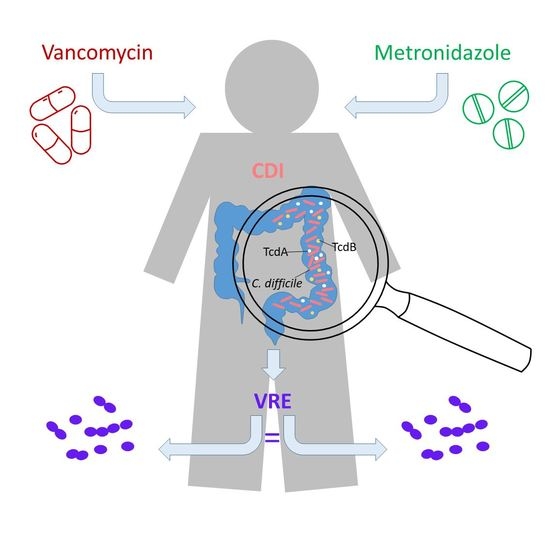
Impact of Clostridioides difficile Therapy on Nosocomial Acquisition of Vancomycin-Resistant Enterococci
Abstract
:1. Introduction
2. Results
2.1. Characteristics of Included Patients and Onset of VRE
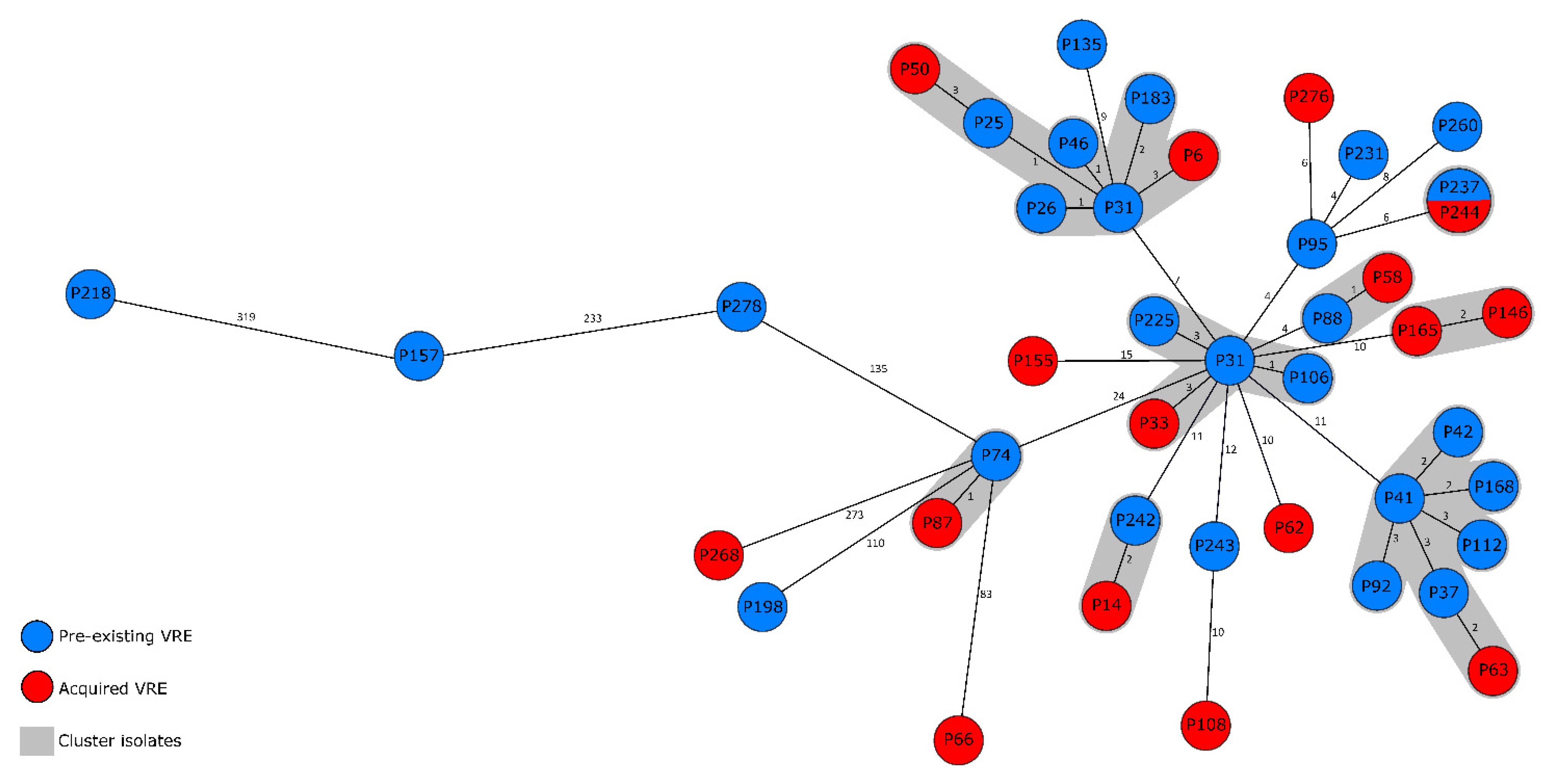
2.2. VRE Genotypes and Genetic Distribution of Strains
3. Discussion
4. Materials and Methods
4.1. Setting and Study Design
4.2. Infection Control Measures
4.3. CDI Diagnostic Procedure
4.4. VRE Screening, Culture, and Antimicrobial Susceptibility Testing
4.5. Whole Genome Sequencing
4.6. Statistical Analysis
4.7. Ethical Statement
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- KISS Krankenhaus-Infektions-Surveillance-System—Modul CDAD-KISS Referenzdaten. Available online: https://www.nrz-hygiene.de/fileadmin/nrz/module/cdad/202001_202012CDAD_Ref.pdf (accessed on 20 October 2021).
- Gesetz zur Verhütung und Bekämpfung von Infektionskrankheiten beim Menschen (Infektionsschutzgesetz-IfSG). Available online: https://www.gesetze-im-internet.de/ifsg/IfSG.pdf (accessed on 20 October 2021).
- Johnson, S.; Lavergne, V.; Skinner, A.M.; Gonzales-Luna, A.J.; Garey, K.W.; Kelly, C.P.; Wilcox, M.H. Clinical Practice Guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 Focused Update Guidelines on Management of Clostridioides difficile Infection in Adults. Clin. Infect. Dis. 2021, 73, e1029–e1044. [Google Scholar] [CrossRef]
- Debast, S.; Bauer, M.; Kuijper, E. European Society of Clinical Microbiology and Infectious Diseases: Update of the Treatment Guidance Document for Clostridium difficile Infection. Clin. Microbiol. Infect. 2014, 20, 1–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drekonja, D.M.; Butler, M.; MacDonald, R.; Bliss, D.; Filice, G.A.; Rector, T.S.; Wilt, T.J. Comparative effectiveness of Clostridium difficile treatments: A systematic review. Ann. Intern. Med. 2011, 155, 839–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, R.L.; Suda, K.J.; Evans, C.T. Antibiotic treatment for Clostridium difficile-associated diarrhoea in adults. Cochrane Database Syst. Rev. 2017, 2017, CD004610. [Google Scholar] [CrossRef]
- Cho, J.M.; Pardi, D.S.; Khanna, S. Update on Treatment of Clostridioides difficile Infection. Mayo Clin. Proc. 2020, 95, 758–769. [Google Scholar] [CrossRef] [PubMed]
- Gerding, D.N. Is there a relationship between vancomycin-resistant enterococcal infection and Clostridium difficile infection? Clin. Infect. Dis. 1997, 25 (Suppl. 2), S206–S210. [Google Scholar] [CrossRef] [Green Version]
- Carmeli, Y.; Samore, M.H.; Huskins, C. The association between antecedent vancomycin treatment and hospital-acquired vancomycin-resistant enterococci: A meta-analysis. Arch. Intern. Med. 1999, 159, 2461–2468. [Google Scholar] [CrossRef] [PubMed]
- Correa-Martinez, C.L.; Stollenwerk, V.B.; Kossow, A.; Schaumburg, F.; Mellmann, A.; Kampmeier, S. Risk Factors for Long-Term Vancomycin-Resistant Enterococci Persistence—A Prospective Longitudinal Study. Microorganisms 2019, 7, 400. [Google Scholar] [CrossRef] [Green Version]
- DiNubile, M.J.; Chow, J.; Satishchandran, V.; Polis, A.; Motyl, M.R.; Abramson, M.A.; Teppler, H. Acquisition of Resistant Bowel Flora during a Double-Blind Randomized Clinical Trial of Ertapenem versus Piperacillin-Tazobactam Therapy for Intraabdominal Infections. Antimicrob. Agents Chemother. 2005, 49, 3217–3221. [Google Scholar] [CrossRef] [Green Version]
- Stevens, V.W.; Khader, K.; Echevarria, K.; Nelson, R.E.; Zhang, Y.; Jones, M.; Timbrook, T.T.; Samore, M.H.; Rubin, M.A. Use of Oral Vancomycin for Clostridioides difficile Infection and the Risk of Vancomycin-Resistant Enterococci. Clin. Infect. Dis. 2019, 71, 645–651. [Google Scholar] [CrossRef]
- Prematunge, C.; MacDougall, C.; Johnstone, J.; Adomako, K.; Lam, F.; Robertson, J.; Garber, G. VRE and VSE Bacteremia Outcomes in the Era of Effective VRE Therapy: A Systematic Review and Meta-analysis. Infect. Control. Hosp. Epidemiol. 2015, 37, 26–35. [Google Scholar] [CrossRef] [Green Version]
- DiazGranados, C.A.; Zimmer, S.M.; Mitchel, K.; Jernigan, J.A. Comparison of Mortality Associated with Vancomycin-Resistant and Vancomycin-Susceptible Enterococcal Bloodstream Infections: A Meta-analysis. Clin. Infect. Dis. 2005, 41, 327–333. [Google Scholar] [CrossRef] [Green Version]
- Zacharioudakis, I.M.; Zervou, F.N.; Ziakas, P.; Rice, L.B.; Mylonakis, E. Vancomycin-Resistant Enterococci Colonization Among Dialysis Patients: A Meta-analysis of Prevalence, Risk Factors, and Significance. Am. J. Kidney Dis. 2015, 65, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou-Olivgeris, M.; Drougka, E.; Fligou, F.; Kolonitsiou, F.; Liakopoulos, A.; Dodou, V.; Anastassiou, E.D.; Petinaki, E.; Marangos, M.; Filos, K.S.; et al. Risk factors for enterococcal infection and colonization by vancomycin-resistant enterococci in critically ill patients. Infection 2014, 42, 1013–1022. [Google Scholar] [CrossRef]
- Erb, S.; Frei, R.; Dangel, M.; Widmer, A.F. Multidrug-Resistant Organisms Detected More Than 48 Hours after Hospital Admission Are Not Necessarily Hospital-Acquired. Infect. Control. Hosp. Epidemiol. 2016, 38, 18–23. [Google Scholar] [CrossRef]
- Suleyman, G.; Alangaden, G.; Bardossy, A.C. The Role of Environmental Contamination in the Transmission of Nosocomial Pathogens and Healthcare-Associated Infections. Curr. Infect. Dis. Rep. 2018, 20, 12. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Monteserin, N.; Larson, E. Temporal trends and risk factors for healthcare-associated vancomycin-resistant enterococci in adults. J. Hosp. Infect. 2016, 94, 236–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ford, C.D.; Lopansri, B.K.; Gazdik, M.A.; Webb, B.; Snow, G.L.; Hoda, D.; Adams, B.; Petersen, F.B. Room contamination, patient colonization pressure, and the risk of vancomycin-resistant Enterococcus colonization on a unit dedicated to the treatment of hematologic malignancies and hematopoietic stem cell transplantation. Am. J. Infect. Control 2016, 44, 1110–1115. [Google Scholar] [CrossRef] [PubMed]
- Babar, S.; El Kurdi, B.; El Iskandarani, M.; Haddad, I.; Imam, Z.; AlOmari, M.; Myers, J.; Moorman, J. Oral vancomycin prophylaxis for the prevention of Clostridium difficile infection: A systematic review and meta-analysis. Infect. Control Hosp. Epidemiol. 2020, 41, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Correa-Martínez, C.L.; Schuler, F.; Kampmeier, S. Sex differences in vancomycin-resistant enterococci bloodstream infections—A systematic review and meta-analysis. Biol. Sex Differ. 2021, 12, 36. [Google Scholar] [CrossRef] [PubMed]
- Remschmidt, C.; Behnke, M.; Kola, A.; Diaz, L.A.P.; Rohde, A.M.; Gastmeier, P.; Schwab, F. The effect of antibiotic use on prevalence of nosocomial vancomycin-resistant enterococci—An ecologic study. Antimicrob. Resist. Infect. Control 2017, 6, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correa-Martinez, C.L.; Tönnies, H.; Froböse, N.J.; Mellmann, A.; Kampmeier, S. Transmission of Vancomycin-Resistant Enterococci in the Hospital Setting: Uncovering the Patient–Environment Interplay. Microorganisms 2020, 8, 203. [Google Scholar] [CrossRef] [Green Version]
- Faron, M.L.; Ledeboer, N.A.; Buchan, B.W. Resistance Mechanisms, Epidemiology, and Approaches to Screening for Vancomycin-Resistant Enterococcus in the Health Care Setting. J. Clin. Microbiol. 2016, 54, 2436–2447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eigenschaften, Häufigkeit und Verbreitung von Vancomycin-resistenten Enterokokken (VRE) in Deutschland. Update 2019/20. Available online: https://www.rki.de/DE/Content/Infekt/EpidBull/Archiv/2021/Ausgaben/27_21.pdf?__blob=publicationFile (accessed on 20 October 2021).
- Flokas, M.E.; Karageorgos, S.A.; Detsis, M.; Alevizakos, M.; Mylonakis, E. Vancomycin-resistant enterococci colonisation, risk factors and risk for infection among hospitalised paediatric patients: A systematic review and meta-analysis. Int. J. Antimicrob. Agents 2017, 49, 565–572. [Google Scholar] [CrossRef]
- Brukner, I.; Oughton, M. A Fundamental Change in Antibiotic Susceptibility Testing Would Better Prevent Therapeutic Failure: From Individual to Population-Based Analysis. Front. Microbiol. 2020, 11, 1820. [Google Scholar] [CrossRef] [PubMed]
- Referenzbericht Universitätsklinikum Münster 2019. Available online: https://www.ukm.de/fileadmin/ukminternet/daten/zentralauftritt/patienten-besucher/Qualtitaet_Hygiene/ukm-qualitaetsbericht-2019.pdf (accessed on 20 October 2021).
- Breakpoint Tables for Interpretation of MICs and Zone Diameters Version 11.0. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_11.0_Breakpoint_Tables.pdf (accessed on 20 October 2021).
- de Been, M.; Pinholt, M.; Top, J.; Bletz, S.; Mellmann, A.; van Schaik, W.; Brouwer, E.; Rogers, M.; Kraat, Y.; Bonten, M.; et al. Core Genome Multilocus Sequence Typing Scheme for High-Resolution Typing of Enterococcus faecium. J. Clin. Microbiol. 2015, 53, 3788–3797. [Google Scholar] [CrossRef] [Green Version]

| Characteristic | Value (%) | |
|---|---|---|
| Metronidazole (n = 37) | Vancomycin (n = 133) | |
| Demographic data | ||
| Median age (years) | 58 | 50 |
| Male gender | 22 (59) | 72 (54) |
| Underlying diseases | ||
| Haemato-oncological diseases | 22 (59) | 57 (43) |
| Immunosuppressive disease | 23 (62) | 57 (43) |
| Hepatic insufficiency | 5 (14) | 12 (9) |
| Liver Transplantation | 1 (3) | 6 (5) |
| Renal insufficiency | 6 (16) | 30 (23) |
| Long term dialysis | 1 (3) | 12 (9) |
| Treatment | ||
| Systemic glucocorticoid treatment | 6 (16) | 36 (27) |
| Non-CDI-specific antibiotic treatment | 20 (54) | 79 (59) |
| Contact to healthcare system | ||
| Average lengths of stay (days) | 67 | 126 |
| Median number of stays | 2 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Correa-Martínez, C.L.; Hagemeier, N.C.J.; Froböse, N.J.; Kampmeier, S. Impact of Clostridioides difficile Therapy on Nosocomial Acquisition of Vancomycin-Resistant Enterococci. Pharmaceuticals 2021, 14, 1066. https://doi.org/10.3390/ph14111066
Correa-Martínez CL, Hagemeier NCJ, Froböse NJ, Kampmeier S. Impact of Clostridioides difficile Therapy on Nosocomial Acquisition of Vancomycin-Resistant Enterococci. Pharmaceuticals. 2021; 14(11):1066. https://doi.org/10.3390/ph14111066
Chicago/Turabian StyleCorrea-Martínez, Carlos L., Niklas C. J. Hagemeier, Neele J. Froböse, and Stefanie Kampmeier. 2021. "Impact of Clostridioides difficile Therapy on Nosocomial Acquisition of Vancomycin-Resistant Enterococci" Pharmaceuticals 14, no. 11: 1066. https://doi.org/10.3390/ph14111066
APA StyleCorrea-Martínez, C. L., Hagemeier, N. C. J., Froböse, N. J., & Kampmeier, S. (2021). Impact of Clostridioides difficile Therapy on Nosocomial Acquisition of Vancomycin-Resistant Enterococci. Pharmaceuticals, 14(11), 1066. https://doi.org/10.3390/ph14111066