Current Strategies and Therapeutic Applications of Mesenchymal Stem Cell-Based Drug Delivery
Abstract
:1. Introduction
2. The Self-Renewal and Differentiation Capabilities of MSCs in MSC-Based Drug Delivery
2.1. Self-Renewal and Differentiation Capabilities of MSCs
2.2. Developing Methods to Study the Differentiation of Human Mesenchymal Stem Cells
3. The Immunosuppressive Functions of MSCs
3.1. Immunosuppressive Functions of MSCs
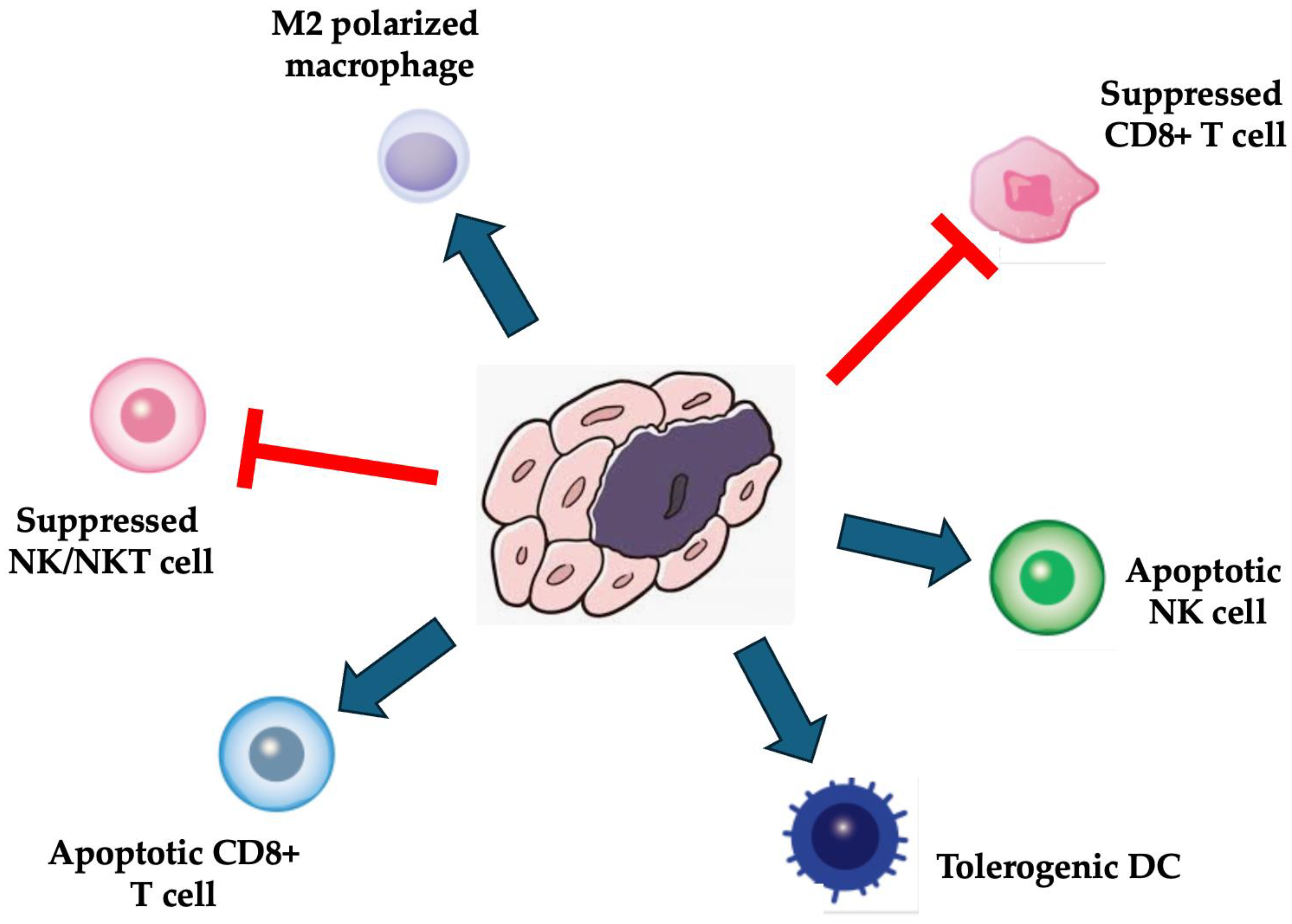
3.2. MSCs-Based Drug Delivery Strategies for Targeting of Tumor Cells
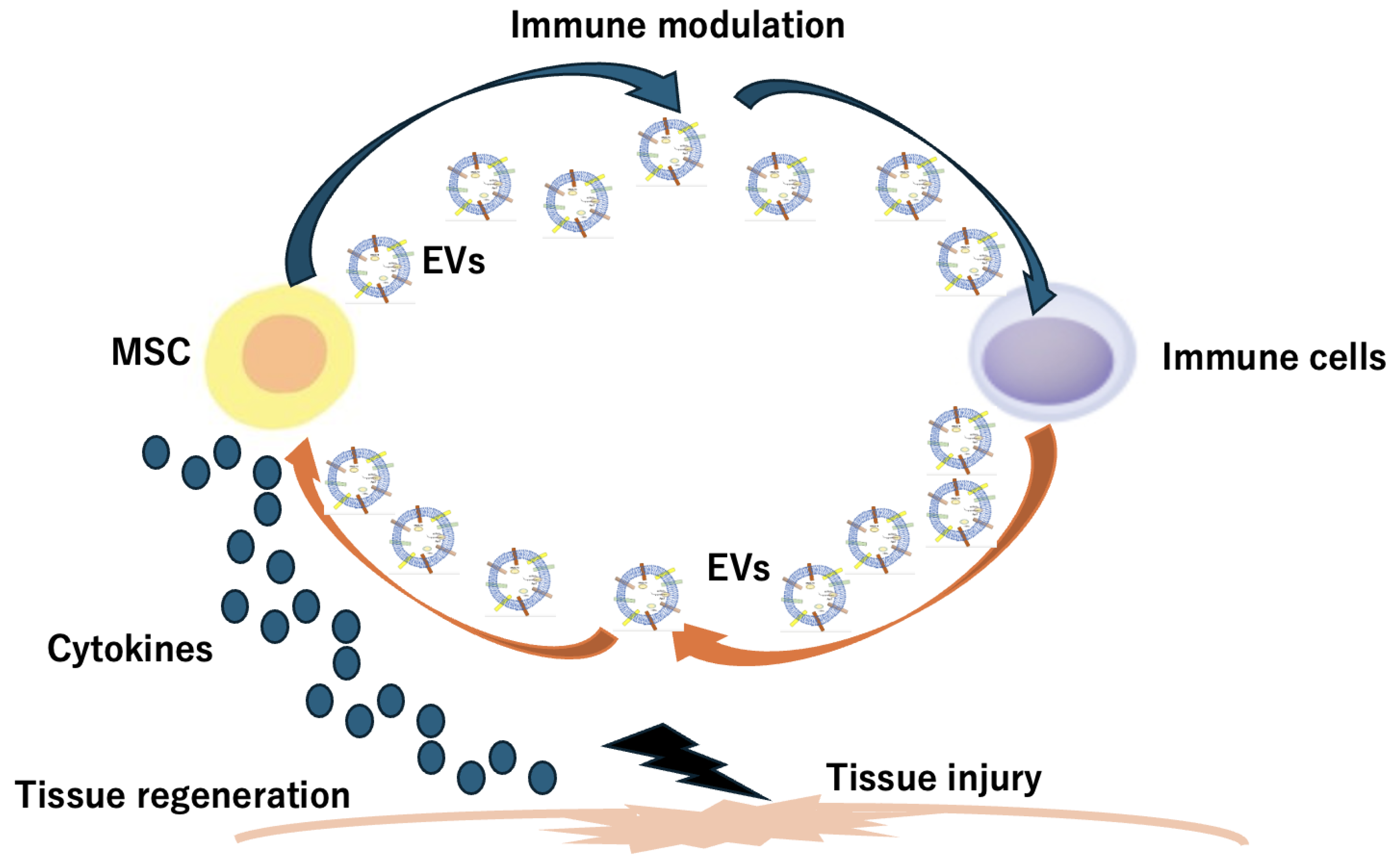
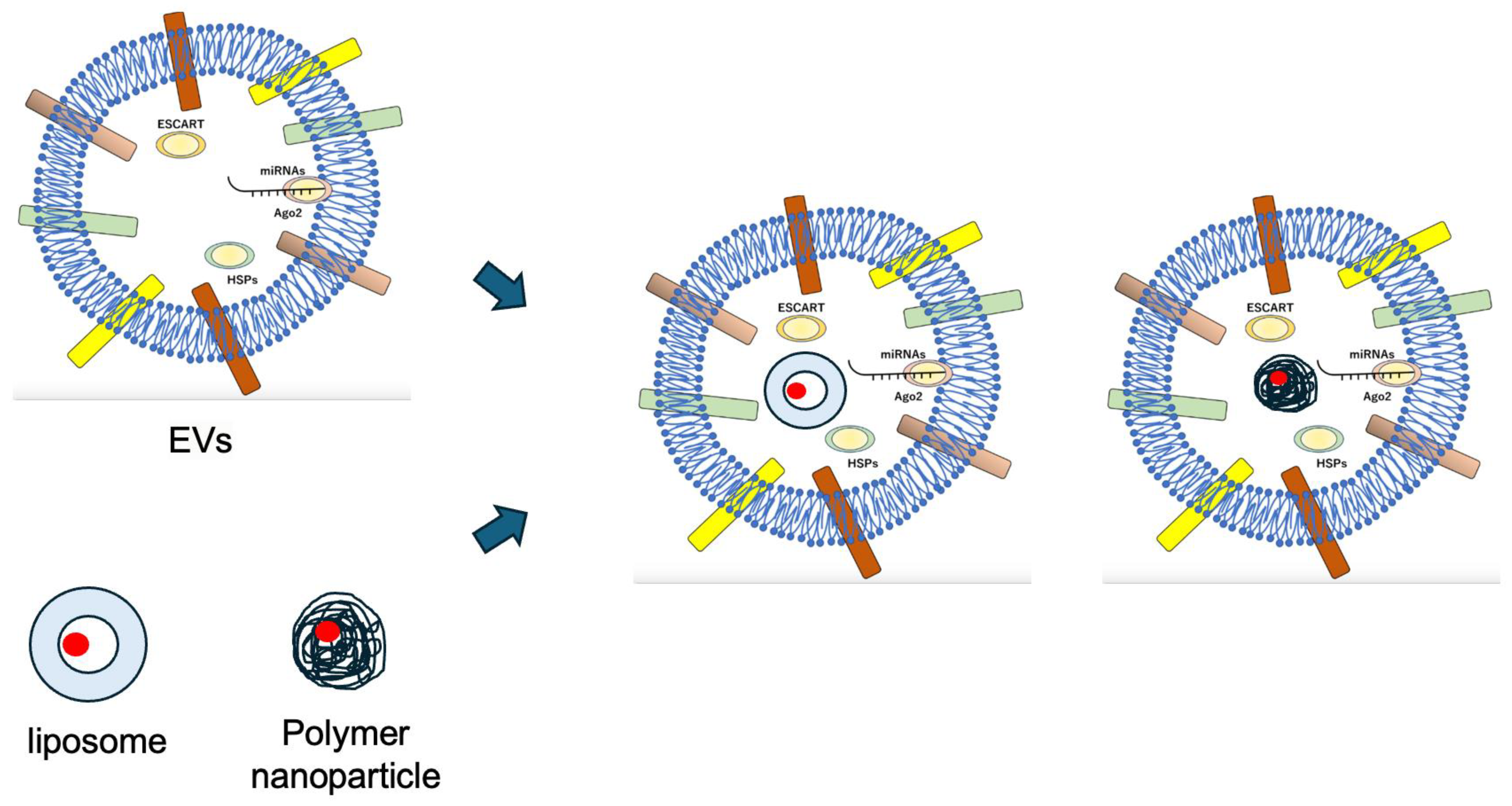
4. Stem Cell-Derived Extracellular Vesicle (EV) and Stem Cell Membrane-Coated Nanoparticles (SCMNPs)
5. Stem Cell-Laden Scaffolds and Scaffold-Free Stem Cell Sheets
6. Therapeutic Applications in MSC-Based Drug Delivery
| MSC Origin | Target Therapy | Registration Year | Drug/Biomolecule Used | Models | Potential Outcomes | References |
|---|---|---|---|---|---|---|
| Cord blood | Type I Diabetes Mellitus (T1DM) | 2014 | streptozotocin-induced diabetic rats | reducing blood glucose levels, improving insulin sensitivity, and inhibiting β-cell apoptosis | [86,87] | |
| Umbilical cord | Chronic kidney injury | 2016 | lipopolysaccharide | models of acute kidney injury and chronic kidney disease | reducing oxidative stress, inflammation, and fibrosis. | [88,89,90] |
| Human umbilical cord | Healing of Macular holes | 2017 | macular degeneration animal models | alleviation of inflammation and damage | [91,92] | |
| Bone marrow | Bronchopulmonary Dysplasia | 2019 | experimental models of bronchopulmonary dysplasia | reducing inflammation, improving alveolarization, and promoting angiogenesis | [93,94,95,96] | |
| Bone marrow | Dystrophic Epidermolysis Bullosa | 2020 | Dystrophic Epidermolysis Bullosa mouse models | promoting wound healing, reducing blister formation, and improving type VII collagen expression | [51,77,97,98] | |
| Adipose tissue | Human Osteochondral Explants | 2020 | in vitro and ex vivo models, including human osteochondral explants | bone and cartilage regeneration, as well as in attenuating osteoarthritis progression | [99,100,101,102,103] | |
| Bone marrow | A Tolerance Clinical Study on Aerosol Inhalation | 2020 | preclinical models | regenerative and anti-inflammatory properties | [77,104,105] | |
| Adipose Tissue | Severe Novel Coronavirus Pneumonia | 2020 | porcine model | reducing virus entry and lung inflammation, anti-inflammatory, immunomodulatory, and regenerative properties | [51,106,107,108] | |
| Adipose Tissue | ARS-CoV-2 Associated PneumoniaSARS-Cov2 pneumonia | 2020 | SARS-CoV-2 pneumonia and ARDS models | reducing lung inflammation, promoting epithelial and endothelial recovery, and enhancing alveolar fluid clearance | [109,110,111] | |
| Human umbilical cord | Dry Eye in Patients With cGVHD | 2020 | animal models | promoting corneal epithelial wound healing by modulating inflammation, promoting angiogenesis, and stimulating stem/progenitor cell proliferation | [112] | |
| Adipose tissue | Periodontitis | 2020 | pre-clinical animal models | alleviating oxidative stress, inhibiting inflammation, and promoting tissue regeneration | [113,114,115,116] | |
| Wharton’s jelly | Chronic Ulcer Wounds | 2020 | preclinical models | accelerating wound healing | [77,117] | |
| Bone marrow | Multiple Organ Dysfunction Syndrome (MODS) After Surgical Repair of Acute Type A Aortic Dissection | 2020 | ischemia-reperfusion injuries | improving organ function (liver, lung, coagulation) and reducing MODS severity | [92] | |
| Adipose tissue | Pulmonary Infection | 2020 | silica | mouse model of silica-induced lung inflammation and fibrosis | reducing collagen fiber content, granuloma size, and the number of macrophages and decreasing the expression levels of pro-inflammatory cytokines IL-1β and TGF-β in the lungs. | [118] |
| Adipose tissue | Alzheimer’s Disease (AD) | 2020 | in vitro and in vivo models of AD | ameliorating AD pathology and neuronal apoptosis | [104,119,120] |
| Target Therapy | MSC Origin | Cargo Type | Cargo | Outcome | Reference |
|---|---|---|---|---|---|
| Breast cancer | Bone marrow | miRNA | miR-100 | Suppressed angiogenesis | [121] |
| Bone marrow | miR-23b | Promoted dormancy | [122] | ||
| Bone marrow | miR-16 | Suppressed angiogenesis | [123] | ||
| Glioma | Bone marrow | miR-124/miR-145 | Decreased migration and self-renewal | [124] | |
| Bone marrow | miR-146b | Inhibited tumor growth | [125] | ||
| Bone marrow | miR-124a | Reduced viability | [121] | ||
| Bone marrow | miR-133b | Inhibited proliferation, invasion, and migration | [126] | ||
| Osteosarcoma | Bone marrow | miR-143 | Suppressed migration | [127] | |
| Hepatocellular carcinoma | Adipose | miR-122 | Growth inhibition | [128] | |
| Prostate cancer | Adipose | miR-145 | Suppressed cancer progression | [129] | |
| Multiple myeloma | Bone marrow | miR-15a | Growth inhibition | [130] | |
| Pancreatic cancer | Bone marrow | miR-1231 | Inhibited cancer activity | [131] | |
| Bone marrow | siRNA | siKrasG12D-1 | Induced apoptosis | [132] | |
| Hepatocellular carcinoma | Bone marrow | siGRP78 | Growth inhibition | [133] |
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, W.; Huang, X. Stem cell-based drug delivery strategy for skin regeneration and wound healing: Potential clinical applications. Inflamm. Regen. 2023, 43, 33. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lu, K.J.; Yu, C.H.; Huang, Q.L.; Du, Y.Z. Nano-drug delivery systems in wound treatment and skin regeneration. J. Nanobiotechnol. 2019, 17, 82. [Google Scholar] [CrossRef] [PubMed]
- Garima; Sharma, D.; Kumar, A.; Mostafavi, E. Extracellular vesicle-based biovectors in chronic wound healing: Biogenesis and delivery approaches. Mol. Ther. Nucleic Acids 2023, 32, 822–840. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, M.; Liang, F.; Li, J. Recent strategies for enhancing the therapeutic efficacy of stem cells in wound healing. Stem Cell Res. Ther. 2021, 12, 588. [Google Scholar] [CrossRef] [PubMed]
- Zeng, N.; Chen, H.; Wu, Y.; Liu, Z. Adipose Stem Cell-Based Treatments for Wound Healing. Front. Cell Dev. Biol. 2022, 9, 821652. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Allabun, S.; Ojo, S.; Alqahtani, M.S.; Shukla, P.K.; Abbas, M.; Wechtaisong, C.; Almohiy, H.M. Enhanced Drug Delivery System Using Mesenchymal Stem Cells and Membrane-Coated Nanoparticles. Molecules 2023, 28, 2130. [Google Scholar] [CrossRef] [PubMed]
- Tashima, T. Mesenchymal Stem Cell (MSC)-Based Drug Delivery into the Brain across the Blood-Brain Barrier. Pharmaceutics 2024, 16, 289. [Google Scholar] [CrossRef] [PubMed]
- Litvinova, L.S.; Shupletsova, V.V.; Khaziakhmatova, O.G.; Daminova, A.G.; Kudryavtseva, V.L.; Yurova, K.A.; Malashchenko, V.V.; Todosenko, N.M.; Popova, V.; Litvinov, R.I.; et al. Human Mesenchymal Stem Cells as a Carrier for a Cell-Mediated Drug Delivery. Front. Bioeng. Biotechnol. 2022, 10, 796111. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.E.; Ayoub, N.; Agrawal, D.K. Mesenchymal stem cells and cutaneous wound healing: Novel methods to increase cell delivery and therapeutic efficacy. Stem Cell Res. Ther. 2016, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Samadi, P.; Saki, S.; Manoochehri, H.; Sheykhhasan, M. Therapeutic Applications of Mesenchymal Stem Cells: A Comprehensive Review. Curr. Stem Cell Res. Ther. 2021, 16, 323–353. [Google Scholar] [CrossRef] [PubMed]
- Jovic, D.; Yu, Y.; Wang, D.; Wang, K.; Li, H.; Xu, F.; Liu, C.; Liu, J.; Luo, Y. A Brief Overview of Global Trends in MSC-Based Cell Therapy. Stem Cell Rev. Rep. 2022, 18, 1525–1545. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, F.; Zhou, Y.; Jin, B.; Sun, Q.; Guo, S. Immunosuppressive Property of MSCs Mediated by Cell Surface Receptors. Front. Immunol. 2020, 11, 1076. [Google Scholar] [CrossRef] [PubMed]
- Tolstova, T.; Dotsenko, E.; Kozhin, P.; Novikova, S.; Zgoda, V.; Rusanov, A.; Luzgina, N. The effect of TLR3 priming conditions on MSC immunosuppressive properties. Stem Cell Res. Ther. 2023, 14, 344. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Jones, T.W.; Dutta, S.; Zhu, Y.; Wang, X.; Narayanan, S.P.; Fagan, S.C.; Zhang, D. Overview and Update on Methods for Cargo Loading into Extracellular Vesicles. Processes 2021, 9, 356. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhya, A.; Tsiapalis, D.; McNamee, N.; Talbot, B.; O’Driscoll, L. Doxorubicin Loading into Milk and Mesenchymal Stem Cells’ Extracellular Vesicles as Drug Delivery Vehicles. Pharmaceutics 2023, 15, 718. [Google Scholar] [CrossRef] [PubMed]
- Damasceno, P.K.F.; de Santana, T.A.; Santos, G.C.; Orge, I.D.; Silva, D.N.; Albuquerque, J.F.; Golinelli, G.; Grisendi, G.; Pinelli, M.; Ribeiro Dos Santos, R.; et al. Genetic Engineering as a Strategy to Improve the Therapeutic Efficacy of Mesenchymal Stem/Stromal Cells in Regenerative Medicine. Front. Cell Dev. Biol. 2020, 8, 737. [Google Scholar] [CrossRef] [PubMed]
- Pawitan, J.A.; Bui, T.A.; Mubarok, W.; Antarianto, R.D.; Nurhayati, R.W.; Dilogo, I.H.; Oceandy, D. Enhancement of the Therapeutic Capacity of Mesenchymal Stem Cells by Genetic Modification: A Systematic Review. Front. Cell Dev. Biol. 2020, 8, 587776. [Google Scholar] [CrossRef] [PubMed]
- Ocansey, D.K.W.; Pei, B.; Yan, Y.; Qian, H.; Zhang, X.; Xu, W.; Mao, F. Improved therapeutics of modified mesenchymal stem cells: An update. J. Transl. Med. 2020, 18, 42. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Liu, Y.; Liu, H.; Li, Y. Genetically modified mesenchymal stem cell therapy for acute respiratory distress syndrome. Stem Cell Res. Ther. 2019, 10, 386. [Google Scholar] [CrossRef] [PubMed]
- Mehrabani, M.; Mohammadyar, S.; Rajizadeh, M.A.; Bejeshk, M.A.; Ahmadi, B.; Nematollahi, M.H.; Mirtajaddini Goki, M.; Bahrampour Juybari, K.; Amirkhosravi, A. Boosting therapeutic efficacy of mesenchymal stem cells in pulmonary fibrosis: The role of genetic modification and preconditioning strategies. Iran. J. Basic Med. Sci. 2023, 26, 1001–1015. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yang, J.; Fang, J.; Zhou, Y.; Candi, E.; Wang, J.; Hua, D.; Shao, C.; Shi, Y. The secretion profile of mesenchymal stem cells and potential applications in treating human diseases. Signal Transduct. Target. Ther. 2022, 7, 92. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Scholtemeijer, M.; Shah, K. Mesenchymal Stem Cell Immunomodulation: Mechanisms and Therapeutic Potential. Trends Pharmacol. Sci. 2020, 41, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Shi, Y. Mesenchymal stem/stromal cells (MSCs): Origin, immune regulation, and clinical applications. Cell. Mol. Immunol. 2023, 20, 555–557. [Google Scholar] [CrossRef] [PubMed]
- Fleifel, D.; Cook, J.G. G1 Dynamics at the Crossroads of Pluripotency and Cancer. Cancers 2023, 15, 4559. [Google Scholar] [CrossRef] [PubMed]
- de Souza Dobuchak, D.; Stricker, P.E.F.; de Oliveira, N.B.; Mogharbel, B.F.; da Rosa, N.N.; Dziedzic, D.S.M.; Irioda, A.C.; Athayde Teixeira de Carvalho, K. The Neural Multilineage Differentiation Capacity of Human Neural Precursors from the Umbilical Cord-Ready to Bench for Clinical Trials. Membranes 2022, 12, 873. [Google Scholar] [CrossRef] [PubMed]
- Labedz-Maslowska, A.; Bryniarska, N.; Kubiak, A.; Kaczmarzyk, T.; Sekula-Stryjewska, M.; Noga, S.; Boruczkowski, D.; Madeja, Z.; Zuba-Surma, E. Multilineage Differentiation Potential of Human Dental Pulp Stem Cells-Impact of 3D and Hypoxic Environment on Osteogenesis In Vitro. Int. J. Mol. Sci. 2020, 21, 6172. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. npj Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Lavrentieva, A.; Hoffmann, A.; Lee-Thedieck, C. Limited Potential or Unfavorable Manipulations? Strategies Toward Efficient Mesenchymal Stem/Stromal Cell Applications. Front. Cell Dev. Biol. 2020, 8, 316. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Suryaprakash, S.; Lao, Y.H.; Leong, K.W. Engineering mesenchymal stem cells for regenerative medicine and drug delivery. Methods 2015, 84, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Vasanthan, J.; Gurusamy, N.; Rajasingh, S.; Sigamani, V.; Kirankumar, S.; Thomas, E.L.; Rajasingh, J. Role of Human Mesenchymal Stem Cells in Regenerative Therapy. Cells 2020, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Chen, X.; Zhang, S.; Fang, J.; Chen, M.; Xu, Y.; Chen, X. Mesenchymal stem cells as a double-edged sword in tumor growth: Focusing on MSC-derived cytokines. Cell Mol. Biol. Lett. 2021, 26, 3. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhang, T.; Huang, T.; Gao, J. Current advances and challenges of mesenchymal stem cells-based drug delivery system and their improvements. Int. J. Pharm. 2021, 600, 120477. [Google Scholar] [CrossRef] [PubMed]
- Tran, C.; Damaser, M.S. Stem cells as drug delivery methods: Application of stem cell secretome for regeneration. Adv. Drug Deliv. Rev. 2015, 82–83, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Weatherall, E.L.; Avilkina, V.; Cortes-Araya, Y.; Dan-Jumbo, S.; Stenhouse, C.; Donadeu, F.X.; Esteves, C.L. Differentiation Potential of Mesenchymal Stem/Stromal Cells Is Altered by Intrauterine Growth Restriction. Front. Vet. Sci. 2020, 7, 558905. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Khor, J.W.; van Neel, T.L.; Tu, W.C.; Berthier, J.; Thongpang, S.; Berthier, E.; Theberge, A.B. Miniaturizing chemistry and biology using droplets in open systems. Nat. Rev. Chem. 2023, 7, 439–455. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.R.; McKenna, E.D.; Mawrie, D.; Papakis, V.; Alessandrini, F.; Anderson, E.N.; Mayers, R.; Ball, H.E.; Kaspi, E.; Lubinski, K.; et al. Loss of function of the ALS-associated NEK1 kinase disrupts microtubule homeostasis and nuclear import. Sci. Adv. 2023, 9, eadi5548. [Google Scholar] [CrossRef] [PubMed]
- Balukoff, N.C.; Ho, J.J.D.; Theodoridis, P.R.; Wang, M.; Bokros, M.; Llanio, L.M.; Krieger, J.R.; Schatz, J.H.; Lee, S. A translational program that suppresses metabolism to shield the genome. Nat. Commun. 2020, 11, 5755. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, M.; Kushwaha, J.; Pandey, K.P.; Rani, J.; Dhoble, A.S. Application of flow cytometry for rapid, high-throughput, multiparametric analysis of environmental microbiomes. J. Microbiol. Methods 2023, 214, 106841. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.W.; Ryu, S.; Kim, D.S.; Sung, K.W.; Koo, H.H.; Yoo, K.H. Strategies to improve the immunosuppressive properties of human mesenchymal stem cells. Stem Cell Res. Ther. 2015, 6, 179. [Google Scholar] [CrossRef] [PubMed]
- Abudurexiti, M.; Zhao, Y.; Wang, X.; Han, L.; Liu, T.; Wang, C.; Yuan, Z. Bio-Inspired Nanocarriers Derived from Stem Cells and Their Extracellular Vesicles for Targeted Drug Delivery. Pharmaceutics 2023, 15, 2011. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Ou, Q.; Shi, S.; Shao, C. Immunomodulatory properties of mesenchymal stem cells/dental stem cells and their therapeutic applications. Cell. Mol. Immunol. 2023, 20, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Musiał-Wysocka, A.; Kot, M.; Majka, M. The Pros and Cons of Mesenchymal Stem Cell-Based Therapies. Cell Transpl. 2019, 28, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.R.R.; Dahlke, M.H. Immunomodulation by Mesenchymal Stem Cells (MSCs): Mechanisms of Action of Living, Apoptotic, and Dead MSCs. Front. Immunol. 2019, 10, 1191. [Google Scholar] [CrossRef] [PubMed]
- Krueger, T.E.G.; Thorek, D.L.J.; Denmeade, S.R.; Isaacs, J.T.; Brennen, W.N. Concise Review: Mesenchymal Stem Cell-Based Drug Delivery: The Good, the Bad, the Ugly, and the Promise. Stem Cells Transl. Med. 2018, 7, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Babajani, A.; Soltani, P.; Jamshidi, E.; Farjoo, M.H.; Niknejad, H. Recent Advances on Drug-Loaded Mesenchymal Stem Cells with Anti-neoplastic Agents for Targeted Treatment of Cancer. Front. Bioeng. Biotechnol. 2020, 8, 748. [Google Scholar] [CrossRef] [PubMed]
- Takayama, Y.; Kusamori, K.; Nishikawa, M. Mesenchymal stem/stromal cells as next-generation drug delivery vehicles for cancer therapeutics. Expert. Opin. Drug Deliv. 2021, 18, 1627–1642. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Huang, X. Stem cell membrane-camouflaged targeted delivery system in tumor. Mater. Today Bio 2022, 16, 100377. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Li, Y.; Sun, Z.; Han, X.; Chen, Y.; Ge, Y.; Mao, Z.; Wang, W. Cell-derived extracellular vesicles and membranes for tissue repair. J. Nanobiotechnol. 2021, 19, 368. [Google Scholar] [CrossRef] [PubMed]
- Liam-Or, R.; Faruqu, F.N.; Walters, A.; Han, S.; Xu, L.; Wang, J.T.; Oberlaender, J.; Sanchez-Fueyo, A.; Lombardi, G.; Dazzi, F.; et al. Cellular uptake and in vivo distribution of mesenchymal-stem-cell-derived extracellular vesicles are protein corona dependent. Nat. Nanotechnol. 2024; in press. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.C.; Cheng, T.S.; Liao, H.J.; Chuang, M.H.; Chen, H.T.; Chen, C.H.; Zhang, K.L.; Chang, C.H.; Lin, P.C.; Huang, C.F. Mesenchymal Stem Cell Secreted-Extracellular Vesicles are Involved in Chondrocyte Production and Reduce Adipogenesis during Stem Cell Differentiation. Tissue Eng. Regen. Med. 2022, 19, 1295–1310. [Google Scholar] [CrossRef] [PubMed]
- Kou, M.; Huang, L.; Yang, J.; Chiang, Z.; Chen, S.; Liu, J.; Guo, L.; Zhang, X.; Zhou, X.; Xu, X.; et al. Mesenchymal stem cell-derived extracellular vesicles for immunomodulation and regeneration: A next generation therapeutic tool? Cell Death Dis. 2022, 13, 580. [Google Scholar] [CrossRef] [PubMed]
- Keshtkar, S.; Azarpira, N.; Ghahremani, M.H. Mesenchymal stem cell-derived extracellular vesicles: Novel frontiers in regenerative medicine. Stem Cell Res. Ther. 2018, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Guan, Y.; Xie, A.; Yan, Z.; Gao, S.; Li, W.; Rao, L.; Chen, X.; Chen, T. Extracellular vesicles: A rising star for therapeutics and drug delivery. J. Nanobiotechnol. 2023, 21, 231. [Google Scholar] [CrossRef] [PubMed]
- Bui, S.; Dancourt, J.; Lavieu, G. Virus-Free Method to Control and Enhance Extracellular Vesicle Cargo Loading and Delivery. ACS Appl. Bio Mater. 2023, 6, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Kumar, S.; Park, J.; Choi, Y.; Clarissa, E.M.; Cho, Y.K. Tonicity-induced cargo loading into extracellular vesicles. Lab Chip 2024, 24, 2069–2079. [Google Scholar] [CrossRef] [PubMed]
- Karnas, E.; Dudek, P.; Zuba-Surma, E.K. Stem cell-derived extracellular vesicles as new tools in regenerative medicine—Immunomodulatory role and future perspectives. Front. Immunol. 2023, 14, 1120175. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Liu, X.; Shi, Y.; Ocansey, D.K.W.; Hu, Y.; Li, X.; Zhang, C.; Xu, W.; Qian, H. Therapeutic Advances of Stem Cell-Derived Extracellular Vesicles in Regenerative Medicine. Cells 2020, 9, 707. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xin, Y.; Cao, H.; Li, W.; Hua, Y.; Webster, T.J.; Zhang, C.; Tang, W.; Liu, Z. Recent advances in mesenchymal stem cell membrane-coated nanoparticles for enhanced drug delivery. Biomater. Sci. 2021, 9, 1088–1103. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Su, Y.Y.; Jiang, X.C.; Gao, J.Q. Cell membrane-coated nanoparticles: A novel multifunctional biomimetic drug delivery system. Drug Deliv. Transl. Res. 2023, 13, 716–737. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Tang, Q.; Xiao, M.; Tong, X.; Yang, T.; Yin, D.; Lei, L.; Li, S. Cell membrane-coated nanomaterials for cancer therapy. Mater. Today Bio 2023, 20, 100633. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Faria, I.; Yousefiasl, S.; Macário-Soares, A.; Pereira-Silva, M.; Peixoto, D.; Zafar, H.; Raza, F.; Faneca, H.; Veiga, F.; Hamblin, M.R.; et al. Stem cell membrane-coated abiotic nanomaterials for biomedical applications. J. Control. Release 2022, 351, 174–197. [Google Scholar] [CrossRef] [PubMed]
- Salerno, A.; Cesarelli, G.; Pedram, P.; Netti, P.A. Modular Strategies to Build Cell-Free and Cell-Laden Scaffolds towards Bioengineered Tissues and Organs. J. Clin. Med. 2019, 8, 1816. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Zhang, H.; Chen, L.; Zhang, M.; Ma, J.; Zhuang, H.; Huan, Z.; Xiao, Y.; Wu, C. Cell-Laden Scaffolds for Vascular-Innervated Bone Regeneration. Adv. Healthc. Mater. 2023, 12, e2201923. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yi, P.; Liu, Z.; Zhang, W.; Mei, L.; Feng, C.; Tu, C.; Li, Z. Stem Cell-Laden Hydrogel-Based 3D Bioprinting for Bone and Cartilage Tissue Engineering. Front. Bioeng. Biotechnol. 2022, 10, 865770. [Google Scholar] [CrossRef] [PubMed]
- Hong, I.S. Enhancing Stem Cell-Based Therapeutic Potential by Combining Various Bioengineering Technologies. Front. Cell Dev. Biol. 2022, 10, 901661. [Google Scholar] [CrossRef] [PubMed]
- Ni, T.; Liu, M.; Zhang, Y.; Cao, Y.; Pei, R. 3D Bioprinting of Bone Marrow Mesenchymal Stem Cell-Laden Silk Fibroin Double Network Scaffolds for Cartilage Tissue Repair. Bioconjug. Chem. 2020, 31, 1938–1947. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Ye, J.; Yuan, F.Z.; Zhang, J.Y.; Chen, Y.R.; Fan, B.S.; Jiang, D.; Jiang, W.B.; Wang, X.; Yu, J.K. Advances of Stem Cell-Laden Hydrogels with Biomimetic Microenvironment for Osteochondral Repair. Front. Bioeng. Biotechnol. 2020, 8, 247. [Google Scholar] [CrossRef] [PubMed]
- De Pieri, A.; Rochev, Y.; Zeugolis, D.I. Scaffold-free cell-based tissue engineering therapies: Advances, shortfalls and forecast. npj Regen. Med. 2021, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.I.; Sakaguchi, K.; Yoshida, A.; Takahashi, H.; Haraguchi, Y.; Shimizu, T. Production of scaffold-free cell-based meat using cell sheet technology. npj Sci. Food 2022, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, Q.; Guo, Z.; Li, Z. Constructing a cell microenvironment with biomaterial scaffolds for stem cell therapy. Stem Cell Res. Ther. 2021, 12, 583. [Google Scholar] [CrossRef] [PubMed]
- Alghuwainem, A.; Alshareeda, A.T.; Alsowayan, B. Scaffold-Free 3-D Cell Sheet Technique Bridges the Gap between 2-D Cell Culture and Animal Models. Int. J. Mol. Sci. 2019, 20, 4926. [Google Scholar] [CrossRef] [PubMed]
- Mito, K.; Lachnish, J.; Le, W.; Chan, C.; Chang, Y.L.; Yao, J. Scaffold-Free Bone Marrow-Derived Mesenchymal Stem Cell Sheets Enhance Bone Formation in a Weight-Bearing Rat Critical Bone Defect Model. Tissue Eng. Part A 2024, 30, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Drewry, M.D.; Dailey, M.T.; Rothermund, K.; Backman, C.; Dahl, K.N.; Syed-Picard, F.N. Promoting and Orienting Axon Extension Using Scaffold-Free Dental Pulp Stem Cell Sheets. ACS Biomater. Sci. Eng. 2022, 8, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Herberg, S.; Varghai, D.; Alt, D.S.; Dang, P.N.; Park, H.; Cheng, Y.; Shin, J.Y.; Dikina, A.D.; Boerckel, J.D.; Rolle, M.W.; et al. Scaffold-free human mesenchymal stem cell construct geometry regulates long bone regeneration. Commun. Biol. 2021, 4, 89. [Google Scholar] [CrossRef] [PubMed]
- Kondo, M.; Kameishi, S.; Grainger, D.W.; Okano, T. Novel therapies using cell sheets engineered from allogeneic mesenchymal stem/stromal cells. Emerg. Top. Life Sci. 2020, 4, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Narita, T.; Shintani, Y.; Ikebe, C.; Kaneko, M.; Campbell, N.G.; Coppen, S.R.; Uppal, R.; Sawa, Y.; Yashiro, K.; Suzuki, K. The use of scaffold-free cell sheet technique to refine mesenchymal stromal cell-based therapy for heart failure. Mol. Ther. 2013, 21, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Massa, M.; Croce, S.; Campanelli, R.; Abbà, C.; Lenta, E.; Valsecchi, C.; Avanzini, M.A. Clinical Applications of Mesenchymal Stem/Stromal Cell Derived Extracellular Vesicles: Therapeutic Potential of an Acellular Product. Diagnostics 2020, 10, 999. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Porada, G.; Atala, A.J.; Porada, C.D. Therapeutic Mesenchymal Stromal Cells for Immunotherapy and for Gene and Drug Delivery. Mol. Ther. Methods Clin. Dev. 2020, 16, 204–224. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.H.; Zhou, Y.; Tabata, Y.; Gao, J.Q. Mesenchymal stem cell-based drug delivery strategy: From cells to biomimetic. J. Control. Release 2019, 294, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.J.; Rim, Y.A.; Nam, Y.; Ju, J.H. Recent Developments in Clinical Applications of Mesenchymal Stem Cells in the Treatment of Rheumatoid Arthritis and Osteoarthritis. Front. Immunol. 2021, 12, 631291. [Google Scholar] [CrossRef] [PubMed]
- Margiana, R.; Markov, A.; Zekiy, A.O.; Hamza, M.U.; Al-Dabbagh, K.A.; Al-Zubaidi, S.H.; Hameed, N.M.; Ahmad, I.; Sivaraman, R.; Kzar, H.H.; et al. Clinical application of mesenchymal stem cell in regenerative medicine: A narrative review. Stem Cell Res. Ther. 2022, 13, 366. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Yuan, Z.; Weng, J.; Pei, D.; Du, X.; He, C.; Lai, P. Challenges and advances in clinical applications of mesenchymal stromal cells. J. Hematol. Oncol. 2021, 14, 24. [Google Scholar] [CrossRef] [PubMed]
- Varkouhi, A.K.; Monteiro, A.P.T.; Tsoporis, J.N.; Mei, S.H.J.; Stewart, D.J.; Dos Santos, C.C. Genetically Modified Mesenchymal Stromal/Stem Cells: Application in Critical Illness. Stem Cell Rev. Rep. 2020, 16, 812–827. [Google Scholar] [CrossRef] [PubMed]
- Hmadcha, A.; Martin-Montalvo, A.; Gauthier, B.R.; Soria, B.; Capilla-Gonzalez, V. Therapeutic Potential of Mesenchymal Stem Cells for Cancer Therapy. Front. Bioeng. Biotechnol. 2020, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.; Arthaud-Day, M.L.; Weiss, M.L. Therapeutic Use of Mesenchymal Stromal Cells: The Need for Inclusive Characterization Guidelines to Accommodate All Tissue Sources and Species. Front. Cell Dev. Biol. 2021, 9, 632717. [Google Scholar] [CrossRef] [PubMed]
- Soltani, S.; Mansouri, K.; Emami Aleagha, M.S.; Moasefi, N.; Yavari, N.; Shakouri, S.K.; Notararigo, S.; Shojaeian, A.; Pociot, F.; Yarani, R. Extracellular Vesicle Therapy for Type 1 Diabetes. Front. Immunol. 2022, 13, 865782. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Sun, Y.; Wu, F.; Xu, W.; Qian, H. Mesenchymal Stem Cell-Derived Extracellular Vesicles: A Potential Therapy for Diabetes Mellitus and Diabetic Complications. Pharmaceutics 2022, 14, 2208. [Google Scholar] [CrossRef] [PubMed]
- Terriaca, S.; Fiorelli, E.; Scioli, M.G.; Fabbri, G.; Storti, G.; Cervelli, V.; Orlandi, A. Endothelial Progenitor Cell-Derived Extracellular Vesicles: Potential Therapeutic Application in Tissue Repair and Regeneration. Int. J. Mol. Sci. 2021, 22, 6375. [Google Scholar] [CrossRef] [PubMed]
- Sadanandan, N.; Lee, J.Y.; Garbuzova-Davis, S. Extracellular vesicle-based therapy for amyotrophic lateral sclerosis. Brain Circ. 2021, 7, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, J.; Hu, J.; Fu, B.; Mao, Z.; Zhang, H.; Cai, G.; Chen, X.; Sun, X. Extracellular vesicles for acute kidney injury in preclinical rodent models: A meta-analysis. Stem Cell Res. Ther. 2020, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Li, X.R.; Zhang, X.M. Mesenchymal stem cell-derived extracellular vesicles as a new therapeutic strategy for ocular diseases. World J. Stem Cells 2020, 12, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Lotfy, A.; AboQuella, N.M.; Wang, H. Mesenchymal stromal/stem cell (MSC)-derived exosomes in clinical trials. Stem Cell Res. Ther. 2023, 14, 66. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Ju, R.; Wang, Y. Mesenchymal Stem Cell-Derived Extracellular Vesicles for the Treatment of Bronchopulmonary Dysplasia. Front. Pediatr. 2022, 10, 852034. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Bellio, M.A.; Benny, M.; Kulandavelu, S.; Chen, P.; Janjindamai, C.; Han, C.; Chang, L.; Sterling, S.; Williams, K.; et al. Mesenchymal Stem Cell-derived Extracellular Vesicles Prevent Experimental Bronchopulmonary Dysplasia Complicated by Pulmonary Hypertension. Stem Cells Transl. Med. 2022, 11, 828–840. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Mulder, C.; Riddle, S.; Song, R.; Yue, D. Mesenchymal stromal/stem cells and bronchopulmonary dysplasia. Front. Cell Dev. Biol. 2023, 11, 1247339. [Google Scholar] [CrossRef] [PubMed]
- Tieu, A.; Hu, K.; Gnyra, C.; Montroy, J.; Fergusson, D.A.; Allan, D.S.; Stewart, D.J.; Thébaud, B.; Lalu, M.M. Mesenchymal stromal cell extracellular vesicles as therapy for acute and chronic respiratory diseases: A meta-analysis. J. Extracell. Vesicles 2021, 10, e12141. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.; Zhang, B.; Wu, C.; Yu, F.; Han, B.; Li, B.; Li, L. Therapeutic roles of mesenchymal stem cell-derived extracellular vesicles in cancer. J. Hematol. Oncol. 2021, 14, 136. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liao, Z.F.; Mo, M.H.; Xiong, X.D. Mesenchymal Stromal Cell-Derived Extracellular Vesicles for Vasculopathies and Angiogenesis: Therapeutic Applications and Optimization. Biomolecules 2023, 13, 1109. [Google Scholar] [CrossRef] [PubMed]
- Gorgun, C.; Palamà, M.E.F.; Reverberi, D.; Gagliani, M.C.; Cortese, K.; Tasso, R.; Gentili, C. Role of extracellular vesicles from adipose tissue- and bone marrow-mesenchymal stromal cells in endothelial proliferation and chondrogenesis. Stem Cells Transl. Med. 2021, 10, 1680–1695. [Google Scholar] [CrossRef] [PubMed]
- Giannasi, C.; Mangiavini, L.; Niada, S.; Colombo, A.; Della Morte, E.; Vismara, V.; Ambrosanio, A.; Savadori, P.; Casati, S.; Peretti, G.M.; et al. Human Osteochondral Explants as an Ex Vivo Model of Osteoarthritis for the Assessment of a Novel Class of Orthobiologics. Pharmaceutics 2022, 14, 1231. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.T.; Hosseini-Beheshti, E.; Afrose, D.; Ding, X.; Xia, B.; Grau, G.E.; Little, C.B.; McClements, L.; Li, J.J. Extracellular Vesicles from Mesenchymal Stromal Cells for the Treatment of Inflammation-Related Conditions. Int. J. Mol. Sci. 2021, 22, 3023. [Google Scholar] [CrossRef] [PubMed]
- Zhi, Z.; Sun, Q.; Tang, W. Research advances and challenges in tissue-derived extracellular vesicles. Front. Mol. Biosci. 2022, 9, 1036746. [Google Scholar] [CrossRef] [PubMed]
- Rosochowicz, M.A.; Lach, M.S.; Richter, M.; Suchorska, W.M.; Trzeciak, T. Conditioned Medium—Is it an Undervalued Lab Waste with the Potential for Osteoarthritis Management? Stem Cell Rev. Rep. 2023, 19, 1185–1213. [Google Scholar] [CrossRef] [PubMed]
- Gowen, A.; Shahjin, F.; Chand, S.; Odegaard, K.E.; Yelamanchili, S.V. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Challenges in Clinical Applications. Front. Cell Dev. Biol. 2020, 8, 149. [Google Scholar] [CrossRef] [PubMed]
- Wiklander, O.P.B.; Brennan, M.Á.; Lötvall, J.; Breakefield, X.O.; El Andaloussi, S. Advances in therapeutic applications of extracellular vesicles. Sci. Transl. Med. 2019, 11, eaav8521. [Google Scholar] [CrossRef] [PubMed]
- Rogers, C.J.; Harman, R.J.; Bunnell, B.A.; Schreiber, M.A.; Xiang, C.; Wang, F.S.; Santidrian, A.F.; Minev, B.R. Rationale for the clinical use of adipose-derived mesenchymal stem cells for COVID-19 patients. J. Transl. Med. 2020, 18, 203. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, X.; Yang, L. Mesenchymal stem cells and their derived small extracellular vesicles for COVID-19 treatment. Stem Cell Res. Ther. 2022, 13, 410. [Google Scholar] [CrossRef] [PubMed]
- Akbari, A.; Rezaie, J. Potential therapeutic application of mesenchymal stem cell-derived exosomes in SARS-CoV-2 pneumonia. Stem Cell Res. Ther. 2020, 11, 356. [Google Scholar] [CrossRef] [PubMed]
- Al-Khawaga, S.; Abdelalim, E.M. Potential application of mesenchymal stem cells and their exosomes in lung injury: An emerging therapeutic option for COVID-19 patients. Stem Cell Res. Ther. 2020, 11, 437. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Nova-Lamperti, E.; Labarca, G.; Kulasinghe, A.; Short, K.R.; Carrión, F.; Salomon, C. Genomic communication via circulating extracellular vesicles and long-term health consequences of COVID-19. J. Transl. Med. 2023, 21, 709. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Guijo, F.; García-Arranz, M.; López-Parra, M.; Monedero, P.; Mata-Martínez, C.; Santos, A.; Sagredo, V.; Álvarez-Avello, J.M.; Guerrero, J.E.; Pérez-Calvo, C.; et al. Adipose-derived mesenchymal stromal cells for the treatment of patients with severe SARS-CoV-2 pneumonia requiring mechanical ventilation. A proof of concept study. EClinicalMedicine 2020, 25, 100454. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Lin, S.; Gao, Y. Mesenchymal Stromal Cell-Based Therapy for Dry Eye: Current Status and Future Perspectives. Cell Transpl. 2022, 31, 9636897221133818. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Wang, L.; Zhang, W.; Liu, B.; Wu, Y.; Pang, J.; Ma, C. The role of extracellular vesicles in periodontitis: Pathogenesis, diagnosis, and therapy. Front. Immunol. 2023, 14, 1151322. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Qi, Y.X.; Zhu, C.H.; Li, A.; Pei, D.D. Mesenchymal stem cell-derived extracellular vesicles for treatment of bone loss within periodontitis in pre-clinical animal models: A meta-analysis. BMC Oral Health 2023, 23, 701. [Google Scholar] [CrossRef] [PubMed]
- Anvari, Y.; Afrashteh, A.; Pourkaveh, S.; Salek, S.B.; Al-Numan, L.; Khademnezhad, S. Emerging role of mesenchymal stem cell-derived extracellular vesicles in periodontal regeneration. J. Taibah Univ. Med. Sci. 2024, 19, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhu, S.; Guo, S.; Tian, W. Mechanisms and clinical application potential of mesenchymal stem cells-derived extracellular vesicles in periodontal regeneration. Stem Cell Res. Ther. 2023, 14, 26. [Google Scholar] [CrossRef] [PubMed]
- Bailey, A.J.M.; Li, H.; Kirkham, A.M.; Tieu, A.; Maganti, H.B.; Shorr, R.; Fergusson, D.A.; Lalu, M.M.; Elomazzen, H.; Allan, D.S. MSC-Derived Extracellular Vesicles to Heal Diabetic Wounds: A Systematic Review and Meta-Analysis of Preclinical Animal Studies. Stem Cell Rev. Rep. 2022, 18, 968–979. [Google Scholar] [CrossRef] [PubMed]
- Bandeira, E.; Oliveira, H.; Silva, J.D.; Menna-Barreto, R.F.S.; Takyia, C.M.; Suk, J.S.; Witwer, K.W.; Paulaitis, M.E.; Hanes, J.; Rocco, P.R.M.; et al. Therapeutic effects of adipose-tissue-derived mesenchymal stromal cells and their extracellular vesicles in experimental silicosis. Respir. Res. 2018, 19, 104. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.G.J.; Vasques, J.F.; da Silva-Junior, A.J.; Gubert, F.; Mendez-Otero, R. Mesenchymal stem cell- and extracellular vesicle-based therapies for Alzheimer’s disease: Progress, advantages, and challenges. Neural Regen. Res. 2023, 18, 1645–1651. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.A.; Lu, C.H.; Ke, C.C.; Liu, R.S. Mesenchymal Stem Cell-Derived Extracellular Vesicle-Based Therapy for Alzheimer’s Disease: Progress and Opportunity. Membranes 2021, 11, 796. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.M.; Hossain, A.; Gumin, J.; Momin, E.N.; Shimizu, Y.; Ledbetter, D.; Shahar, T.; Yamashita, S.; Parker Kerrigan, B.; Fueyo, J.; et al. Mesenchymal stem cells as natural biofactories for exosomes carrying miR-124a in the treatment of gliomas. Neuro-Oncology 2018, 20, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Kosaka, N.; Tominaga, N.; Yoshioka, Y.; Takeshita, F.; Takahashi, R.U.; Yoshida, M.; Tsuda, H.; Tamura, K.; Ochiya, T. Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci. Signal. 2014, 7, ra63. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Park, S.R.; Jung, B.K.; Jeon, Y.K.; Lee, Y.S.; Kim, M.K.; Kim, Y.G.; Jang, J.Y.; Kim, C.W. Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PLoS ONE. 2013, 8, e84256. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.K.; Finniss, S.; Cazacu, S.; Bucris, E.; Ziv-Av, A.; Xiang, C.; Bobbitt, K.; Rempel, S.A.; Hasselbach, L.; Mikkelsen, T.; et al. Mesenchymal stem cells deliver synthetic microRNA mimics to glioma cells and glioma stem cells and inhibit their cell migration and self-renewal. Oncotarget 2013, 4, 346–361. [Google Scholar] [CrossRef] [PubMed]
- Katakowski, M.; Buller, B.; Zheng, X.; Lu, Y.; Rogers, T.; Osobamiro, O.; Shu, W.; Jiang, F.; Chopp, M. Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett. 2013, 335, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhao, G.; Zhang, Y.; Jiang, H.; Wang, W.; Zhao, D.; Hong, J.; Yu, H.; Qi, L. Mesenchymal stem cell-derived exosomal microRNA-133b suppresses glioma progression via Wnt/β-catenin signaling pathway by targeting EZH2. Stem Cell Res. Ther. 2019, 10, 381. [Google Scholar] [CrossRef] [PubMed]
- Shimbo, K.; Miyaki, S.; Ishitobi, H.; Kato, Y.; Kubo, T.; Shimose, S.; Ochi, M. Exosome-formed synthetic microRNA-143 is transferred to osteosarcoma cells and inhibits their migration. Biochem. Biophys. Res. Commun. 2014, 445, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Lou, G.; Song, X.; Yang, F.; Wu, S.; Wang, J.; Chen, Z.; Liu, Y. Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J. Hematol. Oncol. 2015, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Takahara, K.; Ii, M.; Inamoto, T.; Nakagawa, T.; Ibuki, N.; Yoshikawa, Y.; Tsujino, T.; Uchimoto, T.; Saito, K.; Takai, T.; et al. microRNA-145 Mediates the Inhibitory Effect of Adipose Tissue-Derived Stromal Cells on Prostate Cancer. Stem Cells Dev. 2016, 25, 1290–1298. [Google Scholar] [CrossRef] [PubMed]
- Roccaro, A.M.; Sacco, A.; Maiso, P.; Azab, A.K.; Tai, Y.T.; Reagan, M.; Azab, F.; Flores, L.M.; Campigotto, F.; Weller, E.; et al. BM mesenchymal stromal cell-derived exosomes facilitate multiple myeloma progression. J. Clin. Investig. 2013, 123, 1542–1555. [Google Scholar] [CrossRef] [PubMed]
- Shang, S.; Wang, J.; Chen, S.; Tian, R.; Zeng, H.; Wang, L.; Xia, M.; Zhu, H.; Zuo, C. Exosomal miRNA-1231 derived from bone marrow mesenchymal stem cells inhibits the activity of pancreatic cancer. Cancer Med. 2019, 8, 7728–7740. [Google Scholar] [CrossRef] [PubMed]
- Mendt, M.; Kamerkar, S.; Sugimoto, H.; McAndrews, K.M.; Wu, C.C.; Gagea, M.; Yang, S.; Blanko, E.V.R.; Peng, Q.; Ma, X.; et al. Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight 2018, 3, e99263. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, C.; Shi, Y.; Zhao, L. Exosomes derived from siRNA against GRP78 modified bone-marrow-derived mesenchymal stem cells suppress Sorafenib resistance in hepatocellular carcinoma. J. Nanobiotechnol. 2018, 16, 103. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuzaka, Y.; Yashiro, R. Current Strategies and Therapeutic Applications of Mesenchymal Stem Cell-Based Drug Delivery. Pharmaceuticals 2024, 17, 707. https://doi.org/10.3390/ph17060707
Matsuzaka Y, Yashiro R. Current Strategies and Therapeutic Applications of Mesenchymal Stem Cell-Based Drug Delivery. Pharmaceuticals. 2024; 17(6):707. https://doi.org/10.3390/ph17060707
Chicago/Turabian StyleMatsuzaka, Yasunari, and Ryu Yashiro. 2024. "Current Strategies and Therapeutic Applications of Mesenchymal Stem Cell-Based Drug Delivery" Pharmaceuticals 17, no. 6: 707. https://doi.org/10.3390/ph17060707





