A Review of Microsphere Super-Resolution Imaging Techniques
Abstract
1. Introduction
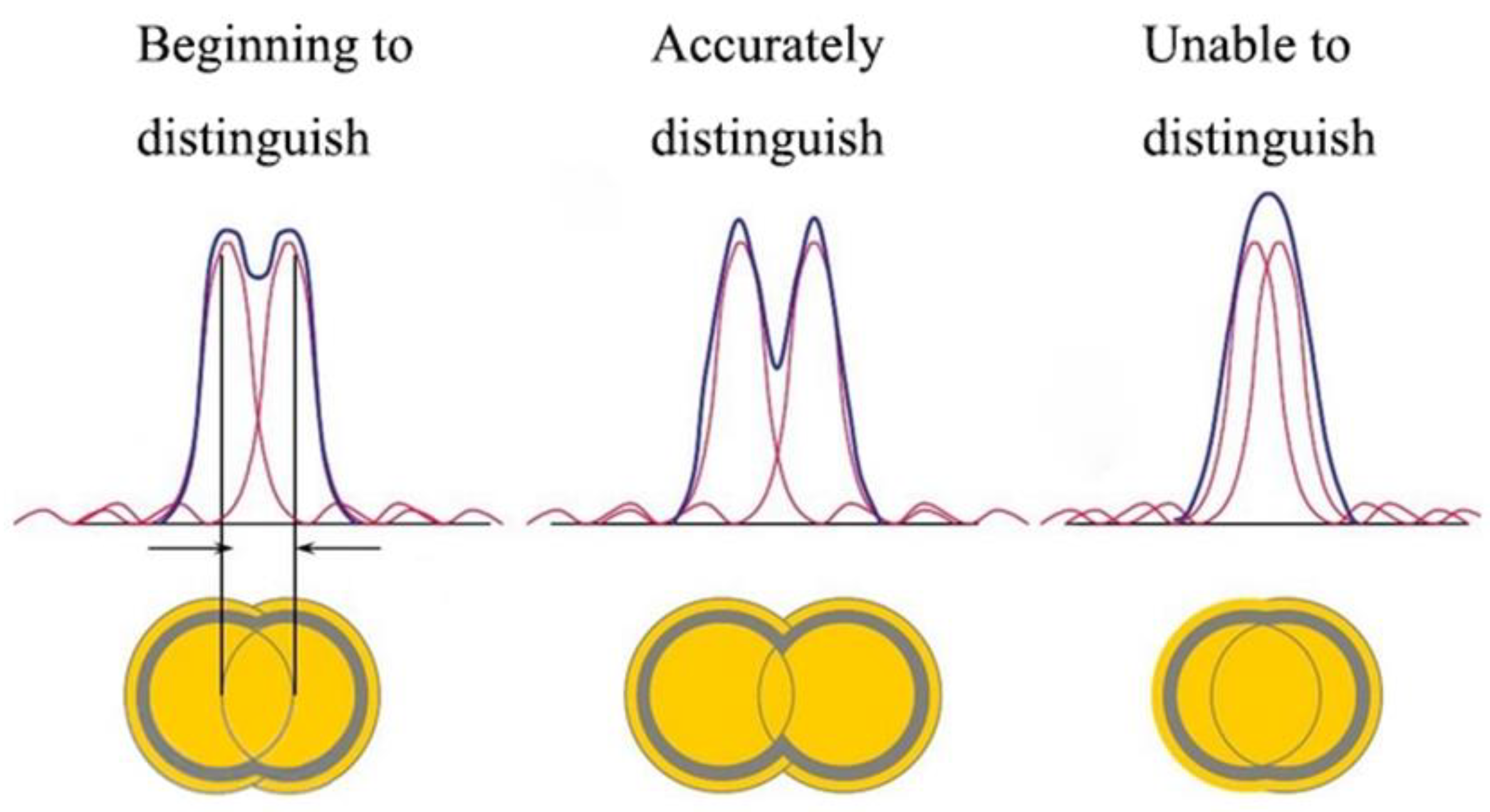
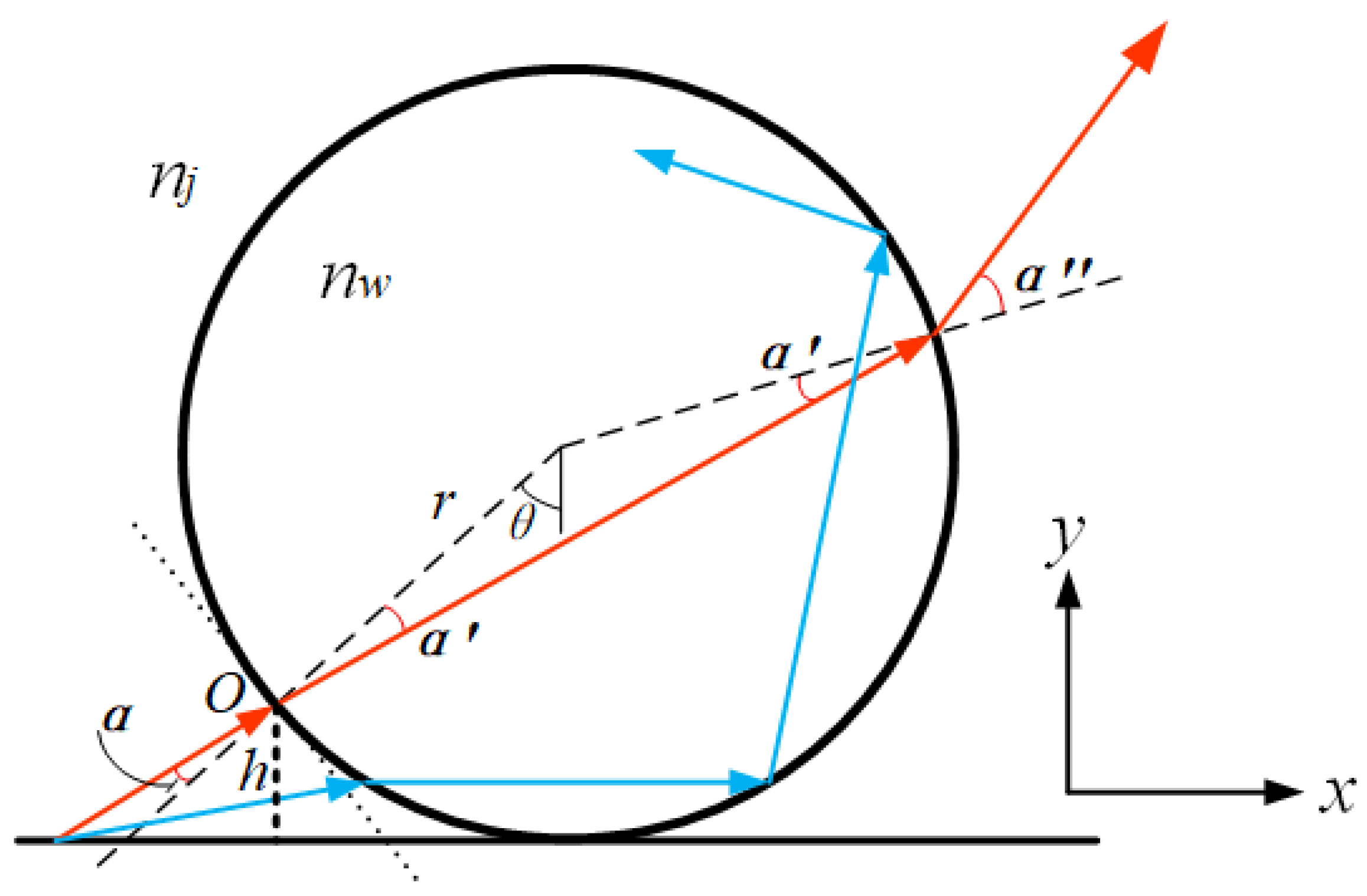
2. Microsphere Super-Resolution Imaging Principle
2.1. Theoretical Analysis of Microsphere Super-Resolution Imaging
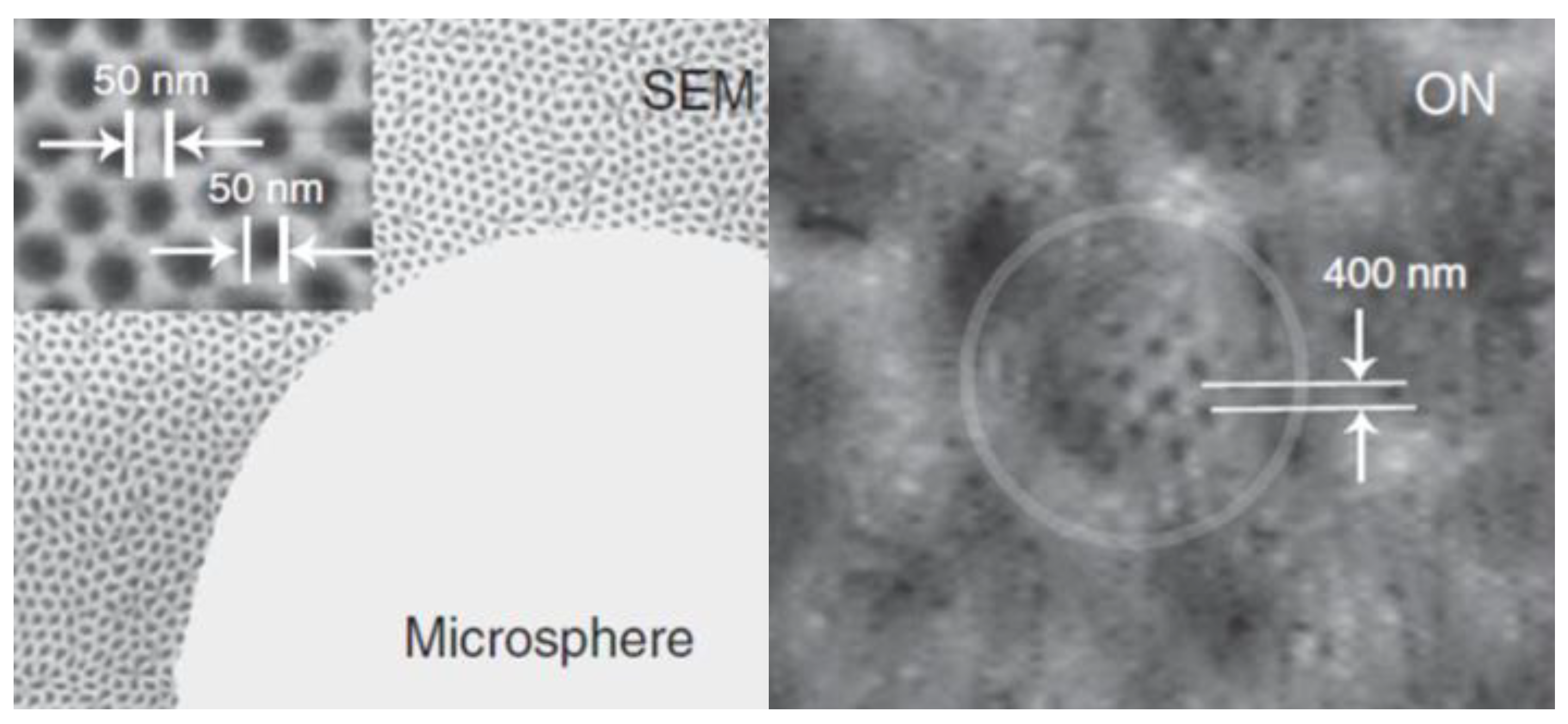
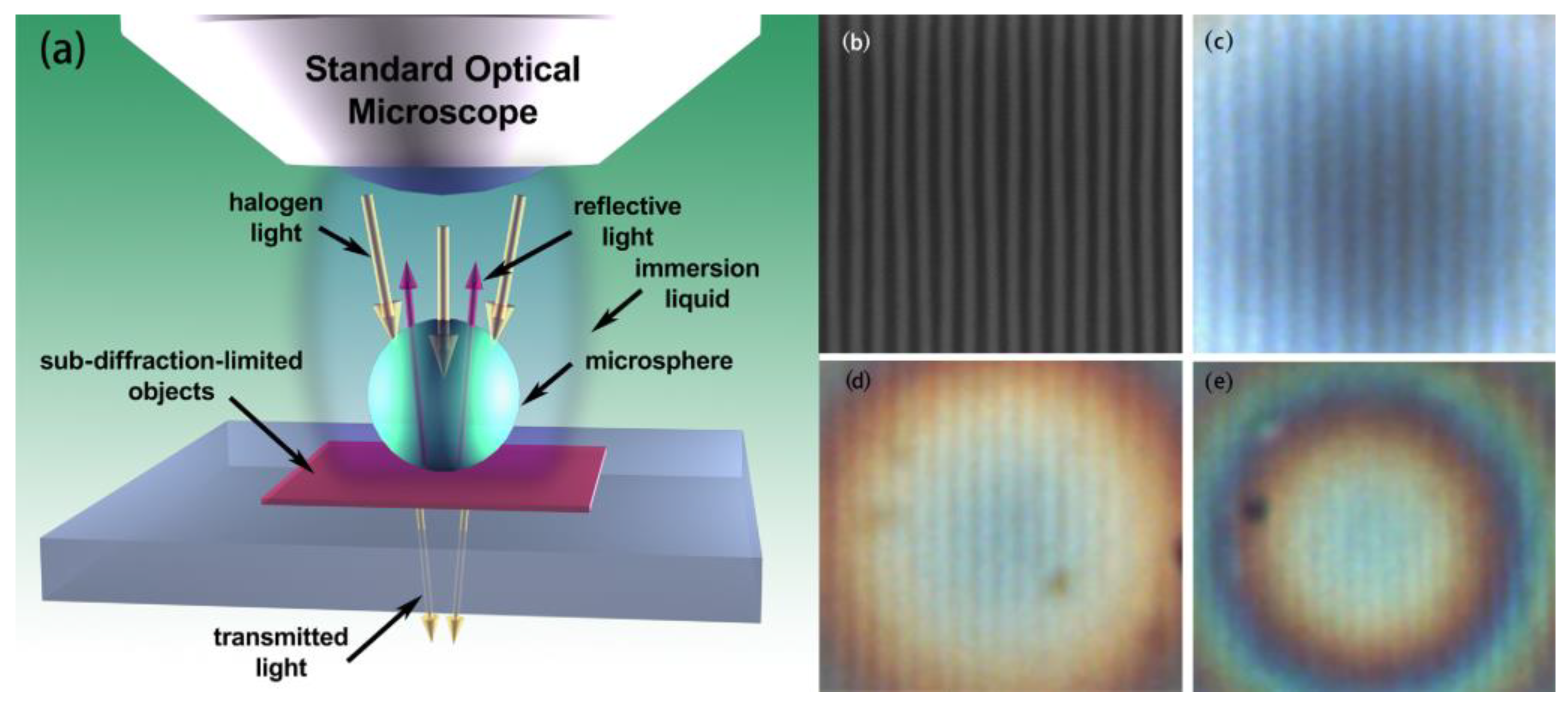
2.2. Microsphere Super-Resolution Imaging Modalities
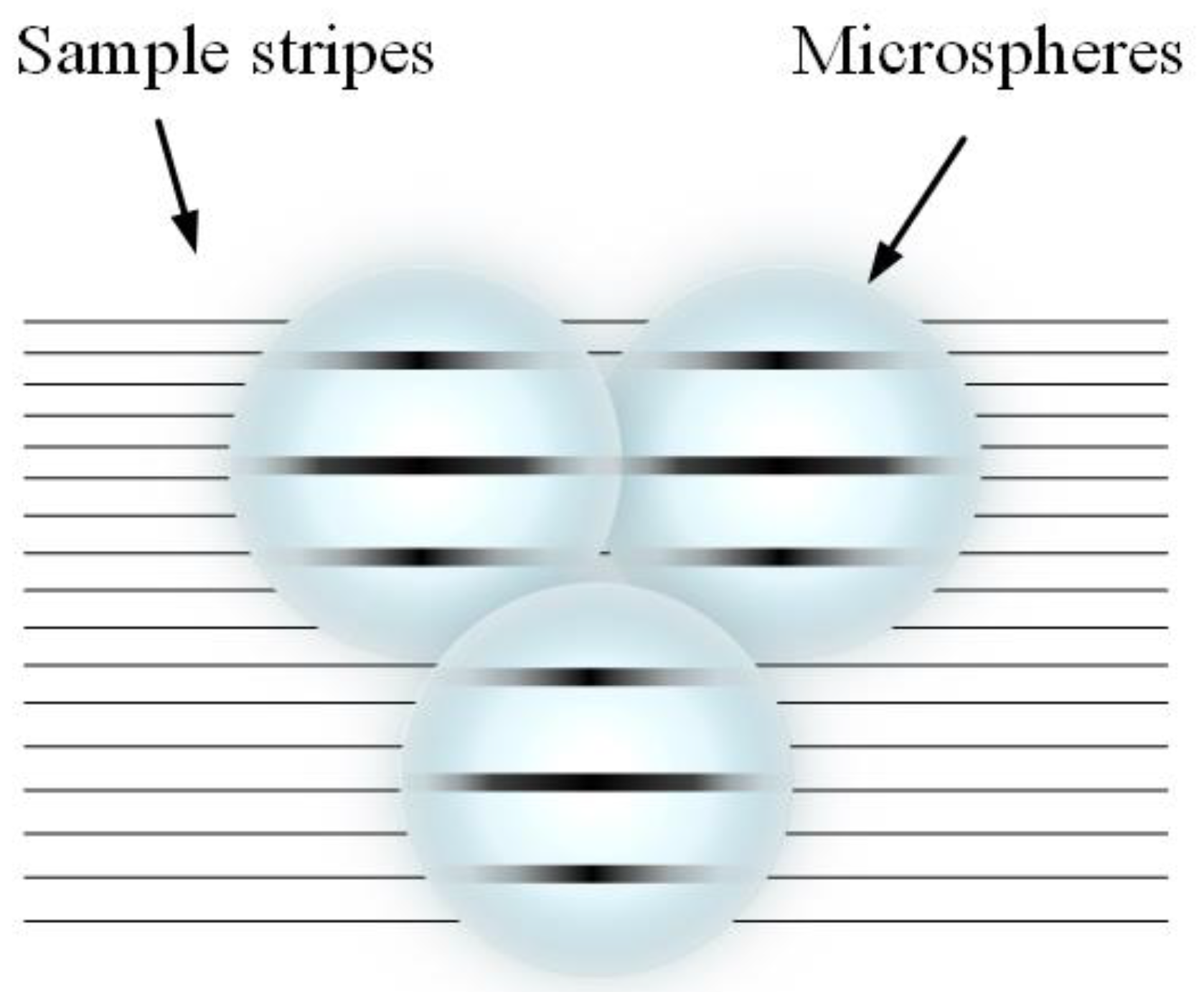
2.2.1. Microsphere-Based Imaging
2.2.2. Immersion Microsphere-Based Imaging
3. Microsphere Material and Preparation Process
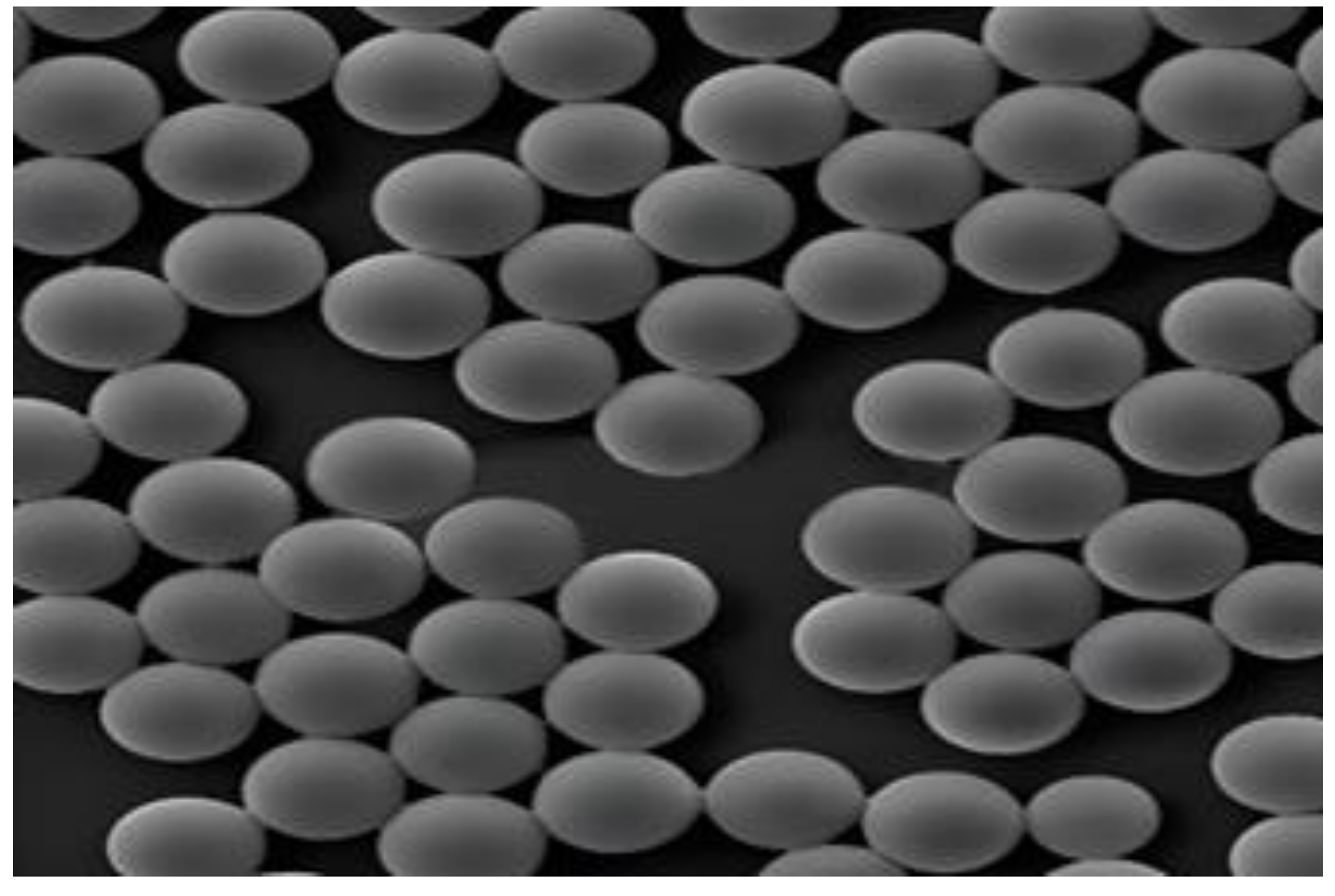
3.1. Microsphere Materials
3.2. Microsphere Preparation Process
4. Microsphere Manipulation
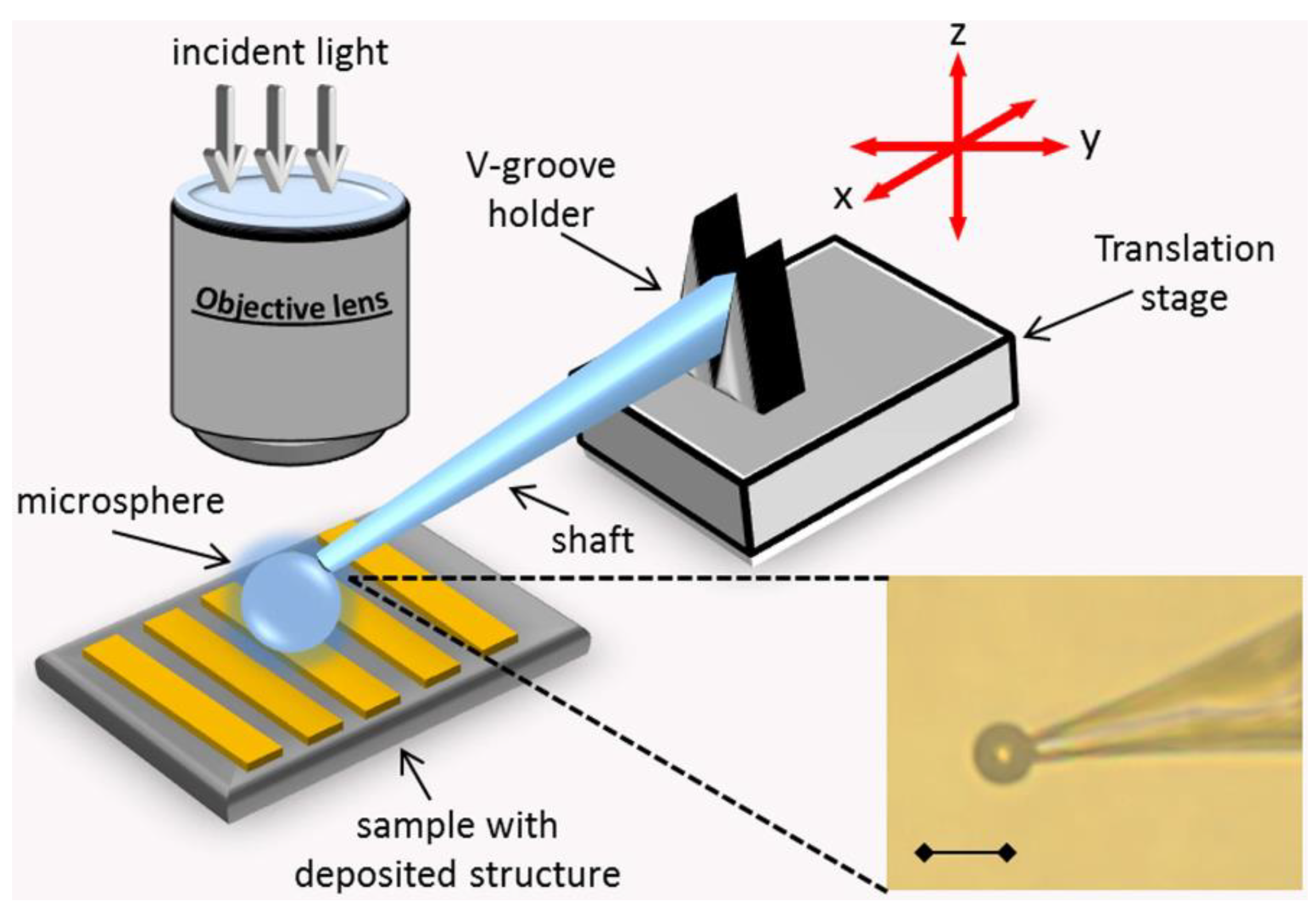
4.1. Mechanical Control Methods
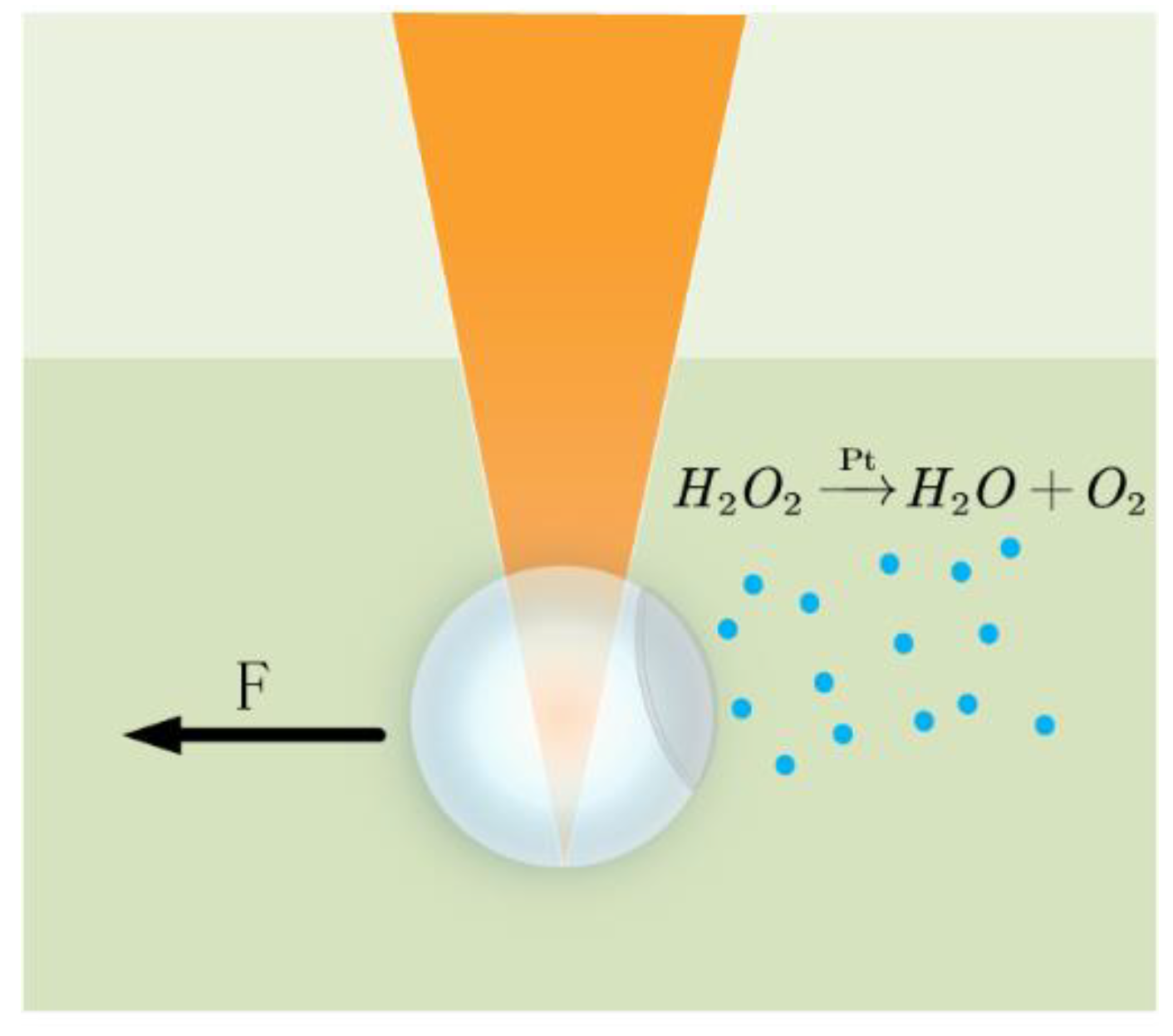
4.2. Chemical Manipulation Methods
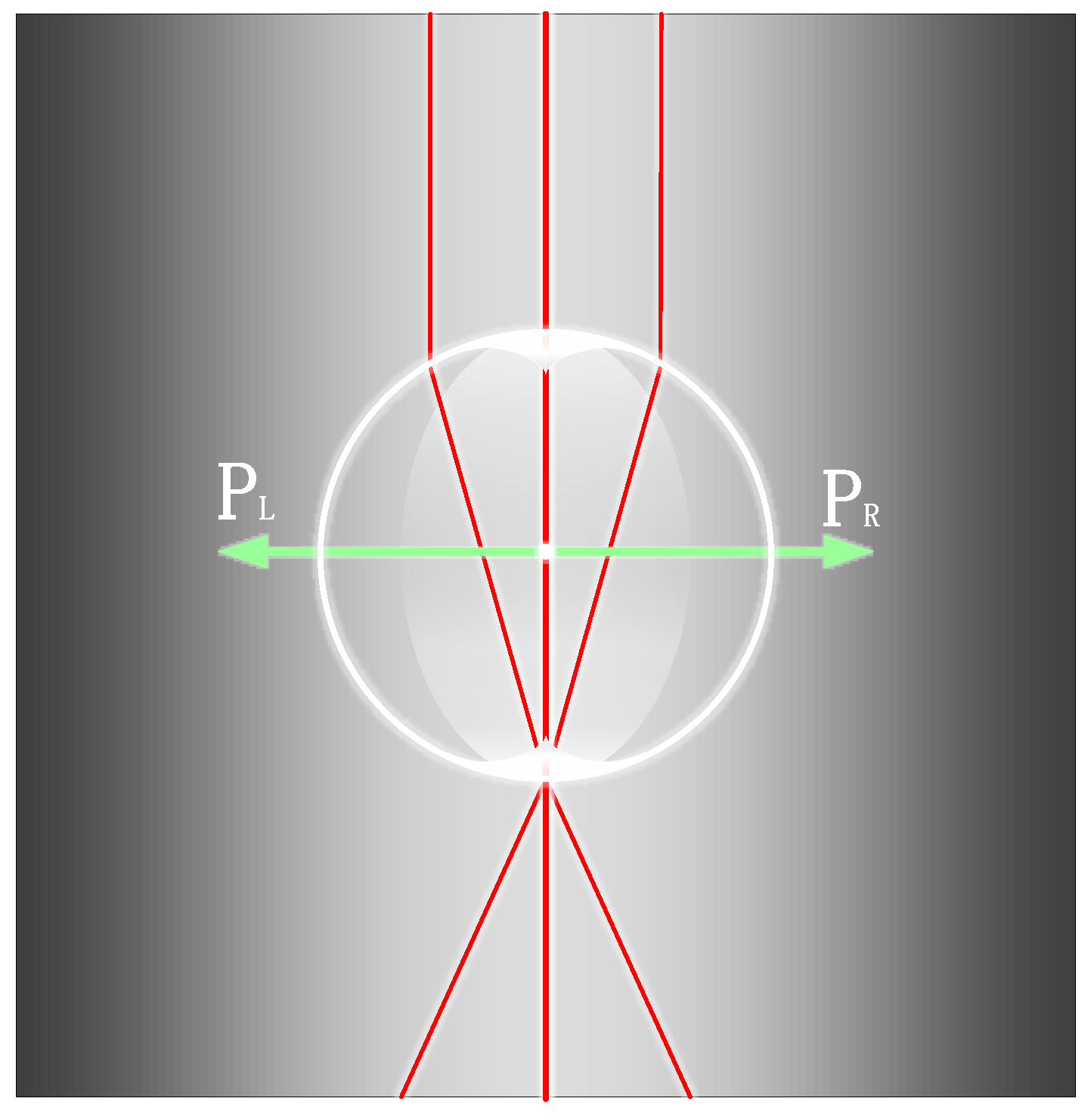
4.3. Optical Manipulation
5. Potential Application Areas
5.1. Nanodevices
- Observation of structural characteristics: The small size of components of nanoscale structures makes observation using traditional methods difficult. Microsphere imaging can help researchers observe and analyze small devices’ appearance, morphology, and size. For example, in 2021, Liu et al. [42] used microsphere-assisted super-resolution imaging technology to perform super-resolution imaging on multi-layer samples, proving that it is capable of fault scanning in the imaging of multi-layer structured samples. The same discovery was made in 2023, when Hong Minghui et al. [43] utilized the superior resolution and label-free properties of optical microsphere nano mirrors to uncover their enormous potential in detecting sample morphologies.
- Defect detection: The manufacture of nanodevices often has unavoidable small defects that are not visible using traditional imaging. Microsphere imaging enables better observation of the details of such tiny devices, with better detection and localization of these defects at the nanometer level. This can help manufacturers identify and resolve potential defects promptly to improve product quality. When using microsphere-assisted super-resolution imaging technology to scan and image samples, researchers can obtain a comprehensive view of the sample, thereby better realizing defect detection [42].
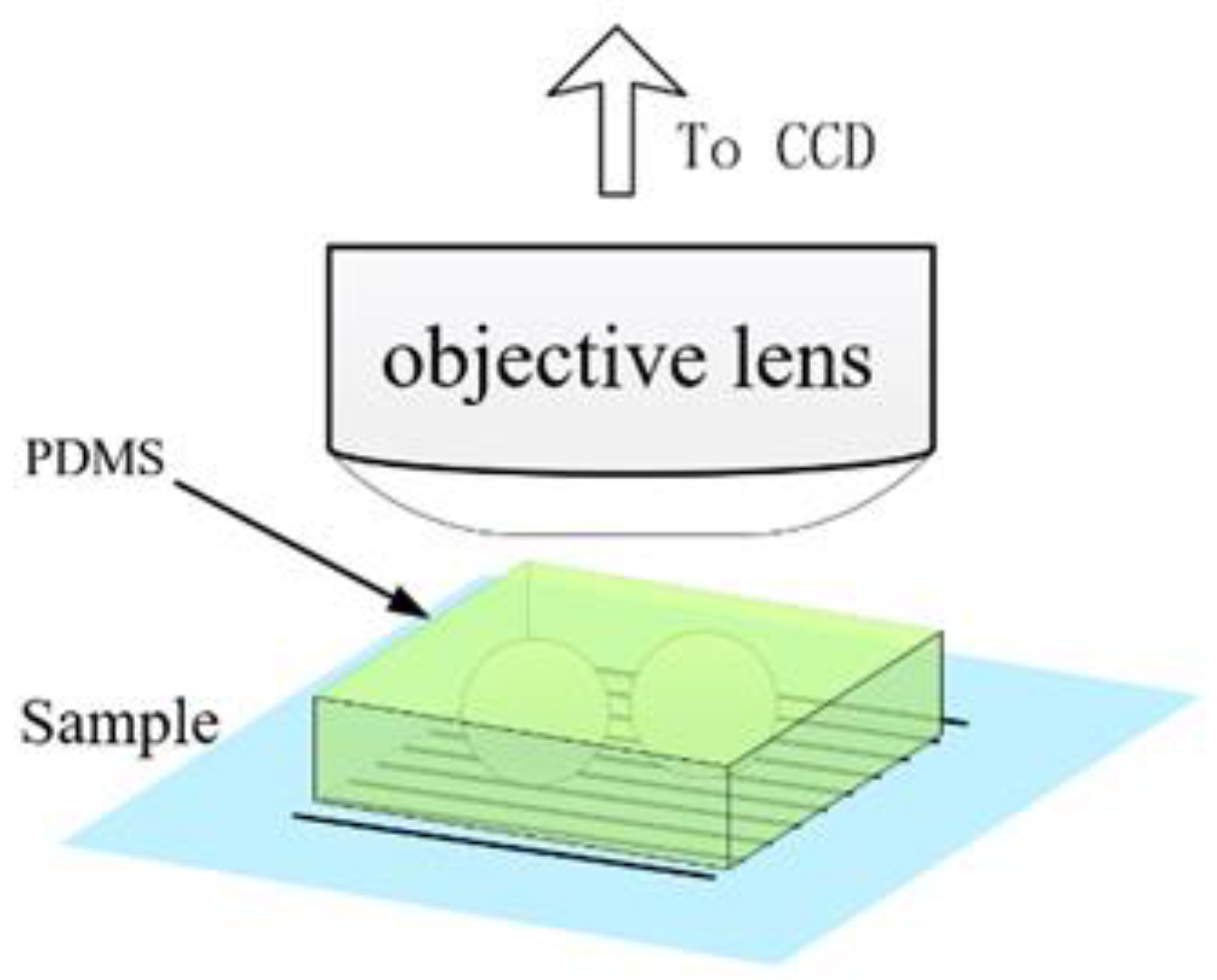
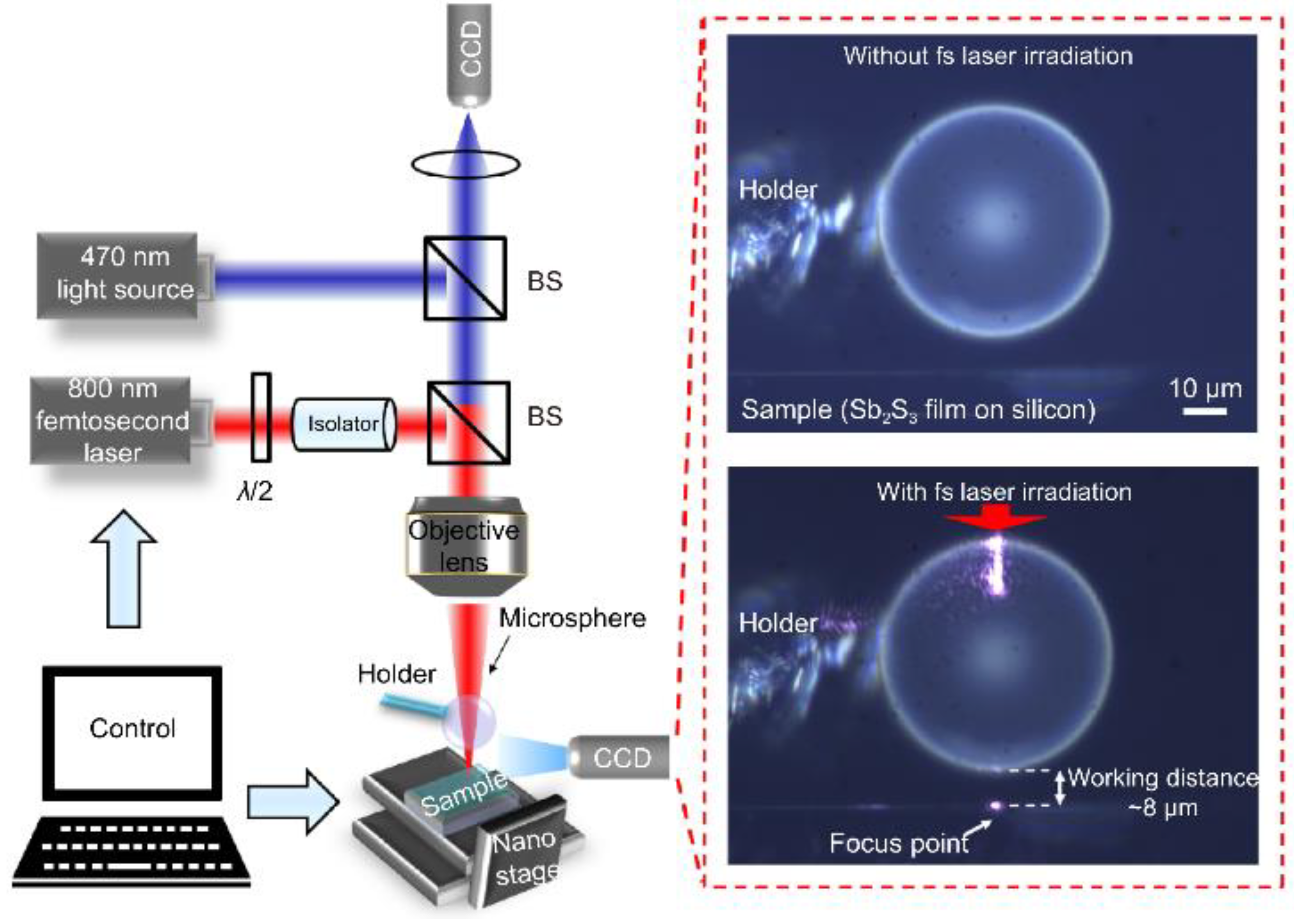
- Materials research: Microsphere imaging can be used to analyze nanomaterials. Nanomaterials have special properties such as quantum effects. Using microspheres, the interaction between nanomaterials and the surrounding environment can be observed and explored to promote the development of nanotechnology. As an example of contemporary research, academician Minghui Hong and the team of Dun Cao [44] jointly reported an ultrafast laser nanopatterning technique using non-contact microspheres in 2023. The experimental setup with a side view of a femtosecond laser beam focusing on the microspheres is shown in Figure 20. The team prepared nanogratings that demonstrated ideal beam diffraction properties. This nanoscale resolution has significant implications for next-generation laser nanolithography in both far field and ambient air.
5.2. Biomedicine
- Cellular imaging: Microsphere imaging is capable of discriminating fine tissue structures, and researchers can use this ability to conduct in-depth studies on the structure and function of cells. For example, in 2010, Arash Darafsheh et al. [45] used a gel-like medium to simulate the optical properties of biological tissues, as shown in Figure 21.
- 2.
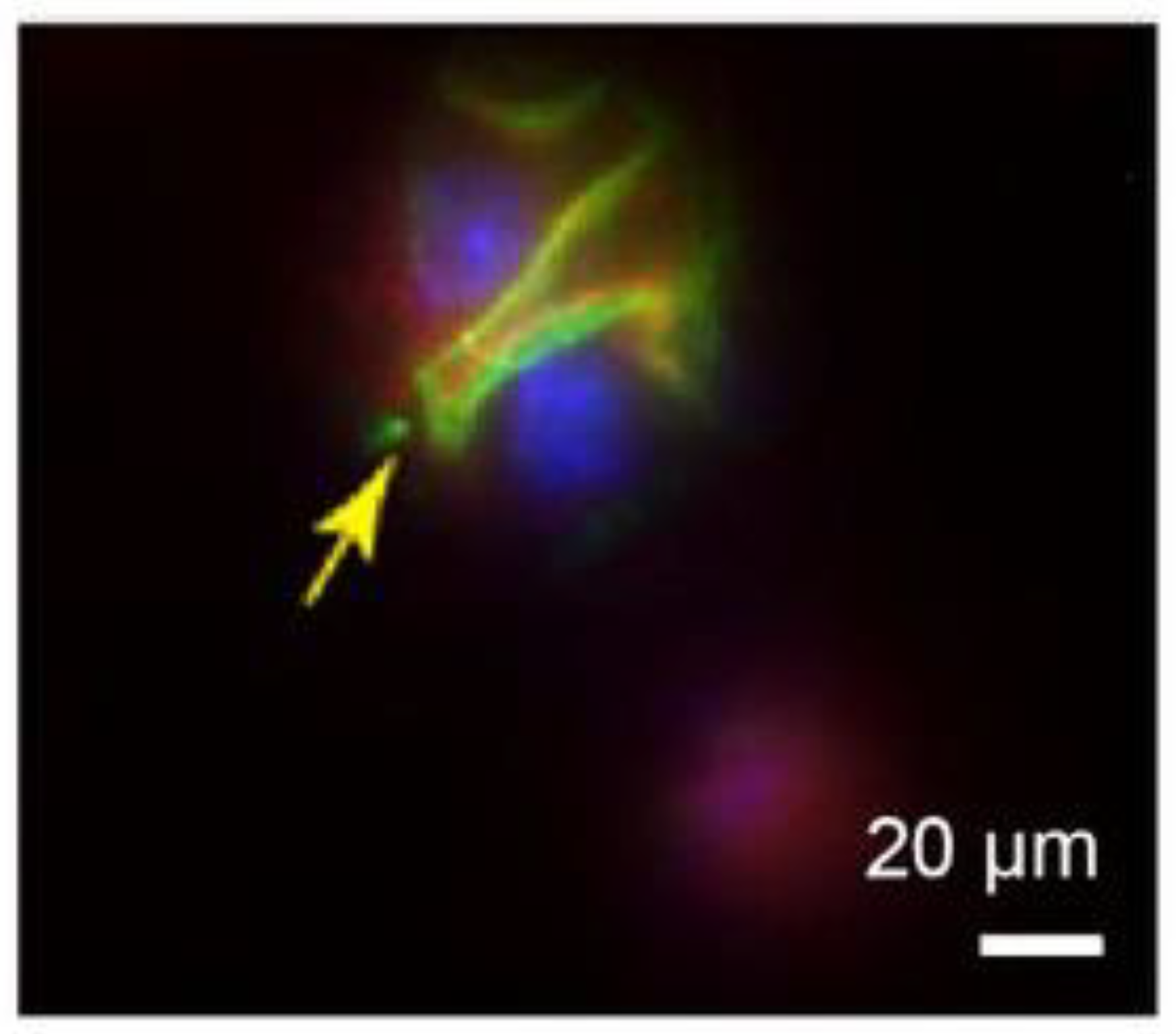
- Disease diagnosis: High-resolution imaging of tissues and organs by microsphere imaging can provide more accurate and detailed information helpful for the diagnosis and assessment of diseases along with the study of physiological and pathological processes of tissues and organs. For example, in 2015, Arash Darafsheh et al. [49] developed a biological super-resolution imaging method using microspheres embedded in cover slips. Immunostaining was performed on renal slices, and significant enhancement of protein distribution patterns was observed in the stained glomeruli using microsphere-assisted super-resolution imaging technology. The stained glomeruli are shown in Figure 23. This method can be used to observe visceral details and diagnose diseases accordingly.
- 3.
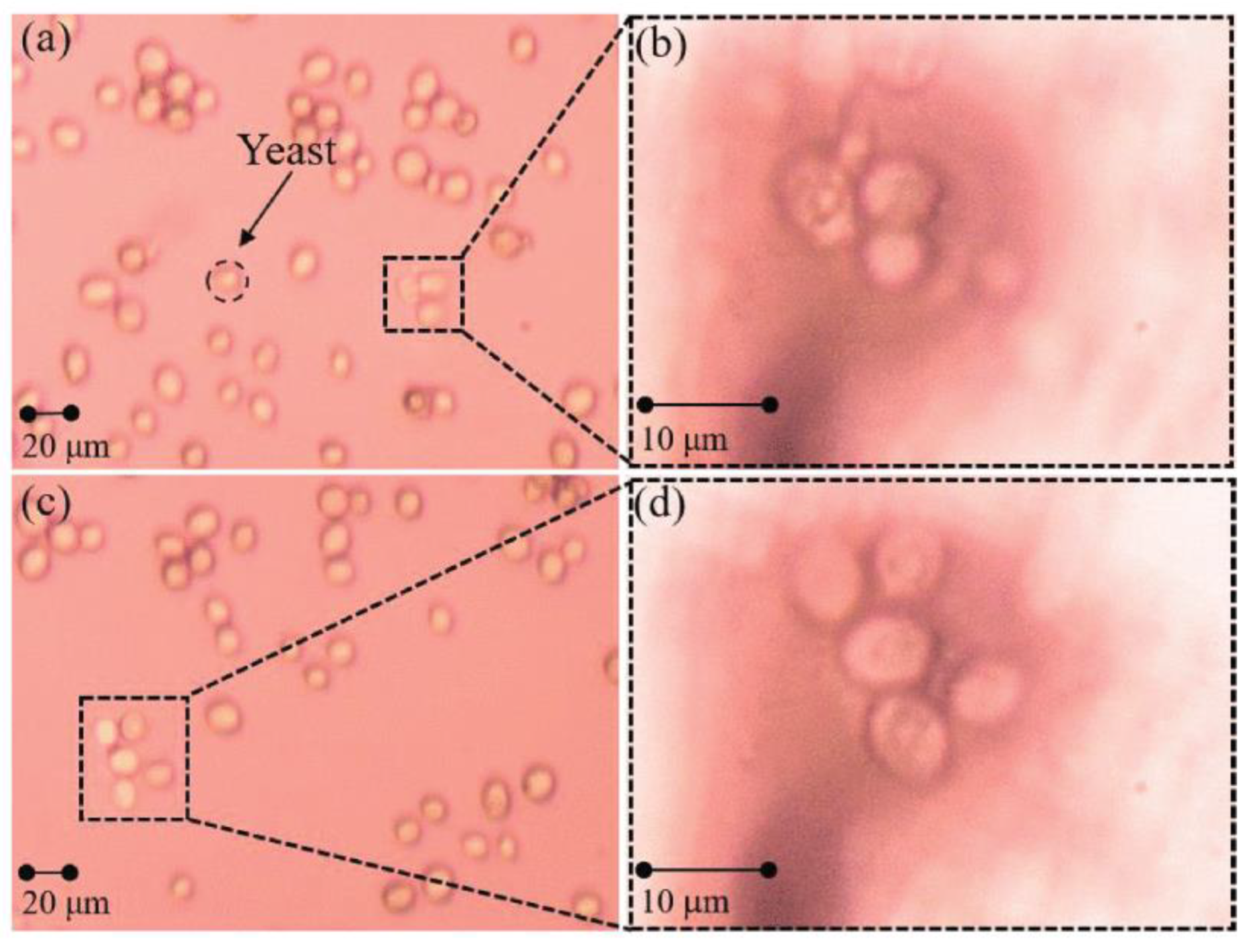
- Biomedical research and innovation: Microsphere imaging technology goes beyond the limits of traditional imaging, improving the contrast and clarity of images and producing higher-resolution output. These improvements help researchers to understand biological mechanisms more fully and to promote the development and innovation in the field of biomedicine. For example, in 2019, Gao Shilin et al. [50] reported that, in addition to biological sectioning, probe-bound microspheres can also be imaged on free cells in water. Using a yeast system as the experimental object, the enlarged results are shown in Figure 24.
5.3. Semiconductors
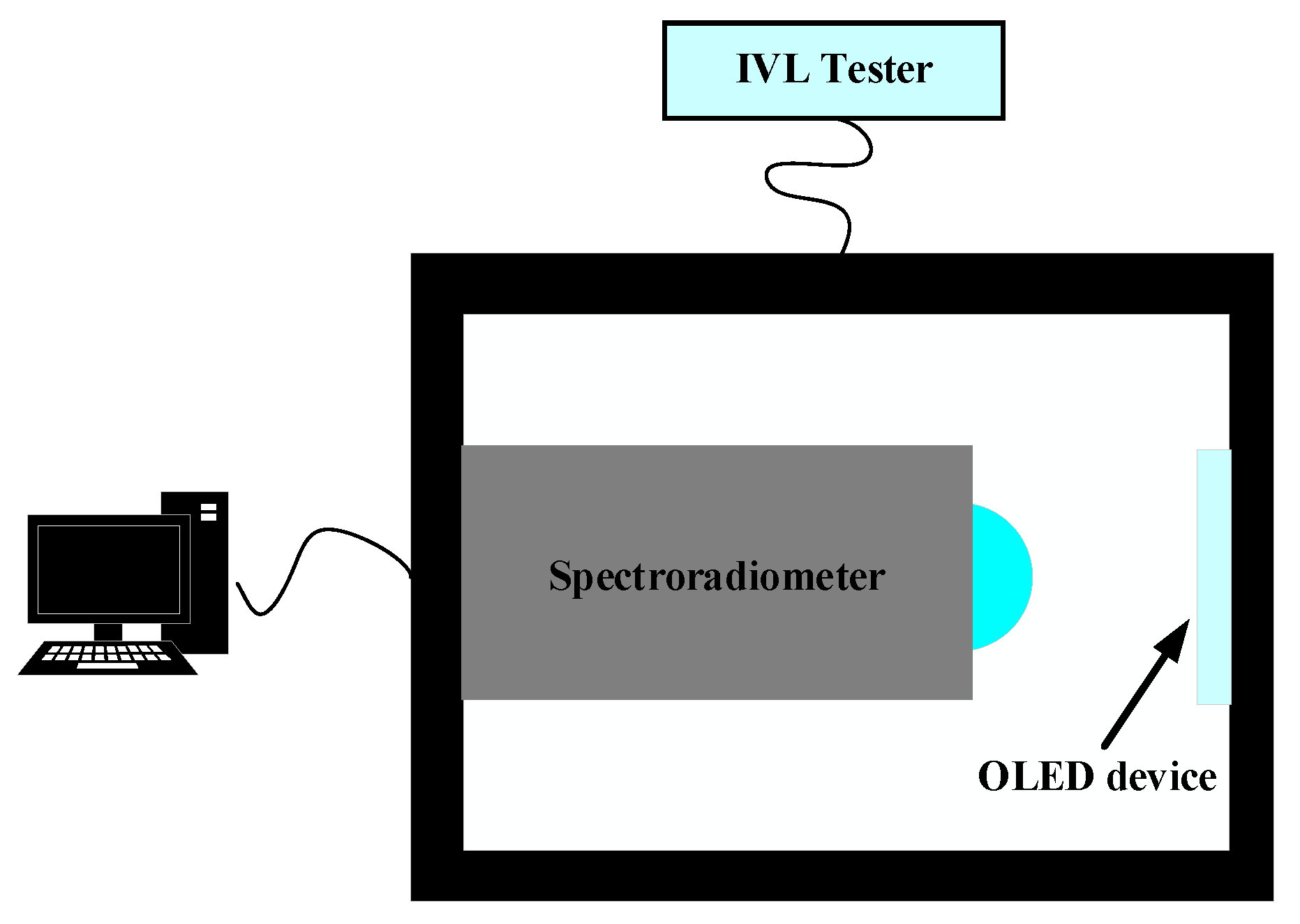
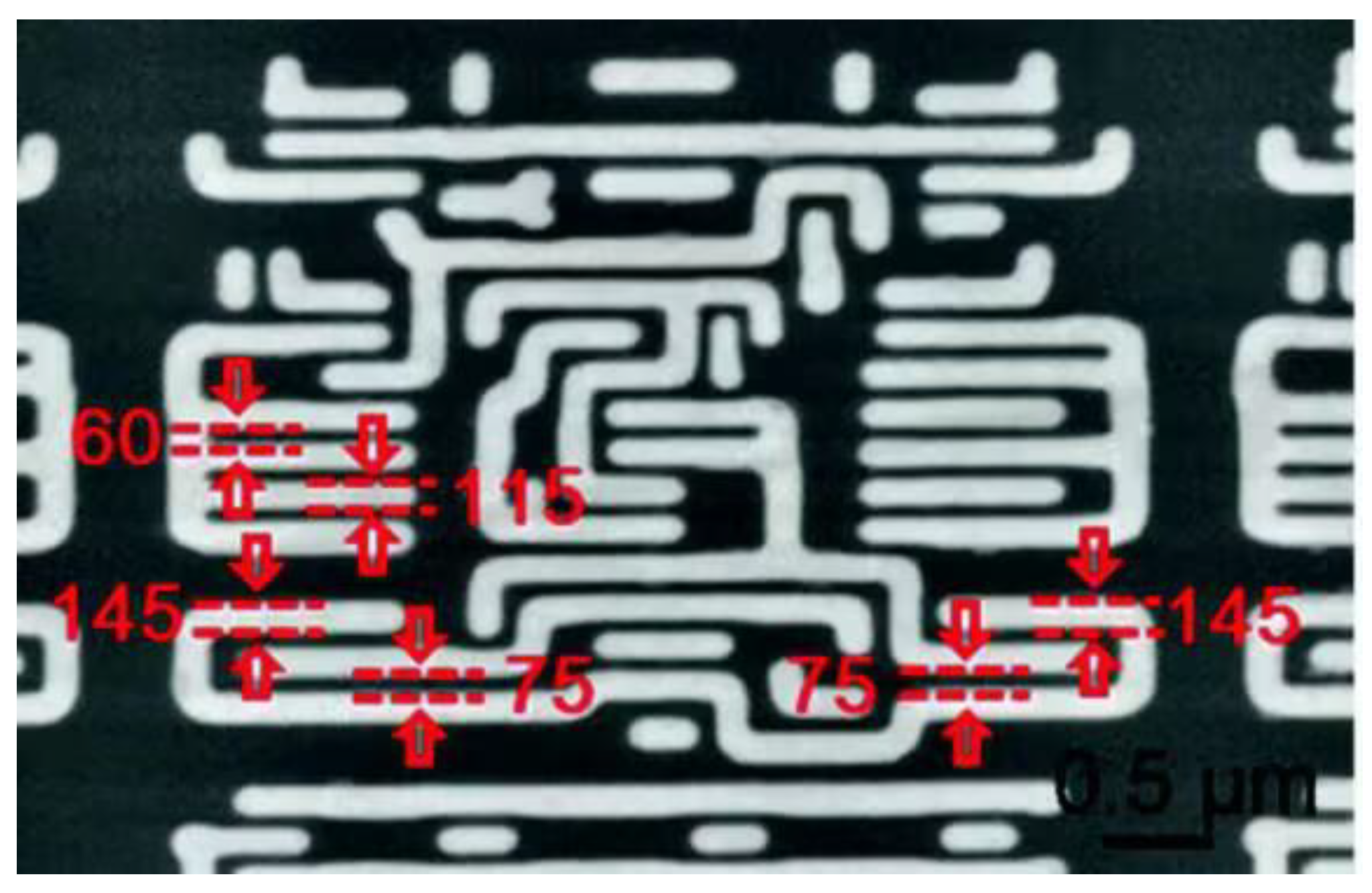
- Chip performance analysis: Microsphere imaging can be used to probe the emission and absorption properties of photons in semiconductor materials, and it can also be used to characterize, analyze, and even visualize chip structures, so as to obtain more accurate information about chip performance. In 2023, Hong Minghui et al. [43] utilized the superior resolution and label-free properties of optical microsphere nano mirrors to detect integrated circuit chips in the semiconductor industry and to detect morphological characterization in the field of biology. It was proven that a microscale composite lens configuration composed of ultra-half microspheres can achieve optical imaging below 3 nm in ambient air when coupled with an objective lens (NA = 10.0), thereby improving the imaging ability of traditional microscopes by about an order of magnitude.
- Assisted lithography: Lithography is an important step in the semiconductor manufacturing process. The use of microsphere imaging can assist lithography to improve the performance of semiconductor devices.
- Wafer surface flatness detection: In the chip manufacturing process, the rough surface of a cut wafer surface needs to be ground and polished in a series of processes before it can be used for subsequent operations. Microsphere imaging has a wide field of view and can effectively examine wafers. For example, Wu et al. [52] demonstrated the use of microsphere lithography technology to manufacture and characterize nanopillar arrays on GaN substrates in their work. This confirms the applicability of microsphere-assisted super-resolution imaging technology on wafers.
6. Research Trends
- Understanding the mechanism of microsphere imaging
- 2.
- Development of more microsphere materials and optimization of preparation processes
- 3.
- Building new types of control methods
- 4.
- Expanding areas of application
- 5.
- Addressing the limitations of microsphere-based super-resolution imaging systems
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abbe, E. Beiträge zur theorie des mikroskops und der mikroskopischen wahrnehmung. Arch. Mikrosk. Anat. 1873, 9, 413–418. [Google Scholar] [CrossRef]
- Rayleigh, L. Investigations in optics, with special reference to the spectroscope. Philos. Mag. J. Sci. 1879, 8, 261–274. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Y.; Guo, Y.; Xie, W.; Yang, Z.; Weng, X.; Yan, W.; Qu, J. Low-power STED nanoscopy based on temporal and spatial modulation. Nano Res. 2022, 15, 3479–3486. [Google Scholar] [CrossRef]
- Liu, Y.; Peng, Z.; Peng, X.; Yan, W.; Yang, Z.; Qu, J. Shedding new lights into STED microscopy: Emerging nanoprobes for imaging. Front. Chem. 2021, 9, 641330. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Hong, B.H.; Kim, W.Y.; Min, S.K.; Kim, Y.; Jouravlev, M.V.; Bose, R.; Kim, K.S.; Hwang, I.-C.; Kaufman, L.J.; et al. Near-field focusing and magnification through self-assembled nanoscale spherical lenses. Nature 2009, 460, 498–501. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, W.; Li, L.; Luk’Yanchuk, B.; Khan, A.; Liu, Z.; Chen, Z.; Hong, M. Optical virtual imaging at 50 nm lateral resolution with a white-light nanoscope. Nat. Commun. 2011, 2, 218. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Li, L.; Ben-Aryeh, Y.; Wang, Z.; Guo, W. Overcoming the diffraction limit induced by microsphere optical nanoscopy. J. Opt. 2013, 15, 125710. [Google Scholar] [CrossRef]
- Lee, S.; Li, L.; Wang, Z. Optical resonances in microsphere photonic nanojets. J. Opt. 2013, 16, 015704. [Google Scholar] [CrossRef]
- Yan, Y.; Li, L.; Feng, C.; Guo, W.; Lee, S.; Hong, M. Microsphere-coupled scanning laser confocal nanoscope for sub-diffraction-limited imaging at 25 nm lateral resolution in the visible spectrum. ACS Nano 2014, 8, 1809–1816. [Google Scholar] [CrossRef]
- Lee, S.; Li, L. Rapid super-resolution imaging of sub-surface nanostructures beyond diffraction limit by high refractive index microsphere optical nanoscopy. Opt. Commun. 2015, 334, 253–257. [Google Scholar] [CrossRef]
- Wang, F.; Liu, L.; Yu, P.; Liu, Z.; Yu, H.; Wang, Y.; Li, W.J. Three-dimensional super-resolution morphology by near-field assisted white-light interferometry. Sci. Rep. 2016, 6, 24703. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, L.; Yu, H.; Wen, Y.; Yu, P.; Liu, Z.; Wang, Y.; Li, W.J. Scanning superlens microscopy for non-invasive large field-of-view visible light nanoscale imaging. Nat. Commun. 2016, 7, 13748. [Google Scholar] [CrossRef] [PubMed]
- Lai HS, S.; Wang, F.; Li, Y.; Jia, B.; Liu, L.; Li, W.J. Super-resolution real imaging in microsphere-assisted microscopy. PLoS ONE 2016, 11, e0165194. [Google Scholar]
- Yan, B.; Wang, Z.; Parker, A.L.; Lai, Y.-K.; Thomas, P.J.; Yue, L.; Monks, J.N. Superlensing microscope objective lens. Appl. Opt. 2017, 56, 3142–3147. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.W.; Zhou, Y.; Wu, M.X.; Hong, M.H. Remote-mode microsphere nano-imaging: New boundaries for optical microscopes. Opto-Electron. Adv. 2018, 1, 170001. [Google Scholar] [CrossRef]
- Yan, B.; Song, Y.; Yang, X.; Xiong, D.; Wang, Z. Unibody microscope objective tipped with a microsphere: Design, fabrication, and application in subwavelength imaging. Appl. Opt. 2020, 59, 2641–2648. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.W.; Zhou, Y.; Zhou, R.; Hong, M.H. Microsphere-toward future of optical microscopes. iScience 2020, 23, 101211. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Ye, A. Microsphere assisted optical super-resolution imaging with narrowband illumination. Opt. Commun. 2021, 485, 126658. [Google Scholar] [CrossRef]
- Lin, Q.W.; Liu, H.M.; Kang, Y.Q. Investigation of far-field super-resolution imaging by microsphere-based optical microscopy. Opt. Lasers Eng. 2021, 144, 106644. [Google Scholar] [CrossRef]
- Wang, J.; Yang, S.; Wang, X.; Cao, Y. Improved performance in microsphere-assisted 2D and 3D imaging by polystyrene microspheres semi-immersed in su-8 resist. AIP Adv. 2022, 12, 035302. [Google Scholar] [CrossRef]
- Hao, X.; Kuang, C.; Liu, X.; Zhang, H.; Li, Y. Microsphere based microscope with optical super-resolution capability. Appl. Phys. Lett. 2011, 99, 203102. [Google Scholar] [CrossRef]
- Darafsheh, A.; Walsh, G.F.; Negro, L.D.; Astratov, V.N. Optical super-resolution by high-index liquid-immersed microspheres. Appl. Phys. Lett. 2012, 101, 141128. [Google Scholar] [CrossRef]
- Lee, S.; Li, L.; Wang, Z.; Guo, W.; Yan, Y.; Wang, T. Immersed transparent microsphere magnifying sub-diffraction-limited objects. Appl. Opt. 2013, 52, 7265–7270. [Google Scholar] [CrossRef]
- Hao, X.; Kuang, C.; Li, Y.; Liu, X.; Ku, Y.; Jiang, Y. Hydrophilic microsphere based mesoscopic-lens microscope (MMM). Opt. Commun. 2012, 285, 4130–4133. [Google Scholar] [CrossRef]
- Allen, K.W.; Farahi, N.; Li, Y.; Limberopoulos, N.I.; Walker, D.E.; Urbas, A.M.; Astratov, V.N. Super-resolution imaging by arrays of high-index spheres embedded in transparent matrices. In Proceedings of the IEEE National Aerospace and Electronics Conference (NAECON), Univ Daytons River Campus, Dayton, OH, USA, 24–27 June 2014; pp. 50–52. [Google Scholar]
- Allen, K.W.; Farahi, N.; Li, Y.; Limberopoulos, N.I.; Walker, D.E.; Urbas, A.M.; Astratov, V.N. Overcoming the diffraction limit of imaging nanoplasmonic arrays by microspheres and microfibers. Opt. Express 2015, 23, 24484–24496. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.W.; Farahi, N.; Li, Y.; Limberopoulos, N.I.; Walker, D.E., Jr.; Urbas, A.M.; Liberman, V.; Astratov, V.N. Super-resolution microscopy by movable thin-films with embedded microspheres: Resolution analysis. Ann. Phys. 2015, 527, 513–522. [Google Scholar] [CrossRef]
- Allen, K.W.; Liberman, V.; Rothschild, M.; Limberopoulos, N.I.; Walker, D.E.; Urbas, A.M.; Astratov, V.N. Deep-UV microsphere-assisted ultramicroscopy. In Proceedings of the 17th International Conference on Transparent Optical Networks (ICTON), Budapest, Hungary, 5–9 July 2015; pp. 1–4. [Google Scholar]
- Pang, H.; Du, C.; Qiu, Q.; Deng, Q.; Zhang, M.; Yin, S. Investigation of microsphere based thin-film for super-resolution imaging. Acta Photonica Sin. 2015, 44, 426004. (In Chinese) [Google Scholar] [CrossRef]
- Darafsheh, A.; Guardiola, C.; Palovcak, A.; Finlay, J.C.; Cárabe, A. Optical super-resolution imaging by high-index microspheres embedded in elastomers. Opt. Lett. 2015, 40, 5–8. [Google Scholar] [CrossRef]
- Yao, L.; Ye, Y.-H.; Ma, H.F.; Cao, L.; Hou, J. Role of the immersion medium in the microscale spherical lens imaging. Opt. Commun. 2015, 335, 23–27. [Google Scholar] [CrossRef]
- Ye, R.; Ye, Y.H.; Ma, H.F.; Ma, J.; Wang, B.; Yao, J.; Liu, S.; Cao, L.; Xu, H.; Zhang, J.Y. Experimental far-field imaging properties of a ~5-μm diameter spherical lens. Opt. Lett. 2013, 38, 1829–1831. [Google Scholar] [CrossRef]
- Ye, R.; Ye, Y.-H.; Ma, H.F.; Cao, L.; Ma, J.; Wyrowski, F.; Shi, R.; Zhang, J.-Y. Experimental imaging properties of immersion microscale spherical lenses. Sci. Rep. 2014, 4, 3769. [Google Scholar] [CrossRef] [PubMed]
- Ling, J.; Li, D.; Wang, X. Influence of refractive index contrast between microsphere lens and immersion medium on imaging resolution. Optik 2023, 291, 171267. [Google Scholar] [CrossRef]
- Yin, J.; Chen, H.; Li, Z.; Qian, X.; Yin, J.; Shi, M.; Zhou, G. Preparation of PS/TiO2 core-shell microspheres and TiO2 hollow shells. J. Mater. Sci. 2003, 38, 4911–4916. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, C.K.; Jang, H.W. Fabrication of a microball lens array for OLEDs fabricated using a monolayer microsphere template. Electron. Mater. Lett. 2013, 9, 39–42. [Google Scholar] [CrossRef]
- Wang, S.Y.; Zhang, H.J.; Zhang, D.X. Location-free optical microscopic imaging method with high-resolution based on microsphere superlenses. Acta Phys. Sin. 2013, 62, 034207. (In Chinese) [Google Scholar] [CrossRef]
- Krivitsky, L.A.; Wang, J.J.; Wang, Z.; Luk’Yanchuk, B. Locomotion of microspheres for super-resolution imaging. Sci. Rep. 2013, 3, 3501. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, D.; Zhang, H.; Han, X.; Xu, R. Super-resolution optical microscopy based on scannable cantilever-combined microsphere. Microsc. Res. Tech. 2015, 78, 1128–1132. [Google Scholar] [CrossRef]
- Li, J.; Liu, W.; Li, T.; Rozen, I.; Zhao, J.; Bahari, B.; Kante, B.; Wang, J. Swimming microrobot optical nanoscopy. Nano Lett. 2016, 16, 6604–6609. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Li, B. Single-cell biomagnifier for optical nanoscopes and nanotweezers. Light Sci. Appl. 2019, 8, 61. [Google Scholar] [CrossRef]
- Liu, C.; Ye, A. Super-resolution imaging on multilayer sample by microsphere-assisted microscope. Optik 2022, 247, 167889. [Google Scholar] [CrossRef]
- Wu, G.; Zhou, Y.; Hong, M. Sub-50 nm optical imaging in ambient air with 10× objective lens enabled by hyper-hemi-microsphere. Light Sci. Appl. 2023, 12, 49. [Google Scholar] [CrossRef]
- Lin, Z.; Liu, K.; Cao, T.; Hong, M. Microsphere femtosecond laser sub-50 nm structuring in far field via non-linear absorption. Opto-Electron. Adv. 2023, 6, 230029. [Google Scholar] [CrossRef]
- Astratov, V.N.; Darafsheh, A.; Kerr, M.D.; Allen, K.W.; Fried, N.M.; Antoszyk, A.N.; Ying, H.S. Photonic nanojets for laser surgery. SPIE Newsroom 2010, 12, 32–34. [Google Scholar] [CrossRef]
- Li, L.; Guo, W.; Yan, Y.; Lee, S.; Wang, T. Label-free super-resolution imaging of adenoviruses by submerged microsphere optical nanoscopy. Light Sci. Appl. 2013, 2, e104. [Google Scholar] [CrossRef]
- Darafsheh, A.; Guardiola, C.; Finlay, J.; Cárabe, A.; Nihalani, D. Simple super-resolution biological imaging. SPIE Newsroom 2015, 29, 1–3. [Google Scholar] [CrossRef]
- Wang, F.; Lai, H.S.S.; Liu, L.; Li, P.; Yu, H.; Liu, Z.; Wang, Y.; Li, W.J. Super-resolution endoscopy for real-time wide-field imaging. Opt. Express 2015, 23, 16803–16811. [Google Scholar] [CrossRef]
- Darafsheh, A.; Guardiola, C.; Nihalani, D.; Lee, D.; Finlay, J.C.; Cárabe, A. Biological super-resolution imaging by using novel microsphere-embedded coverslips. In Proceedings of the Nanoscale Imaging, Sensing, and Actuation for Biomedical Applications XII, San Francisco, CA, USA, 9–12 February 2015; p. 933705. [Google Scholar]
- Gao, S.; Meng, K.; Yang, Z.; Liu, H.; Wang, F.; Sun, L.; Chen, T. The probe-combined microspheres applied in biomedical field for super-resolution imagings and micromanipulations. In Proceedings of the 4th Asia-Pacific Conference on Intelligent Robot Systems (ACIRS), Nagoya, Japan, 13–15 July 2019; pp. 155–158. [Google Scholar]
- Zhu, H.; Yan, B.; Zhou, S.; Wang, Z.; Wu, L. Synthesis and super-resolution imaging performance of a refractive-index-controllable microsphere superlens. J. Mater. Chem. C 2015, 3, 10907–10915. [Google Scholar] [CrossRef]
- Ng, W.N.; Wang, X.H.; Leung, C.H.; Lai, P.T.; Choi, H.W. Nanopatterning GaN with microspheres. In Proceedings of the 7th International Conference on Nitride Semiconductors (ICNS-7), Las Vegas, NV, USA, 16–21 September 2008; pp. 1615–1617. [Google Scholar]










| Super-Resolution Imaging | Imaging Speed | Sample Processing Requirements | Field of Application |
|---|---|---|---|
| STED | Slow | High | Samples are often labeled and fixed for use in areas such as biomedical and materials science. |
| SIM | Fast | Low | Samples are typically less demanding to prepare; suitable for cell biology and neuroscience. |
| SMLM | Slow | High | Often requires special labeling and handling of the sample; suitable for fields such as single molecule level localization tracking. |
| Microspheres | Fast | Low | Suitable for all types of samples, including fixed samples and biological cells. |
| Sphere | n = 1.33 | n = 1.38 | n = 1.548 |
|---|---|---|---|
| SiO2 | 1.8× | 1.67× | 1.44× |
| BaTiO3 | 3.96× | 2.88× | 2.11× |
| Microsphere Types | Transmittance | Index of Refraction | Chemical Inertness | Biocompatibility |
|---|---|---|---|---|
| Silicon dioxide microspheres (SiO2) | Highest | Lowest | Best | Good |
| Barium titanate microspheres (BaTiO3) | Medium | Highest | Bad | Medium |
| Polystyrene Microspheres ((C8H8)n) | High | Low | Good | Bad |
| Preparation Method | Advantages | Disadvantages |
|---|---|---|
| Hydrothermal method | Simple operation, controllable structure, morphology, and purity of the prepared microspheres. | The preparation process requires high temperature and high pressure, and many organic compounds are required as reaction materials, resulting in high cost and energy consumption. The purity and uniformity of microspheres will be affected. |
| Template method | The preparation process is simple, and the morphology and size of the prepared microspheres are controllable, with good performance. Large molds can be used for large-scale preparation. | When preparing microspheres, it is necessary to prepare the mold in advance. If different sizes of microspheres are needed, a separate mold needs to be prepared, which is not easy to adjust, and the disinfection and cleaning of the mold are more complicated. |
| Sol–gel method | Easy to operate, fewer side effects, and easy to control the process. | During the preparation process, microspheres are prone to aggregation and adhesion, and the monodispersity of the prepared microspheres is poor, requiring a longer preparation time. |
| Method | Advantages | Disadvantages |
|---|---|---|
| Mechanical | The microspheres can be precisely maneuvered to the target position above the sample to be observed, flexibly maneuvered to move during observation, and flexibly withdrawn at the end of the observation to avoid contamination of the sample. | These methods have a relatively large number of components and complex processes that result in unpredictable deviations in tool materials and operations. |
| Chemical | It is possible to move the microspheres to the destination location from a distance without contact using a chemical reaction. | The metal layer on the microsphere required by the method reduces image quality. |
| Optical | It is possible to control the movement of microspheres to their destination position without contact and destruction using optical tweezers technology. | Microsphere characteristics such as refractive index affect both optical capture efficiency and imaging performance. Additional problems include a limited imaging field of view, a high cost of equipment, and a complicated optical path design in optical tweezers technology. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, W.; Wang, J.; Yang, Y.; Bu, Y. A Review of Microsphere Super-Resolution Imaging Techniques. Sensors 2024, 24, 2511. https://doi.org/10.3390/s24082511
Jiang W, Wang J, Yang Y, Bu Y. A Review of Microsphere Super-Resolution Imaging Techniques. Sensors. 2024; 24(8):2511. https://doi.org/10.3390/s24082511
Chicago/Turabian StyleJiang, Wenbo, Jingchun Wang, Yidi Yang, and Yun Bu. 2024. "A Review of Microsphere Super-Resolution Imaging Techniques" Sensors 24, no. 8: 2511. https://doi.org/10.3390/s24082511
APA StyleJiang, W., Wang, J., Yang, Y., & Bu, Y. (2024). A Review of Microsphere Super-Resolution Imaging Techniques. Sensors, 24(8), 2511. https://doi.org/10.3390/s24082511






