Polyglactin 910 Meshes Coated with Sustained-Release Cannabigerol Varnish Inhibit Staphylococcus aureus Biofilm Formation and Macrophage Cytokine Secretion: An In Vitro Study
Abstract
:1. Introduction
2. Results
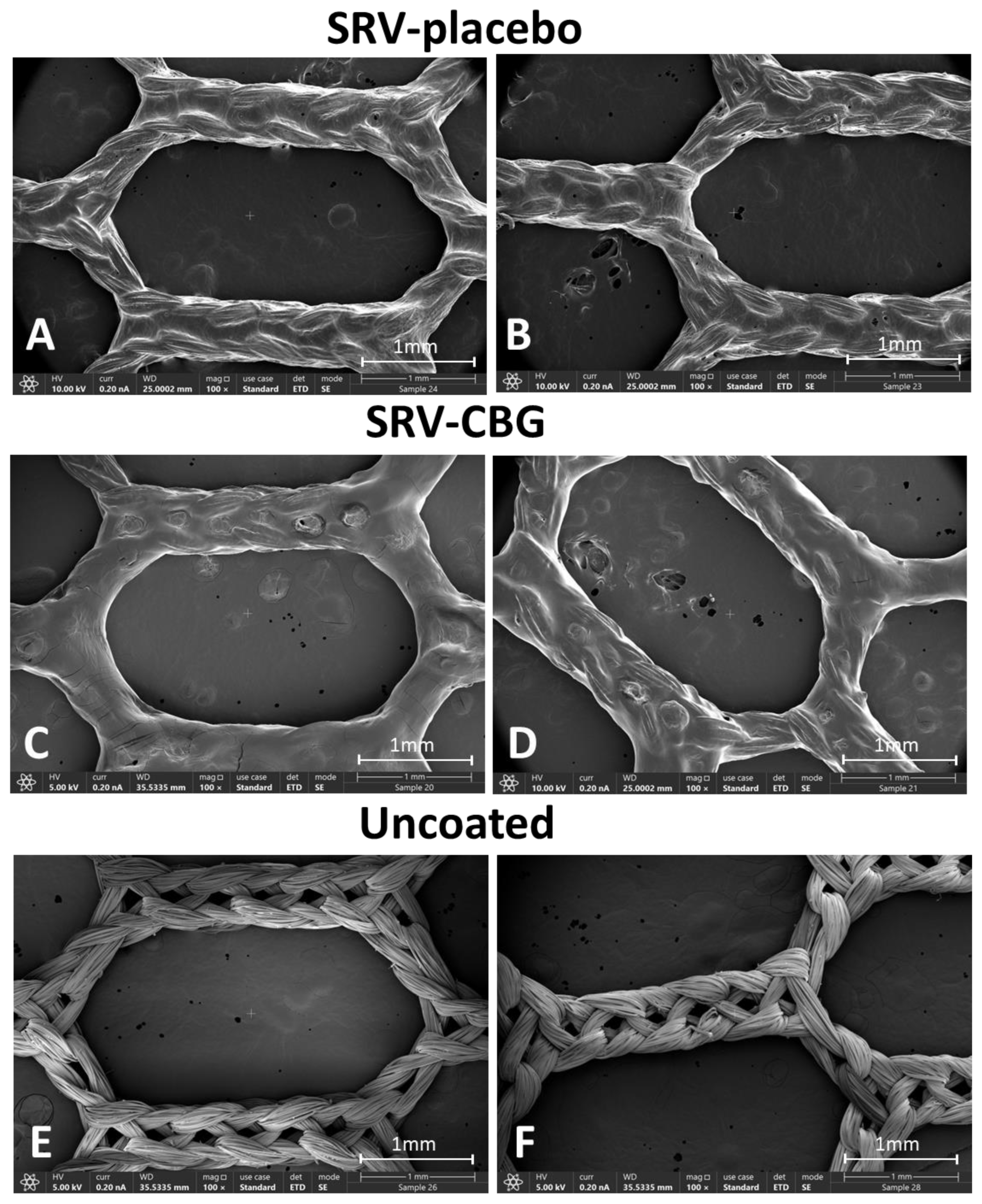
2.1. VICRYL Meshes Coated with SRV-CBG and SRV-Placebo
2.2. Antibacterial Effects of the SRV-CBG-Coated VICRYL Mesh on Staphylococcus aureus
2.3. SRV-CBG-Coated VICRYL Meshes Prevented Biofilm Formation in Its Surroundings
2.4. SRV-CBG-Coated VICRYL Meshes Prevented the Adhesion of S. aureus to the Meshes
2.5. The SRV-CBG-coated Mesh was Not Cytotoxic to Vero Epithelial Cells
2.6. SRV-CBG-coated Mesh Reduces Cytokine Secretion by Macrophages
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Formulation of the Sustained-Release Varnish (SRV)
4.3. Coating the Mesh with the Varnish
4.4. Staphylococcus aureus Growth Conditions
4.5. Determination of Minimum Inhibitory Concentration (MIC) of CBG
4.6. S. aureus Planktonic Growth and Biofilm Formation in the Presence of SRV-Coated Mesh
4.7. Measuring the Metabolic Activity of Planktonic Bacteria
4.8. Determination of S. aureus Biofilm Biomass by Crystal Violet (CV) Staining
4.9. Determination of S. aureus Biofilm Metabolic Activity Using Tetrazolium Reduction Assay
4.10. High-Resolution Scanning Electron Microscopy (HR-SEM)
4.11. Spinning Disk Confocal Microscopy (SDCM)
4.12. Cytotoxicity Assay on Vero Epithelial Cells
4.13. Cultivation of Mouse RAW 264.7 Macrophage Cell Line and the In Vitro Inflammation Model
4.14. Determination of Cytokine Content by Enzyme-Linked Immunosorbent Assay (ELISA)
4.15. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grafmiller, K.T.; Zuckerman, S.T.; Petro, C.; Liu, L.; von Recum, H.A.; Rosen, M.J.; Korley, J.N. Antibiotic-releasing microspheres prevent mesh infection in vivo. J. Surg. Res. 2016, 206, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.R.; Kao, A.M.; Gbozah, K.K.; Heniford, B.T.; Augenstein, V.A. Optimal management of mesh infection: Evidence and treatment options. Int. J. Abdom. Wall Hernia Surg. 2018, 1, 42. [Google Scholar]
- Corduas, F.; Lamprou, D.A.; Mancuso, E. Next-generation surgical meshes for drug delivery and tissue engineering applications: Materials, design and emerging manufacturing technologies. Bio-Des. Manuf. 2021, 4, 278–310. [Google Scholar] [CrossRef]
- Bakri, M.; Lovato, F.C.; Diosti, G.d.M.; Salles, Y.L.S.d.G.; Moreira, P.H.B.; Collaço, L.M.; Czeczko, N.G.; Malafaia, O.; Kubrusly, L.F. Comparative analysis of tissular response after abdominal wall repair using polypropylene mesh and bovine pericardium mesh. Arq. Bras. Cir. Dig. 2022, 34, e1527. [Google Scholar] [CrossRef]
- Wilson, R.B.; Farooque, Y. Risks and prevention of surgical site infection after hernia mesh repair and the predictive utility of ACS-NSQIP. J. Gastrointest. Surg. 2022, 26, 950–964. [Google Scholar] [CrossRef]
- Rastegarpour, A.; Cheung, M.; Vardhan, M.; Ibrahim, M.M.; Butler, C.E.; Levinson, H. Surgical mesh for ventral incisional hernia repairs: Understanding mesh design. Plast. Surg. 2016, 24, 41–50. [Google Scholar] [CrossRef]
- Deeken, C.R.; Matthews, B.D. Characterization of the mechanical strength, resorption properties, and histologic characteristics of a fully absorbable material (Poly-4-hydroxybutyrate—PHASIX Mesh) in a porcine model of hernia repair. Int. Sch. Res. Not. 2013, 2013, 238067. [Google Scholar] [CrossRef]
- Sriussadaporn, S.; Sriussadaporn, S.; Pak-art, R.; Krittayakirana, K.; Prichayuhd, S. Planned ventral hernia with absorbable mesh: A life-saving method in relaparotomy for septic abdomen. J. Med. Assoc. Thai. 2010, 93, 449. [Google Scholar]
- Olavarria, O.A.; Bernardi, K.; Dhanani, N.H.; Lyons, N.B.; Harvin, J.A.; Millas, S.G.; Ko, T.C.; Kao, L.S.; Liang, M.K. Synthetic versus biologic mesh for complex open ventral hernia repair: A pilot randomized controlled trial. Surg. Infect. 2021, 22, 496–503. [Google Scholar] [CrossRef]
- Sanchez, V.M.; Abi-Haidar, Y.E.; Itani, K.M. Mesh infection in ventral incisional hernia repair: Incidence, contributing factors, and treatment. Surg. Infect. 2011, 12, 205–210. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Shao, X.; Cheng, T. The salvage of mesh infection after hernia repair with the use of negative pressure wound therapy (NPWT), a systematic review. ANZ J. Surg. 2022, 92, 2448–2456. [Google Scholar] [CrossRef] [PubMed]
- Guillaume, O.; Pérez-Tanoira, R.; Fortelny, R.; Redl, H.; Moriarty, T.; Richards, R.; Eglin, D.; Puchner, A.P. Infections associated with mesh repairs of abdominal wall hernias: Are antimicrobial biomaterials the longed-for solution? Biomaterials 2018, 167, 15–31. [Google Scholar] [CrossRef]
- Mirel, S.; Pusta, A.; Moldovan, M.; Moldovan, S. Antimicrobial meshes for hernia repair: Current progress and perspectives. J. Clin. Med. 2022, 11, 883. [Google Scholar] [CrossRef]
- Cahill, S.V.; Kwon, H.K.; Back, J.; Lee, I.; Lee, S.; Alder, K.D.; Hao, Z.; Yu, K.E.; Dussik, C.M.; Kyriakides, T.R. Locally delivered adjuvant biofilm-penetrating antibiotics rescue impaired endochondral fracture healing caused by MRSA infection. J. Orthop. Res. 2021, 39, 402–414. [Google Scholar] [CrossRef]
- Karygianni, L.; Ren, Z.; Koo, H.; Thurnheer, T. Biofilm matrixome: Extracellular components in structured microbial communities. Trends Microbiol. 2020, 28, 668–681. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.; Benoliel, C.; Drider, D.; Dhulster, P.; Chihib, N.-E. Biofilm formation and persistence on abiotic surfaces in the context of food and medical environments. Arch. Microbiol. 2014, 196, 453–472. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef]
- Trautner, B.W.; Darouiche, R.O. Role of biofilm in catheter-associated urinary tract infection. Am. J. Infect. Control 2004, 32, 177–183. [Google Scholar] [CrossRef]
- Yin, W.; Wang, Y.; Liu, L.; He, J. Biofilms: The microbial “protective clothing” in extreme environments. Int. J. Mol. Sci. 2019, 20, 3423. [Google Scholar] [CrossRef]
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 2018, 9, 522–554. [Google Scholar] [CrossRef]
- Laschke, M.W.; Häufel, J.M.; Scheuer, C.; Menger, M.D. Angiogenic and inflammatory host response to surgical meshes of different mesh architecture and polymer composition. J. Biomed. Mater. Res. 2009, 91, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.N.; Londono, R.; Tottey, S.; Zhang, L.; Kukla, K.A.; Wolf, M.T.; Daly, K.A.; Reing, J.E.; Badylak, S.F. Macrophage phenotype as a predictor of constructive remodeling following the implantation of biologically derived surgical mesh materials. Acta Biomater. 2012, 8, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Mora-Navarro, C.; Ozpinar, E.W.; Sze, D.; Martin, D.P.; Freytes, D.O. Transcriptome-targeted analysis of human peripheral blood-derived macrophages when cultured on biomaterial meshes. Biomed. Mater. 2021, 16, 025006. [Google Scholar] [CrossRef] [PubMed]
- Gaffney, L.; Warren, P.; Wrona, E.A.; Fisher, M.B.; Freytes, D.O. Macrophages’ role in tissue disease and regeneration. Results Probl. Cell Differ. 2017, 62, 245–271. [Google Scholar] [PubMed]
- Steinberg, D.; Tal, T.; Friedman, M. Sustained-release delivery systems of triclosan for treatment of Streptococcus mutans biofilm. J. Biomed. Mater. Res. 2006, 77, 282–286. [Google Scholar] [CrossRef]
- Steinberg, D.; Friedman, M. Sustained-release drug delivery of antimicrobials in controlling of supragingival oral biofilms. Expert Opin. Drug Deliv. 2017, 14, 571–581. [Google Scholar] [CrossRef]
- Cataldo Russomando, A.; Vogt Sionov, R.; Friedman, M.; Gati, I.; Eliashar, R.; Steinberg, D.; Gross, M. Sinonasal stent coated with slow-release varnish of chlorhexidine has sustained protection against bacterial biofilm growth in the sinonasal cavity: An in vitro study. Pharmaceutics 2021, 13, 1783. [Google Scholar] [CrossRef]
- Shenderovich, J.; Feldman, M.; Kirmayer, D.; Al-Quntar, A.; Steinberg, D.; Lavy, E.; Friedman, M. Local sustained-release delivery systems of the antibiofilm agent thiazolidinedione-8 for prevention of catheter-associated urinary tract infections. Int. J. Pharm. 2015, 485, 164–170. [Google Scholar] [CrossRef]
- Farha, M.A.; El-Halfawy, O.M.; Gale, R.T.; MacNair, C.R.; Carfrae, L.A.; Zhang, X.; Jentsch, N.G.; Magolan, J.; Brown, E.D. Uncovering the hidden antibiotic potential of Cannabis. ACS Infect. Dis. 2020, 6, 338–346. [Google Scholar] [CrossRef]
- Luz-Veiga, M.; Amorim, M.; Pinto-Ribeiro, I.; Oliveira, A.L.; Silva, S.; Pimentel, L.L.; Rodríguez-Alcalá, L.M.; Madureira, R.; Pintado, M.; Azevedo-Silva, J. Cannabidiol and cannabigerol exert antimicrobial activity without compromising skin microbiota. Int. J. Mol. Sci. 2023, 24, 2389. [Google Scholar] [CrossRef]
- Sionov, R.V.; Steinberg, D. Anti-microbial activity of phytocannabinoids and endocannabinoids in the light of their physiological and pathophysiological roles. Biomedicines 2022, 10, 631. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, C.L.R.; Keir-Rudman, S.; Horniman, N.; Clarkson, N.; Page, C. The anti-inflammatory effects of cannabidiol and cannabigerol alone, and in combination. Pulm. Pharmacol. Ther. 2021, 69, 102047. [Google Scholar] [CrossRef] [PubMed]
- Lowin, T.; Tigges-Perez, M.S.; Constant, E.; Pongratz, G. Anti-inflammatory effects of cannabigerol in rheumatoid arthritis synovial fibroblasts and peripheral blood mononuclear cell cultures are partly mediated by TRPA1. Int. J. Mol. Sci. 2023, 24, 855. [Google Scholar] [CrossRef] [PubMed]
- Kogan, N.M.; Lavi, Y.; Topping, L.M.; Williams, R.O.; McCann, F.E.; Yekhtin, Z.; Feldmann, M.; Gallily, R.; Mechoulam, R. Novel CBG derivatives can reduce inflammation, pain and obesity. Molecules 2021, 26, 5601. [Google Scholar] [CrossRef]
- Ruhaak, L.R.; Felth, J.; Karlsson, P.C.; Rafter, J.J.; Verpoorte, R.; Bohlin, L. Evaluation of the cyclooxygenase inhibiting effects of six major cannabinoids isolated from Cannabis sativa. Biol. Pharm. Bull. 2011, 34, 774–778. [Google Scholar] [CrossRef]
- Borrelli, F.; Fasolino, I.; Romano, B.; Capasso, R.; Maiello, F.; Coppola, D.; Orlando, P.; Battista, G.; Pagano, E.; Di Marzo, V. Beneficial effect of the non-psychotropic plant cannabinoid cannabigerol on experimental inflammatory bowel disease. Biochem. Pharmacol. 2013, 85, 1306–1316. [Google Scholar] [CrossRef]
- Nachnani, R.; Raup-Konsavage, W.M.; Vrana, K.E. The pharmacological case for cannabigerol (CBG). Pharmacol. Exp. Ther. 2020, 376, 204–212. [Google Scholar] [CrossRef]
- Calapai, F.; Cardia, L.; Esposito, E.; Ammendolia, I.; Mondello, C.; Lo Giudice, R.; Gangemi, S.; Calapai, G.; Mannucci, C. Pharmacological aspects and biological effects of cannabigerol and its synthetic derivatives. Evid. Based Complement. Altern. Med. 2022, 2022, 3336516. [Google Scholar] [CrossRef]
- di Giacomo, V.; Chiavaroli, A.; Recinella, L.; Orlando, G.; Cataldi, A.; Rapino, M.; Di Valerio, V.; Ronci, M.; Leone, S.; Brunetti, L. Antioxidant and neuroprotective effects induced by cannabidiol and cannabigerol in rat CTX-TNA2 astrocytes and isolated cortexes. Int. J. Mol. Sci. 2020, 21, 3575. [Google Scholar] [CrossRef]
- Aqawi, M.; Sionov, R.V.; Gallily, R.; Friedman, M.; Steinberg, D. Anti-bacterial properties of cannabigerol toward Streptococcus mutans. Front. Microbiol. 2021, 12, 922. [Google Scholar] [CrossRef]
- Appendino, G.; Gibbons, S.; Giana, A.; Pagani, A.; Grassi, G.; Stavri, M.; Smith, E.; Rahman, M.M. Antibacterial cannabinoids from Cannabis sativa: A structure—Activity study. J. Nat. Prod. 2008, 71, 1427–1430. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Sionov, R.V.; Feldman, M.; Smoum, R.; Mechoulam, R.; Steinberg, D. Anandamide alters the membrane properties, halts the cell division and prevents drug efflux in multidrug resistant Staphylococcus aureus. Sci. Rep. 2021, 11, 8690. [Google Scholar] [CrossRef] [PubMed]
- Plymale, M.A.; Davenport, D.L.; Walsh-Blackmore, S.; Hess, J.; Griffiths, W.S.; Plymale, M.C.; Totten, C.F.; Roth, J.S. Costs and complications associated with infected mesh for ventral hernia repair. Surg. Infect. 2020, 21, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, A.; Kallinowski, F.; Köckerling, F. Evidence for replacement of an infected synthetic by a biological mesh in abdominal wall hernia repair. Front. Surg. 2016, 2, 67. [Google Scholar] [CrossRef]
- Jacombs, A.; Karatassas, A.; Klosterhalfen, B.; Richter, K.; Patiniott, P.; Hensman, C. Biofilms and effective porosity of hernia mesh: Are they silent assassins? Hernia 2020, 24, 197–204. [Google Scholar] [CrossRef]
- Pérez-Köhler, B.; Garcia-Moreno, F.; Brune, T.; Pascual, G.; Bellón, J.M. Preclinical bioassay of a polypropylene mesh for hernia repair pretreated with antibacterial solutions of chlorhexidine and allicin: An in vivo study. PLoS ONE 2015, 10, e0142768. [Google Scholar] [CrossRef]
- Sanders, D.; Lambie, J.; Bond, P.; Moate, R.; Steer, J. An in vitro study assessing the effect of mesh morphology and suture fixation on bacterial adherence. Hernia 2013, 17, 779–789. [Google Scholar] [CrossRef]
- Harrell, A.; Novitsky, Y.; Kercher, K.; Foster, M.; Burns, J.; Kuwada, T.; Heniford, B. In vitro infectability of prosthetic mesh by methicillin-resistant Staphylococcus aureus. Hernia 2006, 10, 120–124. [Google Scholar] [CrossRef]
- Cohen, M.S.; Stern, J.M.; Vanni, A.J.; Kelley, R.S.; Baumgart, E.; Field, D.; Libertino, J.A.; Summerhayes, I.C. In vitro analysis of a nanocrystalline silver-coated surgical mesh. Surg. Infect. 2007, 8, 397–404. [Google Scholar] [CrossRef]
- Nergiz Adıgüzel, E.; Esen, E.; Aylaz, G.; Keskinkılıç Yağız, B.; Kıyan, M.; Doğan, A.; Ünal, A.E. Do nano-crystalline silver-coated hernia grafts reduce infection? World J. Surg. 2018, 42, 3537–3542. [Google Scholar] [CrossRef]
- Kiladze, M.; Gogoladze, M.; Chkhikvadze, T. Hernioplasty with new antiseptic polymeric biocomposite meshes. Transl. Clin. Med. 2018, 3, 17–19. [Google Scholar]
- Pérez-Köhler, B.; Benito-Martínez, S.; Rodríguez, M.; García-Moreno, F.; Pascual, G.; Bellón, J. Experimental study on the use of a chlorhexidine-loaded carboxymethylcellulose gel as antibacterial coating for hernia repair meshes. Hernia 2019, 23, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Wiegering, A.; Sinha, B.; Spor, L.; Klinge, U.; Steger, U.; Germer, C.; Dietz, U. Gentamicin for prevention of intraoperative mesh contamination: Demonstration of high bactericide effect (in vitro) and low systemic bioavailability (in vivo). Hernia 2014, 18, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Harth, K.C.; Rosen, M.J.; Thatiparti, T.R.; Jacobs, M.R.; Halaweish, I.; Bajaksouzian, S.; Furlan, J.; von Recum, H.A. Antibiotic-releasing mesh coating to reduce prosthetic sepsis: An in vivo study. J. Surg. Res. 2010, 163, 337–343. [Google Scholar] [CrossRef]
- Reinbold, J.; Hierlemann, T.; Urich, L.; Uhde, A.-K.; Müller, I.; Weindl, T.; Vogel, U.; Schlensak, C.; Wendel, H.P.; Krajewski, S. Biodegradable rifampicin-releasing coating of surgical meshes for the prevention of bacterial infections. Drug Des. Dev. Ther. 2017, 11, 2753–2762. [Google Scholar] [CrossRef]
- Fleisher-Berkovich, S.; Ventura, Y.; Amoyal, M.; Dahan, A.; Feinshtein, V.; Alfahel, L.; Israelson, A.; Bernstein, N.; Gorelick, J.; Ben-Shabat, S. Therapeutic potential of phytocannabinoid cannabigerol for multiple sclerosis: Modulation of microglial activation in vitro and in vivo. Biomolecules 2023, 13, 376. [Google Scholar] [CrossRef]
- Brierley, D.I.; Samuels, J.; Duncan, M.; Whalley, B.J.; Williams, C.M. Cannabigerol is a novel, well-tolerated appetite stimulant in pre-satiated rats. Psychopharmacology 2016, 233, 3603–3613. [Google Scholar] [CrossRef]
- Jastrząb, A.; Jarocka-Karpowicz, I.; Skrzydlewska, E. The origin and biomedical relevance of cannabigerol. Int. J. Mol. Sci. 2022, 23, 7929. [Google Scholar] [CrossRef]
- Aqawi, M.; Sionov, R.V.; Gallily, R.; Friedman, M.; Steinberg, D. Anti-biofilm activity of cannabigerol against Streptococcus mutans. Microorganisms 2021, 9, 2031. [Google Scholar] [CrossRef]
- Aqawi, M.; Sionov, R.V.; Friedman, M.; Steinberg, D. The antibacterial effect of cannabigerol toward Streptococcus mutans is influenced by the autoinducers 21-CSP and AI-2. Biomedicines 2023, 11, 668. [Google Scholar] [CrossRef]
- Kanjickal, D.G.; Lopina, S.T. Modeling of drug release from polymeric delivery systems—A review. Crit. Rev. Ther. Drug Carr. Syst. 2004, 21, 42. [Google Scholar]
- Czerninski, R.; Finfter, O.; Nudelman, Z.; Tal, Y.; Kirmayer, D.; Friedman, M. Overnight use of oral appliance with sirolimus sustained-release varnish delivery system-a clinical note and an observational study. Quintessence Int. 2022, 54, 1–23. [Google Scholar]
- Blaskovich, M.A.; Kavanagh, A.M.; Elliott, A.G.; Zhang, B.; Ramu, S.; Amado, M.; Lowe, G.J.; Hinton, A.O.; Zuegg, J.; Beare, N. The antimicrobial potential of cannabidiol. Commun. Biol. 2021, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Ackland, G.L.; Del Arroyo, A.G.; Yao, S.T.; Stephens, R.C.; Dyson, A.; Klein, N.J.; Singer, M.; Gourine, A.V. Low-molecular-weight polyethylene glycol improves survival in experimental sepsis. Crit. Care Med. 2010, 38, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Cataldo Russomando, A.; Steinberg, D.; Gati, I.; Vogt Sionov, R.; Eliashar, R.; Friedman, M.; Gross, M. Sinonasal stent coated with sustained-release varnish of mometasone furoate inhibits pro-inflammatory cytokine release from macrophages: An in vitro study. Pharmaceutics 2023, 15, 1015. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, X. Sustained release technology and its application in environmental remediation: A review. Int. J. Environ. Res. Public Health 2019, 16, 2153. [Google Scholar] [CrossRef]
- ISO 10993-5; Biological Evaluation of Medical Devices. British Standards: London, UK, 2010.
- Feldman, M.; Gati, I.; Sionov, R.V.; Sahar-Helft, S.; Friedman, M.; Steinberg, D. Potential combinatory effect of cannabidiol and triclosan incorporated into sustained release delivery system against oral Candidiasis. Pharmaceutics 2022, 14, 1624. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, K.; Zhou, X.; Xu, N.; Xu, H.H.; Weir, M.D.; Ge, Y.; Wang, S.; Li, M.; Li, Y. Antibacterial effect of dental adhesive containing dimethylaminododecyl methacrylate on the development of Streptococcus mutans biofilm. Int. J. Mol. Sci. 2014, 15, 12791–12806. [Google Scholar] [CrossRef]
- Aqawi, M.; Gallily, R.; Sionov, R.V.; Zaks, B.; Friedman, M.; Steinberg, D. Cannabigerol prevents quorum sensing and biofilm formation of Vibrio harveyi. Front. Microbiol. 2020, 11, 858. [Google Scholar] [CrossRef]
- Aqawi, M.; Steinberg, D.; Feuerstein, O.; Friedman, M.; Gingichashvili, S. Cannabigerol effect on Streptococcus mutans biofilms—A computational approach to confocal image analysis. Front. Microbiol. 2022, 13, 880993. [Google Scholar] [CrossRef]
- Avraham, M.; Steinberg, D.; Barak, T.; Shalish, M.; Feldman, M.; Sionov, R.V. Improved anti-biofilm effect against the oral cariogenic Streptococcus mutans by combined Triclosan/CBD treatment. Biomedicines 2023, 11, 521. [Google Scholar] [CrossRef] [PubMed]










| S. aureus Strain | MIC (µg/mL) | MBIC (µg/mL) |
|---|---|---|
| MSSA ATCC 25923 | 2 | 2 |
| MRSA ATCC 33592 | 2 | 2 |
| MRSA ATCC 43300 | 2.5 | 2.5 |
| Newman MRSA | 2.5 | 2.5 |
| MDRSA CI-M | 2.5 | 2.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abudalu, M.; Aqawi, M.; Sionov, R.V.; Friedman, M.; Gati, I.; Munz, Y.; Ohana, G.; Steinberg, D. Polyglactin 910 Meshes Coated with Sustained-Release Cannabigerol Varnish Inhibit Staphylococcus aureus Biofilm Formation and Macrophage Cytokine Secretion: An In Vitro Study. Pharmaceuticals 2023, 16, 745. https://doi.org/10.3390/ph16050745
Abudalu M, Aqawi M, Sionov RV, Friedman M, Gati I, Munz Y, Ohana G, Steinberg D. Polyglactin 910 Meshes Coated with Sustained-Release Cannabigerol Varnish Inhibit Staphylococcus aureus Biofilm Formation and Macrophage Cytokine Secretion: An In Vitro Study. Pharmaceuticals. 2023; 16(5):745. https://doi.org/10.3390/ph16050745
Chicago/Turabian StyleAbudalu, Mustafa, Muna Aqawi, Ronit Vogt Sionov, Michael Friedman, Irith Gati, Yaron Munz, Gil Ohana, and Doron Steinberg. 2023. "Polyglactin 910 Meshes Coated with Sustained-Release Cannabigerol Varnish Inhibit Staphylococcus aureus Biofilm Formation and Macrophage Cytokine Secretion: An In Vitro Study" Pharmaceuticals 16, no. 5: 745. https://doi.org/10.3390/ph16050745
APA StyleAbudalu, M., Aqawi, M., Sionov, R. V., Friedman, M., Gati, I., Munz, Y., Ohana, G., & Steinberg, D. (2023). Polyglactin 910 Meshes Coated with Sustained-Release Cannabigerol Varnish Inhibit Staphylococcus aureus Biofilm Formation and Macrophage Cytokine Secretion: An In Vitro Study. Pharmaceuticals, 16(5), 745. https://doi.org/10.3390/ph16050745






