Wound-Healing Potential of Rhoifolin-Rich Fraction Isolated from Sanguisorba officinalis Roots Supported by Enhancing Re-Epithelization, Angiogenesis, Anti-Inflammatory, and Antimicrobial Effects
Abstract
1. Introduction
2. Results
2.1. LC-ESI-MS/MS Analysis of S. officinalis Extract
2.1.1. Flavonoids
2.1.2. Triterpenoids
2.2. Characterization of the Rhoifolin Rich Fraction RRF
2.3. In Vitro Activities
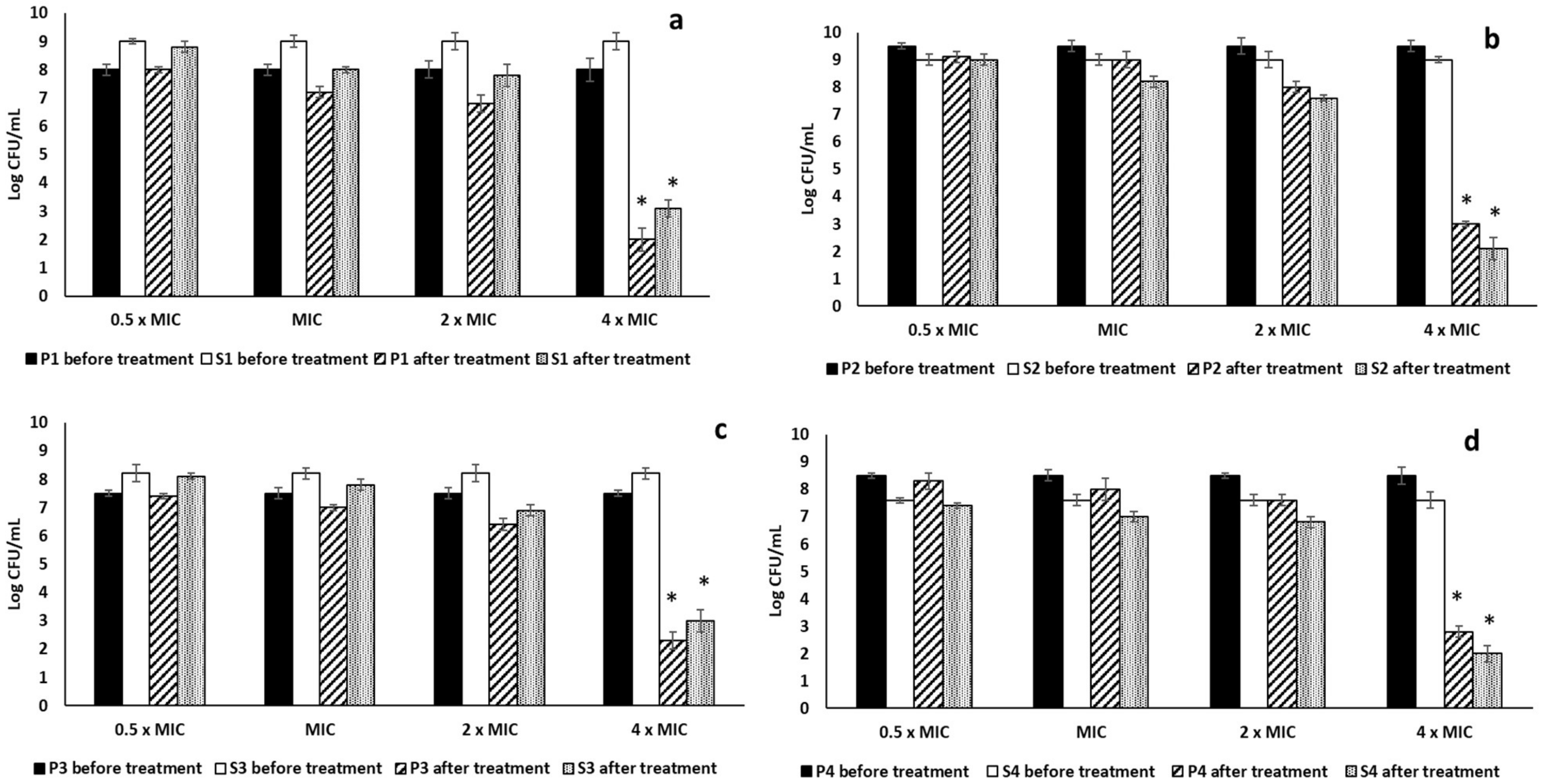
2.3.1. Antibiofilm Activity
2.3.2. Immunomodulatory Activity
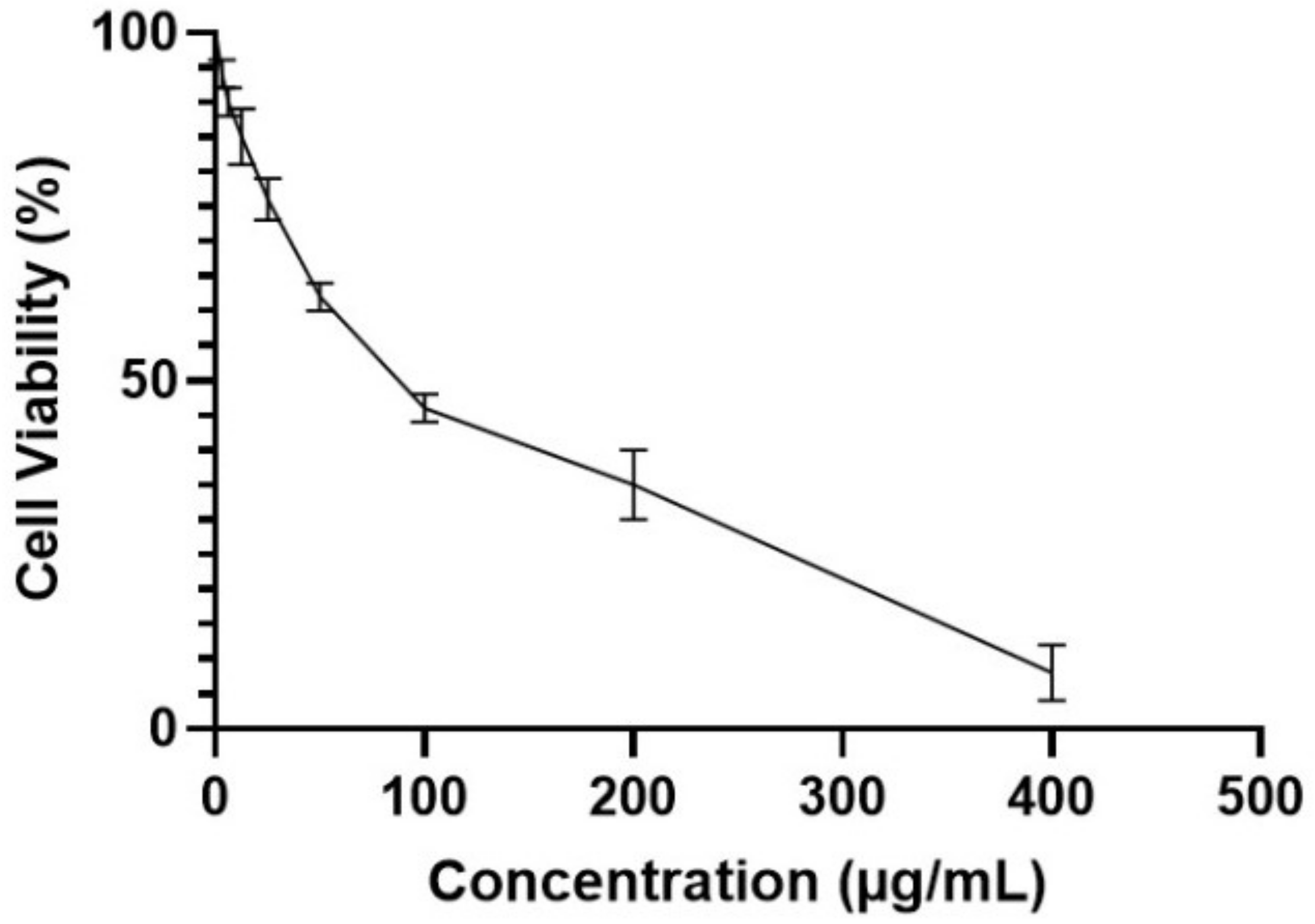
MTT Assay
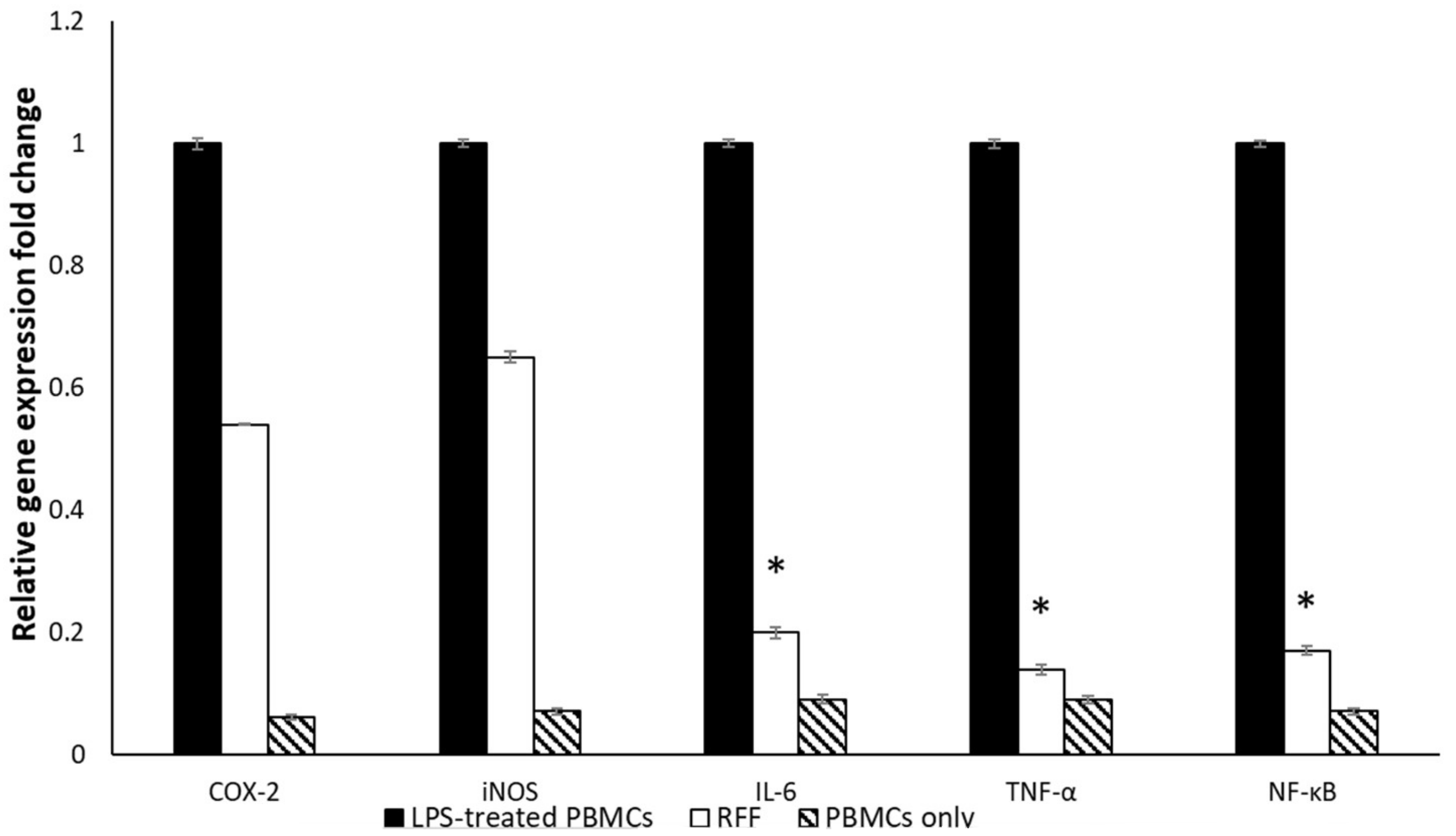
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.4. In Vivo Activities
2.4.1. Wound-Healing Rates
2.4.2. NO Levels
2.4.3. Inflammation Markers
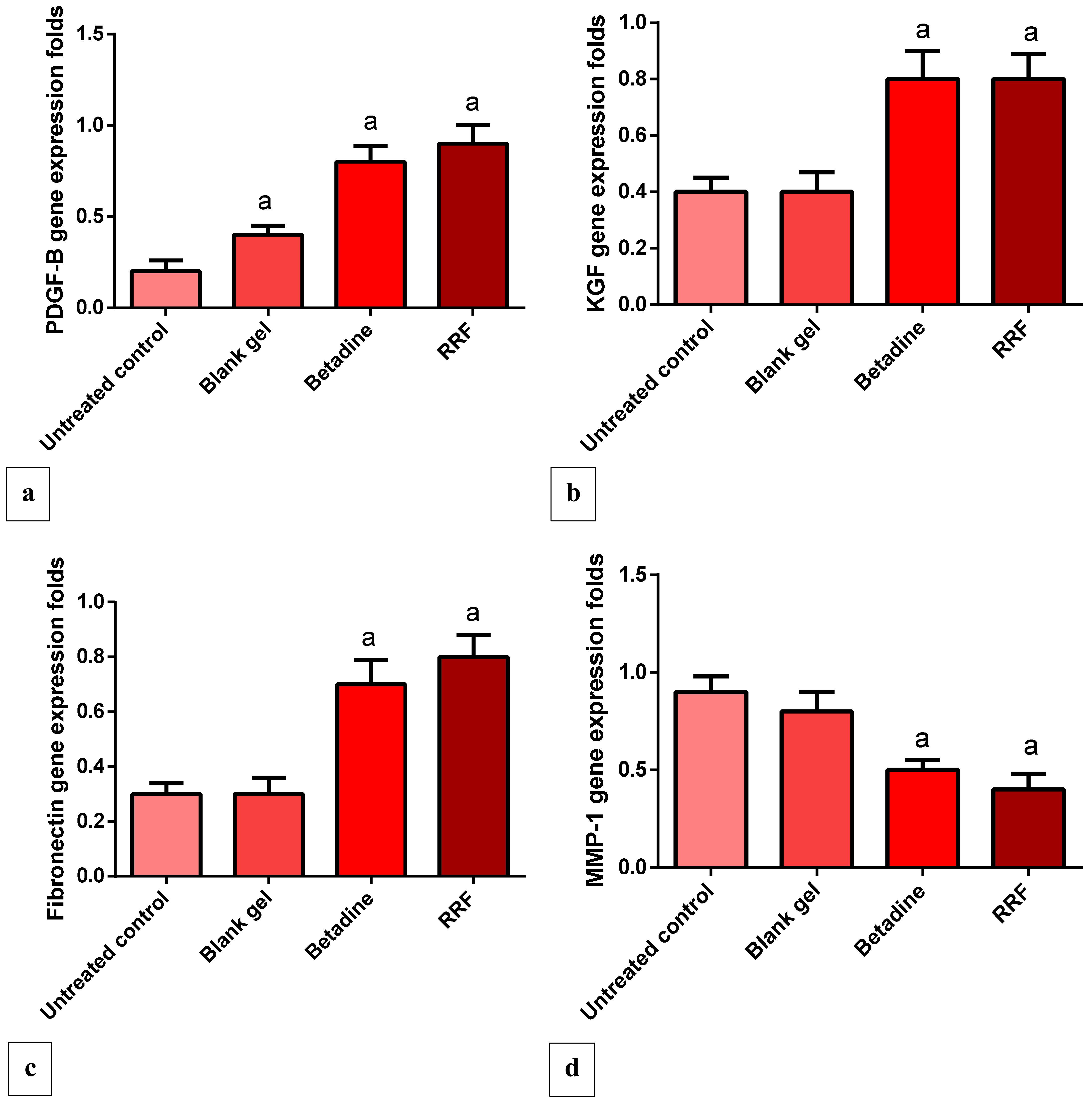
2.4.4. Gene Expression Levels of PDGF, VEGF, KGF, and Fibronectin
2.4.5. Gene Expression Levels of MMP-1 Gene
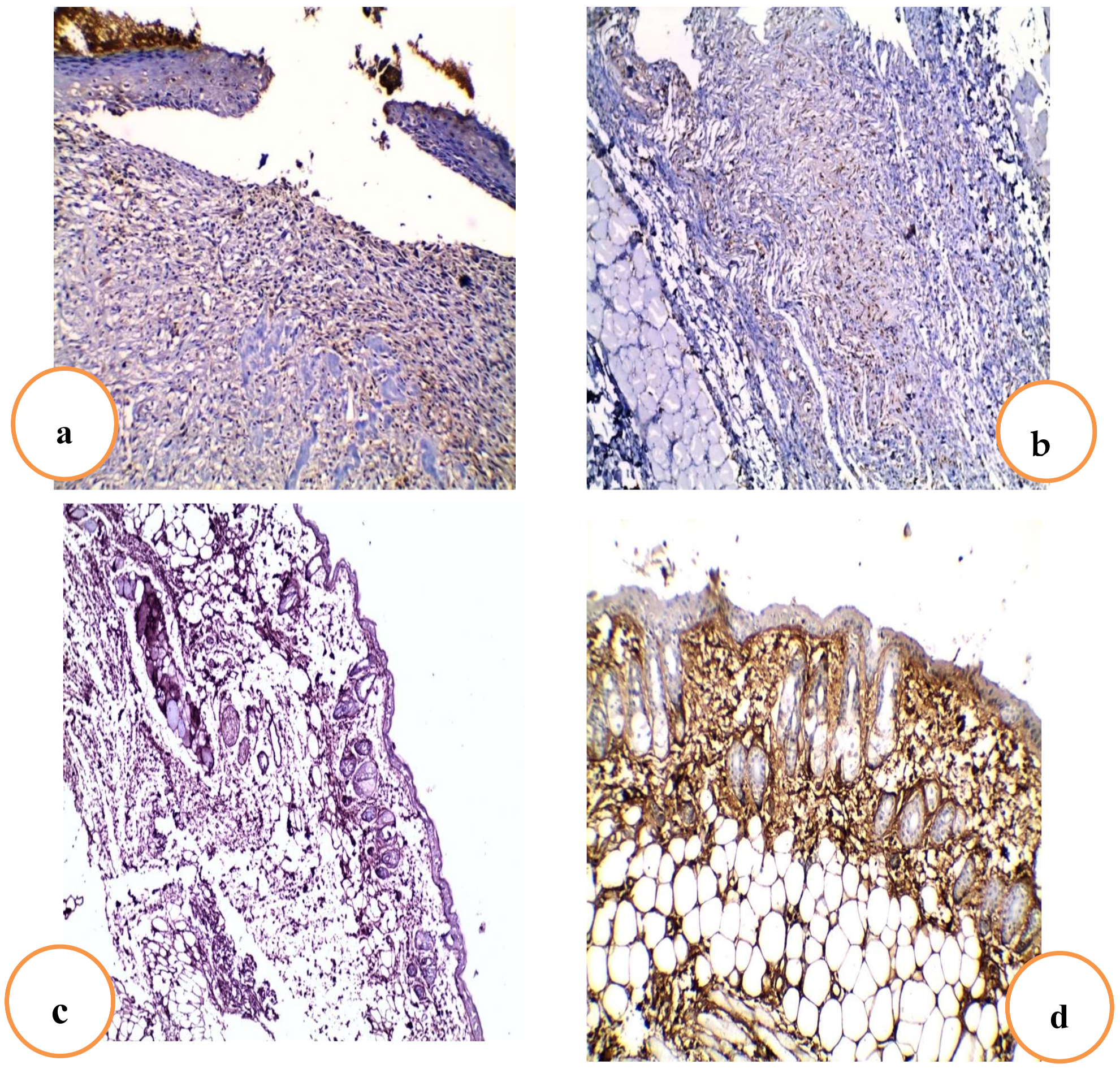
2.4.6. Effects of RRF Topical Treatment on Immunohistochemical Staining of TGF-β
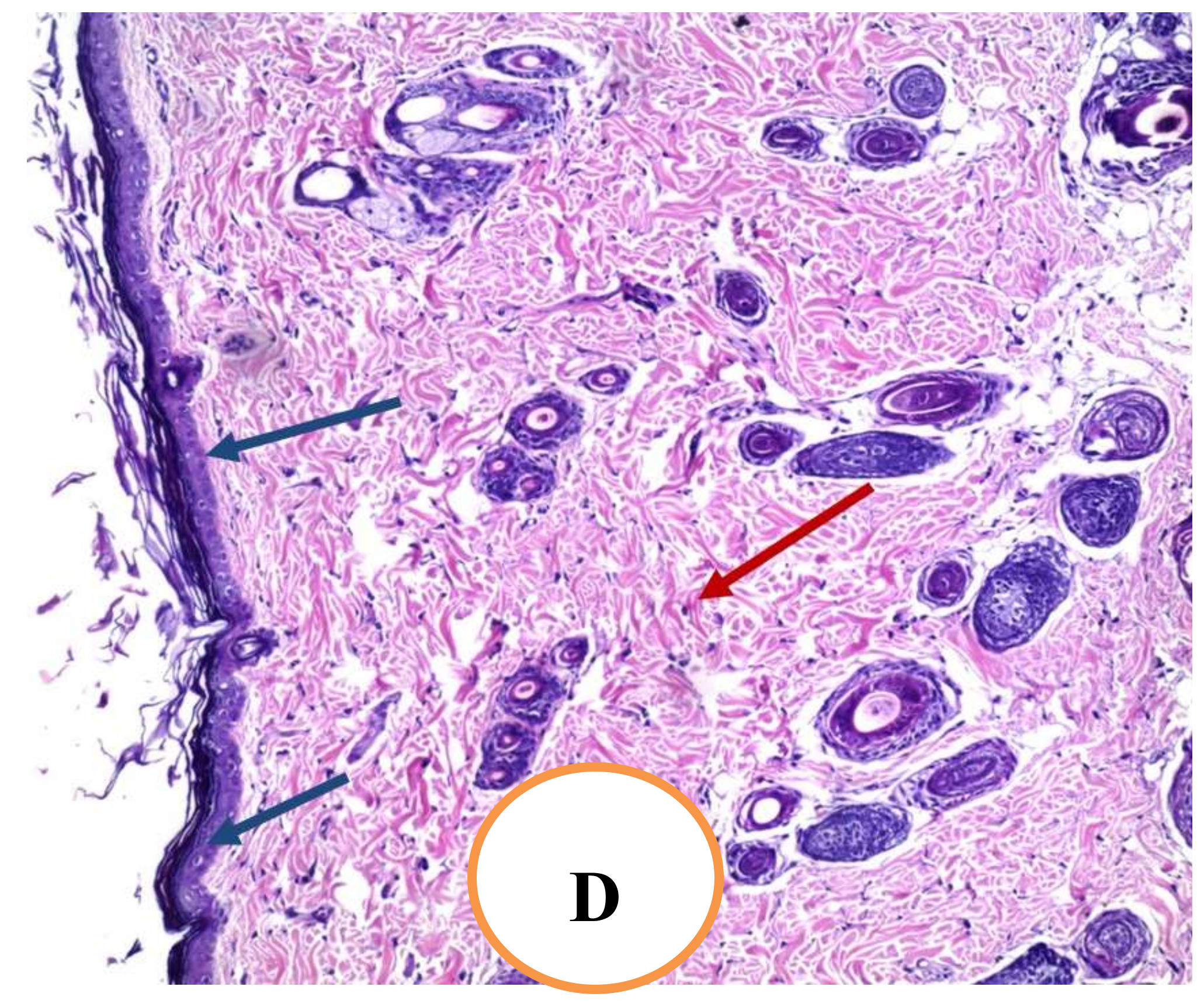
2.4.7. Histopathological Examination of Skin Tissue
3. Discussion
4. Materials and Methods
4.1. Animals and Ethical Approval
4.2. Plant Material
4.3. LC-ESI-MS/MS and Metabolomics Analyses
4.3.1. Sample Preparation and Injection
4.3.2. Acquisition Method and Analytical Parameters
4.3.3. Data Processing
4.4. Isolation of Major Flavonoid Fraction
4.5. Topical Gel Preparation
4.6. Antibiofilm Activities
4.6.1. Isolation and Identification of P. aeruginosa and S. aureus from Wounds
4.6.2. Effect of RRF on Planktonic Cells
4.6.3. Biofilm Formation
4.6.4. Effect on Mono-Species and Dual-Species Biofilms
4.7. Immunomodulatory Activity
4.7.1. Isolation of PBMCs
4.7.2. MTT Cell Viability Assessment
4.7.3. qRT-PCR
4.8. Wound Model and Experimental Groups
4.8.1. Macroscopic Wound Healing
4.8.2. Determination of NO Level
4.8.3. Enzyme-Linked Immunosorbent Assay for IL-6 and IL-1β Levels
4.8.4. Quantitative Real-Time (qRT-PCR) for PDGF, KGF, VEGF, MMP-1, and Fibronectin Genes
4.9. Histopathological Examination of Skin Sections
4.10. Immunohistochemical Staining of TGF-β
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tanrıverdi, S.T.; Suat, B.; Azizoğlu, E.; Köse, F.A. In-vitro evaluation of dexpanthenol-loaded nanofiber mats for wound healing. Trop. J. Pharm. Res. 2018, 17, 387–394. [Google Scholar] [CrossRef]
- Benbow, M. Wound care: Ensuring a holistic and collaborative assessment. Br. J. Community Nurs. 2011, 16 (Suppl. 9), S6–S16. [Google Scholar] [CrossRef]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Siafaka, P.I.; Zisi, A.P.; Exindari, M.K.; Karantas, I.D.; Bikiaris, D.N. Porous dressings of modified chitosan with poly (2-hydroxyethyl acrylate) for topical wound delivery of levofloxacin. Carbohydr. Polym. 2016, 143, 90–99. [Google Scholar] [CrossRef]
- Yadav, M.K.; Chae, S.-W.; Go, Y.Y.; Im, G.J.; Song, J.-J. In vitro multi-species biofilms of methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa and their host interaction during in vivo colonization of an otitis media rat model. Front. Cell. Infect. Microbiol. 2017, 7, 125. [Google Scholar] [CrossRef]
- Chen, J.-f.; Tan, L.; Ju, F.; Kuang, Q.-x.; Yang, T.-l.; Deng, F.; Gu, Y.-c.; Jiang, L.-s.; Deng, Y.; Guo, D.-l. Phenolic glycosides from Sanguisorba officinalis and their anti-inflammatory effects. Nat. Prod. Res. 2020, 1–8. [Google Scholar] [CrossRef]
- Jang, E.; Inn, K.S.; Jang, Y.P.; Lee, K.T.; Lee, J.H. Phytotherapeutic Activities of Sanguisorba officinalis and its Chemical Constituents: A review. Am. J. Chin. Med. 2018, 46, 299–318. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Chung, C.B.; Kim, J.G.; Ko, K.I.; Park, S.H.; Kim, J.H.; Eom, S.Y.; Kim, Y.S.; Hwang, Y.I.; Kim, K.H. Anti-wrinkle activity of ziyuglycoside I isolated from a Sanguisorba officinalis root extract and its application as a cosmeceutical ingredient. Biosci. Biotechnol. Biochem. 2008, 72, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Lachowicz, S.; Oszmiański, J.; Rapak, A.; Ochmian, I. Profile and Content of Phenolic Compounds in Leaves, Flowers, Roots, and Stalks of Sanguisorba officinalis L. Determined with the LC-DAD-ESI-QTOF-MS/MS Analysis and Their in vitro Antioxidant, Antidiabetic, Antiproliferative Potency. Pharmaceuticals 2020, 13, 191. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Yamazaki, K.; Komiya, A.; Aoki, M.; Kasamatsu, S.; Murata, T.; Sayo, T.; Cilek, M.Z.; Okada, Y.; Takahashi, Y. Inhibitory effects of Sanguisorba officinalis root extract on HYBID (KIAA1199)-mediated hyaluronan degradation and skin wrinkling. Int. J. Cosmet. Sci. 2019, 41, 12–20. [Google Scholar] [CrossRef]
- Zhang, F.; Fu, T.-J.; Peng, S.-L.; Liu, Z.-R.; Ding, L.-S. Two New Triterpenoids from the Roots of Sanguisorba officinalis L. J. Integr. Plant Biol. 2005, 47, 251–256. [Google Scholar] [CrossRef]
- Guo, D.-L.; Chen, J.-F.; Tan, L.; Jin, M.-Y.; Ju, F.; Cao, Z.-X.; Deng, F.; Wang, L.-N.; Gu, Y.-C.; Deng, Y. Terpene glycosides from Sanguisorba officinalis and their anti-inflammatory effects. Molecules 2019, 24, 2906. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yang, C.-j.; Wang, L.-q.; Wu, J.; Dai, C.; Yuan, Y.-m.; Li, G.Q.; Yao, M.-c. A tannin compound from Sanguisorba officinalis blocks Wnt/β-catenin signaling pathway and induces apoptosis of colorectal cancer cells. Chin. Med. 2019, 14, 22. [Google Scholar] [CrossRef] [PubMed]
- Su, X.D.; Ali, I.; Arooj, M.; Koh, Y.S.; Yang, S.Y.; Kim, Y.H. Chemical constituents from Sanguisorba officinalis L. and their inhibitory effects on LPS-stimulated pro-inflammatory cytokine production in bone marrow-derived dendritic cells. Arch. Pharmacal Res. 2018, 41, 497–505. [Google Scholar] [CrossRef]
- Zhao, Z.; He, X.; Zhang, Q.; Wei, X.; Huang, L.; Fang, J.C.; Wang, X.; Zhao, M.; Bai, Y.; Zheng, X. Traditional Uses, Chemical Constituents and Biological Activities of Plants from the Genus Sanguisorba L. Am. J. Chin. Med. 2017, 45, 199–224. [Google Scholar] [CrossRef]
- Kim, S.; Oh, S.; Noh, H.B.; Ji, S.; Lee, S.H.; Koo, J.M.; Choi, C.W.; Jhun, H.P. In Vitro Antioxidant and Anti-Propionibacterium acnes Activities of Cold Water, Hot Water, and Methanol Extracts, and Their Respective Ethyl Acetate Fractions, from Sanguisorba officinalis L. Roots. Molecules 2018, 23, 3001. [Google Scholar] [CrossRef]
- Wang, R.; Jin, M.; Jin, C.; Sun, J.; Ye, C.; Zong, T.; Chen, G.; Zhou, W.; Li, G. Three new ursane-type triterpenoids from the roots of Sanguisorba officinalis L. and their cytotoxic activity. Phytochem. Lett. 2019, 32, 96–100. [Google Scholar] [CrossRef]
- Wang, L.; Li, H.; Shen, X.; Zeng, J.; Yue, L.; Lin, J.; Yang, J.; Zou, W.; Li, Y.; Qin, D. Elucidation of the molecular mechanism of Sanguisorba Officinalis L. against leukopenia based on network pharmacology. Biomed. Pharmacother. 2020, 132, 110934. [Google Scholar] [CrossRef]
- Han, J.H.; Kim, M.; Choi, H.-J.; Jin, J.S.; Lee, S.-O.; Bae, S.-J.; Ryu, D.; Ha, K.-T. The Oral Administration of Sanguisorba officinalis Extract Improves Physical Performance through LDHA Modulation. Molecules 2021, 26, 1579. [Google Scholar] [CrossRef]
- Zhang, W.; Peng, C.; Shen, X.; Yuan, Y.; Zhang, W.; Yang, C.; Yao, M. A Bioactive Compound from Sanguisorba officinalis L. Inhibits Cell Proliferation and Induces Cell Death in 5-Fluorouracil-Sensitive/Resistant Colorectal Cancer Cells. Molecules 2021, 26, 3843. [Google Scholar] [CrossRef]
- Elshamy, A.I.; Farrag, A.R.H.; Ayoub, I.M.; Mahdy, K.A.; Taher, R.F.; Gendy, A.E.-N.G.E.; Mohamed, T.A.; Al-Rejaie, S.S.; Ei-Amier, Y.A.; Abd-Eigawad, A.M.; et al. UPLC-qTOF-MS Phytochemical Profile and Antiulcer Potential of Cyperus conglomeratus Rottb. Alcoholic Extract. Molecules 2020, 25, 4234. [Google Scholar] [CrossRef] [PubMed]
- Brinza, I.; Abd-Alkhalek, A.M.; El-Raey, M.A.; Boiangiu, R.S.; Eldahshan, O.A.; Hritcu, L. Ameliorative effects of rhoifolin in scopolamine-induced amnesic zebrafish (Danio rerio) model. Antioxidants 2020, 9, 580. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hui, Q.; Qin, H.; Zhu, W. Studies on chemical constituents of Bidens bipinnata. Chin. Tradit. Herb. Drugs 1992, 23, 229–231. [Google Scholar]
- She, G.; Wang, S.; Liu, B. Dihydrochalcone glycosides from Oxytropis myriophylla. Chem. Cent. J. 2011, 5, 71. [Google Scholar] [CrossRef][Green Version]
- Gawron-Gzella, A.; Witkowska-Banaszczak, E.; Bylka, W.; Dudek-Makuch, M.; Odwrot, A.; Skrodzka, N. Chemical composition, antioxidant and antimicrobial activities of Sanguisorba officinalis L. extracts. Pharm. Chem. J. 2016, 50, 244–249. [Google Scholar] [CrossRef]
- Shi, G.-b.; Wang, B.; Wu, Q.; Wang, T.-c.; Wang, C.-l.; Sun, X.-h.; Zong, W.-t.; Yan, M.; Zhao, Q.-c.; Chen, Y.-f. Evaluation of the wound-healing activity and anti-inflammatory activity of aqueous extracts from Acorus calamus L. Pak. J. Pharm. Sci. 2014, 27, 91–95. [Google Scholar]
- Alsenani, F.; Ashour, A.M.; Alzubaidi, M.A.; Azmy, A.F.; Hetta, M.H.; Abu-Baih, D.H.; Elrehany, M.A.; Zayed, A.; Sayed, A.M.; Abdelmohsen, U.R. Wound Healing Metabolites from Peters’ Elephant-Nose Fish Oil: An In Vivo Investigation Supported by In Vitro and In Silico Studies. Mar. Drugs 2021, 19, 605. [Google Scholar] [CrossRef]
- Okur, M.E.; Karadağ, A.E.; Üstündağ Okur, N.; Özhan, Y.; Sipahi, H.; Ayla, Ş.; Daylan, B.; Demirci, B.; Demirci, F. In vivo wound healing and in vitro anti-inflammatory activity evaluation of Phlomis russeliana extract gel formulations. Molecules 2020, 25, 2695. [Google Scholar] [CrossRef]
- Mihai, M.M.; Preda, M.; Lungu, I.; Gestal, M.C.; Popa, M.I.; Holban, A.M. Nanocoatings for chronic wound repair—modulation of microbial colonization and biofilm formation. Int. J. Mol. Sci. 2018, 19, 1179. [Google Scholar] [CrossRef]
- Brown, M.S.; Ashley, B.; Koh, A. Wearable technology for chronic wound monitoring: Current dressings, advancements, and future prospects. Front. Bioeng. Biotechnol. 2018, 6, 47. [Google Scholar] [CrossRef]
- Kebede, T.; Gadisa, E.; Tufa, A. Antimicrobial activities evaluation and phytochemical screening of some selected medicinal plants: A possible alternative in the treatment of multidrug-resistant microbes. PLoS ONE 2021, 16, e0249253. [Google Scholar] [CrossRef] [PubMed]
- Limoli, D.H.; Jones, C.J.; Wozniak, D.J. Bacterial extracellular polysaccharides in biofilm formation and function. Microbiol. Spectr. 2015, 3, 29. [Google Scholar] [CrossRef] [PubMed]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in inflammatory disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef]
- Bayraktar, R.; Bertilaccio, M.T.S.; Calin, G.A. The interaction between two worlds: MicroRNAs and Toll-like receptors. Front. Immunol. 2019, 10, 1053. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Chen, J.; Ren, G.; Zhang, Y.; Tan, X.; Yang, L. Punicalagin prevents inflammation in LPS-induced RAW264. 7 macrophages by inhibiting FoxO3a/autophagy signaling pathway. Nutrients 2019, 11, 2794. [Google Scholar] [CrossRef]
- Peng, S.; Hu, C.; Liu, X.; Lei, L.; He, G.; Xiong, C.; Wu, W. Rhoifolin regulates oxidative stress and proinflammatory cytokine levels in Freund’s adjuvant-induced rheumatoid arthritis via inhibition of NF-κB. Braz. J. Med. Biol. Res. 2020, 53, e9489. [Google Scholar] [CrossRef]
- Fang, J.; Cao, Z.; Song, X.; Zhang, X.; Mai, B.; Wen, T.; Lin, J.; Chen, J.; Chi, Y.; Su, T. Rhoifolin Alleviates Inflammation of Acute Inflammation Animal Models and LPS-Induced RAW264. 7 Cells via IKKβ/NF-Κb Signaling Pathway. Inflammation 2020, 43, 2191–2201. [Google Scholar] [CrossRef]
- Yan, J.; Ni, B.; Sheng, G.; Zhang, Y.; Xiao, Y.; Ma, Y.; Li, H.; Wu, H.; Tu, C. Rhoifolin Ameliorates Osteoarthritis via Regulating Autophagy. Front. Pharmacol. 2021, 12, 1258. [Google Scholar] [CrossRef]
- Murthuza, S.; Manjunatha, B. In vitro and in vivo evaluation of anti-inflammatory potency of Mesua ferrea, Saraca asoca, Viscum album & Anthocephalus cadamba in murine macrophages raw 264.7 cell lines and Wistar albino rats. Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 719–723. [Google Scholar]
- Okur, M.E.; Karantas, I.D.; Şenyiğit, Z.; Okur, N.Ü.; Siafaka, P.I. Recent trends on wound management: New therapeutic choices based on polymeric carriers. Asian J. Pharm. Sci. 2020, 15, 661–684. [Google Scholar] [CrossRef]
- Eldahshan, O.A.; Azab, S.S. Anti-inflammatory effect of apigenin-7-neohesperidoside (rhoifolin) in carrageenin-induced rat oedema model. J. Appl. Pharm. Sci. 2012, 2, 74. [Google Scholar] [CrossRef]
- Ayla, Ş.; Günal, M.Y.; Sayın Şakul, A.; Biçeroğlu, Ö.; Özdemir, E.M.; Okur, M.E.; Çiçek-Polat, D.; Üstündağ-Okur, N.; Bilgiç, B.E. Effects of Prunus spinosa L. fruits on experimental wound healing. Medeni. Med. J. 2017, 32, 152–158. [Google Scholar] [CrossRef]
- Eldahshan, O.A. Rhoifolin; a potent antiproliferative effect on cancer cell lines. J. Pharm. Res. Int. 2013, 3, 46–53. [Google Scholar] [CrossRef]
- Rousselle, P.; Montmasson, M.; Garnier, C. Extracellular matrix contribution to skin wound re-epithelialization. Matrix Biol. 2019, 75, 12–26. [Google Scholar] [CrossRef]
- Lenselink, E.A. Role of fibronectin in normal wound healing. Int. Wound J. 2015, 12, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Rousselle, P.; Braye, F.; Dayan, G. Re-epithelialization of adult skin wounds: Cellular mechanisms and therapeutic strategies. Adv. Drug Deliv. Rev. 2019, 146, 344–365. [Google Scholar] [CrossRef]
- Johnson, M.B.; Pang, B.; Gardner, D.J.; Niknam-Benia, S.; Soundarajan, V.; Bramos, A.; Perrault, D.P.; Banks, K.; Lee, G.K.; Baker, R.Y. Topical fibronectin improves wound healing of irradiated skin. Sci. Rep. 2017, 7, 3876. [Google Scholar] [CrossRef]
- Chantre, C.O.; Campbell, P.H.; Golecki, H.M.; Buganza, A.T.; Capulli, A.K.; Deravi, L.F.; Dauth, S.; Sheehy, S.P.; Paten, J.A.; Gledhill, K. Production-scale fibronectin nanofibers promote wound closure and tissue repair in a dermal mouse model. Biomaterials 2018, 166, 96–108. [Google Scholar] [CrossRef]
- Patten, J.; Wang, K. Fibronectin in development and wound healing. Adv. Drug Deliv. Rev. 2021, 170, 353–368. [Google Scholar] [CrossRef]
- Reiss, M.J.; Han, Y.-P.; Garcia, E.; Goldberg, M.; Yu, H.; Garner, W.L. Matrix metalloproteinase-9 delays wound healing in a murine wound model. Surgery 2010, 147, 295–302. [Google Scholar] [CrossRef]
- Guo, S.; DiPietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- White, L.A.; Mitchell, T.I.; Brinckerhoff, C.E. Transforming growth factor β inhibitory element in the rabbit matrix metalloproteinase-1 (collagenase-1) gene functions as a repressor of constitutive transcription. Biochim. Biophys. Acta (BBA)-Gene Struct. Expr. 2000, 1490, 259–268. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Khan, R.A.; Abdel-Hafez, A.A.; Abdel-Aziz, M.; Ahmed, E.; Enany, S.; Mahgoub, S.; Al-Rugaie, O.; Alsharidah, M.; Aly, M.S.A.; et al. Phytochemical Profiling, In Vitro and In Silico Anti-Microbial and Anti-Cancer Activity Evaluations and Staph GyraseB and h-TOP-IIβ Receptor-Docking Studies of Major Constituents of Zygophyllum coccineum L. Aqueous-Ethanolic Extract and Its Subsequent Fractions: An Approach to Validate Traditional Phytomedicinal Knowledge. Molecules 2021, 26, 577. [Google Scholar] [PubMed]
- Attallah, N.G.M.; Negm, W.A.; Elekhnawy, E.; Elmongy, E.I.; Altwaijry, N.; El-Haroun, H.; El-Masry, T.A.; El-Sherbeni, S.A. Elucidation of Phytochemical Content of Cupressus macrocarpa Leaves: In Vitro and In Vivo Antibacterial Effect against Methicillin-Resistant Staphylococcus aureus Clinical Isolates. Antibiotics 2021, 10, 890. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, R.S.; El-Banna, A.A. Royal jelly fatty acids bioprofiling using TLC-MS and digital image analysis coupled with chemometrics and non-parametric regression for discovering efficient biomarkers against melanoma. RSC Adv. 2021, 11, 18717–18728. [Google Scholar] [CrossRef]
- Owusu, E.; Ahorlu, M.M.; Afutu, E.; Akumwena, A.; Asare, G.A. Antimicrobial Activity of Selected Medicinal Plants from a Sub-Saharan African Country against Bacterial Pathogens from Post-Operative Wound Infections. Med. Sci. 2021, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Wayne, A.; Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; 20th Informational Supplement; CLSI Document: Malvern, PA, USA, 2017. [Google Scholar]
- El-Banna, T.; Abd El-Aziz, A.; Sonbol, F.; El-Ekhnawy, E. Adaptation of Pseudomonas aeruginosa clinical isolates to benzalkonium chloride retards its growth and enhances biofilm production. Mol. Biol. Rep. 2019, 46, 3437–3443. [Google Scholar] [CrossRef] [PubMed]
- Hacioglu, M.; Oyardi, O.; Bozkurt-Guzel, C.; Savage, P.B. Antibiofilm activities of ceragenins and antimicrobial peptides against fungal-bacterial mono and multispecies biofilms. J. Antibiot. 2020, 73, 455–462. [Google Scholar] [CrossRef]
- Alotaibi, B.; Negm, W.A.; Elekhnawy, E.; El-Masry, T.A.; Elseady, W.S.; Saleh, A.; Alotaibi, K.N.; El-Sherbeni, S.A. Antibacterial, Immunomodulatory, and Lung Protective Effects of Boswelliadalzielii Oleoresin Ethanol Extract in Pulmonary Diseases: In Vitro and In Vivo Studies. Antibiotics 2021, 10, 1444. [Google Scholar] [CrossRef]
- Chan-Zapata, I.; Canul-Canche, J.; Fernández-Martín, K.; Martín-Quintal, Z.; Torres-Romero, J.C.; Lara-Riegos, J.C.; Ramírez-Camacho, M.A.; Arana-Argáez, V.E. Immunomodulatory effects of the methanolic extract from Pouteria campechiana leaves in macrophage functions. Food Agric. Immunol. 2018, 29, 386–399. [Google Scholar] [CrossRef]
- Ezzat, M.I.; Hassan, M.; Abdelhalim, M.A.; El-Desoky, A.M.; Mohamed, S.O.; Ezzat, S.M. Immunomodulatory effect of Noni fruit and its isolates: Insights into cell-mediated immune response and inhibition of LPS-induced THP-1 macrophage inflammation. Food Funct. 2021, 12, 3170–3179. [Google Scholar] [CrossRef] [PubMed]
- Elekhnawy, E.A.; Sonbol, F.I.; Elbanna, T.E.; Abdelaziz, A.A. Evaluation of the impact of adaptation of Klebsiella pneumoniae clinical isolates to benzalkonium chloride on biofilm formation. Egypt. J. Med. Hum. Genet. 2021, 22, 51. [Google Scholar] [CrossRef]
- Tramontina, V.A.; Machado, M.A.N.; Filho, G.d.R.N.; Kim, S.H.; Vizzioli, M.R.; Toledo, S. Effect of bismuth subgallate (local hemostatic agent) on wound healing in rats. Histological and histometric findings. Braz. Dent. J. 2002, 13, 11–16. [Google Scholar] [PubMed]
- Chang, A.C.; Dearman, B.; Greenwood, J.E. A comparison of wound area measurement techniques: Visitrak versus photography. Eplasty 2011, 11, e18. [Google Scholar]
- Miranda, K.; Espey, M.; Wink, D. Unique oxidative mechanisms for the reactive nitrogen oxide species. Nitric Oxide Biol. Chem. 2001, 5, 5–62. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Cakin, M.C.; Ozdemir, B.; Kaya-Dagistanli, F.; Arkan, H.; Bahtiyar, N.; Anapali, M.; Akbas, F.; Onaran, I. Evaluation of the in vivo wound healing potential of the lipid fraction from activated platelet-rich plasma. Platelets 2020, 31, 513–520. [Google Scholar] [CrossRef]
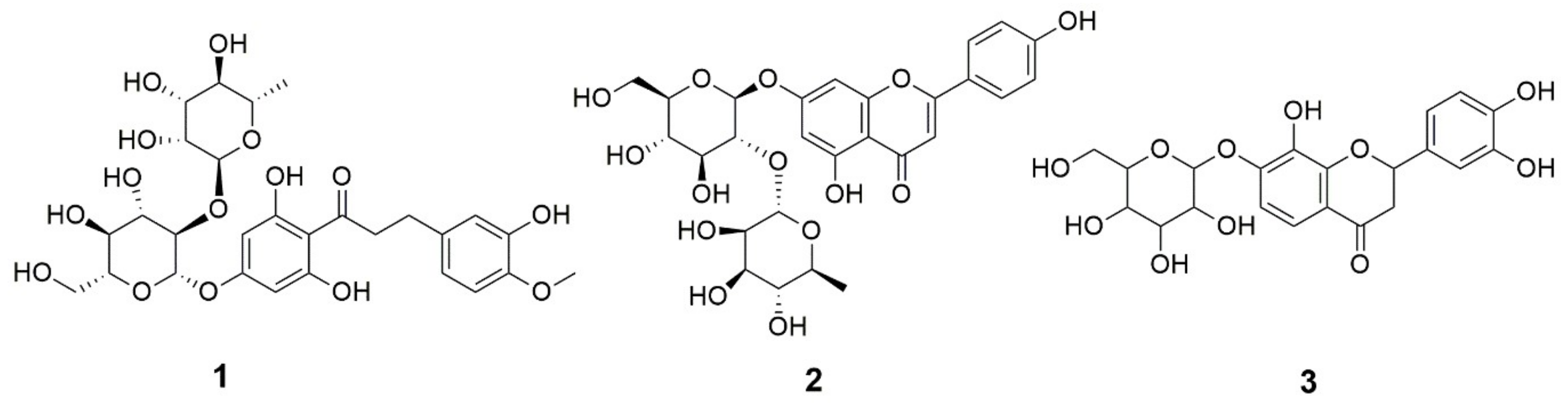



| No. | Peak Area (%) | Identified Metabolite | RT (min) | Molecular Formula | [M − H]− m/z | [M + H]+ m/z | MS2 Fragments (m/z) | Ref | |
|---|---|---|---|---|---|---|---|---|---|
| Phenolic acids/glycoside | |||||||||
| 1 | 0.11 | Syringoylmalic acid | 2.7 | C13H14O9 | 315.070 | 125.0, 153.0, 169.1, 297.1 | [21] | ||
| 2 | 0.07 | Rosmarinic acid | 4.1 | C18H16O8 | 359.096 | 150.9, 169.0, 188.9, 314.9 | --- * | ||
| 3 | 0.01 | Homogenentisic acid | 4.46 | C8H8O4 | 167.034 | 82.9, 109.0, 122.9, 122.9, 149.0 | --- | ||
| 4 | 0.2 | Syringic acid | 7.21 | C9H10O5 | 199.059 | 59.0, 65.0, 95.0, 107.0, 123.0, 125.0, 135.0, 139.0, 140.0, 152.0, 167.0 | [21] | ||
| 5 | 0.22 | 1-O-β-D-glucopyranosyl sinapate | 14.32 | C17H22O10 | 387.178 | 77.0, 93.0, 105.0, 119.0, 121.0, 147.0 | --- | ||
| 6 | 0.21 | Unknown phenolic acids/glycoside | 21.61 | C16H24O7 | 329.159 | 111.0, 129.0, 139.0, 157.0, 185.0 | --- | ||
| Alkaloids and related metabolites | |||||||||
| 7 | 0.07 | Harmaline | 1.21 | C13H14N2O | 215.099 | 127.1, 144.0, 157.0, 183.0 | --- | ||
| 8 | 0.03 | 3-Methyl xanthine | 7.21 | C6H6N4O2 | 167.034 | 60.0, 149.0, 152.0 | --- | ||
| Flavonoids and related metabolites | |||||||||
| 9 | 0.02 | Quercetin-3-D-xyloside | 1.34 | C20H18O11 | 433.038 | 299.9, 300.9, 366.9 | --- | ||
| 10 | 0.12 | Epigallocatechin | 2.71 | C15H14O7 | 305.063 | 125.0, 165.0, 179.0, 221.0, 261.0 | --- | ||
| 11 | 0.07 | Eriodictyol-7-O-glucoside | 3.71 | C21H22O11 | 449.110 | 229.0, 259.0, 269.0, 274.9, 287.0 | --- | ||
| 12 | 0.81 | Procyanidin B2 | 4.29 | C30H26O12 | 577.131 | 125.0, 137.0, 161.0, 205.0, 245.0, 247.0, 273.0, 275.0, 287.0, 289.0, 299.0, 339.0, 381.1, 407.0, 425.0, 451.1, 559.1 | --- | ||
| 13 | 0.02 | Procyanidin C1 | 4.47 | C45H38O18 | 865.208 | 287.0, 413.1, 425.1, 575.1, 575.1 577.1, 695.1, 713.1, 713.1, 739.1 | --- | ||
| 14 | 3.3 | (-)-Epicatechin | 4.62 | C15H14O6 | 289.071 | 57.0, 81.0, 83.0, 95.1, 97.0, 108.0, 123.0, 125.0, 135.0, 137.0, 139.0, 149.0, 151.0, 161.0, 162.0, 164.0, 165.0, 167.0, 175.0, 179.0, 187.0, 188.0, 202.0, 203.0, 205.0, 220.9, 221.0, 227.0, 230.0, 247.0 | --- | ||
| 15 | 0.51 | Catechin | 4.73 | C15H14O6 | 291.085 | 68.0 77.0, 91.0, 93.0, 105.0, 111.0, 115.0, 119.0, 123.0, 127.0, 133.0, 137.0, 137.0, 139.0, 143.0, 147.0 151.0, 161.0, 163.0, 165.0, 177.0 179.0, 189.0, 207.0, 249.0, 273.0 | --- | ||
| 16 | 0.2 | 3,5,7-trihydroxy-4′-methoxyflavone | 5.12 | C16H12O6 | 298.982 | 79.9, 181.0, 283.9 | --- | ||
| 17 | 0.003 | Luteolin-6-C- Glucoside | 5.2 | C21H20O11 | 447.094 | 174.9, 299.9, 303.0, 315.0, 327.0, 378.9, 401.1 | --- | ||
| 18 | 0.12 | Procyanidin B1 | 5.46 | C30H26O12 | 577.134 | 125.0, 165.0, 287.0, 289.0, 299.0, 381.1, 407.1, 425.1, 451.1 | --- | ||
| 19 | 0.02 | Naringenin-7-O- Glucoside | 5.8 | C21H22O10 | 433.114 | 123.0, 135.0, 163.0, 188.9, 237.0, 253.0, 271.0, 296.9 | --- | ||
| 20 | 0.2 | Isookanin-7-glucoside | 5.85 | C21H22O11 | 449.109 | 150.9, 178.9, 259.0, 269.0, 287.0 | --- | ||
| 21 | 0.06 | Kaempferol-3- Glucuronide | 6.53 | C21H18O12 | 461.072 | 188.9, 256.9, 313.9, 324.9, 328.0, 329.0, 392.8 | --- | ||
| 22 | 0.01 | Quercetin-3- Arabinoside | 7.17 | C20H18O11 | 435.164 | 273.0, 302.9, 303.0 | --- | ||
| 23 | 0.03 | Phlorizin | 7.77 | C21H24O10 | 435.129 | 167.0, 180.0, 271.0, 273.0 | --- | ||
| 24 | 0.03 | Isorhamnetin-3-O-glucoside | 6.78 | C22H22O12 | 477.142 | 163.0, 169.0, 313.0, 324.9, 364.8, 432.8 | --- | ||
| 25 | 0.01 | 4,5′-dihydroxy-3-methoxy-3′-glucopyranosylstilbene | 6.85 | C21H24O9 | 419.099 | 259.0, 282.9, 287.0, 351.0 | --- | ||
| 26 | 0.003 | Rhoifolin (Apigenin 7-O-neohesperidoside) | 7.24 | C27H30O14 | 577.213 | 112.9, 356.9 | --- | ||
| 27 | 0.004 | Neohesperidin dihydrochalcone | 8.08 | C28H36O15 | 611.141 | 400.8, 520.8, 565.0 | --- | ||
| 28 | 0.03 | Kaempferol-3-O-α-L-arabinoside | 8.13 | C20H18O10 | 417.117 | 119.0, 218.9, 255.0, 280.9, 286.9, 354.9 | --- | ||
| 29 | 0.01 | 4-deoxyphloridzin | 8.29 | C21H24O9 | 419.133 | 151.0, 257.0, 351.0 | --- | ||
| 30 | 0.02 | Naringenin | 9.94 | C15H12O5 | 271.062 | 93.0, 119.0, 151.0, 225.1, 253.0 | --- | ||
| 31 | 0.19 | 4,4′-Di-O-methylellagic acid | 10.22 | C16H10O8 | 331.042 | 225.0, 245.0, 270.0011:54 271.0, 299.0, 300.9, 316.0 | --- | ||
| 32 | 0.08 | 3,3′,4′,5-tetrahydroxy-7-methoxyflavone | 10.23 | C16H12O7 | 316.971 | 317.0 | --- | ||
| 33 | 0.01 | Apigenin | 10.39 | C15H10O5 | 269.043 | 117.0, 269.0, 269.2, 269.2 | --- | ||
| 34 | 0.003 | Cyanidin-3-O-(2″-O-β-xylopyranosyl-β-glucopyranoside) | 12.43 | C26H29O15 | 581.079 | 564.1 | --- | ||
| 35 | 0.01 | Luteolin | 15.93 | C15H10O6 | 287.200 | 137.0, 203.1, 272.1 | --- | ||
| 36 | 0.02 | 3′-Methoxy-4′,5,7-trihydroxyflavonol | 16.87 | C16H12O7 | 317.056 | 299.2, 302.0 | --- | ||
| 37 | 0.01 | E-3,4,5′-Trihydroxy-3′-glucopyranosylstilbene | 19.85 | C20H22O9 | 405.171 | 390.1 | --- | ||
| 38 | 0.04 | 3,5,7-trihydroxy-4′-methoxyflavone | 20.11 | C16H12O6 | 301.141 | 161.0, 285.0 | --- | ||
| Triterpenoids | |||||||||
| 39 | 0.1 | 3-Oxo-15α, 19α-dihydroxyurs-12-en-28-oic acid or 3-oxo-7β, 19α-dihydroxyurs-12-en-28-oic acid | 11.29 | C30H46O5 | 485.328 | 354.9, 372.9, 405.3, 423.3, 455.3 | [17] | ||
| 40 | 0.02 | Ziyuglycoside I | 11.35 | C41H66O13 | 765.481 | 585.3, 601.4, 603.3, 604.3 | [8] | ||
| 41 | 0.1 | Unknown triterpenoid | 11.96 | C31H50O8 | 549.339 | 421.3, 501.7, 503.3 | |||
| 42 | 0.11 | Lup-12-en-15α,19β-diol-3,11-dioxo-28-oic acid | 13.41 | C30H44O6 | 501.319 | 231.1, 341.2, 437.3, 455.3, 465.3, 483.3 | [11] | ||
| 43 | 0.3 | Euscaphic acid or Arjunic acid | 13.96 | C30H48O5 | 488.347 | 424.3, 487.3, 488.3 | [16] | ||
| 44 | 0.25 | 3-Oxo-23-hydroxyurs-12-en-28-oic acid | 14.22 | C30H46O4 | 471.348 | 213.1, 285.2, 407.3, 425.3, 453.3 | [17] | ||
| 45 | 0.07 | Unknown | 15.81 | C30H42O5 | 483.310 | 185.1, 213.1, 233.1, 419.2, 465.2 | |||
| 46 | 0.05 | Sanguisorbigenin | 18.88 | C30H46O3 | 455.351 | 187.1, 189.1, 191.1, 201.1, 409.3, 437.3 | [9] | ||
| 47 | 0.11 | 18,19-Seco,1β-hydroxyl-3,19-dioxo-urs-11,13(18)-dien-28-oic acid | 18.98 | C30H44O5 | 485.326 | 187.1, 205.1, 235.1, 367.2, 421.3, 439.3, 467.3 | [17] | ||
| 48 | 0.54 | Unknown | 19.74 | C30H44O4 | 469.330 | 147.1, 283.2, 351.2, 405.3, 423.3 | |||
| 49 | 0.23 | Fupenzic acid | 20.35 | C30H44O3 | 453.337 | 119.1, 133.1, 145.1, 173.1, 175.1, 177.1, 205.1, 259.1, 389.3 | [17] | ||
| 50 | 0.02 | Ursolic acid | 22.3 | C30H48O3 | 455.355 | 180.9, 248.9, 250.9, 318.9, 409.2 | [17] | ||
| Fatty acids | |||||||||
| 51 | 0.32 | Linoleic acid | 21.92 | C18H32O2 | 279.234 | 210.9 | --- | ||
| 52 | 0.7 | Glyceryl palmitate | 23.29 | C19H38O4 | 331.286 | 57.0, 71.0, 85.0, 95.0, 109.0, 123.1, 239.2, 313.2 | --- | ||
| 53 | 0.07 | Glyceryl 2-linolenate | 23.31 | C21H36O4 | 353.263 | --- | |||
| 54 | 0.06 | Oleic acid | 24.19 | C18H34O2 | 281.251 | 213.2, 280.3 | --- | ||
| Others | |||||||||
| 55 | 0.01 | D-Carnitine | 1.2 | C7H15NO3 | 162.112 | 55.0, 59.0, 73.9, 103.0, 127.0 | --- | ||
| 56 | 0.33 | 7-(α-D-Glucopyranosyloxy)-2,3,4,5,6-pentahydroxyheptanoic acid | 1.24 | C13H24O13 | 387.114 | 89.0, 161.0, 179.0, 251.0, 258.9, 263.0, 323.1, 341.1 | --- | ||
| 57 | 0.002 | Unknown thioglycoside | 6.16 | C12H23NO10S3 | 436.088 | 304.0, 388.0 | --- | ||
| 58 | 1.1 | Zingiberoside A | 9.51 | C22H38O12 | 7.6 | 493.225 | 89.0, 131.0, 149.0, 179.0, 191.0, 221.0, 251.0, 288.9, 311.0, 315.1, 356.9, 430.8, 447.2 | --- | |
| 59 | 0.2 | Decanoylsucrose | 10.35 | C22H40O12 | 495.243 | 99.0, 119.0, 131.0, 317.1, 449.2 | --- | ||
| 60 | 0.08 | γ-Terpinene | 10.76 | C10H16 | 137.132 | 67.0, 77.0, 110.0, 122.0 | --- | ||
| 61 | 0.03 | Esculin | 14.75 | C15H16O9 | 339.196 | 189.0, 255.2, 270.9, 324.1 | --- | ||
| 62 | 0.12 | Unknown | 16.87 | C18H14O3 | 279.102 | 149.0, 190.0, 205.1, 233.0, 261.0 | |||
| 63 | 1.44 | Unknown | 20.86 | C19H18O3 | 295.133 | 178.0, 191.0, 192.0, 206.1, 207.0, 207.1, 219.0, 221.1, 235.0 249.1, 252.0, 262.1, 265.0, 266.0, 277.1, 280.0 | |||
| VEGF Gene Expression Folds | IL-6 Level (pg/mg Tissue) | IL-1β Level (pg/mg Tissue) | Skin NO Content (nmol/g Tissue) | |
|---|---|---|---|---|
| Untreated control | 0.4 ± 0.06 | 350.6 ± 8.65 | 442.3 ± 12.3 | 45.48 ± 3.65 |
| Control vehicle | 0.5 ± 0.09 | 356.3 ± 9.63 | 440 ± 10.9 | 48.53 ± 4.5 |
| Betadine | 1.1 ± 0.13 a | 145.3 ± 5.6 a | 136.8 ± 7.8 a | 20.12 ± 2.85 a |
| Rhoifolin rich fraction (RRF) | 1 ± 0.07 a | 70.1 ± 6.7a | 101.2 ± 8.12 a | 18.4 ± 2.12 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Negm, W.A.; El-Kadem, A.H.; Elekhnawy, E.; Attallah, N.G.M.; Al-Hamoud, G.A.; El-Masry, T.A.; Zayed, A. Wound-Healing Potential of Rhoifolin-Rich Fraction Isolated from Sanguisorba officinalis Roots Supported by Enhancing Re-Epithelization, Angiogenesis, Anti-Inflammatory, and Antimicrobial Effects. Pharmaceuticals 2022, 15, 178. https://doi.org/10.3390/ph15020178
Negm WA, El-Kadem AH, Elekhnawy E, Attallah NGM, Al-Hamoud GA, El-Masry TA, Zayed A. Wound-Healing Potential of Rhoifolin-Rich Fraction Isolated from Sanguisorba officinalis Roots Supported by Enhancing Re-Epithelization, Angiogenesis, Anti-Inflammatory, and Antimicrobial Effects. Pharmaceuticals. 2022; 15(2):178. https://doi.org/10.3390/ph15020178
Chicago/Turabian StyleNegm, Walaa A., Aya H. El-Kadem, Engy Elekhnawy, Nashwah G. M. Attallah, Gadah Abdulaziz Al-Hamoud, Thanaa A. El-Masry, and Ahmed Zayed. 2022. "Wound-Healing Potential of Rhoifolin-Rich Fraction Isolated from Sanguisorba officinalis Roots Supported by Enhancing Re-Epithelization, Angiogenesis, Anti-Inflammatory, and Antimicrobial Effects" Pharmaceuticals 15, no. 2: 178. https://doi.org/10.3390/ph15020178
APA StyleNegm, W. A., El-Kadem, A. H., Elekhnawy, E., Attallah, N. G. M., Al-Hamoud, G. A., El-Masry, T. A., & Zayed, A. (2022). Wound-Healing Potential of Rhoifolin-Rich Fraction Isolated from Sanguisorba officinalis Roots Supported by Enhancing Re-Epithelization, Angiogenesis, Anti-Inflammatory, and Antimicrobial Effects. Pharmaceuticals, 15(2), 178. https://doi.org/10.3390/ph15020178










