IL12/23 Blockade with Ustekinumab as a Treatment for Immune-Related Cutaneous Adverse Events
Abstract
:1. Introduction
2. Results
2.1. Demographics and Oncologic History
2.2. Clinicopathologic Characteristics
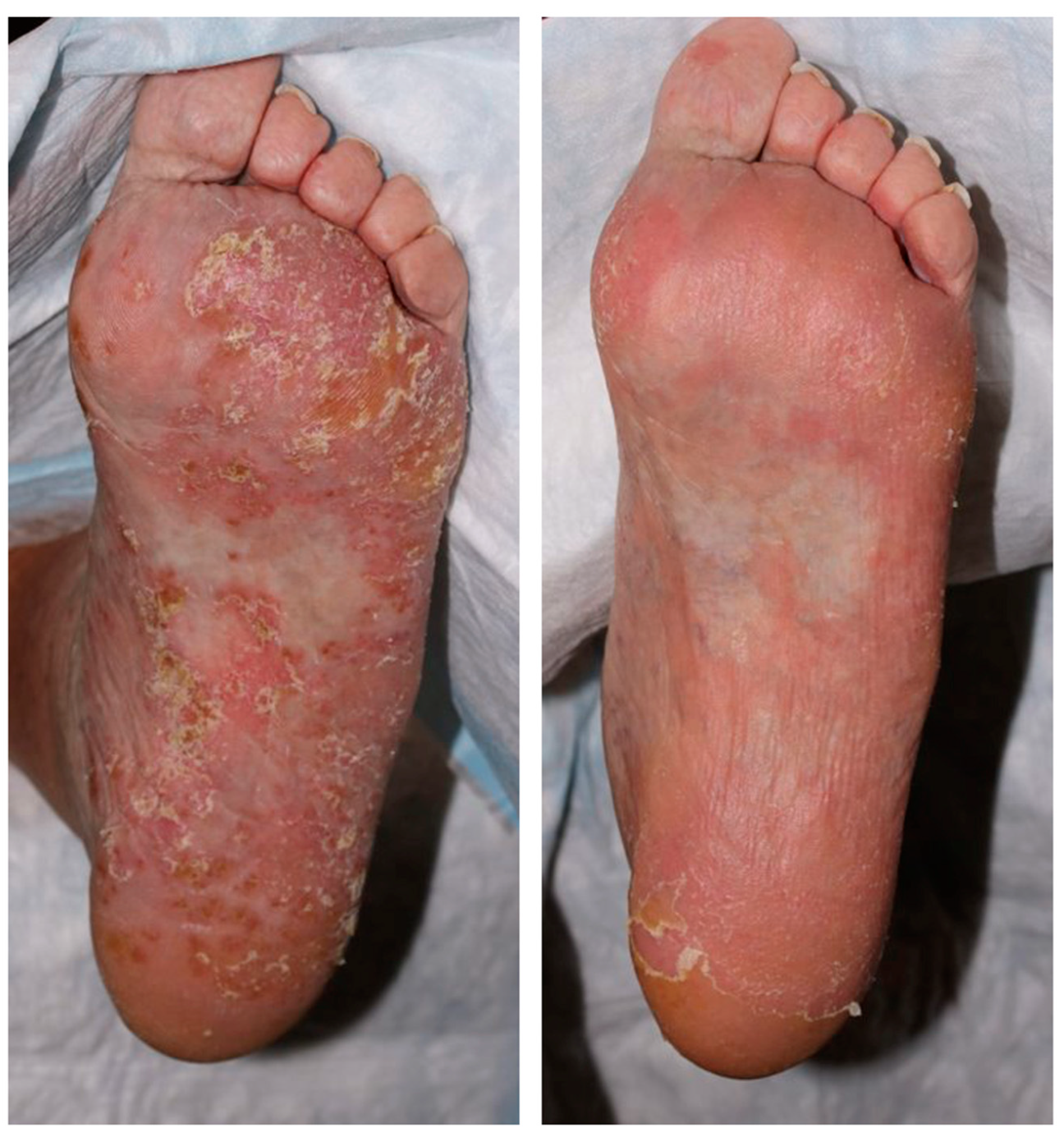
2.3. Clinical Response to UST
2.4. Changes in Supportive Management of ircAEs
2.5. Safety of Ustekinumab
2.6. Laboratory Values
2.7. Anti-Tumor Response and Cancer Progression
3. Discussion
3.1. Clinical Response to Ustekinumab
3.2. Mechanism of Ustekinumab for ircAE Treatment
3.3. Safety of Ustekinumab
4. Materials and Methods
4.1. Patients
4.2. Study Outcomes
4.3. Statistical Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, M.D.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.W.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.; Alekseev, B.; Rha, S.Y.; Porta, C.; Eto, M.; Powles, T.; Grünwald, V.; Hutson, T.E.; Kopyltsov, E.; Méndez-Vidal, M.J.; et al. Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. N. Engl. J. Med. 2021, 384, 1289–1300. [Google Scholar] [CrossRef]
- Shah, N.J.; Sura, S.D.; Shinde, R.; Shi, J.; Singhal, P.K.; Robert, N.J.; Vogelzang, N.J.; Perini, R.F.; Motzer, R.J. Real-world Treatment Patterns and Clinical Outcomes for Metastatic Renal Cell Carcinoma in the Current Treatment Era. Eur. Urol. Open Sci. 2023, 49, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F.; et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2017, 377, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.J.; Naidoo, J.; Santomasso, B.D.; Lacchetti, C.; Adkins, S.; Anadkat, M.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J. Clin. Oncol. 2021, 39, 4073–4126. [Google Scholar] [CrossRef]
- Wang, E.; Kraehenbuehl, L.; Ketosugbo, K.; Kern, J.A.; Lacouture, M.E.; Leung, D.Y.M. Immune-related cutaneous adverse events due to checkpoint inhibitors. Ann. Allergy Asthma Immunol. 2021, 126, 613–622. [Google Scholar] [CrossRef]
- Lacouture, M.; Sibaud, V. Toxic Side Effects of Targeted Therapies and Immunotherapies Affecting the Skin, Oral Mucosa, Hair, and Nails. Am. J. Clin. Dermatol. 2018, 19 (Suppl. S1), 31–39. [Google Scholar] [CrossRef]
- Shah, N.J.; Lacouture, M.E. Dermatologic immune-related adverse events to checkpoint inhibitors in cancer. J. Allergy Clin. Immunol. 2023, 151, 407–409. [Google Scholar] [CrossRef]
- Kuo, A.M.; Markova, A. High Grade Dermatologic Adverse Events Associated With Immune Checkpoint Blockade for Cancer. Front. Med. 2022, 9, 898790. [Google Scholar] [CrossRef]
- Feagan, B.G.; Sandborn, W.J.; Gasink, C.; Jacobstein, D.; Lang, Y.; Friedman, J.R.; Blank, M.A.; Johanns, J.; Gao, L.L.; Miao, Y.; et al. Ustekinumab as Induction and Maintenance Therapy for Crohn’s Disease. N. Engl. J. Med. 2016, 375, 1946–1960. [Google Scholar] [CrossRef]
- Sands, B.E.; Sandborn, W.J.; Panaccione, R.; O’Brien, C.D.; Zhang, H.; Johanns, J.; Adedokun, O.J.; Li, K.; Peyrin-Biroulet, L.; Van Assche, G.; et al. Ustekinumab as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2019, 381, 1201–1214. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, C.E.; Strober, B.E.; van de Kerkhof, P.; Ho, V.; Fidelus-Gort, R.; Yeilding, N.; Guzzo, C.; Xia, Y.; Zhou, B.; Li, S.; et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N. Engl. J. Med. 2010, 362, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, D.L.; Juhl, C.B.; Chen, I.M.; Kellermann, L.; Nielsen, O.H. Immune checkpoint Inhibitor-Induced diarrhea and Colitis: Incidence and Management. A systematic review and Meta-analysis. Cancer Treat. Rev. 2022, 109, 102440. [Google Scholar] [CrossRef]
- Thomas, A.S.; McQuade, J.; Altan, M.; Wang, Y. S1778 IL12/23 Blockade Therapy for Refractory Immune Checkpoint Inhibitor Colitis: A Case Series. Off. J. Am. Coll. Gastroenterol. ACG 2021, 116, S786. [Google Scholar] [CrossRef]
- Buchman, A.L. Side effects of corticosteroid therapy. J. Clin. Gastroenterol. 2001, 33, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Rice, J.B.; White, A.G.; Scarpati, L.M.; Wan, G.; Nelson, W.W. Long-term Systemic Corticosteroid Exposure: A Systematic Literature Review. Clin. Ther. 2017, 39, 2216–2229. [Google Scholar] [CrossRef]
- Warrington, T.P.; Bostwick, J.M. Psychiatric adverse effects of corticosteroids. Mayo Clin. Proc. 2006, 81, 1361–1367. [Google Scholar] [CrossRef]
- Arbour, K.C.; Mezquita, L.; Long, N.; Rizvi, H.; Auclin, E.; Ni, A.; Martínez-Bernal, G.; Ferrara, R.; Lai, W.V.; Hendriks, L.E.L.; et al. Impact of Baseline Steroids on Efficacy of Programmed Cell Death-1 and Programmed Death-Ligand 1 Blockade in Patients With Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 2872–2878. [Google Scholar] [CrossRef]
- Fucà, G.; Galli, G.; Poggi, M.; Lo Russo, G.; Proto, C.; Imbimbo, M.; Ferrara, R.; Zilembo, N.; Ganzinelli, M.; Sica, A.; et al. Modulation of peripheral blood immune cells by early use of steroids and its association with clinical outcomes in patients with metastatic non-small cell lung cancer treated with immune checkpoint inhibitors. ESMO Open 2019, 4, e000457. [Google Scholar] [CrossRef]
- Nikita, N.; Banks, J.; Keith, S.W.; Song, A.; Johnson, J.M.; Wilson, M.; Sharma, S.; Lu-Yao, G. Is Timing of Steroid Exposure Prior to Immune Checkpoint Inhibitor Initiation Associated with Treatment Outcomes in Melanoma? A Population-Based Study. Cancers 2022, 14, 1296. [Google Scholar] [CrossRef] [PubMed]
- Iorgulescu, J.B.; Gokhale, P.C.; Speranza, M.C.; Eschle, B.K.; Poitras, M.J.; Wilkens, M.K.; Soroko, K.M.; Chhoeu, C.; Knott, A.; Gao, Y.; et al. Concurrent Dexamethasone Limits the Clinical Benefit of Immune Checkpoint Blockade in Glioblastoma. Clin. Cancer Res. 2021, 27, 276–287. [Google Scholar] [CrossRef]
- Petrelli, F.; Signorelli, D.; Ghidini, M.; Ghidini, A.; Pizzutilo, E.G.; Ruggieri, L.; Cabiddu, M.; Borgonovo, K.; Dognini, G.; Brighenti, M.; et al. Association of Steroids use with Survival in Patients Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Cancers 2020, 12, 546. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Fonacier, L.S. Adverse Effects of Nonsystemic Steroids (Inhaled, Intranasal, and Cutaneous): A Review of the Literature and Suggested Monitoring Tool. Curr. Allergy Asthma Rep. 2016, 16, 44. [Google Scholar] [CrossRef]
- Evoy, K.E.; Sadrameli, S.; Contreras, J.; Covvey, J.R.; Peckham, A.M.; Morrison, M.D. Abuse and Misuse of Pregabalin and Gabapentin: A Systematic Review Update. Drugs 2021, 81, 125–156. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Smyth, M.J.; Teng, M.W.L. Interleukin (IL)-12 and IL-23 and Their Conflicting Roles in Cancer. Cold Spring Harb. Perspect. Biol. 2018, 10, a028530. [Google Scholar] [CrossRef]
- Lim, S.Y.; Lee, J.H.; Gide, T.N.; Menzies, A.M.; Guminski, A.; Carlino, M.S.; Breen, E.J.; Yang, J.Y.H.; Ghazanfar, S.; Kefford, R.F.; et al. Circulating Cytokines Predict Immune-Related Toxicity in Melanoma Patients Receiving Anti-PD-1-Based Immunotherapy. Clin. Cancer Res. 2019, 25, 1557–1563. [Google Scholar] [CrossRef]
- Kulig, P.; Musiol, S.; Freiberger, S.N.; Schreiner, B.; Gyülveszi, G.; Russo, G.; Pantelyushin, S.; Kishihara, K.; Alessandrini, F.; Kündig, T.; et al. IL-12 protects from psoriasiform skin inflammation. Nat. Commun. 2016, 7, 13466. [Google Scholar] [CrossRef]
- Liu, T.; Li, S.; Ying, S.; Tang, S.; Ding, Y.; Li, Y.; Qiao, J.; Fang, H. The IL-23/IL-17 Pathway in Inflammatory Skin Diseases: From Bench to Bedside. Front. Immunol. 2020, 11, 594735. [Google Scholar] [CrossRef]
- Chan, T.C.; Hawkes, J.E.; Krueger, J.G. Interleukin 23 in the skin: Role in psoriasis pathogenesis and selective interleukin 23 blockade as treatment. Ther. Adv. Chronic Dis. 2018, 9, 111–119. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Rebuck, R.; Wang, Y.; Zou, B.; Adedokun, O.J.; Gasink, C.; Sands, B.E.; Hanauer, S.B.; Targan, S.; Ghosh, S.; et al. Five-Year Efficacy and Safety of Ustekinumab Treatment in Crohn’s Disease: The IM-UNITI Trial. Clin. Gastroenterol. Hepatol. 2022, 20, 578–590.e574. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, J.; Matsuura, M.; Hisamatsu, T. Safety evaluation of ustekinumab for moderate-to-severe ulcerative colitis. Expert. Opin. Drug Saf. 2022, 21, 1–8. [Google Scholar] [CrossRef]
- Teng, M.W.; Vesely, M.D.; Duret, H.; McLaughlin, N.; Towne, J.E.; Schreiber, R.D.; Smyth, M.J. Opposing roles for IL-23 and IL-12 in maintaining occult cancer in an equilibrium state. Cancer Res. 2012, 72, 3987–3996. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, A.; Ständer, S.; Berneburg, M.; Böhm, M.; Kulms, D.; van Steeg, H.; Grosse-Heitmeyer, K.; Krutmann, J.; Schwarz, T. Interleukin-12 suppresses ultraviolet radiation-induced apoptosis by inducing DNA repair. Nat. Cell Biol. 2002, 4, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, D.A.; Walterscheid, J.P.; Ullrich, S.E. Reversal of ultraviolet radiation-induced immune suppression by recombinant interleukin-12: Suppression of cytokine production. Immunology 2000, 101, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Langowski, J.L.; Zhang, X.; Wu, L.; Mattson, J.D.; Chen, T.; Smith, K.; Basham, B.; McClanahan, T.; Kastelein, R.A.; Oft, M. IL-23 promotes tumour incidence and growth. Nature 2006, 442, 461–465. [Google Scholar] [CrossRef]
- Chan, I.H.; Jain, R.; Tessmer, M.S.; Gorman, D.; Mangadu, R.; Sathe, M.; Vives, F.; Moon, C.; Penaflor, E.; Turner, S.; et al. Interleukin-23 is sufficient to induce rapid de novo gut tumorigenesis, independent of carcinogens, through activation of innate lymphoid cells. Mucosal Immunol. 2014, 7, 842–856. [Google Scholar] [CrossRef]
- Teng, M.W.; Andrews, D.M.; McLaughlin, N.; von Scheidt, B.; Ngiow, S.F.; Möller, A.; Hill, G.R.; Iwakura, Y.; Oft, M.; Smyth, M.J. IL-23 suppresses innate immune response independently of IL-17A during carcinogenesis and metastasis. Proc. Natl. Acad. Sci. USA 2010, 107, 8328–8333. [Google Scholar] [CrossRef]
- Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf (accessed on 11 September 2023).


| All Patients (n = 14) | Responders to Ustekinumab (n = 10) | Non-Responders to Ustekinumab (n = 4) | p-Value | ||
|---|---|---|---|---|---|
| Age | Median (IQR) | 71 (10) | 71 (24) | 72 (12) | 0.515 |
| Sex, n (%) | Female | 6 (42.9) | 3 (75) | 3 (30) | 0.125 |
| Male | 8 (57.1) | 1 (25) | 7 (70) | ||
| Race, n (%) | White | 14 (100) | 4 (100) | 10 (100) | -- |
| Non-Hispanic | 14 (100) | 4 (100) | 10 (100) | -- | |
| Cancer type, n (%) | Breast cancer | 1 (7.1) | 1 (25) | 0 (0) | 0.708 |
| Lymphoma | 1 (7.1) | 0 (0) | 1 (10) | ||
| Melanoma | 4 (28.6) | 1 (25) | 3 (30) | ||
| Pancreatic | 1 (7.1) | 0 (0) | 1 (10) | ||
| Genitourinary | 7 (14.3) | 1 (25) | 1 (10) | ||
| ICB type, n (%) | Atezolizumab | 1 (7.1) | 0 (0) | 1 (10) | 0.375 |
| Avelumab | 1 (7.1) | 0 (0) | 1 (10) | ||
| Nivolumab | 3 (21.4) | 2 (50) | 1 (10) | ||
| Pembrolizumab | 9 (64.3) | 2 (50) | 7 (70) |
| All Patients (n = 14) | Responders to Ustekinumab (n = 10) | Non-Responders to Ustekinumab (n = 4) | p-Value | ||
|---|---|---|---|---|---|
| IBC infusions before ircAE | Median (IQR) | 3.5 (4) | 3 (4) | 13 (27) | 0.226 |
| Days from ICB to ircAE | Median (IQR) | 47.5 (112) | 31.5 (70) | 122 (663) | 0.296 |
| ircAE phenotype, n (%) | Psoriasiform | 12 (85.7) | 9 (90) | 3 (75) | 0.689 |
| Morbilliform | 1 (7.1) | 0 (0) | 1 (25) | ||
| Pyoderma gangrenosum | 1 (7.1) | 1 (10) | 0 (0) | ||
| Baseline ircAE CTCAE 5.0 grade, n (%) | 1 | 3 (21.4) | 2 (20) | 1 (25 | 0.869 |
| 2 | 6 (42.9) | 4 (40) | 2 (50) | ||
| 3 | 5 (35.7) | 4 (40) | 1 (25) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, S.L.; Maier, T.; Moy, A.P.; Dusza, S.; Faleck, D.M.; Shah, N.J.; Lacouture, M.E. IL12/23 Blockade with Ustekinumab as a Treatment for Immune-Related Cutaneous Adverse Events. Pharmaceuticals 2023, 16, 1548. https://doi.org/10.3390/ph16111548
Gu SL, Maier T, Moy AP, Dusza S, Faleck DM, Shah NJ, Lacouture ME. IL12/23 Blockade with Ustekinumab as a Treatment for Immune-Related Cutaneous Adverse Events. Pharmaceuticals. 2023; 16(11):1548. https://doi.org/10.3390/ph16111548
Chicago/Turabian StyleGu, Stephanie L., Tara Maier, Andrea P. Moy, Stephen Dusza, David M. Faleck, Neil J. Shah, and Mario E. Lacouture. 2023. "IL12/23 Blockade with Ustekinumab as a Treatment for Immune-Related Cutaneous Adverse Events" Pharmaceuticals 16, no. 11: 1548. https://doi.org/10.3390/ph16111548
APA StyleGu, S. L., Maier, T., Moy, A. P., Dusza, S., Faleck, D. M., Shah, N. J., & Lacouture, M. E. (2023). IL12/23 Blockade with Ustekinumab as a Treatment for Immune-Related Cutaneous Adverse Events. Pharmaceuticals, 16(11), 1548. https://doi.org/10.3390/ph16111548






