The Antiviral Role of Galectins toward Influenza A Virus Infection—An Alternative Strategy for Influenza Therapy
Abstract
1. Introduction
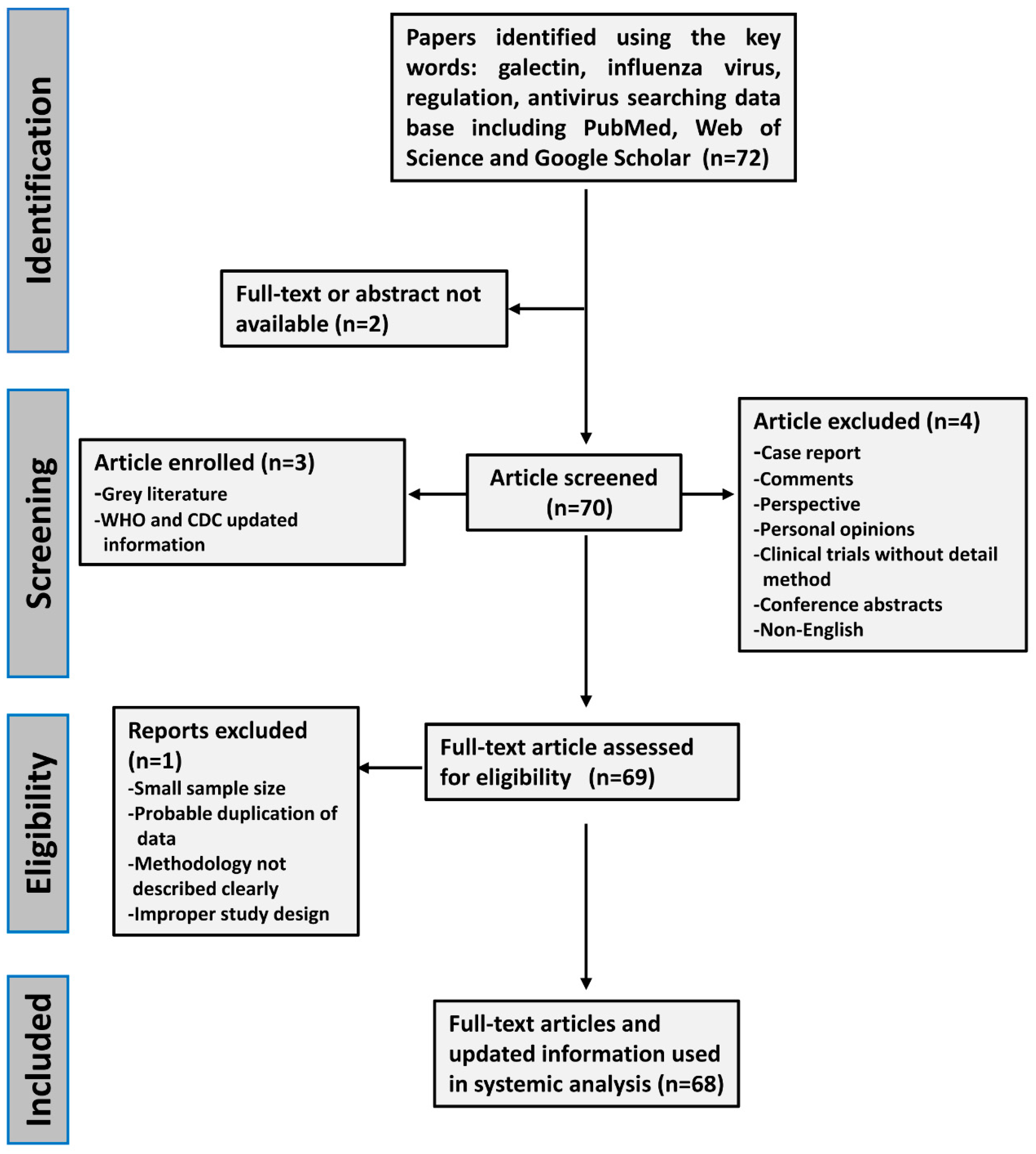
2. Methods
3. Results
3.1. Galectin-1 (Gal-1)
3.1.1. Basic Information of Gal-1
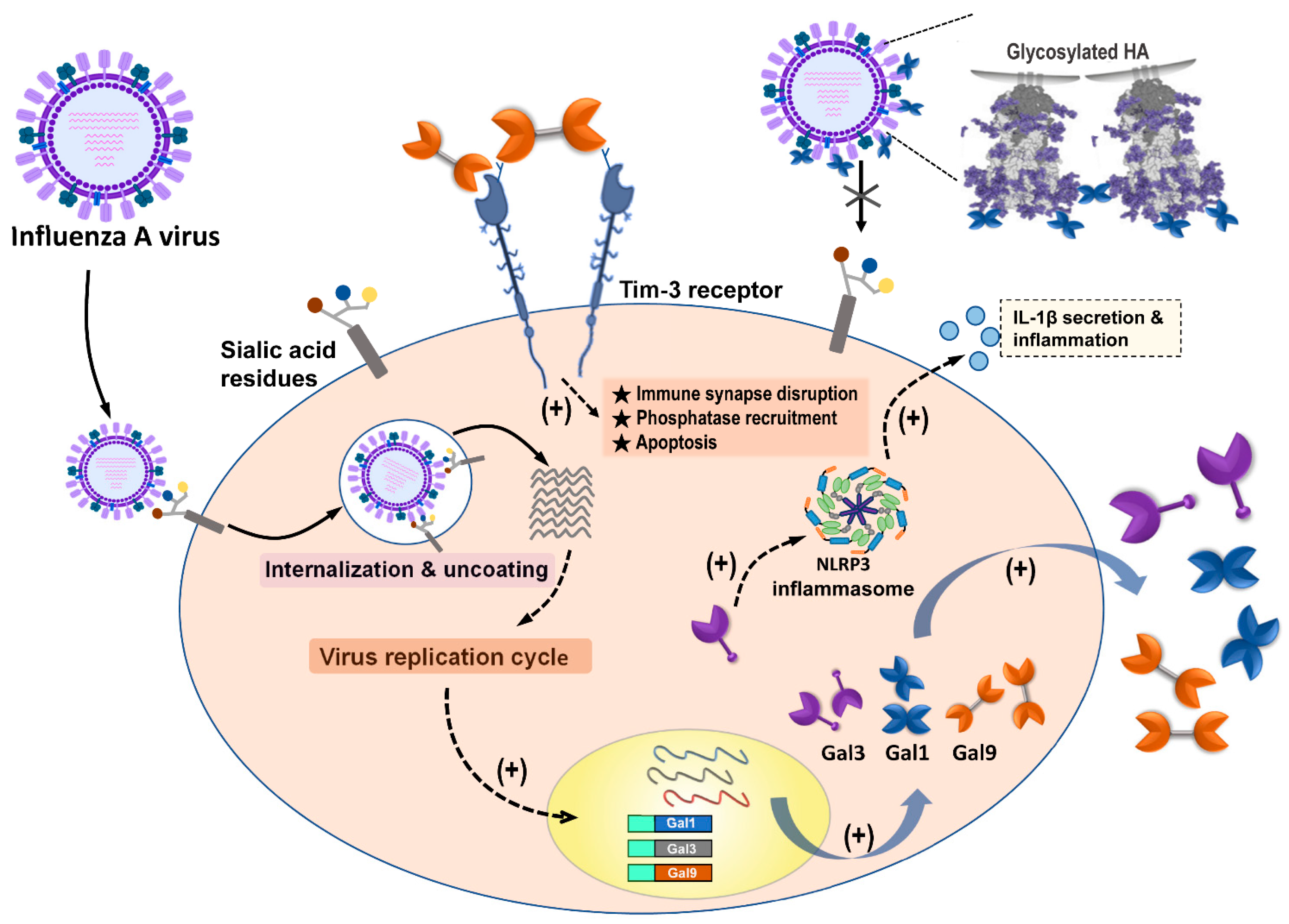
3.1.2. Gal-1 Blocks the Interaction between Influenza Virus and Sialic Acid Receptors
3.1.3. Gal-1 Participates in Influenza Virus-Induced Cell Apoptosis
3.1.4. Gal-1 Treatment Enhances Immune Response and Ameliorates Influenza Virus-Induced Patho Genesis
3.1.5. The Polymorphism of LGALS1 Gene Correlates with Resistance of IAV Infection in Humans
3.2. Galectin-3 (Gal-3)
3.2.1. Basic Information of Gal-3
3.2.2. Gal-3 Expression Augment Expression of Antiviral Genes to Fight against IAV Infection
3.2.3. Gal-3 Participates in the Activation of NLRP3 Inflammasome during IAV Infection
3.2.4. Gal-3 Ameliorates the Immunopathogenesis of IAVs and Streptococcus pneumoniae Coinfection In Vivo
3.2.5. Gal-3 Could Bind to HA of IAVs and Desialylated Airway Epithelial Cells
3.3. Galectin-9 (Gal-9)
3.3.1. Basic Information of Gal-9
3.3.2. Gal-9 Binds to IAVs to Block Virus Attachment
3.3.3. Gal-9/Tim-3 Signaling Control IAV-Infected Cells
3.3.4. Plasma Gal-9 Could Be a Biomarker for IAV Infection
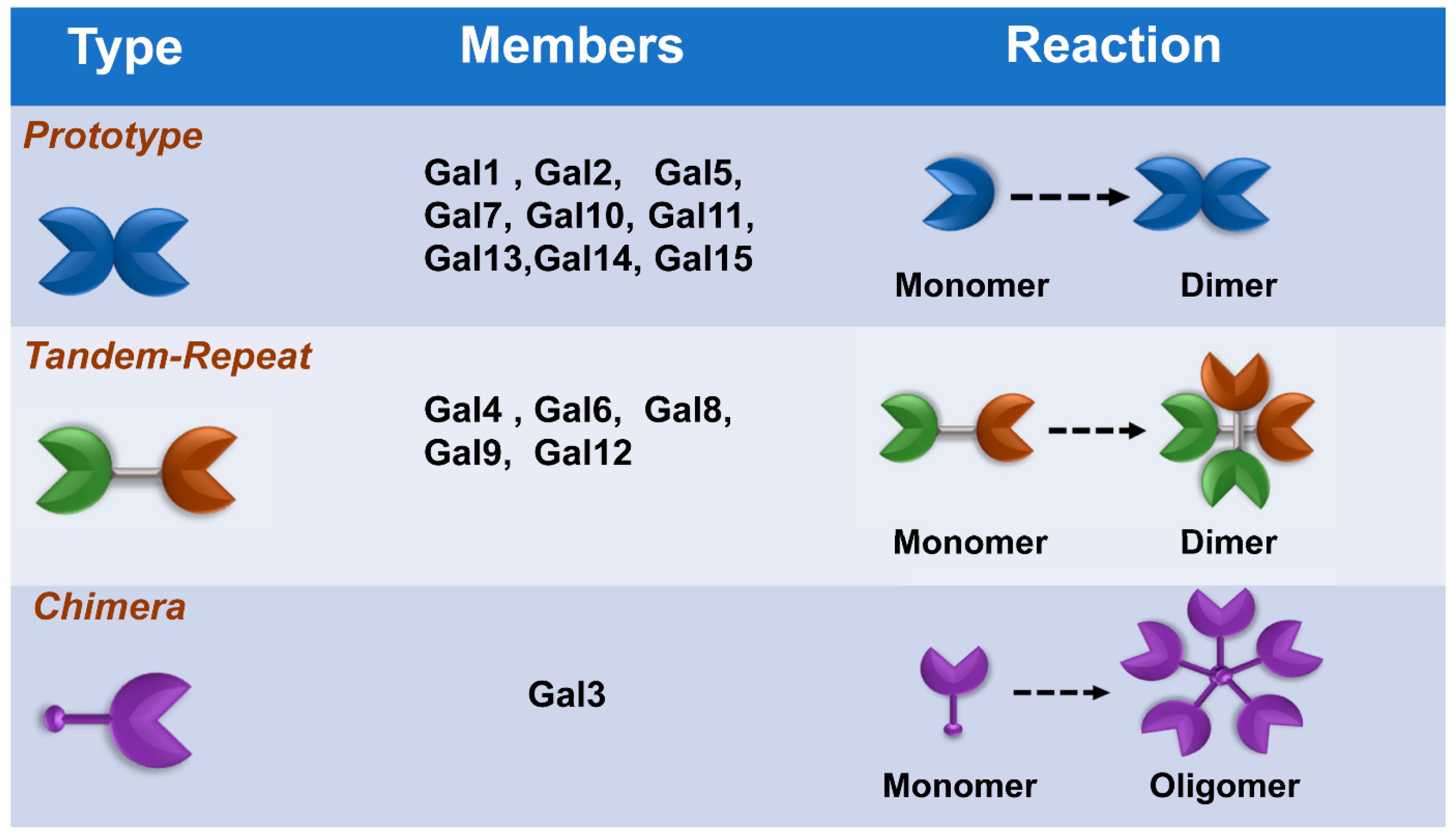
4. Other Galectins
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bouvier, N.M.; Palese, P. The biology of influenza viruses. Vaccine 2008, 26, D49–D53. [Google Scholar] [CrossRef]
- Erbelding, E.J.; Post, D.J.; Stemmy, E.J.; Roberts, P.C.; Augustine, A.D.; Ferguson, S.; Paules, C.I.; Graham, B.S.; Fauci, A.S. A universal influenza vaccine: The strategic plan for the National Institute of Allergy and Infectious Diseases. J. Infect. Dis. 2018, 218, 347–354. [Google Scholar] [CrossRef] [PubMed]
- WHO. Influenza (Seasonal). Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 6 November 2018).
- Iuliano, A.D.; Roguski, K.M.; Chang, H.H.; Muscatello, D.J.; Palekar, R.; Tempia, S.; Cohen, C.; Gran, J.M.; Schanzer, D.; Cowling, B.J.; et al. Estimates of global seasonal influenza-associated respiratory mortality: A modelling study. Lancet 2018, 391, 1285–1300. [Google Scholar] [CrossRef]
- Van Kerkhove, M.D.; Hirve, S.; Koukounari, A.; Mounts, A.W.; H1N1pdm Serology Working Group. Estimating age-specific cumulative incidence for the 2009 influenza pandemic: A meta-analysis of A(H1N1)pdm09 serological studies from 19 countries. Influenza Other Respir. Viruses 2013, 7, 872–886. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, K.; Viboud, C.; Simonsen, L. Antibody response to influenza vaccination in the elderly: A quantitative review. Vaccine 2006, 24, 1159–1169. [Google Scholar] [CrossRef]
- Soema, P.C.; Kompier, R.; Amorij, J.P.; Kersten, G.F. Current and next generation influenza vaccines: Formulation and production strategies. Eur. J. Pharm. Biopharm. 2015, 94, 251–263. [Google Scholar] [CrossRef]
- Lewnard, J.A.; Cobey, S. Immune history and influenza vaccine effectiveness. Vaccines 2018, 6, 28. [Google Scholar] [CrossRef]
- CDC. Seasonal Flu Vaccine Effectiveness Studies. Available online: https://www.cdc.gov/flu/vaccines-work/effectiveness-studies.htm (accessed on 11 December 2020).
- Grohskopf, L.A.; Alyanak, E.; Broder, K.R.; Blanton, L.H.; Fry, A.M.; Jernigan, D.B.; Atmar, R.L. Prevention and control of seasonal influenza with vaccines: Recommendations of the advisory committee on immunization practices—United States, 2020–21 influenza season. MMWR Recomm. Rep. 2020, 69, 1–24. [Google Scholar] [CrossRef]
- Hussain, M.; Galvin, H.D.; Haw, T.Y.; Nutsford, A.N.; Husain, M. Drug resistance in influenza A virus: The epidemiology and management. Infect. Drug Resist. 2017, 10, 121–134. [Google Scholar] [CrossRef]
- Gupta, G.S.G.A.; Gupta, R.K. Lectins: An overview. In Animal Lectins: Forms, Functions and Clinical Applications; Gupta, G.S., Ed.; Springer: New York, NY, USA, 2012; Volume 1, p. 1108. [Google Scholar]
- Liu, Y.; Liu, J.; Pang, X.; Liu, T.; Ning, Z.; Cheng, G. The roles of direct recognition by animal lectins in antiviral immunity and viral pathogenesis. Molecules 2015, 20, 2272–2295. [Google Scholar] [CrossRef]
- Mason, C.P.; Tarr, A.W. Human lectins and their roles in viral infections. Molecules 2015, 20, 2229–2271. [Google Scholar] [CrossRef]
- Wang, S.F.; Huang, J.C.; Lee, Y.M.; Liu, S.J.; Chan, Y.J.; Chau, Y.P.; Chong, P.; Chen, Y.M. DC-SIGN mediates avian H5N1 influenza virus infection in cis and in trans. Biochem. Biophys. Res. Commun. 2008, 373, 561–566. [Google Scholar] [CrossRef]
- Machala, E.A.; McSharry, B.P.; Rouse, B.T.; Abendroth, A.; Slobedman, B. Gal power: The diverse roles of galectins in regulating viral infections. J. Gen. Virol. 2019, 100, 333–349. [Google Scholar] [CrossRef]
- Wang, W.H.; Lin, C.Y.; Chang, M.R.; Urbina, A.N.; Assavalapsakul, W.; Thitithanyanont, A.; Chen, Y.H.; Liu, F.T.; Wang, S.F. The role of galectins in virus infection—A systemic literature review. J. Microbiol. Immunol. Infect. 2020, 53, 925–935. [Google Scholar] [CrossRef]
- Teichberg, V.I.; Silman, I.; Beitsch, D.D.; Resheff, G. A beta-D-galactoside binding protein from electric organ tissue of Electrophorus electricus. Proc. Natl. Acad. Sci. USA 1975, 72, 1383–1387. [Google Scholar] [CrossRef] [PubMed]
- Houzelstein, D.; Goncalves, I.R.; Fadden, A.J.; Sidhu, S.S.; Cooper, D.N.; Drickamer, K.; Leffler, H.; Poirier, F. Phylogenetic analysis of the vertebrate galectin family. Mol. Biol. Evol. 2004, 21, 1177–1187. [Google Scholar] [CrossRef]
- Vasta, G.R.; Ahmed, H.; Nita-Lazar, M.; Banerjee, A.; Pasek, M.; Shridhar, S.; Guha, P.; Fernandez-Robledo, J.A. Galectins as self/non-self recognition receptors in innate and adaptive immunity: An unresolved paradox. Front. Immunol. 2012, 3, 199. [Google Scholar] [CrossRef]
- Cummings, R.D.; Liu, F.T.; Vasta, G.R. Galectins. In Essentials of Glycobiology, 3rd ed.; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Darvill, A.G., Kinoshita, T., Packer, N.H., Prestegard, J.H., et al., Eds.; Cold Spring Harbor: New York, NY, USA, 2017; pp. 469–480. [Google Scholar]
- Vasta, G.R. Roles of galectins in infection. Nat. Rev. Microbiol. 2009, 7, 424–438. [Google Scholar] [CrossRef]
- Ayona, D.; Fournier, P.E.; Henrissat, B.; Desnues, B. Utilization of Galectins by Pathogens for Infection. Front Immunol 2020, 11, 1877. [Google Scholar] [CrossRef]
- Yang, Z.-S.; Lin, C.-Y.; Huang, S.-W.; Wang, W.-H.; Urbina, A.N.; Tseng, S.-P.; Lu, P.-L.; Chen, Y.-H.; Wang, S.-F. Regulatory roles of galectins on influenza A virus and their potential as a therapeutic strategy. Biomed. Pharmacother. 2021, 139, 111713. [Google Scholar] [CrossRef]
- Bao, J.; Wang, X.; Liu, S.; Zou, Q.; Zheng, S.; Yu, F.; Chen, Y. Galectin-1 ameliorates influenza A H1N1pdm09 virus-induced acute lung injury. Front. Microbiol. 2020, 11, 1293. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Ouellet, M.; Mercier, S.; Pelletier, I.; Bounou, S.; Roy, J.; Hirabayashi, J.; Sato, S.; Tremblay, M.J. Galectin-1 acts as a soluble host factor that promotes HIV-1 infectivity through stabilization of virus attachment to host cells. J. Immunol. 2005, 174, 4120–4126. [Google Scholar] [CrossRef]
- Yang, M.L.; Chen, Y.H.; Wang, S.W.; Huang, Y.J.; Leu, C.H.; Yeh, N.C.; Chu, C.Y.; Lin, C.C.; Shieh, G.S.; Chen, Y.L.; et al. Galectin-1 binds to influenza virus and ameliorates influenza virus pathogenesis. J. Virol. 2011, 85, 10010–10020. [Google Scholar] [CrossRef]
- Chernyy, E.S.; Rapoport, E.M.; Andre, S.; Kaltner, H.; Gabius, H.J.; Bovin, N.V. Galectins promote the interaction of influenza virus with its target cell. Biochemistry 2011, 76, 958–967. [Google Scholar] [CrossRef]
- Fang, S.; Zhang, K.; Wang, T.; Wang, X.; Lu, X.; Peng, B.; Wu, W.; Zhang, R.; Chen, S.; Zhang, R.; et al. Primary study on the lesions and specific proteins in BEAS-2B cells induced with the 2009 A (H1N1) influenza virus. Appl. Microbiol. Biotechnol. 2014, 98, 9691–9701. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, J.; Cheng, Z.; Yang, S.; Chu, H.; Fan, Y.; Li, C.; Wong, B.H.; Zheng, S.; Zhu, Y.; et al. Functional variants regulating LGALS1 (Galectin 1) expression affect human susceptibility to influenza A(H7N9). Sci. Rep. 2015, 5, 8517. [Google Scholar] [CrossRef]
- Krzeslak, A.; Lipińska, A. Galectin-3 as a multifunctional protein. Cell. Mol. Biol. Lett. 2004, 9, 305–328. [Google Scholar]
- Suthahar, N.; Meijers, W.C.; Sillje, H.H.W.; Ho, J.E.; Liu, F.T.; de Boer, R.A. Galectin-3 activation and inhibition in heart failure and cardiovascular disease: An update. Theranostics 2018, 8, 593–609. [Google Scholar] [CrossRef]
- Menon, R.P.; Hughes, R.C. Determinants in the N-terminal domains of galectin-3 for secretion by a novel pathway circumventing the endoplasmic reticulum-Golgi complex. Eur. J. Biochem. 1999, 264, 569–576. [Google Scholar] [CrossRef]
- Dong, R.; Zhang, M.; Hu, Q.; Zheng, S.; Soh, A.; Zheng, Y.; Yuan, H. Galectin-3 as a novel biomarker for disease diagnosis and a target for therapy (Review). Int. J. Mol. Med. 2018, 41, 599–614. [Google Scholar] [CrossRef]
- Xue, J.; Fu, C.; Cong, Z.; Peng, L.; Peng, Z.; Chen, T.; Wang, W.; Jiang, H.; Wei, Q.; Qin, C. Galectin-3 promotes caspase-independent cell death of HIV-1-infected macrophages. FEBS J. 2017, 284, 97–113. [Google Scholar] [CrossRef]
- Wang, S.-F.; Tsao, C.-H.; Lin, Y.-T.; Hsu, D.K.; Chiang, M.-L.; Lo, C.-H.; Chien, F.-C.; Chen, P.; Chen, Y.-M.A.; Chen, H.-Y.; et al. Galectin-3 promotes HIV-1 budding via association with Alix and Gag p6. Glycobiology 2014, 24, 1022–1035. [Google Scholar] [CrossRef]
- Li, S.-W.; Yang, T.-C.; Lai, C.-C.; Huang, S.-H.; Liao, J.-M.; Wan, L.; Lin, Y.-J.; Lin, C.-W. Antiviral activity of aloe-emodin against influenza A virus via galectin-3 up-regulation. Eur. J. Pharmacol. 2014, 738, 125–132. [Google Scholar] [CrossRef]
- WHO. Influenza (Avian and Other Zoonotic). 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(avian-and-other-zoonotic) (accessed on 13 November 2018).
- Lupfer, C.; Malik, A.; Kanneganti, T.D. Inflammasome control of viral infection. Curr. Opin. Virol. 2015, 12, 38–46. [Google Scholar] [CrossRef]
- Chen, Y.J.; Wang, S.F.; Weng, I.C.; Hong, M.H.; Lo, T.H.; Jan, J.T.; Hsu, L.C.; Chen, H.Y.; Liu, F.T. Galectin-3 enhances avian H5N1 influenza A virus-induced pulmonary inflammation by promoting NLRP3 inflammasome activation. Am. J. Pathol. 2018, 188, 1031–1042. [Google Scholar] [CrossRef]
- Nita-Lazar, M.; Banerjee, A.; Feng, C.; Vasta, G.R. Galectins regulate the inflammatory response in airway epithelial cells exposed to microbial neuraminidase by modulating the expression of SOCS1 and RIG1. Mol. Immunol. 2015, 68, 194–202. [Google Scholar] [CrossRef]
- Wang, H.; Huang, W.; Orwenyo, J.; Banerjee, A.; Vasta, G.R.; Wang, L.X. Design and synthesis of glycoprotein-based multivalent glyco-ligands for influenza hemagglutinin and human galectin-3. Bioorg. Med. Chem. 2013, 21, 2037–2044. [Google Scholar] [CrossRef]
- Nita-Lazar, M.; Banerjee, A.; Feng, C.; Amin, M.N.; Frieman, M.B.; Chen, W.H.; Cross, A.S.; Wang, L.X.; Vasta, G.R. Desialylation of airway epithelial cells during influenza virus infection enhances pneumococcal adhesion via galectin binding. Mol. Immunol. 2015, 65, 1–16. [Google Scholar] [CrossRef]
- Heusschen, R.; Schulkens, I.A.; van Beijnum, J.; Griffioen, A.W.; Thijssen, V.L. Endothelial LGALS9 splice variant expression in endothelial cell biology and angiogenesis. Biochim. Biophys. Acta 2014, 1842, 284–292. [Google Scholar] [CrossRef][Green Version]
- Leffler, H.; Carlsson, S.; Hedlund, M.; Qian, Y.; Poirier, F. Introduction to galectins. Glycoconj. J. 2002, 19, 433–440. [Google Scholar] [CrossRef]
- Wada, J.; Kanwar, Y.S. Identification and characterization of galectin-9, a novel beta-galactoside-binding mammalian lectin. J. Biol. Chem. 1997, 272, 6078–6086. [Google Scholar] [CrossRef]
- Hirashima, M.; Kashio, Y.; Nishi, N.; Yamauchi, A.; Imaizumi, T.A.; Kageshita, T.; Saita, N.; Nakamura, T. Galectin-9 in physiological and pathological conditions. Glycoconj. J. 2002, 19, 593–600. [Google Scholar] [CrossRef]
- Lai, J.H.; Luo, S.F.; Wang, M.Y.; Ho, L.J. Translational implication of galectin-9 in the pathogenesis and treatment of viral infection. Int. J. Mol. Sci. 2017, 18, 2108. [Google Scholar] [CrossRef]
- Merani, S.; Chen, W.; Elahi, S. The bitter side of sweet: The role of galectin-9 in immunopathogenesis of viral infections. Rev. Med. Virol. 2015, 25, 175–186. [Google Scholar] [CrossRef]
- Hattori, T.A.T.; Fujioka, Y.; Maruyama, J.; Nakayama, Y.; Ohba, Y.; Niki, T.; Miyazaki, T.; Hirashima, M.; Kida, H. Inhibition of influenza A virus infection by Galectin-9. Jpn. J. Vet. Res. 2013, 61, 5–18. [Google Scholar]
- Zhu, C.; Anderson, A.C.; Schubart, A.; Xiong, H.; Imitola, J.; Khoury, S.J.; Zheng, X.X.; Strom, T.B.; Kuchroo, V.K. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat. Immunol. 2005, 6, 1245–1252. [Google Scholar] [CrossRef]
- Vega-Carrascal, I.; Reeves, E.P.; McElvaney, N.G. The role of TIM-containing molecules in airway disease and their potential as therapeutic targets. J. Inflamm. Res. 2012, 5, 77–87. [Google Scholar]
- Van de Weyer, P.S.; Muehlfeit, M.; Klose, C.; Bonventre, J.V.; Walz, G.; Kuehn, E.W. A highly conserved tyrosine of Tim-3 is phosphorylated upon stimulation by its ligand galectin-9. Biochem. Biophys. Res. Commun. 2006, 351, 571–576. [Google Scholar] [CrossRef]
- Sharma, S.; Sundararajan, A.; Suryawanshi, A.; Kumar, N.; Veiga-Parga, T.; Kuchroo, V.K.; Thomas, P.G.; Sangster, M.Y.; Rouse, B.T. T cell immunoglobulin and mucin protein-3 (Tim-3)/galectin-9 interaction regulates influenza A virus-specific humoral and CD8 T-cell responses. Proc. Natl. Acad. Sci. USA 2011, 108, 19001–19006. [Google Scholar] [CrossRef]
- Katoh, S.; Ikeda, M.; Shimizu, H.; Mouri, K.; Obase, Y.; Kobashi, Y.; Fukushima, K.; Hirashima, M.; Oka, M. Increased levels of plasma galectin-9 in patients with influenza virus infection. Tohoku J. Exp. Med. 2014, 232, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.Y.; Rabinovich, G.A.; Liu, F.T. Galectins: Structure, function and therapeutic potential. Expert Rev. Mol. Med. 2008, 10, e17. [Google Scholar] [CrossRef]
- Vasta, G.R. Galectins as pattern recognition receptors: Structure, function, and evolution. Adv. Exp. Med. Biol. 2012, 946, 21–36. [Google Scholar] [PubMed]
- Arthur, C.M.; Baruffi, M.D.; Cummings, R.D.; Stowell, S.R. Evolving mechanistic insights into galectin functions. Methods Mol. Biol. 2015, 1207, 1–35. [Google Scholar] [PubMed]
- Oda, Y.; Herrmann, J.; Gitt, M.A.; Turck, C.W.; Burlingame, A.L.; Barondes, S.H.; Leffler, H. Soluble lactose-binding lectin from rat intestine with two different carbohydrate-binding domains in the same peptide chain. J. Biol. Chem. 1993, 268, 5929–5939. [Google Scholar] [CrossRef]
- Plans, P. Recommendations for the prevention and treatment of influenza using antiviral drugs based on cost-effectiveness. Expert Rev. Pharmacoecon. Outcomes Res. 2008, 8, 563–573. [Google Scholar] [CrossRef]
- McKimm-Breschkin, J.L. Influenza neuraminidase inhibitors: Antiviral action and mechanisms of resistance. Influenza Other Respir. Viruses 2013, 7, 25–36. [Google Scholar] [CrossRef]
- Matos, A.R.; Resende, P.C.; Miranda, M.D.; Garcia, C.C.; Caetano, B.C.; Lopes, J.C.O.; Debur, M.C.; Cury, A.L.F.; Vianna, L.A.; Lima, M.C.; et al. Susceptibility of Brazilian influenza A(H1N1)pdm09 viruses to neuraminidase inhibitors in the 2014–2016 seasons: Identification of strains bearing mutations associated with reduced inhibition profile. Antivir. Res. 2018, 154, 35–43. [Google Scholar] [CrossRef]
- Matrosovich, M.; Klenk, H.D. Natural and synthetic sialic acid-containing inhibitors of influenza virus receptor binding. Rev. Med. Virol. 2003, 13, 85–97. [Google Scholar] [CrossRef]
- Skehel, J.J.; Stevens, D.J.; Daniels, R.S.; Douglas, A.R.; Knossow, M.; Wilson, I.A.; Wiley, D.C. A carbohydrate side chain on hemagglutinins of Hong Kong influenza viruses inhibits recognition by a monoclonal antibody. Proc. Natl. Acad. Sci. USA 1984, 81, 1779–1783. [Google Scholar] [CrossRef]
- Wilson, I.A.; Skehel, J.J.; Wiley, D.C. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature 1981, 289, 366–373. [Google Scholar] [CrossRef] [PubMed]
- To, K.K.; Ng, K.H.; Que, T.L.; Chan, J.M.; Tsang, K.Y.; Tsang, A.K.; Chen, H.; Yuen, K.Y. Avian influenza A H5N1 virus: A continuous threat to humans. Emerg. Microbes Infect. 2012, 1, e25. [Google Scholar] [CrossRef] [PubMed]
- Sehrawat, S.; Suryawanshi, A.; Hirashima, M.; Rouse, B.T. Role of Tim-3/galectin-9 inhibitory interaction in viral-induced immunopathology: Shifting the balance toward regulators. J. Immunol. 2009, 182, 3191–3201. [Google Scholar] [CrossRef] [PubMed]

| Galectin | Antiviral Effects | Target Virus | Ref. |
|---|---|---|---|
| Gal-1 directly binds to the envelope glycoproteins of influenza virus and constrain the viral hemagglutination activity and infectivity. | A/WSN/1933(H1N1) | [28] | |
| Galectin-1 | Recombinant Gal-1 (rGal-1) treatment reduced mice fatality via mediating the expression of cytokines and chemokines. | 2009 influenza A H1N1 subtype (H1N1pdm09) | [25] |
| Gal-1 participated in regulation of cytopathic processes by H1N1pdm09 virus to induce an arrest of the cell cycle at the G0/G1 phase. | H1N1pdm09 | [30] | |
| Gal-1 expression was correlated with the differential susceptibility to H7N9 influenza via extracellular matrix (ECM)-receptor interaction and mitogen-activated protein kinase (MAPK) signaling. | Human H7N9 isolates | [31] | |
| IAVs and S. pneumoniae coinfection induced the secretion of Gal-1 to the epithelial cell surface and further modulated the expression of SOCS1 and RIG1 and activate ERK, AKT, or JAK/STAT1 signaling pathways. | H1N1pdm09 | [42] | |
| Galectin-3 | Aloe-emodin treatment ameliorated influenza H1N1 virus infection via up-regulation of Gal-3 expression to further trigger antiviral genes expression | A/Taiwan/CMUH01/2007(H1N1) | [38] |
| Gal-3 enhances effects of H5N1 promoting host inflammatory response by up-regulating IL-1β via NLRP3. | A/Vietnam/1204/03 | [41] | |
| IAVs and S. pneumoniae coinfection induced the secretion of Gal-3 to the epithelial cell surface and further modulated the expression of SOCS1 and RIG1, and activate ERK, AKT, or JAK/STAT1 signaling pathways. | H1N1pdm09 | [42] | |
| Gal-3 preferred binding to desialylated multivalent glycoligands. | A/PuertoRico/08/1934 (H1N1) | [43] | |
| Galectin-9 | Gal-9 inhibited the infection of IAVs via Gal-9 binding to influenza virus particles to inhibit virus attachment. | A/Puerto Rico/8/34 (H1N1); Aichi/2/68 (H3N2); A/Hong Kong/483/97 (H5N1) | [51] |
| Virus-specific CD8 T cells upregulate Tim-3 expression and Gal-9/Tim-3 interaction induce cell apoptosis after IAV infection from in vitro and ex vivo assays. | HK/×31 (H3N2) A/Puerto Rico/8/34 (H1N1)) | [55] | |
| Influenza virus infection induces plasma Gal-9 expression, suggesting Gal-9 as a possible biomarker for influenza. | Seasonal influenza virus | [56] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-Y.; Yang, Z.-S.; Wang, W.-H.; Urbina, A.N.; Lin, Y.-T.; Huang, J.C.; Liu, F.-T.; Wang, S.-F. The Antiviral Role of Galectins toward Influenza A Virus Infection—An Alternative Strategy for Influenza Therapy. Pharmaceuticals 2021, 14, 490. https://doi.org/10.3390/ph14050490
Lin C-Y, Yang Z-S, Wang W-H, Urbina AN, Lin Y-T, Huang JC, Liu F-T, Wang S-F. The Antiviral Role of Galectins toward Influenza A Virus Infection—An Alternative Strategy for Influenza Therapy. Pharmaceuticals. 2021; 14(5):490. https://doi.org/10.3390/ph14050490
Chicago/Turabian StyleLin, Chih-Yen, Zih-Syuan Yang, Wen-Hung Wang, Aspiro Nayim Urbina, Yu-Ting Lin, Jason C. Huang, Fu-Tong Liu, and Sheng-Fan Wang. 2021. "The Antiviral Role of Galectins toward Influenza A Virus Infection—An Alternative Strategy for Influenza Therapy" Pharmaceuticals 14, no. 5: 490. https://doi.org/10.3390/ph14050490
APA StyleLin, C.-Y., Yang, Z.-S., Wang, W.-H., Urbina, A. N., Lin, Y.-T., Huang, J. C., Liu, F.-T., & Wang, S.-F. (2021). The Antiviral Role of Galectins toward Influenza A Virus Infection—An Alternative Strategy for Influenza Therapy. Pharmaceuticals, 14(5), 490. https://doi.org/10.3390/ph14050490







