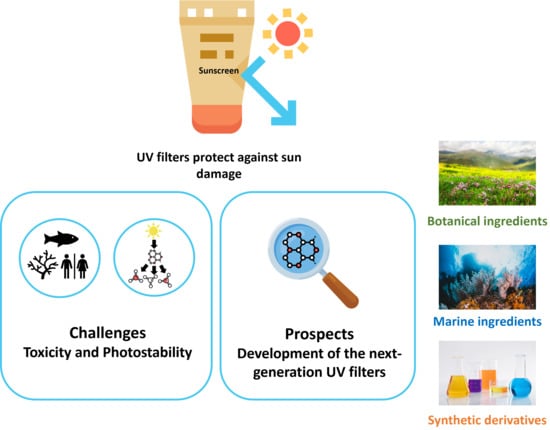
UV Filters: Challenges and Prospects
Abstract
:1. Introduction
2. Challenges
2.1. Photostability
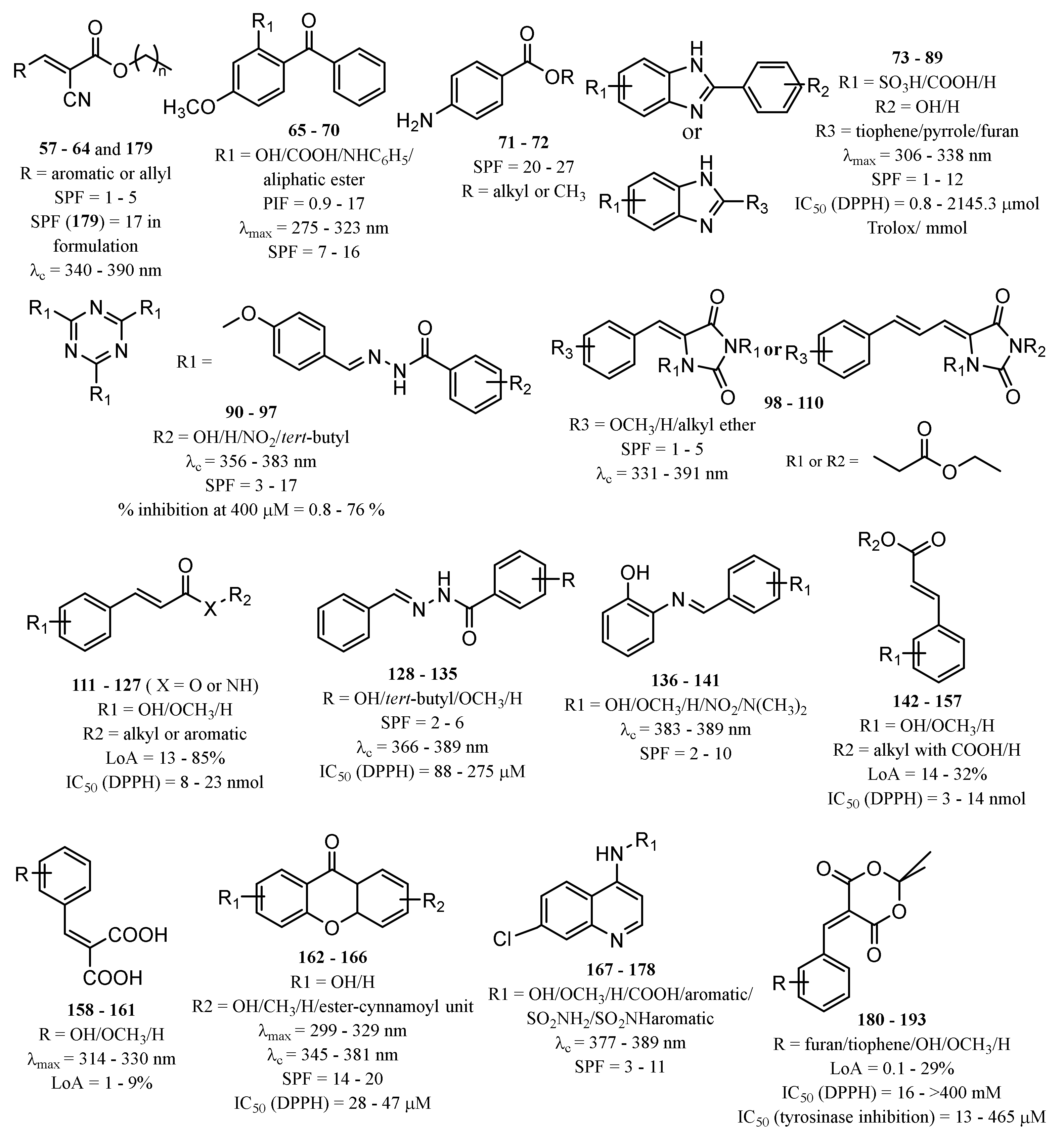
Strategies to Improve the Photostability of Organic UV Filters
| UV Filter | Conditions | Photodegradation Products | Reference |
|---|---|---|---|
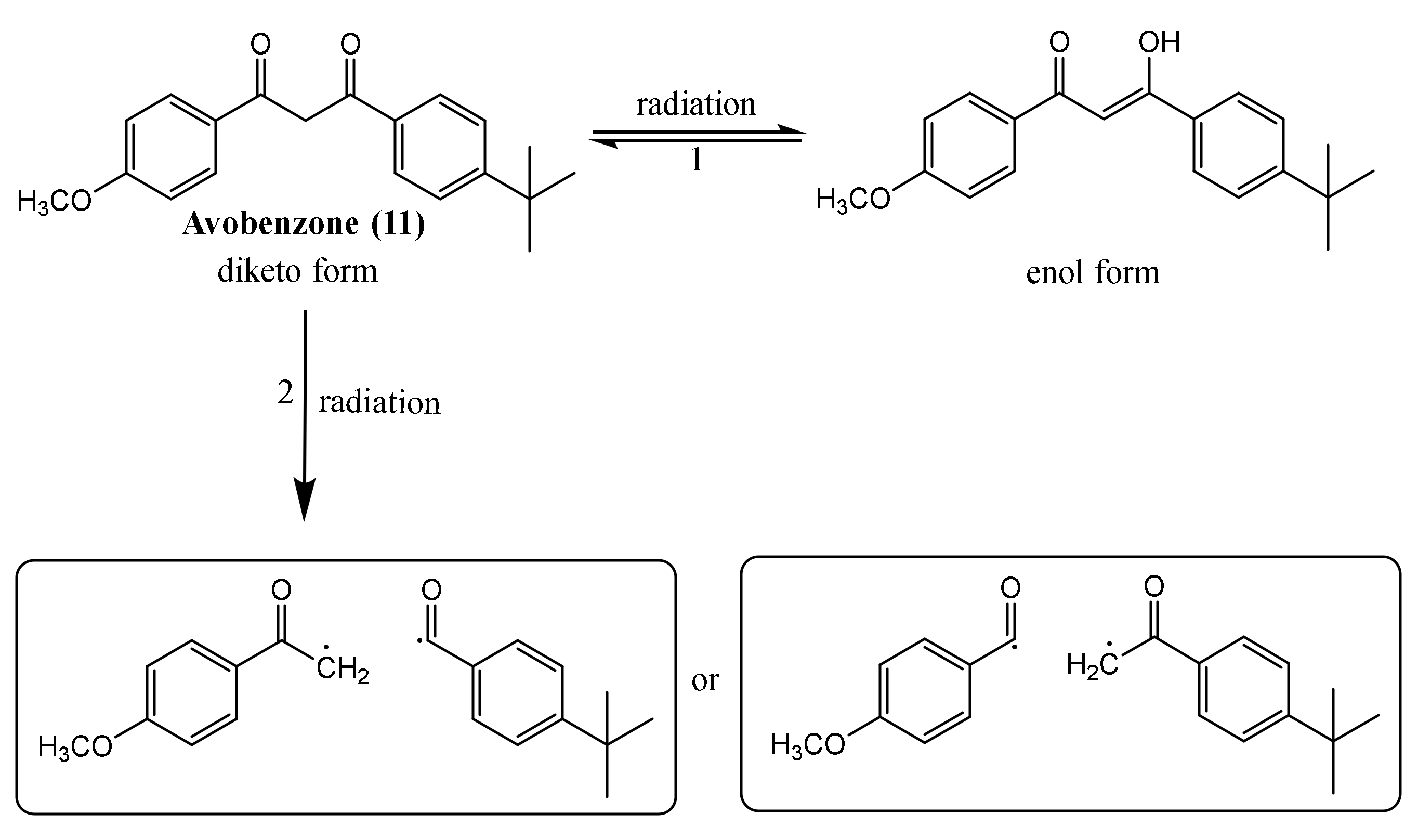
| Avobenzone (11) | Time: 100 h Lamp: mercury vapour immersion Dose: 100 J/cm2 Solvent: cyclohexane | 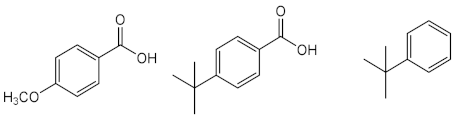 | [33] |
| Time: 8 h Lamp: SOL 500 Dose: 692 J/cm2 Solvent: ethyl acetate |  | [31] | |
| Time: 8 h Lamp: SOL 500 Dose: 692 J/cm2 Solvent: cyclohexane | 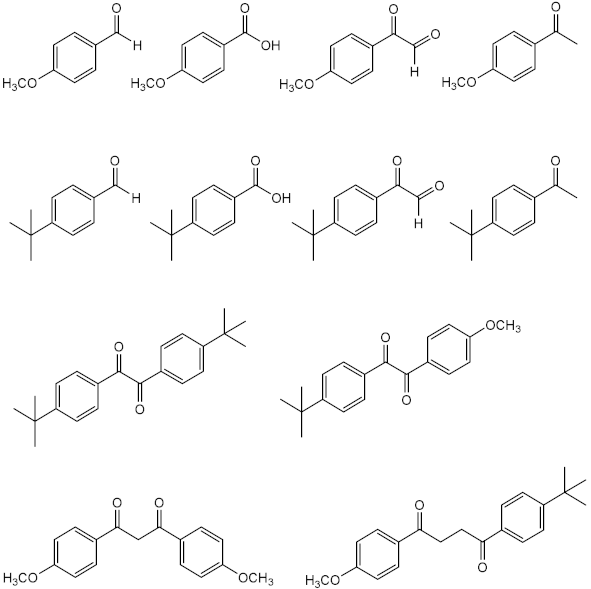 | ||
| Time: 8 h Lamp: SOL 500 Dose: 692 J/cm2 Solvent: dimethylsulfoxide (DMSO) | Did not occurred degradation but the UV filter photoisomerised | ||
| Time: 2 h Lamp: Xenon Dose: 60 kJ/m2 Solvent: water | 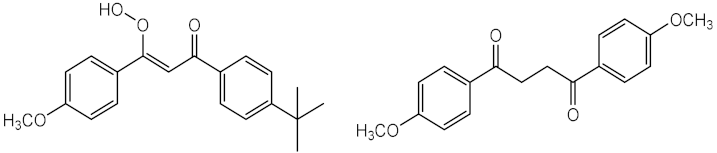 | [50] | |
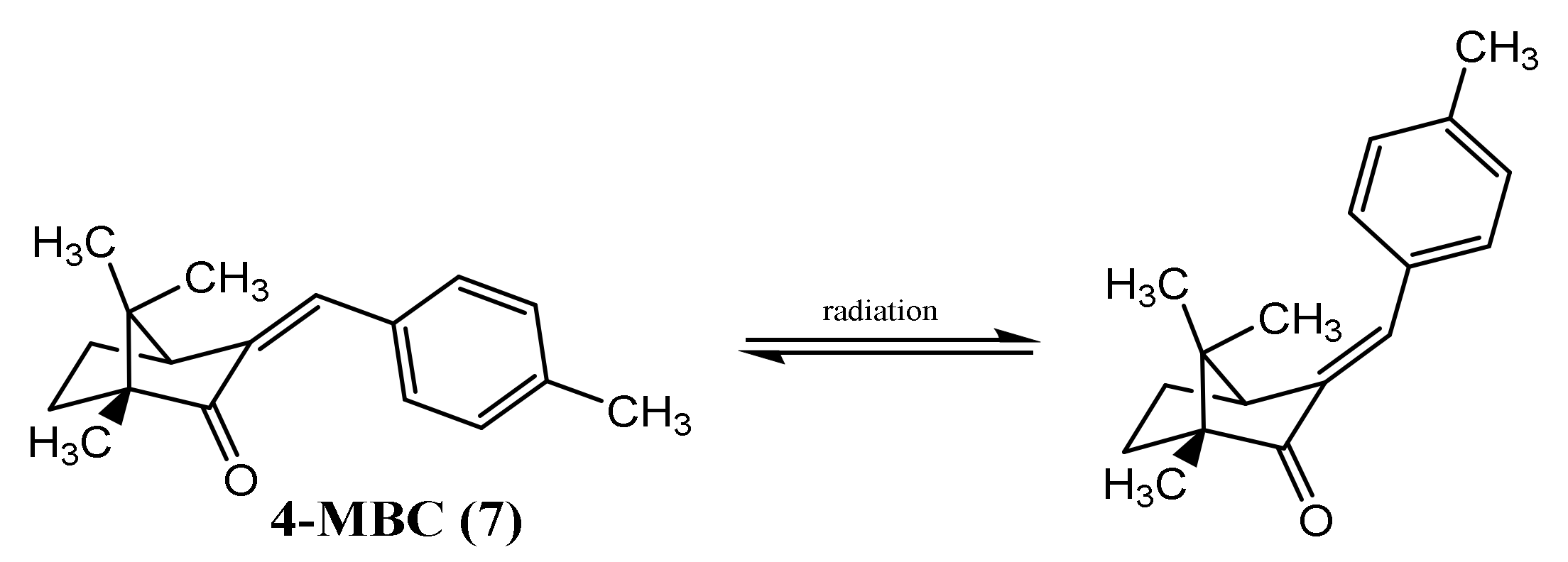
| 2-ethylhexyl-4-dimethylaminobenzoate (Padimate O) (24) | Time: 20 min Lamp: UVASUN 2000 Dose: 100 J/cm2 Solvent: petroleum jelly |  | [30] |
| Time: 140 h Lamp: mercury vapour immersion Dose: 100 J/cm2 Solvent: cyclohexane | 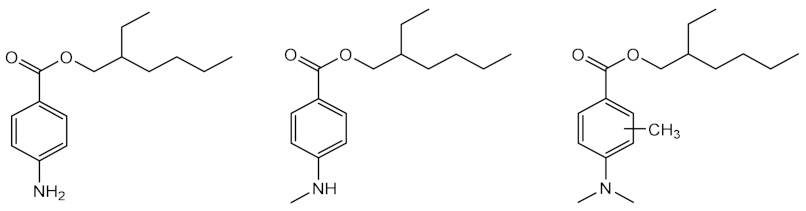 | [33] |
2.2. Toxicity (Human and Environmental)
2.2.1. Human Safety
2.2.2. Environmental Safety
Corals
Other Marine Organisms
3. Prospects
3.1. Nature as a Source of Potential Photoprotective Agents and UV Filters
| Organism and Species | Main Identified Secondary Metabolites | Activity | Values | References |
|---|---|---|---|---|
| Botanical Extracts and Metabolites | ||||
| Methanolic extract of grape seeds (from Village Farm and Winery; Nakhon Ratchasima, Thailand) | (+)-catechin (36) and (-)-epicatechin (37) (determined by HPLC)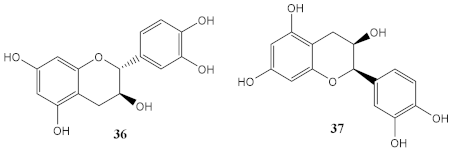 | Photoprotective (% cell viability) | At 25 μg/mL 10 J/cm2 (110%) 20 J/cm2 (68%) | [99] |
| Photodegradation | 36 = 35.1%; 37 = 31.3% Combination with UV filter: 36 (4.6%); 37 (7.0%) | |||
| Hydroethanolic extract of Vitis vinifera L. | Flavonoids, phenolic compounds, procyanidins, among others (determined by HPLC) | Antioxidant (DPPH) at 1mg/mL | 707.00 ± 0.03 µmol/g (pH = 5) 1098.00 ± 0.01 µmol/g (pH = 7) | [100] |
| Photoprotection | SPF = 20–76 λc = 360–381 nm (pH = 5) | |||
| Ethanolic commercial extract of olive leaves | 20% of oleuropein (38) | Antioxidant (DPPH) | 38: IC50 = 11.75 ± 1.01 μg/mL Extract: IC50 = 13.8 ± 0.8 μg/mL | [101] |
| Photoprotective | λmax = 376 nm SPF = 22 | |||
| Ethanolic Extract of varied Lippia species (L. brasiliensis, L. rotundifolia, L. rubella and L. sericea) | Phenols and flavonoids | Antioxidant (DPPH) | IC50 = 0.604 mg/mL | [102] |
| Photoprotective | SPF = 1.7–7.6 (formulation with 10% of the extract) λc = 375 nm | |||
| Ethanolic extract of Amazonian Cecropia obtusa leaves | Polyphenols | Antioxidant | IC50 = 1.63 µg/mL (DPPH) IC50 = 0.34 µg/mL(O2−) IC50 = 0.55 µg/mL(1O2) | [103] |
| Photoprotective | SPF = 16 | |||
| Cytotoxicity (HaCaT keratinocyte cell line) | At 20 µg/mL: cell viability = 100% | |||
| Ethanolic extract of Acacia catechu heartwood | - | Photoprotective | SPF = 24–30 | [104] |
| Hydroalcoholic extract of five wild Brazilian bamboo species (Chusqueaspp., Aulonemia aristulata, and Merostachys pluriflora) | Phenolic compounds | Antioxidant (DPPH) | IC50 = 137.55–260 μg/mL | [105] |
| Photoprotective | SPF (before irradiation) = 34–86 SPF (after irradiation) = 14–44 | |||
| Dichloromethane/acetone (1:1) extract from Lasallia pustulata | Lichenic metabolites, being gyrophoric acid (39) identified by HPLC  | Antioxidant (DPPH) | 25 % at 500 µg/mL | [106] |
| Photoprotective | λmax = 300 nm SPF = 5.03 | |||
| Cytotoxicity (HaCaT keratinocytes cell line) | IC50 = 168 ± 33 µg/mL (before radiation) IC50 > 200 µg/mL (after radiation) | |||
| Wood powder | - | Photoprotective | SPF = 11 (formulation) SPF = 37 (formulation + 5% of wood powder) | [107] |
| Ethanolic extracts of Alpinia galanga, Curcuma longa and Aloe vera | Flavonoids, phenols and terpenoids | Photoprotective | SPF = 18.2 (extract of C. longa) λmax = 290 nm (C. longa) SPF = 15.1 (A. galanga) λmax = 290 nm (A. galanga) | [108] |
| Coconut oil | High quantity of saturated fatty acids | Photoprotective | λmax = 205 nm (coconut oil) λmax = 320 nm (coconut oil + BP-3) | [109] |
| Resveratrol (40) and ethanolic extract of green tea | Resveratrol (40) | Antioxidant (DPPH) | IC50 = 38.67–85.44 % (resveratrol) IC50 = 37.41–77.50 % (green tea extract) | [110] |
| Photoprotective | λmax = 310 nm (40) λmax = 270 nm (green tea) SPF = 9.35 (40) SPF = 14.59 (green tea extract) SPF = 16.91 (40 and green tea extract) | |||
| Marine Organisms Extracts and Metabolites | ||||
| Methanolic extract of red macroalgae Curdiea racovitzae and Iridaea cordata | MAAs, with major quantity of palythine (41), asterina-330 (42), and shinorine (43)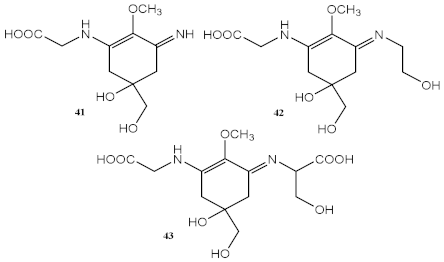 | Antioxidant (DPPH) | IC50 = 970.00 μg/mL (C. racovitzae) IC50 = 2960.00 μg/mL (I. cordata) | [119] |
| Photoprotective | λmax = 320 nm (both) λc = 356 nm (C. racovitzae) λc = 347 nm (I. cordata) | |||
| Cytotoxicity (HaCaT keratinocytes cell line) | At 1 mg/mL % cell viability = 89 (C. racovitzae) % cell viability = 73 (I. cordata) | |||
| Ethanolic extract of brown macroalgae Sargassum cristafolium | Palythine (41) | Photoprotective | λc = 370 nm | [120] |
| Methanolic extract red alga Corallina pilulifera | - | Antioxidant (DPPH) | At 200 mg/mL: 80% scaveging activity | [127] |
| Metabolite from extracts of cyanobacteria Stigonema sp., Scytonema sp. and Lyngbya sp. | Scytonemin (44)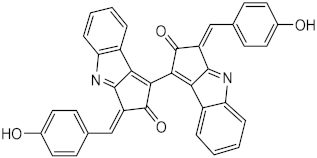 | Photoprotective | λmax = 252, 278, 300, 386 nm | [121] |
| Metabolites from aqueous methanolic extract of cyanobacteria Microcystis aeruginosa | MAAs shinorine (43) and porphyra-334 (45)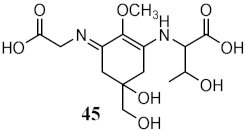 | Photoprotective | λmax = 334 nm | [118] |
| Metabolites from ethyl acetate extract of marine fungi Penicillium echinulatum | Quinolinic Alkaloids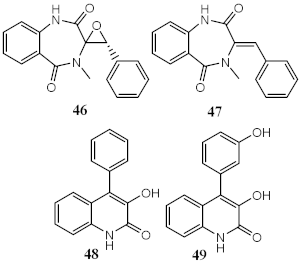 | Photoprotective | λmax = 287 (48) λc = 335 nm (48) λmax = 330 (49) λc = 334 nm (49) | [123] |
| Phototoxicity (HaCaT keratinocytes cells) | Reduction of ROS (43%) at 200 µg/mL (49) | |||
| Metabolites from dichloromethane/methanol (2:1) extract of algae Bostrychia radicans -associated fungi Annulohypoxylon stygium | 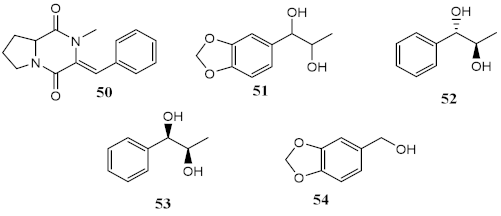 | Phototoxicity (3T3 murine fibroblasts) | PIF = 1.00 (50 and 51) PIF = 5.2 (54) | [124] |
| Metabolite from ethanolic extract of plant Thalassia testudinum | Thalassiolin B (55) | Antioxidant (DPPH) | IC50 = 100 μg/mL | [125] |
| Repair of Acute UVB-Damaged Skin | Skin damage suppression (with 55 at 240 μg/cm2) = 90% | |||
| Platyfish Xiphophorus metabolite | Melanin (56) | Photo-repair of the skin | Stimulate the production of melanin, which reduced the formation of pyrimidine dimers. | [126] |
3.2. Synthetic Derivatives with Photoprotective and UV Filter Activity
3.2.1. Inorganic UV Filters
3.2.2. Organic UV Filters
New Synthetic Derivatives Inspired by Commercialised UV Filters
Nature-Inspired Synthetised Compounds
Other New Synthetic Derivatives
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jussila, A.; Huotari-Orava, R.; Ylianttila, L.; Partonen, T.; Snellman, E. Narrow-band ultraviolet B radiation induces the expression of beta-endorphin in human skin in vivo. J. Photochem. Photobiol. B 2016, 155, 104–108. [Google Scholar] [CrossRef]
- Dahmane, R.; Pandel, R.; Trebse, P.; Poljsak, B. The role of sun exposure in skin aging. In Sun Exposure: Risk Factors, Protection Practices and Health Effects; Nova Science Publishers, Inc.: New York, NY, USA, 2015; pp. 1–40. [Google Scholar]
- Labille, J.; Catalano, R.; Slomberg, D.; Motellier, S.; Pinsino, A.; Hennebert, P.; Santaella, C.; Bartolomei, V. Assessing Sunscreen Lifecycle to Minimize Environmental Risk Posed by Nanoparticulate UV-Filters—A Review for Safer-by-Design Products. Front. Environ. Sci. 2020, 8, 00101. [Google Scholar] [CrossRef]
- Ma, Y.; Yoo, J. History of sunscreen: An updated view. J. Cosmet. Dermatol. 2021, 20, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- Pawlowski, S.; Petersen-Thiery, M. Sustainable sunscreens: A challenge between performance, animal testing ban, and human and environmental safety. In Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2020; Volume 94, pp. 185–207. [Google Scholar]
- Abiola, T.T.; Whittock, A.L.; Stavros, V.G. Unravelling the Photoprotective Mechanisms of Nature-Inspired Ultraviolet Filters Using Ultrafast Spectroscopy. Molecules 2020, 25, 3945. [Google Scholar] [CrossRef] [PubMed]
- L’Oreal. Garnier Ambre Solaire. 2020. Available online: https://www.loreal.com/it-it/italy/press-release/group/garnier-ambre-solaire/ (accessed on 3 August 2021).
- Roelandts, R.; Vanhee, J.; Bonamie, A.; Kerkhofs, L.; Degreef, H. A Survey of Ultraviolet Absorbers in Commercially Available Sun Products. Int. J. Dermatol. 1983, 22, 247–255. [Google Scholar] [CrossRef]
- Regulation (EC) No. 1223/2009 of the European Parliament and of the Council: Current Consolidated Version. Off. J. Eur. Union, (Last Current Consolidated Version Published on 1 October 2021); Available online: https://eur-lex.europa.eu/eli/reg/2009/1223/oj (accessed on 26 December 2021).
- Bissonnette, R. Update on Sunscreens. Skin Ther. Lett. 2008. Available online: https://www.skintherapyletter.com/sunscreen/advances-update/ (accessed on 1 October 2021).
- Chen, L.L.; Tooley, I.; Wang, S.Q. Nanotechnology in photoprotection. In Nanotechnology in Dermatology; Springer: New York, NY, USA, 2013; pp. 229–236. [Google Scholar]
- Valle-Sistac, J.; Molins-Delgado, D.; Díaz, M.; Ibáñez, L.; Barceló, D.; Silvia Díaz-Cruz, M. Determination of parabens and benzophenone-type UV filters in human placenta: First description of the existence of benzyl paraben and benzophenone-4. Environ. Int. 2016, 88, 243–249. [Google Scholar] [CrossRef]
- Rehfeld, A.; Egeberg, D.L.; Almstrup, K.; Petersen, J.H.; Dissing, S.; Skakkebæk, N.E. EDC IMPACT: Chemical UV filters can affect human sperm function in a progesterone-like manner. Endocr. Connect. 2018, 7, 16–25. [Google Scholar] [CrossRef] [Green Version]
- Bachelot, M.; Li, Z.; Munaron, D.; Le Gall, P.; Casellas, C.; Fenet, H.; Gomez, E. Organic UV filter concentrations in marine mussels from French coastal regions. Sci. Total Environ. 2012, 420, 273–279. [Google Scholar] [CrossRef]
- He, H.; Li, A.; Li, S.; Tang, J.; Li, L.; Xiong, L. Natural components in sunscreens: Topical formulations with sun protection factor (SPF). Biomed. Pharmacother. 2021, 134, 111161. [Google Scholar] [CrossRef]
- Giraldo, A.; Montes, R.; Rodil, R.; Quintana, J.B.; Vidal-Liñán, L.; Beiras, R. Ecotoxicological Evaluation of the UV Filters Ethylhexyl Dimethyl p-Aminobenzoic Acid and Octocrylene Using Marine Organisms Isochrysis galbana, Mytilus galloprovincialis and Paracentrotus lividus. Arch. Environ. Contam. Toxicol. 2017, 72, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Lozano, C.; Givens, J.; Stien, D.; Matallana-Surget, S.; Lebaron, P. Bioaccumulation and toxicological effects of uv-filters on marine species. In Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2020; Volume 94, pp. 85–130. [Google Scholar]
- Bahia, M.F. Proteção Solar—Actualização, 1st ed.; Universidade do Porto: Porto, Portugal, 2003. [Google Scholar]
- Stiefel, C.; Schwack, W. Reactions of cosmetic UV filters with skin proteins: Model studies of esters with primary amines. Trends Photochem. Photobiol. 2013, 15, 105–116. [Google Scholar]
- Kockler, J.; Oelgemöller, M.; Robertson, S.; Glass, B.D. Photostability of sunscreens. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 91–110. [Google Scholar] [CrossRef]
- Cefali, L.C.; Ataide, J.A.; Moriel, P.; Foglio, M.A.; Mazzola, P.G. Plant-based active photoprotectants for sunscreens. Int. J. Cosmet. Sci. 2016, 38, 346–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vega, J.; Bonomi-Barufi, J.; Gomez-Pinchetti, J.L.; Figueroa, F.L. Cyanobacteria and Red Macroalgae as Potential Sources of Antioxidants and UV Radiation-Absorbing Compounds for Cosmeceutical Applications. Mar. Drugs 2020, 18, 659. [Google Scholar] [CrossRef] [PubMed]
- Solano, F. Photoprotection and skin pigmentation: Melanin-related molecules and some other new agents obtained from natural sources. Molecules 2020, 25, 1537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peyrot, C.; Mention, M.M.; Fournier, R.; Brunissen, F.; Couvreur, J.; Balaguer, P.; Allais, F. Expeditious and sustainable two-step synthesis of sinapoyl-l-malate and analogues: Towards non-endocrine disruptive bio-based and water-soluble bioactive compounds. Green Chem. 2020, 22, 6510–6518. [Google Scholar] [CrossRef]
- Reis, J.S.; Corrêa, M.A.; Ribeiro, C.A.; Dos Santos, J.L. Synthesis and evaluation of 1,3,5-triazine derivatives as sunscreens useful to prevent skin cancer. Bioorg. Med. Chem. Lett. 2019, 29, 126755. [Google Scholar] [CrossRef]
- Popiół, J.; Gunia-Krzyżak, A.; Słoczyńska, K.; Koczurkiewicz-Adamczyk, P.; Piska, K.; Wójcik-Pszczoła, K.; Żelaszczyk, D.; Krupa, A.; Żmudzki, P.; Marona, H.; et al. The involvement of xanthone and (E)-cinnamoyl chromophores for the design and synthesis of novel sunscreening agents. Int. J. Mol. Sci. 2021, 22, 34. [Google Scholar] [CrossRef]
- Herzog, B.; Wehrle, M.; Quass, K. Photostability of UV absorber systems in sunscreens. Photochem. Photobiol. 2009, 85, 869–878. [Google Scholar] [CrossRef]
- Bonda, C.A.; Lott, D. Sunscreen photostability. In Principles and Practice of Photoprotection; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 247–273. [Google Scholar]
- Afonso, S.; Horita, K.; Sousa E Silva, J.P.; Almeida, I.F.; Amaral, M.H.; Lobão, P.A.; Costa, P.C.; Miranda, M.S.; Esteves Da Silva, J.C.G.; Sousa Lobo, J.M. Photodegradation of avobenzone: Stabilization effect of antioxidants. J. Photochem. Photobiol. B Biol. 2014, 140, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Tarras-Wahlberg, N.; Stenhagen, G.; Larko, O.; Rosen, A.; Wennberg, A.M.; Wennerstrom, O. Changes in ultraviolet absorption of sunscreens after ultraviolet irradiation. J. Investig. Dermatol. 1999, 113, 547–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwack, W.; Rudolph, T. Photochemistry of dibenzoyl methane UVA filters—Part 1. J. Photochem. Photobiol. B Biol. 1995, 28, 229–234. [Google Scholar] [CrossRef]
- Downs, C.A.; DiNardo, J.C.; Stien, D.; Rodrigues, A.M.S.; Lebaron, P. Benzophenone Accumulates over Time from the Degradation of Octocrylene in Commercial Sunscreen Products. Chem. Res. Toxicol. 2021, 34, 1046–1054. [Google Scholar] [CrossRef]
- Roscher, N.M.; Lindemann, M.K.O.; Bin Kong, S.; Cho, C.G.; Jiang, P. Photodecomposition of several compounds commonly used as sunscreen agents. J. Photochem. Photobiol. A Chem. 1994, 80, 417–421. [Google Scholar] [CrossRef]
- Duarte, J.; Almeida, I.F.; Costa, M.; Da Silva, E.S.; Faria, J.L.; Sousa Lobo, J.M.; Costa, P.C.; Scalia, S. Alginate microparticles as carriers for the UV filter 2-ethylhexyl 4-methoxycinnamate: Influence on photostability. Int. J. Cosmet. Sci. 2019, 41, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Chatelain, E.; Gabard, B. Photostabilization of butyl methoxydibenzoylmethane (Avobenzone) and ethylhexyl methoxycinnamate by bis-ethylhexyloxyphenol methoxyphenyl triazine (Tinosorb S), a new UV broadband filter. Photochem. Photobiol. 2001, 74, 401–406. [Google Scholar] [CrossRef]
- Kockler, J.; Oelgemöller, M.; Robertson, S.; Glass, B.D. Influence of titanium dioxide particle size on the photostability of the chemical UV-filters butyl methoxy dibenzoylmethane and octocrylene in a microemulsion. Cosmetics 2014, 1, 128–139. [Google Scholar] [CrossRef] [Green Version]
- Freitas, J.V.; Lopes, N.P.; Gaspar, L.R. Photostability evaluation of five UV-filters, trans-resveratrol and beta-carotene in sunscreens. Eur. J. Pharm. Sci. 2015, 78, 79–89. [Google Scholar] [CrossRef]
- Karpkird, T.; Khunsakorn, R.; Noptheeranuphap, C.; Midpanon, S. Inclusion complexes and photostability of UV filters and curcumin with beta-cyclodextrin polymers: Effect on cross-linkers. J. Incl. Phenom. Macrocycl. Chem. 2018, 91, 37–45. [Google Scholar] [CrossRef]
- Paris, C.; Lhiaubet-Vallet, V.; Jimenez, O.; Trullas, C.; Miranda, M.A. A blocked diketo form of avobenzone: Photostability, photosensitizing properties and triplet quenching by a triazine-derived UVB-filter. Photochem. Photobiol. 2009, 85, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Coelho, L.; Almeida, I.F.; Sousa Lobo, J.M.; Sousa, E.S.J.P. Photostabilization strategies of photosensitive drugs. Int. J. Pharm. 2018, 541, 19–25. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, C.A.; Dario, M.F.; Sarruf, F.D.; Mariz, I.F.A.; Velasco, M.V.R.; Rosado, C.; Baby, A.R. Safety and efficacy evaluation of gelatin-based nanoparticles associated with UV filters. Colloids Surf. B Biointerfaces 2016, 140, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Graziola, F.; Candido, T.M.; De Oliveira, C.A.; Peres, D.D.; Issa, M.G.; Mota, J.; Rosado, C.; Consiglieri, V.O.; Kaneko, T.M.; Velasco, M.V.R.; et al. Gelatin-based microspheres crosslinked with glutaraldehyde and rutin oriented to cosmetics. Bras. J. Pharm. Sci. 2016, 52, 603–612. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, C.A.D.; Peres, D.D.; Graziola, F.; Chacra, N.A.B.; Araújo, G.L.B.D.; Flórido, A.C.; Mota, J.; Rosado, C.; Velasco, M.V.R.; Rodrigues, L.M.; et al. Cutaneous biocompatible rutin-loaded gelatin-based nanoparticles increase the SPF of the association of UVA and UVB filters. Eur. J. Pharm. Sci. 2016, 81, 1–9. [Google Scholar] [CrossRef]
- Al-Rawashdeh, N.A.F.; Al-Sadeh, K.S.; Al-Bitar, M.B. Physicochemical study on microencapsulation of hydroxypropyl-β-cyclodextrin in dermal preparations. Drug Dev. Ind. Pharm. 2010, 36, 688–697. [Google Scholar] [CrossRef]
- Scalia, S.; Casolari, A.; Iaconinoto, A.; Simeoni, S. Comparative studies of the influence of cyclodextrins on the stability of the sunscreen agent, 2-ethylhexyl-p-methoxycinnamate. J. Pharm. Biomed. Anal. 2002, 30, 1181–1189. [Google Scholar] [CrossRef]
- Scalia, S.; Tursilli, R.; Iannuccelli, V. Complexation of the sunscreen agent, 4-methylbenzylidene camphor with cyclodextrins: Effect on photostability and human stratum corneum penetration. J. Pharm. Biomed. Anal. 2007, 44, 29–34. [Google Scholar] [CrossRef]
- Shokri, J.; Hasanzadeh, D.; Ghanbarzadeh, S.; Dizadji-Ilkhchi, M.; Adibkia, K. The effect of beta-cyclodextrin on percutaneous absorption of commonly used Eusolex® sunscreens. Drug Res. 2013, 63, 591–596. [Google Scholar] [CrossRef]
- Gonzalez, H.; Tarras-Wahlberg, N.; Stromdahl, B.; Juzeniene, A.; Moan, J.; Larko, O.; Rosen, A.; Wennberg, A.M. Photostability of commercial sunscreens upon sun exposure and irradiation by ultraviolet lamps. BMC Dermatol. 2007, 7, 1. [Google Scholar] [CrossRef] [Green Version]
- Shaath, N.A. Ultraviolet filters. Photochem. Photobiol. Sci. 2010, 9, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Huong, S.P.; Rocher, E.E.; Fourneron, J.-D.; Charles, L.; Monnier, V.; Bun, H.; Andrieu, V. Photoreactivity of the sunscreen butylmethoxydibenzoylmethane (DBM) under various experimental conditions. J. Photochem. Photobiol. A Chem. 2008, 196, 106–112. [Google Scholar] [CrossRef]
- Juliano, C.; Magrini, G.A. Cosmetic ingredients as emerging pollutants of environmental and health concern. A mini-review. Cosmetics 2017, 4, 11. [Google Scholar] [CrossRef]
- Bordalo, D.; Leite, C.; Almeida, Â.; Soares, A.M.V.M.; Pretti, C.; Freitas, R. Impacts of UV filters in Mytilus galloprovincialis: Preliminary data on the acute effects induced by environmentally relevant concentrations. Sustainability 2020, 12, 6852. [Google Scholar] [CrossRef]
- Picot Groz, M.; Martinez Bueno, M.J.; Rosain, D.; Fenet, H.; Casellas, C.; Pereira, C.; Maria, V.; Bebianno, M.J.; Gomez, E. Detection of emerging contaminants (UV filters, UV stabilizers and musks) in marine mussels from Portuguese coast by QuEChERS extraction and GC-MS/MS. Sci. Total Environ. 2014, 493, 162–169. [Google Scholar] [CrossRef]
- Cunha, S.C.; Fernandes, J.O.; Vallecillos, L.; Cano-Sancho, G.; Domingo, J.L.; Pocurull, E.; Borrull, F.; Maulvault, A.L.; Ferrari, F.; Fernandez-Tejedor, M.; et al. Co-occurrence of musk fragrances and UV-filters in seafood and macroalgae collected in European hotspots. Environ. Res. 2015, 143, 65–71. [Google Scholar] [CrossRef]
- Gadelha, J.R.; Rocha, A.C.; Camacho, C.; Eljarrat, E.; Peris, A.; Aminot, Y.; Readman, J.W.; Boti, V.; Nannou, C.; Kapsi, M.; et al. Persistent and emerging pollutants assessment on aquaculture oysters (Crassostrea gigas) from NW Portuguese coast (Ria De Aveiro). Sci. Total Environ. 2019, 666, 731–742. [Google Scholar] [CrossRef]
- Mitchelmore, C.L.; He, K.; Gonsior, M.; Hain, E.; Heyes, A.; Clark, C.; Younger, R.; Schmitt-Kopplin, P.; Feerick, A.; Conway, A.; et al. Occurrence and distribution of UV-filters and other anthropogenic contaminants in coastal surface water, sediment, and coral tissue from Hawaii. Sci. Total Environ. 2019, 670, 398–410. [Google Scholar] [CrossRef]
- Shick, J.M.; Lesser, M.P.; Jokiel, P.L. Effects of ultraviolet radiation on corals and other coral reef organisms. Glob. Chang. Biol. 1996, 2, 527–545. [Google Scholar] [CrossRef]
- Wijgerde, T.; van Ballegooijen, M.; Nijland, R.; van der Loos, L.; Kwadijk, C.; Osinga, R.; Murk, A.; Slijkerman, D. Adding insult to injury: Effects of chronic oxybenzone exposure and elevated temperature on two reef-building corals. Sci. Total Environ. 2020, 733, 139030. [Google Scholar] [CrossRef]
- Corinaldesi, C.; Marcellini, F.; Nepote, E.; Damiani, E.; Danovaro, R. Impact of inorganic UV filters contained in sunscreen products on tropical stony corals (Acropora spp.). Sci. Total Environ. 2018, 637–638, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Cahova, J.; Blahova, J.; Marsalek, P.; Doubkova, V.; Franc, A.; Garajová, M.; Tichy, F.; Mares, J.; Svobodova, Z. The biological activity of the organic UV filter ethylhexyl methoxycinnamate in rainbow trout (Oncorhynchus mykiss). Sci. Total Environ. 2021, 774, 145570. [Google Scholar] [CrossRef] [PubMed]
- Gago-Ferrero, P.; Díaz-Cruz, M.S.; Barceló, D. UV filters bioaccumulation in fish from Iberian river basins. Sci. Total Environ. 2015, 518–519, 518–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balmer, M.E.; Buser, H.R.; Müller, M.D.; Poiger, T. Occurrence of some organic UV filters in wastewater, in surface waters, and in fish from Swiss lakes. Environ. Sci. Technol. 2005, 39, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Gago-Ferrero, P.; Alonso, M.B.; Bertozzi, C.P.; Marigo, J.; Barbosa, L.; Cremer, M.; Secchi, E.R.; Azevedo, A.; Lailson-Brito, J., Jr.; Torres, J.P.M.; et al. First determination of UV filters in marine mammals. octocrylene levels in Franciscana dolphins. Environ. Sci. Technol. 2013, 47, 5619–5625. [Google Scholar] [CrossRef]
- Wawrzynczak, A.; Feliczak-Guzik, A.; Nowak, I. Nanosunscreens: From nanoencapsulated to nanosized cosmetic active forms. In Nanobiomaterials in Galenic Formulations and Cosmetics: Applications of Nanobiomaterials; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 25–46. [Google Scholar]
- Wang, S.Q.; Balagula, Y.; Osterwalder, U. Photoprotection: A Review of the Current and Future Technologies. Dermatol. Ther. 2010, 23, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Janjua, N.R.; Mogensen, B.; Andersson, A.M.; Petersen, J.H.; Henriksen, M.; Skakkebaek, N.E.; Wulf, H.C. Systemic absorption of the sunscreens benzophenone-3, octyl-methoxycinnamate, and 3-(4-methyl-benzylidene) camphor after whole-body topical application and reproductive hormone levels in humans. J. Invest. Dermatol. 2004, 123, 57–61. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, I.; Fenollosa, R.; Meseguer, F. Silicon Microspheres as UV, Visible and Infrared Filters for Cosmetics. Available online: https://www.cosmeticsandtoiletries.com/formulating/function/uvfilter/premium-Silicon-Microspheres-as-UV-Visible-and-Infrared-Filters-for-Cosmetics-208701831.html (accessed on 12 July 2021).
- Ambrogi, V.; Latterini, L.; Marmottini, F.; Pagano, C.; Ricci, M. Mesoporous silicate MCM-41 as a particulate carrier for octyl methoxycinnamate: Sunscreen release and photostability. J. Pharm. Sci. 2013, 102, 1468–1475. [Google Scholar] [CrossRef]
- Revised Guidance Document 150 on Standardised Test Guidelines for Evaluating Chemicals for Endocrine Disruption; OECD Publishing: Paris, France, 2018.
- Witorsch, R.J.; Thomas, J.A. Personal care products and endocrine disruption: A critical review of the literature. Crit. Rev. Toxicol. 2010, 40, 515563. [Google Scholar] [CrossRef]
- Rehfeld, A.; Dissing, S.; Skakkebæk, N.E. Chemical UV filters mimic the effect of progesterone on Ca2+ signaling in human sperm cells. Endocrinology 2016, 157, 4297–4308. [Google Scholar] [CrossRef] [Green Version]
- Ponzo, O.J.; Silvia, C. Evidence of reproductive disruption associated with neuroendocrine changes induced by UV-B filters, phtalates and nonylphenol during sexual maturation in rats of both gender. Toxicology 2013, 311, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Axelstad, M.; Boberg, J.; Hougaard, K.S.; Christiansen, S.; Jacobsen, P.R.; Mandrup, K.R.; Nellemann, C.; Lund, S.P.; Hass, U. Effects of pre- and postnatal exposure to the UV-filter Octyl Methoxycinnamate (OMC) on the reproductive, auditory and neurological development of rat offspring. Toxicol. Appl. Pharmacol. 2011, 250, 278–290. [Google Scholar] [CrossRef] [PubMed]
- Ozáez, I.; Martínez-Guitarte, J.L.; Morcillo, G. Effects of in vivo exposure to UV filters (4-MBC, OMC, BP-3, 4-HB, OC, OD-PABA) on endocrine signaling genes in the insect Chironomus riparius. Sci. Total Environ. 2013, 456, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Li, A.J.; Law, J.C.F.; Chow, C.H.; Huang, Y.; Li, K.; Leung, K.S.Y. Joint Effects of Multiple UV Filters on Zebrafish Embryo Development. Environ. Sci. Technol. 2018, 52, 9460–9467. [Google Scholar] [CrossRef] [PubMed]
- Li, V.W.T.; Tsui, M.P.M.; Chen, X.; Hui, M.N.Y.; Jin, L.; Lam, R.H.W.; Yu, R.M.K.; Murphy, M.B.; Cheng, J.; Lam, P.K.S.; et al. Effects of 4-methylbenzylidene camphor (4-MBC) on neuronal and muscular development in zebrafish (Danio rerio) embryos. Environ. Sci. Pollut. Res. 2016, 23, 8275–8285. [Google Scholar] [CrossRef]
- Lorigo, M.; Mariana, M.; Cairrao, E. Photoprotection of ultraviolet-B filters: Updated review of endocrine disrupting properties. Steroids 2018, 131, 46–58. [Google Scholar] [CrossRef]
- Balázs, A.; Krifaton, C.; Orosz, I.; Szoboszlay, S.; Kovács, R.; Csenki, Z.; Urbányi, B.; Kriszt, B. Hormonal activity, cytotoxicity and developmental toxicity of UV filters. Ecotoxicol. Environ. Saf. 2016, 131, 45–53. [Google Scholar] [CrossRef]
- Chen, T.H.; Hsieh, C.Y.; Ko, F.C.; Cheng, J.O. Effect of the UV-filter benzophenone-3 on intra-colonial social behaviors of the false clown anemonefish (Amphiprion ocellaris). Sci. Total Environ. 2018, 644, 1625–1629. [Google Scholar] [CrossRef]
- Carvalhais, A.; Pereira, B.; Sabato, M.; Seixas, R.; Dolbeth, M.; Marques, A.; Guilherme, S.; Pereira, P.; Pacheco, M.; Mieiro, C. Mild effects of sunscreen agents on a marine flatfish: Oxidative stress, energetic profiles, neurotoxicity and behaviour in response to titanium dioxide nanoparticles and oxybenzone. Int. J. Mol. Sci. 2021, 22, 1567. [Google Scholar] [CrossRef]
- Pablos, M.V.; Garcia-Hortiguela, P.; Fernandez, C. Acute and chronic toxicity of emerging contaminants, alone or in combination, in Chlorella vulgaris and Daphnia magna. Environ. Sci. Pollut. Res. Int. 2015, 22, 5417–5424. [Google Scholar] [CrossRef]
- Elder, D.P.; Snodin, D.J. Drug substances presented as sulfonic acid salts: Overview of utility, safety and regulation. J. Pharm. Pharmacol. 2009, 61, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Lozano, C.; Matallana-Surget, S.; Givens, J.; Nouet, S.; Arbuckle, L.; Lambert, Z.; Lebaron, P. Toxicity of UV filters on marine bacteria: Combined effects with damaging solar radiation. Sci. Total Environ. 2020, 722, 1377803. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Law, J.C.F.; Lam, T.K.; Leung, K.S.Y. Risks of organic UV filters: A review of environmental and human health concern studies. Sci. Total Environ. 2021, 755, 142486. [Google Scholar] [CrossRef] [PubMed]
- Pico, Y.; Belenguer, V.; Corcellas, C.; Diaz-Cruz, M.S.; Eljarrat, E.; Farre, M.; Gago-Ferrero, P.; Huerta, B.; Navarro-Ortega, A.; Petrovic, M.; et al. Contaminants of emerging concern in freshwater fish from four Spanish Rivers. Sci. Total Environ. 2019, 659, 1186–1198. [Google Scholar] [CrossRef]
- Duis, K.; Junker, T.; Coors, A. Review of the environmental fate and effects of two UV filter substances used in cosmetic products. Sci. Total Environ. 2022, 808, 151931. [Google Scholar] [CrossRef]
- Mitchelmore, C.L.; Burns, E.E.; Conway, A.; Heyes, A.; Davies, I.A. A Critical Review of Organic Ultraviolet Filter Exposure, Hazard, and Risk to Corals. Environ. Toxicol. Chem. 2021. [Google Scholar] [CrossRef]
- Tsui, M.M.P.; Lam, J.C.W.; Ng, T.Y.; Ang, P.O.; Murphy, M.B.; Lam, P.K.S. Occurrence, Distribution, and Fate of Organic UV Filters in Coral Communities. Environ. Sci. Technol. 2017, 51, 4182–4190. [Google Scholar] [CrossRef]
- He, T.; Tsui, M.M.P.; Tan, C.J.; Ng, K.Y.; Guo, F.W.; Wang, L.H.; Chen, T.H.; Fan, T.Y.; Lam, P.K.S.; Murphy, M.B. Comparative toxicities of four benzophenone ultraviolet filters to two life stages of two coral species. Sci. Total Environ. 2019, 651, 2391–2399. [Google Scholar] [CrossRef]
- Downs, C.A.; Kramarsky-Winter, E.; Segal, R.; Fauth, J.; Knutson, S.; Bronstein, O.; Ciner, F.R.; Jeger, R.; Lichtenfeld, Y.; Woodley, C.M.; et al. Toxicopathological Effects of the Sunscreen UV Filter, Oxybenzone (Benzophenone-3), on Coral Planulae and Cultured Primary Cells and Its Environmental Contamination in Hawaii and the U.S. Virgin Islands. Arch. Environ. Contam. Toxicol. 2016, 70, 265–288. [Google Scholar] [CrossRef]
- The Senate, TWENTY-NINTH LEGISLATURE, State of Hawaii. Available online: https://www.capitol.hawaii.gov/session2018/bills/SB2571_.HTM (accessed on 29 April 2021).
- Danovaro, R.; Bongiorni, L.; Corinaldesi, C.; Giovannelli, D.; Damiani, E.; Astolfi, P.; Greci, L.; Pusceddu, A. Sunscreens cause coral bleaching by promoting viral infections. Environ. Health Perspect. 2008, 116, 441–447. [Google Scholar] [CrossRef] [Green Version]
- Thorel, E.; Clergeaud, F.; Jaugeon, L.; Rodrigues, A.M.S.; Lucas, J.; Stien, D.; Lebaron, P. Effect of 10 UV filters on the brine shrimp Artemia salina and themarinemicroalga Tetraselmis sp. Toxics 2020, 8, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, F.; He, Y.; Gin, K.H. Evaluating the joint toxicity of two benzophenone-type UV filters on the green alga Chlamydomonas reinhardtii with response surface methodology. Toxics 2018, 6, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyd, A.; Stewart, C.B.; Philibert, D.A.; How, Z.T.; El-Din, M.G.; Tierney, K.B.; Blewett, T.A. A burning issue: The effect of organic ultraviolet filter exposure on the behaviour and physiology of Daphnia magna. Sci. Total Environ. 2021, 750, 141707. [Google Scholar] [CrossRef] [PubMed]
- Quintaneiro, C.; Teixeira, B.; Benedé, J.L.; Chisvert, A.; Soares, A.M.V.M.; Monteiro, M.S. Toxicity effects of the organic UV-filter 4-Methylbenzylidene camphor in zebrafish embryos. Chemosphere 2019, 218, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Pawlowski, S.; Herzog, B.; Sohn, M.; Petersen-Thiery, M.; Acker, S. EcoSun Pass: A tool to evaluate the ecofriendliness of UV filters used in sunscreen products. Int. J. Cosmet. Sci. 2021, 43, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J.; Passeron, T.; Gilaberte, Y.; Granger, C.; Leone, G.; Narda, M.; Schalka, S.; Trullas, C.; Masson, P.; Lim, H.W. Photoprotection of the future: Challenges and opportunities. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Yarovaya, L.; Waranuch, N.; Wisuitiprot, W.; Khunkitti, W. Effect of grape seed extract on skin fibroblasts exposed to UVA light and its photostability in sunscreen formulation. J. Cosmet. Dermatol. 2020, 20, 1271–1282. [Google Scholar] [CrossRef]
- Hubner, A.; Sobreira, F.; Neto, A.V.; de Oliveira Pinto, C.A.S.; Dario, M.F.; Díaz, I.E.C.; Lourenço, F.R.; Rosado, C.; Baby, A.R.; Bacchi, E.M. The synergistic behavior of antioxidant phenolic compounds obtained fromwinemaking waste’s valorization, increased the efficacy of a sunscreen system. Antioxidants 2019, 8, 530. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, A.C.P.; Paiva, J.P.; Diniz, R.R.; dos Anjos, V.M.; Silva, A.B.S.M.; Pinto, A.V.; dos Santos, E.P.; Leitão, A.C.; Cabral, L.M.; Rodrigues, C.R.; et al. Photoprotection assessment of olive (Olea europaea L.) leaves extract standardized to oleuropein: In vitro and in silico approach for improved sunscreens. J. Photochem. Photobiol. B Biol. 2019, 193, 162–171. [Google Scholar] [CrossRef]
- Polonini, H.C.; Brandão, M.A.F.; Raposo, N.R.B. A natural broad-spectrum sunscreen formulated from the dried extract of Brazilian Lippia sericea as a single UV filter. RSC Adv. 2014, 4, 62566–62575. [Google Scholar] [CrossRef]
- Alves, G.D.A.D.; de Souza, R.O.; Rogez, H.; Masaki, H.; Fonseca, M.J.V. Cecropia obtusa, an Amazonian ethanolic extract, exhibits photochemoprotective effect in vitro and balances the redox cellular state in response to UV radiation. Ind. Crops Prod. 2016, 94, 893–902. [Google Scholar] [CrossRef]
- Anurukvorakun, O.; Boonruang, R.; Lahpun, N. Formulation strategy, stability issues, safety and efficacy evaluations of Acacia catechu whitening cream. Int. J. Appl. Pharm. 2019, 11, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Wróblewska, K.B.; Baby, A.R.; Grombone Guaratini, M.T.; Moreno, P.R.H. In vitro antioxidant and photoprotective activity of five native Brazilian bamboo species. Ind. Crops Prod. 2019, 130, 208–215. [Google Scholar] [CrossRef]
- Lohezic-Le Devehat, F.; Legouin, B.; Couteau, C.; Boustie, J.; Coiffard, L. Lichenic extracts and metabolites as UV filters. J. Photochem. Photobiol. B Biol. 2013, 120, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Lee, S.H.; Won, K. Wood Powder as a New Natural Sunscreen Ingredient. Biotechnol. Bioprocess Eng. 2019, 24, 258–263. [Google Scholar] [CrossRef]
- Rasheed, A.; Shama, S.N.; Mohanalakshmi, S.; Ravichandran, V. Formulation, characterization and in vitro evaluation of herbal sunscreen lotion. Orient. Pharm. Exp. Med. 2012, 12, 241–246. [Google Scholar] [CrossRef]
- Widiyati, E. Determination of ultraviolet filter activity on coconut oil cosmetic cream. AIP Conf. Proc. 2017, 1868, 020004. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, S.; Sherje, A.P. Development of resveratrol and green tea sunscreen formulation for combined photoprotective and antioxidant properties. J. Drug Deliv. Sci. Technol. 2020, 60, 102000. [Google Scholar] [CrossRef]
- L’Alloret, F.; Candau, D.; Seité, S.; Pygmalion, M.J.; Ruiz, L.; Josso, M.; Meaudre, H.; Gauchet, L.; Pena, A.M.; Colonna, A. New combination of ultraviolet absorbers in an oily emollient increases sunscreen efficacy and photostability. Dermatol. Ther. 2012, 2, 4. [Google Scholar] [CrossRef] [Green Version]
- Montenegro, L.; Santagati, L.M. Use of vegetable oils to improve the sun protection factor of sunscreen formulations. Cosmetics 2019, 6, 25. [Google Scholar] [CrossRef] [Green Version]
- Mota, M.D.; Costa, R.Y.S.; Guedes, A.A.S.; Silva, L.C.R.C.E.; Chinalia, F.A. Guava-fruit extract can improve the UV-protection efficiency of synthetic filters in sun cream formulations. J. Photochem. Photobiol. B Biol. 2019, 201, 111639. [Google Scholar] [CrossRef] [PubMed]
- Milani, L.P.G.; Garcia, N.O.S.; Morais, M.C.; Dias, A.L.S.; Oliveira, N.L.; Conceição, E.C. Extract from byproduct Psidium guajava standardized in ellagic acid: Additivation of the in vitro photoprotective efficacy of a cosmetic formulation. Rev. Bras. Farmacogn. 2018, 28, 692–696. [Google Scholar] [CrossRef]
- Niculae, G.; Lacatusu, I.; Badea, N.; Stan, R.; Vasile, B.S.; Meghea, A. Rice bran and raspberry seed oil-based nanocarriers with self-antioxidative properties as safe photoprotective formulations. Photochem. Photobiol. Sci. 2014, 13, 703–716. [Google Scholar] [CrossRef]
- Almeida, W.A.D.S.; Antunes, A.D.S.; Penido, R.G.; Correa, H.S.D.G.; Nascimento, A.M.D.; Andrade, Â.L.; Santos, V.R.; Cazati, T.; Amparo, T.R.; Souza, G.H.B.D.; et al. Photoprotective activity and increase of SPF in sunscreen formulation using lyophilized red propolis extracts from Alagoas. Rev. Bras. Farmacogn. 2019, 29, 373–380. [Google Scholar] [CrossRef]
- Llewellyn, C.A.; Airs, R.L. Distribution and abundance of MAAs in 33 species of microalgae across 13 classes. Mar. Drugs 2010, 8, 1273–1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z. Occurrence of mycosporine-like amino acids (MAAs) in the bloom-forming cyanobacterium Microcystis aeruginosa. J. Plankton Res. 2004, 26, 963–966. [Google Scholar] [CrossRef] [Green Version]
- Rangel, K.C.; Villela, L.Z.; Pereira, K.D.C.; Colepicolo, P.; Debonsi, H.M.; Gaspar, L.R. Assessment of the photoprotective potential and toxicity of Antarctic red macroalgae extracts from Curdiea racovitzae and Iridaea cordata for cosmetic use. Algal Res. 2020, 50, 101984. [Google Scholar] [CrossRef]
- Prasedya, E.S.; Syafitri, S.M.; Geraldine, B.A.F.D.; Hamdin, C.D.; Frediansyah, A.; Miyake, M.; Kobayashi, D.; Hazama, A.; Sunarpi, H. UVA photoprotective activity of brown macroalgae Sargassum cristafolium. Biomedicines 2019, 7, 77. [Google Scholar] [CrossRef] [Green Version]
- Proteau, P.J.; Gerwick, W.H.; Garcia-Pichel, F.; Castenholz, R. The structure of scytonemin, an ultraviolet sunscreen pigment from the sheaths of cyanobacteria. Experientia 1993, 49, 825–829. [Google Scholar] [CrossRef]
- Kang, M.R.; Jo, S.A.; Lee, H.; Yoon, Y.D.; Kwon, J.H.; Yang, J.W.; Choi, B.J.; Park, K.H.; Lee, M.Y.; Lee, C.W.; et al. Inhibition of Skin Inflammation by Scytonemin, an Ultraviolet Sunscreen Pigment. Mar. Drugs 2020, 18, 300. [Google Scholar] [CrossRef]
- Teixeira, T.R.; Rangel, K.C.; Tavares, R.S.N.; Kawakami, C.M.; Dos Santos, G.S.; Maria-Engler, S.S.; Colepicolo, P.; Gaspar, L.R.; Debonsi, H.M. In Vitro Evaluation of the Photoprotective Potential of Quinolinic Alkaloids Isolated from the Antarctic Marine Fungus Penicillium echinulatum for Topical Use. Mar. Biotechnol. 2021, 23, 357–372. [Google Scholar] [CrossRef] [PubMed]
- Maciel, O.M.C.; Tavares, R.S.N.; Caluz, D.R.E.; Gaspar, L.R.; Debonsi, H.M. Photoprotective potential of metabolites isolated from algae-associated fungi Annulohypoxylon stygium. J. Photochem. Photobiol. B Biol. 2018, 178, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Regalado, E.L.; Rodriguez, M.; Menendez, R.; Concepcion, A.A.; Nogueiras, C.; Laguna, A.; Rodriguez, A.A.; Williams, D.E.; Lorenzo-Luaces, P.; Valdes, O.; et al. Repair of UVB-damaged skin by the antioxidant sulphated flavone glycoside thalassiolin B isolated from the marine plant Thalassia testudinum Banks ex Konig. Mar. Biotechnol. 2009, 11, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.E.; Setlow, R.B. Ultraviolet Radiation-induced DNA Damage and Its Photorepair in the Skin of the Platyfish Xiphophorus. Cancer Res. 1993, 53, 2249–2255. [Google Scholar]
- Ryu, B.; Qian, Z.-J.; Kim, M.-M.; Nam, K.W.; Kim, S.-K. Anti-photoaging activity and inhibition of matrix metalloproteinase (MMP) by marine red alga, Corallina pilulifera methanol extract. Radiat. Phys. Chem. 2009, 78, 98–105. [Google Scholar] [CrossRef]
- Yabe, S.; Sato, T. Cerium oxide for sunscreen cosmetics. J. Solid State Chem. 2003, 171, 7–11. [Google Scholar] [CrossRef]
- Seixas, V.C.; Serra, O.A. Stability of sunscreens containing CePO4: Proposal for a new inorganic UV filter. Molecules 2014, 19, 9907–9925. [Google Scholar] [CrossRef] [Green Version]
- Polonini, H.C.; Lopes, R.S.; Beatriz, A.; Gomes, R.S.; Silva, A.O.; De Lima, R.V.; Nunes, G.A.; Brandão, M.A.F.; Raposo, N.R.B.; De Lima, D.P. Synthesis and evaluation of octocrylene-inspired compounds for Uv-filter activity. Quim. Nova 2014, 37, 1004–1009. [Google Scholar] [CrossRef]
- González, M.T.P.; Fumagalli, F.; Benevenuto, C.G.; da Silva Emery, F.; Gaspar, L.R. Novel benzophenone-3 derivatives with promising potential as UV filters: Relationship between structure, photoprotective potential and phototoxicity. Eur. J. Pharm. Sci. 2017, 101, 200–210. [Google Scholar] [CrossRef]
- Gonçalves, M.C.; Rossoni, J.V., Jr.; Rabelo, A.C.S.; Costa, D.C.; Cazati, T.; Taylor, J.G.; Dos Santos, V.M.R. Preliminary studies of the cytotoxicity and photoprotective properties of benzophenone and lactone derivatives. Rev. Virtual Quim. 2018, 10, 600–608. [Google Scholar] [CrossRef]
- Abbas, N.; Mahmood, Z.; Manzoor, S.; Akhtar, Z.; Tariq, M. Synthesis, characterization and sunscreen protection factor of novel synthesized Para Amino Benzoic Acid (PABA) esters. J. Chem. Soc. Pak. 2019, 41, 701–706. [Google Scholar]
- Bino, A.; Baldisserotto, A.; Scalambra, E.; Dissette, V.; Vedaldi, D.E.; Salvador, A.; Durini, E.; Manfredini, S.; Vertuani, S. Design, synthesis and biological evaluation of novel hydroxy-phenyl-1H-benzimidazoles as radical scavengers and UV-protective agents. J. Enzyme Inhib. Med. Chem. 2017, 32, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Djuidje, E.N.; Durini, E.; Sciabica, S.; Serra, E.; Balzarini, J.; Liekens, S.; Manfredini, S.; Vertuani, S.; Baldisserotto, A. Skin damages—Structure activity relationship of benzimidazole derivatives bearing a 5-membered ring system. Molecules 2020, 25, 4324. [Google Scholar] [CrossRef] [PubMed]
- Popiół, J.; Gunia-Krzyzak, A.; Piska, K.; Zelaszczyk, D.; Koczurkiewicz, P.; Słoczynska, K.; Wójcik-Pszczoła, K.; Krupa, A.; Kryczyk-Poprawa, A.; Zesławska, E.; et al. Discovery of novel UV-filters with favorable safety profiles in the 5-arylideneimidazolidine-2,4-dione derivatives group. Molecules 2019, 24, 2321. [Google Scholar] [CrossRef] [Green Version]
- Peyrot, C.; Mention, M.M.; Brunissen, F.; Allais, F. Sinapic acid esters: Octinoxate substitutes combining suitable uv protection and antioxidant activity. Antioxidants 2020, 9, 782. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.S.; Corrêa, M.A.; Chung, M.C.; Dos Santos, J.L. Synthesis, antioxidant and photoprotection activities of hybrid derivatives useful to prevent skin cancer. Bioorg. Med. Chem. 2014, 22, 2733–2738. [Google Scholar] [CrossRef] [PubMed]
- Polonini, H.C.; Lima, L.L.; Gonçalves, K.M.; Do Carmo, A.M.R.; Da Silva, A.D.; Raposo, N.R.B. Photoprotective activity of resveratrol analogues. Bioorg. Med. Chem. 2013, 21, 964–968. [Google Scholar] [CrossRef]
- Horbury, M.D.; Holt, E.L.; Mouterde, L.M.M.; Balaguer, P.; Cebrian, J.; Blasco, L.; Allais, F.; Stavros, V.G. Towards symmetry driven and nature inspired UV filter design. Nat. Commun. 2019, 10, 4748. [Google Scholar] [CrossRef] [Green Version]
- Rioux, B.; Peyrot, C.; Mention, M.M.; Brunissen, F.; Allais, F. Sustainable synthesis of p-hydroxycinnamic diacids through proline-mediated knoevenagel condensation in Ethanol: An access to potent phenolic UV filters and radical scavengers. Antioxidants 2020, 9, 331. [Google Scholar] [CrossRef] [Green Version]
- Resende, D.I.S.P.; Almeida, M.C.; Maciel, B.; Carmo, H.; Lobo, J.S.; Pozzo, C.D.; Cravo, S.M.; Rosa, G.P.; Kane-Pagès, A.; Do Carmo Barreto, M.; et al. Efficacy, stability, and safety evaluation of new polyphenolic xanthones towards identification of bioactive compounds to fight skin photoaging. Molecules 2020, 25, 2782. [Google Scholar] [CrossRef]
- Polonini, H.C.; Dias, R.M.P.; Souza, I.O.; Gonçalves, K.M.; Gomes, T.B.B.; Raposo, N.R.B.; Da Silva, A.D. Quinolines derivatives as novel sunscreening agents. Bioorg. Med. Chem. Lett. 2013, 23, 4506–4510. [Google Scholar] [CrossRef] [PubMed]
- Giacomo, B.; Luca, B.; Nicola, L.; Luigi, R. Development of an innovative and eco-friendly UV radiation absorber, based on furan moieties. Cosmetics 2020, 7, 6. [Google Scholar] [CrossRef] [Green Version]
- Peyrot, C.; Mention, M.M.; Brunissen, F.; Balaguer, P.; Allais, F. Innovative bio-based organic UV-A and blue light filters from Meldrum’s acid. Molecules 2020, 25, 2178. [Google Scholar] [CrossRef] [PubMed]

| Marine Organism | Species | UV Filter | Concentration | Negative Effects | Reference |
|---|---|---|---|---|---|
| Corals | Pocillopora damicornis, Seriatopora caliendrum, Stylophora pistillata, Acropora spp. | Benzophenone-3 (10) | 31.8 ng/g | Not described | [88] |
| Acropora spp. | Zinc Oxide (35) | - | Coral bleaching | [59] | |
| Algae | Tetraselmis sp. | Homosalate (8) | 1 mg/L | Growth inhibition | [93] |
| - | Changes in cell morphology | ||||
| Benzophenone-3 (10) | |||||
| Avobenzone (11) | |||||
| Chlamydomonas reinhardtii | Benzophenone-1 | 5 mg/L | Growth inhibition Decrease of photosynthetic pigments | [94] | |
| Benzophenone-3 (10) | |||||
| Brine Shrimp | Artemia salina | Homosalate (8) | 2 mg/L | Induce mortality in 54% | [93] |
| Avobenzone (11) | Induce mortality in 64% | ||||
| Octocrylene (30) | Induce mortality in 88% | ||||
| Crustaceans | Daphnia magna | Avobenzone (11) | 200 μg/L | Induce metabolic disruption Reduce ability to detect light stimuli Behavioural changes | [95] |
| Octocrylene (30) | 200 μg/L | ||||
| Dolphins | Pontoporia blainvillei | Octocrylene (30) | 782 ng/g | Bioaccumulation and biomagnification | [63] |
| Fish | Danio rerio | 3-(4-methylbenzylidene) camphor (7) | 0.19–0.77 mg/L | Induce malformations Decrease heart rate Affecting sexual differentiation Induce neurotoxicity | [96] |
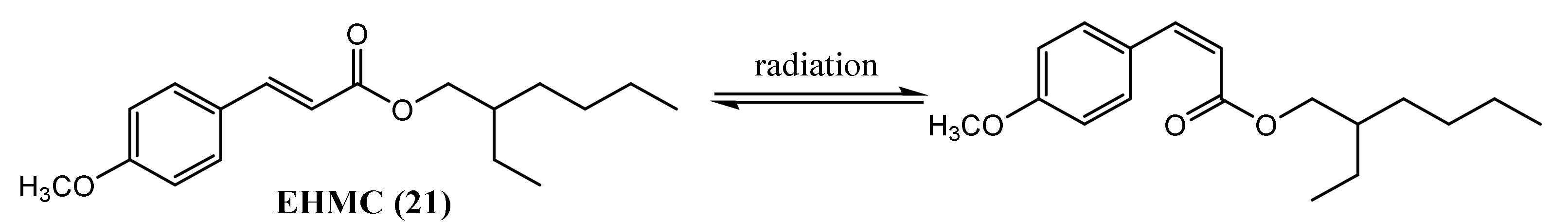
| Oncorhynchus mykiss | Ethylhexyl methoxycinnamate (21) | 96.0–395.6 μg/kg | Changes in metabolic pathway Increasing of leukocytes Oxidative stress | [60] | |
| Mussels | Mytilus galloprovincialis | Benzophenone-3 (10) | 100 µg/L | Affect the metabolic activity | [52] |
| 1000 µg/L | Induce cellular damage | ||||
| Ethylhexyl methoxycinnamate (21) | 3992 ng/g | Not described | [53] | ||
| Padimate O (24) | 833 ng/g | ||||
| Octocrylene (30) | 1765 ng/g | ||||
| Ethylhexyl methoxycinnamate (21) | 3–256 ng/g | Bioaccumulation | [14] | ||
| Octocrylene (30) | 2–7112 ng/g | ||||
| Mytilus edulis | Ethylhexyl methoxycinnamate (21) | 3–256 ng/g | Bioaccumulation | [14] | |
| Octocrylene (30) | 2–7112 ng/g |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jesus, A.; Sousa, E.; Cruz, M.T.; Cidade, H.; Lobo, J.M.S.; Almeida, I.F. UV Filters: Challenges and Prospects. Pharmaceuticals 2022, 15, 263. https://doi.org/10.3390/ph15030263
Jesus A, Sousa E, Cruz MT, Cidade H, Lobo JMS, Almeida IF. UV Filters: Challenges and Prospects. Pharmaceuticals. 2022; 15(3):263. https://doi.org/10.3390/ph15030263
Chicago/Turabian StyleJesus, Ana, Emília Sousa, Maria T. Cruz, Honorina Cidade, José M. Sousa Lobo, and Isabel F. Almeida. 2022. "UV Filters: Challenges and Prospects" Pharmaceuticals 15, no. 3: 263. https://doi.org/10.3390/ph15030263
APA StyleJesus, A., Sousa, E., Cruz, M. T., Cidade, H., Lobo, J. M. S., & Almeida, I. F. (2022). UV Filters: Challenges and Prospects. Pharmaceuticals, 15(3), 263. https://doi.org/10.3390/ph15030263