Flexible Gas Sensor Based on PANI/WO3/CuO for Room-Temperature Detection of H2S
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of WO3 Microspheres
2.2. Synthesis of WO3/CuO Nanocomposite
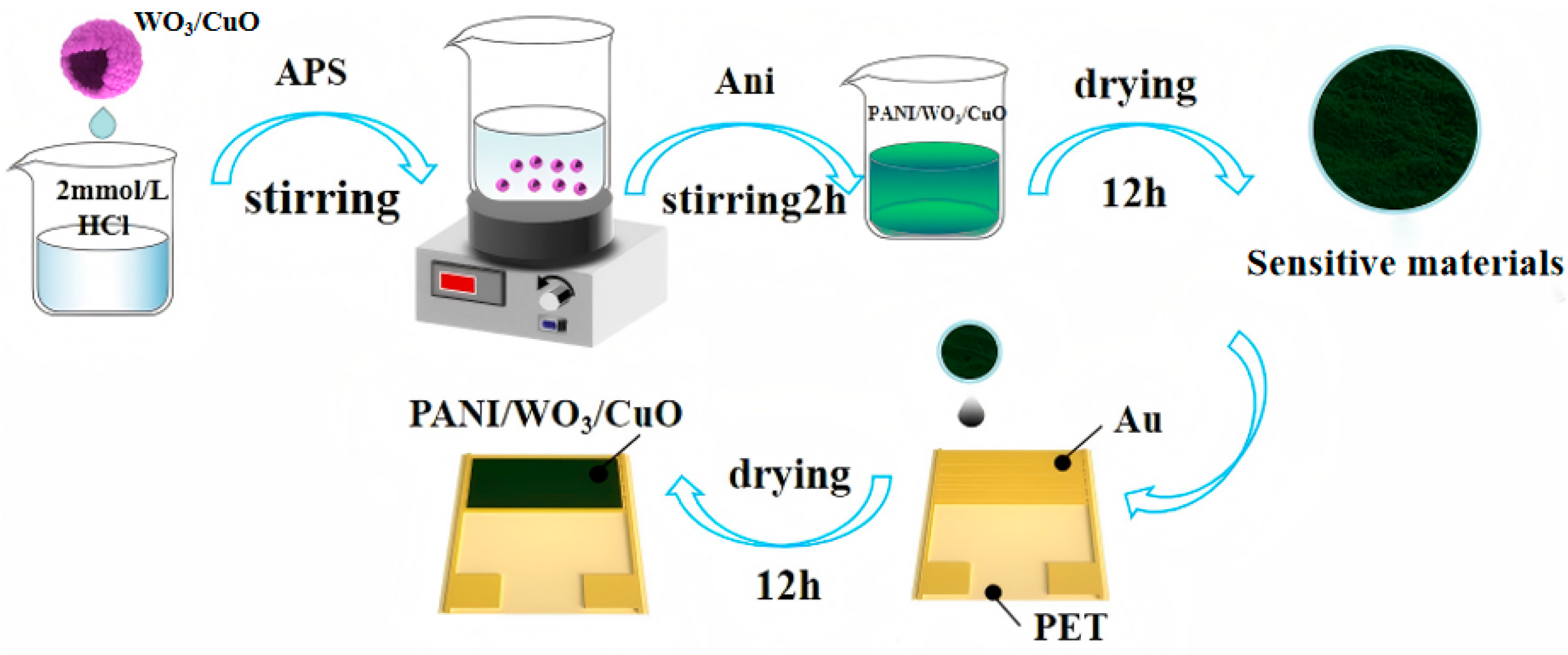
2.3. Synthesis of PANI/WO3/CuO Nanocomposites and Preparation of Sensitive Elements
3. Results
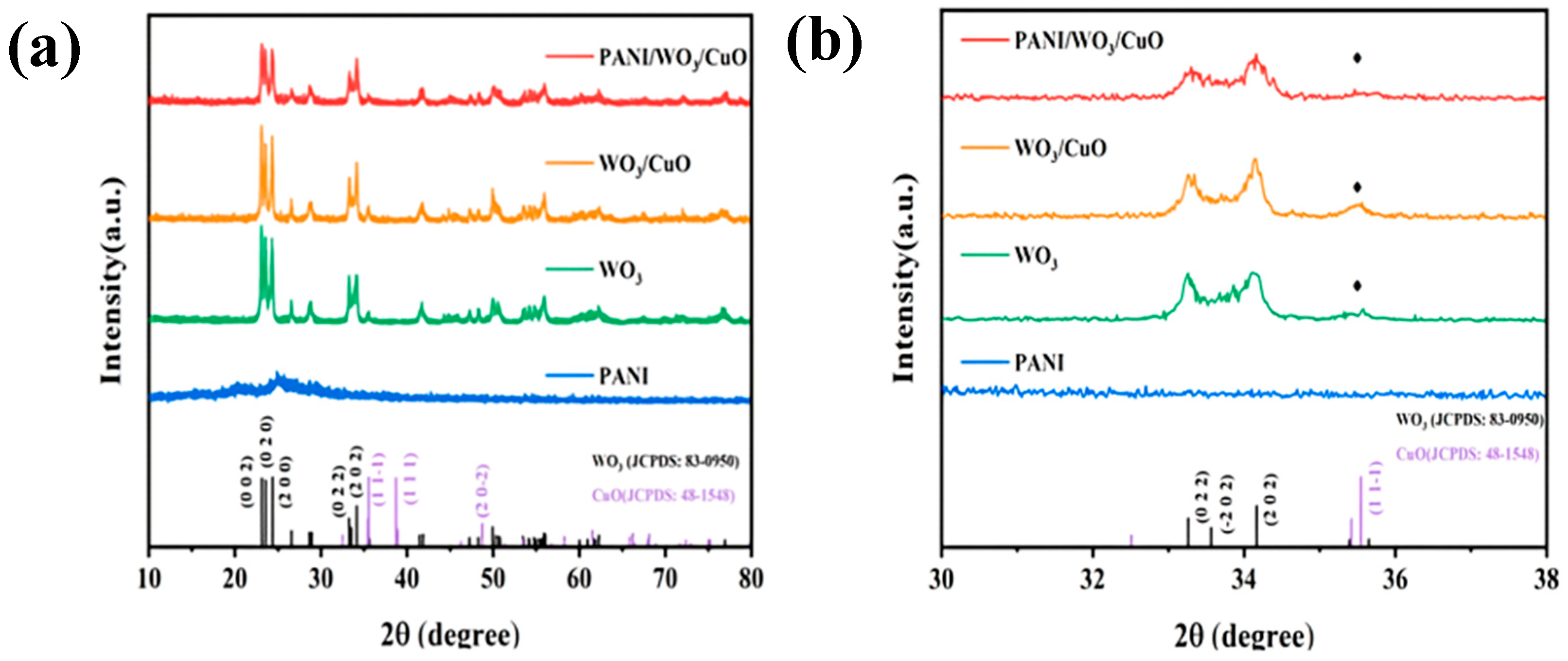
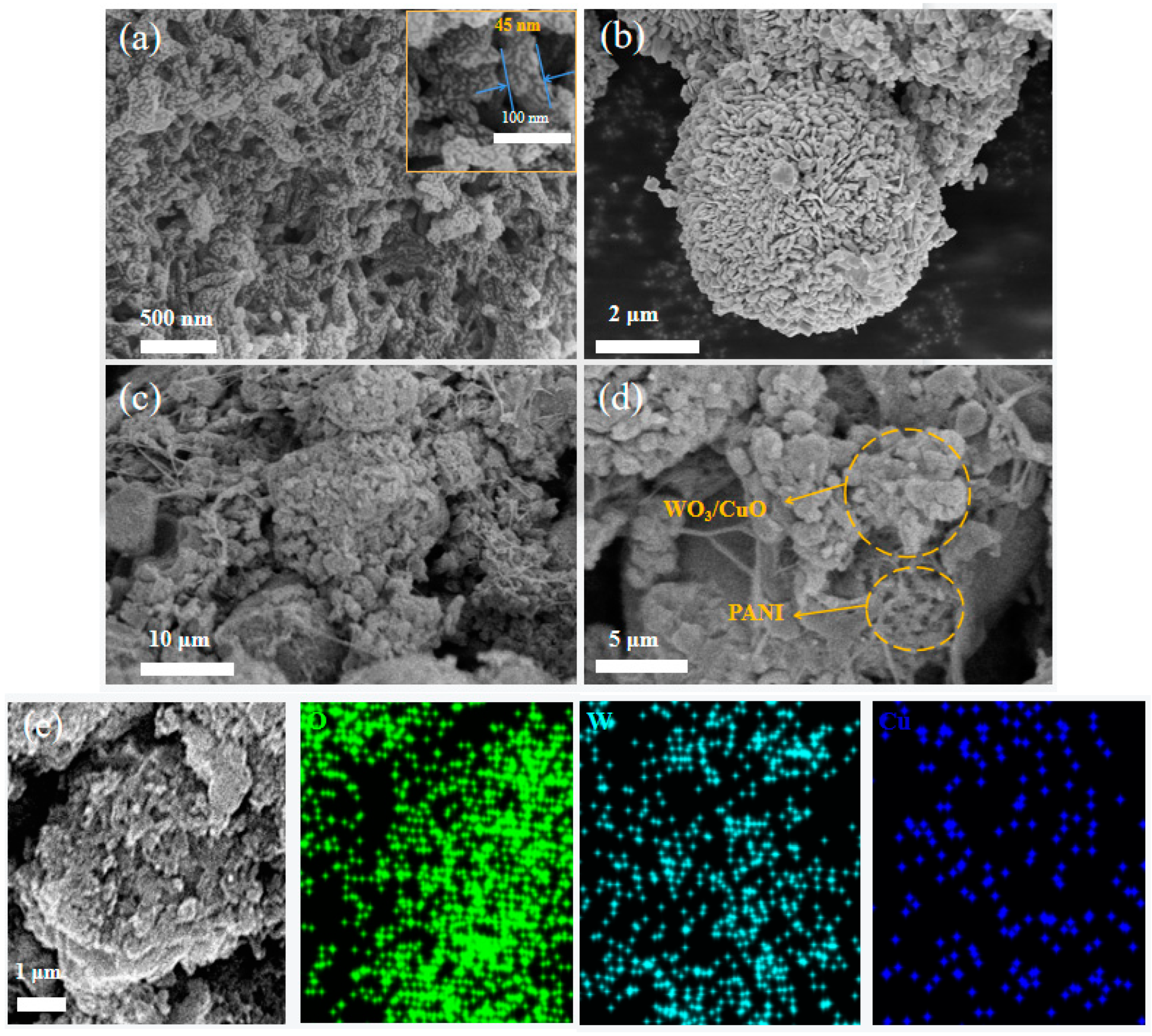
3.1. Materials Characterizations
3.2. H2S-Sensing Performance
3.2.1. Response/Recovery Characteristics
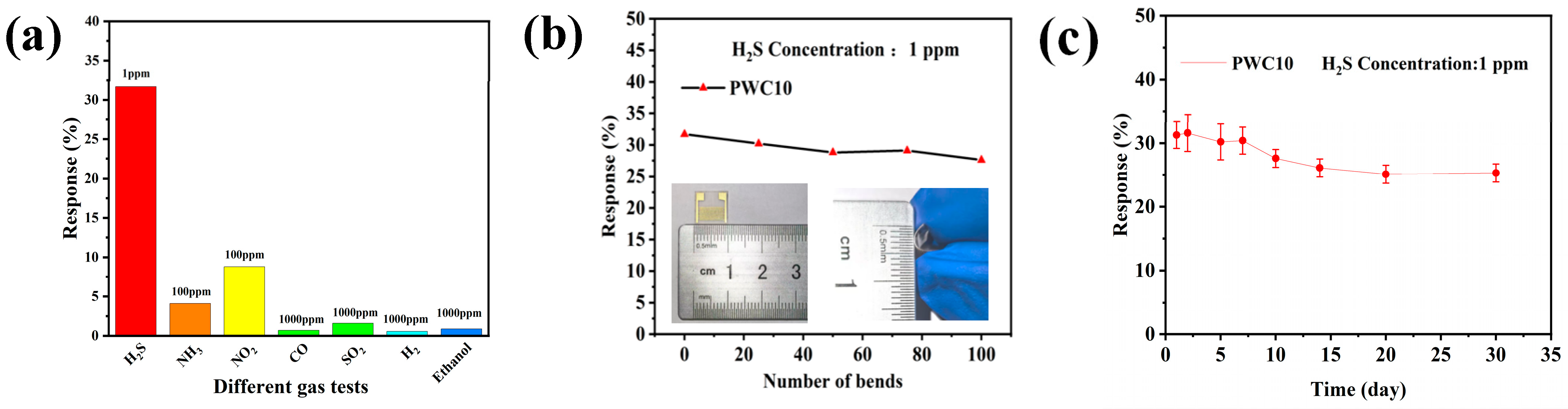
3.2.2. Selectivity and Stability
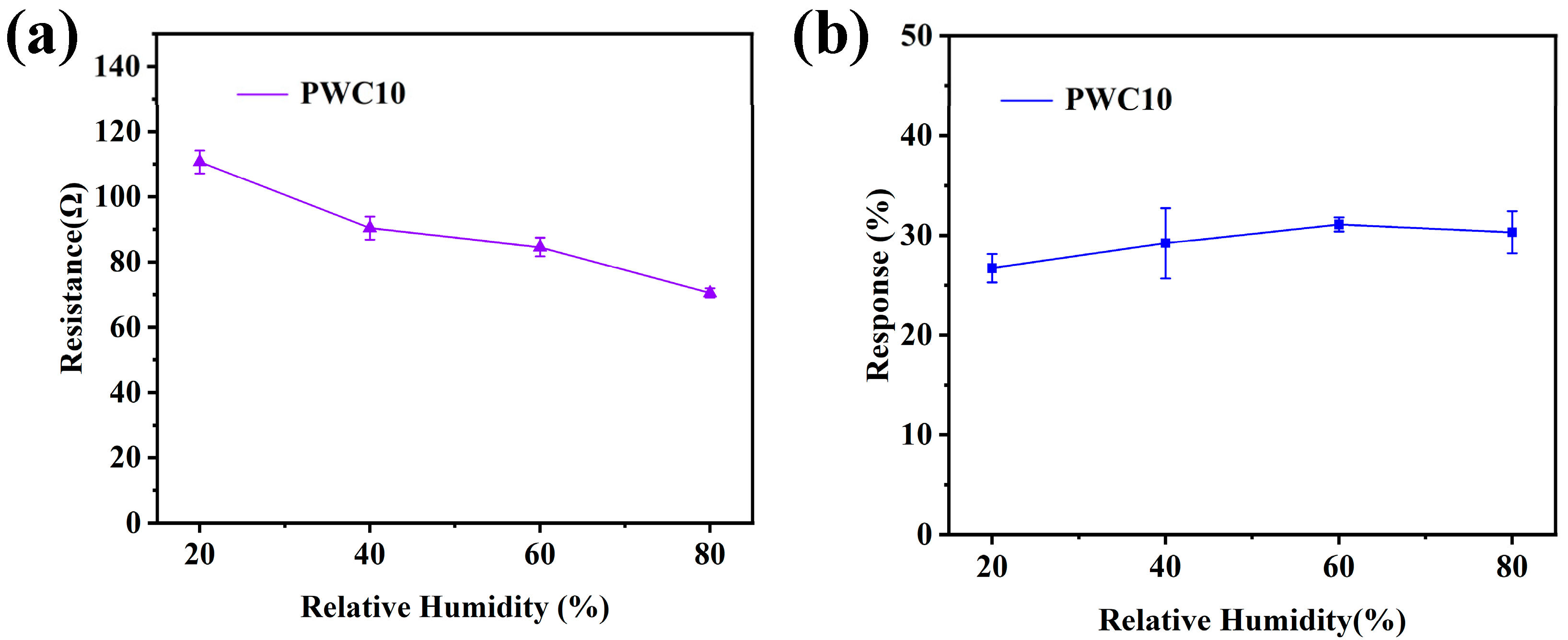
3.2.3. Humidity Effect
3.3. H2S Gas-Sensing Mechanism
3.3.1. Interface Activation Mechanism of Polyaniline
3.3.2. Redox Synergy of CuO/WO3 Heterojunction
3.3.3. Oxygen-Vacancy-Mediated Deep-Purification Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simonton, D.S.; King, S. Hydrogen Sulfide Formation and Potential Health Consequences in Coal Mining Regions. Water Qual. Expo. Health 2013, 5, 85–92. [Google Scholar] [CrossRef]
- Ma, Q.; Yang, Z.; Zhang, L.; Lin, R.; Wang, X. Generation of hydrogen sulfide during the thermal enhanced oil recovery process under superheated steam conditions. RSC Adv. 2019, 9, 33990–33996. [Google Scholar] [CrossRef]
- Du, Y.; Zhao, X.; Xu, Y.; Ma, W.; Wang, G.; Zhao, Z.; Du, D. Innovative hydrogen sulfide generation from natural pyrrhotite: A green solution for acid wastewater treatment with resource recovery benefits. Process Saf. Environ. Prot. 2025, 194, 96–106. [Google Scholar] [CrossRef]
- Pham, C.H.; Saggar, S.; Berben, P.; Palmada, T.; Ross, C. Removing Hydrogen Sulfide Contamination in Biogas Produced from Animal Wastes. J. Environ. Qual. 2019, 48, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Godoi, A.F.L.; Grasel, A.M.; Polezer, G.; Brown, A.; Potgieter-Vermaak, S.; Scremim, D.C.; Yamamoto, C.I.; Godoi, R.H.M. Human exposure to hydrogen sulphide concentrations near wastewater treatment plants. Sci. Total Environ. 2017, 610–611, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Lu, Z.; Wang, Z.; Zeng, W.; Zhou, Q. Highly Sensitive SF6 Decomposition Byproducts Sensing Platform Based on CuO/ZnO Heterojunction Nanofibers. Chemosensors 2023, 11, 58. [Google Scholar] [CrossRef]
- Hedlund, F.H. Confined space hazards: Plain seawater, an insidious source of hydrogen sulfide. J. Occup. Environ. Hyg. 2023, 20, 322–328. [Google Scholar] [CrossRef]
- Liu, D.; Wang, Q.; Wu, J.; Liu, Y. A review of sorbents for high-temperature hydrogen sulfide removal from hot coal gas. Environ. Chem. Lett. 2019, 17, 259–276. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, Z.G.; Wu, Z.H. Molecular and experimental study on hydrogen sulfide formation mechanism during Chang 7 type-II oil shale kerogen pyrolysis. Fuel 2023, 340, 127552. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, J.; Li, M.; Li, Z.; Yuan, Y.; Yang, X.; Guo, L. Highly efficient toluene gas sensor based on spinel structured hollow urchin-like core-shell ZnFe2O4 spheres. Sens. Actuators B Chem. 2021, 349, 130734. [Google Scholar] [CrossRef]
- Platonov, V.; Rumyantseva, M. Electrospun ZnO/MOx nanocomposites as sensitive materials for biomarker gas sensors: Role of MOx in C1–C4 short-chain fatty acids detection. Sens. Actuators B Chem. 2025, 433, 137535. [Google Scholar] [CrossRef]
- Dube, A.; Malode, S.J.; Alshehri, M.A.; Shetti, N.P. Recent advances in the development of electrochemical sensors for detecting pesticides. J. Ind. Eng. Chem. 2025, 144, 77–99. [Google Scholar] [CrossRef]
- Verhoeff, D.; Schreuders, H.; Bannenberg, L. Tantalum-palladium alloy based optical micro-mirror hydrogen sensor. Sens. Actuators B Chem. 2025, 428, 137229. [Google Scholar] [CrossRef]
- Holicky, M.; Fenech-Salerno, B.; Dabas, R.; Teenan, O.; Li, X.; Steer, I.; Higgins, C.A.; Akhavani, M.; Gadegaard, N.; Kamaly, N.; et al. Wearable microneedle graphene field-effect transistor sensors. 2D Mater. 2025, 12, 025019. [Google Scholar] [CrossRef]
- Zhang, F.; Lin, Q.; Han, F.; Wang, Z.; Tian, B.; Zhao, L.; Dong, T.; Jiang, Z. A fexible and wearable NO2 gas detection and early warning device based on a spraying process and an interdigital elec-trode at room temperature. Microsyst. Nanoeng. 2022, 8, 40. [Google Scholar] [CrossRef]
- Gao, Z.; Lou, Z.; Chen, S.; Li, L.; Jiang, K.; Fu, Z.; Han, W.; Shen, G. Fiber gas sensor-integrated smart face mask for room-temperature distinguishing of target gases. Nano Res. 2018, 11, 511–519. [Google Scholar] [CrossRef]
- Li, W.; Chen, R.; Qi, W.; Cai, L.; Sun, Y.; Sun, M.; Li, C.; Yang, X.; Xiang, L.; Xie, D.; et al. Reduced graphene oxide/mesoporous ZnO nss hybrid fbers for fexible, stretchable, twisted, and wearable NO2 E-textile gas sensor. ACS Sens. 2019, 4, 2809–2818. [Google Scholar] [CrossRef] [PubMed]
- Tanguy, N.R.; Thompson, M.; Yan, N. A review on advances in application of polyaniline for ammonia detection. Sens. Actuators B Chem. 2018, 257, 1044–1064. [Google Scholar] [CrossRef]
- Macdiarmid, A.G.; Chiang, J.C.; Richter, A.F. Polyaniline: A new concept in conducting polymers. Synth. Met. 1987, 18, 285–290. [Google Scholar] [CrossRef]
- Sen, T.; Mishra, S.; Shimpi, N.G. Synthesis and sensing applications of polyaniline nanocomposites: A review. RSC Adv. 2016, 6, 42196–42222. [Google Scholar] [CrossRef]
- Liu, C.; Hayashi, K.; Toko, K. Au nanoparticles decorated polyaniline nanofiber sensor for detecting volatile sulfur compounds in expired breath. Sens. Actuators B Chem. 2012, 161, 504–509. [Google Scholar] [CrossRef]
- Akber, H.J.; Razeg, K.H.; Ibrahim, I.M. Sensing Characteristics of Nanostructured PANI/Ag Thin Films as H2S Gas Sensor. IOP Conf. Ser. Mater. Sci. Eng. 2020, 928, 072146. [Google Scholar] [CrossRef]
- Zhang, D.; Fan, X.; Hao, X.; Dong, G. Facile Fabrication of Polyaniline Nanocapsule Modified Zinc Oxide Hexagonal Microdiscs for H2S Gas Sensing Applications. Ind. Eng. Chem. Res. 2019, 58, 1906–1913. [Google Scholar] [CrossRef]
- Zhang, B.; Shang, F.; Shi, X.; Yao, R.; Wei, F.; Hou, X.; Li, W.; Zhang, J. Polyaniline/CuO Nanoparticle Composites for Use in Selective H2S Sensors. ACS Appl. Nano Mater. 2023, 6, 18413–18425. [Google Scholar] [CrossRef]
- Kumawat, M.; Thapliyal, D.; Verros, G.D.; Arya, R.K.; Barman, S.; Halder, G.; Shandilya, P. PANI-Based Hydrogen Sulfide Gas Sensors. Coatings 2022, 12, 186. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, Z.; Zong, X. Flexible and highly sensitive H2S gas sensor based on in-situ polymerized SnO2/rGO/PANI ternary nanocomposite with application in halitosis diagnosis. Sens. Actuators B Chem. 2019, 289, 32–41. [Google Scholar] [CrossRef]
- Bibi, A.; Rubio, Y.R.M.; Santiago, K.S.; Jia, H.W.; Ahmed, M.M.; Lin, Y.F.; Yeh, J.M. H2S-Sensing Studies Using Interdigitated Electrode with Spin-Coated Carbon Aerogel-Polyaniline Composites. Polymers 2021, 13, 1457. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Zou, X.; Dong, Y.; Cui, Y. Analysis of the effect of visible light photocatalytic reduction of Cr (VI) in industrial wastewater by LaVO4/WO3 composite nanosheets synthesized by hydrothermal method. J. Water Resour. Water Eng. 2019, 30, 116–122. [Google Scholar]
- Zhou, C.; Xu, L.; Song, J.; Xing, R.; Xu, S.; Liu, D.; Song, H. Ultrasensitive non-enzymatic glucose sensor based on three-dimensional network of ZnO-CuO hierarchical nanocomposites by electrospinning. Sci. Rep. 2014, 4, 7382. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.; Xiao, D.; Wang, S.; Zhang, T.; Yang, X.; Heng, S.; Sun, M. CuO/WO3 hollow microsphere P-Nheterojunction sensor for continuous cycle detection of H2S gas. Sens. Actuators B Chem. 2023, 374, 132823. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Jiang, Y.; Duan, Z.; Liu, B.; Zhao, Q.; Wang, S.; Yuan, Z.; Tai, H. Ultrasensitive flexible NH3 gas sensor based on polyaniline/SrGe4O9 nanocomposite with ppt-level detection ability at room temperature. Sens. Actuators B Chem. 2020, 319, 128293. [Google Scholar] [CrossRef]
- Feng, X.; Li, R.; Ma, Y.; Chen, R.; Shi, N.; Fan, Q.; Huang, W. One-Step Electrochemical Synthesis ofGraphene/Polyaniline Composite Film and Its Applications. Adv. Funct. Mater. 2011, 21, 2989–2996. [Google Scholar] [CrossRef]
- Li, S.; Diao, Y.; Yang, Z.; He, J.; Wang, J.; Liu, C.; Liu, F.; Lu, H.; Yan, X.; Sun, P.; et al. Enhanced room temperature gas sensor based on Au-loaded mesoporous In2O3 nanospheres@polyaniline core-shell nanohybrid assembled on flexible PET substrate for NH3 detection. Sens. Actuators B Chem. 2018, 276, 526–533. [Google Scholar] [CrossRef]
- Ji, H.; Zeng, W.; Li, Y. Gas sensing mechanisms of metal oxide semiconductors: A focus review. Nanoscale 2019, 11, 22664–22684. [Google Scholar] [CrossRef] [PubMed]
- Shaik, R.; Kampara, R.K.; Kumar, A.; Sharma, C.S.; Kumar, M. Metal oxide nanofibers based chemiresistive H2S gas sensors. Coord. Chem. Rev. 2022, 471, 214752. [Google Scholar] [CrossRef]
- Degler, D.; Rank, S.; Mueller, S.; Pereira de Carvalho, H.W.; Grunwaldt, J.D.; Weimar, U. Gold-loaded tin dioxide gas sensing materials: Mechanistic insights and the role of gold dispersion. ACS Sens. 2016, 1, 1322–1329. [Google Scholar] [CrossRef]
- Cho, S.; Lee, J.S.; Jun, J.; Kim, S.G.; Jang, J. Fabrication of water-dispersible and highly conductive PSS-doped PANI/graphene nanocomposites using a high molecular weight PSS dopant and their application in H2S detection. Nanoscale 2014, 6, 15181–15195. [Google Scholar] [CrossRef]
- Gautam, S.K.; Panda, S. Effect of moisture and molecular weight of polyaniline on H2S sensing characteristics. Sens. Actuators B Chem. 2021, 344, 130323. [Google Scholar] [CrossRef]
- El-Shazly, A.H.; Elkady, M.; Abdelraheem, A. Investigating the Adsorption Behavior of Polyaniline and Its Clay Nanocomposite towards Ammonia Gas. Polymers 2022, 14, 4533. [Google Scholar] [CrossRef]
- Li, S.; Liu, A.; Yang, Z.; Zhao, L.; Wang, J.; Liu, F.; You, R.; He, J.; Wang, C.; Yan, X.; et al. Design and preparation of the WO3 hollow spheres@PANI conducting films for room temperature flexible NH3 sensing device. Sens. Actuators B Chem. 2019, 289, 252–259. [Google Scholar] [CrossRef]
- Jain, R.K.; Khanna, A. CuO-doped WO3 thin film H2S sensors. Sens. Actuators B Chem. 2021, 343, 130153. [Google Scholar] [CrossRef]
- Ramgir, N.S.; Goyal, C.; Sharma, P.; Goutam, U.; Bhattacharya, S.; Datta, N.; Kaur, M.; Debnath, A.; Aswal, D.; Gupta, S. Selective H2S sensing characteristics of CuO modified WO3 thin films. Sens. Actuators B Chem. 2013, 188, 525–532. [Google Scholar] [CrossRef]
- He, M.; Xie, L.; Zhao, X.; Hu, X.; Li, S.; Zhu, Z.G. Highly sensitive and selective H2S gas sensors based on flower-like WO3/CuO composites operating at low/room temperature. J. Alloys Compd. 2019, 788, 36–43. [Google Scholar] [CrossRef]



| Type | Materials | OT (°C) | Gas (ppm) | LOD (ppm) | Response | Tres (s) | Trec (s) | Ref. |
|---|---|---|---|---|---|---|---|---|
| metal | PANI/AuNPs | 200 | 1 | - | 48% | - | - | [21] |
| PANI/Ag | RT | 25 | - | 73.35% | 0.82 | 0.81 | [22] | |
| metal oxides | PANI/ZnO | RT | 50 | 0.1 | 40.5% | 63 | 12 | [23] |
| PANI/CuO | RT | 25 | - | 188% | >300 | - | [24] | |
| PANI/WO3/CuCl2 | RT | 1.156 | 0.3 | 93.62% | 67.9 | 250 | [25] | |
| PANI/WO3/CuO | RT | 1 | 0.1 | 31.3% | 353 | 4958 | This work | |
| carbon materials | PANI/SnO2/rGO | RT | 5 | 0.05 | 91.11% | 80 | 88 | [26] |
| PANI/CA | RT | 50 | 1 | 24.64 (Ra/Rg) | 1 | 1065 | [27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Liu, Y.; Wang, Y.; Li, Z.; Xiao, D.; Zhou, T.; Sun, M. Flexible Gas Sensor Based on PANI/WO3/CuO for Room-Temperature Detection of H2S. Sensors 2025, 25, 2640. https://doi.org/10.3390/s25092640
Zhang D, Liu Y, Wang Y, Li Z, Xiao D, Zhou T, Sun M. Flexible Gas Sensor Based on PANI/WO3/CuO for Room-Temperature Detection of H2S. Sensors. 2025; 25(9):2640. https://doi.org/10.3390/s25092640
Chicago/Turabian StyleZhang, Dongxiang, Yingmin Liu, Yang Wang, Zhi Li, Dongkun Xiao, Tianhong Zhou, and Mojie Sun. 2025. "Flexible Gas Sensor Based on PANI/WO3/CuO for Room-Temperature Detection of H2S" Sensors 25, no. 9: 2640. https://doi.org/10.3390/s25092640
APA StyleZhang, D., Liu, Y., Wang, Y., Li, Z., Xiao, D., Zhou, T., & Sun, M. (2025). Flexible Gas Sensor Based on PANI/WO3/CuO for Room-Temperature Detection of H2S. Sensors, 25(9), 2640. https://doi.org/10.3390/s25092640





