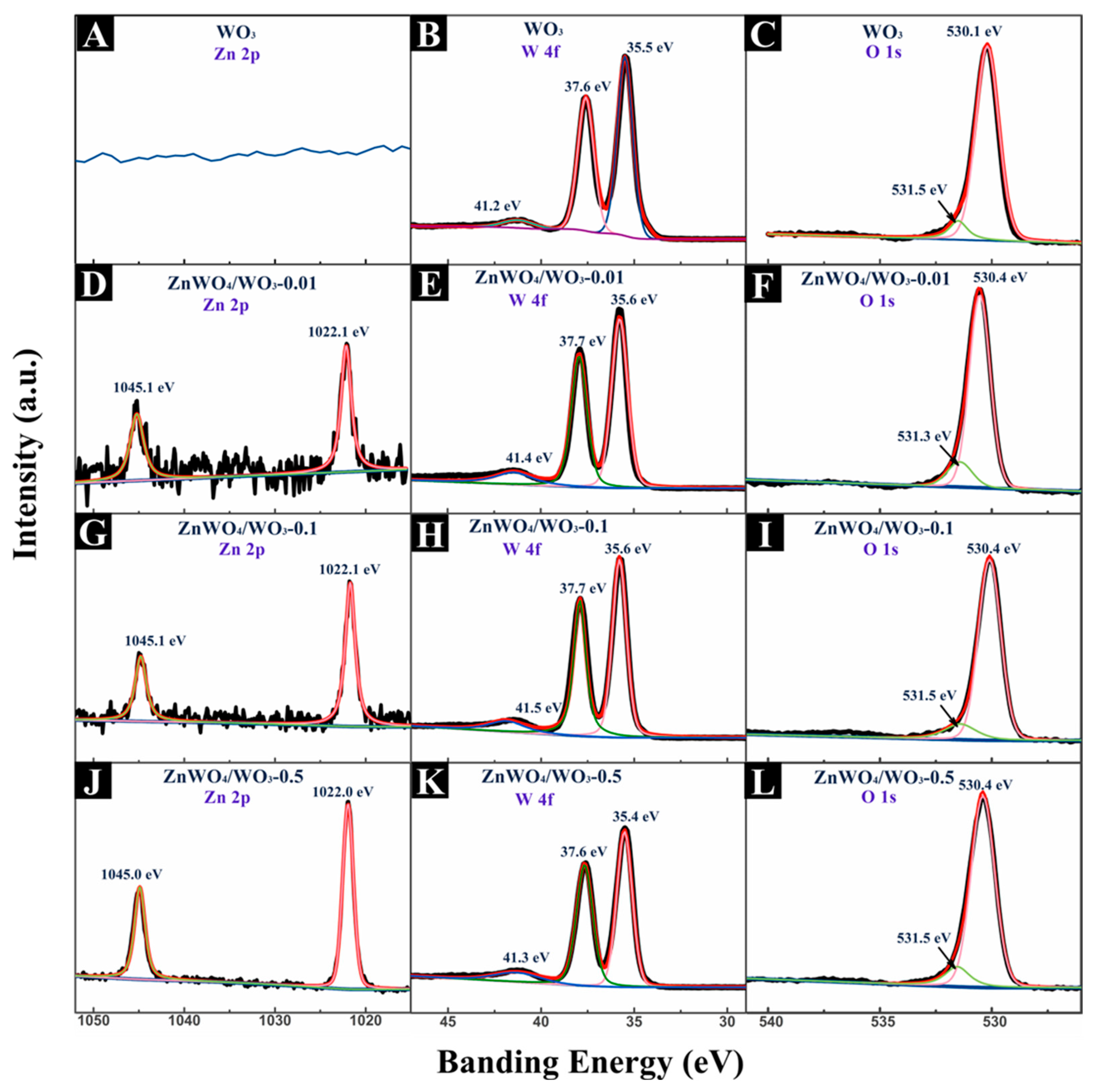
2.4. Microscopic Characterization
2.5. Gas Sensing Measurement
3. Gas Sensing Properties
4. Gas Sensing Mechanism
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, M.; Luo, N.; Chen, Y.; Fan, Y.; Wang, X.; Xu, J. Fast-response MEMS xylene gas sensor based on CuO/WO3 hierarchical structure. J. Hazard. Mater. 2022, 429, 127471. [Google Scholar] [CrossRef] [PubMed]
- Staerz, A.; Liu, Y.; Geyik, U.; Brinkmann, H.; Weimar, U.; Zhang, T.; Barsan, N. The effect of platinum loading on WO3 based sensors. Sens. Actuators B Chem. 2019, 291, 378–384. [Google Scholar] [CrossRef]
- Korotcenkov, G. Gas response control through structural and chemical modification of metal oxide films: State of the art and approaches. Sens. Actuators B Chem. 2005, 107, 209–232. [Google Scholar] [CrossRef]
- Liang, Y.; Yang, Y.; Zou, C.; Xu, K.; Luo, X.; Luo, T.; Li, J.; Yang, Q.; Shi, P.; Yuan, C. 2D ultra-thin WO3 nanoplates with dominant {002} crystal facets for high-performance xylene sensing and methyl orange photocatalytic degradation. J. Alloys Compd. 2019, 783, 848–854. [Google Scholar] [CrossRef]
- Wang, X.; Han, W.; Yang, J.; Jiang, B.; Cheng, P.; Wang, Y.; Sun, P.; Zhang, H.; Sun, Y.; Lu, G. Facet effect on diverse WO3 morphologies and ideal work function for ppb-level triethylamine detection. Sens. Actuators B Chem. 2022, 363, 131849. [Google Scholar] [CrossRef]
- Gui, Y.; Yang, L.; Tian, K.; Zhang, H.; Fang, S. P-type Co3O4 nanoarrays decorated on the surface of n-type flower-like WO3 nanoplates for high-performance gas sensing. Sens. Actuators B Chem. 2019, 288, 104–112. [Google Scholar] [CrossRef]
- Dong, C.; Zhao, R.; Yao, L.; Ran, Y.; Zhang, X.; Wang, Y. A review on WO3 based gas sensors: Morphology control and enhanced sensing properties. J. Alloys Compd. 2020, 820, 153194. [Google Scholar] [CrossRef]
- Degler, D.; Pereira de Carvalho, H.W.; Kvashnina, K.; Grunwaldt, J.-D.; Weimar, U.; Barsan, N. Structure and chemistry of surface-doped Pt:SnO2 gas sensing materials. RSC Adv. 2016, 6, 28149–28155. [Google Scholar] [CrossRef]
- Cui, Y.; Pan, L.; Chen, Y.; Afzal, N.; Ullah, S.; Liu, D.; Wang, L.; Zhang, X.; Zou, J.-J. Defected ZnWO4-decorated WO3 nanorod arrays for efficient photoelectrochemical water splitting. RSC Adv. 2019, 9, 5492–5500. [Google Scholar] [CrossRef]
- Li, W.; Cao, L.; Kong, X.; Huang, J.; Yao, C.; Fei, J.; Li, J. In situ synthesis and photocatalytic performance of WO3/ZnWO4 composite powders. RSC Adv. 2016, 6, 23783–23789. [Google Scholar] [CrossRef]
- Sheng, Z.; Ma, D.; He, Q.; Wu, K.; Yang, L. Mechanism of photocatalytic toluene oxidation with ZnWO4: A combined experimental and theoretical investigation. Catal. Sci. Technol. 2019, 9, 5692–5697. [Google Scholar] [CrossRef]
- Li, W.; Xu, H.; Zhai, T.; Yu, H.; Chen, Z.; Qiu, Z.; Song, X.; Wang, J.; Cao, B. Enhanced triethylamine sensing properties by designing Au@SnO2/MoS2 nanostructure directly on alumina tubes. Sens. Actuators B Chem. 2017, 253, 97–107. [Google Scholar] [CrossRef]
- Pan, H.; Wang, C.; Zhang, Z.; Li, Y.; Hou, X.; Zheng, W.; Liu, X.; Wan, Y.; Zhang, J. Oxygen vacancy-enriched ALD NiO sub-50 nm thin films for enhanced triethylamine detection. Appl. Phys. Lett. 2022, 121, 111603. [Google Scholar] [CrossRef]
- Tian, K.; Yang, X.-Y.; Ren, X.-N.; Huang, Y.-X.; Li, H.-Y.; Gui, Y. Metal–Organic Framework-Derived Hierarchical Nanostructured Ni0.7Zn0.3O Hollow Porous Microspheres for Selective and Sensitive aniline Sensing. ACS Appl. Nano Mater. 2023, 6, 5508–5516. [Google Scholar] [CrossRef]
- Tian, K.; Zhang, W.; Sun, S.-N.; Xing, L.; Li, Z.-Y.; Zhang, T.-T.; Yang, N.-N.; Kuang, B.-B.; Li, H.-Y. Design and fabrication of spinel nanocomposites derived from perovskite hydroxides as gas sensing layer for volatile organic compounds detection. Sens. Actuators B Chem. 2021, 329, 129076. [Google Scholar] [CrossRef]
- Guillén, C.; Herrero, J. Amorphous WO3-x thin films with color characteristics tuned by the oxygen vacancies created during reactive DC sputtering. J. Mater. Sci. Technol. 2021, 78, 223–228. [Google Scholar] [CrossRef]
- Bai, X.; Wang, L.; Zhu, Y. Visible Photocatalytic Activity Enhancement of ZnWO4 by Graphene Hybridization. ACS Catal. 2012, 2, 2769–2778. [Google Scholar] [CrossRef]
- Jung, J.; Jeong, J.R.; Dang Van, C.; Yoo, H.Y.; Lee, M.H. Morphology-Controlled ZnO@ZnWO4 Hetero-Nanostructures for Efficient Photooxidation of Water in Near-Neutral pH. ACS Appl. Mater. Interfaces 2024, 16, 4700–4707. [Google Scholar] [CrossRef]
- Sun, D.; Wang, Q.; Wang, W.; Chen, Y.; Ruan, S. Hollow ZnWO4/In2O3 Nanotubes for Ultrasensitive and Rapid Trace Detection of Triethylamine. ACS Appl. Nano Mater. 2023, 6, 10581–10589. [Google Scholar] [CrossRef]
- Bai, S.; Yin, P.; Zhao, Y.; Tang, P.; Luo, R.; Li, D.; Chen, A. rGO-Modified ZnWO4/WO3 Nanocomposite for Detection of NH3. J. Phys. Chem. C 2023, 127, 9315–9326. [Google Scholar] [CrossRef]
- Kumar, G.M.; Lee, D.J.; Jeon, H.C.; Ilanchezhiyan, P.; Young, K.D.; Won, K.T. One dimensional ZnWO4 nanorods coupled with WO3 nanoplates heterojunction composite for efficient photocatalytic and photoelectrochemical activity. Ceram. Int. 2022, 48, 4332–4340. [Google Scholar] [CrossRef]
- Wei, L.; Zhang, H.; Cao, J. Electrospinning of Ag/ZnWO4/WO3 composite nanofibers with high visible light photocatalytic activity. Mater. Lett. 2019, 236, 171–174. [Google Scholar] [CrossRef]
- Huang, Y.; Gao, Y.; Zhang, Q.; Cao, J.-J.; Huang, R.-J.; Ho, W.; Lee, S.C. Hierarchical porous ZnWO4 microspheres synthesized by ultrasonic spray pyrolysis: Characterization, mechanistic and photocatalytic NOx removal studies. Appl. Catal. A: Gen. 2016, 515, 170–178. [Google Scholar] [CrossRef]
- Leonard, K.C.; Nam, K.M.; Lee, H.C.; Kang, S.H.; Park, H.S.; Bard, A.J. ZnWO4/WO3 Composite for Improving Photoelectrochemical Water Oxidation. J. Phys. Chem. C 2013, 117, 15901–15910. [Google Scholar] [CrossRef]
- Li, H.; Chu, S.; Ma, Q.; Wang, J.; Che, Q.; Wang, G.; Yang, P. Hierarchical WO3/ZnWO4 1D fibrous heterostructures with tunable in-situ growth of WO3 nanoparticles on surface for efficient low concentration HCHO detection. Sens. Actuators B Chem. 2019, 286, 564–574. [Google Scholar]
- Zhang, M.; Zhao, Z.; Hui, B.; Sun, J.; Sun, J.; Tian, W.; Zhang, Z.; Zhang, K.; Xia, Y. Carbonized polymer dots activated hierarchical tungsten oxide for efficient and stable triethylamine sensor. J. Hazard. Mater. 2021, 416, 126161. [Google Scholar] [CrossRef]
- Bai, S.; Zuo, Y.; Zhang, K.; Zhao, Y.; Luo, R.; Li, D.; Chen, A. WO3-ZnFe2O4 heterojunction and rGO decoration synergistically improve the sensing performance of triethylamine. Sens. Actuators B Chem. 2021, 347, 130619. [Google Scholar] [CrossRef]
- Han, Y.; Liu, Y.; Su, C.; Chen, X.; Zeng, M.; Hu, N.; Su, Y.; Zhou, Z.; Wei, H.; Yang, Z. Sonochemical synthesis of hierarchical WO3 flower-like spheres for highly efficient triethylamine detection. Sens. Actuators B Chem. 2020, 306, 127536. [Google Scholar] [CrossRef]
- Wang, X.; Han, W.; Yang, J.; Cheng, P.; Wang, Y.; Feng, C.; Wang, C.; Zhang, H.; Sun, Y.; Lu, G. Conductometric ppb-level triethylamine sensor based on macroporous WO3-W18O49 heterostructures functionalized with carbon layers and PdO nanoparticles. Sens. Actuators B Chem. 2022, 361, 131707. [Google Scholar] [CrossRef]
- Tian, W.; Wang, Y.; Zhang, Y.; Cao, J.; Guan, R.-F. WO3 Nanoflakes Coupled with Hexagonal Boron Nitride Nanoplates for Triethylamine Sensing. ACS Appl. Nano Mater. 2021, 4, 6316–6327. [Google Scholar] [CrossRef]
- Hu, Q.; He, J.; Chang, J.; Gao, J.; Huang, J.; Feng, L. Needle-Shaped WO3 Nanorods for Triethylamine Gas Sensing. ACS Appl. Nano Mater. 2020, 3, 9046–9054. [Google Scholar] [CrossRef]
- Zhai, C.; Zhu, M.; Jiang, L.; Yang, T.; Zhao, Q.; Luo, Y.; Zhang, M. Fast triethylamine gas sensing response properties of nanoplates assembled WO3 hollow microspheres. Appl. Sur. Sci. 2019, 463, 1078–1084. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, K.; Zhang, X.; Wang, B.; Xu, X.; Du, X.; Yang, C.; Zhang, K. Interfacial energy barrier tuning of hierarchical Bi2O3/WO3 heterojunctions for advanced triethylamine sensor. J. Adv. Ceram. 2022, 11, 1860–1872. [Google Scholar] [CrossRef]
- Zeb, S.; Yang, Z.; Hu, R.; Umair, M.; Naz, S.; Cui, Y.; Qin, C.; Liu, T.; Jiang, X. Electronic structure and oxygen vacancy tuning of Co&Ni co-doped W18O49 nanourchins for efficient TEA gas sensing. Chem. Eng. J. 2023, 465, 142815–142851. [Google Scholar]
- Dong, J.; Liu, H.; Sun, C.; Shao, J.; Wang, M.; He, P.; Qi, Y.; Pan, G.; Yang, X. Low-temperature and high-efficient detection of triethylamine based on Pt/PtO2 loaded WO3 gas sensors. J. Alloy. Compd. 2023, 966, 171642. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, Q.; Hao, H.; Lin, H.; Gao, W.; Yan, S. Hydrophobic modification of WO3/WS2 heterostructure for construction of humidity immune TEA sensors. Mater. Chem. Phys. 2023, 294, 126993. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, B.; Lv, L.; Shao, J.; Du, Y.; Li, Y.; Chang, W. Enhanced triethylamine sensing characteristics of In-doped WO3 cubic nanoblocks at low operating temperature. Vacuum 2023, 218, 112640. [Google Scholar] [CrossRef]
- Li, J.; Guo, C.; Li, L.; Gu, Y.; BoK-Hee, K.; Huang, J. In Situ Fabrication of 1D WO3 Nanorod/2D ZnWO4 Nanosheet Heterojunction for Enhanced Photoelectrochemical Performance. Catal. Lett. 2022, 152, 1611–1620. [Google Scholar] [CrossRef]



| Sensor | R2 | K | n |
|---|---|---|---|
| WO3 | 0.9998 | 3.830 | 0.543 |
| ZnWO4/WO3-0.01 | 0.9997 | 8.491 | 0.761 |
| ZnWO4/WO3-0.1 | 0.9999 | 7.957 | 0.693 |
| ZnWO4/WO3-0.5 | 0.9999 | 10.651 | 0.586 |
| Material | Operation Condition | Concentration (ppm) | Response | tres/trec (s) | LOD (ppb) |
|---|---|---|---|---|---|
| Hierarchical CPDs/WO3 [26] | 140 °C | 40 | 14.6 | 267/340 | 46 |
| 0.8% rGO-WO3-ZnFe2O4 [27] | 130 °C | 10 | 26.92 | 51/144 | 20 |
| Hierarchical WO3 Flower-like Spheres [28] | 205 °C | 10 | 11.6 | 3/55 | 83 |
| Micro-flower WO3 [5] | 325 °C | 1 | 2.2 | 3/5 | 50 |
| Carbon Modified WO3-W18O49 with PdO [29] | 325 °C | 100 | 35.7 | 1/2 | 50 |
| WO3/h-BN-5wt% [30] | 260 °C | 500 | 390.6 | 8/60 | 41 |
| Needle-Shaped WO3 [31] | 250 °C | 1 | 6.4 | 45/78 | 1000 |
| WO3 Hollow Microspheres [32] | 220 °C | 60 | 16 | 1.5/22 | 50,000 |
| Hierarchical Bi2O3/WO3 [33] | 140 °C | 50 | 9.2 | 89/162 | 32 |
| Co&Ni Co-Doped W18O49 Nanourchins [34] | 250 °C | 50 | 154 | 16/13 | 1000 |
| Layered Pt/PtO2-WO3 [35] | 137.5 °C | 50 | 3323.5 | 16/262 | 100 |
| WO3/WS2 [36] | 240 °C | 50 | 21.81 | −/− | 3000 |
| In-doped WO3 cubic nanoblocks [37] | 115 °C | 50 | 11.2 | 11/40 | 1000 |
| ZnWO4/WO3-0.01 (This work) | 250 °C | 10 | 44.86 | 16/122 | 93 |
| 0.2 | 2.41 | 32/188 | |||
| ZnWO4/WO3-0.1 (This work) | 250 °C | 10 | 38.72 | 13/113 | 81 |
| 0.2 | 2.89 | 26/178 | |||
| ZnWO4/WO3-0.5 (This work) | 250 °C | 10 | 40.75 | 10/89 | 31 |
| 0.2 | 3.62 | 24/47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, K.; Yang, K.; Ren, X.; Miao, Y.; Liu, M.; Su, M.; Wu, J.; Sun, Y.; Xu, P. Improving Triethylamine-Sensing Performance of WO3 Nanoplates through In Situ Heterojunction Construction. Sensors 2024, 24, 5606. https://doi.org/10.3390/s24175606
Tian K, Yang K, Ren X, Miao Y, Liu M, Su M, Wu J, Sun Y, Xu P. Improving Triethylamine-Sensing Performance of WO3 Nanoplates through In Situ Heterojunction Construction. Sensors. 2024; 24(17):5606. https://doi.org/10.3390/s24175606
Chicago/Turabian StyleTian, Kuan, Kai Yang, Xuening Ren, Yuxin Miao, Mengyao Liu, Mingxing Su, Jiawen Wu, Yu’an Sun, and Pengcheng Xu. 2024. "Improving Triethylamine-Sensing Performance of WO3 Nanoplates through In Situ Heterojunction Construction" Sensors 24, no. 17: 5606. https://doi.org/10.3390/s24175606






