Apigenin Ameliorates Hyperuricemia and Renal Injury through Regulation of Uric Acid Metabolism and JAK2/STAT3 Signaling Pathway
Abstract
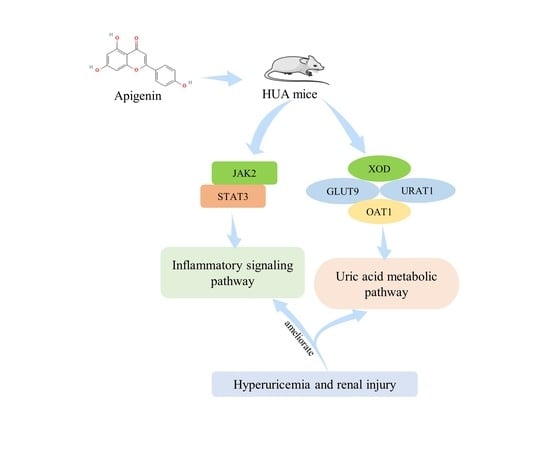
:1. Introduction
2. Results
2.1. Apigenin Reduces Serum Levels of UA, CRE, and BUN, and Increases Urine Levels of UA and CRE in HUA Mice
2.2. Apigenin Ameliorates Renal Pathological Microstructures and Urine Protein Levels in HUA Mouse
2.3. HUA Exposure and Apigenin Intervention Do Not Affect Liver Function and Pathological Microstructures in Mice
2.4. Apigenin Suppresses Serum Inflammatory Response in HUA Mice
2.5. Apigenin Decreases Liver XOD Levels and Renal Expression Levels of GLUT9 and URAT1, and Increases Renal Expression Levels of OAT1 in HUA Mouse
2.6. Interactions between Apigenin Molecule with UA Transporters and JAK2 Protein by Molecular Docking
2.7. Apigenin Inhibits JAK2/STAT3 Signaling Pathway in HUA Mouse
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Animals and Treatments
4.3. Biochemical Analysis
4.4. H&E and Masson Trichome Staining
4.5. IHC Staining
4.6. Western Blot Assay
4.7. Molecular Docking
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Su, H.Y.; Yang, C.; Liang, D.; Liu, H.F. Research Advances in the Mechanisms of Hyperuricemia-Induced Renal Injury. BioMed Res. Int. 2020, 2020, 5817348. [Google Scholar] [CrossRef] [PubMed]
- Ichida, K.; Matsuo, H.; Takada, T.; Nakayama, A.; Murakami, K.; Shimizu, T.; Yamanashi, Y.; Kasuga, H.; Nakashima, H.; Nakamura, T.; et al. Decreased extra-renal urate excretion is a common cause of hyperuricemia. Nat. Commun. 2012, 3, 764. [Google Scholar] [CrossRef] [PubMed]
- Ragab, G.; Elshahaly, M.; Bardin, T. Gout: An old disease in new perspective—A review. J. Adv. Res. 2017, 8, 495–511. [Google Scholar] [CrossRef] [PubMed]
- Goli, P.; Riahi, R.; Daniali, S.S.; Pourmirzaei, M.; Kelishadi, R. Association of serum uric acid concentration with components of pediatric metabolic syndrome: A systematic review and meta-analysis. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2020, 25, 43. [Google Scholar] [CrossRef]
- Saito, Y.; Tanaka, A.; Node, K.; Kobayashi, Y. Uric acid and cardiovascular disease: A clinical review. J. Cardiol. 2021, 78, 51–57. [Google Scholar] [CrossRef]
- Yu, H.; Liu, X.; Song, Y.; Cheng, J.; Bao, H.; Qin, L.; Zhou, X.; Wang, L.; Peng, A. Safety and Efficacy of Benzbromarone and Febuxostat in Hyperuricemia Patients with Chronic Kidney Disease: A Prospective Pilot Study. Clin. Exp. Nephrol. 2018, 22, 1324–1330. [Google Scholar] [CrossRef]
- Chen, S.; Yang, H.; Chen, Y.; Wang, J.; Xu, L.; Miao, M.; Xu, C. Association between serum uric acid levels and dyslipidemia in Chinese adults: A cross-sectional study and further meta-analysis. Medicine 2020, 99, e19088. [Google Scholar] [CrossRef]
- Yanai, H.; Adachi, H.; Hakoshima, M.; Katsuyama, H. Molecular Biological and Clinical Understanding of the Pathophysiology and Treatments of Hyperuricemia and Its Association with Metabolic Syndrome, Cardiovascular Diseases and Chronic Kidney Disease. Int. J. Mol. Sci. 2021, 22, 9221. [Google Scholar] [CrossRef]
- Ji, X.; Leng, X.Y.; Dong, Y.; Ma, Y.H.; Xu, W.; Cao, X.P.; Hou, X.H.; Dong, Q.; Tan, L.; Yu, J.T. Modifiable risk factors for carotid atherosclerosis: A meta-analysis and systematic review. Ann. Transl. Med. 2019, 7, 632. [Google Scholar] [CrossRef]
- Van der Schaft, N.; Brahimaj, A.; Wen, K.X.; Franco, O.H.; Dehghan, A. The association between serum uric acid and the incidence of prediabetes and type 2 diabetes mellitus: The Rotterdam Study. PLoS ONE 2017, 12, e0179482. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Z.; Luo, J.; Jiang, Y.; Li, L.; Chen, Y.; Zhang, L.; Huang, Q.; Cao, Y.; Zhou, P.; et al. Apigenin ameliorates hyperuricemic nephropathy by inhibiting URAT1 and GLUT9 and relieving renal fibrosis via the Wnt/β-catenin pathway. Phytomed. Int. J. Phytother. Phytopharm. 2021, 87, 153585. [Google Scholar] [CrossRef] [PubMed]
- Dehlin, M.; Jacobsson, L.; Roddy, E. Global epidemiology of gout: Prevalence, incidence, treatment patterns and risk factors. Nat. Rev. Rheumatol. 2020, 16, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Chalès, G. How should we manage asymptomatic hyperuricemia? Jt. Bone Spine 2019, 86, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Danve, A.; Sehra, S.T.; Neogi, T. Role of diet in hyperuricemia and gout. Best Pract. Res. Clin. Rheumatol. 2021, 35, 101723. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.J.; Anoopkumar, K.; Krishnan, V. Asymptomatic hyperuricemia: Is it time to intervene? Clin. Rheumatol. 2017, 36, 2637–2644. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, X.; Wu, J.; Huang, Z.; Zhao, Z.; Zhang, X.; Xue, Y.; Wan, W.; Li, C.; Zhang, W.; et al. Prevalence of Hyperuricemia Among Chinese Adults: Findings From Two Nationally Representative Cross-Sectional Surveys in 2015–16 and 2018–19. Front. Immunol. 2021, 12, 791983. [Google Scholar] [CrossRef]
- Endocrinology, C.S.O. Chinese guidelines for the diagnosis and treatment of hyperuricemia and gout (2019). Chin. J. Endocrinol. Metab. 2020, 36, 1–13. [Google Scholar]
- Fontana, R.J.; Li, Y.J.; Phillips, E.; Saeed, N.; Barnhart, H.; Kleiner, D.; Hoofnagle, J. Allopurinol hepatotoxicity is associated with human leukocyte antigen Class I alleles. Liver Int. Off. J. Int. Assoc. Study Liver 2021, 41, 1884–1893. [Google Scholar] [CrossRef]
- Zhang, S.; Xie, Q.; Xie, S.; Chen, J.; Deng, Q.; Zhong, L.; Guo, J.; Yu, Y. The association between urate-lowering therapies and treatment-related adverse events, liver damage, and major adverse cardiovascular events (MACE): A network meta-analysis of randomized trials. Pharmacotherapy 2021, 41, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Nie, Y.; Chang, Y.; Zeng, S.; Liang, C.; Zheng, X.; Xiao, D.; Zhan, S.; Zheng, Q. Protective effects of Rhizoma smilacis glabrae extracts on potassium oxonate- and monosodium urate-induced hyperuricemia and gout in mice. Phytomed. Int. J. Phytother. Phytopharm. 2019, 59, 152772. [Google Scholar] [CrossRef]
- Müller, C.M.d.S.; Coelho, G.B.; Araújo, M.C.d.P.M.; Saúde-Guimarães, D.A. Lychnophora pinaster ethanolic extract and its chemical constituents ameliorate hyperuricemia and related inflammation. J. Ethnopharmacol. 2019, 242, 112040. [Google Scholar] [CrossRef]
- Kanemitsu, T.; Tsurudome, Y.; Kusunose, N.; Oda, M.; Matsunaga, N.; Koyanagi, S.; Ohdo, S. Periodic variation in bile acids controls circadian changes in uric acid via regulation of xanthine oxidase by the orphan nuclear receptor PPARα. J. Biol. Chem. 2017, 292, 21397–21406. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, J.; Tang, L.; Li, P.; Ru, J.; Bai, Y. Withaferin A protects against hyperuricemia induced kidney injury and its possible mechanisms. Bioengineered 2021, 12, 589–600. [Google Scholar] [CrossRef]
- Lee, T.H.; Chen, J.J.; Wu, C.Y.; Yang, C.W.; Yang, H.Y. Hyperuricemia and Progression of Chronic Kidney Disease: A Review from Physiology and Pathogenesis to the Role of Urate-Lowering Therapy. Diagnostics 2021, 11, 1674. [Google Scholar] [CrossRef]
- Xu, L.; Lin, G.; Yu, Q.; Li, Q.; Mai, L.; Cheng, J.; Xie, J.; Liu, Y.; Su, Z.; Li, Y. Anti-Hyperuricemic and Nephroprotective Effects of Dihydroberberine in Potassium Oxonate- and Hypoxanthine-Induced Hyperuricemic Mice. Front. Pharm. 2021, 12, 645879. [Google Scholar] [CrossRef]
- Zhao, L.; Li, Y.; Yao, D.; Sun, R.; Liu, S.; Chen, X.; Lin, C.; Huang, J.; Wang, J.; Li, G. Pharmacological Basis for Use of a Novel Compound in Hyperuricemia: Anti-Hyperuricemic and Anti-Inflammatory Effects. Front. Pharmacol. 2021, 12, 772504. [Google Scholar] [CrossRef]
- Liu, L.; Jiang, S.; Liu, X.; Tang, Q.; Chen, Y.; Qu, J.; Wang, L.; Wang, Q.; Wang, Y.; Wang, J.; et al. Inflammatory Response and Oxidative Stress as Mechanism of Reducing Hyperuricemia of Gardenia jasminoides-Poria cocos with Network Pharmacology. Oxid. Med. Cell. Longev. 2021, 2021, 8031319. [Google Scholar] [CrossRef]
- Jung, S.W.; Kim, S.M.; Kim, Y.G.; Lee, S.H.; Moon, J.Y. Uric acid and inflammation in kidney disease. Am. J. Physiol. Ren. Physiol. 2020, 318, F1327–F1340. [Google Scholar] [CrossRef]
- Pan, J.; Zhang, C.; Shi, M.; Guo, F.; Liu, J.; Li, L.; Ren, Q.; Tao, S.; Tang, M.; Ye, H.; et al. Ethanol extract of Liriodendron chinense (Hemsl.) Sarg barks attenuates hyperuricemic nephropathy by inhibiting renal fibrosis and inflammation in mice. J. Ethnopharmacol. 2021, 264, 113278. [Google Scholar] [CrossRef]
- Ren, Q.; Tao, S.; Guo, F.; Wang, B.; Yang, L.; Ma, L.; Fu, P. Natural flavonol fisetin attenuated hyperuricemic nephropathy via inhibiting IL-6/JAK2/STAT3 and TGF-β/SMAD3 signaling. Phytomed. Int. J. Phytother. Phytopharm. 2021, 87, 153552. [Google Scholar] [CrossRef]
- Magoro, T.; Dandekar, A.; Jennelle, L.T.; Bajaj, R.; Lipkowitz, G.; Angelucci, A.R.; Bessong, P.O.; Hahn, Y.S. IL-1β/TNF-α/IL-6 inflammatory cytokines promote STAT1-dependent induction of CH25H in Zika virus-infected human macrophages. J. Biol. Chem. 2019, 294, 14591–14602. [Google Scholar] [CrossRef]
- Nie, Q.; Liu, M.; Zhang, Z.; Zhang, X.; Wang, C.; Song, G. The effects of hyperuricemia on endothelial cells are mediated via GLUT9 and the JAK2/STAT3 pathway. Mol. Biol. Rep. 2021, 48, 8023–8032. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, J.; Zhang, N.; Chen, J. Astilbin improves potassium oxonate-induced hyperuricemia and kidney injury through regulating oxidative stress and inflammation response in mice. Biomed. Pharmacother. 2016, 83, 975–988. [Google Scholar] [CrossRef]
- Chesworth, R.; Gamage, R.; Ullah, F.; Sonego, S.; Millington, C.; Fernandez, A.; Liang, H.; Karl, T.; Münch, G.; Niedermayer, G.; et al. Spatial Memory and Microglia Activation in a Mouse Model of Chronic Neuroinflammation and the Anti-inflammatory Effects of Apigenin. Front. Neurosci. 2021, 15, 699329. [Google Scholar] [CrossRef]
- Mo, S.F.; Zhou, F.; Lv, Y.Z.; Hu, Q.H.; Zhang, D.M.; Kong, L.D. Hypouricemic action of selected flavonoids in mice: Structure-activity relationships. Biol. Pharm. Bull. 2007, 30, 1551–1556. [Google Scholar] [CrossRef]
- Adachi, S.I.; Oyama, M.; Kondo, S.; Yagasaki, K. Comparative effects of quercetin, luteolin, apigenin and their related polyphenols on uric acid production in cultured hepatocytes and suppression of purine bodies-induced hyperuricemia by rutin in mice. Cytotechnology 2021, 73, 343–351. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Du, Y.; Chen, Z.; Guo, J.; Weng, X.; Wang, X.; Wang, M.; Chen, D.; Liu, X. Effects of apigenin pretreatment against renal ischemia/reperfusion injury via activation of the JAK2/STAT3 pathway. Biomed. Pharmacother. 2017, 95, 1799–1808. [Google Scholar] [CrossRef]
- Perez-Ruiz, F.; Becker, M.A. Inflammation: A possible mechanism for a causative role of hyperuricemia/gout in cardiovascular disease. Curr. Med. Res. Opin. 2015, 31 (Suppl. 2), 9–14. [Google Scholar] [CrossRef]
- Qian, X.; Jiang, Y.; Luo, Y.; Jiang, Y. The Anti-hyperuricemia and Anti-inflammatory Effects of Atractylodes Macrocephala in Hyperuricemia and Gouty Arthritis Rat Models. Comb. Chem. High Throughput Screen. 2022, in press, 1–15. [Google Scholar] [CrossRef]
- Méndez Landa, C.E. Renal Effects of Hyperuricemia. Contrib. Nephrol. 2018, 192, 8–16. [Google Scholar] [CrossRef]
- Zheng, L.; Wen, X.L.; Dai, Y.C. Mechanism of Jianpi Qingchang Huashi Recipe in treating ulcerative colitis: A study based on network pharmacology and molecular docking. World J. Clin. Cases 2021, 9, 7653–7670. [Google Scholar] [CrossRef]
- Xian, M.; Ji, S.; Chen, C.; Liang, S.; Wang, S. Sparganin A alleviates blood stasis syndrome and its key targets by molecular docking. RSC Adv. 2019, 9, 37978–37985. [Google Scholar] [CrossRef]
- Ragunathan, A.; Malathi, K.; Ramaiah, S.; Anbarasu, A. FtsA as a cidal target for Staphylococcus aureus: Molecular docking and dynamics studies. J. Cell. Biochem. 2018, 120, 7751–7758. [Google Scholar] [CrossRef]
- Desai, V.; Desai, S.; Gaonkar, S.N.; Palyekar, U.; Joshi, S.D.; Dixit, S.K. Novel quinoxalinyl chalcone hybrid scaffolds as enoyl ACP reductase inhibitors: Synthesis, molecular docking and biological evaluation. Bioorg. Med. Chem. Lett. 2017, 27, 2174–2180. [Google Scholar] [CrossRef]
- Shi, M.; Lin, Z.; Ye, L.; Chen, X.; Zhang, W.; Zhang, Z.; Luo, F.; Liu, Y.; Shi, M. Estrogen receptor-regulated SOCS3 modulation via JAK2/STAT3 pathway is involved in BPF-induced M1 polarization of macrophages. Toxicology 2020, 433–434, 152404. [Google Scholar] [CrossRef]
- Shan, R.; Ning, Y.; Ma, Y.; Gao, X.; Zhou, Z.; Jin, C.; Wu, J.; Lv, J.; Li, L. Incidence and Risk Factors of Hyperuricemia among 2.5 Million Chinese Adults during the Years 2017–2018. Int. J. Environ. Res. Public Health 2021, 18, 2360. [Google Scholar] [CrossRef]
- Jiao, H.; Zhang, Y.; Yan, Z.; Wang, Z.G.; Liu, G.; Minshall, R.D.; Malik, A.B.; Hu, G. Caveolin-1 Tyr14 phosphorylation induces interaction with TLR4 in endothelial cells and mediates MyD88-dependent signaling and sepsis-induced lung inflammation. J. Immunol. (Baltimore Md. 1950) 2013, 191, 6191–6199. [Google Scholar] [CrossRef]
- Wu, Q.; Li, W.; Zhao, J.; Sun, W.; Yang, Q.; Chen, C.; Xia, P.; Zhu, J.; Zhou, Y.; Huang, G.; et al. Apigenin ameliorates doxorubicin-induced renal injury via inhibition of oxidative stress and inflammation. Biomed Pharm. 2021, 137, 111308. [Google Scholar] [CrossRef]
- Wang, E.; Chen, F.; Hu, X.; Yuan, Y. Protective effects of apigenin against furan-induced toxicity in mice. Food Funct. 2014, 5, 1804–1812. [Google Scholar] [CrossRef]
- Li, P.; Bukhari, S.N.A.; Khan, T.; Chitti, R.; Bevoor, D.B.; Hiremath, A.R.; SreeHarsha, N.; Singh, Y.; Gubbiyappa, K.S. Apigenin-Loaded Solid Lipid Nanoparticle Attenuates Diabetic Nephropathy Induced by Streptozotocin Nicotinamide Through Nrf2/HO-1/NF-kB Signalling Pathway. Int. J. Nanomed. 2020, 15, 9115–9124. [Google Scholar] [CrossRef]
- Chen, S.M.; Jahejo, A.R.; Nabi, F.; Ahmed, S.; Zhao, J.F.; Yu, J.; Zhang, C.L.; Ning, G.B.; Zhang, D.; Raza, S.H.A.; et al. Janus kinase/signal transducer and activator of transcription signaling pathway-related genes STAT3, SOCS3 and their role in thiram induced tibial dyschondroplasia chickens. Res. Vet. Sci. 2021, 136, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhao, H.; Wang, P.; Wang, J.; Zou, L. The roles of SOCS3 and STAT3 in bacterial infection and inflammatory diseases. Scand. J. Immunol. 2018, 88, e12727. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.Y.; Sp, N.; Jo, E.S.; Rugamba, A.; Kim, H.D.; Kim, I.H.; Park, J.C.; Bae, S.W.; Jang, K.J.; Yang, Y.M. Non-toxic sulfur inhibits LPS-induced inflammation by regulating TLR-4 and JAK2/STAT3 through IL-6 signaling. Mol. Med. Rep. 2021, 24, 485. [Google Scholar] [CrossRef]
- Seo, H.S.; Ku, J.M.; Choi, H.S.; Woo, J.K.; Lee, B.H.; Kim, D.S.; Song, H.J.; Jang, B.H.; Shin, Y.C.; Ko, S.G. Apigenin overcomes drug resistance by blocking the signal transducer and activator of transcription 3 signaling in breast cancer cells. Oncol. Rep. 2017, 38, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Baskin, R.; Majumder, A.; Sayeski, P.P. The recent medicinal chemistry development of Jak2 tyrosine kinase small molecule inhibitors. Curr. Med. Chem. 2010, 17, 4551–4558. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Mishra, S.K.; Noel, S.; Sharma, S.; Rath, S.K. Acute exposure of apigenin induces hepatotoxicity in Swiss mice. PLoS ONE 2012, 7, e31964. [Google Scholar] [CrossRef]
- Lin, G.; Yu, Q.; Xu, L.; Huang, Z.; Mai, L.; Jiang, L.; Su, Z.; Xie, J.; Li, Y.; Liu, Y.; et al. Berberrubine attenuates potassium oxonate- and hypoxanthine-induced hyperuricemia by regulating urate transporters and JAK2/STAT3 signaling pathway. Eur. J. Pharmacol. 2021, 912, 174592. [Google Scholar] [CrossRef]
- Wu, Y.L.; Chen, J.F.; Jiang, L.Y.; Wu, X.L.; Liu, Y.H.; Gao, C.J.; Wu, Y.; Yi, X.Q.; Su, Z.R.; Cai, J.; et al. The Extract of Sonneratia apetala Leaves and Branches Ameliorates Hyperuricemia in Mice by Regulating Renal Uric Acid Transporters and Suppressing the Activation of the JAK/STAT Signaling Pathway. Front. Pharmacol. 2021, 12, 698219. [Google Scholar] [CrossRef]
- Xiangxiang, Y.F.Z.; Minxia, P.; Li, B.; Suhong, C.; Guiyuan, L.V. Comparative Study of Hyperuricemia Models in Mice with Different Modeling Factors. Zhejiang Chin. Med. Univ. 2018, 42, 142–148. [Google Scholar] [CrossRef]
- Liu, X.Y.; Zhu, X.J.; Guo, J.; Wu, H.W.; Wang, Y.W. The Effect of Different Strains on Mouse Hyperucicemia Model. Prog. Mod. Biomed. 2011, 11, 4824–4827. [Google Scholar] [CrossRef]
- Lucas, T.; Riis, J.L.; Buchalski, Z.; Drolet, C.E.; Dawadi, A.; Granger, D.A. Reactivity of salivary uric acid in response to social evaluative stress in African Americans. Biol. Psychol. 2020, 153, 107882. [Google Scholar] [CrossRef] [PubMed]
- Goodman, A.M.; Wheelock, M.D.; Harnett, N.G.; Mrug, S.; Granger, D.A.; Knight, D.C. The hippocampal response to psychosocial stress varies with salivary uric acid level. Neuroscience 2016, 339, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, R.; Ulrich, H.; Zarate, C.A., Jr.; Machado-Vieira, R. Purinergic system dysfunction in mood disorders: A key target for developing improved therapeutics. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2015, 57, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, C.; Duan, S.; Yuan, X.; Liang, J.; Hou, S. Curcumin attenuates potassium oxonate-induced hyperuricemia and kidney inflammation in mice. Biomed. Pharm. 2019, 118, 109195. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, X.; Zhu, H.; Wang, J.; Ma, J.; Gu, M. Apigenin Protects Against Renal Tubular Epithelial Cell Injury and Oxidative Stress by High Glucose via Regulation of NF-E2-Related Factor 2 (Nrf2) Pathway. Med. Sci. Monit. 2019, 25, 5280–5288. [Google Scholar] [CrossRef]
- Seo, H.S.; Ku, J.M.; Choi, H.S.; Woo, J.K.; Jang, B.H.; Go, H.; Shin, Y.C.; Ko, S.G. Apigenin induces caspase-dependent apoptosis by inhibiting signal transducer and activator of transcription 3 signaling in HER2-overexpressing SKBR3 breast cancer cells. Mol. Med. Rep. 2015, 12, 2977–2984. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Tian, T.; Zheng, Y.; Zhou, L.; Dai, C.; Wang, M.; Lin, S.; Deng, Y.; Hao, Q.; Zhai, Z.; et al. Scutellarin inhibits proliferation and invasion of hepatocellular carcinoma cells via down-regulation of JAK2/STAT3 pathway. J. Cell. Mol. Med. 2019, 23, 3040–3044. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, B.; Zhao, X.; Lin, Y.; Wang, J.; Wang, X.; Hu, N.; Wang, S. Chlorogenic acid supplementation ameliorates hyperuricemia, relieves renal inflammation, and modulates intestinal homeostasis. Food Funct. 2021, 12, 5637–5649. [Google Scholar] [CrossRef] [PubMed]
- An, J.H.; Li, C.Y.; Chen, C.Y.; Wu, J.B.; Shen, H. Raloxifene Protects Cisplatin-Induced Renal Injury in Mice via Inhibiting Oxidative Stress. OncoTargets Ther. 2021, 14, 4879–4890. [Google Scholar] [CrossRef]
- Chen, B.; Wei, J.; Zhu, R.; Zhang, H.; Xia, B.; Liu, Y.; Dai, X.; Ye, Z.; Tian, Y.; Li, R.; et al. Fructus Ligustri Lucidi aqueous extract promotes calcium balance and short-chain fatty acids production in ovariectomized rats. J. Ethnopharmacol. 2021, 279, 114348. [Google Scholar] [CrossRef]









| Protein | XOD | GLUT9 | URAT1 | OAT1 | JAK2 |
|---|---|---|---|---|---|
| Crystal structure | 1FIQ | 4GBY | AF-Q96S37-F1 | AF-Q4U2R8-F1 | 3UGC |
| Total score | 6.20 | 3.87 | 4.90 | 6.14 | 6.48 |
| Grash score | −1.24 | −0.76 | −0.34 | −0.65 | −1.38 |
| Polar score | 3.89 | 1.16 | 2.49 | 2.61 | 3.50 |
| C-score score | 3 | 5 | 5 | 3 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Gao, H.; Zhang, Y.; Wang, S.; Lu, M.; Dai, X.; Liu, Y.; Shi, H.; Xu, T.; Yin, J.; et al. Apigenin Ameliorates Hyperuricemia and Renal Injury through Regulation of Uric Acid Metabolism and JAK2/STAT3 Signaling Pathway. Pharmaceuticals 2022, 15, 1442. https://doi.org/10.3390/ph15111442
Liu T, Gao H, Zhang Y, Wang S, Lu M, Dai X, Liu Y, Shi H, Xu T, Yin J, et al. Apigenin Ameliorates Hyperuricemia and Renal Injury through Regulation of Uric Acid Metabolism and JAK2/STAT3 Signaling Pathway. Pharmaceuticals. 2022; 15(11):1442. https://doi.org/10.3390/ph15111442
Chicago/Turabian StyleLiu, Tianyuan, Huimin Gao, Yueyi Zhang, Shan Wang, Meixi Lu, Xuan Dai, Yage Liu, Hanfen Shi, Tianshu Xu, Jiyuan Yin, and et al. 2022. "Apigenin Ameliorates Hyperuricemia and Renal Injury through Regulation of Uric Acid Metabolism and JAK2/STAT3 Signaling Pathway" Pharmaceuticals 15, no. 11: 1442. https://doi.org/10.3390/ph15111442
APA StyleLiu, T., Gao, H., Zhang, Y., Wang, S., Lu, M., Dai, X., Liu, Y., Shi, H., Xu, T., Yin, J., Gao, S., Wang, L., & Zhang, D. (2022). Apigenin Ameliorates Hyperuricemia and Renal Injury through Regulation of Uric Acid Metabolism and JAK2/STAT3 Signaling Pathway. Pharmaceuticals, 15(11), 1442. https://doi.org/10.3390/ph15111442