Revealing the Mechanism of Hemerocallis citrina Baroni in Depression Treatment Through Integrated Network Pharmacology and Transcriptomic Analysis
Abstract
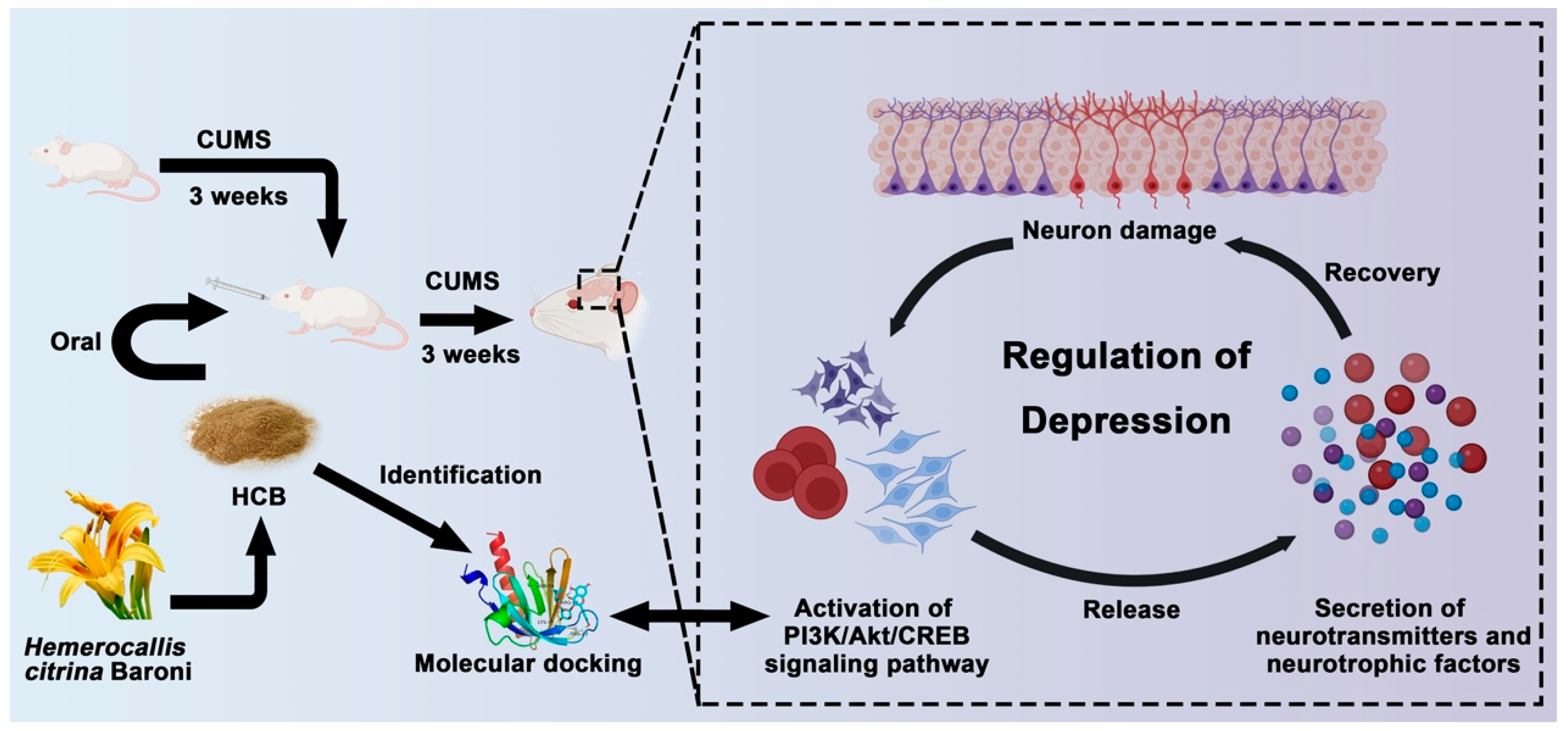
:1. Introduction
2. Results
2.1. Identification of the Constituents in HCB
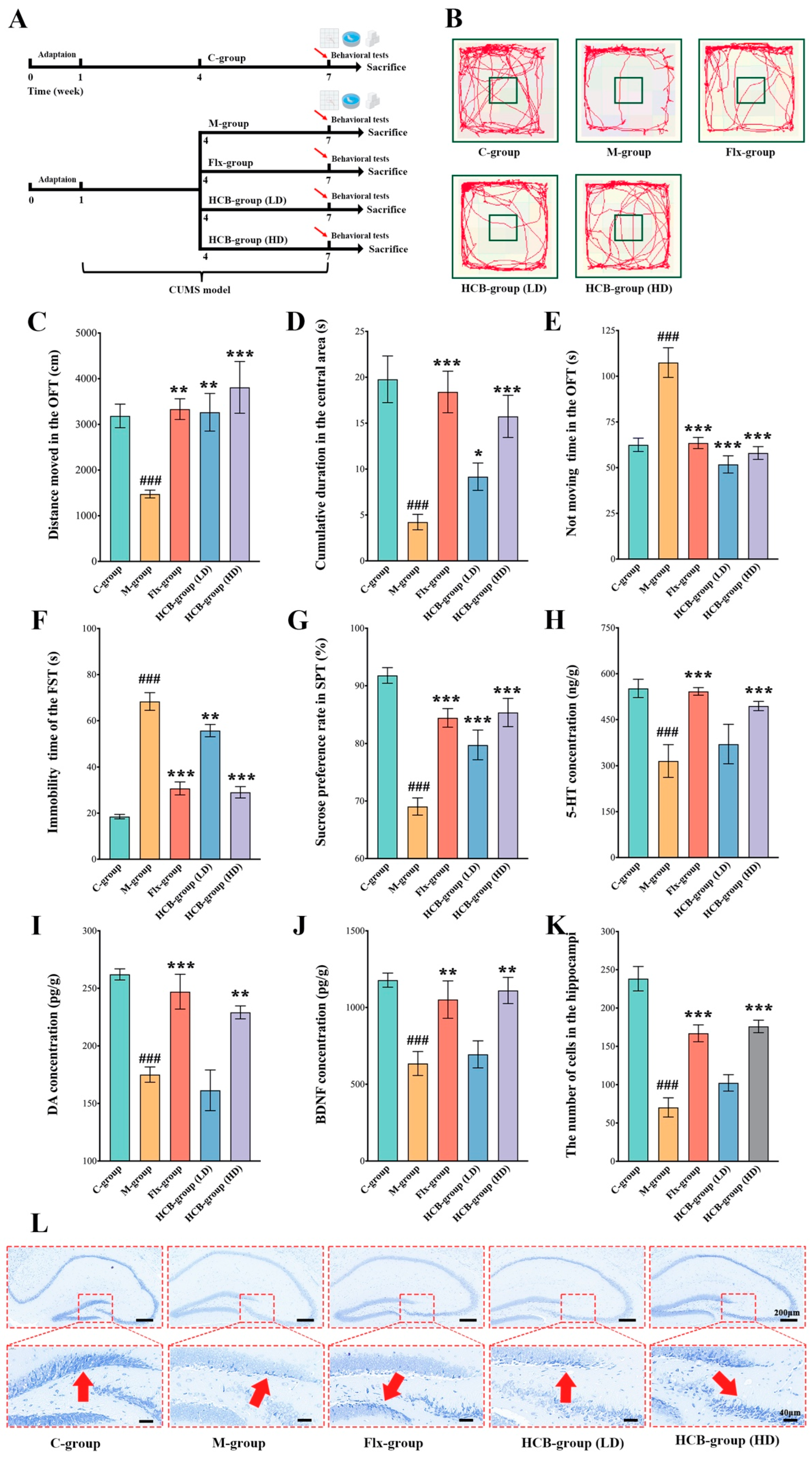
2.2. HCB Attenuates CUMS-Induced Depression
2.3. Network Pharmacology Analysis of HCB’s Interaction with Depression
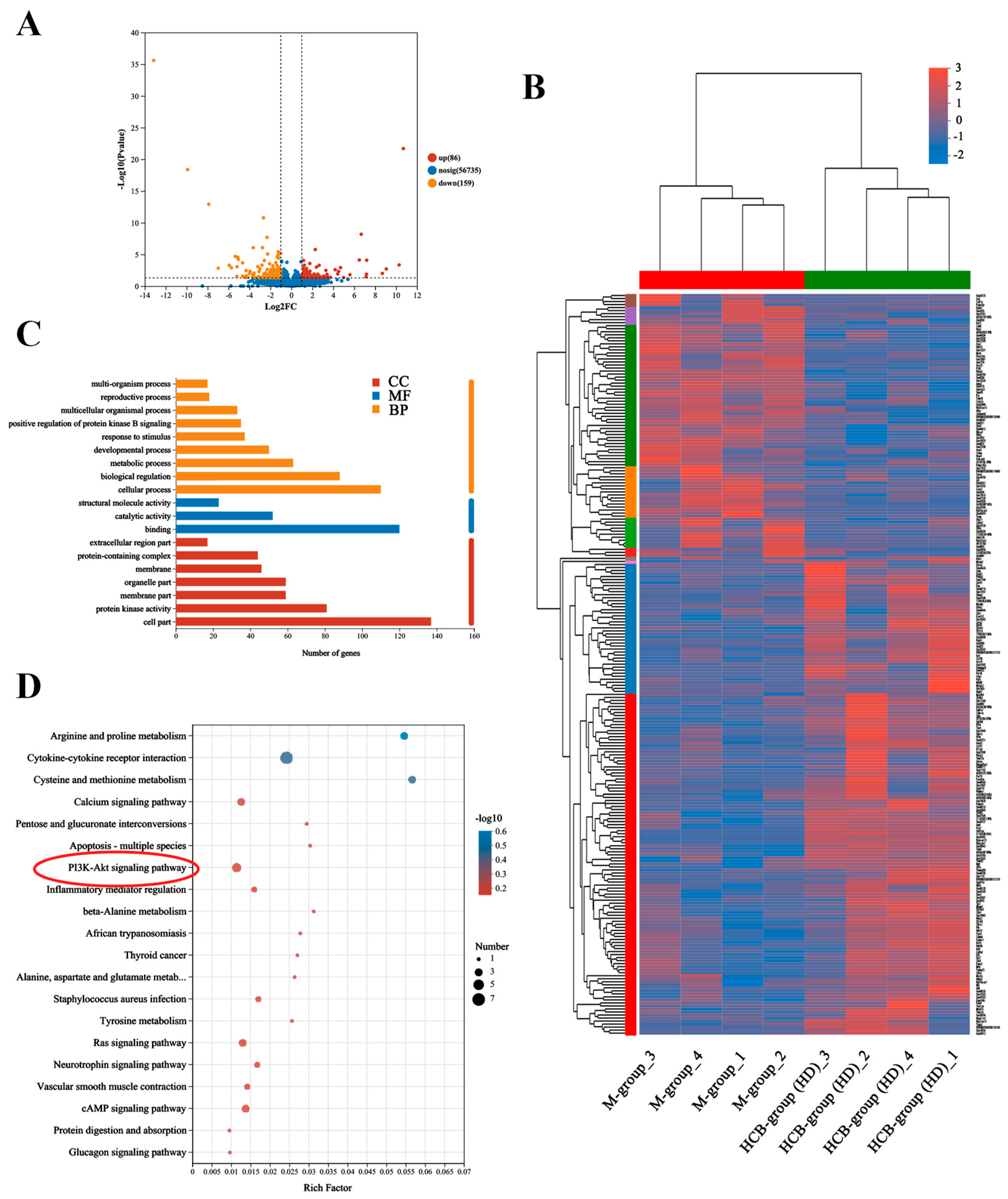
2.4. Potential Mechanism of HCB on Depression by RNA Sequencing
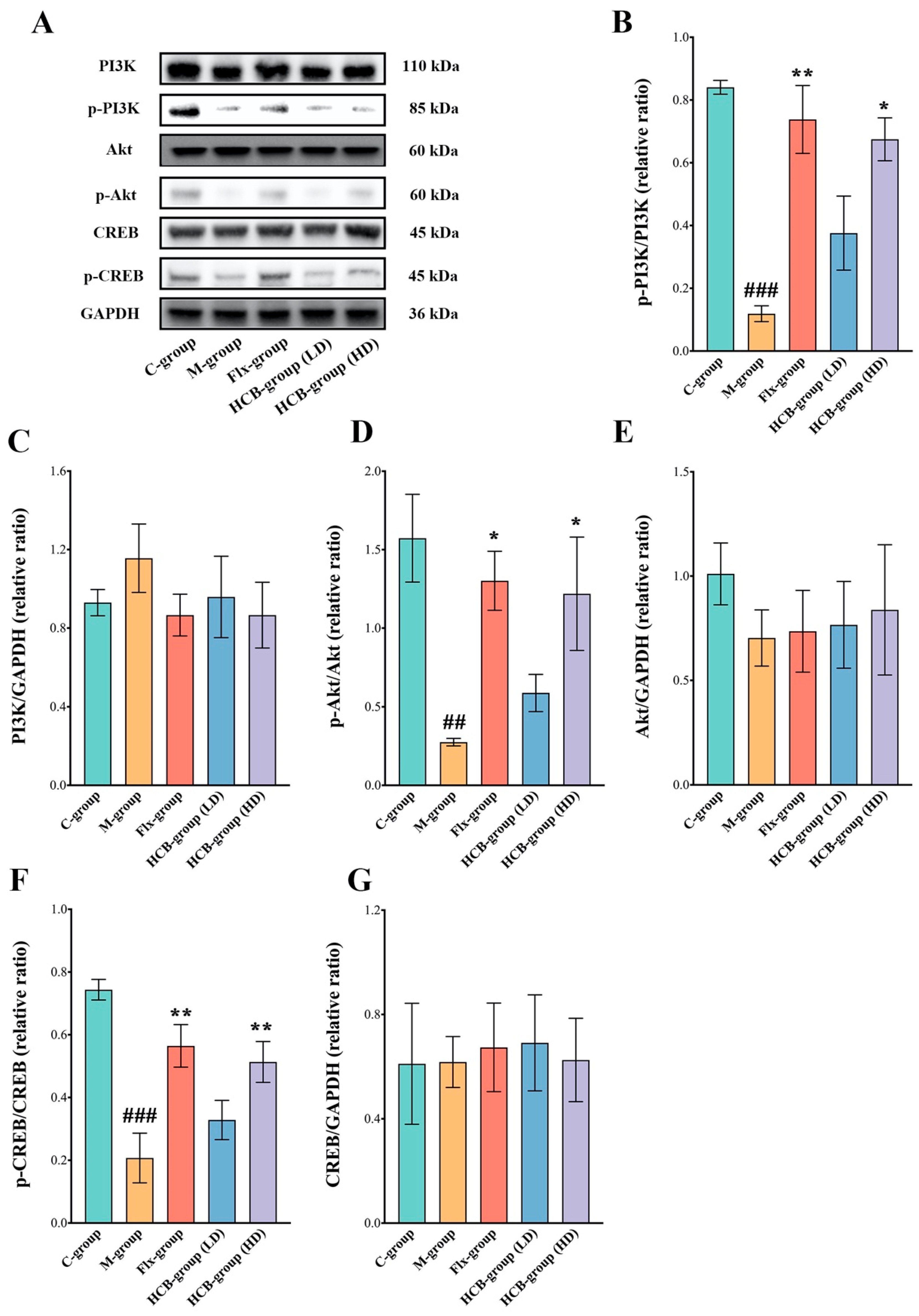
2.5. HCB Regulates the PI3K/Akt/CREB Signaling Pathway
3. Discussion
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Preparation of HCB
4.3. UHPLC-Q-Orbitrap HRMS Analysis
4.4. Network Pharmacology Analysis
4.5. Molecular Docking
4.6. Animals
4.7. CUMS Model and Grouping
4.8. Behavioral Tests
4.9. Preparation of Tissue Samples
4.10. Nissl Staining
4.11. ELISA Test
4.12. Western Blot
4.13. RNA Sequencing of the Hippocampus
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Campos, A.I.; Thompson, P.M.; Veltman, D.J.; Pozzi, E.; van Veltzen, L.S.; Jahanshad, N.; Adams, M.J.; Baune, B.T.; Berger, K.; Brosch, K.; et al. Brain Correlates of Suicide Attempt in 18,925 Participants Across 18 International Cohorts. Biol. Psychiatry 2021, 90, 243–252. [Google Scholar] [CrossRef]
- Gaynes, B.N.; Lux, L.; Gartlehner, G.; Asher, G.; Forman-Hoffman, V.; Green, J.; Boland, E.; Weber, R.P.; Randolph, C.; Bann, C.; et al. Defining treatment-resistant depression. Depress Anxiety 2020, 37, 134–145. [Google Scholar] [CrossRef]
- Barnett, R. Depression. Lancet 2019, 393, 2113. [Google Scholar] [CrossRef]
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef]
- Oliva, V.; Lippi, M.; Paci, R.; Del Fabro, L.; Delvecchio, G.; Brambilla, P.; De Ronchi, D.; Fanelli, G.; Serretti, A. Gastrointestinal side effects associated with antidepressant treatments in patients with major depressive disorder: A systematic review and meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 109, 110266. [Google Scholar] [CrossRef]
- Hou, Y.; Jiang, J.G. Origin and concept of medicine food homology and its application in modern functional foods. Food Funct. 2013, 4, 1727–1741. [Google Scholar] [CrossRef]
- Fu, R.; Wang, X.; Zhao, B.; Yang, M.; Gou, J.; Tian, C.Y. Hemecitones A and B: Two phenanthrenes with cytotoxicity from Hemerocallis fulva (L.) L. Nat. Prod. Res. 2022, 36, 1266–1272. [Google Scholar] [CrossRef]
- Szewczyk, K.; Miazga-Karska, M.; Pietrzak, W.; Komsta, L.; Krzeminska, B.; Grzywa-Celinska, A. Phenolic Composition and Skin-Related Properties of the Aerial Parts Extract of Different Hemerocallis Cultivars. Antioxidants 2020, 9, 690. [Google Scholar] [CrossRef]
- Guo, A.; Li, S.; Yang, Y.; Hou, F.; Wu, J.; Gao, Y.; Xing, G. Lecithin extraction optimisation and synthesis in Hemerocallis citrina Baroni. Scientia Horticulturae 2022, 293, 110682. [Google Scholar] [CrossRef]
- Lin, S.H.; Chang, H.C.; Chen, P.J.; Hsieh, C.L.; Su, K.P.; Sheen, L.Y. The Antidepressant-like Effect of Ethanol Extract of Daylily Flowers (Jin Zhen Hua) in Rats. J. Tradit. Complement. Med. 2013, 3, 53–61. [Google Scholar] [CrossRef]
- Yi, L.T.; Li, J.; Li, H.C.; Zhou, Y.; Su, B.F.; Yang, K.F.; Jiang, M.; Zhang, Y.T. Ethanol extracts from Hemerocallis citrina attenuate the decreases of brain-derived neurotrophic factor, TrkB levels in rat induced by corticosterone administration. J. Ethnopharmacol. 2012, 144, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhong, X.; Jiang, Y.; Yu, L.; Huang, X.; Dong, Z.; Yang, S.; He, W.; Zeng, J.; Qing, Z. Systematic identification metabolites of Hemerocallis citrina Borani by high-performance liquid chromatography/quadrupole-time-of-flight mass spectrometry combined with a screening method. J. Pharm. Biomed. Anal. 2020, 186, 113314. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Chen, Q.; Li, Z.; Zhou, Z. An evidence update on the protective mechanism of tangeretin against neuroinflammation based on network pharmacology prediction and transcriptomic analysis. Eur. J. Pharmacol. 2021, 906, 174094. [Google Scholar] [CrossRef]
- Blardi, P.; de Lalla, A.; Auteri, A.; Iapichino, S.; Dell’Erba, A.; Castrogiovanni, P. Plasma catecholamine levels after fluoxetine treatment in depressive patients. Neuropsychobiology 2005, 51, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Grace, A.A. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat. Rev. Neurosci. 2016, 17, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Jans, L.A.; Riedel, W.J.; Markus, C.R.; Blokland, A. Serotonergic vulnerability and depression: Assumptions, experimental evidence and implications. Mol. Psychiatry 2007, 12, 522–543. [Google Scholar] [CrossRef]
- Chen, A.; Xiong, L.J.; Tong, Y.; Mao, M. Neuroprotective effect of brain-derived neurotrophic factor mediated by autophagy through the PI3K/Akt/mTOR pathway. Mol. Med. Rep. 2013, 8, 1011–1016. [Google Scholar] [CrossRef]
- Zhuo, J.; Chen, B.; Sun, C.; Jiang, T.; Chen, Z.; Liu, Y.; Nie, J.; Yang, H.; Zheng, J.; Lai, X.; et al. Patchouli alcohol protects against chronic unpredictable mild stress-induced depressant-like behavior through inhibiting excessive autophagy via activation of mTOR signaling pathway. Biomed. Pharmacother. 2020, 127, 110115. [Google Scholar] [CrossRef]
- Zeng, J.; Ji, Y.; Luan, F.; Hu, J.; Rui, Y.; Liu, Y.; Rao, Z.; Liu, R.; Zeng, N. Xiaoyaosan ethyl acetate fraction alleviates depression-like behaviors in CUMS mice by promoting hippocampal neurogenesis via modulating the IGF-1Rbeta/PI3K/Akt signaling pathway. J. Ethnopharmacol. 2022, 288, 115005. [Google Scholar] [CrossRef]
- Arai, K.; Lo, E.H. Astrocytes protect oligodendrocyte precursor cells via MEK/ERK and PI3K/Akt signaling. J. Neurosci. Res. 2010, 88, 758–763. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, Y.; Shi, X.; Gong, F.; Xiong, X.; Kang, X.; Xing, G.; Li, S. The numerical classification and grading standards of daylily (Hemerocallis) flower color. PLoS ONE 2019, 14, e0216460. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Wang, K.Z.; Lu, C.; Dong, L.M.; Le Zhai, J.; Liao, Y.H.; Aibai, S.; Yang, Y.; Liu, X.M. Antidepressant-like effects and cognitive enhancement of the total phenols extract of Hemerocallis citrina Baroni in chronic unpredictable mild stress rats and its related mechanism. J. Ethnopharmacol. 2016, 194, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Tunc-Ozcan, E.; Peng, C.Y.; Zhu, Y.; Dunlop, S.R.; Contractor, A.; Kessler, J.A. Activating newborn neurons suppresses depression and anxiety-like behaviors. Nat. Commun. 2019, 10, 3768. [Google Scholar] [CrossRef] [PubMed]
- Morais, M.; Santos, P.A.; Mateus-Pinheiro, A.; Patricio, P.; Pinto, L.; Sousa, N.; Pedroso, P.; Almeida, S.; Filipe, A.; Bessa, J.M. The effects of chronic stress on hippocampal adult neurogenesis and dendritic plasticity are reversed by selective MAO-A inhibition. J. Psychopharmacol. 2014, 28, 1178–1183. [Google Scholar] [CrossRef]
- Koolschijn, P.C.; van Haren, N.E.; Lensvelt-Mulders, G.J.; Hulshoff Pol, H.E.; Kahn, R.S. Brain volume abnormalities in major depressive disorder: A meta-analysis of magnetic resonance imaging studies. Hum. Brain Mapp. 2009, 30, 3719–3735. [Google Scholar] [CrossRef]
- Zarneshan, S.N.; Fakhri, S.; Khan, H. Targeting Akt/CREB/BDNF signaling pathway by ginsenosides in neurodegenerative diseases: A mechanistic approach. Pharmacol. Res. 2022, 177, 106099. [Google Scholar] [CrossRef]
- Karege, F.; Perroud, N.; Burkhardt, S.; Fernandez, R.; Ballmann, E.; La Harpe, R.; Malafosse, A. Alterations in phosphatidylinositol 3-kinase activity and PTEN phosphatase in the prefrontal cortex of depressed suicide victims. Neuropsychobiology 2011, 63, 224–231. [Google Scholar] [CrossRef]
- Guo, L.T.; Wang, S.Q.; Su, J.; Xu, L.X.; Ji, Z.Y.; Zhang, R.Y.; Zhao, Q.W.; Ma, Z.Q.; Deng, X.Y.; Ma, S.P. Baicalin ameliorates neuroinflammation-induced depressive-like behavior through inhibition of toll-like receptor 4 expression via the PI3K/AKT/FoxO1 pathway. J. Neuroinflamm. 2019, 16, 95. [Google Scholar] [CrossRef]
- Jia, N.; Sun, Q.; Su, Q.; Dang, S.; Chen, G. Taurine promotes cognitive function in prenatally stressed juvenile rats via activating the Akt-CREB-PGC1alpha pathway. Redox Biol. 2016, 10, 179–190. [Google Scholar] [CrossRef]
- Liu, Z.; Qi, Y.; Cheng, Z.; Zhu, X.; Fan, C.; Yu, S.Y. The effects of ginsenoside Rg1 on chronic stress induced depression-like behaviors, BDNF expression and the phosphorylation of PKA and CREB in rats. Neuroscience 2016, 322, 358–369. [Google Scholar] [CrossRef]
- Zaletel, I.; Filipovic, D.; Puskas, N. Hippocampal BDNF in physiological conditions and social isolation. Rev. Neurosci. 2017, 28, 675–692. [Google Scholar] [CrossRef] [PubMed]
- Ramezany Yasuj, S.; Nourhashemi, M.; Keshavarzi, S.; Motaghinejad, M.; Motevalian, M. Possible Role of Cyclic AMP Response Element Binding/Brain-Derived Neurotrophic Factor Signaling Pathway in Mediating the Pharmacological Effects of Duloxetine against Methamphetamine Use-Induced Cognitive Impairment and Withdrawal-Induced Anxiety and Depression in Rats. Adv. Biomed. Res. 2019, 8, 11. [Google Scholar] [CrossRef]
- Ahmed, S.; Kwatra, M.; Gawali, B.; Panda, S.R.; Naidu, V.G.M. Potential role of TrkB agonist in neuronal survival by promoting CREB/BDNF and PI3K/Akt signaling in vitro and in vivo model of 3-nitropropionic acid (3-NP)-induced neuronal death. Apoptosis 2021, 26, 52–70. [Google Scholar] [CrossRef]
- Yang, Y.; Li, F.; Yan, M.; Chen, S.; Cai, D.; Liu, X.; Han, N.; Yuan, Z.; Lu, J.; Zhang, Y.; et al. Revealing the Toxicity-Enhancing Essence of Glycyrrhiza on Genkwa Flos Based on Ultra-high-performance Liquid Chromatography Coupled with Quadrupole-Orbitrap High-Resolution Mass Spectrometry and Self-Assembled Supramolecular Technology. Front. Chem. 2021, 9, 740952. [Google Scholar] [CrossRef]
- Guo, X.; Wu, Y.; Zhang, C.; Wu, L.; Qin, L.; Liu, T. Network Pharmacology Analysis of ZiShenWan for Diabetic Nephropathy and Experimental Verification of Its Anti-Inflammatory Mechanism. Drug Des. Devel Ther. 2021, 15, 1577–1594. [Google Scholar] [CrossRef]
- Sukalovic, V.; Soskic, V.; Sencanski, M.; Andric, D.; Kostic-Rajacic, S. Determination of key receptor-ligand interactions of dopaminergic arylpiperazines and the dopamine D2 receptor homology model. J. Mol. Model. 2013, 19, 1751–1762. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Gao, C.S.; Zhang, H.; Yang, J.; Wang, Y.P.; Pan, L.B.; Yu, H.; He, C.Y.; Luo, H.B.; Zhao, Z.X.; et al. Morinda officinalis oligosaccharides increase serotonin in the brain and ameliorate depression via promoting 5-hydroxytryptophan production in the gut microbiota. Acta Pharm. Sin. B 2022, 12, 3298–3312. [Google Scholar] [CrossRef]
- Gu, L.; Liu, Y.J.; Wang, Y.B.; Yi, L.T. Role for monoaminergic systems in the antidepressant-like effect of ethanol extracts from Hemerocallis citrina. J. Ethnopharmacol. 2012, 139, 780–787. [Google Scholar] [CrossRef]
- Yu, Z.; Kong, D.; Liang, Y.; Zhao, X.; Du, G. Protective effects of VMY-2-95 on corticosterone-induced injuries in mice and cellular models. Acta Pharm. Sin. B 2021, 11, 1903–1913. [Google Scholar] [CrossRef]
- Böck, P. Improved Nissl method to stain formaldehyde or glutaraldehyde-fixed material. Acta Neuropathol. 1979, 46, 243–244. [Google Scholar] [CrossRef]
- Le, T.T.; Savitz, J.; Suzuki, H.; Misaki, M.; Teague, T.K.; White, B.C.; Marino, J.H.; Wiley, G.; Gaffney, P.M.; Drevets, W.C.; et al. Identification and replication of RNA-Seq gene network modules associated with depression severity. Transl. Psychiatry 2018, 8, 180. [Google Scholar] [CrossRef] [PubMed]
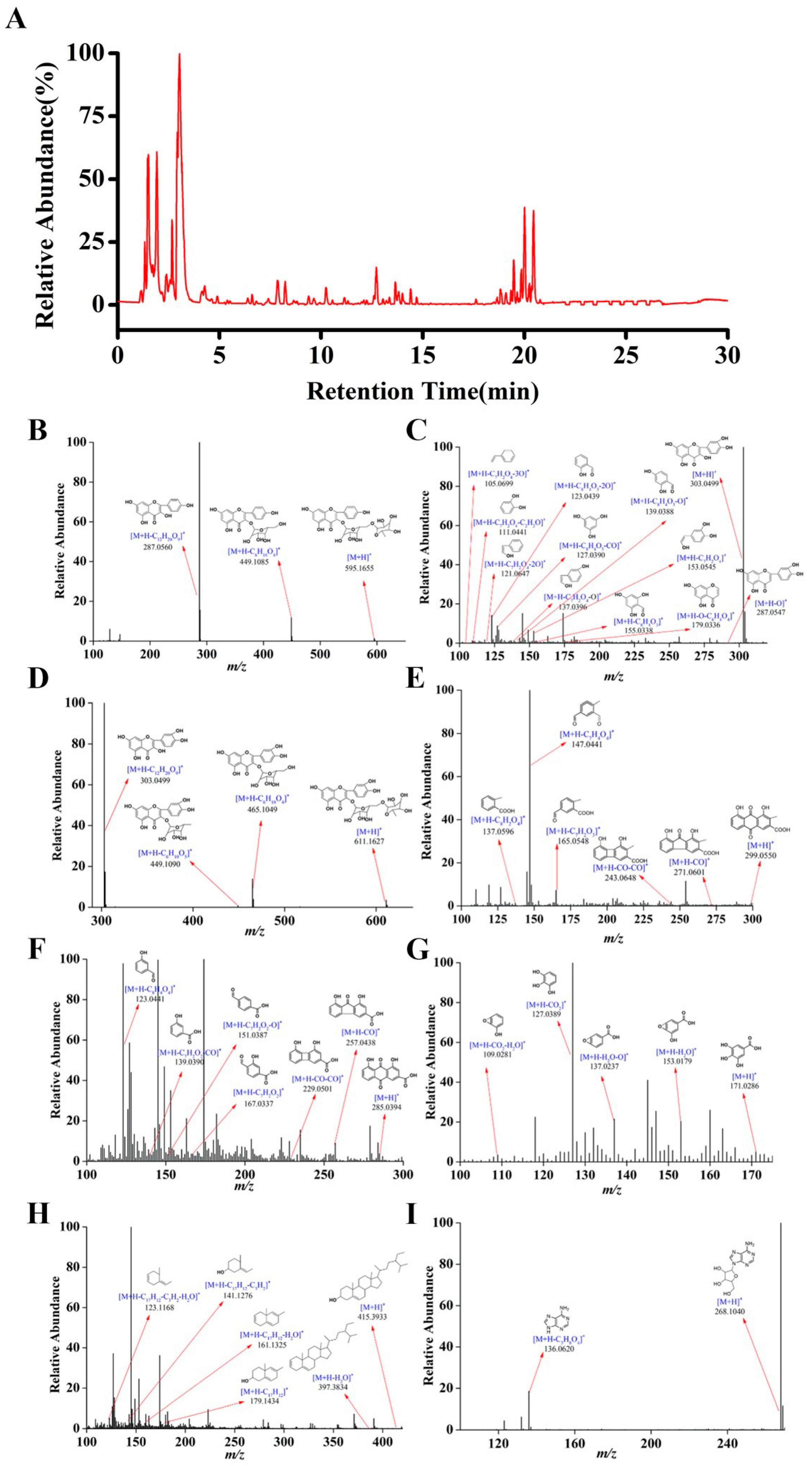

| Constituents | Formula | Retention Time (min) | Identity | Precursor Ion | Fragment Ions (m/z) | ||
|---|---|---|---|---|---|---|---|
| Experimental (m/z) | Theoretical (m/z) | Mass Accuracy (△ppm) | |||||
| Clionasterol | C29H50O | 0.32 | [M+H]+ | 415.3934 | 415.3933 | −0.24 | 397.3834, 179.1434, 123.1168 |
| Quinic acid | C7H12O6 | 1.43 | [M+H]+ | 193.0708 | 193.0707 | 0.52 | 175.0600, 157.0494, 133.0495, 115.0389 |
| Kwansonine A | C16H26N2O11 | 1.44 | [M+H]+ | 423.1609 | 423.1609 | 0.00 | 261.1081, 245.1131, 243.0973, 217.1178, 202.1068, 163.0712, 146.0447, 145.0607, 144.0658 |
| Oxypinnatanine | C10H16N2O6 | 1.50 | [M+H]+ | 261.1083 | 261.1081 | 0.77 | 246.0971, 245.1132, 230.1023, 215.1021, 200.0911, 187.1077, 163.0712, 146.0447, 130.0498 |
| Syringic acid | C9H10O5 | 1.97 | [M+H]+ | 199.0602 | 199.0601 | 0.50 | 183.0652, 169.0494, 153.0541, 151.0389, 139.0388, 123.0439 |
| Pinnatannine | C10H16N2O5 | 2.07 | [M+H]+ | 245.1134 | 245.1132 | 0.82 | 228.0856, 227.1021, 128.0704, 100.0759, 84.0449 |
| Oxypinnatanine A | C10H16N2O5 | 2.36 | [M+H]+ | 245.1132 | 245.1132 | 0.00 | 230.1023, 228.0863, 215.1029, 201.1233, 198.0763, 186.1128, 156.1022, 144.0655, 116.0707 |
| Longitubanine A | C10H16N2O2 | 2.40 | [M+H]+ | 245.1130 | 245.1132 | −0.82 | 227.1026, 210.0765, 201.1238, 172.0966, 163.0716, 156.1020, 145.0607, 120.0655, 100.0757 |
| Adenosine | C10H13N5O4 | 2.67 | [M+H]+ | 268.1040 | 268.1040 | 0.00 | 136.0620 |
| Kwansonine B | C16H26N2O10 | 3.25 | [M+H]+ | 407.1659 | 407.1660 | −0.25 | 391.1699, 325.1236, 245.1129, 229.1178, 163.0711, 147.0762 |
| Longitubanine B | C10H16N2O4 | 3.28 | [M+H]+ | 229.1182 | 229.1183 | −0.44 | 214.1070, 170.1172, 147.0764, 132.0655, 130.0499, 128.0704, 117.0545, 104.0704 |
| Kwansonine C | C16H26N2O10 | 3.48 | [M+H]+ | 407.1660 | 407.1660 | 0.00 | 391.1700, 307.1130, 245.1131, 229.1180, 163.0712, 145.0608 |
| Fuluanine A | C9H13NO5 | 5.46 | [M+H]+ | 216.0869 | 216.0866 | 1.39 | 198.0763, 186.0760, 118.0499 |
| Vanillic acid | C8H8O4 | 5.49 | [M+H]+ | 169.0497 | 169.0495 | 1.18 | 153.0547, 139.0390, 125.0598, 123.0440, 109.0648 |
| Chlorogenic acid | C16H18O9 | 6.39 | [M+H]+ | 355.1024 | 355.1024 | 0.00 | 337.0901, 165.0542, 163.0390, 145.0283, 137.0595, 135.0442, 117.0336 |
| Cryptochlorogenic acid | C16H18O9 | 6.39 | [M+H]+ | 355.1024 | 355.1024 | 0.00 | 337.0901, 165.0542, 163.0390, 145.0283, 137.0595, 135.0442, 117.0336 |
| Neochlorogenic acid | C16H18O9 | 6.39 | [M+H]+ | 355.1024 | 355.1024 | 0.00 | 337.0901, 165.0542, 163.0390, 145.0283, 137.0595, 135.0442, 117.0336 |
| Salidroside | C14H20O7 | 6.88 | [M+H]+ | 301.1280 | 301.1282 | −0.66 | 285.1331, 153.0910, 149.0962, 139.0754, 123.0805, 107.0856 |
| Icariside D2 | C14H20O7 | 6.89 | [M+H]+ | 301.1282 | 301.1281 | 0.00 | 283.1176, 265.1069, 235.0963, 139.0755, 107.0857 |
| 7-hydroxycoumarin | C9H6O3 | 6.93 | [M+H]+ | 163.0386 | 163.0390 | −2.45 | 147.0436, 145.0284, 135.0445, |
| 1′,2′,3′,4′-tetraphydro-5′-deoxypinnatanine | C10H20N2O4 | 7.42 | [M+H]+ | 233.1503 | 233.1496 | 3.00 | 216.1234, 215.1384, 146.0450, 84.0442, 73.0283 |
| 3-O-p-coumaroylquinic acid | C16H18O8 | 7.93 | [M+H]+ | 339.1075 | 339.1064 | 0.29 | 323.1125, 247.0817, 193.0711, 175.0604, 165.0546, 157.0496, 143.0708, 139.0390, 121.0650 |
| 4-O-p-coumaroylquinic acid | C16H18O8 | 7.93 | [M+H]+ | 339.1075 | 339.1064 | 0.29 | 247.0806, 193.0709, 175.0601, 165.0545, 157.0495, 147.0443, 139.0389, 121.0648, 101.0598 |
| Quercetin-3,7-2-O-glucose | C27H30O17 | 8.20 | [M+H]+ | 627.1568 | 627.1556 | 1.91 | 465.1039, 303.0493 |
| Isoquercetin | C21H20O12 | 8.34 | [M+H]+ | 465.1031 | 465.1028 | 0.65 | 303.0507, 127.0398 |
| Quercetin 3-O-rutinoside-7-glucoside | C33H40O21 | 8.59 | [M+H]+ | 773.2130 | 773.2135 | 0.65 | 627.1515, 611.1633, 465.1054, 303.0501 |
| 4-O-caffeoyl-quinic acid | C16H18O9 | 8.61 | [M+H]+ | 355.1023 | 355.1024 | −0.28 | 339.1080, 337.0914, 293.1022, 193.0712, 181.0497, 175.0603, 163.0390, 145.0287, 113.0596 |
| Methyl chlorogenate | C17H20O9 | 8.75 | [M+H]+ | 369.1180 | 369.1180 | 0.00 | 355.1023, 339.1080, 195.0653, 175.0604, 177.0545, 163.0385, 157.0497, 131.0706, 121.0650 |
| 3-O-feruloylquinic acid | C17H20O9 | 8.75 | [M+H]+ | 433.1133 | 433.1129 | 0.92 | 271.0601, 153.0181, 127.0389 |
| Gallic acid | C7H6O5 | 9.21 | [M+H]+ | 171.0286 | 171.0288 | −1.17 | 153.0179, 137.0237, 127.0389, 109.0281 |
| Hemerocallone | C18H14O6 | 9.87 | [M+H]+ | 327.0871 | 327.0863 | 2.44 | 165.0549, 163.0752, 137.0601, 127.0391 |
| Puerarin | C21H20O9 | 10.08 | [M+H]+ | 417.1176 | 417.1180 | −0.96 | 255.0647, 165.0544, 163.0392, 149.0599, 139.0388, 123.0442 |
| 2-hydroxychrysophanol | C15H10O5 | 10.24 | [M+H]+ | 271.0601 | 271.0601 | 0.00 | 243.0648, 215.0704, 153.0548, 135.0442, 125.0599, 109.0648 |
| Aloe emodin | C15H10O5 | 10.32 | [M+H]+ | 271.0602 | 271.0601 | 0.37 | 243.0658, 137.0596, 123.0441, 107.0490 |
| Kwanzoquinone G | C16H10O6 | 10.34 | [M+H]+ | 299.0550 | 299.0550 | 0.00 | 271.0601, 243.0648, 165.0548, 147.0441, 137.0596 |
| 4-O-caffeoylshikimic acid | C16H16O8 | 10.60 | [M+H]+ | 337.0912 | 337.0918 | −1.78 | 319.0812, 229.0708, 185.0812, 181.0496, 174.0530, 159.0657, 149.0956, 131.0705, 111.0440 |
| Ferulic acid | C10H10O4 | 10.62 | [M+H]+ | 195.0661 | 195.0652 | 4.69 | 177.0554, 149.0590, 125.0598, 95.0496, 79.0540 |
| Phenethyl-β-D-glu | C14H20O6 | 11.48 | [M+H]+ | 285.1329 | 285.1333 | −1.40 | 249.1117, 181.1222, 149.0962, 147.0806, 123.0805, 105.0699 |
| Catechin | C15H14O6 | 12.35 | [M+H]+ | 291.0858 | 291.0863 | –1.72 | 183.0653, 169.0860, 167.0704, 153.0909, 137.0962, 109.0649 |
| Chrysoobtusin | C17H14O5 | 12.61 | [M+H]+ | 299.0913 | 299.0914 | −0.33 | 271.0967, 181.0859, 151.0756, 123.0440, 121.0649 |
| Quercetin | C15H10O7 | 12.68 | [M+H]+ | 303.0499 | 303.0499 | 0.00 | 287.0547, 179.0336, 155.0338, 139.0388, 123.0439, 105.0699 |
| Rutin | C27H30O16 | 12.71 | [M+H]+ | 611.1627 | 611.1607 | 3.27 | 465.1049, 449.1090, 303.0499 |
| Rhein | C15H8O6 | 12.72 | [M+H]+ | 285.0394 | 285.0394 | 0.00 | 257.0438, 229.0501, 167.0337, 151.0387, 139.0390, 123.0441 |
| Hyperoside | C21H20O12 | 13.21 | [M+H]+ | 465.1021 | 465.1028 | −1.51 | 303.0496 |
| Kaempferol | C15H10O6 | 13.66 | [M+H]+ | 287.0541 | 287.0550 | −3.14 | 231.0642, 153.0180 |
| Kaempferol-3-rutinoside | C27H30O15 | 13.35 | [M+H]+ | 595.1655 | 595.1657 | −0.34 | 449.1085, 287.0560 |
| Kaempferol-3-O-glucosyl | C21H20O11 | 13.66 | [M+H]+ | 449.1086 | 449.1078 | 1.78 | 287.0561, 127.0380 |
| Kwanzoquinone E | C15H10O6 | 13.66 | [M+H]+ | 287.0541 | 287.0550 | −3,14 | 241.0475, 231.0642, 213.0549, 121.0299, 107.0482 |
| Guajavarin | C20H18O11 | 13.74 | [M+H]+ | 435.0920 | 435.0922 | −0.46 | 303.0495, 287.0556, 195.0295, 155.0337, 137.0596, 121.0651 |
| Kwanzoquinone F | C21H20O11 | 13.78 | [M+H]+ | 449.1094 | 449.1078 | 3.56 | 287.0551, 259.0609, 257.0439, 201.0547, 169.0498, 139.0391, 123.0441, 121.0287 |
| Isorhamnetin-3-glucopyranoside | C22H22O12 | 14.08 | [M+H]+ | 479.1158 | 479.1184 | −5.43 | 317.0662, 127.0390 |
| Hesperidin | C28H34O15 | 14.30 | [M+H]+ | 611.1964 | 611.1970 | –0.98 | 327.1297, 303.0865, 273.0761, 181.0495, 165.0761, 125.0597 |
| Kaempferol 3-α-arabinopyranoside | C20H18O10 | 14.47 | [M+H]+ | 419.0972 | 419.0973 | −0.24 | 287.0555, 195.0294, 155.0337, 139.0391, 127.0390, 111.0441 |
| Huanghua anthraquinone | C16H12O6 | 15.13 | [M+H]+ | 301.0706 | 301.0707 | −0.33 | 285.0757, 273.0759, 245.0804, 183.0652, 167.0703, 155.0704, 139.0753, 109.0649, 107.0493 |
| Chrysophanic acid | C15H10O4 | 15.91 | [M+H]+ | 255.0652 | 255.0652 | 0.00 | 199.0753, 183.0803, 137.0595, 123.0441, 121.0647, 109.0648 |
| 3′-methoxy puerarin | C22H22O10 | 18.04 | [M+H]+ | 447.1290 | 447.1280 | 0.89 | 285.0766 |
| 3α-acetyl-11-oxo-12-ursene-24-carboxylic acid | C33H48O5 | 19.03 | [M+H]+ | 513.3583 | 513.3575 | 1.55 | 455.3509, 281.1744, 235.2056, 223.1695, 219.2110 |
| 11α-hydroxy-3-hexanoyl-β-boswellic acid | C32H50O5 | 19.92 | [M+H]+ | 515.3731 | 515.3731 | 0.00 | 281.1749, 235.2052, 223.1693, 211.2060, 185.1537 |
| Kwanzoquinone A | C18H14O4 | 21.17 | [M+H]+ | 295.0976 | 295.0965 | 3.05 | 179.0706, 149.0600, 137.0602, 121.0652 |
| Kwanzoquinone B | C18H14O4 | 21.17 | [M+H]+ | 295.0976 | 295.0965 | 3.05 | 179.0706, 149.0600, 137.0602, 121.0652 |
| α-boswellic acid | C30H48O3 | 22.44 | [M+H]+ | 457.3676 | 457.3676 | 0.00 | 461.3972, 441.3719, 439.3560, 237.1485, 221.1535, 219.2106, 191.1789 |
| β-boswellic acid | C30H48O3 | 22.44 | [M+H]+ | 457.3676 | 457.3676 | 0.00 | 461.3972, 441.3719, 439.3560, 237.1485, 221.1535, 219.2106, 191.1789 |
| No. | Name | Degree | Betweenness Centrality | Closeness Centrality |
|---|---|---|---|---|
| 1 | Quercetin | 38 | 0.2635 | 0.6213 |
| 2 | Kaempferol | 22 | 0.0794 | 0.4740 |
| 3 | Clionasterol | 5 | 0.0102 | 0.3786 |
| 4 | Guajavarin | 5 | 0.0029 | 0.3786 |
| 5 | Isoquercetin | 5 | 0.0029 | 0.3786 |
| 6 | Hyperoside | 5 | 0.0029 | 0.3786 |
| 7 | Adenosine | 5 | 0.0077 | 0.3786 |
| 8 | Kaempferol-3-rutinoside | 4 | 0.0029 | 0.3742 |
| 9 | Rutin | 4 | 0.0029 | 0.3742 |
| 10 | Chrysophanic acid | 2 | 0.0020 | 0.3657 |
| 11 | Gallic acid | 2 | 4.4434 | 0.3657 |
| 12 | Kwanzoquinone G | 2 | 0.0012 | 0.3657 |
| 13 | Rhein | 2 | 0.0012 | 0.3657 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, S.; Lu, J.; Gu, Y.; Zhang, Y.; Wang, C.; Gao, F.; Dai, Z.; Xu, S.; Zhang, J.; Yang, Y.; et al. Revealing the Mechanism of Hemerocallis citrina Baroni in Depression Treatment Through Integrated Network Pharmacology and Transcriptomic Analysis. Pharmaceuticals 2024, 17, 1704. https://doi.org/10.3390/ph17121704
Gao S, Lu J, Gu Y, Zhang Y, Wang C, Gao F, Dai Z, Xu S, Zhang J, Yang Y, et al. Revealing the Mechanism of Hemerocallis citrina Baroni in Depression Treatment Through Integrated Network Pharmacology and Transcriptomic Analysis. Pharmaceuticals. 2024; 17(12):1704. https://doi.org/10.3390/ph17121704
Chicago/Turabian StyleGao, Shan, Jihui Lu, Yixiao Gu, Yaozhi Zhang, Cheng Wang, Feng Gao, Ziqi Dai, Shujing Xu, Jindong Zhang, Yuqin Yang, and et al. 2024. "Revealing the Mechanism of Hemerocallis citrina Baroni in Depression Treatment Through Integrated Network Pharmacology and Transcriptomic Analysis" Pharmaceuticals 17, no. 12: 1704. https://doi.org/10.3390/ph17121704
APA StyleGao, S., Lu, J., Gu, Y., Zhang, Y., Wang, C., Gao, F., Dai, Z., Xu, S., Zhang, J., Yang, Y., & Lei, H. (2024). Revealing the Mechanism of Hemerocallis citrina Baroni in Depression Treatment Through Integrated Network Pharmacology and Transcriptomic Analysis. Pharmaceuticals, 17(12), 1704. https://doi.org/10.3390/ph17121704







