Sensing Utilities of Cesium Lead Halide Perovskites and Composites: A Comprehensive Review
Abstract
1. Introduction
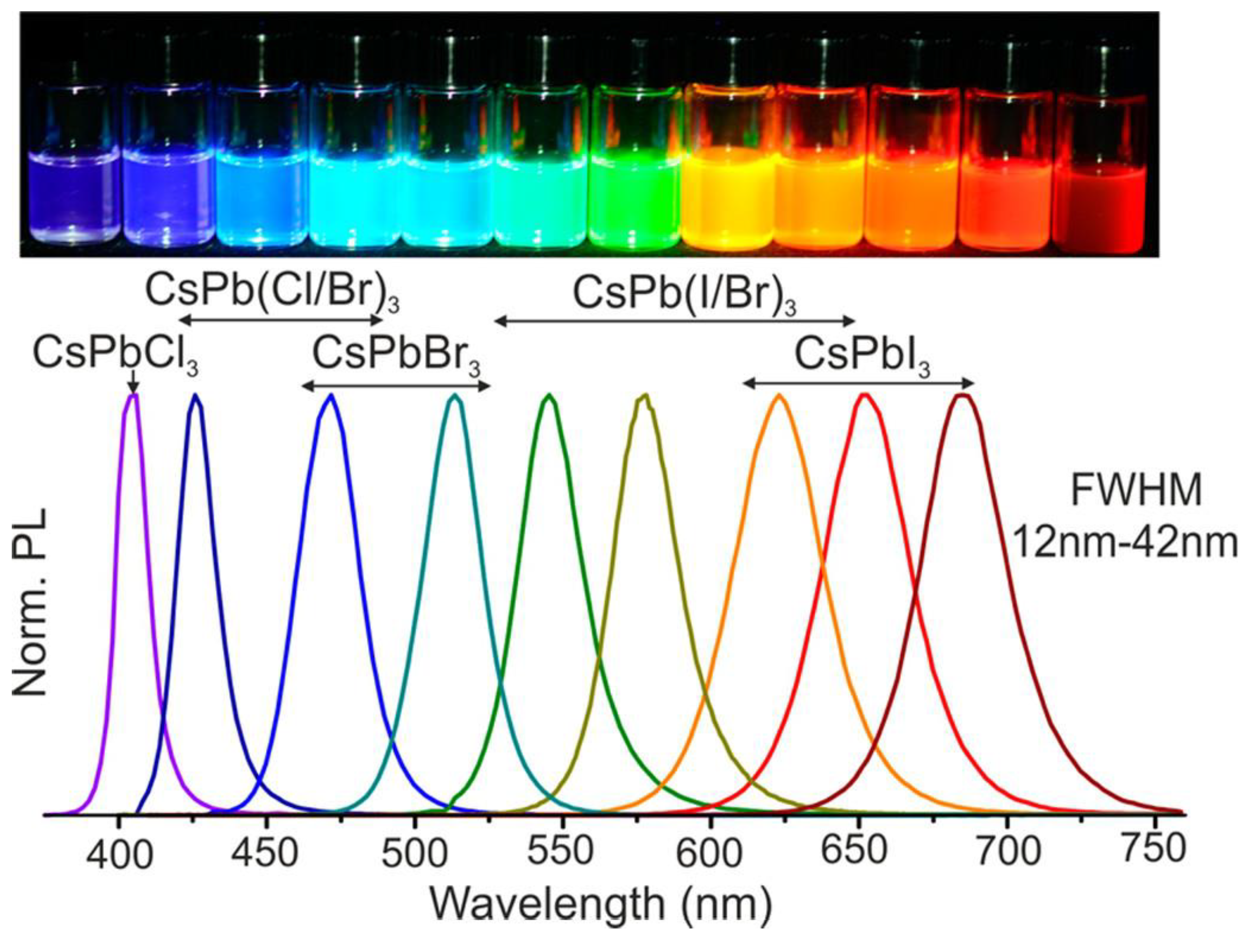
2. Role of Structural Stability and Optoelectronic Properties in Sensors
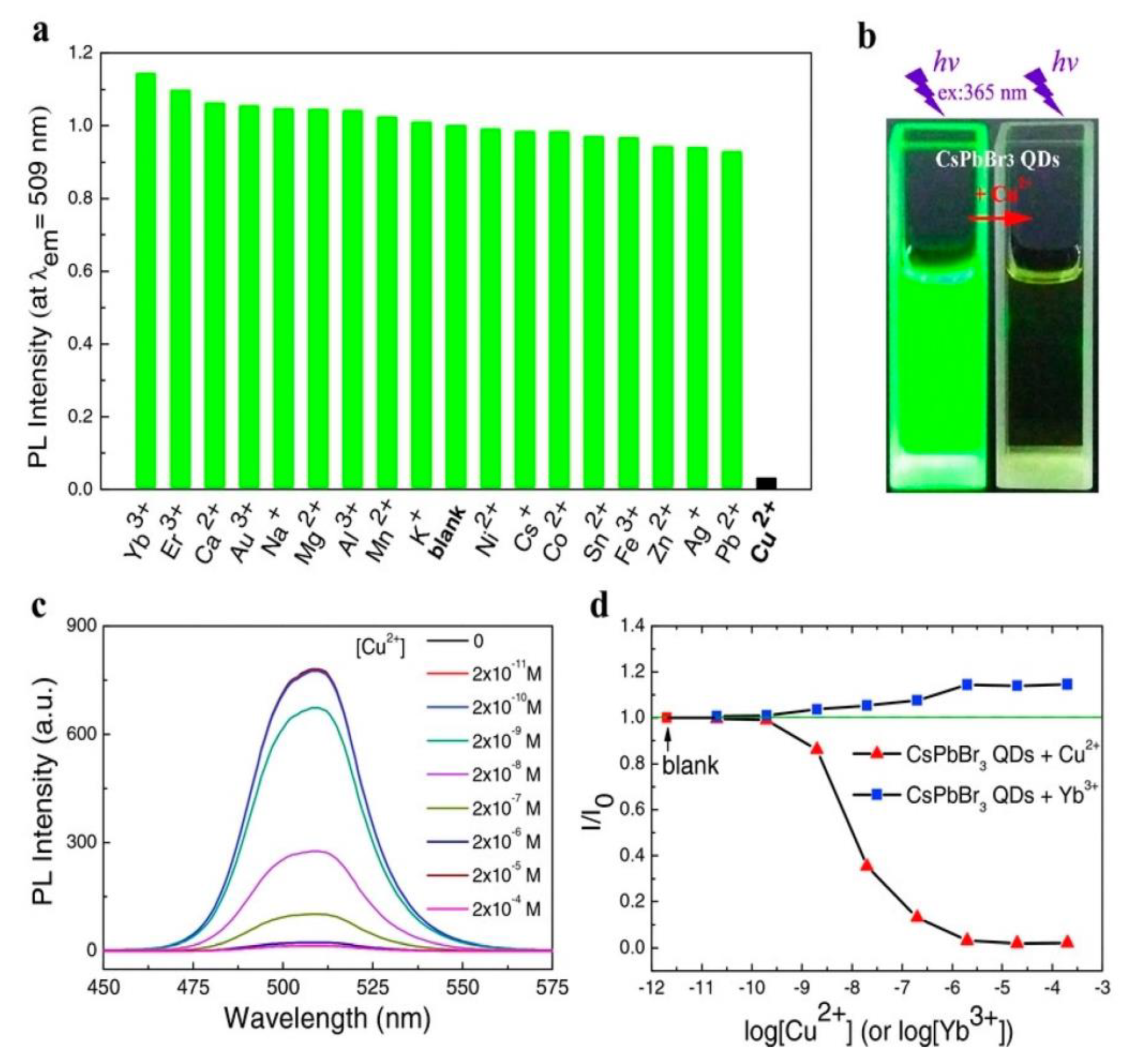
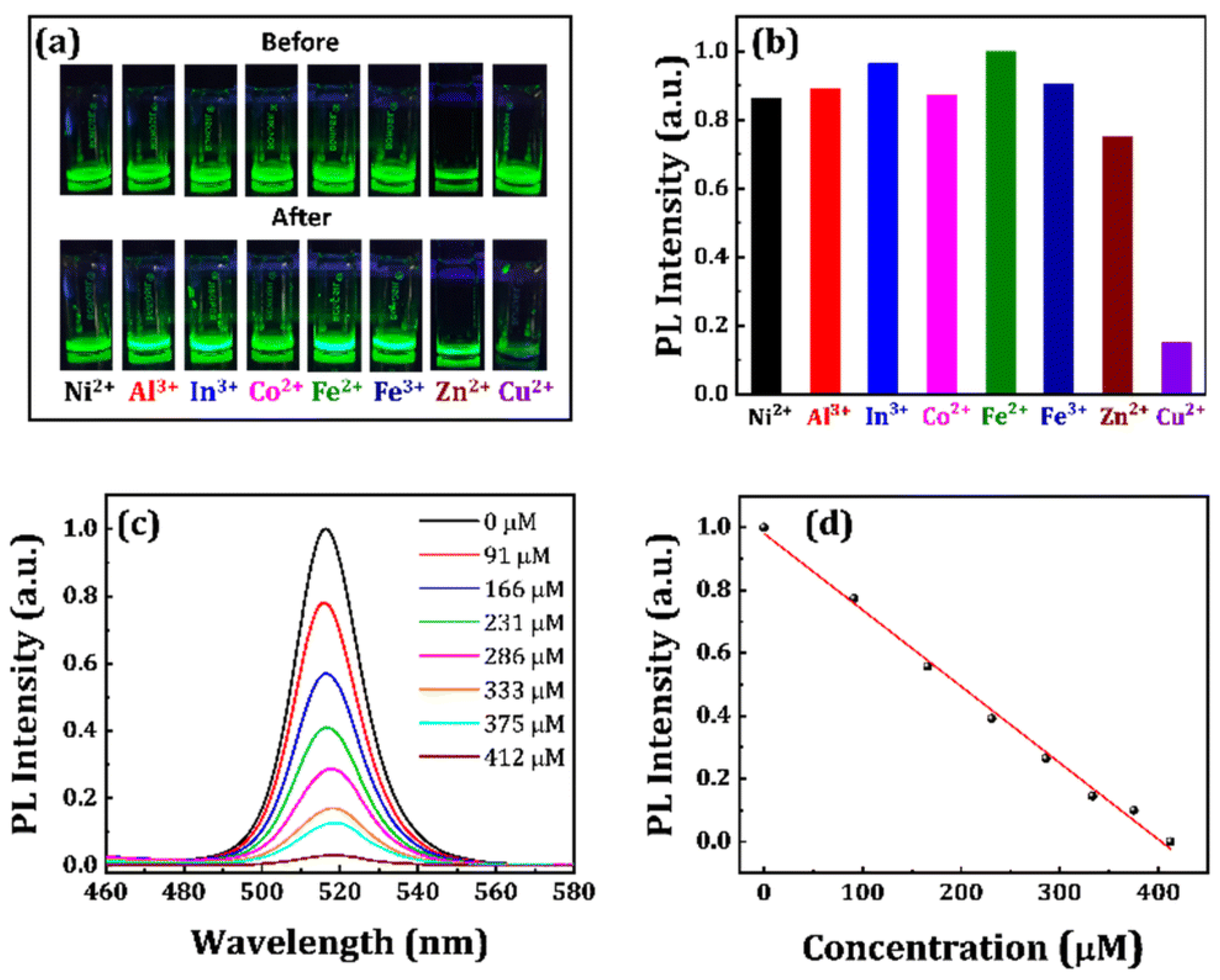
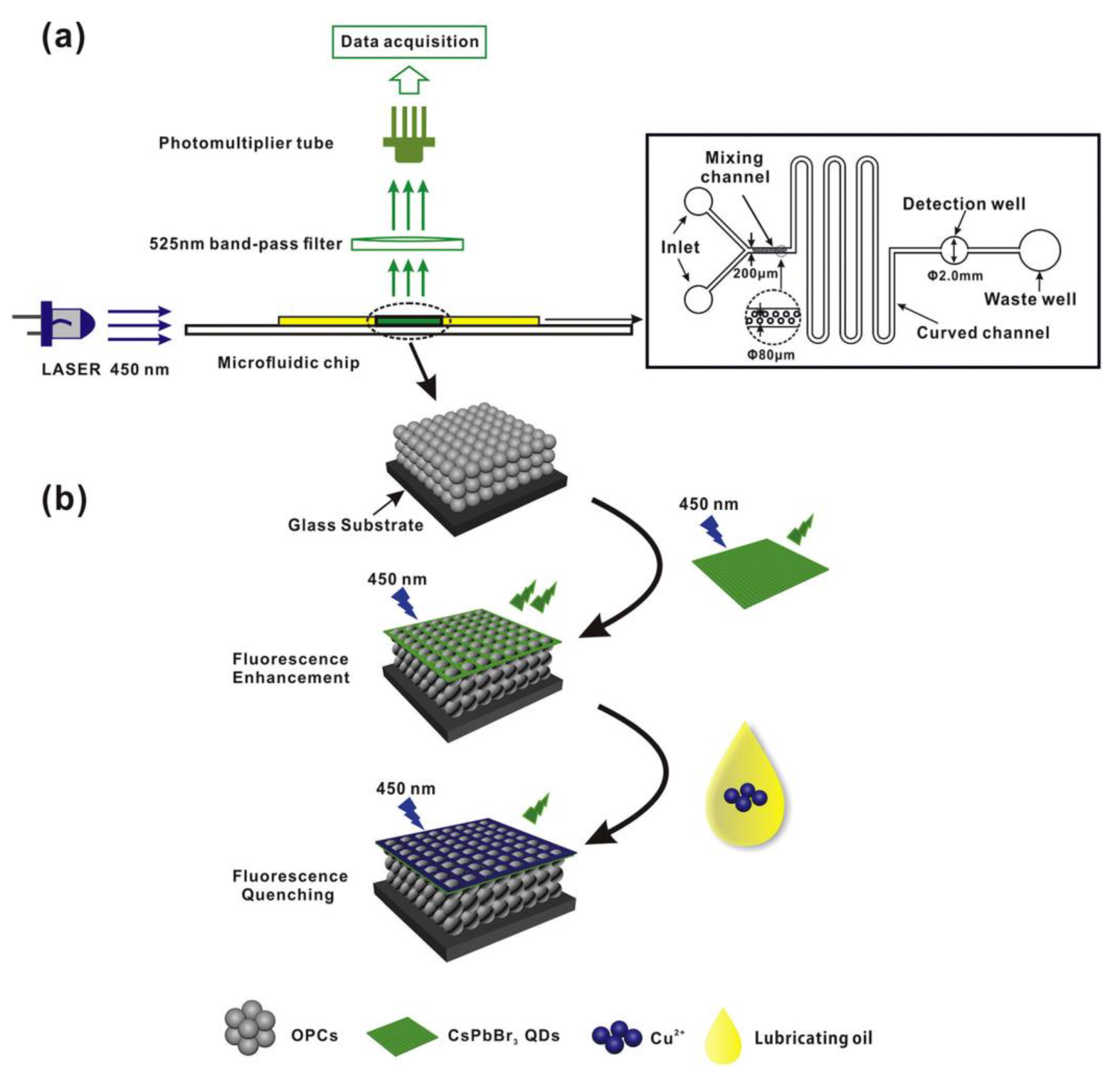
3. CsPbX3 (X = Cl, Br, and I) and Composites toward Metal Ion Detection
Critical Comments on CsPbX3 (X = Cl, Br, and I)-Based Metal Ion Detection
4. Anion Detection by CsPbX3 (X = Cl, Br, and I) and Composites
Critical View on CsPbX3 (X = Cl, Br, and I)-Based Anion Sensors
5. CsPbX3 (X = Cl, Br, and I) and Composites for the Recognition of Chemicals and Explosives
Critical View on CsPbX3 (X = Cl, Br, and I)-Based Chemical and Explosive Sensors
6. CsPbX3 (X = Cl, Br, and I) and Composites for the Quantification of Gaseous Analytes and Volatile Organic Compounds (VOCs)
Critical View on the Detection of CsPbX3 (X = Cl, Br, and I)-Based Gases and VOCs
7. Humidity, Temperature, and Radiation/Photodetection by CsPbX3 (X = Cl, Br, and I) and Composites
Critical View on the Detection of CsPbX3 (X = Cl, Br, and I)-Based Humidity, Temperature, and Radiation/Photodetection
8. CsPbX3 (X = Cl, Br, and I) and Composites in the Detection of Bioanalytes, Drugs, Fungicides, and Pesticides
Critical View on the Detection of CsPbX3 (X = Cl, Br, and I)-Based Bioanalytes, Drugs, Fungicides, and Pesticides
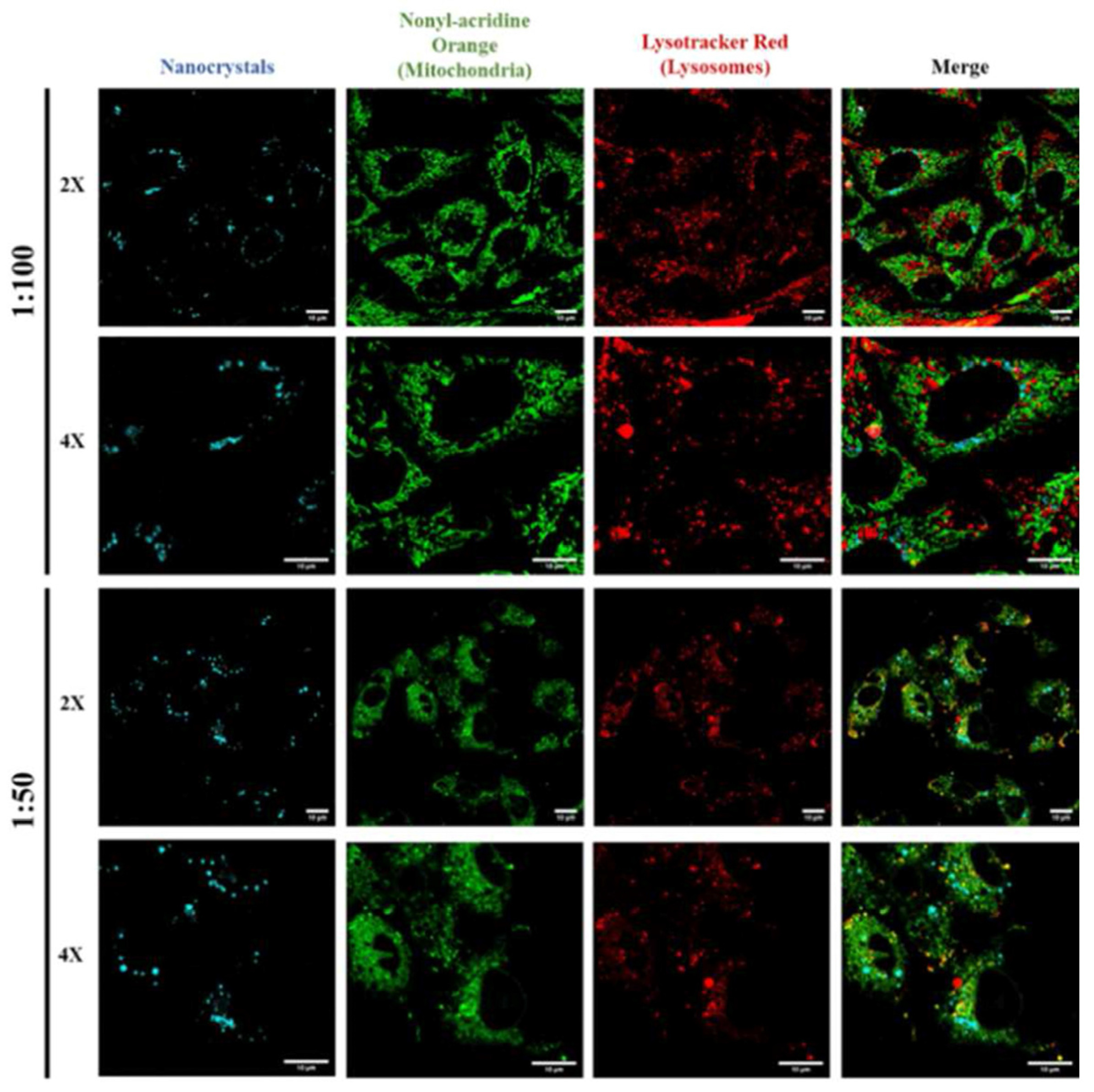
9. Cellular Imaging Applications of CsPbX3 (X = Cl, Br, and I) and Composites
Critical View on CsPbX3 (X = Cl, Br, and I)-Based Cellular Imaging
10. Advantages and Limitations
10.1. Advantages
- Due to the unique structural features, tuning the photophysical properties of CsPbX3 (X = Cl, Br, and I) and anion exchange can result in red, green, and blue emission with an enhanced PLQY (reaches up to 98%); therefore, PL-based sensors with relevant colorimetric responses can benefit from the above properties.
- The greater carrier mobility of CsPbX3 (X = Cl, Br, and I) can be adjusted by combining with other semiconducting materials (such as MoS2, graphene, mxenes, etc.), which is advantageous to the fabrication of heterojunction devices and electrodes for photo/chemoresistive and electrochemical detection of a specific analyte [222,223,224,225].
- The capping and encapsulation of the proposed CsPbX3 (X = Cl, Br, and I) system can enhance water stability, which allows for long-term tracking of bioanalytes.
10.2. Limitations
- Many stable CsPbX3 (X = Cl, Br, and I) and composites are fabricated using multiple synthetic steps, which require precision optimization. However, optimizing synthesis processes is time-consuming, which restricts the use of CsPbX3 (X = Cl, Br, and I) as sensor probes.
- To investigate the precise underlying mechanism of the sensing response to a specific analyte, supporting lines of evidence through methods, such as TEM, XPS, dynamic light scattering spectra (DLS), Zeta potential, etc., are necessary. Thus, the cost-effectiveness of research is questionable, which limits such sensor development in developing or underdeveloped countries.
- Since real water samples may contain certain numbers of ionic species, the reliability of metal ion quantification by CsPbX3 (X = Cl, Br, and I) and composites in real samples is still questionable. This limits the application of CsPbX3 (X = Cl, Br, and I) probes toward the detection of metal ions and anions.
11. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Butt, M.A.; Voronkov, G.S.; Grakhova, E.P.; Kutluyarov, R.V.; Kazanskiy, N.L.; Khonina, S.N. Environmental Monitoring: A Comprehensive Review on Optical Waveguide and Fiber-Based Sensors. Biosensors 2022, 12, 1038. [Google Scholar] [CrossRef] [PubMed]
- Dhall, S.; Mehta, B.R.; Tyagi, A.K.; Sood, K. A Review on Environmental Gas Sensors: Materials and Technologies. Sens. Inter. 2021, 2, 100116. [Google Scholar] [CrossRef]
- Gavrilaș, S.; Ursachi, C.Ș.; Perța-Crișan, S.; Munteanu, F.-D. Recent Trends in Biosensors for Environmental Quality Monitoring. Sensors 2022, 22, 1513. [Google Scholar] [CrossRef] [PubMed]
- Shellaiah, M.; Sun, K.-W. Review on Anti-Aggregation-Enabled Colorimetric Sensing Applications of Gold and Silver Nanoparticles. Chemosensors 2022, 10, 536. [Google Scholar] [CrossRef]
- Kannan, P.; Maduraiveeran, G. Metal Oxides Nanomaterials and Nanocomposite-Based Electrochemical Sensors for Healthcare Applications. Biosensors 2023, 13, 542. [Google Scholar] [CrossRef]
- Shellaiah, M.; Sun, K.W. Review on Carbon Dot-Based Fluorescent Detection of Biothiols. Biosensors 2023, 13, 335. [Google Scholar] [CrossRef]
- Shakeel, A.; Rizwan, K.; Farooq, U.; Iqbal, S.; Iqbal, T.; Awwad, N.S.; Ibrahium, H.A. Polymer-based Nanocomposites: A Strategic Tool for Detection of Toxic Pollutants in Environmental Matrices. Chemosphere 2022, 303, 134923. [Google Scholar] [CrossRef]
- Cho, I.-H.; Kim, D.H.; Park, S. Electrochemical Biosensors: Perspective on Functional Nanomaterials for On-Site Analysis. Biomater. Res. 2020, 24, 6. [Google Scholar] [CrossRef] [PubMed]
- Shellaiah, M.; Sun, K.W. Review on Sensing Applications of Perovskite Nanomaterials. Chemosensors 2020, 8, 55. [Google Scholar] [CrossRef]
- George, K.J.; Halali, V.V.; Sanjayan, C.G.; Suvina, V.; Sakar, M.; Balakrishna, R.G. Perovskite Nanomaterials as Optical and Electrochemical Sensors. Inorg. Chem. Front. 2020, 7, 2702–2725. [Google Scholar] [CrossRef]
- Xiang, W.; Liu, S.; Tress, W. A Review on the Stability of Inorganic Metal Halide Perovskites: Challenges and Opportunities for Stable Solar Cells. Energy Environ. Sci. 2021, 14, 2090–2113. [Google Scholar] [CrossRef]
- Xun, J.; Deng, J.; Shen, W.; Li, M.; He, R. Rapid Synthesis of Highly Stable All-Inorganic Perovskite Nanocrystals Exhibiting Strong Blue Luminescence. J. Alloys Comp. 2021, 872, 159612. [Google Scholar] [CrossRef]
- Sanjayan, C.G.; Jyothi, M.S.; Balakrishna, R.G. Stabilization of CsPbBr3 Quantum Dots for Photocatalysis, Imaging and Optical Sensing in Water and Biological Medium: A Review. J. Mater. Chem. C 2022, 10, 6935–6956. [Google Scholar]
- Zhu, Z.; Sun, Q.; Zhang, Z.; Dai, J.; Xing, G.; Li, S.; Huang, X.; Huang, W. Metal halide perovskites: Stability and sensing-ability. J. Mater. Chem. C 2018, 6, 10121–10137. [Google Scholar] [CrossRef]
- Jia, D.; Xu, M.; Mu, S.; Ren, W.; Liu, C. Recent Progress of Perovskite Nanocrystals in Chem/Bio-Sensing. Biosensors 2022, 12, 754. [Google Scholar] [CrossRef] [PubMed]
- Casanova-Chafer, J.; Garcia-Aboal, R.; Atienzar, P.; Bittencourt, C.; Llobet, E. Perovskite@Graphene Nanohybrids for Breath Analysis: A Proof-of-Concept. Chemosensors 2021, 9, 215. [Google Scholar] [CrossRef]
- Aamir, M.; Shahiduzzaman, M.; Taima, T.; Akhtar, J.; Nunzi, J.-M. It is an All-Rounder! On the Development of Metal Halide Perovskite-Based Fluorescent Sensors and Radiation Detectors. Adv. Opt. Mater. 2021, 9, 2101276. [Google Scholar] [CrossRef]
- Bu, H.; He, C.; Xu, Y.; Xing, L.; Liu, X.; Ren, S.; Yi, S.; Chen, L.; Wu, H.; Zhang, G.; et al. Emerging New-Generation Detecting and Sensing of Metal Halide Perovskites. Adv. Electron. Mater. 2022, 8, 2101204. [Google Scholar] [CrossRef]
- Kailasa, S.K.; Vajubhai, G.N.; Koduru, J.R.; Park, T.J.; Hussain, C.M. Recent Progress on the Modifications of Ultra-Small Perovskite Nanomaterials for Sensing Applications. TrAC Trends Anal. Chem. 2021, 144, 116432. [Google Scholar] [CrossRef]
- Liu, P.; Yu, B.; Cai, W.; Yao, X.; Chang, K.; Zhao, X.; Si, Z.; Deng, W.; Zhou, Y.; Zhou, G.; et al. Air-Stable High-PLQY Cesium Lead Halide Perovskites for Laser-Patterned Displays. J. Mater. Chem. C 2023, 11, 2282–2290. [Google Scholar] [CrossRef]
- Ghosh, D.; Ali, M.Y.; Chaudhary, D.K.; Bhattacharyya, S. Dependence of Halide Composition on the Stability of Highly Efficient All-Inorganic Cesium Lead Halide Perovskite Quantum Dot Solar Cells. Solar Energy Mater. Solar Cells 2018, 185, 28–35. [Google Scholar] [CrossRef]
- Unger, E.L.; Kegelmann, L.; Suchan, K.; Sörell, D.; Korte, L.; Albrecht, S. Roadmap and Roadblocks For the Band Gap Tunability of Metal Halide Perovskites. J. Mater. Chem. A 2017, 5, 11401–11409. [Google Scholar] [CrossRef]
- Li, Y.; Lu, Y.; Huo, X.; Wei, D.; Meng, J.; Dong, J.; Qiao, B.; Zhao, S.; Xu, Z.; Song, D. Bandgap Tuning Strategy by Cations and Halide Ions of Lead Halide Perovskites Learned from Machine Learning. RSC Adv. 2021, 11, 15688–15694. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Yun, Y.; Yang, S.W.; Oh, H.G.; Jeon, A.Y.; Nam, Y.; Heo, Y.-W.; Chae, W.-S.; Lee, S. Ternary Diagrams of Phase, Stability, and Optical Properties of Cesium Lead Mixed-Halide Perovskites. Acta Mater. 2023, 246, 118661. [Google Scholar] [CrossRef]
- Tai, M.; Lau, C.F.J.; Lin, H.; Wang, Z. Advances in Phase Stability of Cesium Lead Halide Perovskites. Sol. RRL 2020, 4, 2000495. [Google Scholar] [CrossRef]
- Yang, D.; Cao, M.; Zhong, Q.; Li, P.; Zhang, X.; Zhang, Q. All-Inorganic Cesium Lead Halide Perovskite Nanocrystals: Synthesis, Surface Engineering and Applications. J. Mater. Chem. C 2019, 7, 757–789. [Google Scholar] [CrossRef]
- Jung, H.-S.; Cho, J.; Neuman, K.C. Highly Stable Cesium Lead Bromide Perovskite Nanocrystals for Ultra-Sensitive and Selective Latent Fingerprint Detection. Anal. Chim. Acta 2021, 1181, 338850. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Feng, S.; Yang, Q.; Yi, F.; Li, X.; Yuan, Y.; Wang, C.; Yan, H. Promoting Charge Separation in A Composite of δ-CsPbI3 and Covalent Organic Frameworks. J. Mater. Chem. C 2023, 11, 7570–7574. [Google Scholar] [CrossRef]
- Cao, L.; Liu, X.; Li, Y.; Li, X.; Du, L.; Chen, S.; Zhao, S.; Wang, C. Recent Progress in All-Inorganic Metal Halide Nanostructured Perovskites: Materials Design, Optical Properties, and Application. Front. Phys. 2020, 16, 33201. [Google Scholar] [CrossRef]
- Skurlov, I.D.; Sokolova, A.V.; Tatarinov, D.A.; Parfenov, P.S.; Kurshanov, D.A.; Ismagilov, A.O.; Koroleva, A.V.; Danilov, D.V.; Zhizhin, E.V.; Mikushev, S.V.; et al. Engineering the Optical Properties of CsPbBr3 Nanoplatelets through Cd2+ Doping. Materials 2022, 15, 7676. [Google Scholar] [CrossRef]
- Akkerman, Q.A.; D’Innocenzo, V.; Accornero, S.; Scarpellini, A.; Petrozza, A.; Prato, M.; Manna, L. Tuning the Optical Properties of Cesium Lead Halide Perovskite Nanocrystals by Anion Exchange Reactions. J. Am. Chem. Soc. 2015, 137, 10276–10281. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Feng, J.; Yang, Z.; Liu, Y.; Liu, S. Halide Perovskite: A Promising Candidate for Next-Generation X-Ray Detectors. Adv. Sci. 2023, 10, 2205536. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Chou, H.-L.; Lin, J.-C.; Lee, Y.-C.; Pao, C.-W.; Chen, J.-L.; Chang, C.-C.; Chi, R.-Y.; Kuo, T.-R.; Lu, C.-W.; et al. Enhanced Luminescence and Stability of Cesium Lead Halide Perovskite CsPbX3 Nanocrystals by Cu2+-Assisted Anion Exchange Reactions. J. Phys. Chem. C 2019, 123, 2353–2360. [Google Scholar] [CrossRef]
- Chistyakov, A.A.; Zvaigzne, M.A.; Nikitenko, V.R.; Tameev, A.R.; Martynov, I.L.; Prezhdo, O.V. Optoelectronic Properties of Semiconductor Quantum Dot Solids for Photovoltaic Applications. J. Phys. Chem. Lett. 2017, 8, 4129–4139. [Google Scholar] [CrossRef] [PubMed]
- Peltonen, L.; Strachan, C. Understanding Critical Quality Attributes for Nanocrystals from Preparation to Delivery. Molecules 2015, 20, 22286–22300. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Zhang, H.; Sun, C.; Yin, C.; Lv, B.; Zhang, C.; Yu, W.W.; Wang, X.; Zhang, Y.; Xiao, M. Superior Optical Properties of Perovskite Nanocrystals as Single Photon Emitters. ACS Nano 2015, 9, 12410–12416. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.; Bain, D.; Patra, A. An Overview on the Current Understanding of the Photophysical Properties of Metal Nanoclusters and Their Potential Applications. Nanoscale 2019, 11, 22685–22723. [Google Scholar] [CrossRef] [PubMed]
- Shellaiah, M.; Chen, T.H.; Simon, T.; Li, L.-C.; Sun, K.W.; Ko, F.-H. An Affordable Wet Chemical Route to Grow Conducting Hybrid Graphite-Diamond Nanowires: Demonstration by A Single Nanowire Device. Sci. Rep. 2017, 7, 11243. [Google Scholar] [CrossRef] [PubMed]
- Shellaiah, M.; Chen, Y.-C.; Simon, T.; Li, L.-C.; Sun, K.W.; Ko, F.-H. Effect of Metal Ions on Hybrid Graphite-Diamond Nanowire Growth: Conductivity Measurements from a Single Nanowire Device. Nanomaterials 2019, 9, 415. [Google Scholar] [CrossRef] [PubMed]
- Zhai, W.; Lin, J.; Li, C.; Hu, S.; Huang, Y.; Yu, C.; Wen, Z.; Liu, Z.; Fang, Y.; Tang, C. Solvothermal Synthesis of Cesium Lead Halide Perovskite Nanowires with Ultra-High Aspect Ratios for High-Performance Photodetectors. Nanoscale 2018, 10, 21451–21458. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, C.; Zhang, G.; Chen, Y.; Shang, F.; Xia, Q.; Yang, W. Full review: The Progress and Developing Trends of Nanosheet-Based Sensing Applications. Coord. Chem. Rev. 2021, 433, 213742. [Google Scholar] [CrossRef]
- Lv, L.; Xu, Y.; Fang, H.; Luo, W.; Xu, F.; Liu, L.; Wang, B.; Zhang, X.; Yang, D.; Hu, W.; et al. Generalized Colloidal Synthesis of High-Quality, Two-Dimensional Cesium Lead Halide Perovskite Nanosheets and Their Applications in Photodetectors. Nanoscale 2016, 8, 13589–13596. [Google Scholar] [CrossRef]
- Khan, Y.; Sadia, H.; Ali Shah, S.Z.; Khan, M.N.; Shah, A.A.; Ullah, N.; Ullah, M.F.; Bibi, H.; Bafakeeh, O.T.; Khedher, N.B.; et al. Classification, Synthetic, and Characterization Approaches to Nanoparticles, and Their Applications in Various Fields of Nanotechnology: A Review. Catalysts 2022, 12, 1386. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of Heavy Metal Ions from Wastewater: A Comprehensive and Critical Review. Npj Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Kumar, E.; Bhatnagar, A.; Hogland, W.; Marques, M.; Sillanpää, M. Interaction of Anionic Pollutants with Al-based Adsorbents in Aqueous media—A Review. Chem. Eng. J. 2014, 241, 443–456. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, X.; Khan, A.; Wang, P.; Liu, Y.; Alsaedi, A.; Hayat, T.; Wang, X. Environmental Remediation and Application of Nanoscale Zero-Valent Iron and Its Composites for the Removal of Heavy Metal Ions: A Review. Environ. Sci. Technol. 2016, 50, 7290–7304. [Google Scholar] [CrossRef] [PubMed]
- Idris, A.O.; Orimolade, B.; Dennany, L.; Mamba, B.; Azizi, S.; Kaviyarasu, K.; Maaza, M. A Review on Monitoring of Organic Pollutants in Wastewater Using Electrochemical Approach. Electrocatalysis 2023, 14, 659–687. [Google Scholar] [CrossRef]
- Kim, C.; Eom, J.B.; Jung, S.; Ji, T. Detection of Organic Compounds in Water by an Optical Absorbance Method. Sensors 2016, 16, 61. [Google Scholar] [CrossRef]
- To, K.C.; Ben-Jaber, S.; Parkin, I.P. Recent Developments in the Field of Explosive Trace Detection. ACS Nano 2020, 14, 10804–10833. [Google Scholar] [CrossRef] [PubMed]
- Onyancha, R.B.; Ukhurebor, K.E.; Aigbe, U.O.; Osibote, O.A.; Kusuma, H.S.; Darmokoesoemo, H.; Balogun, V.A. A systematic review on the detection and monitoring of toxic gases using carbon nanotube-based biosensors. Sens. Bio-Sens. Res. 2021, 34, 100463. [Google Scholar] [CrossRef]
- Mirzaei, A.; Leonardi, S.G.; Neri, G. Detection of Hazardous Volatile Organic Compounds (VOCs) by Metal Oxide Nanostructures-based Gas Sensors: A Review. Ceram. Inter. 2016, 42, 15119–15141. [Google Scholar] [CrossRef]
- Wang, H.; Sun, Y.; Chen, J.; Wang, F.; Han, R.; Zhang, C.; Kong, J.; Li, L.; Yang, J. A Review of Perovskite-Based Photodetectors and Their Applications. Nanomaterials 2022, 12, 4390. [Google Scholar] [CrossRef] [PubMed]
- Arneodo, F.; Di Giovanni, A.; Marpu, P. A Review of Requirements for Gamma Radiation Detection in Space Using CubeSats. Appl. Sci. 2021, 11, 2659. [Google Scholar] [CrossRef]
- Arman Kuzubasoglu, B.; Kursun Bahadir, S. Flexible Temperature Sensors: A Review. Sens. Actuators A 2020, 315, 112282. [Google Scholar] [CrossRef]
- Wang, T.; Ramnarayanan, A.; Cheng, H. Real-Time Analysis of Bioanalytics in Healthcare, Food, Zoology and Botany. Sensors 2018, 18, 5. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.R.; Chand, R.; Kumar, S.; Mittal, N.; Srinivasan, S.; Rajabzadeh, A.R. Recent Biosensing Advances in the Rapid Detection of Illicit Drugs. TrAC Trends Anal. Chem. 2020, 131, 116006. [Google Scholar] [CrossRef]
- Gikas, G.D.; Parlakidis, P.; Mavropoulos, T.; Vryzas, Z. Particularities of Fungicides and Factors Affecting Their Fate and Removal Efficacy: A Review. Sustainability 2022, 14, 4056. [Google Scholar] [CrossRef]
- Zamora-Sequeira, R.; Starbird-Pérez, R.; Rojas-Carillo, O.; Vargas-Villalobos, S. What are the Main Sensor Methods for Quantifying Pesticides in Agricultural Activities? A Review. Molecules 2019, 24, 2659. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Feng, Y.; Li, F.; Lin, F.; Wang, Y.; Chen, X.; Xie, R. Sensing Studies and Applications Based on Metal Halide Perovskite Materials: Current Advances and Future Perspectives. TrAC Trends Anal. Chem. 2021, 134, 116127. [Google Scholar] [CrossRef]
- Bui, T.H.; Shin, J.H. Perovskite Materials for Sensing Applications: Recent Advances and Challenges. Microchem. J. 2023, 191, 108924. [Google Scholar] [CrossRef]
- Wang, B.; Yang, X.; Chen, S.; Lu, S.; Zhao, S.; Qian, Q.; Cai, W.; Wang, S.; Zang, Z. Flexible Perovskite Scintillators and Detectors for X-Ray Detection. iScience 2022, 25, 105593. [Google Scholar] [CrossRef] [PubMed]
- Lian, H.; Li, Y.; Saravanakumar, S.; Jiang, H.; Li, Z.; Wang, J.; Xu, L.; Zhao, W.; Han, G. Metal Halide Perovskite Quantum Dots for Amphiprotic Bio-Imaging. Coord. Chem. Rev. 2022, 452, 214313. [Google Scholar] [CrossRef]
- Yang, T.; Li, F.; Zheng, R. Recent Progress on Cesium Lead Halide Perovskites for Photodetection Applications. ACS Appl. Electron. Mater. 2019, 1, 1348–1366. [Google Scholar] [CrossRef]
- Cheng, L.; Chi, J.; Su, M.; Song, Y. Interface Engineering of Perovskite Nanocrystals: Challenges and Opportunities for Biological Imaging and Detection. J. Mater. Chem. C 2023, 11, 7970–7981. [Google Scholar] [CrossRef]
- Plesko, S.; Kind, R.; Roos, J. Structural Phase Transitions in CsPbCl3 and RbCdCl3. J. Phys. Soc. Jpn. 1978, 45, 553–557. [Google Scholar] [CrossRef]
- Peña, M.A.; Fierro, J.L.G. Chemical Structures and Performance of Perovskite Oxides. Chem. Rev. 2001, 101, 1981–2018. [Google Scholar] [CrossRef] [PubMed]
- Rodová, M.; Brožek, J.; Knížek, K.; Nitsch, K. Phase Transitions in Ternary Cesium Lead Bromide. J. Therm. Anal. Calorim. 2003, 71, 667–673. [Google Scholar] [CrossRef]
- Tsvetkov, D.S.; Mazurin, M.O.; Sereda, V.V.; Ivanov, I.L.; Malyshkin, D.A.; Zuev, A.Y. Formation Thermodynamics, Stability, and Decomposition Pathways of CsPbX3 (X = Cl, Br, I) Photovoltaic Materials. J. Phys. Chem. C 2020, 124, 4252–4260. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, Y. Chemical Stability and Instability of Inorganic Halide Perovskites. Energy Environ. Sci. 2019, 12, 1495–1511. [Google Scholar] [CrossRef]
- Haeger, T.; Heiderhoff, R.; Riedl, T. Thermal Properties of Metal-Halide Perovskites. J. Mater. Chem. C 2020, 8, 14289–14311. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Huang, S.; Zhang, Y.; Lv, H.; Ge, D.; Gu, Y.; Jiang, M. Cesium Lead Bromide Perovskite Nanocrystals for the Visual Detection of Chloride Ions: A Review. J. Solid State Chem. 2024, 329, 124418. [Google Scholar] [CrossRef]
- Shen, J.; Zhu, Q. Stability Strategies of Perovskite Quantum Dots and Their Extended Applications in Extreme Environment: A Review. Mater. Res. Bull. 2022, 156, 111987. [Google Scholar] [CrossRef]
- Kang, Y.; Han, S. Intrinsic Carrier Mobility of Cesium Lead Halide Perovskites. Phys. Rev. Appl. 2018, 10, 044013. [Google Scholar] [CrossRef]
- Kawano, S.; Tadano, T.; Iikubo, S. Effect of Halogen Ions on the Low Thermal Conductivity of Cesium Halide Perovskite. J. Phys. Chem. C 2021, 125, 91–97. [Google Scholar] [CrossRef]
- Ripka, E.G.; Deschene, C.R.; Franck, J.M.; Bae, I.-T.; Maye, M.M. Understanding the Surface Properties of Halide Exchanged Cesium Lead Halide Nanoparticles. Langmuir 2018, 34, 11139–11146. [Google Scholar] [CrossRef] [PubMed]
- Grisorio, R.; Di Clemente, M.E.; Fanizza, E.; Allegretta, I.; Altamura, D.; Striccoli, M.; Terzano, R.; Giannini, C.; Irimia-Vladu, M.; Suranna, G.P. Exploring the Surface Chemistry of Cesium Lead Halide Perovskite Nanocrystals. Nanoscale 2019, 11, 986–999. [Google Scholar] [CrossRef] [PubMed]
- Akkerman, Q.A.; Rainò, G.; Kovalenko, M.V.; Manna, L. Genesis, Challenges and Opportunities for Colloidal Lead Halide Perovskite Nanocrystals. Nat. Mater. 2018, 17, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Kazes, M.; Udayabhaskararao, T.; Dey, S.; Oron, D. Effect of Surface Ligands in Perovskite Nanocrystals: Extending in and Reaching out. Acc. Chem. Res. 2021, 54, 1409–1418. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Hu, S.; Shao, H.; Li, L.; Chen, W.; Dong, B.; Xu, L.; Xu, W.; Zhou, D.; Wu, Z.; et al. Introducing Ytterbium Acetate to Luminescent CsPbCl3 Nanocrystals for Enhanced Sensitivity of Cu2+ Detection. Inorg. Chem. Front. 2022, 9, 44–50. [Google Scholar] [CrossRef]
- Sheng, X.; Liu, Y.; Wang, Y.; Li, Y.; Wang, X.; Wang, X.; Dai, Z.; Bao, J.; Xu, X. Cesium Lead Halide Perovskite Quantum Dots as a Photoluminescence Probe for Metal Ions. Adv. Mater. 2017, 29, 1700150. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tang, X.; Zhu, T.; Deng, M.; Ikechukwu, I.P.; Huang, W.; Yin, G.; Bai, Y.; Qu, D.; Huang, X.; et al. All-Inorganic CsPbBr3 Perovskite Quantum Dots as A Photoluminescent Probe for Ultrasensitive Cu2+ Detection. J. Mater. Chem. C 2018, 6, 4793–4799. [Google Scholar] [CrossRef]
- Kar, M.R.; Patel, U.; Bhaumik, S. Highly Stable and Water Dispersible Polymer-Coated CsPbBr3 Nanocrystals for Cu-Ion Detection in Water. Mater. Adv. 2022, 3, 8629–8638. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, W.; Yu, L.; Lian, S.; Xie, Q. Perovskite Quantum Dots as a Fluorescent Probe for Metal Ion Detection in Aqueous Solution via Phase Transfer. Mater. Lett. 2021, 282, 128654. [Google Scholar] [CrossRef]
- Song, L.; Zhang, X.; Lin, M.; Guo, L.; Xu, S.; Sun, J.; Zhang, J.; Chen, B. Excellent Long-Wavelength-Pass Filters of CsPbBr3 and CsPb(Cl/Br)3 Quantum Dot Glasses by A Cu2+ Quenching Strategy. J. Opt. Soc. Am. B 2022, 39, 2120–2128. [Google Scholar] [CrossRef]
- Li, H.; Yin, W.; Ng, C.K.; Huang, R.; Du, S.; Sharma, M.; Li, B.; Yuan, G.; Michalska, M.; Matta, S.K.; et al. Macroporous Perovskite Nanocrystal Composites for Ultrasensitive Copper Ion Detection. Nanoscale 2022, 14, 11953–11962. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Pan, X.; Xu, S.; Liu, Z.; Wang, J.; Yu, K.; Wang, C.; Yuan, H.; Wu, S. Fluorescence-Enhanced Microfluidic Sensor for Highly Sensitive In-Situ Detection of Copper Ions in Lubricating Oil. Mater. Design 2020, 191, 108693. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, S.; Liu, Z.; Yu, K.; Wang, C.; Wu, S.; Wang, J.; Pan, X. Fluorescence Enhanced Microfluidic Sensor with CsPbI3 Probe for Lubricant Copper Ions On-Site Rapid Detection Based on SiO2 Inverse Opal Photonic Crystals. J. Lumines. 2021, 238, 118276. [Google Scholar] [CrossRef]
- Zhang, P.; Li, S.; Xie, H.; Liu, Z.; Li, S. Organic Cross-Linker-Reinforced Small-Sized CsPbBr3@Silica Nanoparticles for Fluorescence Detection of Copper and Sulfide Ions. J. Mater. Sci. 2022, 57, 11871–11881. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.; Huang, J.; Cai, J.; Zhu, J.; Yang, X.; Shen, J.; Li, C. Perovskite Quantum Dots Encapsulated in Electrospun Fiber Membranes as Multifunctional Supersensitive Sensors for Biomolecules, Metal Ions and pH. Nanoscale Horiz. 2017, 2, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Lahkar, S.; Saikia, P.; Mohanta, D.; Das, J.; Dolui, S.K. Stable and Highly Luminescent CsPbX3 (X = Br, Br/Cl) Perovskite Quantum Dot Embedded Into Zinc(II) Imidazole-4,5-dicarboxylate Metal-organic Framework as A Luminescent Probe for Metal Ion Detection. Mater. Chem. Phys. 2023, 295, 127093. [Google Scholar] [CrossRef]
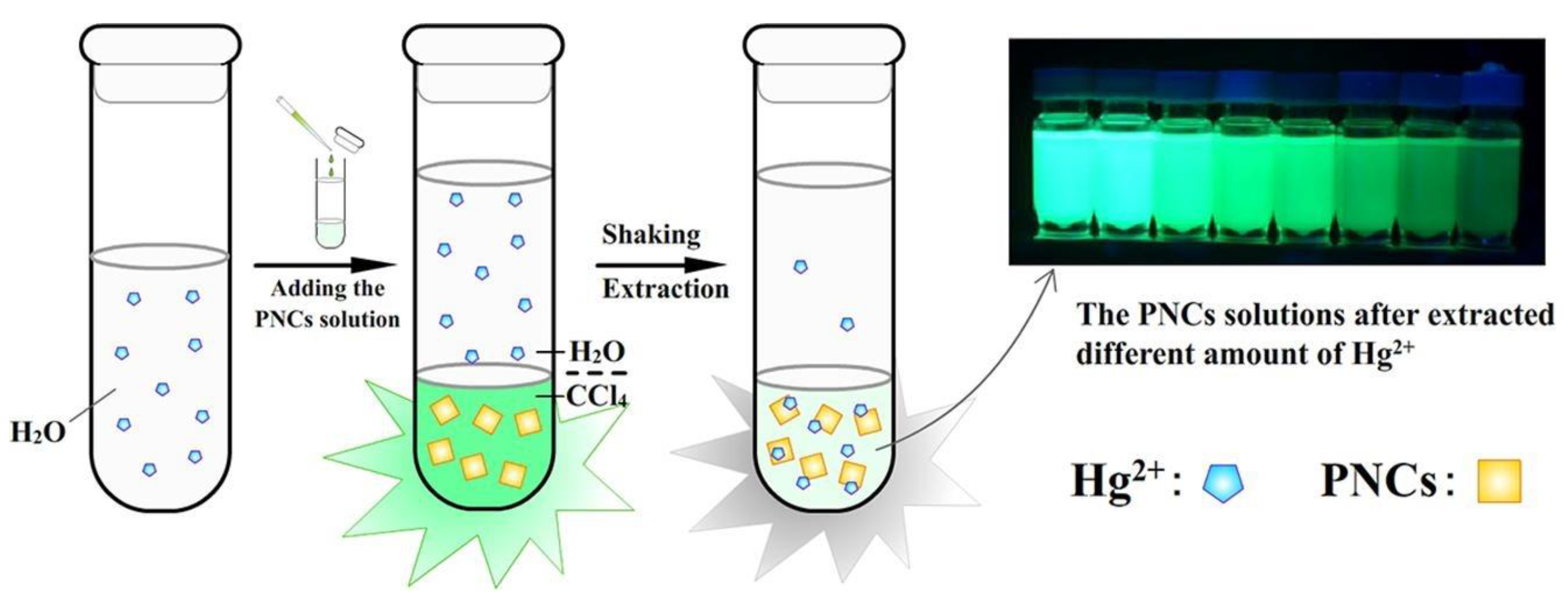
- Wang, J.; Hu, Y.-L.; Zhao, R.-X.; Wen, Q.-L.; Wang, J.; Yang, N.; Ling, J.; Huang, C.Z.; Cao, Q. Liquid-Liquid Extraction and Visual Detection of Hg2+ in Aqueous Solution by Luminescent CsPbBr3 Perovskite Nanocrystals. Microchem. J. 2021, 170, 106769. [Google Scholar] [CrossRef]
- Jiang, W.; Xu, Y.; Wang, L.; Chen, L.; Li, S. Ultrasensitive Detection of Mercury(ii) in Aqueous Solutions Via the Spontaneous Precipitation of CsPbBr3 Crystallites. Dalton Trans. 2022, 51, 12996–13002. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Sun, L.; Wang, Y.; Jin, D.; Xu, Q.; Hu, X. Polymer Surface Ligand and Silica Coating Induced Highly Stable Perovskite Nanocrystals with Enhanced Aqueous Fluorescence for Efficient Hg2+ and Glutathione Detection. Analyst 2021, 146, 6798–6807. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Ye, S.; Song, J.; Qu, J. Large-Scale Synthesis of Cesium Lead Halide Perovskite Nanocrystals for Zinc Ion Detection. J. Nanoparticle Res. 2020, 22, 153. [Google Scholar] [CrossRef]
- George, J.K.; Ramu, S.; Halali, V.V.; Balakrishna, R.G. Inner Filter Effect as a Boon in Perovskite Sensing Systems to Achieve Higher Sensitivity Levels. ACS Appl. Mater. Interfaces 2021, 13, 57264–57273. [Google Scholar] [CrossRef] [PubMed]
- Halali, V.V.; Shwetha Rani, R.; Geetha Balakrishna, R.; Budagumpi, S. Ultra-Trace Level Chemosensing of Uranyl Ions; Scuffle Between Electron and Energy Transfer from Perovskite Quantum Dots to Adsorbed Uranyl Ions. Microchem. J. 2020, 156, 104808. [Google Scholar] [CrossRef]
- Ray, S.; Mohapatra, A.; Bhaumik, S. Synthesis of Highly Stable Double-Coated Zn-Doped Cesium Lead Bromide Nanocrystals for Indium Ion Detection in Water. Mater. Adv. 2022, 3, 4684–4692. [Google Scholar] [CrossRef]
- Pandey, P.; Sengupta, A.; Parmar, S.; Bansode, U.; Gosavi, S.; Swarnkar, A.; Muduli, S.; Mohite, A.D.; Ogale, S. CsPbBr3–Ti3C2Tx MXene QD/QD Heterojunction: Photoluminescence Quenching, Charge Transfer, and Cd Ion Sensing Application. ACS Appl. Nano Mater. 2020, 3, 3305–3314. [Google Scholar] [CrossRef]
- Hsieh, C.-W.; Singh, R.K.; Som, S.; Lu, C.-H. Detection of Fe (III) Using APTES-Coated CsPbBr3–CsPb2Br5 Perovskite Quantum Dots. Chem. Eng. J. Adv. 2022, 12, 100358. [Google Scholar] [CrossRef]
- Jan, Q.; Nabi, S.; Ahmad Sofi, F.; Ahmad Bhat, M. CsPbBr3 Perovskite Nanoplatelets: Excellent Probes for Spectrofluorimetric Sensing of Chloride and Arsenite. Spectrochim. Acta A 2022, 270, 120749. [Google Scholar] [CrossRef] [PubMed]
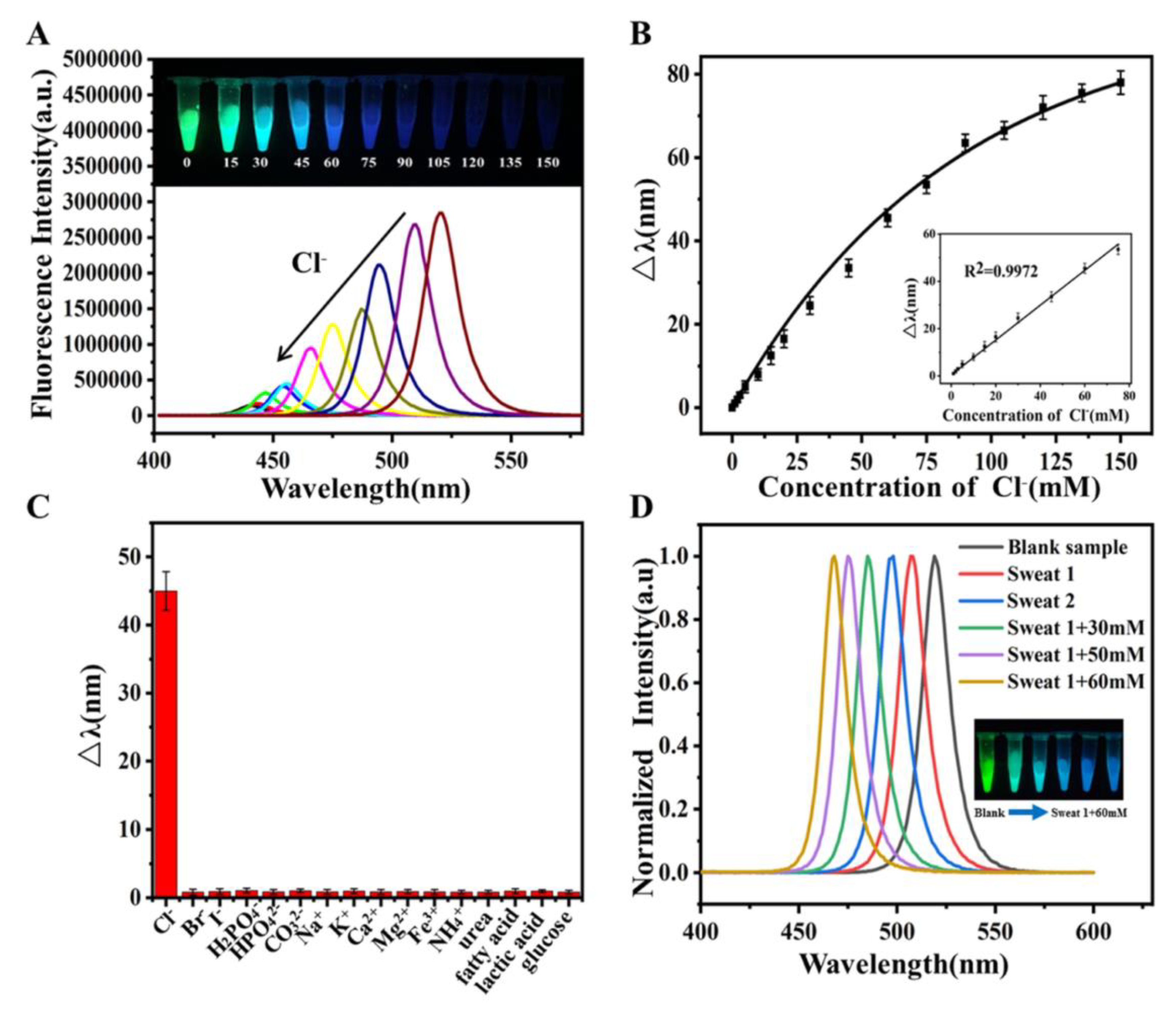
- Huang, G.-B.; Guo, Z.-Y.; Ye, T.-X.; Zhang, C.; Zhou, Y.-M.; Yao, Q.-H.; Chen, X. Colorimetric Determination of Chloridion in Domestic Water Based on the Wavelength Shift of CsPbBr3 Perovskite Nanocrystals via Halide Exchange. J. Anal. Test. 2021, 5, 3–10. [Google Scholar] [CrossRef]
- Shu, Y.; Wang, Y.; Guan, J.; Ji, Z.; Xu, Q.; Hu, X. Amphiphilic Polymer Ligand-Assisted Synthesis of Highly Luminescent and Stable Perovskite Nanocrystals for Sweat Fluorescent Sensing. Anal. Chem. 2022, 94, 5415–5424. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Feng, Y.; Huang, Y.; Yao, Q.; Huang, G.; Zhu, Y.; Chen, X. Colorimetric Sensing of Chloride in Sweat Based on Fluorescence Wavelength Shift Via Halide Exchange of CsPbBr3 Perovskite Nanocrystals. Microchim. Acta 2021, 188, 2. [Google Scholar] [CrossRef] [PubMed]
- Vasavi Dutt, V.G.; Akhil, S.; Mishra, N. Cesium Lead Bromide Perovskite Nanocrystals as a Simple and Portable Spectrochemical Probe for Rapid Detection of Chlorides. ChemistrySelect 2021, 6, 8171–8176. [Google Scholar] [CrossRef]
- Zhang, P.; Xiong, C.; Liu, Z.; Chen, H.; Li, S. CsPbBr3 Nanocrystals as Luminescent Probe for In Situ Detection of Chloride and Iodide Ions in Water. Microchem. J. 2023, 190, 108651. [Google Scholar] [CrossRef]
- Chen, X.; Hu, H.; Xia, Z.; Gao, W.; Gou, W.; Qu, Y.; Ma, Y. CsPbBr3 Perovskite Nanocrystals as Highly Selective and Sensitive Spectrochemical Probes for Gaseous HCl Detection. J. Mater. Chem. C 2017, 5, 309–313. [Google Scholar] [CrossRef]
- Sandeep, K.; Hamida, K.T. CsPbBr3 Perovskite–Coated Paper Substrate for the Cost-Effective Detection of Fluoride, Chloride, and Iodide Ions in Water. Phys. Status Solidi A 2021, 218, 2100101. [Google Scholar] [CrossRef]
- Li, H.; Li, F.; Huang, Y.; Zhang, L.; Ye, M.; Jin, J.; Chen, X. Photoluminescence Sensing of Chloride Ions in Sea Sand Using Alcohol-Dispersed CsPbBr3@SiO2 Perovskite Nanocrystal Composites. Chemosensors 2022, 10, 170. [Google Scholar] [CrossRef]
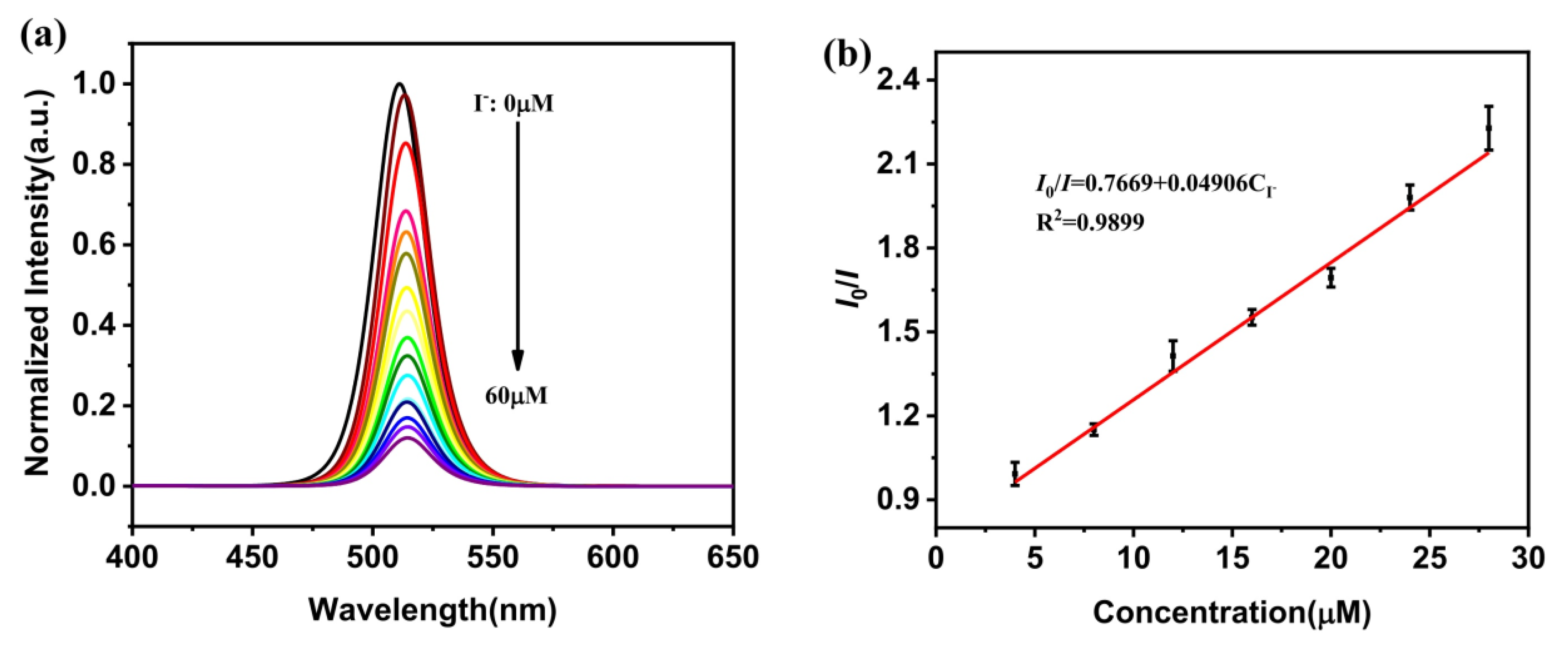
- Fu, Y.-B.; Wen, Q.-L.; Ding, H.-T.; Yang, N.; Chai, X.-Y.; Zhang, Y.; Ling, J.; Shi, Y.-G.; Cao, Q. Green and simple synthesis of NH2-functionalized CsPbBr3 perovskite nanocrystals for detection of iodide ion. Microchem. J. 2022, 182, 107892. [Google Scholar] [CrossRef]
- Park, B.; Kang, S.-M.; Lee, G.-W.; Kwak, C.H.; Rethinasabapathy, M.; Huh, Y.S. Fabrication of CsPbBr3 Perovskite Quantum Dots/Cellulose-Based Colorimetric Sensor: Dual-Responsive On-Site Detection of Chloride and Iodide Ions. Ind. Eng. Chem. Res. 2020, 59, 793–801. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, X.; Zhou, L.L.; Liu, B.; Chen, Z.; Zuo, X. A Colorimetric Sensor Array for Rapid Discrimination of Edible Oil Species Based on A Halogen Ion Exchange Reaction Between CsPbBr3 and Iodide. Analyst 2022, 147, 404–409. [Google Scholar] [CrossRef] [PubMed]
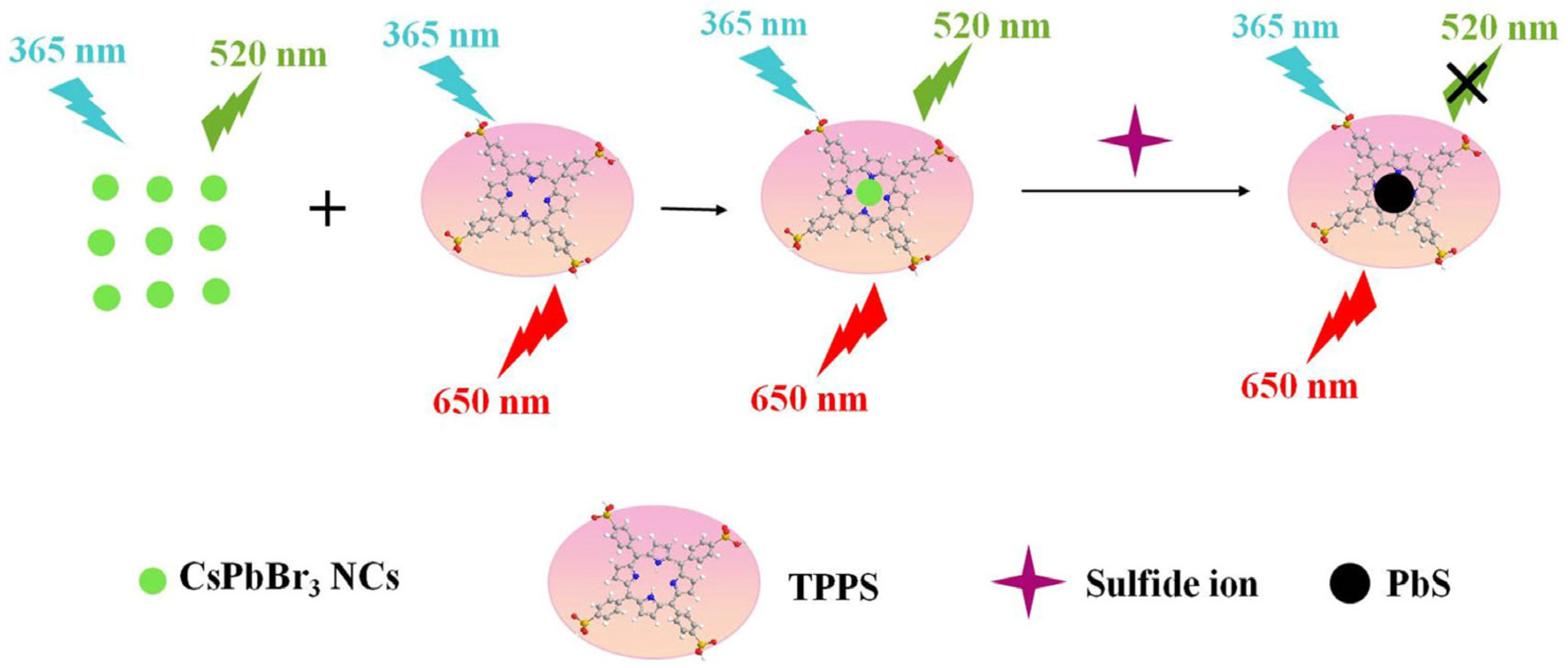
- Wang, H.; Li, Q.; Niu, X.; Du, J. Tetraphenylporphyrin-Modified Perovskite Nanocrystals Enable Ratiometric Fluorescent Determination of Sulfide Ion in Water Samples. J. Mater. Sci. 2021, 56, 15029–15039. [Google Scholar] [CrossRef]
- Yin, W.; Li, H.; Chesman, A.S.R.; Tadgell, B.; Scully, A.D.; Wang, M.; Huang, W.; McNeill, C.R.; Wong, W.W.H.; Medhekar, N.V.; et al. Detection of Halomethanes Using Cesium Lead Halide Perovskite Nanocrystals. ACS Nano 2021, 15, 1454–1464. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Qin, Y.; Sui, R.; Wang, G.; Zhang, X.; Liu, X.; Pei, J.; Liu, D.; Chen, Z. CH3I Sensing Using Yttrium Single Atom-Doped Perovskite Nanocrystals. Nano Res. 2023, 16, 10429–10435. [Google Scholar] [CrossRef]
- Xie, L.; Zan, J.; Yang, Z.; Wu, Q.; Chen, X.; Ou, X.; Lin, C.; Chen, Q.; Yang, H. A Perovskite-Based Paper Microfluidic Sensor for Haloalkane Assays. Front. Chem. 2021, 9, 682006. [Google Scholar] [CrossRef] [PubMed]
- Saikia, P.; Kumar Dolui, S.; Pran Mahanta, S. CsPbBr3 Perovskites: A Dual Fluorescence Sensor to Distinguish Ethanol from Methanol. Spectrochim. Acta A 2023, 291, 122309. [Google Scholar] [CrossRef] [PubMed]
- Bahtiar, A.; Ginanjar, G.G. Colorimetric Sensor for Measuring Bleaching Benzoyl Peroxide (BPO) Content in Wheat Flour Based on Perovskite CsPbBr3 Nanocrystal: A Preliminary Study. J. Phys. Conf. Ser. 2022, 2376, 012012. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, Y.; Li, F.; Zhang, L.; You, L.; Guo, Z.; Huang, Y.; Zhao, L.; Chen, X. Colorimetric Sensing of Benzoyl Peroxide Based on the Emission Wavelength-Shift of CsPbBr3 Perovskite Nanocrystals. Chemosensors 2021, 9, 319. [Google Scholar] [CrossRef]
- Huangfu, C.; Feng, L. High-Performance Fluorescent Sensor Based on CsPbBr3 Quantum Dots for Rapid Analysis of Total Polar Materials in Edible Oils. Sens. Actuators B 2021, 344, 130193. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, Y.; Shi, L.; Fan, Y. Perovskite Nanomaterial-Engineered Multiplex-Mode Fluorescence Sensing of Edible Oil Quality. Anal. Chem. 2021, 93, 11033–11042. [Google Scholar] [CrossRef] [PubMed]
- Aamir, M.; Khan, M.D.; Sher, M.; Bhosale, S.V.; Malik, M.A.; Akhtar, J.; Revaprasadu, N. A Facile Route to Cesium Lead Bromoiodide Perovskite Microcrystals and Their Potential Application as Sensors for Nitrophenol Explosives. Eur. J. Inorg. Chem. 2017, 2017, 3755–3760. [Google Scholar] [CrossRef]
- Chen, X.; Sun, C.; Liu, Y.; Yu, L.; Zhang, K.; Asiri, A.M.; Marwani, H.M.; Tan, H.; Ai, Y.; Wang, X.; et al. All-Inorganic Perovskite Quantum Dots CsPbX3 (Br/I) for Highly Sensitive and Selective Detection of Explosive Picric Acid. Chem. Eng. J. 2020, 379, 122360. [Google Scholar] [CrossRef]
- Aznar-Gadea, E.; Sanchez-Alarcon, I.; Soosaimanickam, A.; Rodriguez-Canto, P.J.; Perez-Pla, F.; Martínez-Pastor, J.P.; Abargues, R. Molecularly Imprinted Nanocomposites of CsPbBr3 Nanocrystals: An Approach Towards Fast and Selective Gas Sensing of Explosive Taggants. J. Mater. Chem. C 2022, 10, 1754–1766. [Google Scholar] [CrossRef]
- Huang, Z.-H.; Layek, M.; Li, C.-F.; Lee, K.-M.; Huang, Y.-C. Cesium Lead Bromide Nanocrystals: Synthesis, Modification, and Application to O2 Sensing. Sensors 2022, 22, 8853. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Li, F.; Lai, Z.; Cai, Z.; Wang, Y.; Wolfbeis, O.S.; Chen, X. MnII-Doped Cesium Lead Chloride Perovskite Nanocrystals: Demonstration of Oxygen Sensing Capability Based on Luminescent Dopants and Host-Dopant Energy Transfer. ACS Appl. Mater. Interfaces 2018, 10, 23335–23343. [Google Scholar] [CrossRef] [PubMed]
- Brintakis, K.; Gagaoudakis, E.; Kostopoulou, A.; Faka, V.; Argyrou, A.; Binas, V.; Kiriakidis, G.; Stratakis, E. Ligand-Free All-Inorganic Metal Halide Nanocubes for Fast, Ultra-Sensitive and Self-Powered Ozone Sensors. Nanoscale Adv. 2019, 1, 2699–2706. [Google Scholar] [CrossRef]
- Park, B.; Kang, H.; Han, S.; Kim, H.-U.; Cho, Y.; Huh, Y.S.; Kang, S.-M. The Fabrication of Cesium Lead Bromide-Coated Cellulose Nanocomposites and Their Effect on the Detection of Nitrogen Gas. Sensors 2022, 22, 7737. [Google Scholar] [CrossRef] [PubMed]
- Chizhov, A.S.; Rumyantseva, M.N.; Drozdov, K.A.; Krylov, I.V.; Batuk, M.; Hadermann, J.; Filatova, D.G.; Khmelevsky, N.O.; Kozlovsky, V.F.; Maltseva, L.N.; et al. Photoresistive Gas Sensor Based on Nanocrystalline ZnO Sensitized with Colloidal Perovskite CsPbBr3 Nanocrystals. Sens. Actuators B 2021, 329, 129035. [Google Scholar] [CrossRef]
- Yueyue, L.; Siqi, S.; Yilin, W.; Fengmin, L.; Hongtao, W.; Jihao, B.; Min, L.; Geyu, L. CsPbBr3 Quantum Dots Enhanced ZnO Sensing to NO2 at room temperature. Sens. Actuators B 2022, 368, 132189. [Google Scholar] [CrossRef]
- Lu, Z.; Lou, C.; Cheng, A.; Zhang, J.; Sun, J. A Sensitive and Ultrafast FA0.83Cs0.17PbI3 Perovskite Sensor for NO2 Detection at Room Temperature. J. Alloys Comp. 2022, 919, 165831. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Lin, P.; Wu, D.; Shi, Z.; Chen, X.; Xu, T.; Wang, X.; Tian, Y.; Li, X. All-Inorganic CsPbBr3/Cs4PbBr6 Perovskite/ZnO for Detection of NO with Enhanced Response and Low-Work Temperature. ChemistrySelect 2021, 6, 9657–9662. [Google Scholar] [CrossRef]
- Chen, C.; Cai, Q.; Luo, F.; Dong, N.; Guo, L.; Qiu, B.; Lin, Z. Sensitive Fluorescent Sensor for Hydrogen Sulfide in Rat Brain Microdialysis via CsPbBr3 Quantum Dots. Anal. Chem. 2019, 91, 15915–15921. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Zhang, Y.; Zu, Y.; Li, S.; Chen, Y.; Chen, Z.; Huang, D.; Qiu, B.; Lin, Z. Quick Preparation of Water-Soluble Perovskite Nanocomposite Via Cetyltrimethylammonium Bromide and Its Application. Microchim. Acta 2022, 189, 68. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, Y.; Zu, Y.; Chen, Y.; Luo, F.; Huang, D.; Weng, Z.; Qiu, B.; Lin, Z. Photothermal Sensor Based on Water-Soluble CsPbBr3@Sulfobutylether-β-Cyclodextrins Nanocomposite Using A Thermometer as Readout. Sens. Actuators B 2022, 355, 131301. [Google Scholar] [CrossRef]
- Shan, H.; Xuan, W.; Li, Z.; Hu, D.; Gu, X.; Huang, S. Room-Temperature Hydrogen Sulfide Sensor Based on Tributyltin Oxide Functionalized Perovskite CsPbBr3 Quantum Dots. ACS Appl. Nano Mater. 2022, 5, 6801–6809. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, M.; Bo, R.; Barugkin, C.; Zheng, J.; Ma, Q.; Huang, S.; Ho-Baillie, A.W.Y.; Catchpole, K.R.; Tricoli, A. Superior Self-Powered Room-Temperature Chemical Sensing with Light-Activated Inorganic Halides Perovskites. Small 2018, 14, 1702571. [Google Scholar] [CrossRef] [PubMed]
- Xuan, W.; Shan, H.; Hu, D.; Zhu, L.; Guan, T.; Zhao, Y.; Qiang, Y.; Song, J.; Zhang, J.; Sui, M.; et al. In-Situ Synthesis of Stable ZnO-Coated CsPbBr3 Nanocrystals for Room-Temperature Heptanal Sensors. Mater. Today Chem. 2022, 26, 101155. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, M.; Fu, X.; Fusco, Z.; Bo, R.; Xing, B.; Nguyen, H.T.; Barugkin, C.; Zheng, J.; Lau, C.F.J.; et al. Light-Activated Inorganic CsPbBr2I Perovskite for Room-Temperature Self-powered Chemical Sensing. Phys. Chem. Chem. Phys. 2019, 21, 24187–24193. [Google Scholar] [CrossRef]
- Liu, J.; Peng, Y.; Zhu, B.; Li, Y.; Zhang, L.; Yu, J. ZIF-8 derived ZnO-CsPbBr3 Polyhedrons for Efficient Triethylamine Detection. Sens. Actuators B 2022, 357, 131366. [Google Scholar] [CrossRef]
- Xu, X.; Wang, S.; Chen, Y.; Liu, W.; Wang, X.; Jiang, H.; Ma, S.; Yun, P. CsPbBr3-Based Nanostructures for Room-Temperature Sensing of Volatile Organic Compounds. ACS Appl. Mater. Interfaces 2022, 14, 39524–39534. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, X.; Liu, W.; Wang, S.; Jiang, H.; Ma, S.; Yuan, F.; Ma, N. Ambient Stable CsPbBr3/ZnO Nanostructures for Ethanolamine Sensing. ACS Appl. Nano Mater. 2022, 5, 15030–15041. [Google Scholar] [CrossRef]
- Lin, L.; Qiu, L.; Li, F.; You, C.; Zhu, Y.; Yao, Q.; Zeng, Y.; Wang, Y.; Li, J.; Chen, X. Stable Electrochemiluminescence of CsPbBr3 Perovskite Nanocrystals Assisted by Graphene Oxide for Ultrasensitive Sensing. ACS Appl. Nano Mater. 2021, 4, 8823–8833. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, X.; Huang, J.; Wu, R.; Leng, Y.; Chen, Z. CsPbBr3 and CsPbBr3/SiO2 Nanocrystals as a Fluorescence Sensing Platform for High-Throughput Identification of Multiple Thiophene Sulfides. Anal. Chem. 2022, 94, 5946–5952. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Hao, M.; Song, Y.; Dang, S.; Liu, X.; Dong, Q. Dynamic Passivation in Perovskite Quantum Dots for Specific Ammonia Detection at Room Temperature. Small 2020, 16, 1904462. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, J.; Zhang, X.; Jian, J.; Zou, J.; Jin, Q.; Zhang, X. The Ammonia Detection of Cesium Lead Halide Perovskite Quantum Dots in Different Halogen Ratios at Room Temperature. Opt. Mater. 2022, 134, 113155. [Google Scholar] [CrossRef]
- Huangfu, C.; Wang, Y.; Wang, Z.; Hu, Q.; Feng, L. A Stable and Humidity Resistant NH3 Sensor Based on Luminous CsPbBr3 Perovskite Nanocrystals. Talanta 2023, 253, 124070. [Google Scholar] [CrossRef]
- Park, B.; Kim, S.; Kwak, C.H.; Shanmugam, K.R.; Han, Y.-K.; Cho, Y.; Huh, Y.S. Visual Colorimetric Detection of Ammonia Under Gaseous and Aqueous State: Approach on Cesium Lead Bromide Perovskite-Loaded Porous Electrospun Nanofibers. J. Ind. Eng. Chem. 2021, 97, 515–522. [Google Scholar] [CrossRef]
- He, X.; Yu, C.; Yu, M.; Lin, J.; Li, Q.; Fang, Y.; Liu, Z.; Xue, Y.; Huang, Y.; Tang, C. Synthesis of Perovskite CsPbBr3 Quantum Dots/Porous Boron Nitride Nanofiber Composites with Improved Stability and Their Reversible Optical Response to Ammonia. Inorg. Chem. 2020, 59, 1234–1241. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhao, C.; Hu, M.; Pan, A.; Xiong, W.; Chen, Y. CsPbBr3 Perovskite Quantum Dots Grown Within Fe-Doped Zeolite X with Improved Stability for Sensitive NH3 Detection. Nanoscale 2023, 15, 5705–5711. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhu, H.; Chen, J.; Hautzinger, M.P.; Zhu, X.Y.; Jin, S. Metal Halide Perovskite Nanostructures for Optoelectronic Applications and the Study of Physical Properties. Nat. Rev. Mater. 2019, 4, 169–188. [Google Scholar] [CrossRef]
- Haque, M.A.; Syed, A.; Akhtar, F.H.; Shevate, R.; Singh, S.; Peinemann, K.-V.; Baran, D.; Wu, T. Giant Humidity Effect on Hybrid Halide Perovskite Microstripes: Reversibility and Sensing Mechanism. ACS Appl. Mater. Interfaces 2019, 11, 29821–29829. [Google Scholar] [CrossRef] [PubMed]
- Petrila, I.; Tudorache, F. Influence of Sb3+ Cations on the Structural, Magnetic and Electrical Properties of AlFeO3 Multiferroic Perovskite with Humidity Sensors Applicative Characteristics. Materials 2022, 15, 8369. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yu, L.; Wei, Z.; Xu, Q.; Zhou, W.; Xiao, Y. Dual-Response Ratiometric Fluorescence-Based Ligand-Functionalized CsPbBr3 Perovskite Quantum Dots for Sensitive Detection of Trace Water in Edible Oils. Sens. Actuators B 2022, 366, 132010. [Google Scholar] [CrossRef]
- Xiang, X.; Ouyang, H.; Li, J.; Fu, Z. Humidity-Sensitive CsPbBr3 Perovskite-Based Photoluminescent Sensor for Detecting Water Content in Herbal Medicines. Sens. Actuators B 2021, 346, 130547. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, J.; Sun, X.; Wu, Y.; Wang, L.; Meng, G.; Kuang, D.; Guo, X.; Qu, W.; Du, B.; et al. An Excellent Impedance-Type Humidity Sensor Based on Halide Perovskite CsPbBr3 Nanoparticles for Human Respiration Monitoring. Sens. Actuators B 2021, 337, 129772. [Google Scholar] [CrossRef]
- Liu, D.; Yang, P.; Zhang, Y.; Chen, Y.; Dai, H.; Li, T.; Xue, R.; Chen, J.; Su, Y.; Chen, Z. Effects of Microdefects and Grain Size on the Phase Transition Properties of Nano-VO2(M). J. Solid State Chem. 2020, 288, 121450. [Google Scholar] [CrossRef]
- Huang, S.; Li, Z.; Wang, B.; Zhu, N.; Zhang, C.; Kong, L.; Zhang, Q.; Shan, A.; Li, L. Morphology Evolution and Degradation of CsPbBr3 Nanocrystals under Blue Light-Emitting Diode Illumination. ACS Appl. Mater. Interfaces 2017, 9, 7249–7258. [Google Scholar] [CrossRef]
- Lu, C.-H.; Biesold-McGee, G.V.; Liu, Y.; Kang, Z.; Lin, Z. Doping and Ion Substitution in Colloidal Metal Halide Perovskite Nanocrystals. Chem. Soc. Rev. 2020, 49, 4953–5007. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Zhou, X.; Jiang, S.; Xiang, G.; Li, L.; Li, Y.; Jing, C.; Ling, F.; Wang, Y.; Xiao, P. Dual-Mode Luminescence Temperature Sensing Performance of Manganese (II) Doped CsPbCl3 Perovskite Quantum Dots. Ceram. Inter. 2022, 48, 33645–33652. [Google Scholar] [CrossRef]
- Zhuang, B.; Liu, Y.; Yuan, S.; Huang, H.; Chen, J.; Chen, D. Glass Stabilized Ultra-Stable Dual-Emitting Mn-Doped Cesium Lead Halide Perovskite Quantum Dots for Cryogenic Temperature Sensing. Nanoscale 2019, 11, 15010–15016. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Liu, W.; Wang, Q.; Han, Q.; Yang, Q. Temperature-Dependent Photoluminescence of Pure and Mn-Doped CsPbCl3 Nanocrystals. J. Alloys Comp. 2019, 787, 165–172. [Google Scholar] [CrossRef]
- Yu, Y.; Shao, G.; Ding, L.; Zhang, H.; Liang, X.; Liu, J.; Xiang, W. Ultra-Stable Eu3+-Doped CsPbCl2Br1 Perovskite Quantum Dots Glass for Optical Temperature Sensing. J. Rare Earths 2021, 39, 1497–1505. [Google Scholar] [CrossRef]
- Li, X.; Yu, Y.; Hong, J.; Feng, Z.; Guan, X.; Chen, D.; Zheng, Z. Optical Temperature Sensing of Eu3+-Doped Oxyhalide Glasses Containing CsPbBr3 Perovskite Quantum Dots. J. Lumines. 2020, 219, 116897. [Google Scholar] [CrossRef]
- Huang, Y.; Lai, Z.; Jin, J.; Lin, F.; Li, F.; Lin, L.; Tian, D.; Wang, Y.; Xie, R.; Chen, X. Ultrasensitive Temperature Sensing Based on Ligand-Free Alloyed CsPbClxBr3−x Perovskite Nanocrystals Confined in Hollow Mesoporous Silica with High Density of Halide Vacancies. Small 2021, 17, 2103425. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Lin, J.; Huang, Y.; Zhang, L.; Jiang, Y.; Tian, D.; Lin, F.; Wang, Y.; Chen, X. High Sensitivity Ratiometric Fluorescence Temperature Sensing Using the Microencapsulation of CsPbBr3 and K2SiF6:Mn4+ Phosphor. Chin. Chem. Lett. 2022, 33, 4798–4802. [Google Scholar] [CrossRef]
- Lu, P.; Lin, L.; Li, Z.; Feng, Z.; Yang, Y.; Cai, J.; Wang, Z.; Zheng, Z. Highly Sensitive Temperature Sensing of Compound of CsPbBr3 Perovskite Quantum Dots and NaYF4:Ho3+ Nanoparticles. Optik 2021, 246, 167794. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, Y.; Xia, G.; Zou, J.; Deng, W.; Tian, F.; Deng, J. Metal–Semiconductor–Metal-Nanostructured CsPbBr3 Crystal Detector for Long-Term Stable α-Particle Detection. ACS Appl. Nano Mater. 2022, 5, 16039–16044. [Google Scholar] [CrossRef]
- Toufanian, R.; Swain, S.; Becla, P.; Motakef, S.; Datta, A. Cesium Lead Bromide Semiconductor Radiation Detectors: Crystal Growth, Detector Performance and Polarization. J. Mater. Chem. C 2022, 10, 12708–12714. [Google Scholar] [CrossRef]
- Feng, Y.; Pan, L.; Wei, H.; Liu, Y.; Ni, Z.; Zhao, J.; Rudd, P.N.; Cao, L.R.; Huang, J. Low Defects Density CsPbBr3 Single Crystals Grown by An Additive Assisted Method for Gamma-Ray Detection. J. Mater. Chem. C 2020, 8, 11360–11368. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, F.; Lu, Y.; Sun, Q.; Xu, Y.; Zhang, B.-B.; Jie, W.; Kanatzidis, M.G. High-Sensitivity X-Ray Detectors Based on Solution-Grown Caesium Lead Bromide Single Crystals. J. Mater. Chem. C 2020, 8, 1248–1256. [Google Scholar] [CrossRef]
- Peng, J.; Xia, C.Q.; Xu, Y.; Li, R.; Cui, L.; Clegg, J.K.; Herz, L.M.; Johnston, M.B.; Lin, Q. Crystallization of CsPbBr3 Single Crystals in Water for X-Ray Detection. Nat. Commun. 2021, 12, 1531. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Li, F.; Bai, R.; Zhang, X.; Sun, Q.; Jie, W.; Xu, Y. Investigation of LiF Interlayer on Charge Collection Efficiency and Leakage Current in CsPbBr3 Radiation Detector. IEEE Trans. Electron. Devices 2022, 69, 6837–6842. [Google Scholar] [CrossRef]
- Xiang, L.; Huang, X.; Wang, Y.; Xin, Z.; Chai, G.; Xu, Y.; Wang, K.; Chen, J.; Liu, C.; Wang, X.; et al. X-ray Sensitive Hybrid Organic Photodetectors with Embedded CsPbBr3 Perovskite Quantum Dots. Org. Electron. 2021, 98, 106306. [Google Scholar] [CrossRef]
- Jiang, J.; Xiang, M.; Fan, K.; Bao, C.; Xin, D.; Pan, Z.; Fei, L.; Huang, H.; Zhou, L.; Yao, K.; et al. Synergistic Strain Engineering of Perovskite Single Crystals for Highly Stable and Sensitive X-Ray Detectors with Low-Bias Imaging and Monitoring. Nat. Photonics 2022, 16, 575–581. [Google Scholar] [CrossRef]
- Abiedh, K.; Dhanabalan, B.; Kutkan, S.; Lauciello, S.; Pasquale, L.; Toma, A.; Salerno, M.; Arciniegas, M.P.; Hassen, F.; Krahne, R. Surface-Dependent Properties and Tunable Photodetection of CsPbBr3 Microcrystals Grown on Functional Substrates. Adv. Optical Mater. 2022, 10, 2101807. [Google Scholar] [CrossRef]
- Chen, F.; Li, C.; Shang, C.; Wang, K.; Huang, Q.; Zhao, Q.; Zhu, H.; Ding, J. Ultrafast Response of Centimeter Scale Thin CsPbBr3 Single Crystal Film Photodetector for Optical Communication. Small 2022, 18, 2203565. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zu, Z.; Zang, Z.; Hu, Z.; Hu, W.; Yao, Z.; Chen, W.; Li, S.; Han, S.; Zhou, M. CsPbBr3/Reduced Graphene Oxide Nanocomposites and Their Enhanced Photoelectric Detection Application. Sens. Actuators B 2017, 245, 435–440. [Google Scholar] [CrossRef]
- Tang, J.-F.; Sie, Y.-D.; Tseng, Z.-L.; Lin, J.-H.; Chen, L.-C.; Hsu, C.-L. Perovskite Quantum Dot–ZnO Nanowire Composites for Ultraviolet–Visible Photodetectors. ACS Appl. Nano Mater. 2022, 5, 7237–7245. [Google Scholar] [CrossRef]
- Liu, S.; Liu, X.; Zhu, Z.; Wang, S.; Gu, Y.; Shan, F.; Zou, Y. Improved Flexible ZnO/CsPbBr3/Graphene UV Photodetectors with Interface Optimization by Solution Process. Mater. Res. Bull. 2020, 130, 110956. [Google Scholar] [CrossRef]
- Niu, X.; Gao, H.; Du, J. CsPbBr3 Perovskite Nanocrystals Decorated with Cu Nanoclusters for Ratiometric Detection of Glucose. ACS Appl. Nano Mater. 2022, 5, 2350–2357. [Google Scholar] [CrossRef]
- Chen, X.; Li, D.; Pan, G.; Zhou, D.; Xu, W.; Zhu, J.; Wang, H.; Chen, C.; Song, H. All-Inorganic Perovskite Quantum Dot/TiO2 Inverse Opal Electrode Platform: Stable and Efficient Photoelectrochemical Sensing of Dopamine Under Visible Irradiation. Nanoscale 2018, 10, 10505–10513. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Cui, S.; Yang, X.; Yang, G.; Zhu, Y.; Wang, Y.; Qiu, D. CsPbCl3 Perovskite Quantum Dots/TiO2 Inverse Opal Photonic Crystals for Efficient Photoelectrochemical Detection of alpha-fetoprotein. J. Phys. D Appl. Phys. 2019, 52, 415101. [Google Scholar] [CrossRef]
- Saikia, P.; Nath, J.; Dolui, S.K.; Mahanta, S.P. Cesium Lead Bromide as A Colorimetric and Fluorometric Sensing Platform for the Selective Detection of Uric Acid. New J. Chem. 2023, 47, 7425–7431. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Li, Q.; Du, J. Substrate-Free Fluorescence Ratiometric Detection of Serum Acetylcholinesterase Activity with A Self-Assembled CsPbBr3 Perovskite Nanocrystals/Tetraphenylporphyrin Tetrasulfonic Acid Nanocomposite. Talanta 2022, 250, 123746. [Google Scholar] [CrossRef]
- Kim, H.-R.; Bong, J.-H.; Park, J.-H.; Song, Z.; Kang, M.-J.; Son, D.H.; Pyun, J.-C. Cesium Lead Bromide (CsPbBr3) Perovskite Quantum Dot-Based Photosensor for Chemiluminescence Immunoassays. ACS Appl. Mater. Interfaces 2021, 13, 29392–29405. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.-L.; Yang, N.; Zhao, R.-X.; Fu, Y.-B.; Ling, J.; Xie, X.-G.; Cao, Q. A Water-Soluble Luminescent Cesium-Lead Perovskite Nanocrystal Probe for Sensitive Detection of Penicillamine. Dyes Pig. 2022, 205, 110537. [Google Scholar] [CrossRef]
- Jiang, X.; Zeng, H.; Duan, C.; Hu, Q.; Wu, Q.; Yu, Y.; Yang, X. One-Pot Synthesis of Stable and Functional Hydrophilic CsPbBr3 Perovskite Quantum Dots for “Turn-On” Fluorescence Detection of Mycobacterium Tuberculosis. Dalton Trans. 2022, 51, 3581–3589. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Kim, H.-R.; Kim, M.-J.; Song, Z.; Kang, M.-J.; Son, D.H.; Pyun, J.-C. Defect-Passivated Photosensor Based on Cesium Lead Bromide (CsPbBr3) Perovskite Quantum Dots for Microbial Detection. ACS Appl. Mater. Interfaces 2023, 15, 56702–56716. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Wu, M.-X.; Ding, S.-N. Anodic Electrochemiluminescence From CsPbBr3 Perovskite Quantum Dots for An Alkaline Phosphatase Assay. Chem. Commun. 2020, 56, 8099–8102. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Tian, T.; Zeng, Y.; Zhu, S.; Li, C.; Yin, Y.; Li, G. One-Step Assay of Pore-Forming Biotoxins Based on Biomimetic Perovskite Nanocrystals. Sens. Actuators B 2021, 338, 129839. [Google Scholar] [CrossRef]
- Li, M.; Wang, Y.; Hu, H.; Feng, Y.; Zhu, S.; Li, C.; Feng, N. A Dual-Readout Sandwich Immunoassay Based on Biocatalytic Perovskite Nanocrystals for Detection of Prostate Specific Antigen. Biosens. Bioelectron. 2022, 203, 113979. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Sun, A.; Dong, X.; Sun, Y.; Qin, M.; Ding, N.; Zhang, Y.; Wang, Z. High-Stable Perovskite Nanocrystal Fluorescent Probe-Based Aptasensor for Ultrasensitive Detection of Peanut Allergen Ara h1. Sens. Actuators B 2023, 379, 133232. [Google Scholar] [CrossRef]
- Wang, T.; Wei, X.; Zong, Y.; Zhang, S.; Guan, W. An Efficient and Stable Fluorescent Sensor Based on APTES-Functionalized CsPbBr3 Perovskite Quantum Dots for Ultrasensitive Tetracycline Detection in Ethanol. J. Mater. Chem. C 2020, 8, 12196–12203. [Google Scholar] [CrossRef]
- Wei, X.; Li, Y.; Wang, T.; Guo, J.; Zhang, S.; Wang, X.; Liu, X.; Wang, F. Design and Implementation of Fluorescence Sensor Based on All-Inorganic Perovskite Quantum Dots for Rapid Detection of Tetracycline in Water. J. Lumines. 2022, 252, 119344. [Google Scholar] [CrossRef]
- Thuy, T.T.; Huy, B.T.; Kumar, A.P.; Lee, Y.-I. Highly Stable Cs4PbBr6/CsPbBr3 Perovskite Nanoparticles as A New Fluorescence Nanosensor for Selective Detection of Trace Tetracycline in Food Samples. J. Ind. Eng. Chem. 2021, 104, 437–444. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, S.; Wang, T.; Chen, A.; Guo, J.; Bai, B.; Yang, S.; Li, Y.; Wang, X.; Liu, X. Molecularly Imprinted CsPbBr3 Quantum Dot-Based Fluorescent Sensor for Trace Tetracycline Detection in Aqueous Environments. J. Mater. Chem. C 2022, 10, 8432–8440. [Google Scholar] [CrossRef]
- Wang, W.; Deng, P.; Liu, X.; Ma, Y.; Yan, Y. A CsPbBr3 Quantum Dots/Ultra-Thin BN Fluorescence Sensor for Stability and Highly Sensitive Detection of Tetracycline. Microchem. J. 2021, 162, 105876. [Google Scholar] [CrossRef]
- Shi, X.; Kralj, M.; Zhang, Y. Colorimetric Paper Test Strips Based on Cesium Lead Bromide Perovskite Nanocrystals for Rapid Detection of Ciprofloxacin Hydrochloride. J. Phys. Condens. Matter. 2022, 34, 304002. [Google Scholar] [CrossRef]
- Han, J.; Sharipov, M.; Hwang, S.; Lee, Y.; Huy, B.T.; Lee, Y.-I. Water-Stable Perovskite-Loaded Nanogels Containing Antioxidant Property for Highly Sensitive and Selective Detection of Roxithromycin in Animal-Derived Food Products. Sci. Rep. 2022, 12, 3147. [Google Scholar] [CrossRef]
- Salari, R.; Amjadi, M.; Hallaj, T. Perovskite Quantum Dots as A Chemiluminescence Platform for Highly Sensitive Assay of Cefazolin. Spectrochim. Acta A 2023, 285, 121845. [Google Scholar] [CrossRef] [PubMed]
- She, W.-Z.; Zhao, R.-X.; Liu, J.-Z.; Zhang, H.-C.; Li, R.S.; Liu, M.-T.; Zhou, C.-H.; Ling, J.; Cao, Q. Selective Detection of Folic Acid Using a Water-Stable Fluorescent CsPbBr3/Cs4PbBr6 Perovskite Nanocrystal Probe. Chemosensors 2023, 11, 19. [Google Scholar] [CrossRef]
- He, J.; Yu, L.; Jiang, Y.; Lü, L.; Han, Z.; Zhao, X.; Xu, Z. Encoding CsPbX3 Perovskite Quantum Dots with Different Colors in Molecularly Imprinted Polymers as Fluorescent Probes for the Quantitative Detection of Sudan I in Food Matrices. Food Chem. 2023, 402, 134499. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, H.; Yue, X.; Du, J. Perovskite Nanocrystals Fluorescence Nanosensor for Ultrasensitive Detection of Trace Melamine in Dairy Products by the Manipulation of Inner Filter Effect of Gold Nanoparticles. Talanta 2020, 211, 120705. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Tong, P.; Zhang, L.; Luo, Z.; Fu, C.; Tang, D.; Zhang, Y. Photoelectrochemical Immunoassay of Aflatoxin B1 in Foodstuff Based on Amorphous TiO2 and CsPbBr3 Perovskite Nanocrystals. Analyst 2019, 144, 4880–4886. [Google Scholar] [CrossRef]
- Chen, S.; Huang, M.; Huang, M.; Feng, L. Fluorometric Determination of Ziram Using CsPbBr3 Quantum Dots. Microchim. Acta 2021, 188, 390. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Guo, M.; Tan, J.; Geng, Y.; Wu, J.; Tang, Y.; Su, C.; Lin, C.C.; Liang, Y. Novel Fluorescence Sensor Based on All-Inorganic Perovskite Quantum Dots Coated with Molecularly Imprinted Polymers for Highly Selective and Sensitive Detection of Omethoate. ACS Appl. Mater. Interfaces 2018, 10, 39056–39063. [Google Scholar] [CrossRef] [PubMed]
- Vajubhai, G.N.; Chetti, P.; Kailasa, S.K. Perovskite Quantum Dots for Fluorescence Turn-Off Detection of the Clodinafop Pesticide in Food Samples via Liquid–Liquid Microextraction. ACS Appl. Nano Mater. 2022, 5, 18220–18228. [Google Scholar] [CrossRef]
- Tan, L.; Guo, M.; Tan, J.; Geng, Y.; Huang, S.; Tang, Y.; Su, C.; Lin, C.; Liang, Y. Development of High-Luminescence Perovskite Quantum Dots Coated with Molecularly Imprinted Polymers for Pesticide Detection by Slowly Hydrolysing the Organosilicon Monomers In-Situ. Sens. Actuators B 2019, 291, 226–234. [Google Scholar] [CrossRef]
- Huang, S.; Tan, L.; Zhang, L.; Wu, J.; Zhang, L.; Tang, Y.; Wang, H.; Liang, Y. Molecularly Imprinted Mesoporous Silica Embedded with Perovskite CsPbBr3 Quantum Dots for the Fluorescence Sensing of 2,2-Dichlorovinyl Dimethyl Phosphate. Sens. Actuators B 2020, 325, 128751. [Google Scholar] [CrossRef]
- Getachew, G.; Korupalli, C.; Rasal, A.S.; Dirersa, W.B.; Fahmi, M.Z.; Chang, J.-Y. Highly Luminescent, Stable, and Red-Emitting CsMgxPb1−xI3 Quantum Dots for Dual-Modal Imaging-Guided Photodynamic Therapy and Photocatalytic Activity. ACS Appl. Mater. Interfaces 2022, 14, 278–296. [Google Scholar] [CrossRef] [PubMed]
- Kar, M.R.; Chakraborty, R.; Patel, U.; Chakraborty, R.; Ray, S.; Acharya, T.K.; Goswami, C.; Bhaumik, S. Impact of Zn-Doping on the Composition, Stability, Luminescence Properties of Silica-Coated All-Inorganic Cesium Lead Bromide Nanocrystals and Their Biocompatibility. Mater. Today Chem. 2022, 23, 100753. [Google Scholar] [CrossRef]
- Kumar, P.; Patel, M.; Park, C.; Han, H.; Jeong, B.; Kang, H.; Patel, R.; Koh, W.-G.; Park, C. Highly Luminescent Biocompatible CsPbBr3@SiO2 Core–Shell Nanoprobes for Bioimaging and Drug Delivery. J. Mater. Chem. B 2020, 8, 10337–10345. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.K.; Giovanni, D.; He, H.; Sum, T.C.; Yong, K.-T. Water-Stable All-Inorganic Perovskite Nanocrystals with Nonlinear Optical Properties for Targeted Multiphoton Bioimaging. ACS Appl. Nano Mater. 2021, 4, 9022–9033. [Google Scholar] [CrossRef]
- Talianov, P.M.; Peltek, O.O.; Masharin, M.; Khubezhov, S.; Baranov, M.A.; Drabavičius, A.; Timin, A.S.; Zelenkov, L.E.; Pushkarev, A.P.; Makarov, S.V.; et al. Halide Perovskite Nanocrystals with Enhanced Water Stability for Upconversion Imaging in a Living Cell. J. Phys. Chem. Lett. 2021, 12, 8991–8998. [Google Scholar] [CrossRef]
- Yan, Q.-B.; Bao, N.; Ding, S.-N. Thermally Stable and Hydrophilic CsPbBr3/mPEG-NH2 Nanocrystals with Enhanced Aqueous Fluorescence for Cell Imaging. J. Mater. Chem. B 2019, 7, 4153–4160. [Google Scholar] [CrossRef]
- Wang, F.; Wang, H.; Ali, A.; Zhang, Y.; Cui, X.; Liu, Y. In-Situ One-Step Electrospray Fabrication of Polyvinylidene Fluoride Encapsulated CsPbBr3 Spheres with High Stability and Cell Imaging Application. Inorg. Chem. Commun. 2019, 106, 99–103. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Liao, Q.; Xu, Z.; Li, H.; Zheng, L.; Fu, H. Embedding Perovskite Nanocrystals into a Polymer Matrix for Tunable Luminescence Probes in Cell Imaging. Adv. Funct. Mater. 2017, 27, 1604382. [Google Scholar] [CrossRef]
- Wu, D.; Chi, J.; Zhang, M.; Cheng, L.; Wang, X.; Fan, J.; Huang, Z.; Wang, H.; Xie, H.; Pan, Q.; et al. Water-Dispersing Perovskite Probes for the Rapid Imaging of Glioma Cells. Adv. Opt. Mater. 2022, 10, 2101835. [Google Scholar] [CrossRef]
- Avugadda, S.K.; Castelli, A.; Dhanabalan, B.; Fernandez, T.; Silvestri, N.; Collantes, C.; Baranov, D.; Imran, M.; Manna, L.; Pellegrino, T.; et al. Highly Emitting Perovskite Nanocrystals with 2-Year Stability in Water through an Automated Polymer Encapsulation for Bioimaging. ACS Nano 2022, 16, 13657–13666. [Google Scholar] [CrossRef]
- Lou, S.; Zhou, Z.; Xuan, T.; Li, H.; Jiao, J.; Zhang, H.; Gautier, R.; Wang, J. Chemical Transformation of Lead Halide Perovskite into Insoluble, Less Cytotoxic, and Brightly Luminescent CsPbBr3/CsPb2Br5 Composite Nanocrystals for Cell Imaging. ACS Appl. Mater. Interfaces 2019, 11, 24241–24246. [Google Scholar] [CrossRef] [PubMed]
- Shellaiah, M.; Sun, K.W. Inorganic-Diverse Nanostructured Materials for Volatile Organic Compound Sensing. Sensors 2021, 21, 633. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.; Jagtap, S. Nanomaterials-Based Resistive Sensors for Detection of Environmentally Hazardous H2S Gas. J. Electron. Mater. 2021, 50, 2531–2555. [Google Scholar] [CrossRef]
- Liu, X.; Huang, L.; Qian, K. Nanomaterial-Based Electrochemical Sensors: Mechanism, Preparation, and Application in Biomedicine. Adv. NanoBiomed. Res. 2021, 1, 2000104. [Google Scholar] [CrossRef]
- Shellaiah, M.; Sun, K.W. Diamond-Based Electrodes for Detection of Metal Ions and Anions. Nanomaterials 2022, 12, 64. [Google Scholar] [CrossRef]
- Xiao, L.; Huang, L.; Su, W.; Wang, T.; Liu, H.; Wei, Z.; Fan, H. Efficiency Enhancement Strategies for Stable Bismuth-Based Perovskite and Its Bioimaging Applications. Int. J. Mol. Sci. 2023, 24, 4711. [Google Scholar] [CrossRef] [PubMed]
- Shellaiah, M.; Awasthi, K.; Chandran, S.; Aazaad, B.; Sun, K.W.; Ohta, N.; Wu, S.-P.; Lin, M.-C. Methylammonium Tin Tribromide Quantum Dots for Heavy Metal Ion Detection and Cellular Imaging. ACS Appl. Nano Mater. 2022, 5, 2859–2874. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, F.; Wang, Y.; Li, H.; Zhang, J.; Sun, Z.; Jiang, Y. Fluorescent Organic Small Molecule Probes for Bioimaging and Detection Applications. Molecules 2022, 27, 8421. [Google Scholar] [CrossRef] [PubMed]
- Shellaiah, M.; Chen, H.-C.; Awasthi, K.; Aazaad, B.; Sun, K.W.; Ohta, N.; Lin, M.-C. An AIE Active Anthracene-Based Schiff Base Probe for “Turn-On” Detection of Cu2+ Ions: Demonstrations with Nanostructural Investigations, DFT, Cellular Imaging, and Real Water Analysis. J. Mol. Struct. 2024, 1301, 137347. [Google Scholar] [CrossRef]
- Shellaiah, M.; Sun, K.W. Conjugation of Cysteamine Functionalized Nanodiamond to Gold Nanoparticles for pH Enhanced Colorimetric Detection of Cr3+ Ions Demonstrated by Real Water Sample Analysis. Spectrochim. Acta A 2023, 286, 121962. [Google Scholar] [CrossRef] [PubMed]
- Shellaiah, M.; Simon, T.; Thirumalaivasan, N.; Sun, K.W.; Ko, F.-H.; Wu, S.-P. Cysteamine-Capped Gold-Copper Nanoclusters for Fluorometric Determination and Imaging of Chromium(VI) and Dopamine. Microchim. Acta 2019, 186, 788. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-Y.; Shellaiah, M.; Sun, K.W. Structural, Photophysical, and Transport Properties of Porous MAPbBr3 Single Nanowire and Nanodevice for Electrochemical Sensing. J. Electrochem. Soc. 2023, 170, 077505. [Google Scholar] [CrossRef]
- Rahman, M.M.; Ahmed, J.; Asiri, A.M. A Glassy Carbon Electrode Modified with γ-Ce2S3-Decorated CNT Nanocomposites for Uric Acid Sensor Development: A Real Sample Analysis. RSC Adv. 2017, 7, 14649–14659. [Google Scholar] [CrossRef]
- Wen, Y.; Zhang, P.; Sharma, V.K.; Ma, X.; Zhou, H.-C. Metal-Organic Frameworks for Environmental Applications. Cell Rep. Phys. Sci. 2021, 2, 100348. [Google Scholar] [CrossRef]
- Li, J.; Zhang, L.; Li, P.; Zhang, Y.; Dong, C. One Step Hydrothermal Synthesis of Carbon Nanodots to Realize the Fluorescence Detection of Picric Acid in Real Samples. Sens. Actuators B 2018, 258, 580–588. [Google Scholar] [CrossRef]
- Galisteo-López, J.F.; Anaya, M.; Calvo, M.E.; Míguez, H. Environmental Effects on the Photophysics of Organic–Inorganic Halide Perovskites. J. Phys. Chem. Lett. 2015, 6, 2200–2205. [Google Scholar] [CrossRef] [PubMed]
- Kachhap, S.; Singh, S.; Singh, A.K.; Singh, S.K. Lanthanide-Doped Inorganic Halide Perovskites (CsPbX3): Novel Properties and Emerging Applications. J. Mater. Chem. C 2022, 10, 3647–3676. [Google Scholar] [CrossRef]
- De Giorgi, M.L.; Milanese, S.; Klini, A.; Anni, M. Environment-Induced Reversible Modulation of Optical and Electronic Properties of Lead Halide Perovskites and Possible Applications to Sensor Development: A Review. Molecules 2021, 26, 705. [Google Scholar] [CrossRef] [PubMed]
- Fransson, E.; Wiktor, J.; Erhart, P. Phase Transitions in Inorganic Halide Perovskites from Machine-Learned Potentials. J. Phys. Chem. C 2023, 127, 13773–13781. [Google Scholar] [CrossRef]
- Steele, J.A.; Lai, M.; Zhang, Y.; Lin, Z.; Hofkens, J.; Roeffaers, M.B.J.; Yang, P. Phase Transitions and Anion Exchange in All-Inorganic Halide Perovskites. Acc. Mater. Res. 2020, 1, 3–15. [Google Scholar] [CrossRef]






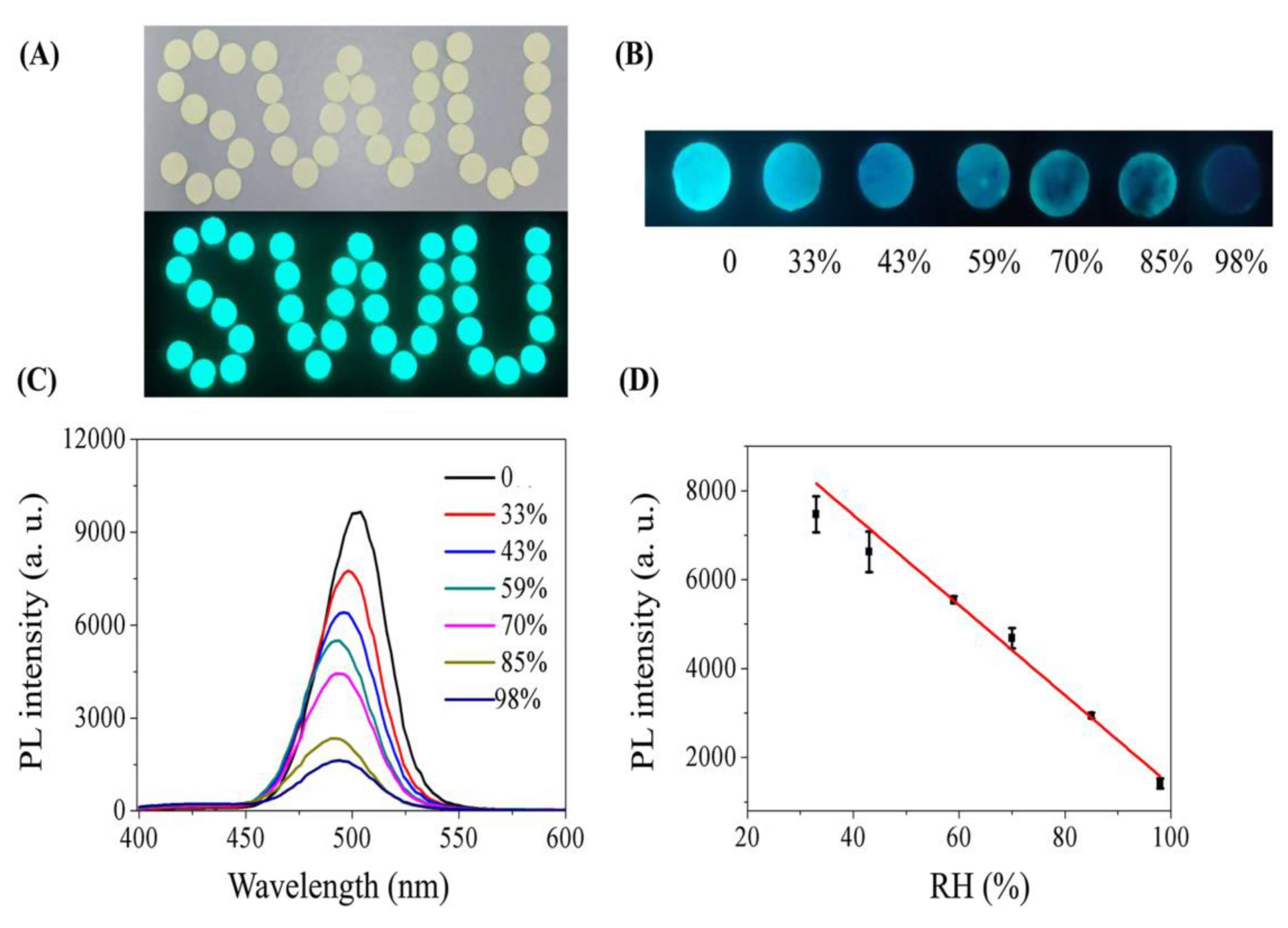

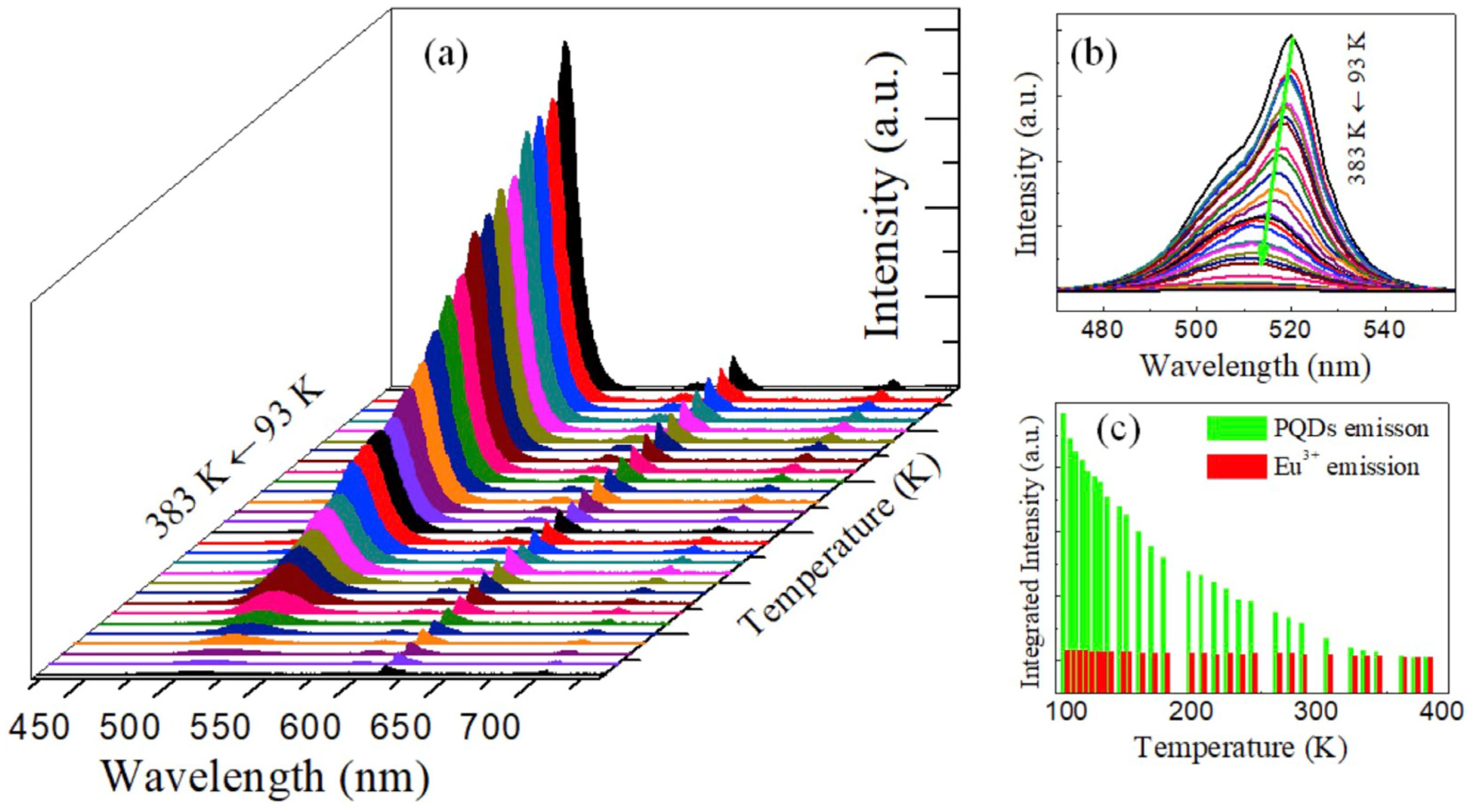
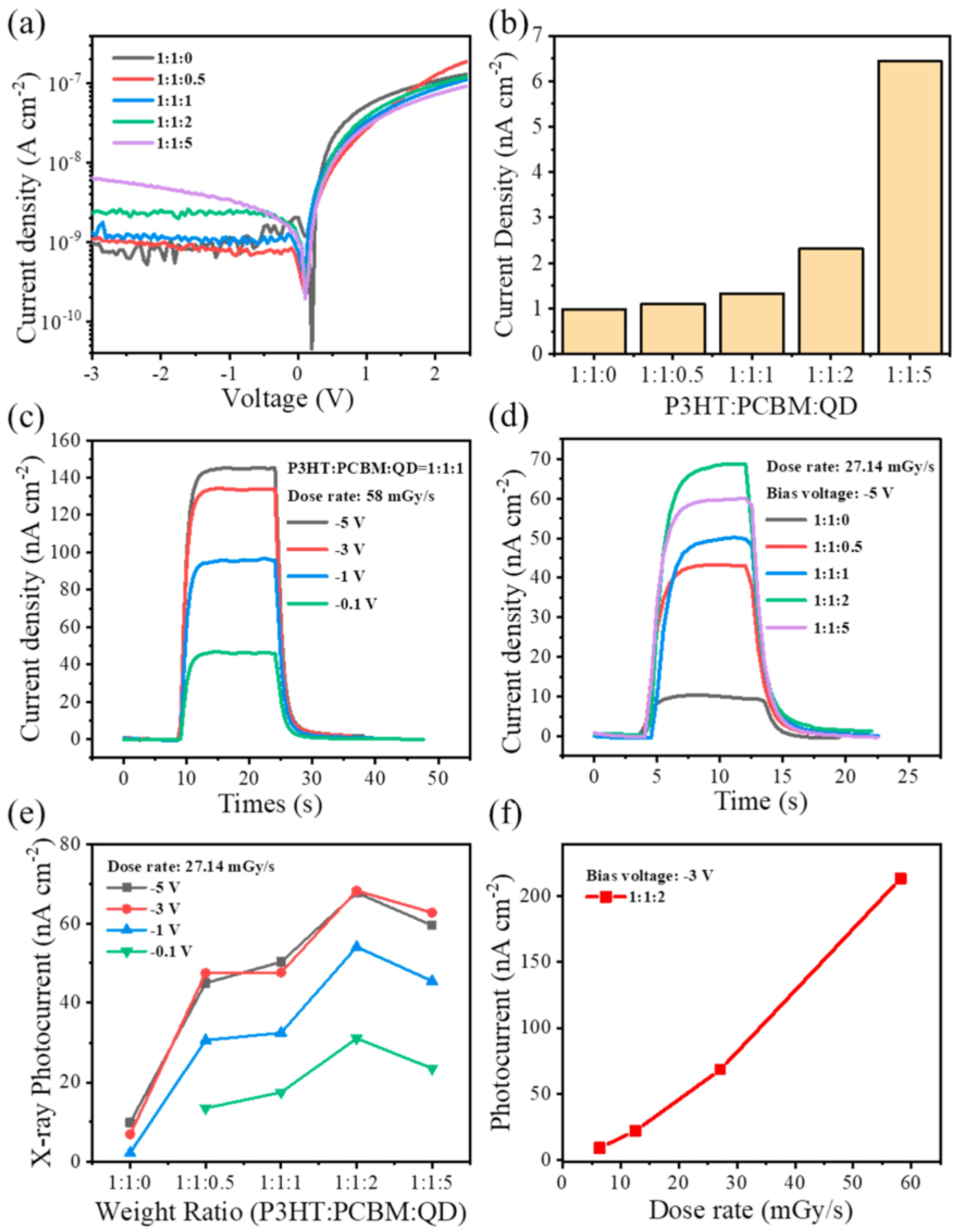



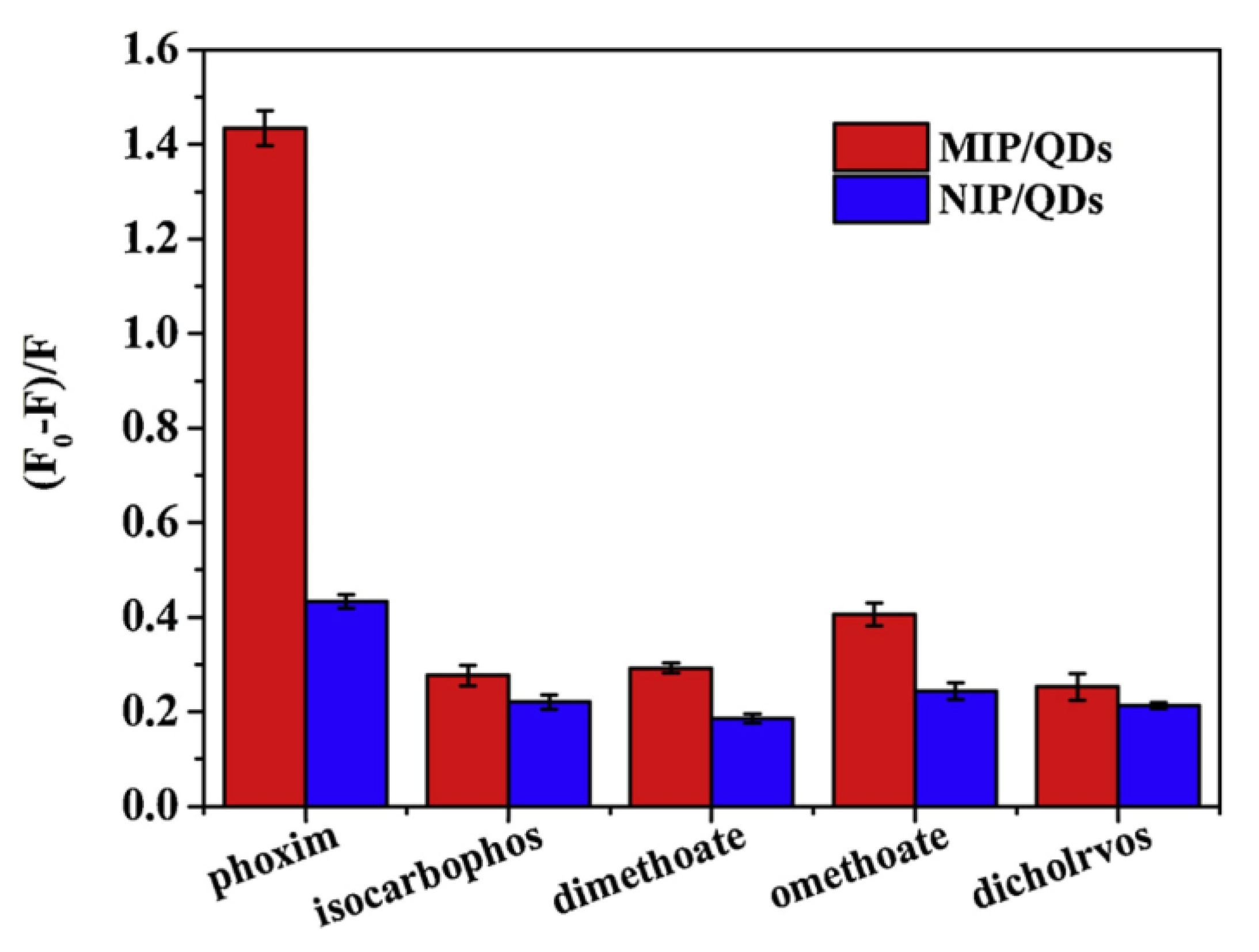
| Composition | Synthetic Route; PLQY (%) | Analyte | Method of Detection | Linear Regression | Detection Limit (LOD) | Applications | Ref. |
|---|---|---|---|---|---|---|---|
| CsPbCl3 NCs and CsPbCl3 NWs | Hot-injection method; 2.1% and 17.3% | Cu2+ | PL quenching | 0–1 µM | 0.06 nM | NA | [80] |
| CSPbBr3 QDs | Hot-injection method; 63% | Cu2+ | PL quenching | 2 nM–2 µM | 2 nM | Edible oils | [81] |
| CSPbBr3 QDs | Hot-injection method; 90% | Cu2+ | PL quenching | 0–100 nM | 0.1 nM | NA | [82] |
| Silica-coated CsPbCl3 NCs | ligand-assisted reprecipitation (LARP) method; 93% | Cu2+ | PL quenching | 0–412 µM | 18.6 µM | Natural water systems | [83] |
| CsPbBr3 QDs | Hot-injection method; NA | Cu2+ | PL quenching | 1 µM–10 mM | NA | NA | [84] |
| CsPbBr3-(SH) polyHIPE composite | Hot-injection method; ~98% | Cu2+ | PL quenching | 10 fM–10 mM | 10 fM | NA | [86] |
| PMMA OPCs/CsPbBr3 QD composites | Hot-injection method; NA | Cu2+ | PL quenching/Microfluidic detection | 1 nM–10 mM | 0.4 nM | Lubricating oils | [87] |
| CsPbI3 QD/S iO2 IOPCs | Hot-injection method; NA | Cu2+ | PL quenching/Microfluidic detection | 0–20 nM and 20–50 nM | 0.34 nM | Lubricating oils | [88] |
| CsPbBr3@SiO2-E NPs | Hot injection followed by 3-step synthetic modification; 90% | Cu2+/S2− | PL quenching/PL Recovery | 0–5 µM and 5–10 µM (for Cu2+) and 0–120 µM (for S2−) | 0.16 µM (for Cu2+) and 8.8 µM (for S2−) | NA | [89] |
| CsPbBr3 QD/PMMA fiber membranes | Hot injection/Electrospinning method; 88% | Cu2+ | PL quenching | 1 fM–1 M | 1 fM | NA | [90] |
| CsPbBr3@MOF QDs | two step surfactant free procedure; 39.2% | Cu2+ | PL quenching | 100–600 nM | 63 nM | NA | [91] |
| CsPbBr3 NCs | Hot-injection method; NA | Hg2+ | PL quenching | 50 nM–10 µM | 35.65 nM | NA | [92] |
| CsPbBr3 Crystals | two-step precipitation method; NA | Hg2+ | PL quenching | 5–100 nM | 0.1 nM | NA | [93] |
| CsPbBr3-mPEG@SiO2 NCs | Ligand engineering and silica encapsulation method; 67.5% | Hg2+/GSH | PL quenching/PL Recovery | 0.1–50 nM (for Hg2+) and 1–10 µM (for GSH) | 0.08 nM (for Hg2+) and 0.19 µM (for GSH) | Tap water and Serum analysis | [94] |
| CsPbBr3 NCs | Nucleation growth synthesis; >89% | Zn2+ | PL quenching | 0–40 µM | NA | NA | [95] |
| alpha-amino butyric acid (A-ABA)-capped CsPbBr3 QDs (M PQDs) | Hot-injection method; NA | Co2+ | PL quenching | 0–100 nM | 0.8 µM | NA | [96] |
| CsPbBr3 QDs | Hot-injection method; NA | UO22+ | PL quenching | 0–3.3 µM | 83.33 nM | NA | [97] |
| PVP shell-grown silica-coated Zn-doped CsPbBr3 NCs | Hot-injection method; 88% | In3+ | PL quenching | 0–104 µM | 11 µM | NA | [98] |
| CsPbBr3−Ti3C2Tx MXene QD/QD heterojunction | Hot-injection method; NA | Cd2+ | PL quenching | 99–590 µM | 99 µM | NA | [99] |
| APTES-coated CsPbBr3–CsPb2Br5 QDs | ligand-assisted reprecipitation method; NA | Fe3+ | PL quenching | 10 µM–10 mM | 10 µM | NA | [100] |
| Composition | Synthetic Route; PLQY (%) | Analyte | Method of Detection | Linear Regression | Detection Limit (LOD) | Applications | Ref. |
|---|---|---|---|---|---|---|---|
| CsPbBr3 nanoplatelets | Hot-injection method; 83.7% | Cl− and As3+ | PL peak shift | 0.2–0.4 nM and 6.4–58 nM | 28 pM and 1 nM | NA | [101] |
| CsPbBr3 QDs | Hot-injection method; 87% | Cl− | PL peak shift | 10–200 µM | 4 µM | Real-time water analysis | [102] |
| CsPbBr3 NCs | Ligand-assisted synthesis; >40% | Cl− | PL peak shift | 1–80 mM | 0.34 mM | Human sweat sample analysis | [103] |
| CsPbBr3 NCs | Hot-injection method; NA | Cl− | PL peak shift | 10–130 mM | 3 mM | Human sweat sample analysis | [104] |
| CsPbBr3 NCs | Hot-injection method; NA | Cl− | PL peak shift | 100 µM–10 mM | 100 µM | Glass/Paper-strip analysis | [105] |
| β-cyclodextrin stabilized, Arginine added CsPbBr3 NCs (ACD-PNCs) | Ligand-assisted synthesis; 82% | Cl− and I− | PL peak shift | 0.04–0.8 mM and 0.04–1.16 mM | 3.2 µM and 9 µM | Human saliva, sweat, and test-strip analysis | [106] |
| CsPbBr3@SiO2 NCs | Room-temperature synthesis; NA | Cl− | PL peak shift | 0–3% | 0.05 mg/g | Sand Analysis | [109] |
| NH2-functionalized CsPbBr3 NCs | Hot-injection method; NA | Cl− | PL Quenching | 4–28 µM | 1 µM | NA | [110] |
| CsPbBr3 QD/Cellulose composite | Hot-injection method; NA | Cl− and I− | PL peak shift | 0.1 mM–1 M | 2.56 mM (For Cl−) and 4.11 mM (For I−) | Real-time water analysis | [111] |
| Tetraphenylporphyrin tetrasulfonic acid (TPPS)-modified CsPbBr3 NCs | Hot-injection method followed by compositing; NA | S2− | PL Quenching | 0.2–15 nM | 0.05 nM | Real-time water analysis | [113] |
| Composition | Synthetic Route; PLQY (%) | Analyte | Method of Detection | Linear Regression | Detection Limit (LOD) | Applications | Ref. |
|---|---|---|---|---|---|---|---|
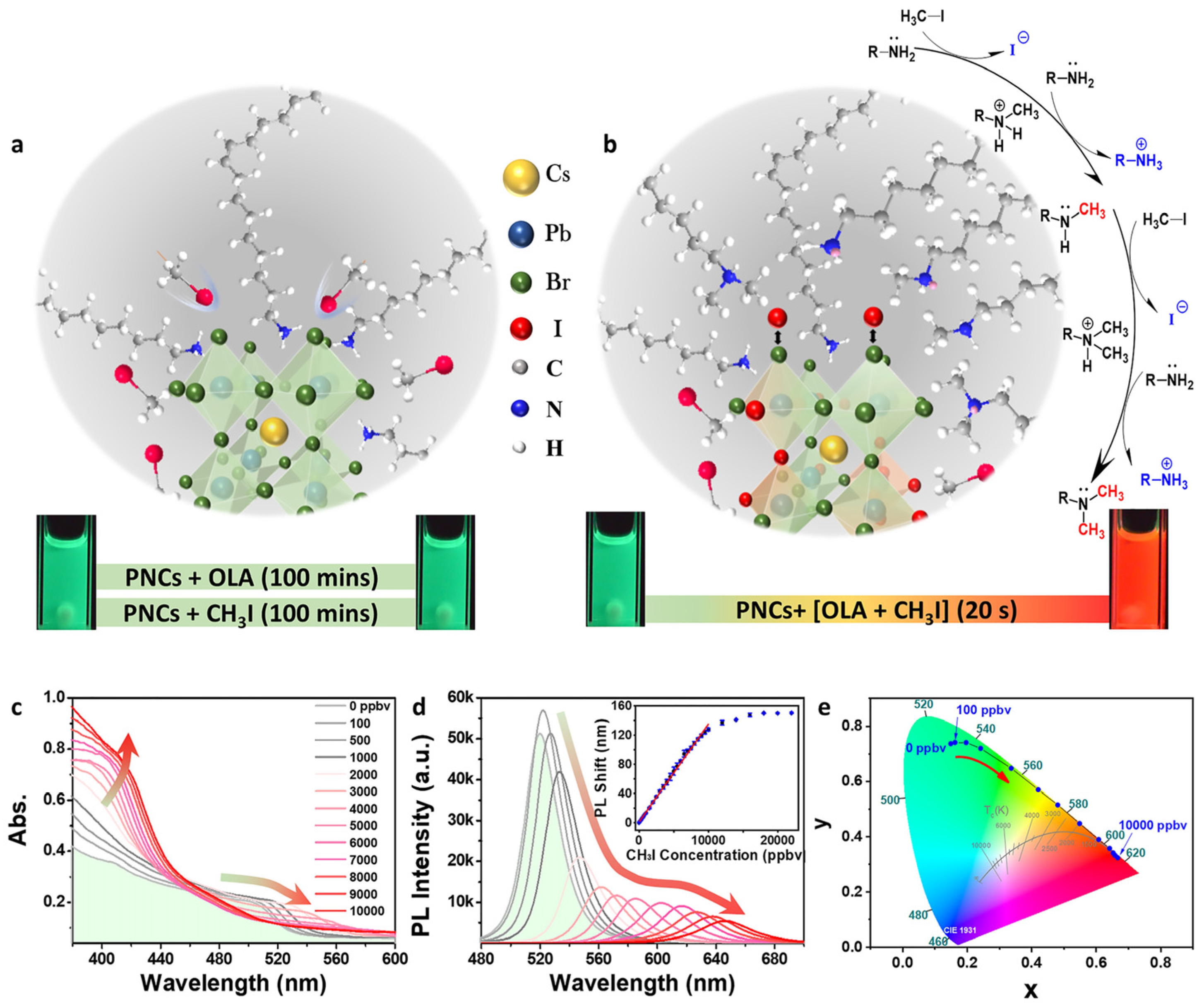
| CsPbBr3 NCs | Hot-injection method; NA | CH3I | PL peak shift | 0.7–70 µM | 0.2 ± 0.07 µM | NA | [114] |
| Yttrium single-atom-doped CsPbBr3 NCs | Hot-injection method; NA | CH3I | PL peak shift | 5.6–157 µM | 0.3 µM | NA | [115] |
| CsPbX3 (X = Cl, Br, or I) NCs | Hot-injection method; NA | CH2Cl2 and CH2Br2 | PL peak shift | 0–0.9 M and 7.2–21 mM | 48 mM and 1.7 mM | Microfluidic application | [116] |
| CsPbBr3 NCs | NA | Benzoyl peroxide | Peak shift and Ratiometric detection | NA | NA | Food sample analysis | [118] |
| CsPbBr3 NCs | Hot-injection method; 87% | Benzoyl peroxide | Peak shift and Ratiometric detection | 0 µM–120 µM | 0.13 µM | Food sample analysis | [119] |
| CsPbBr2I microcrystals | Hot-injection method; NA | Nitrophenol | PL Quenching | 0.1–0.6 mM | NA | NA | [122] |
| CsPbBr3 and CsPbI3 QDs | Hot-injection method; 52.88% and 46.18%, respectively | Picric acid | PL Quenching | 0–180 nM and 0–270 nM, respectively | 0.8 nM and 1.9 nM, respectively | Paper-strip analysis | [123] |
| Composition | Synthetic Route; PLQY (%) | Analyte | Method of Detection | Linear Regression | Detection Limit (LOD) | Applications | Ref. |
|---|---|---|---|---|---|---|---|
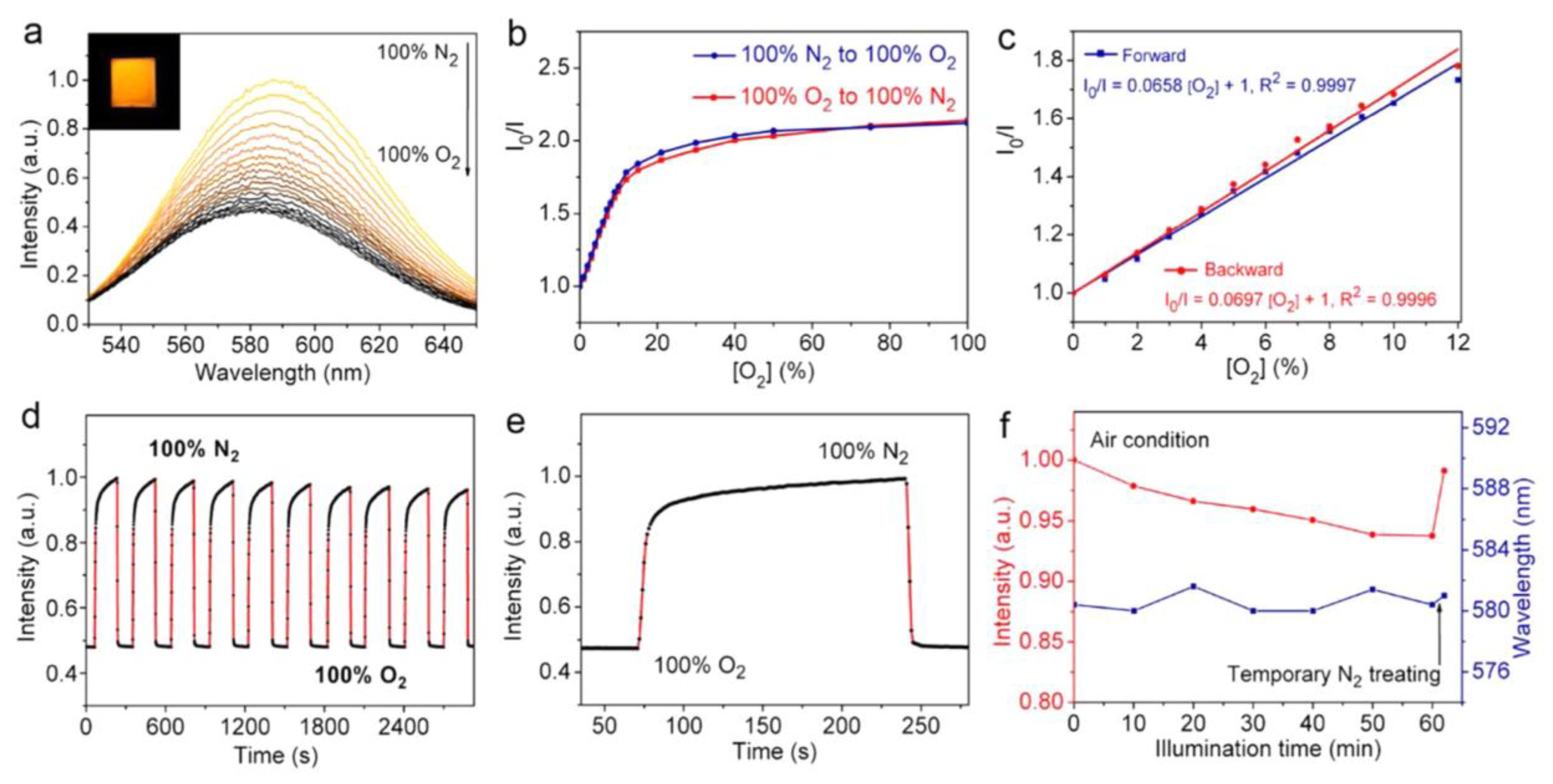
| Mn:CsPbCl3 NCs | Heat-up strategy; NA | O2 | PL quenching | 0–12% | NA | NA | [126] |
| CsPbBr3 NFs | Hot-injection method; NA | N2 | PL quenching | 1–20 ppm | 1 ppm | NA | [128] |
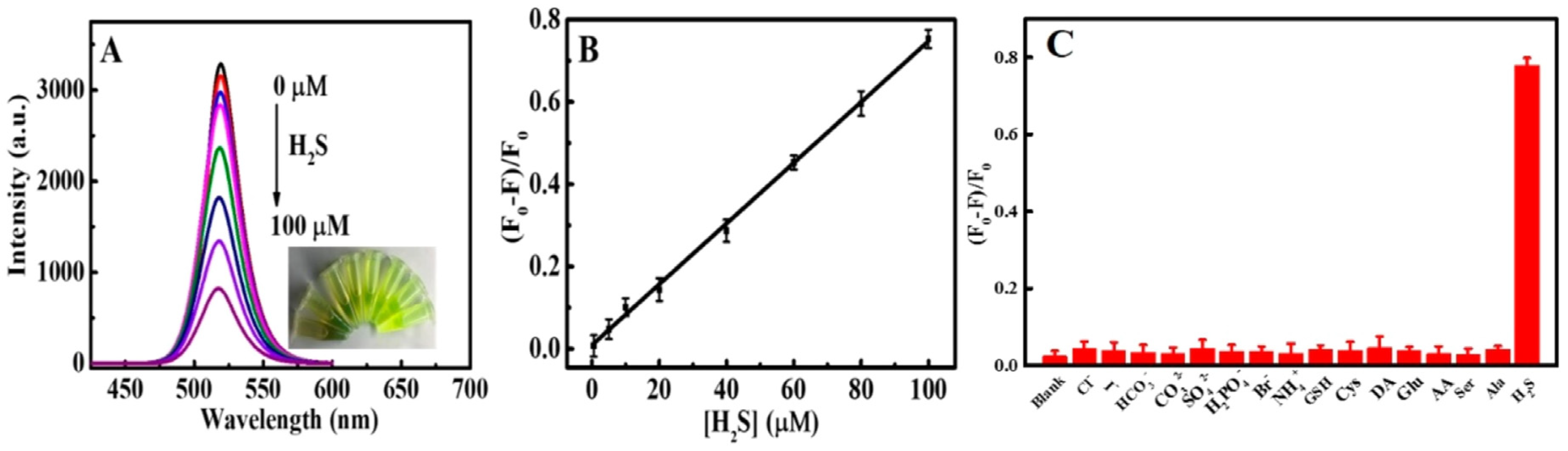
| CsPbBr3 QDs | Sonication Method; NA | H2S | PL quenching | 0–100 µM | 0.18 µM | Rat brain studies | [133] |
| CsPbBr3@CMO | Sonication followed by compositing method; NA | H2S | PL quenching | 0.15–105 µM | 53 nM | Rat brain studies | [134] |
| CsPbBr3@SBE-β-CD nanocomposite | Sonication followed by compositing method; NA | H2S | PL quenching | 0.5 µM–6 mM | 0.19 µM | Zebrafish studies | [136] |
| CsPbBr3/NCM composite | Hot injection followed by EDC−NHS method; NA | tripropylamine (TPrA) and Cesium oleate | Electrochemiluminescence (ECL) signals | 10 mM and NA | NA and 1 aM | NA | [143] |
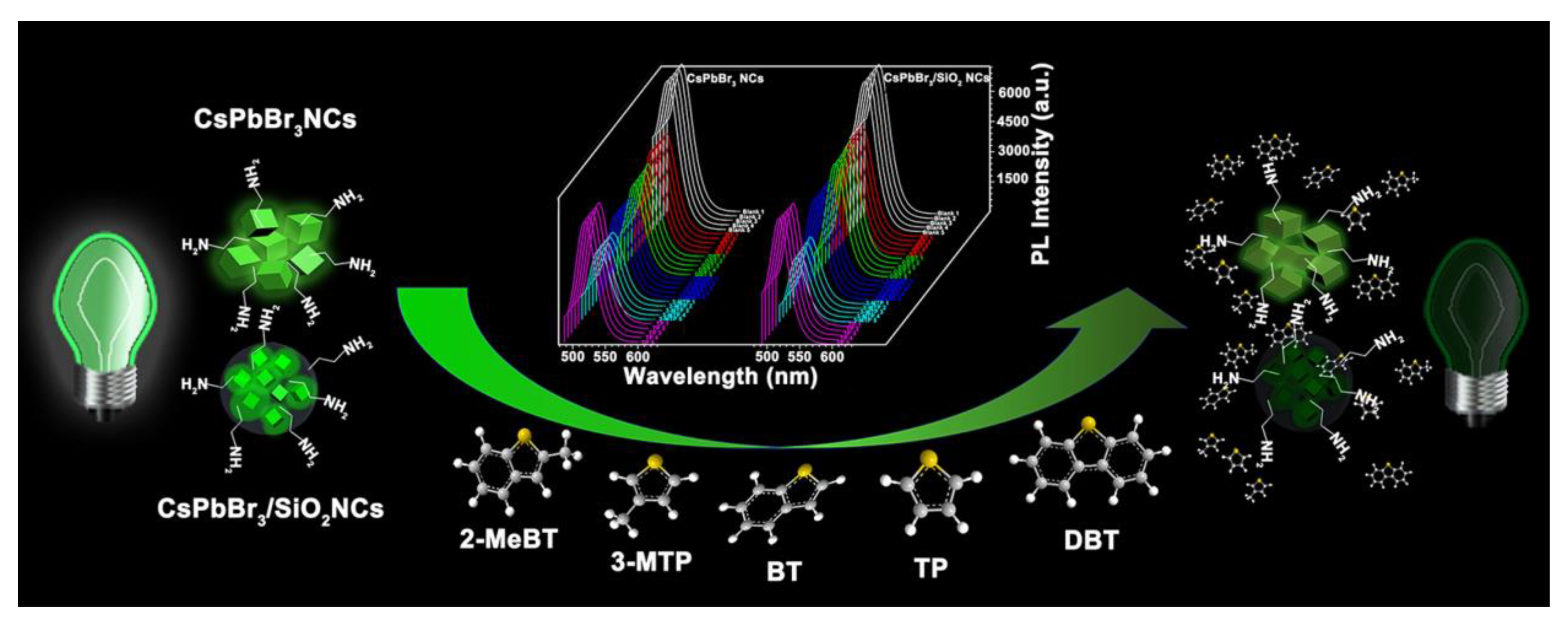
| CsPbBr3/SiO2 NCs | Precursor injection followed by compositing method; NA | Thiophene Sulfides | PL quenching | 10–50 ppm | ≈10 ppm | NA | [144] |
| CsPbBr3 QD film | Hot-injection method; NA | NH3 | PL enhancement | 25–300 ppm | 8.85 ppm | Film-based sensor study | [145] |
| CsPbX3 (X = Cl, Br, I or mixed halogen) QD film | Hot-injection method; NA | NH3 | PL enhancement | 25–200 ppm | ≈20 ppm | Film-based sensor study | [146] |
| CsPbBr3–SiO2 nanocomposites on PVDF membrane | controllable strategy; NA | NH3 | PL quenching | 2160–3600 ppm | NA | Test paper study | [147] |
| CsPbBr3 NFs | Hot-injection method followed by electrospinning; NA | NH3 | PL quenching | 528 µM–1.76 mM | <0.5 mM | NA | [148] |
| CsPbBr3/BNNF composites | Hot-injection method followed by compositing method; ~54% | NH3 | PL quenching | NA | NA | NA | [149] |
| CsPbX3 (X = Cl, Br, and I) QDs @Fe/X-n | Hydrothermal crystallization followed by in situ growth of QDs; NA | NH3 | PL quenching | 0–10 mL | NA | NA | [150] |
| Composition | Synthetic Route; PLQY (%) | Analyte | Method of Detection | Linear Regression | Detection Limit (LOD) | Applications | Ref. |
|---|---|---|---|---|---|---|---|
| CsPbBr3@Cu nanohybrid | In situ synthesis; NA | H2O2 and Glucose | Ratiometric PL response | 0.2–100 µM and 2–120 µM | 0.07 µM and 0.8 µM | Human serum analysis | [181] |
| TiO2/CsPbBr1.5I1.5 composite film | Slow volatilization method; NA | Dopamine (DA) | Photoelectrochemical (PEC) detection | 0.1–250 μM | 12 nM | Human serum analysis | [182] |
| TiO2 IOPCs/CsPbCl3 | slow volatilization method; NA | alpha-fetoprotein (AFP) | Photoelectrochemical (PEC) detection | 0.08–980 ng/mL | 30 pg/mL | NA | [183] |
| CsPbBr3 microcrystals | one-pot synthesis method; 60% | Uric acid (UA) | PL quenching | 3.1 nM–1.33 µM | 0.063 ppm | Human blood serum analysis | [184] |
| CsPbBr3 NC-TPPS nanocomposite | Self-assembly strategy; 60% | Acetylcholinesterase (AChE) | PL quenching | 0.05–1.0 U/L | 0.0042 U/L | Human serum analysis | [185] |
| CsPbX3 (X = Br/I) PNCs | Anion exchange method; NA | Penicillamine | PL enhancement | 5.0–35.0 nM | 1.19 nM and 5. 47 nM | NA | [187] |
| CsPbBr3 QD-DNA/MoS2 NS | One-pot synthetic method; NA | Mycobacterium tuberculosis (Mtb) | PL enhancement | 0.2–4.0 nM | 51.9 pM | Clinical tuberculosis pathogen analysis | [188] |
| CsPbBr3 NCs@PL | Film hydration method; NA | Pore-forming biotoxins | PL quenching | 50 nM–150 µM | 50 nM | Bacterial study | [191] |
| Phospholipid-coated CsPbBr3 NCs | Film hydration method; NA | Prostate-specific antigen (PSA) | PL enhancement and colorimetric sensing | 0.01–80 ng/mL and 0.1–15 ng/mL | 0.081 ng/mL and 0.29 ng/mL | Clinical sample analysis | [192] |
| Apt-PNCs@cDNA-MNPs | One-pot synthesis, magnetic stirring, and sonication; NA | Peanut allergen Ara h1 | PL enhancement | 0.1–100 ng/mL | 0.04 ng/mL | Food sample analysis | [193] |
| APTES-functionalized CsPbBr3 QDs | Slow hydrolysis of the capping agent; 46.86% | Tetracycline | PL quenching | 0.5–15.0 µM | 76 nM | Soil sample analysis | [194] |
| LMSNs@CsPbBr3 QDs | Water emulsion followed by homogeneous mixing; ~54% | Tetracycline | PL quenching | 0.7–15 µM | 93 nM | Water sample analysis | [195] |
| Cs4PbBr6/CsPbBr3 NPs | Temperature-controlled synthesis; NA | Tetracycline | PL quenching | 0.4–10 µM | 76 nM | Food sample analysis | [196] |
| Molecularly imprinted CsPbBr3 QDs | Water emulsion followed by homogeneous mixing; NA | Tetracycline | PL quenching | 0.2–5 µM | 28 nM | Water sample analysis | [197] |
| CsPbBr3@BN | Hot injection followed by calcination; NA | Tetracycline | PL quenching | 0–99 µM | 14.6 µM | Honey and milk samples analysis | [198] |
| CsPbBr3 NCs | Hot-injection method; NA | Ciprofloxacin hydrochloride | PL peak shift | 0.8–50 mM | 0.1 mM | Paper-strip analysis | [199] |
| CsPbBr3-loaded MIP nanogels | In situ hot-injection method; NA | Roxithromycin | PL quenching | 100 pM–100 nM | 20.6 pM | Animal-derived food product analysis | [200] |
| CsPbBr3 QDs | ligand-assisted reprecipitation method; 42% | Cefazolin | Chemiluminescence | 25–300 nM | 9.6 nM | Human plasma, urine, water, and milk samples analysis | [201] |
| Water-stable fluorescent CsPbBr3/Cs4PbBr6 NCs | Water emulsion method; NA | Folic acid | PL quenching | 10–800 µM | 1.695 µM | Urine sample analysis | [202] |
| MIP-CsPbX3 (X = Cl, Br, and I) fluorescent-encoding microspheres | Encoding of MIPs with CsPbX3 QDs: NA | Sudan I | PL quenching | 2–604 nM | 1.21 nM | Food sample analysis | [203] |
| CsPbBr3 NCs@BaSO4 | Aqueous emulsion process; 80.3% | Melamine | PL enhancement | 5 nM–5 µM | 0.42 nM | Spiked dairy sample analysis | [204] |
| CsPbBr3/a-TiO2/FTO | Hot injection followed by compositing; NA | Aflatoxin B1 (AFB1) | Photoelectrochemical immunoassay | 32 pM–48 nM | 9 pM | Food sample analysis | [205] |
| CsPbBr3 QDs | Room-temperature-controlled synthesis; 96% | Ziram | PL quenching | 0.1–50 ppm | 0.086 ppm | Food sample analysis | [206] |
| CsPbBr3 QDs coated MIPs | Slow hydrolysis of the capping agent; 92% | Omethoate | PL quenching | 0–1.9 µM | 88 nM | soil and cabbage samples analysis | [207] |
| CsPbI3 QDs | Microwave synthesis; 27% | Clodinafop | PL quenching | 0.1–5 μM | 34.7 nM | Food sample analysis | [208] |
| MIP/CsPbBr3 QD composite | Hot injection followed by self-assembly method; NA | Phoxim | PL quenching | 16.8–335.4 nM | 4.9 nM | Food sample analysis | [209] |
| MIP-mesoporous silica-embedded CsPbBr3 QDs | Multiple synthetic methods; NA | Dichlorvos | PL quenching | 23–110 nM | 5.7 nM | Food sample analysis | [210] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shellaiah, M.; Sun, K.W.; Thirumalaivasan, N.; Bhushan, M.; Murugan, A. Sensing Utilities of Cesium Lead Halide Perovskites and Composites: A Comprehensive Review. Sensors 2024, 24, 2504. https://doi.org/10.3390/s24082504
Shellaiah M, Sun KW, Thirumalaivasan N, Bhushan M, Murugan A. Sensing Utilities of Cesium Lead Halide Perovskites and Composites: A Comprehensive Review. Sensors. 2024; 24(8):2504. https://doi.org/10.3390/s24082504
Chicago/Turabian StyleShellaiah, Muthaiah, Kien Wen Sun, Natesan Thirumalaivasan, Mayank Bhushan, and Arumugam Murugan. 2024. "Sensing Utilities of Cesium Lead Halide Perovskites and Composites: A Comprehensive Review" Sensors 24, no. 8: 2504. https://doi.org/10.3390/s24082504
APA StyleShellaiah, M., Sun, K. W., Thirumalaivasan, N., Bhushan, M., & Murugan, A. (2024). Sensing Utilities of Cesium Lead Halide Perovskites and Composites: A Comprehensive Review. Sensors, 24(8), 2504. https://doi.org/10.3390/s24082504









