Lipid-Based Nanoparticles in Delivering Bioactive Compounds for Improving Therapeutic Efficacy
Abstract
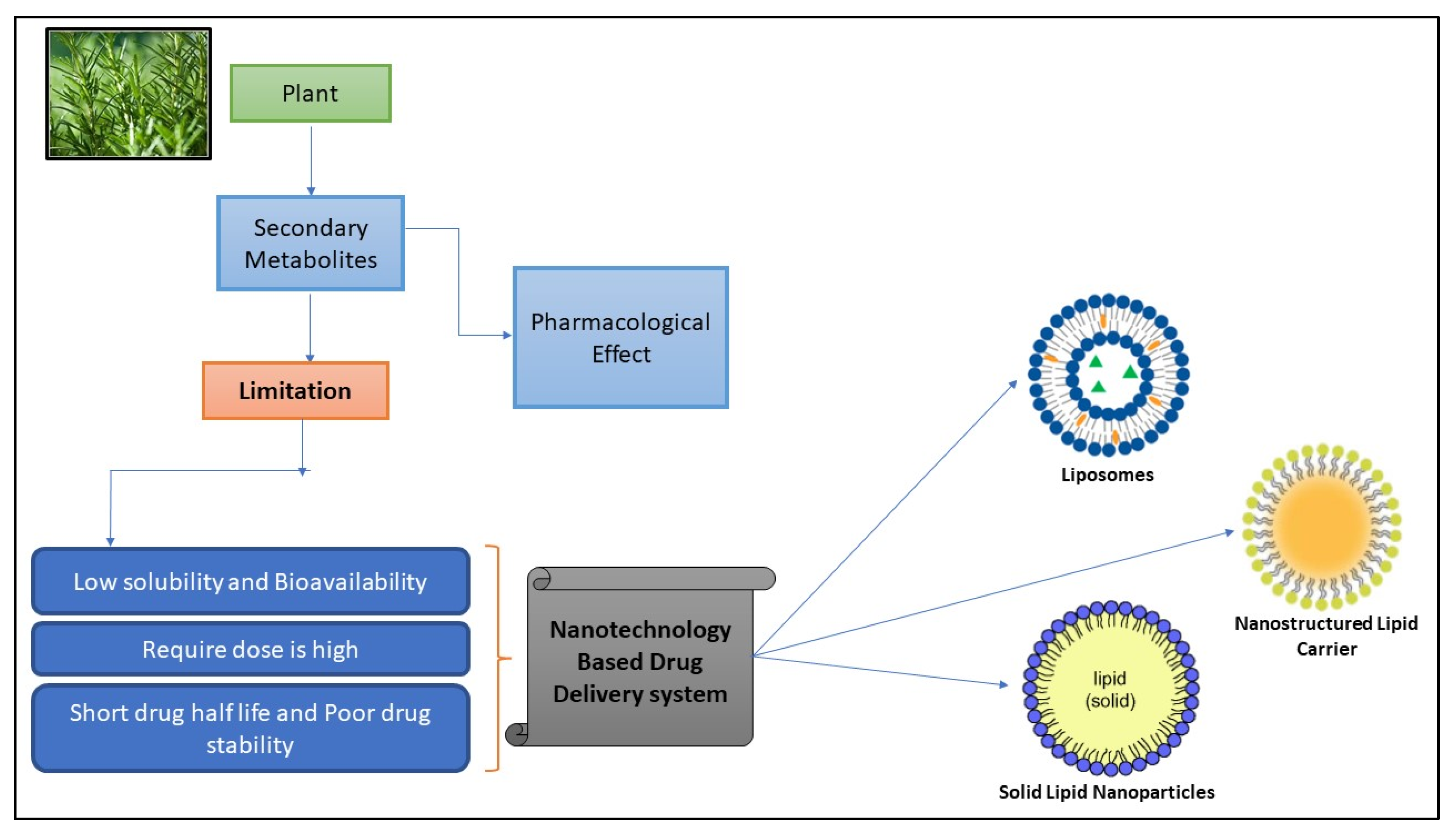
:1. Introduction
2. Lipid Based Nanoparticles
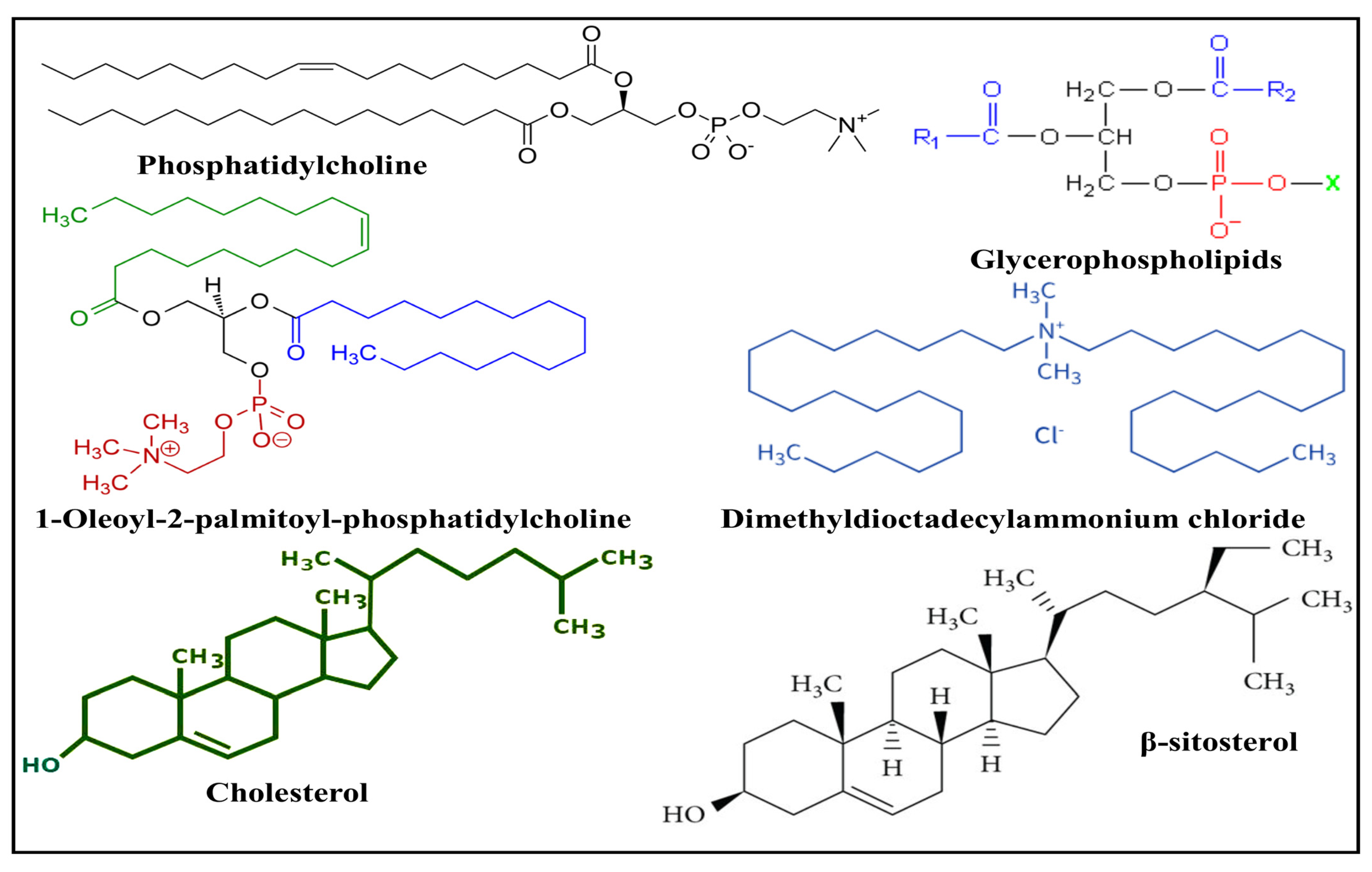
3. Lipids and Phospholipids Used in the Fabrication of Nanoparticles
3.1. Natural Lipids
3.2. Synthetic Lipids
3.3. Steroids
| Lipid Carrier | Drug Delivery | Bioactive Compounds | Preparation Method/Composition | Critical Attributes and Outcomes | Reference |
|---|---|---|---|---|---|
| Dipalmitoyl phosphatidylcholine | NLC-skin | Quercetin | Melt emulsification method followed by ultrasonication | Higher drug loading capacity makes them more suitable for topical skin administration and ensures longer colloidal stability which also shows a positive impact on skin permeability. | [38] |
| Soy-l-α-phosphatidylcholine | Injectable liposomes | Paclitaxel | Cell disruptor-type sonicator | Improved bioavailability, solubility, biodistribution, and intracellular uptake of Paclitaxel. | [39] |
| 1,2-distearoyl-sn-glycero-3-phosphoethanolamine | Liposomes | Naringin | Thin-film hydration | Improved solubility as well as dissolution, enhanced permeability and retention effect in Rheumatoid Arthritis treatment | [40] |
| Glycerol monostearate and medium-chain triglycerides | NLC | Curcumin | Emulsification | The encapsulation of curcumin in an NLC was increased, resulting in an increase in its antimalarial activity as well as increased colloidal stability. | [41] |
| Egg phosphatidylcholine | Intranasal Liposomes | Quercetin | Thin film hydration | Intranasal liposomes showed better solubility and dissolution at a lower dose than the other treatments. The best cognitive and anxiolytic effects for intranasal QU liposomes may be attributed to the alteration of various neurotransmitters. | [42] |
| Dipalmitoylphosphatidylcholine and Dipalmitoyl-sn-glycero-3-phosphoglycerol | Liposomes | curcumin | Conventional thin film hydration technique | Curcumin-loaded liposomes exhibited sustained release and substantial antibacterial activity against Gram-positive bacteria. | [42] |
4. Natural Bioactive Compounds in Lipid-Based Drug Delivery Systems
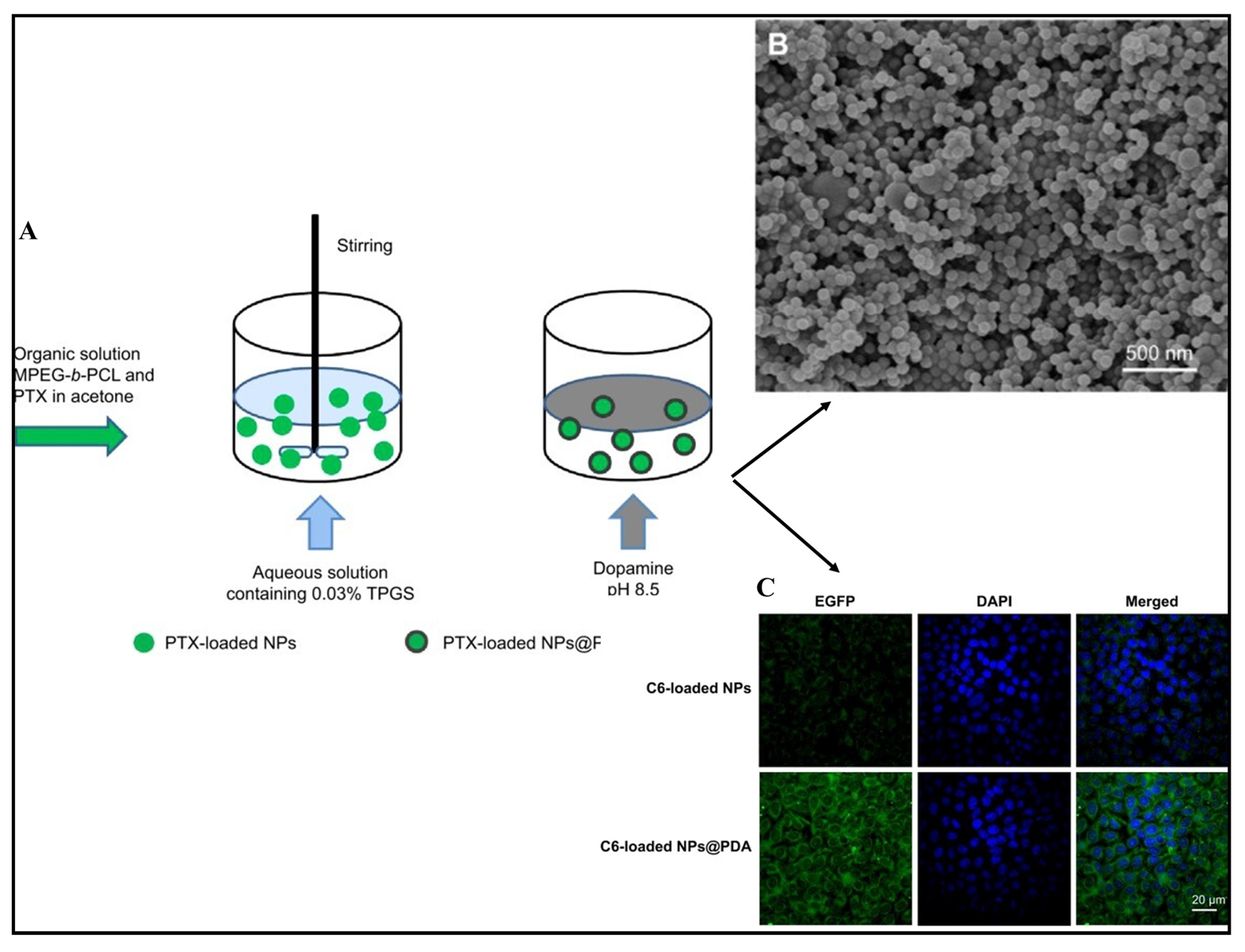
4.1. Paclitaxel
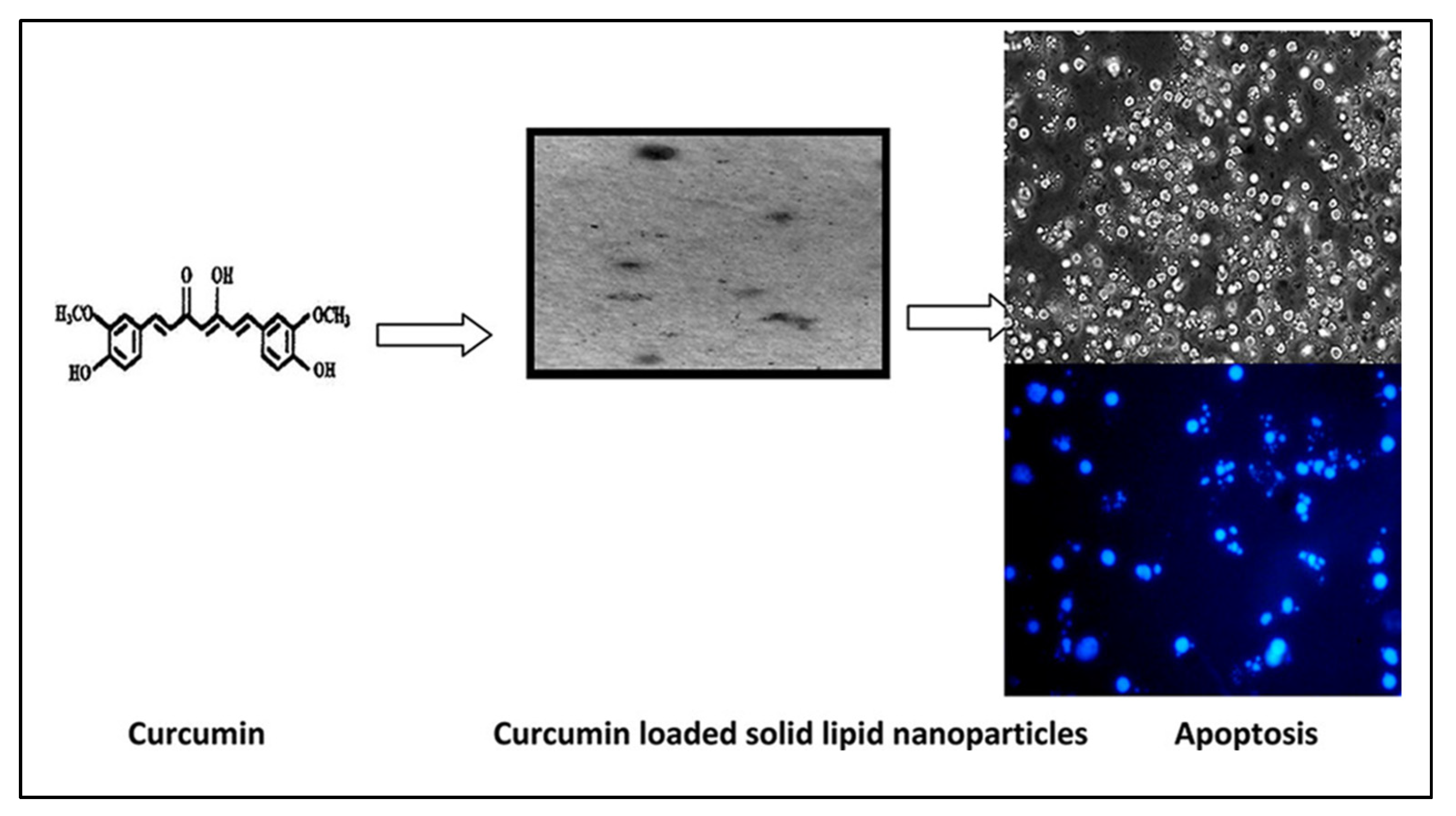
4.2. Curcumin
4.3. Rhodomyrtone
4.4. Quercetin
4.5. Kaempferol
4.6. Resveratrol
4.7. Epigallocatechin-3-gallate
4.8. Silymarin
4.9. Saponins
4.10. Oridonin
5. Safety Evaluation of Lipid-Based Nanoparticles Encapsulating Bioactive Compounds
5.1. Preclinical and Clinical Aspects of Lipid-Based Nanoparticles Encapsulating Bioactive Compounds
5.2. Patented Lipid-Based Nanoparticles Encapsulating Bioactive Compounds
6. Conclusions, Future Prospects, and Challenges
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Saudagar, R.B.; Saokar, S. Anti-inflammatory natural compounds from herbal and marine origin. J. Drug Deliv. Ther. 2019, 9, 669–672. [Google Scholar]
- Akbari, B.; Baghaei-Yazdi, N.; Bahmaie, M.; Mahdavi Abhari, F. The role of plant-derived natural antioxidants in reduction of oxidative stress. BioFactors 2022, 48, 611–633. [Google Scholar] [CrossRef] [PubMed]
- Kaur, C.; Kapoor, H.C. Antioxidants in fruits and vegetables–the millennium’s health. Int. J. Food Sci. Technol. 2001, 36, 703–725. [Google Scholar]
- Gunes-Bayir, A.; Mendes, B.; Dadak, A. The Integral Role of Diets Including Natural Products to Manage Rheumatoid Arthritis: A Narrative Review. Curr. Issues Mol. Biol. 2023, 45, 5373–5388. [Google Scholar] [CrossRef] [PubMed]
- Simonen, P.; Öörni, K.; Sinisalo, J.; Strandberg, T.E.; Wester, I.; Gylling, H. High cholesterol absorption: A risk factor of atherosclerotic cardiovascular diseases? Atherosclerosis 2023, 376, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Muyumba, N.; Mutombo, S.; Sheridan, H.; Nachtergael, A.; Duez, P. Quality control of herbal drugs and preparations: The methods of analysis, their relevance and applications. Talanta Open 2021, 4, 100070. [Google Scholar] [CrossRef]
- da Silva, P.B.; dos Santos Ramos, M.A.; Bonifacio, B.V.; Negri, K.M.S.; Sato, M.R.; Bauab, T.M.; Chorilli, M. Nanotechnological strategies for vaginal administration of drugs—A review. J. Biomed. Nanotechnol. 2014, 10, 2218–2243. [Google Scholar] [CrossRef] [PubMed]
- Dewi, M.K.; Chaerunisaa, A.Y.; Muhaimin, M.; Joni, I.M. Improved Activity of Herbal Medicines through Nanotechnology. Nanomaterials 2022, 12, 4073. [Google Scholar] [CrossRef]
- Chittasupho, C.; Chaobankrang, K.; Sarawungkad, A.; Samee, W.; Singh, S.; Hemsuwimon, K.; Okonogi, S.; Kheawfu, K.; Kiattisin, K.; Chaiyana, W. Antioxidant, Anti-Inflammatory and Attenuating Intracellular Reactive Oxygen Species Activities of Nicotiana tabacum var. Virginia Leaf Extract Phytosomes and Shape Memory Gel Formulation. Gels 2023, 9, 78. [Google Scholar] [CrossRef]
- Singh, S.; Ushir, Y.V.; Prajapati, B.G. Phytosomes and Herbosomes: A Vesicular Drug Delivery System for Improving the Bioavailability of Natural Products. In Lipid-Based Drug Delivery Systems: Principles and Applications; Prajapati, B., Ed.; Jenny Stanford Publishing: London, UK, 2023. [Google Scholar]
- Gabizon, A.; Catane, R.; Uziely, B.; Kaufman, B.; Safra, T.; Cohen, R.; Martin, F.; Huang, A.; Barenholz, Y. Prolonged circulation time and enhanced accumulation in malignant exudates of doxorubicin encapsulated in polyethylene-glycol coated liposomes. Cancer Res. 1994, 54, 987–992. [Google Scholar]
- Barenholz, Y.C. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in cancer treatment: From preclinical studies to clinical practice. Front. Pharmacol. 2020, 10, 1614. [Google Scholar] [CrossRef]
- Uddin, M.J.; Mohite, P.; Munde, S.; Ade, N.; Oladosu, T.A.; Chidrawar, V.R.; Patel, R.; Bhattacharya, S.; Paliwal, H.; Singh, S. Extracellular vesicles: The future of therapeutics and drug delivery systems. Intell. Pharm. 2024, in press. [Google Scholar] [CrossRef]
- Baldassari, S.; Balboni, A.; Drava, G.; Donghia, D.; Canepa, P.; Ailuno, G.; Caviglioli, G. Phytochemicals and Cancer Treatment: Cell-Derived and Biomimetic Vesicles as Promising Carriers. Pharmaceutics 2023, 15, 1445. [Google Scholar] [CrossRef]
- Reolon, J.B.; Sari, M.H.M.; Marchiori, C.; Dallabrida, K.G.; Santos, J.A.R.d.; Almeida, I.d.F.R.d.; Alves, F.M.S.; Bonini, J.S.; Ferreira, L.M. Herbal drugs-loaded soft nanoparticles for treating skin disorders: Where do we stand? Ind. Crops Prod. 2023, 206, 117602. [Google Scholar] [CrossRef]
- Fahr, A.; van Hoogevest, P.; May, S.; Bergstrand, N.; Leigh, M.L. Transfer of lipophilic drugs between liposomal membranes and biological interfaces: Consequences for drug delivery. Eur. J. Pharm. Sci. 2005, 26, 251–265. [Google Scholar] [CrossRef]
- Singh, S.; Dodiya, T.R.; Dodiya, R.; Ushir, Y.V.; Widodo, S. Lipid Nanoparticulate Drug Delivery Systems: A Revolution in Dosage Form Design and Development. In Drug Carriers; IntechOpen: London, UK, 2022. [Google Scholar]
- Patel, P.; Pal, R.; Butani, K.; Singh, S.; Prajapati, B.G. Nanomedicine-fortified cosmeceutical serums for the mitigation of psoriasis and acne. Nanomedicine 2023, 18, 1769–1793. [Google Scholar] [CrossRef] [PubMed]
- Mohite, P.; Singh, S.; Pawar, A.; Sangale, A.; Prajapati, B.G. Lipid-based oral formulation in capsules to improve the delivery of poorly water-soluble drugs. Front. Drug Deliv. 2023, 3, 1–20. [Google Scholar] [CrossRef]
- Ajeeshkumar, K.K.; Aneesh, P.A.; Raju, N.; Suseela, M.; Ravishankar, C.N.; Benjakul, S. Advancements in liposome technology: Preparation techniques and applications in food, functional foods, and bioactive delivery: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1280–1306. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, D.; Chilkapalli, S.C.; Prajapati, B.G.; Rodriques, P.; Patel, R.; Singh, S.; Bhattacharya, S. The Astonishing Accomplishment of Biological Drug Delivery using Lipid Nanoparticles: An Ubiquitous Review. Curr. Pharm. Biotechnol. 2024, 25. [Google Scholar] [CrossRef]
- Kapoor, D.U.; Gaur, M.; Parihar, A.; Prajapati, B.G.; Singh, S.; Patel, R.J. Phosphatidylcholine (PCL) fortified nano-phytopharmaceuticals for improvement of therapeutic efficacy. EXCLI J. 2023, 22, 880–903. [Google Scholar] [PubMed]
- Nakhaei, P.; Margiana, R.; Bokov, D.O.; Abdelbasset, W.K.; Jadidi Kouhbanani, M.A.; Varma, R.S.; Marofi, F.; Jarahian, M.; Beheshtkhoo, N. Liposomes: Structure, biomedical applications, and stability parameters with emphasis on cholesterol. Front. Bioeng. Biotechnol. 2021, 9, 748. [Google Scholar] [CrossRef] [PubMed]
- Pavelić, Ž.; Škalko-Basnet, N.; Jalšenjak, I. Characterisation and in vitro evaluation of bioadhesive liposome gels for local therapy of vaginitis. Int. J. Pharm. 2005, 301, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Grazia Calvagno, M.; Celia, C.; Paolino, D.; Cosco, D.; Iannone, M.; Castelli, F.; Doldo, P.; Fresta, M. Effects of lipid composition and preparation conditions on physical-chemical properties, technological parameters and in vitro biological activity of gemcitabine-loaded liposomes. Curr. Drug Deliv. 2007, 4, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, composition, types, and clinical applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef] [PubMed]
- Large, D.E.; Abdelmessih, R.G.; Fink, E.A.; Auguste, D.T. Liposome composition in drug delivery design, synthesis, characterization, and clinical application. Adv. Drug Deliv. Rev. 2021, 176, 113851. [Google Scholar] [CrossRef]
- Daraee, H.; Etemadi, A.; Kouhi, M.; Alimirzalu, S.; Akbarzadeh, A. Application of liposomes in medicine and drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 381–391. [Google Scholar] [CrossRef]
- Monteiro, N.; Martins, A.; Reis, R.L.; Neves, N.M. Liposomes in tissue engineering and regenerative medicine. J. R. Soc. Interface 2014, 11, 20140459. [Google Scholar] [CrossRef]
- Abdalla, A.M.; Hossain, S.; Azad, A.T.; Petra, P.M.I.; Begum, F.; Eriksson, S.G.; Azad, A. Nanomaterials for solid oxide fuel cells: A review. Renew. Sustain. Energy Rev. 2018, 82, 353–368. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Zhang, T.; Wang, C.; Huang, Z.; Luo, X.; Deng, Y. A review on phospholipids and their main applications in drug delivery systems. Asian J. Pharm. Sci. 2015, 10, 81–98. [Google Scholar] [CrossRef]
- van der Veen, J.N.; Kennelly, J.P.; Wan, S.; Vance, J.E.; Vance, D.E.; Jacobs, R.L. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim. Biophys. Acta (BBA)-Biomembr. 2017, 1859, 1558–1572. [Google Scholar] [CrossRef] [PubMed]
- van Hoogevest, P.; Wendel, A. The use of natural and synthetic phospholipids as pharmaceutical excipients. Eur. J. Lipid Sci. Technol. 2014, 116, 1088–1107. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Ribeiro, A.M. Bilayer-forming synthetic lipids: Drugs or carriers? Curr. Med. Chem. 2003, 10, 2425–2446. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Kampf, N.; Goldberg, R.; Driver, M.J.; Klein, J. Poly-phosphocholinated Liposomes Form Stable Superlubrication Vectors. Langmuir 2019, 35, 6048–6054. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, A.A.; Balanč, B.D.; Ota, A.; Ahlin Grabnar, P.; Djordjević, V.B.; Šavikin, K.P.; Bugarski, B.M.; Nedović, V.A.; Poklar Ulrih, N. Comparative effects of cholesterol and β-sitosterol on the liposome membrane characteristics. Eur. J. Lipid Sci. Technol. 2018, 120, 1800039. [Google Scholar] [CrossRef]
- Lúcio, M.; Giannino, N.; Barreira, S.; Catita, J.; Gonçalves, H.; Ribeiro, A.; Fernandes, E.; Carvalho, I.; Pinho, H.; Cerqueira, F. Nanostructured lipid carriers enriched hydrogels for skin topical administration of quercetin and omega-3 fatty acid. Pharmaceutics 2023, 15, 2078. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.-S.; Choi, J.Y.; Kim, J.O.; Lee, M.-K.; Kim, S.H.; Lim, S.-J. Development of paclitaxel-loaded liposomal nanocarrier stabilized by triglyceride incorporation. Int. J. Nanomed. 2016, 11, 4465–4477. [Google Scholar]
- Mohanty, S.; Sahoo, A.K.; Konkimalla, V.B.; Pal, A.; Si, S.C. Naringin in combination with isothiocyanates as liposomal formulations potentiates the anti-inflammatory activity in different acute and chronic animal models of rheumatoid arthritis. ACS Omega 2020, 5, 28319–28332. [Google Scholar] [CrossRef]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, pharmaceutical, nutraceutical, and analytical aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef]
- Priprem, A.; Watanatorn, J.; Sutthiparinyanont, S.; Phachonpai, W.; Muchimapura, S. Anxiety and cognitive effects of quercetin liposomes in rats. Nanomed. Nanotechnol. Biol. Med. 2008, 4, 70–78. [Google Scholar] [CrossRef]
- Alves, R.C.; Fernandes, R.P.; Eloy, J.O.; Salgado, H.R.N.; Chorilli, M. Characteristics, properties and analytical methods of paclitaxel: A review. Crit. Rev. Anal. Chem. 2018, 48, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-Y.; Lee, C.K.; Lee, Y.-B. Preparation and evaluation of PEGylated and folate-PEGylated liposomes containing paclitaxel for lymphatic delivery. J. Nanomater. 2015, 16, 36. [Google Scholar] [CrossRef]
- Ruttala, H.B.; Ko, Y.T. Liposomal co-delivery of curcumin and albumin/paclitaxel nanoparticle for enhanced synergistic antitumor efficacy. Colloids Surf. B Biointerfaces 2015, 128, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Peng, L.; Chen, H.; Li, Q. Surface modification of MPEG-b-PCL-based nanoparticles via oxidative self-polymerization of dopamine for malignant melanoma therapy. Int. J. Nanomed. 2015, 10, 2985. [Google Scholar]
- Zhao, P.; Wang, H.; Yu, M.; Liao, Z.; Wang, X.; Zhang, F.; Ji, W.; Wu, B.; Han, J.; Zhang, H.; et al. Paclitaxel loaded folic acid targeted nanoparticles of mixed lipid-shell and polymer-core: In vitro and in vivo evaluation. Eur. J. Pharm. Biopharm. 2012, 81, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Goyal, A.; Sönmez Gürer, E.; Algın Yapar, E.; Garg, M.; Sood, M.; Sindhu, R.K. Bioactive loaded novel nano-formulations for targeted drug delivery and their therapeutic potential. Pharmaceutics 2022, 14, 1091. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Lecouturier, N.; Vroman, B.; Jérôme, C.; Marchand-Brynaert, J.; Feron, O.; Préat, V. Paclitaxel-loaded PEGylated PLGA-based nanoparticles: In vitro and in vivo evaluation. J. Control. Release 2009, 133, 11–17. [Google Scholar] [CrossRef]
- Dattani, S.; Li, X.; Lampa, C.; Lechuga-Ballesteros, D.; Barriscale, A.; Damadzadeh, B.; Jasti, B.R. A comparative study on micelles, liposomes and solid lipid nanoparticles for paclitaxel delivery. Int. J. Pharm. 2023, 631, 122464. [Google Scholar] [CrossRef]
- Duan, R.; Li, C.; Wang, F.; Yangi, J.-C. Polymer–lipid hybrid nanoparticles-based paclitaxel and etoposide combinations for the synergistic anticancer efficacy in osteosarcoma. Colloids Surf. B Biointerfaces 2017, 159, 880–887. [Google Scholar] [CrossRef]
- Godara, S.; Lather, V.; Kirthanashri, S.; Awasthi, R.; Pandita, D. Lipid-PLGA hybrid nanoparticles of paclitaxel: Preparation, characterization, in vitro and in vivo evaluation. Mater. Sci. Eng. C 2020, 109, 110576. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, J.; Feng, S.-S. Nanoparticles of lipid monolayer shell and biodegradable polymer core for controlled release of paclitaxel: Effects of surfactants on particles size, characteristics and in vitro performance. Int. J. Pharm. 2010, 395, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Singh, S.; Singh, S.; Sheth, N.; Gendle, R. Development and characterization of curcumin loaded transfersome for transdermal delivery. J. Pharm. Sci. Res. 2009, 1, 71. [Google Scholar]
- Panahi, Y.; Alishiri, G.H.; Parvin, S.; Sahebkar, A. Mitigation of systemic oxidative stress by curcuminoids in osteoarthritis: Results of a randomized controlled trial. J. Diet. Suppl. 2016, 13, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Marton, L.T.; Pescinini-e-Salzedas, L.M.; Camargo, M.E.C.; Barbalho, S.M.; Haber, J.F.d.S.; Sinatora, R.V.; Detregiachi, C.R.P.; Girio, R.J.; Buchaim, D.V.; Cincotto dos Santos Bueno, P. The effects of curcumin on diabetes mellitus: A systematic review. Front. Endocrinol. 2021, 12, 669448. [Google Scholar] [CrossRef] [PubMed]
- Srimal, R.; Dhawan, B. Pharmacology of diferuloyl methane (curcumin), a non-steroidal anti-inflammatory agent. J. Pharm. Pharmacol. 1973, 25, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Jakubczyk, K.; Drużga, A.; Katarzyna, J.; Skonieczna-Żydecka, K. Antioxidant potential of curcumin—A meta-analysis of randomized clinical trials. Antioxidants 2020, 9, 1092. [Google Scholar] [CrossRef] [PubMed]
- García-Niño, W.R.; Pedraza-Chaverrí, J. Protective effect of curcumin against heavy metals-induced liver damage. Food Chem. Toxicol. 2014, 69, 182–201. [Google Scholar] [CrossRef]
- Liu, H.-T.; Ho, Y.-S. Anticancer effect of curcumin on breast cancer and stem cells. Food Sci. Hum. Wellness 2018, 7, 134–137. [Google Scholar] [CrossRef]
- Hussain, Y.; Alam, W.; Ullah, H.; Dacrema, M.; Daglia, M.; Khan, H.; Arciola, C.R. Antimicrobial potential of curcumin: Therapeutic potential and challenges to clinical applications. Antibiotics 2022, 11, 322. [Google Scholar] [CrossRef]
- Cole, G.M.; Teter, B.; Frautschy, S.A. Neuroprotective effects of curcumin. Mol. Targets Ther. Uses Curcumin Health Dis. 2007, 595, 197–212. [Google Scholar]
- Flora, G.; Gupta, D.; Tiwari, A. Nanocurcumin: A promising therapeutic advancement over native curcumin. Crit. Rev. ™ Ther. Drug Carr. Syst. 2013, 30, 331–368. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Advances in nanoparticle and microparticle delivery systems for increasing the dispersibility, stability, and bioactivity of phytochemicals. Biotechnol. Adv. 2020, 38, 107287. [Google Scholar] [CrossRef]
- Chittasupho, C.; Srisawad, K.; Arjsri, P.; Phongpradist, R.; Tingya, W.; Ampasavate, C.; Dejkriengkraikul, P. Targeting Spike Glycoprotein S1 Mediated by NLRP3 Inflammasome Machinery and the Cytokine Releases in A549 Lung Epithelial Cells by Nanocurcumin. Pharmaceuticals 2023, 16, 862. [Google Scholar] [CrossRef] [PubMed]
- Quirós-Fallas, M.I.; Wilhelm-Romero, K.; Quesada-Mora, S.; Azofeifa-Cordero, G.; Vargas-Huertas, L.F.; Alvarado-Corella, D.; Mora-Román, J.J.; Vega-Baudrit, J.R.; Navarro-Hoyos, M.; Araya-Sibaja, A.M. Curcumin Hybrid Lipid Polymeric Nanoparticles: Antioxidant Activity, Immune Cellular Response, and Cytotoxicity Evaluation. Biomedicines 2022, 10, 2431. [Google Scholar] [CrossRef] [PubMed]
- Naziris, N.; Sekowski, S.; Olchowik-Grabarek, E.; Buczkowski, A.; Balcerzak, Ł.; Chrysostomou, V.; Pispas, S.; Małecka, M.; Bryszewska, M.; Ionov, M. Biophysical interactions of mixed lipid-polymer nanoparticles incorporating curcumin: Potential as antibacterial agent. Biomater. Adv. 2023, 144, 213200. [Google Scholar] [CrossRef] [PubMed]
- Vandita, K.; Shashi, B.; Santosh, K.G.; Pal, K.I. Enhanced apoptotic effect of curcumin loaded solid lipid nanoparticles. Mol. Pharm. 2012, 9, 3411–3421. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.S.; Morris, A.; Billa, N.; Leong, C.-O. An evaluation of curcumin-encapsulated chitosan nanoparticles for transdermal delivery. Aaps Pharmscitech 2019, 20, 69. [Google Scholar] [CrossRef]
- Chirio, D.; Gallarate, M.; Peira, E.; Battaglia, L.; Serpe, L.; Trotta, M. Formulation of curcumin-loaded solid lipid nanoparticles produced by fatty acids coacervation technique. J. Microencapsul. 2011, 28, 537–548. [Google Scholar] [CrossRef]
- Caon, T.; Mazzarino, L.; Simões, C.M.O.; Senna, E.L.; Silva, M.A.S. Lipid-and polymer-based nanostructures for cutaneous delivery of curcumin. AAPS PharmSciTech 2017, 18, 920–925. [Google Scholar] [CrossRef]
- Singh, S.; Supaweera, N.; Nwabor, O.F.; Chaichompoo, W.; Suksamrarn, A.; Chittasupho, C.; Chunglok, W. Poly (vinyl alcohol)-gelatin-sericin copolymerized film fortified with vesicle-entrapped demethoxycurcumin/bisdemethoxycurcumin for improved stability, antibacterial, anti-inflammatory, and skin tissue regeneration. Int. J. Biol. Macromol. 2024, 258, 129071. [Google Scholar] [CrossRef]
- Nwabor, O.F.; Singh, S. A Systematic Review on Rhodomyrtus Tomentosa (Aiton) Hassk: A Potential Source of Pharmacological Relevant Bioactive Compounds with Prospects as Alternative Remedies in Varied Medical Conditions. Int. J. Pharm. Sci. Nanotechnol. (IJPSN) 2022, 15, 5875–5891. [Google Scholar] [CrossRef]
- Huang, L.; Matsuo, M.; Calderón, C.; Fan, S.-H.; Ammanath, A.V.; Fu, X.; Li, N.; Luqman, A.; Ullrich, M.; Herrmann, F. Molecular basis of rhodomyrtone resistance in Staphylococcus aureus. Mbio 2022, 13, e03833-21. [Google Scholar] [CrossRef] [PubMed]
- Chorachoo, J.; Amnuaikit, T.; Voravuthikunchai, S.P. Liposomal encapsulated rhodomyrtone: A novel antiacne drug. Evid. -Based Complement. Altern. Med. 2013, 2013, 157635. [Google Scholar] [CrossRef] [PubMed]
- Ontong, J.C.; Singh, S.; Siriyong, T.; Voravuthikunchai, S.P. Transferosomes stabilized hydrogel incorporated rhodomyrtone-rich extract from Rhodomyrtus tomentosa leaf fortified with phosphatidylcholine for the management of skin and soft-tissue infections. Biotechnol. Lett. 2024, 46, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Eichenseher, J. Peptic Ulcer Disease. In Integrative Medicine, 4th ed.; Rakel, D., Ed.; Elsevier: Philadelphia, PA, USA, 2018; Chapter 43. [Google Scholar]
- Allgrove, J.E.; Davison, G. Chocolate/Cocoa Polyphenols and Oxidative Stress. In Polyphenols: Mechanisms of Action in Human Health and Disease; Elsevier: Hoboken, NJ, USA, 2018; pp. 207–219. [Google Scholar]
- Shawky, S.; Makled, S.; Awaad, A.; Boraie, N. Quercetin Loaded Cationic Solid Lipid Nanoparticles in a Mucoadhesive In Situ Gel—A Novel Intravesical Therapy Tackling Bladder Cancer. Pharmaceutics 2022, 14, 2527. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Hou, Y.; Song, X.; Wang, P.; Li, Y. Cholate-modified polymer-lipid hybrid nanoparticles for oral delivery of quercetin to potentiate the antileukemic effect. Int. J. Nanomed. 2019, 14, 4045–4057. [Google Scholar] [CrossRef] [PubMed]
- Elmowafy, M.; Alruwaili, N.K.; Ahmad, N.; Kassem, A.M.; Ibrahim, M.F. Quercetin-Loaded Mesoporous Silica Nanoparticle–Based Lyophilized Tablets for Enhanced Physicochemical Features and Dissolution Rate: Formulation, Optimization, and In Vitro Evaluation. AAPS PharmSciTech 2022, 24, 6. [Google Scholar] [CrossRef]
- Chittasupho, C.; Junmahasathien, T.; Chalermmongkol, J.; Wongjirasakul, R.; Leesawat, P.; Okonogi, S. Suppression of Intracellular Reactive Oxygen Species in Human Corneal Epithelial Cells via the Combination of Quercetin Nanoparticles and Epigallocatechin Gallate and In Situ Thermosensitive Gel Formulation for Ocular Drug Delivery. Pharmaceuticals 2021, 14, 679. [Google Scholar] [CrossRef]
- Kumar, R.; Choudhary, D.K.; Debnath, M. Development of BSA conjugated on modified surface of quercetin-loaded lipid nanocarriers for breast cancer treatment. Mater. Res. Express 2020, 7, 015411. [Google Scholar] [CrossRef]
- Patil, N.L.; Mahajan, H.S. Quercetin loaded nanostructured lipid carriers for nose to brain delivery: In vitro and in vivo studies. Am. J. Adv. Drug Deliv. 2018, 6, 9–20. [Google Scholar] [CrossRef]
- Hariyadi, D.M.; Prestisya, I.; Suhariyono, G.; Miatmoko, A.; Rosita, N.; Rahmadi, M. Characterization of dry powder inhaler quercetin solid lipid microparticle (SLM) as lung delivery system: Effect of polymer concentration. Egypt. J. Chem. 2022, 65, 281–289. [Google Scholar]
- Tefas, L.R.; Muntean, D.-M.; Vlase, L.; Porfire, A.S.; Achim, M.; Tomuță, I. Quercetin-loaded liposomes: Formulation optimization through a D-optimal experimental design. Farmacia 2015, 63, 26–31. [Google Scholar]
- Chen, A.Y.; Chen, Y.C. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013, 138, 2099–2107. [Google Scholar] [CrossRef] [PubMed]
- Silva dos Santos, J.; Goncalves Cirino, J.P.; de Oliveira Carvalho, P.; Ortega, M.M. The pharmacological action of kaempferol in central nervous system diseases: A review. Front. Pharmacol. 2021, 11, 565700. [Google Scholar] [CrossRef]
- Kazmi, I.; Al-Abbasi, F.A.; Afzal, M.; Altayb, H.N.; Nadeem, M.S.; Gupta, G. Formulation and evaluation of Kaempferol loaded nanoparticles against experimentally induced hepatocellular carcinoma: In vitro and in vivo studies. Pharmaceutics 2021, 13, 2086. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Zhang, J.; Ju, J.; Wu, Y.; Zhang, Y.; Zhan, L.; Li, C.; Wang, Y. Preparation, characterization, and evaluation of the antitumor effect of kaempferol nanosuspensions. Drug Deliv. Transl. Res. 2023, 13, 2885–2902. [Google Scholar] [CrossRef] [PubMed]
- Ilk, S.; Saglam, N.; Özgen, M. Kaempferol loaded lecithin/chitosan nanoparticles: Preparation, characterization, and their potential applications as a sustainable antifungal agent. Artif. Cells Nanomed. Biotechnol. 2017, 45, 907–916. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A double-edged sword in health benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef]
- Rius, C.; Abu-Taha, M.; Hermenegildo, C.; Piqueras, L.; Cerda-Nicolas, J.-M.; Issekutz, A.C.; Estañ, L.; Cortijo, J.; Morcillo, E.J.; Orallo, F. Trans-but not Cis-resveratrol impairs angiotensin-II–mediated vascular inflammation through inhibition of NF-κB activation and peroxisome proliferator-activated receptor-γ upregulation. J. Immunol. 2010, 185, 3718–3727. [Google Scholar] [CrossRef]
- Soleas, G.J.; Diamandis, E.P.; Goldberg, D.M. Resveratrol: A molecule whose time has come? And gone? Clin. Biochem. 1997, 30, 91–113. [Google Scholar] [CrossRef]
- Park, E.-J.; Pezzuto, J.M. The pharmacology of resveratrol in animals and humans. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2015, 1852, 1071–1113. [Google Scholar] [CrossRef] [PubMed]
- Heredia, A.; Davis, C.; Redfield, R. Synergistic inhibition of HIV-1 in activated and resting peripheral blood mononuclear cells, monocyte-derived macrophages, and selected drug-resistant isolates with nucleoside analogues combined with a natural product, resveratrol. JAIDS J. Acquir. Immune Defic. Syndr. 2000, 25, 246–255. [Google Scholar] [CrossRef]
- Loópez-Nicolaás, J.M.; Garciía-Carmona, F. Aggregation state and p K a values of (E)-resveratrol as determined by fluorescence spectroscopy and UV-visible absorption. J. Agric. Food Chem. 2008, 56, 7600–7605. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhou, Y.; Su, Y.; Li, S.; Dong, J.; He, Q.; Cao, Y.; Lu, T.; Qin, L. Resveratrol-loaded solid lipid nanoparticle supplementation ameliorates physical fatigue by improving mitochondrial quality control. Crystals 2019, 9, 559. [Google Scholar] [CrossRef]
- Isailović, B.D.; Kostić, I.T.; Zvonar, A.; Đorđević, V.B.; Gašperlin, M.; Nedović, V.A.; Bugarski, B.M. Resveratrol loaded liposomes produced by different techniques. Innov. Food Sci. Emerg. Technol. 2013, 19, 181–189. [Google Scholar] [CrossRef]
- Kim, S.; Ng, W.K.; Dong, Y.; Das, S.; Tan, R.B. Preparation and physicochemical characterization of trans-resveratrol nanoparticles by temperature-controlled antisolvent precipitation. J. Food Eng. 2012, 108, 37–42. [Google Scholar] [CrossRef]
- Alves, M.G.; Silva, B.M.; Oliveira, P.F. Nutritional factors and male reproduction. Encycl. Reprod. 2018, 1, 458–464. [Google Scholar]
- Bansal, M.; Singh, N.; Pal, S.; Dev, I.; Ansari, K.M. Chemopreventive role of dietary phytochemicals in colorectal cancer. Adv. Mol. Toxicol. 2018, 12, 69–121. [Google Scholar]
- Zhang, J.; Nie, S.; Wang, S. Nanoencapsulation enhances epigallocatechin-3-gallate stability and its antiatherogenic bioactivities in macrophages. J. Agric. Food Chem. 2013, 61, 9200–9209. [Google Scholar] [CrossRef]
- Farabegoli, F.; Granja, A.; Magalhães, J.; Purgato, S.; Voltattorni, M.; Pinheiro, M. Epigallocatechin-3-gallate Delivered in Nanoparticles Increases Cytotoxicity in Three Breast Carcinoma Cell Lines. ACS Omega 2022, 7, 41872–41881. [Google Scholar] [CrossRef]
- Hajipour, H.; Hamishehkar, H.; Nazari Soltan Ahmad, S.; Barghi, S.; Maroufi, N.F.; Taheri, R.A. Improved anticancer effects of epigallocatechin gallate using RGD-containing nanostructured lipid carriers. Artif. Cells Nanomed. Biotechnol. 2018, 46, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Vásquez, M.J.; Plascencia-Jatomea, M.; Sánchez-Valdes, S.; Tanori-Córdova, J.C.; Castillo-Yañez, F.J.; Quintero-Reyes, I.E.; Graciano-Verdugo, A.Z. Characterization of epigallocatechin-gallate-grafted chitosan nanoparticles and evaluation of their antibacterial and antioxidant potential. Polymers 2021, 13, 1375. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Xu, Y.; Yin, J.-F.; Jin, J.; Jiang, Y.; Du, Q. Improving the effectiveness of (−)-epigallocatechin gallate (EGCG) against rabbit atherosclerosis by EGCG-loaded nanoparticles prepared from chitosan and polyaspartic acid. J. Agric. Food Chem. 2014, 62, 12603–12609. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Yan, H.; Yang, H.-J.; Kim, H.W.; Wan, X.; Lee, J.; Ko, S. Synthesis and controlled-release properties of chitosan/β-Lactoglobulin nanoparticles as carriers for oral administration of epigallocatechin gallate. Food Sci. Biotechnol. 2016, 25, 1583–1590. [Google Scholar] [CrossRef]
- Gillessen, A.; Schmidt, H.H.-J. Silymarin as supportive treatment in liver diseases: A narrative review. Adv. Ther. 2020, 37, 1279–1301. [Google Scholar] [CrossRef] [PubMed]
- Javed, S.; Kohli, K.; Ali, M. Reassessing bioavailability of silymarin. Altern. Med. Rev. 2011, 16, 239. [Google Scholar] [PubMed]
- Javed, S.; Kohli, K.; Ali, M. Patented bioavailability enhancement techniques of silymarin. Recent Pat. Drug Deliv. Formul. 2010, 4, 145–152. [Google Scholar] [CrossRef]
- Liang, J.; Liu, Y.; Liu, J.; Li, Z.; Fan, Q.; Jiang, Z.; Yan, F.; Wang, Z.; Huang, P.; Feng, N. Chitosan-functionalized lipid-polymer hybrid nanoparticles for oral delivery of silymarin and enhanced lipid-lowering effect in NAFLD. J. Nanobiotechnol. 2018, 16, 64. [Google Scholar] [CrossRef]
- Snima, K.; Arunkumar, P.; Jayakumar, R.; Lakshmanan, V.-K. Silymarin encapsulated poly (D, L-lactic-co-glycolic acid) nanoparticles: A prospective candidate for prostate cancer therapy. J. Biomed. Nanotechnol. 2014, 10, 559–570. [Google Scholar] [CrossRef]
- Hirlekar, R.; Patil, E.; Bhairy, S. Solid nanostructured lipid carriers loaded with silymarin for oral delivery: Formulation development and evaluation. Curr. Trends Pharm. Pharm. Chem. 2021, 3, 56–67. [Google Scholar] [CrossRef]
- Azadpour, M.; Farajollahi, M.M.; Dariushnejad, H.; Varzi, A.M.; Varezardi, A.; Barati, M. Effects of synthetic silymarin-PLGA nanoparticles on M2 polarization and inflammatory cytokines in LPS-treated murine peritoneal macrophages. Iran. J. Basic Med. Sci. 2021, 24, 1446. [Google Scholar] [PubMed]
- El-Sherbiny, I.M.; Abdel-Mogib, M.; Dawidar, A.-A.M.; Elsayed, A.; Smyth, H.D. Biodegradable pH-responsive alginate-poly (lactic-co-glycolic acid) nano/micro hydrogel matrices for oral delivery of silymarin. Carbohydr. Polym. 2011, 83, 1345–1354. [Google Scholar] [CrossRef]
- Podolak, I.; Galanty, A.; Sobolewska, D. Saponins as cytotoxic agents: A review. Phytochem. Rev. 2010, 9, 425–474. [Google Scholar] [CrossRef] [PubMed]
- Rejinold, N.S.; Muthunarayanan, M.; Muthuchelian, K.; Chennazhi, K.; Nair, S.V.; Jayakumar, R. Saponin-loaded chitosan nanoparticles and their cytotoxicity to cancer cell lines in vitro. Carbohydr. Polym. 2011, 84, 407–416. [Google Scholar] [CrossRef]
- Van de Ven, H.; Vermeersch, M.; Vandenbroucke, R.; Matheeussen, A.; Apers, S.; Weyenberg, W.; De Smedt, S.; Cos, P.; Maes, L.; Ludwig, A. Intracellular drug delivery in Leishmania-infected macrophages: Evaluation of saponin-loaded PLGA nanoparticles. J. Drug Target. 2012, 20, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, C.-T.; Ma, W.; Xie, X.; Huang, Q. Oridonin: A review of its pharmacology, pharmacokinetics and toxicity. Front. Pharmacol. 2021, 12, 645824. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Bi, Y.; Xu, Y.; Zhang, Z.; Xu, W.; Zhang, S.; Chen, J. Oridonin inhibits the migration and epithelial-to-mesenchymal transition of small cell lung cancer cells by suppressing FAK-ERK1/2 signalling pathway. J. Cell. Mol. Med. 2020, 24, 4480–4493. [Google Scholar] [CrossRef]
- Guo, W.; Zheng, P.; Zhang, J.; Ming, L.; Zhou, C.; Zhang, S. Oridonin suppresses transplant rejection by depleting T cells from the periphery. Int. Immunopharmacol. 2013, 17, 1148–1154. [Google Scholar] [CrossRef]
- Cummins, C.B.; Wang, X.; Sommerhalder, C.; Bohanon, F.J.; Nunez Lopez, O.; Tie, H.-Y.; Rontoyanni, V.G.; Zhou, J.; Radhakrishnan, R.S. Natural compound oridonin inhibits endotoxin-induced inflammatory response of activated hepatic stellate cells. BioMed Res. Int. 2018, 2018, 6137420. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, P.-X.; Han, C.-H.; Wei, D.; Qiao, T.; Peng, B.; Liu, K.; Zheng, J.; Liu, W. Oridonin protects the lung against hyperoxia-induced injury in a mouse model. Undersea Hyperb. Med. 2017, 44, 33–38. [Google Scholar] [CrossRef]
- Liu, H.; Gu, C.; Liu, M.; Liu, G.; Wang, Y. NEK7 mediated assembly and activation of NLRP3 inflammasome downstream of potassium efflux in ventilator-induced lung injury. Biochem. Pharmacol. 2020, 177, 113998. [Google Scholar] [CrossRef]
- Wen, F.; Zhuge, W.; Wang, J.; Lu, X.; You, R.; Liu, L.; Zhuge, Q.; Ding, S. Oridonin prevents insulin resistance–mediated cognitive disorder through PTEN/Akt pathway and autophagy in minimal hepatic encephalopathy. J. Cell. Mol. Med. 2020, 24, 61–78. [Google Scholar] [CrossRef]
- Xing, J.; Zhang, D.; Tan, T. Studies on the oridonin-loaded poly (D, L-lactic acid) nanoparticles in vitro and in vivo. Int. J. Biol. Macromol. 2007, 40, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Tan, T.; Gao, L. Preparation of oridonin-loaded solid lipid nanoparticles and studies on them in vitro and in vivo. Nanotechnology 2006, 17, 5821. [Google Scholar] [CrossRef]
- Toragall, V.; Jayapala, N.; Muthukumar, S.; Vallikanan, B. Biodegradable chitosan-sodium alginate-oleic acid nanocarrier promotes bioavailability and target delivery of lutein in rat model with no toxicity. Food Chem. 2020, 330, 127195. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Duan, J.; Yu, Y.; Li, Y.; Sun, Z. Silica nanoparticle-induced blockage of autophagy leads to autophagic cell death in HepG2 cells. J. Biomed. Nanotechnol. 2017, 13, 485–499. [Google Scholar] [CrossRef]
- Freyre-Fonseca, V.; Delgado-Buenrostro, N.L.; Gutiérrez-Cirlos, E.B.; Calderón-Torres, C.M.; Cabellos-Avelar, T.; Sánchez-Pérez, Y.; Pinzón, E.; Torres, I.; Molina-Jijón, E.; Zazueta, C. Titanium dioxide nanoparticles impair lung mitochondrial function. Toxicol. Lett. 2011, 202, 111–119. [Google Scholar] [CrossRef]
- Roberts, M.; Mohammed, Y.; Pastore, M.; Namjoshi, S.; Yousef, S.; Alinaghi, A.; Haridass, I.; Abd, E.; Leite-Silva, V.; Benson, H. Topical and cutaneous delivery using nanosystems. J. Control. Release 2017, 247, 86–105. [Google Scholar] [CrossRef]
- Din, F.u.; Choi, J.Y.; Kim, D.W.; Mustapha, O.; Kim, D.S.; Thapa, R.K.; Ku, S.K.; Youn, Y.S.; Oh, K.T.; Yong, C.S. Irinotecan-encapsulated double-reverse thermosensitive nanocarrier system for rectal administration. Drug Deliv. 2017, 24, 502–510. [Google Scholar] [CrossRef]
- Yoshimura, K.; Aoki, H.; Teruyama, C.; Iijima, M.; Tsutsumi, H.; Kuroda, S.I.; Hamano, K. A novel hybrid drug delivery system for treatment of aortic aneurysms. Int. J. Mol. Sci. 2020, 21, 5538. [Google Scholar] [CrossRef]
- Sim, T.; Han, S.M.; Lim, C.; Won, W.R.; Lee, E.S.; Youn, Y.S.; Oh, K.T. A pH-sensitive polymer for cancer targeting prepared by one-step modulation of functional side groups. Macromol. Res. 2019, 27, 795–802. [Google Scholar] [CrossRef]
- Yu, M.; Ji, N.; Wang, Y.; Dai, L.; Xiong, L.; Sun, Q. Starch-based nanoparticles: Stimuli responsiveness, toxicity, and interactions with food components. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1075–1100. [Google Scholar] [CrossRef]
- Anh, N.; Cuong, N.; Hoang, N. Formation of biodegradable copolymeric nanoparticles for anticancer drug delivery. In Proceedings of the Third International Conference on the Development of Biomedical Engineering in Vietnam: BME2010, Ho Chi Minh City, Vietnam, 11–14 January 2010; pp. 203–206. [Google Scholar]
- Martirosyan, D.; Lampert, T.; Ekblad, M. Classification and regulation of functional food proposed by the Functional Food Center. Funct. Food Sci. 2022, 2, 25–46. [Google Scholar] [CrossRef]
- Rajput, S.K.; Gullaiya, S.; Nagpal, D. Herbal Nanoparticle Based Targeted Drug Delivery for Alcohol Intoxication. Patent IN2960/DEL/2014, 2014. [Google Scholar]
- Xue, M.; Jiang, Z.-Z.; Wu, T.; Li, J.; Zhang, L.; Zhao, Y.; Li, X.-J.; Zhang, L.-Y.; Yang, S.-Y. Anti-inflammatory effects and hepatotoxicity of Tripterygium-loaded solid lipid nanoparticles on adjuvant-induced arthritis in rats. Phytomedicine 2012, 19, 998–1006. [Google Scholar] [CrossRef]
- Vanderbist, F.; Baudier, P.; Amighi, K.; Pilcer, G. Improved Pharmaceutical Dry Powder Compositions for Inhalation. EP 2 050 437 A1, 15 October 2007. [Google Scholar]
- Müller, R.H.; Wissing, S.; Mäder, K. UV Radiation Reflecting or Absorbing Agents, Protecting against Harmful UV Radiation and Reinforcing the Natural Skin Barrier. US Patent CN1372452A, 2 October 2004. [Google Scholar]
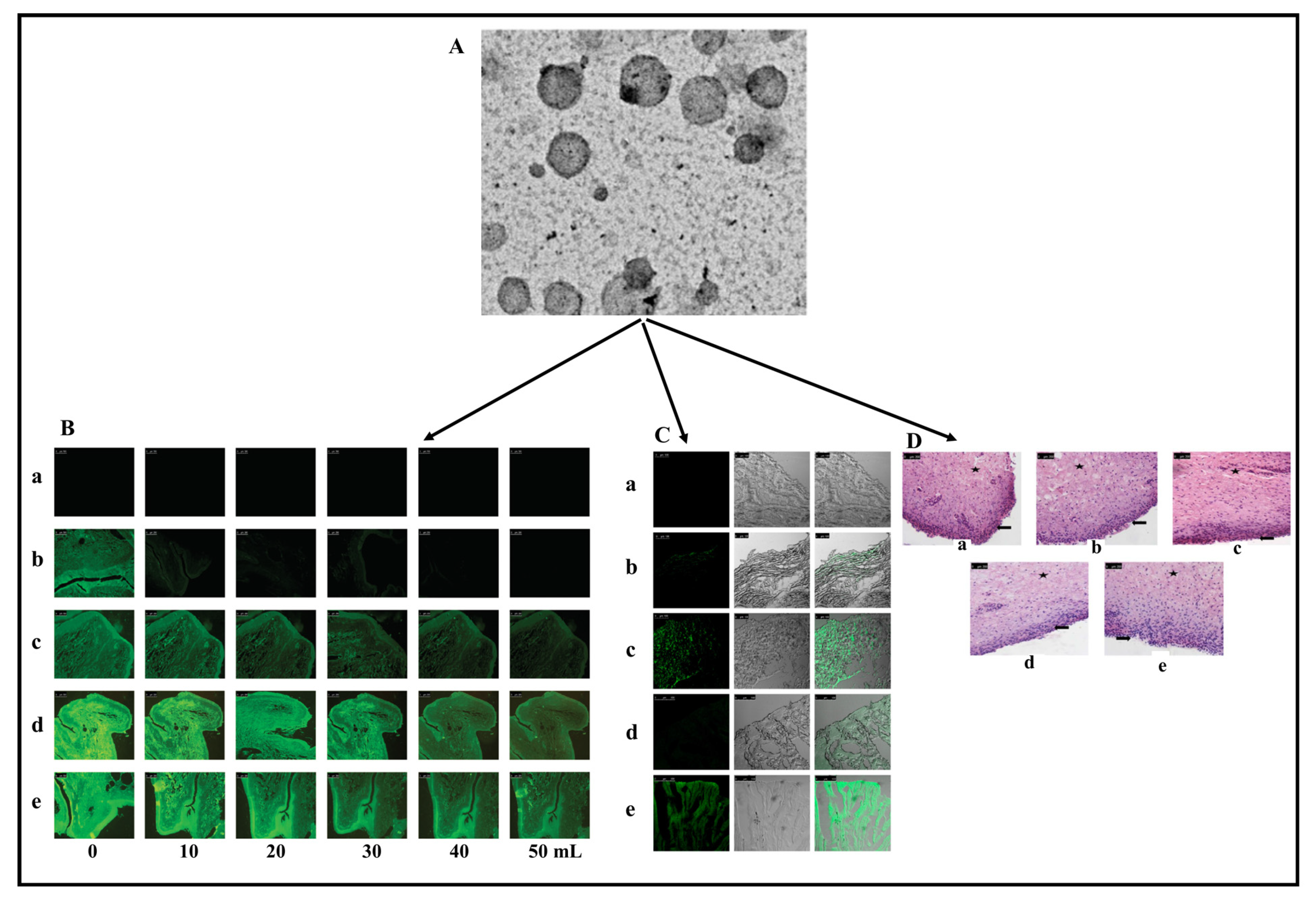
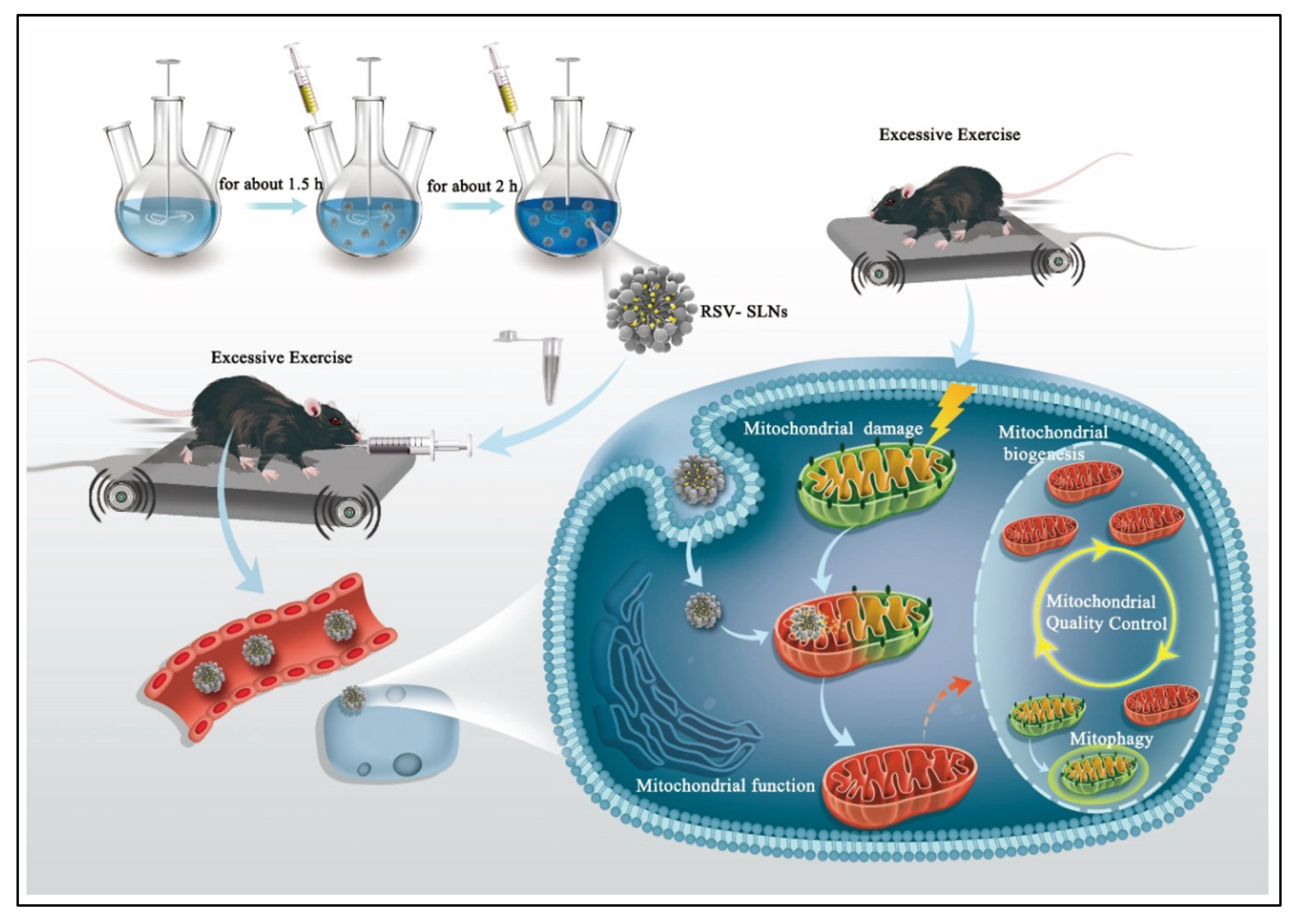
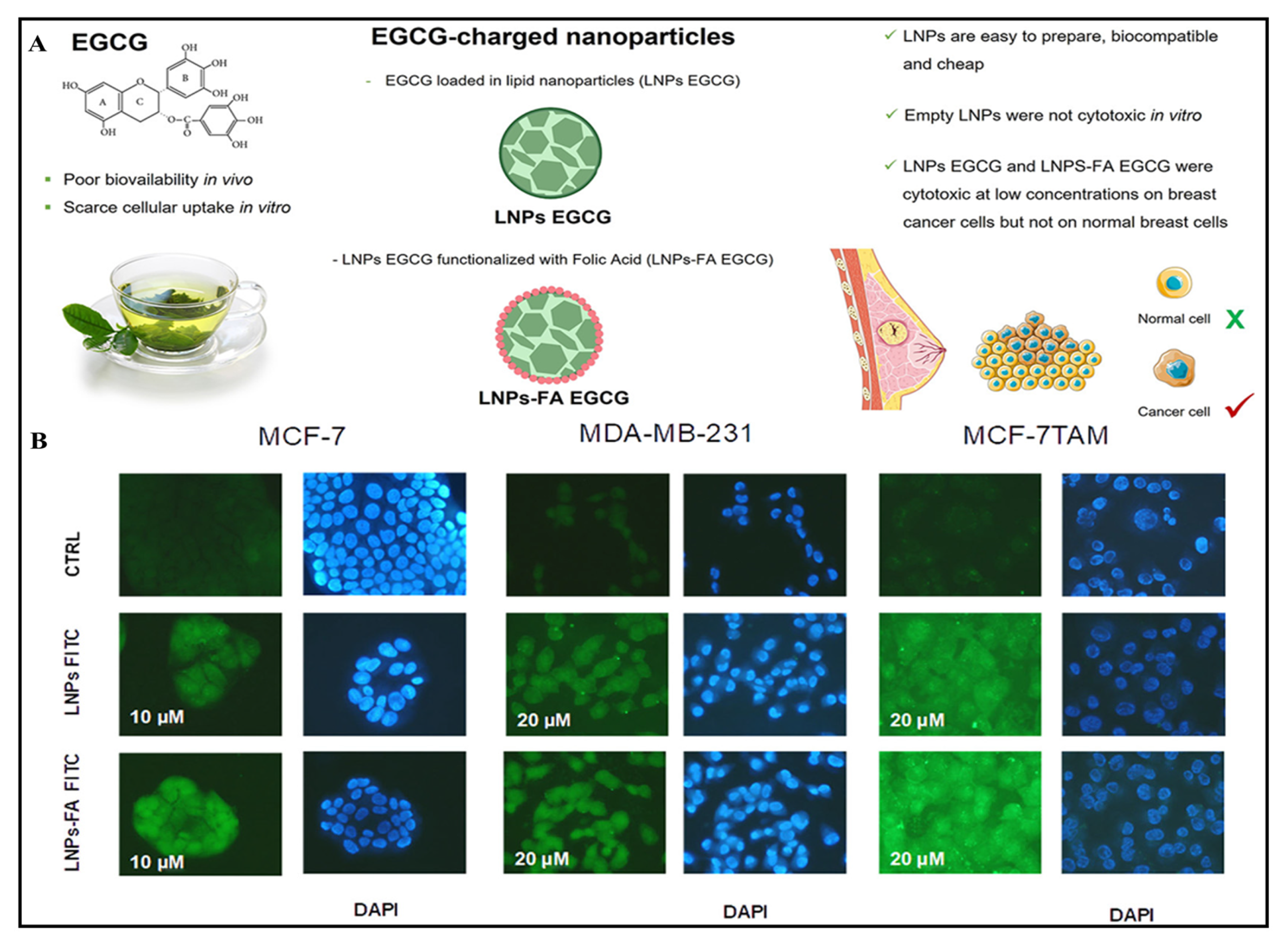
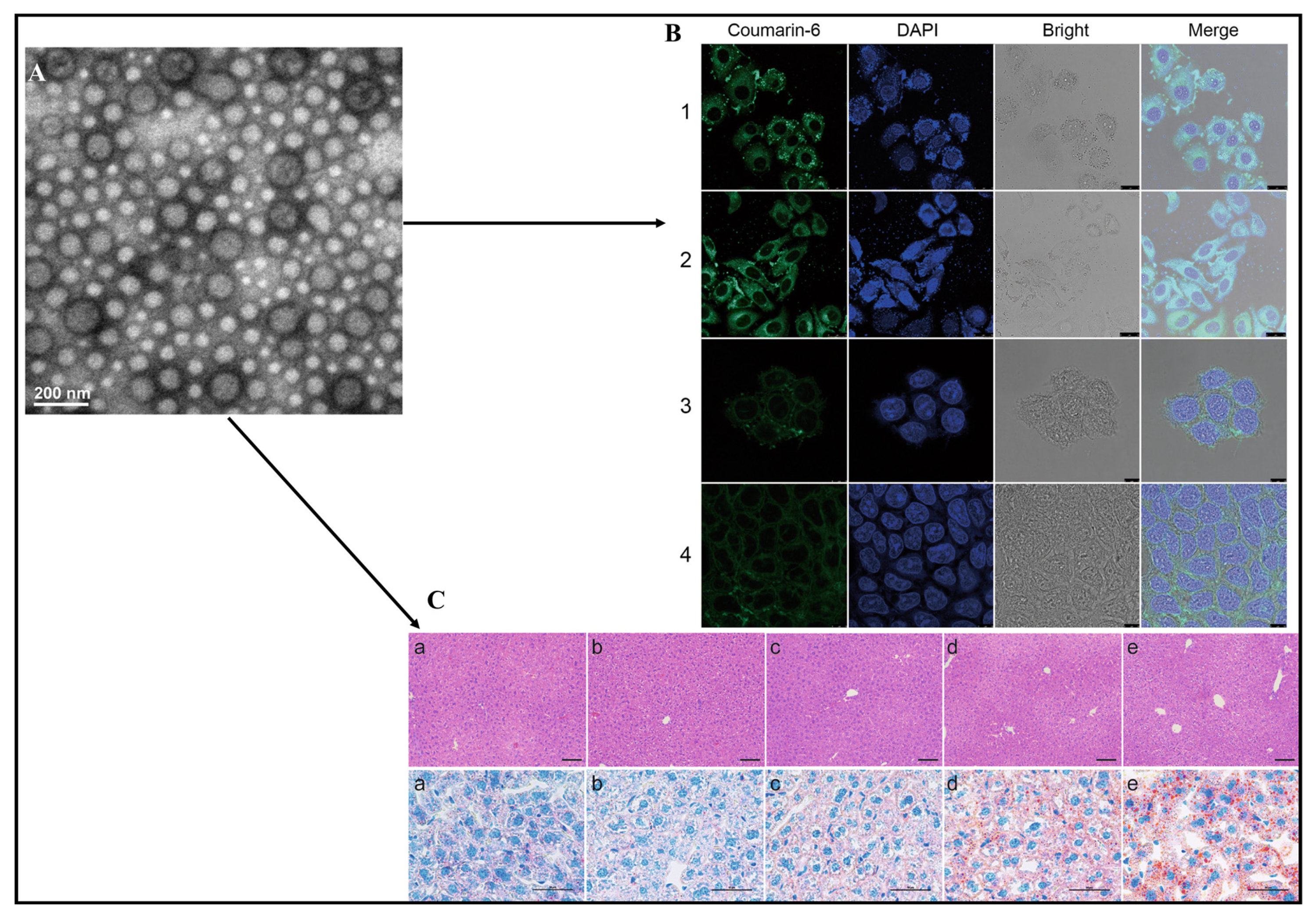
| Patented Lipid-Based Nanoparticles Encapsulating Bioactive Compound | Site | Applications | Country of Patent | Reference |
|---|---|---|---|---|
| Floating pills contain SLNs loaded with an extract of the Ficus benjamina aerial part. | Oral | IN2960/DEL/2014 | India | [139] |
| Increased oral bioavailability of Tripterygium glycoside due to increased dissolvability | Oral | CN200910034968 | China | [140] |
| Nanoparticles fabricated using cacao butter can be used to administer medications parenterally. | Parenteral | WO2009102121 | Korea | [141] |
| Delivery of nanoparticles improves the stability and effectiveness of UV filters. | Dermal | WO0103652 | Canada | [142] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, P.; Garala, K.; Singh, S.; Prajapati, B.G.; Chittasupho, C. Lipid-Based Nanoparticles in Delivering Bioactive Compounds for Improving Therapeutic Efficacy. Pharmaceuticals 2024, 17, 329. https://doi.org/10.3390/ph17030329
Patel P, Garala K, Singh S, Prajapati BG, Chittasupho C. Lipid-Based Nanoparticles in Delivering Bioactive Compounds for Improving Therapeutic Efficacy. Pharmaceuticals. 2024; 17(3):329. https://doi.org/10.3390/ph17030329
Chicago/Turabian StylePatel, Priya, Kevinkumar Garala, Sudarshan Singh, Bhupendra G. Prajapati, and Chuda Chittasupho. 2024. "Lipid-Based Nanoparticles in Delivering Bioactive Compounds for Improving Therapeutic Efficacy" Pharmaceuticals 17, no. 3: 329. https://doi.org/10.3390/ph17030329
APA StylePatel, P., Garala, K., Singh, S., Prajapati, B. G., & Chittasupho, C. (2024). Lipid-Based Nanoparticles in Delivering Bioactive Compounds for Improving Therapeutic Efficacy. Pharmaceuticals, 17(3), 329. https://doi.org/10.3390/ph17030329









