Abstract
Venous thromboembolism (VTE) represents one of the leading causes of death during pregnancy. The greatest risk for it is the presence of medical or family history of VTE, stillbirth, cesarean section and selected thrombophilia. Appropriate thromboprophylaxis has the potential to decrease the risk of VTE in at-risk pregnant patients by 60–70%. Based on this, the authors reviewed the PubMed, Web of Science and Scopus databases to identify the possibilities of thromboprophylaxis in pregnant patients with a high risk of VTE. Moreover, they summarized its management in specific situations, such as cesarean delivery or neuraxial blockade. Currently, low-molecular-weight heparins (LMWH) are the preferred drugs for anticoagulant thromboprophylaxis in the course of pregnancy and postpartum due to easy administration and a lower rate of adverse events.
1. Introduction
The first well-described case of deep vein thrombosis (DVT) was documented in the Middle Ages, when in 1271, Raoul noticed a unilateral edema of the ankle extending to the leg. During the Renaissance, pregnancy-related DVT was one of the leading causes of DVT at that time. It was presumed that postpartum DVT was developed due to the retention of unconsumed milk in the lower extremities [1].
Currently, the risk of venous thromboembolism (VTE) is higher in women than in men. Along with the use of combined oral contraceptives, pregnancy belongs to the clinical states increasing the risk of thromboembolic complications in women of childbearing age [2].
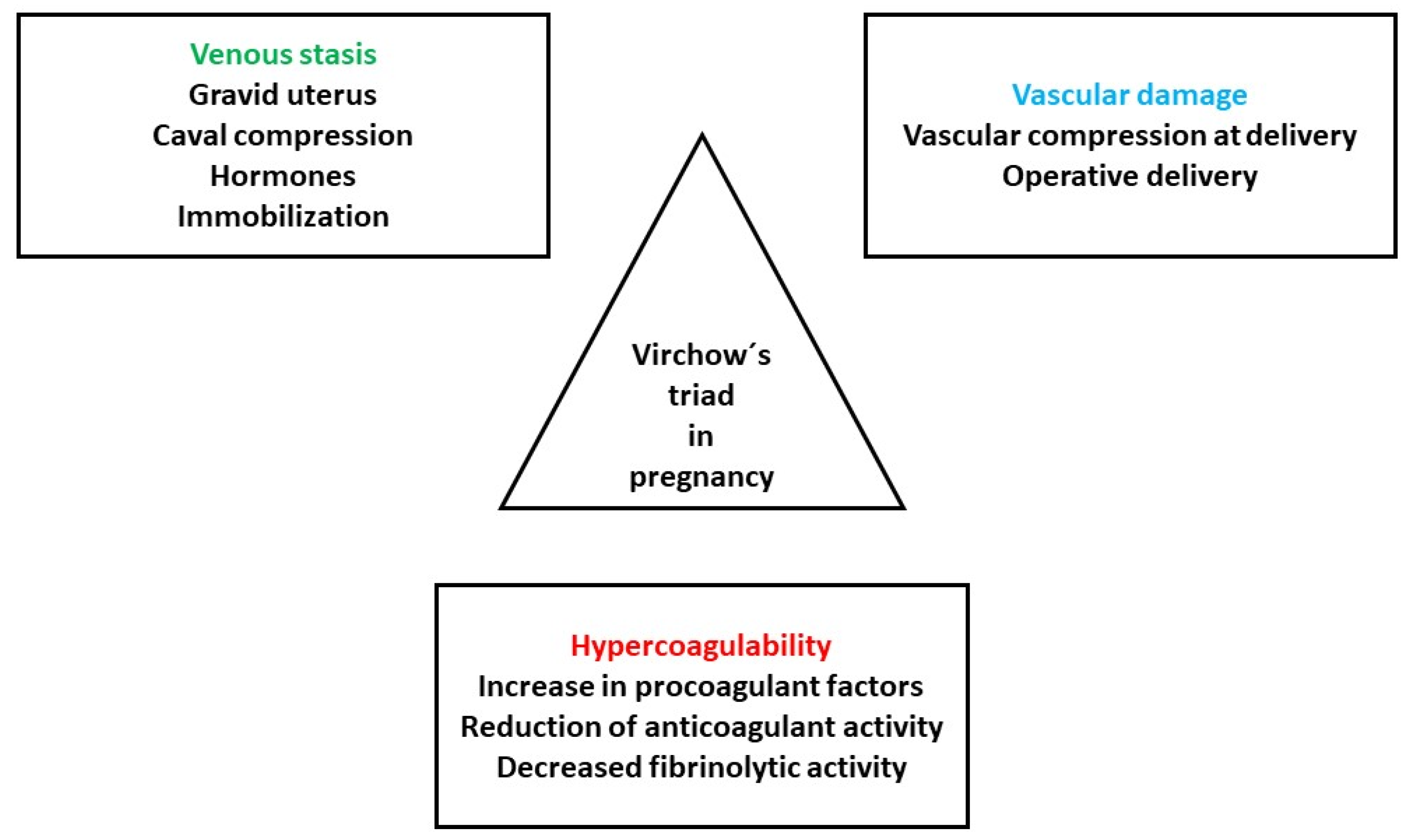
Pregnancy influences all three elements of the Virchow’s triad: hypercoagulability, endothelial dysfunction and hemodynamic alterations (Figure 1) [3].
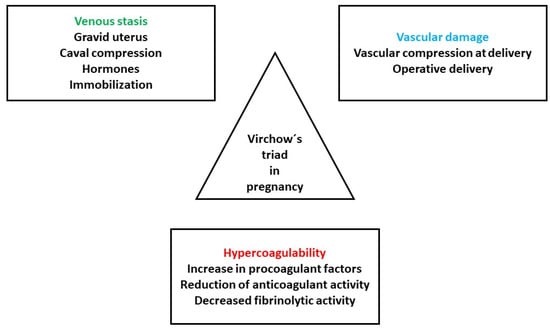
Figure 1.
Pathogenesis of VTE in pregnancy. Adapted from [3].
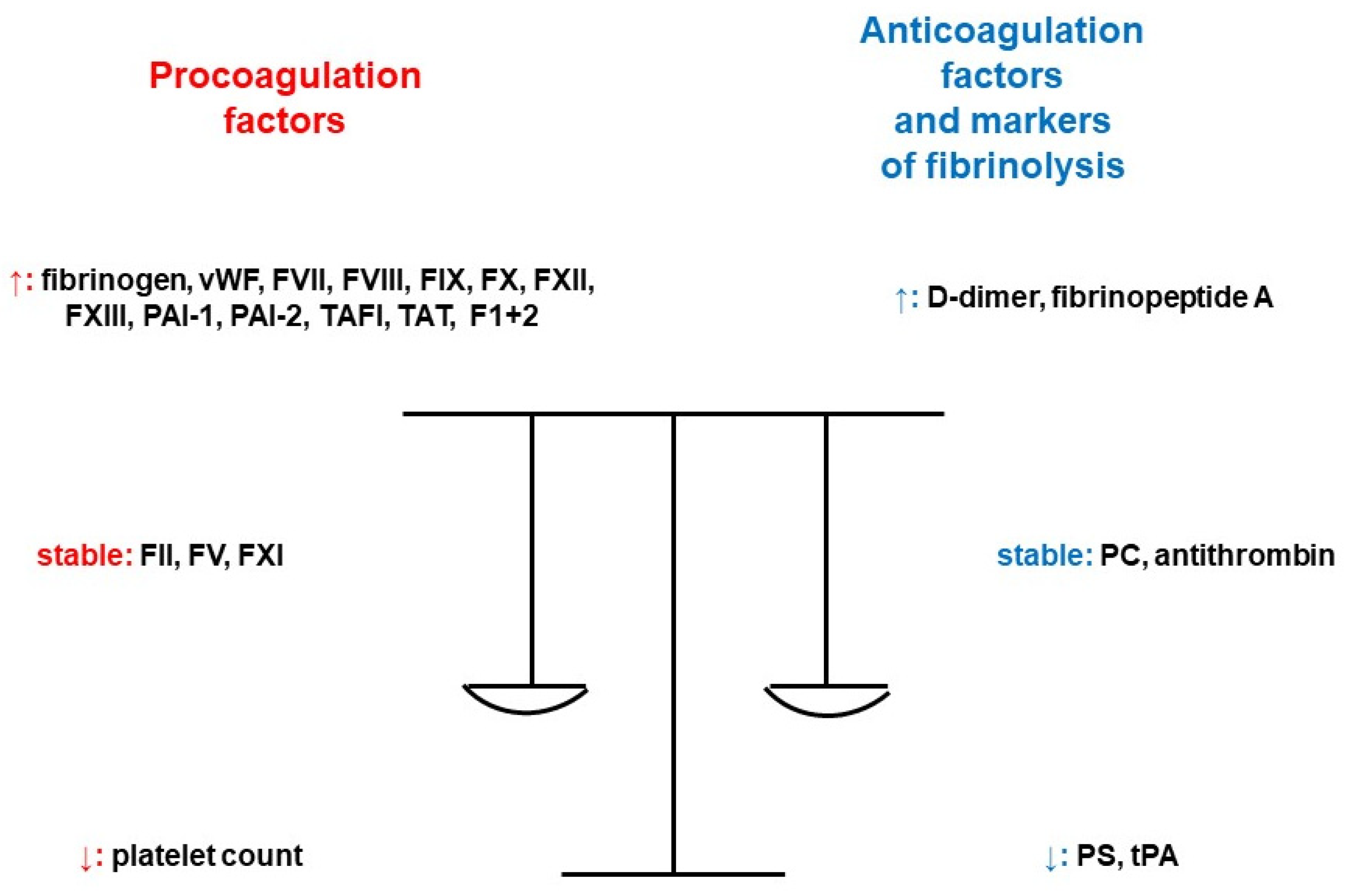
Hypercoagulability during pregnancy is a consequence of increased or decreased activity and quantity of coagulation factors, and it is increased 5–10-fold in comparison to nonpregnant levels [4]. There is an increased activity of fibrinogen, coagulation factors VII (FVII), VIII (FVIII), IX (FIX), X (FX), XII (FXII), XIII (FXIII) and von Willebrand factor (vWF). Approximately 50% more molecules of fibrinogen are produced. Levels of factor II (FII) and V (FV) do not change significantly during pregnancy, and factor XI (FXI) activity decreases by 20–30% of up to 40% of the reference range. As one of the most important natural inhibitors of coagulation, antithrombin is not affected by hormones. It only slightly decreases by 15% in the last weeks of pregnancy. The synthesis of protein C (PC) is different depending on the gestational age, with an increase in the second trimester, a decrease in the third trimester, and another increase during the immediate postpartum period. There is a gradual decrease in protein S (PS) function, continuing for 2 months postpartum and longer in the case of breastfeeding. Regarding the fibrinolytic system, D-dimers increase throughout the pregnancy. The increased formation of thrombin is maximal at term and contributes to the prevention of massive hemorrhage during the delivery. It is associated with a significant increase in the levels of markers of coagulation activation (fragments of prothrombin 1+2 and thrombin–antithrombin complexes). Pregnancy provokes a decrease in tissue plasminogen activator (tPA), an increase in plasminogen activator inhibitor (PAI)-1 activity and the synthesis of PAI-2 by the placenta. Conclusively, there is the development of hypofibrinolysis [5]. The levels of novel coagulation markers plasmin-α2 plasmin inhibitor complex (PIC), thrombin–antithrombin complex (TAT), thrombomodulin (TM) and tissue plasminogen activator/plasminogen activator inhibitor compound (tPAI-C) in pregnant patients increase significantly throughout the pregnancy and gradually return to normal ranges after delivery. Their reference intervals in healthy pregnant women by trimester were established according to Clinical and Laboratory Standards Institute Document C28-A3c, thus contributing to the judgement of prothrombotic risk [6]. Additionally, after the first trimester, platelet aggregation also increases [7]. Figure 2 provides a summary of the influence of pregnancy on the markers of hemostasis [8].
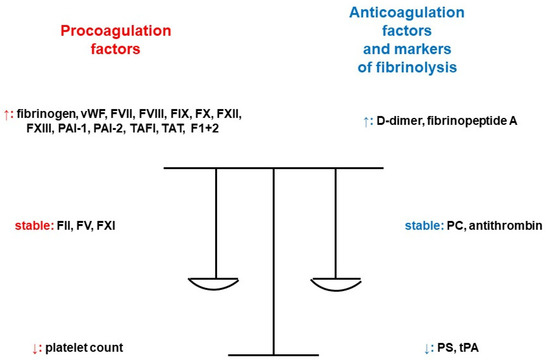
Figure 2.
Pregnancy and its impact on the markers of hemostasis [8]. Legend: F1+2; prothrombin fragment 1+2, FII; coagulation factor II, FV; coagulation factor V, FVII; coagulation factor VII, FVIII; coagulation factor VIII, FIX; coagulation factor IX, FX; coagulation factor X, FXI; coagulation factor XI, FXII; coagulation factor XII, FXIII; coagulation factor XIII, PAI-1; plasminogen activator inhibitor-1, PAI-2; plasminogen activator inhibitor 2, PC; protein C, PS; protein S, TAFI; thrombin-activatable fibrinolysis inhibitor, TAT; thrombin–antithrombin complex, tPA; tissue plasminogen activator, vWF; von Willebrand factor (adapted from [8]).
The endothelium is composed of the cells lining the blood vessels. Endothelial cells are covered by the endothelial glycocalyx (EG), which prevents platelet and leukocyte adhesion, provides antithrombotic activity and also maintains tissue integrity. It is located on the luminal surface of endothelial cells. Injury of the EG is related to clinical conditions like inflammation, thrombosis, preeclampsia or fetal growth restriction (FGR) [9].
Uteroplacental circulation is formed at the beginning of the second trimester. The functional adaptation of uterine arteries and the remodeling of spiral arteries cause a change in the uteroplacental circulation to a high-flow and low-resistance system. This process is supported mainly by estrogens [10].
Described changes of circulation in pregnancy lead to a variety of changes in pharmacokinetics of anticoagulants. This is due to an increase in maternal plasma volume that results in increased distribution volume for water-soluble drugs and reduced peak and steady-state drug concentrations. There is also an increase in renal blood flow, and thus an increased glomerular filtration rate, from the second trimester, leading to quicker clearance of drugs excreted by the kidneys. Last but not least, during pregnancy, we can observe an increase in the free fraction of highly protein-bound agents because of lower concentrations of albumin in pregnancy associated with the placental and fetal metabolic effects [7].
VTE represents a leading cause of death of pregnant and postpartum women. The risk of VTE is higher in the course of pregnancy when compared with the nonpregnant state and peaks in the postpartum period: pooled incidence rates are 1.2 (95% confidence interval (CI): 1.0–1.4) and 4.2 (95% CI: 2.4–7.6) per 1000 persons [11]. The pooled incidence rate for DVT is 1.1 (1.0–1.3) per 1000 patients and 0.3 (0.2–0.4) per 1000 persons for pulmonary embolism (PE) [12]. However, the VTE risk remains increased during the 12 weeks after delivery. The majority of the cases are attributed to DVT, but PE is of greater concern, because it causes 10–15% of all pregnancy-associated maternal deaths in developed countries [13].
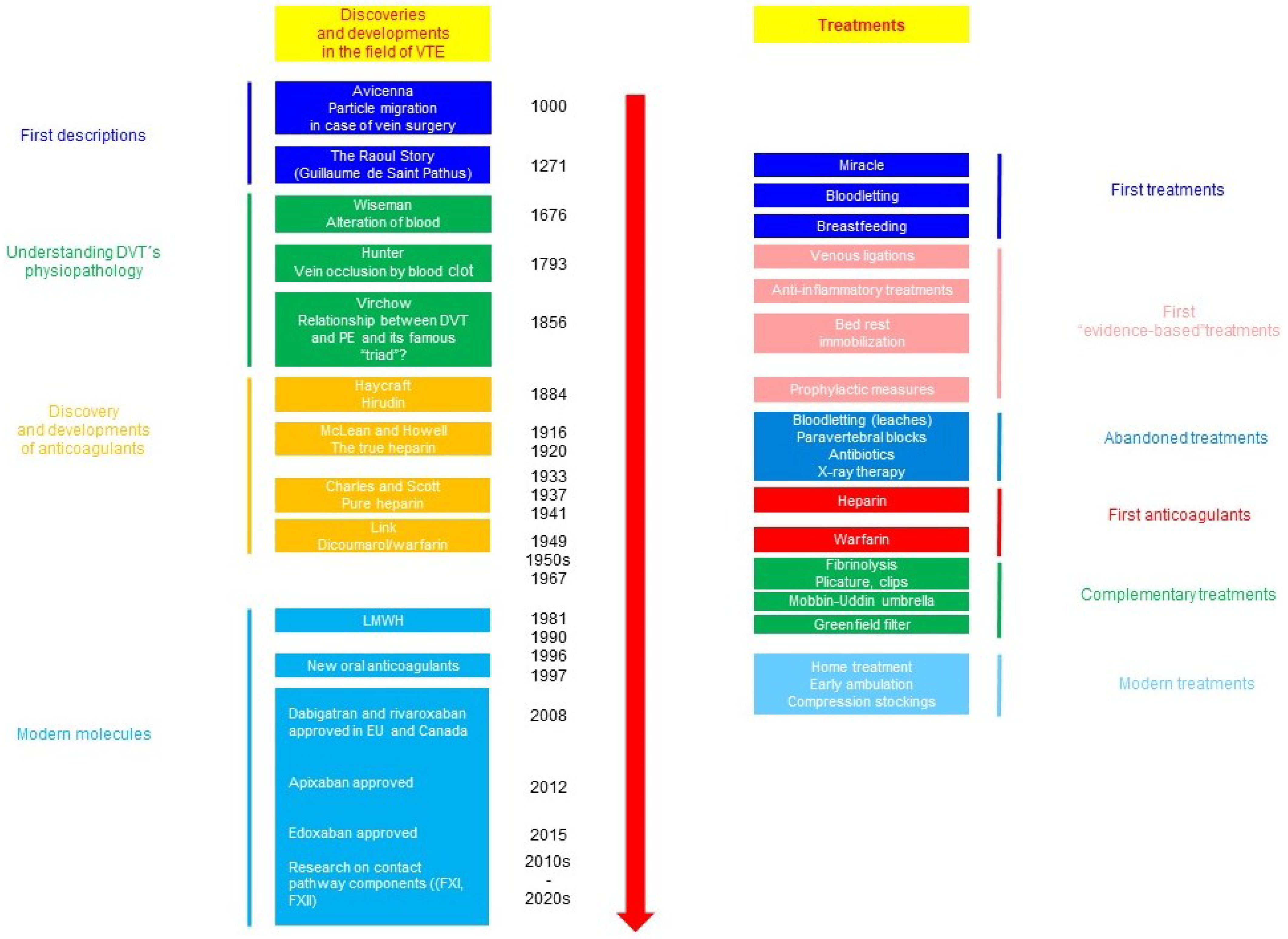
The cornerstone of the prevention of VTE-related maternal death is a mechanic and pharmacologic thromboprophylaxis. The history of the management of VTE is outlined in Figure 3 [1,14].
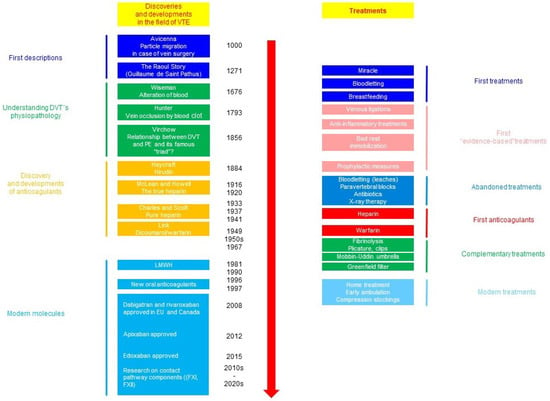
Figure 3.
Outline of the history of advances in the management of thromboembolic complications [1,14].
The use of low-molecular-weight heparin (LMWH) has the potential to reduce the risk of VTE by 60–70% in at-risk pregnant patients [15].
Based on this knowledge, the authors aimed to summarize the current approaches for thromboprophylaxis during at-risk pregnancy and to fill the gap in the existing literature regarding the optimal dose of thromboprophylaxis, the duration of an increased risk of VTE and the interaction of risk factors of thromboembolic complications in pregnancy. We also examined specific aspects of thromboprophylaxis in high-risk pregnancy, such as possibilities of monitoring its effectiveness, or the use of thromboprophylaxis in the setting of cesarean section and neuraxial anesthesia.
2. Methods
This article is written in the form of a non-systematic (narrative) review. We performed a search in the medical literature databases PubMed Central, Web of Science and Scopus. To identify the most appropriate articles from the recent sources that were processed in the form of presented narrative review articles, we used the keywords “pregnancy”, “venous thromboembolism” and “anticoagulant thromboprophylaxis”.
Wherever possible, we aimed to review the information from articles published during the last five years. We included articles found by searching the databases that were written mainly in the English language or translated to English from another original language. We excluded all the studies performed only in a nonpregnant population. Regarding quality assessment, we preferred information obtained in randomized controlled trials and systematic reviews.
3. Which Women Are “At Risk” and Why?
3.1. Previous Thromboembolic Episode
The incidence of recurrent VTE during pregnancy is approximately 7.6% [16,17,18], and this risk is increased more than 3-fold when compared with a nonpregnant state. However, this information was obtained in a retrospective study performed only in 109 patients who had at least one pregnancy following VTE [19]. The risk of antepartum recurrent VTE is increased in patients with a history of two or more previous thromboembolic events, antiphospholipid syndrome or paradoxically long-term anticoagulation. It is disputed that antepartum thromboprophylaxis with even an intermediate dose of LMWH might not be sufficient in this population [17]. Recurrent VTE is a potentially life-threatening clinical condition that increases the probability of the development of a post-thrombotic syndrome [19].
3.2. Family History of Venous Thromboembolism
Several studies have shown that positive family history of VTE along with thrombophilia are strong risk factors for the development of VTE, and especially PE, in women during pregnancy. These factors increase the prothrombotic risk from 3.7-fold to 8.5-fold [20,21,22,23].
Some studies providing this information are retrospective [20], with features of post hoc analyses [22]. Potential confounding with other unmeasured factors could not be considered here [20], and the presence of a control group might be beneficial [22].
On the other hand, the report of Jerjes-Sánchez et al. is based on a multicentric study performed in patients with VTE associated with pregnancy included in the Global Anticoagulant Registry in the FIELD (GARFIELD)-VTE [21]. Additionally, the systematic review of Nanne Croles et al. identified the largest number of studies investigating thrombophilia and pregnancy-associated VTE, with the inclusion of 41,297 pregnancies. It did not take into account changes in the methodology of diagnosis of thrombophilia [23].
3.3. Smoking Prior to or during Pregnancy
Tobacco increases the risk of VTE by several mechanisms—it raises levels of fibrinogen, tissue factor, FII, FV, FVIII, FX, FXIII and homocysteine [24]. Smoking is also a risk factor of pregnancy-associated VTE [25]. The adjusted odds ratio (AOR) for smoking 10–30 cigarettes/day before or during the course of pregnancy as a risk factor of VTE is 2.1 (95% CI 1.3–3.4) [26].
3.4. Obesity
It is suggested that obesity predisposes to venous stasis and alters the coagulation system by impairment of the fibrinolytic activity and an increase in the levels of D-dimers, fibrinogen, FVIII and FIX. Additionally, overweight patients have higher concentrations of C-reactive protein, promoting low-grade systemic inflammation [27]. Women with a BMI of 30–35 kg/m2 (category of high normal BMI) have an adjusted hazard ratio (HR) of 2.35 (95% CI 2.04–2.70), and patients with ≥35 kg/m2 (considered as severely obese) have a HR of 3.47 (95% CI 2.82–4.25). Therefore, there is a significantly increased long-term risk of post-pregnancy VTE [28].
These results were obtained in a study that included a large study population of 1,068,040 patients and near-complete coverage with a long-term prospective follow-up. However, the authors did not consider subsequent pregnancies and changes in BMI during follow-up. A further limitation is the fact that the diagnoses were not verified [28].
3.5. Age
The risk of VTE increases with age. It is correlated with a simultaneous enhancement of coagulation activation, represented by increased levels of coagulation activation peptides and decreased fibrinolytic activity—states aggravated by the existence of prothrombotic comorbidities [29]. The risk of postpartum VTE is increased in women aged over 35 years. On the contrary, age ≥ 40 years (adjusted OR 1.67) is a risk factor of PE in the postpartum period to a lesser extent. Before undergoing IVF, it is recommended to assess the risk factors of thromboembolic complications, including increased age (>40 years) [30].
3.6. Parity with Three or More Pregnancies
Multiparity with a history of three or more pregnancies was evaluated as one of the most important risk factors for VTE not only during hospitalization in pregnancy (OR 3.5, 95% CI 3.0–4.0) [31,32], but also throughout the pregnancy [33].
However, the incidence of VTE might have been higher due to the impossibility of contacting some of the patients [31].
Multiple pregnancies increase the risk of VTE [31,34,35,36,37], with an aOR of 2.7 (95% CI 1.6–4.5) [32]. However, some studies providing this fact were retrospective [35,36] or performed in the form of survey [34].
3.7. Anemia
Iron deficiency is a relatively unknown but also important risk factor for VTE. It induces thrombocytosis, which predisposes to hypercoagulability [38]. The adjusted OR for VTE is 2.6 (95% CI is 2.2–2.9) [26].
3.8. Varicose Veins
Varicose veins are typical for intimal hyperplastic areas and plaque that is infiltrated with leukocytes and mast cells. In pregnancy, type III collagen and elastin are reduced, and elastin fibers are fragmented [39]. The resulting abnormal blood flow and endothelial damage leads to an increased expression of tissue factor, PAI-1 and vascular cell adhesion molecule 1 (VCAM-1). Simultaneously, we can detect the activation of platelets, leukocytes, tissue factor-positive microvesicles and neutrophil extracellular traps (NETs) [40]. In the pathogenesis of varicose veins, further molecules have been involved: FoxC2-Delta-like ligand 4 (Dll4) pathway signaling; lnc-RNA, affecting the proliferation and migration of human saphenous vein smooth muscle cells through annexin A2; hypoxia-inducible factor target genes, such as GLUT1, CA9, vascular endothelial growth factor (VEGF) and BNIP3, which are upregulated; increased matrix metalloproteinase 2, 9 and 13 expression; tissue inhibitors of these matrix metalloproteinases; increased transforming growth factor β levels and dysregulation in the expression of the molecules, such as endothelin 1; laminin, intercellular adhesion molecule 1 (ICAM-1); endothelial cell-leukocyte adhesion molecule 1 (ELAM-1); angiotensin-converting enzyme, L-selectin; and proapoptotic regulators Bax and poly-ADP-ribose polymerase or cyclic guanosine monophosphate [41].
Varicose veins are a frequent finding in women with two or more pregnancies. Enhanced engorgement and impaired blood flow through them during pregnancy predispose the patient to thrombophlebitis [42]. Gross varicose veins were identified as a significant and often underestimated risk factor of VTE, with aOR of 2.69 (95% CI 1.53–4.7) [20,32,34,43,44]. A strength of the study by Sultan et al. [44] is the large database of patients, containing 376 154 pregnancies between the years of 1995 and 2009. However, it lacked validation studies of obstetric complications, and there was a scarcity of data estimating their prevalence in the global population. Moreover, data from other mentioned studies were retrospective [42] or obtained in a survey format [34].
3.9. Preeclampsia
Preeclampsia occurs in up to 8% of pregnancies. The risk of VTE is attributed to the increased expression of procoagulant factors, attenuation of endogenous natural inhibitors of coagulation, endothelial dysfunction, and enhanced platelet activity, and it is most prominent in the postpartum period [45]. As further pathogenetic characteristics, the function of cell-free DNA and NETs was recently proposed [46]. This fact is useful because of the involvement of agents such as vitamin D, nuclear factor kappa B inhibitors and acetylsalicylic acid in the treatment of NETosis in preeclampsia [47]. Attention should also be paid to the fact that preeclampsia and arterial hypertension increase the risk of VTE in the course of pregnancy and the postpartum period and even in the 13 years after [48,49]. The aOR for the risk of VTE in these patients is 3.1 (95% CI 1.8–5.3) [32]. The limitations of the study providing this conclusion were the inclusion criteria of patients with VTE during pregnancy who received LMWH, which could have contributed to an underestimation of the incidence of thromboembolic complications; a lack of information about potential death or arterial thrombotic events; and an inconsistent definition of hypertension during pregnancy and preeclampsia [48].
Additionally, the second of these studies did not include pregnancy loss developed before 20 weeks of gestation. Moreover, data on ultrasonography, blood samples and history of the patients were not fully available [49].
Preeclampsia is also considered to be a risk factor for arterial diseases [50].
3.10. Weight Gain
A retrospective study by Wu et al. showed that higher weight gain in the course of pregnancy contributes to increased risk of VTE [51], especially when it is more than 21 kg, with aOR of 1.6 (95% CI 1.1–2.6) [26,32].
3.11. Stillbirth
Stillbirth is another feature associated with an increased risk of VTE [52]. It is one of the strongest predictors of this complication in the postpartum period (incidence rate ratio (IRR) 6.2) [44,52,53,54].
3.12. Preterm Delivery
The delivery of a newborn before term is considered to be an intermediate or even strong risk factor for maternal postpartum VTE [44,55]. The strengths of this study were its population-based design, the high validity of newborn birth weight measurements and the sample size; limitations were the inclusion of hospitalized events, a lack of data about thrombophilia and thromboprophylaxis, the possibility of residual confounding and incomplete data on BMI [55]. Preterm delivery increases the risk of thromboembolic complications by approximately three times (aOR 2.7, 95% CI (2–6.6)) [26].
3.13. Cesarean Section
Cesarean section increases the risk of VTE. There are also further factors, such as prothrombotic effects of surgical delivery lasting more than 30 min or weight gain during pregnancy [56]. When compared with women who had a spontaneous vaginal delivery, cesarean section increases the risk of VTE by four-fold [57], and the aOR is 2.1 (95% CI 1.8–2.4) [32].
3.14. Peripartum Hemorrhage
Postpartum hemorrhage is considered to be significant when the estimated volume of blood loss is more than 1000 mL, and this represents an aOR of 4.1 (95% CI 2.3–7.3) [26,58]. However, the data on immobilization have only been addressed in a limited number of case-control studies up to now [58].
3.15. Postpartum Infection
The incidence of postpartum infection increases with the presence of smoking, employment, obesity, BMI > 30, anemia, diabetes mellitus, age of the woman being more than 35 years, the degree of the perineal wound, the use of equipment for delivery and its duration and the frequency of vaginal examinations. It confers an aOR for VTE of 4.1 (2.9–5.7). There have been a limited number of studies investigating the effectiveness of antibiotic prophylaxis in women following vaginal delivery [32,59]. In the puerperium, infections increase the risk of thrombosis by four-fold [60].
3.16. Transfusion
Red blood cell transfusion represents a relative risk of VTE of 3.9, and the administration of other transfusions or infusions of procoagulant fractions represents a risk of 8.2 [61].
3.17. In Vitro Fertilization
The hyperestrogenism resulting from ovarian stimulation causes an increase in the activity of procoagulant factors, a decrease in the levels of natural anticoagulants and reduced fibrinolytic capacity. It is rare to develop VTE before the use of chorionic gonadotropin (hCG). Only 3% of arterial thrombosis and VTE occurred before the administration of final oocyte maturation trigger [62]. The procedure of in vitro fertilization (IVF) is associated with an aOR of 2.7 (95% CI 2.1–3.6) [32]. Therefore, several prothrombotic risk factors during the IVF cycle in the development of VTE have been identified—aggressive stimulation, increased estradiol levels, the use of hCG and exogenous estrogen for frozen embryo transfer, ovarian hyperstimulation syndrome (OHSS) and multiple pregnancies [62].
3.18. Ovarian Hyperstimulation Syndrome
OHSS is the most severe complication of IVF. Young age, polycystic ovarian syndrome (PCOS), low BMI, a rapid increase in serum estradiol, a higher number of growing follicles at the time of triggering and many oocytes retrieved are its risk factors. OHSS consists of several components, such as ovarian enlargement, fluid shift into the third space and its resulting hemoconcentration, hypovolemia, renal failure, serosal effusions and hypercoagulable state [63]. The risk of VTE in patients undergoing IVF complicated by OHSS is increased by 100 times when compared to background risk [64]. The aOR for VTE is 2.7 (2.1–3.6) [32].
3.19. Antepartum Immobilization
Immobility increases the risk of VTE by 1.5–2-fold [60]. Such risk, related to the venous stasis, is dependent on the BMI of pregnant woman—when her pre-pregnancy BMI is ≥25 kg/m2, the aOR is 62.3 (95% CI 11.5–337). When pre-pregnancy BMI is <25 kg/m2, the aOR is 7.7 (95% CI 3.2–19) [26,32].
Generally, there is still a lack of adequate study data. Strategies for the prevention of VTE in pregnancy are often deduced from case-control or observational studies, with extrapolations from suggestions proposed for non-pregnant patients. A complex, multidisciplinary approach is thus often required, predominantly in the peripartum period [26].
4. When to Use Antithrombotic Prophylaxis
The risk of thromboembolic complications should be evaluated during pregnancy and also in the postpartum period.
Different guidelines provide various stratification systems for the need for thromboprophylaxis, and a summary of these is outlined in Table 1 [65,66,67,68].

Table 1.
Summary of risk stratification strategies for thromboprophylaxis in pregnancy according to the selected guidelines [65,66,67,68].
Furthermore, according to the Asian venous thromboembolism guidelines adapted from the guidelines of the American College of Chest Physicians (ACCP), the American Heart Association and the American Stroke Association (AHA/ASA), the American Society for Metabolic and Bariatric Surgery (ASMBS), the RCOG and the European Society for Medical Oncology (ESMO), patients with four or more risk factors ought to be evaluated for a prophylactic dose of LMWH throughout the pregnancy and for six weeks postpartum [69].
Thus, there is a difference in the situation when the patient has a history of unprovoked VTE or an episode of VTE associated with transient risk factors (Table 2) [70].

Table 2.
Summary of recommendations regarding the circumstances of previous VTE in pregnant patients [70].
5. How Important Is the Presence of Thrombophilia and Why?
Table 3 provides a summary of the guidelines focused on the prevention of VTE in pregnant women with inherited thrombophilia [32,71].

Table 3.
Summary of recommendations for thromboprophylaxis in pregnancy according to the presence of thrombophilic state [32,71].
In the case of the co-existence of thrombophilic states, ASH guidelines recommend thromboprophylaxis either during ante- and postpartum period regardless of family history of VTE [71].
6. What Is the Form of Anticoagulant Thromboprophylaxis and Which Dose Should Be Used?
Generally, apart from sufficient fluid intake and exercise, ways of non-pharmacologic prophylaxis of VTE are the use of thromboembolic-deterrent stockings, graduated compression stockings and an intermittent pneumatic compression or sequential compression device. However, there is limited evidence related to the use of these aids in the pregnancy and postpartum period. Before prescribing pharmacologic thromboprophylaxis, several features have to be taken into account (Table 4) [67].

Table 4.
Aspects of pharmacologic thromboprophylaxis for further consideration [67].
6.1. Unfractionated Heparin
6.1.1. Pharmacokinetics in Pregnancy
In pregnant patients receiving a single weight-based dose of UFH (9500 ± 640 IU), a lower peak plasma concentration (0.11 ± 0.017 vs. 0.23 ± 0.036 IU/mL) and a shorter time to such peak plasma concentration (113 ± 20 vs. 222 ± 20 min) were detected. Plasma heparin concentration designated as the area under the curve for pregnant patients is 55% that of the values of nonpregnant subjects. Moreover, pregnant women have a non-significant increase from their baseline activated partial thromboplastin time (aPTT). Additionally, no significant increase in anti-Xa activity in pregnant patients administered a single dose of UFH 7500 IU before cesarean delivery and after NB can be measured.
These findings might be attributed to increased binding of circulating heparin-neutralizing proteins and increased levels of FVIII and fibrinogen in pregnant patients. Moreover, in the third trimester, there might be a higher heparin requirement, or potentially a lower requirement, because of a decrease in placental heparinase activity due to placental aging. Conclusively, in pregnant patients treated with a single dose of UFH 5000–10,000 IU, the peak plasma heparin concentration might be at or below the plasma heparin concentration detected 6 h after this UFH dose in nonpregnant individuals [7].
6.1.2. Dosages

Table 5.
Dosing regimens of UFH [70].

Table 6.
Dosing regimens of UFH according to the weight of the pregnant patient [67].
6.2. Low-Molecular-Weight Heparin
6.2.1. Pharmacokinetics in Pregnancy
The group of drugs entitled LMWH possess favorable properties, such as easy administration, an improved bioavailability and safety and more predictable pharmacokinetics. Moreover, a decreased occurrence of osteoporosis, heparin-induced thrombocytopenia and bleeding complications in comparison to UFH were reported [7]. Further advantages of LMWH include a longer half-life, predictable treatment response and less allergic reactions [70].
6.2.2. Enoxaparin
The volume of distribution and its clearance increase during pregnancy. The first of these characteristics increases 49%, with the largest proportion in the third trimester, and resolves a few days after delivery. Clearance increases 48% with changes in glomerular filtration rate and normalizes 2 weeks following delivery. It seems that the peak anti-Xa activity is lower in pregnant patients in comparison to nonpregnant women. The maximum plasma concentration and anti-Xa activity are lower in the course of pregnancy when compared to the levels achieved six weeks after delivery [7].
6.2.3. Dalteparin
The mean maximal concentration (0.21 ± 0.05 vs. 0.49 ± 0.13 anti-Xa IU/mL) and area under the curve (1.97 ± 0.46 IU vs. 3.23 ± 0.85 h/mL) detected up to 24 h from the administration of this drug are also lower when compared to nonpregnant women. The average time to achieve peak concentration is 3 h. The mean half-life for a morning dose is 4.9, and for evening dose, it is 3.9 h. In pregnancy, there are significantly lower mean anti-Xa activities in comparison to postpartum, with the lowest level at the 36th week of gestation [7].
6.2.4. Tinzaparin
Peak anti-Xa activity measured 4 h after its administration is commonly below 0.1 IU/mL, and 24 h anti-Xa activity detected at 36 weeks of gestation compared to 28 weeks is reduced [7].

Table 7.
Dosages of LMWHs [65,66,67,70,72,73,74].
6.2.5. Protamine Sulfate to Reverse Anticoagulation with Low-Molecular-Weight Heparin and Unfractionated Heparin
Protamine sulfate might lead to full reversal of UFH and 60–80% reversal of LMWH. One milligram of intravenous protamine can neutralize 100 IU of intravenous heparin. Reversal of subcutaneous heparin might require repeated doses of protamine (its half-life is approximately 7 min). Adverse effects in pregnant women are hypotension from histamine release, hypersensitivity reaction or even anaphylaxis, noncardiogenic pulmonary edema, pulmonary hypertension, thrombocytopenia, impaired platelet aggregation, decreased thrombin effect and fibrinogen precipitation [7].
6.3. Fondaparinux
This pentasaccharide crosses the placental barrier, so it is not recommended during the first trimester. However, reports of its use in more advanced stages of pregnancy exist. It is mainly used in pregnant patients with contraindication to heparin due to thrombocytopenia or allergic reaction. Moreover, fondaparinux is considered safe during breastfeeding [70,76]. The suggested dose according to clinical trials performed in nonpregnant women is 2.5 mg daily [72].
6.4. Danaparoid
Danaparoid represents another heparanoid molecule that is—like fondaparinux—recommended in pregnant women with allergy to LMWHs or HIT, because it does not cross-react with HIT antibodies [72]. Additionally, this LMWH derivative does not cross the placenta [65]. According to the SOGC guidelines, its recommended prophylactic dose in pregnancy is 750 IU administered subcutaneously twice a day [72].
6.5. Vitamin K antagonists
Vitamin K antagonists (VKAs) are able to cross the placental barrier and lead to fetal abnormalities (member and nasal hypoplasia, scoliosis, chondral calcification, schizencephaly and fetus intracranial hemorrhage) from the 6th week of gestation. Additionally, their use in the third trimester contributes to peripartum fetal hemorrhage, and its administration in the second trimester is correlated with neurologic impairment. However, warfarin is safe during breastfeeding [70].
Regarding the use of VKA during pregnancy, several situations might develop:
- -
- In patients requiring long-term treatment with VKA who are attempting pregnancy, it is suggested to substitute LMWH for VKA only when pregnancy is achieved. LMWH should be administered in adjusted doses or 75% of therapeutic doses;
- -
- Another option is to switch to UFH or LMWH until the 13th week of gestation, with substitution by VKA until close to delivery when UFH or LMWH is restored;
- -
- In pregnant patients with previous VTE, postpartum thromboprophylaxis with prophylactic or intermediate dose of LMWH or VKA targeted at the International Normalized Ratio (INR) 2.0–3.0 is recommended;
- -
- In pregnant patients without a history of VTE, homozygous for factor V Leiden or the prothrombin G20210A mutation and with a positive family history for VTE, except antepartum prophylactic- or intermediate-dose LMWH, postpartum thromboprophylaxis with continuing LMWH or VKA targeted at INR 2.0–3.0 is recommended;
- -
- In pregnant patients without personal and family history of VTE, homozygous for factor V Leiden or the prothrombin G20210A mutation, postpartum thromboprophylaxis for 6 weeks with prophylactic- or intermediate-dose LMWH or VKA targeted at INR 2.0–3.0 is suggested;
- -
- In asymptomatic pregnant patients with all other thrombophilic states and a positive family history for VTE, postpartum thromboprophylaxis with prophylactic or intermediate dose of LMWH or, in those who do not have PC or PS deficiency, VKA targeted at INR 2.0–3.0 is suggested [68].
6.6. Direct Oral Anticoagulants
Due to absence of data about the safety and effectiveness of direct oral anticoagulants (DOACs) during pregnancy and breastfeeding, their use in these settings is not currently recommended [70]. A summary of the characteristics of antithrombotic drugs used in pregnancy is available in Table 8 [67,74,76].

Table 8.
The most important features of antithrombotic drugs used in pregnancy and during postpartum period [67,74,76].
7. Further Aspects Related to Thromboprophylaxis That Are Useful for Clinical Practice
7.1. How to Monitor the Effectiveness of Anticoagulant Thromboprophylaxis
aPTT is the standard coagulation parameter used for the evaluation of the effectiveness of UFH and anti-Xa activity for the monitoring of the safety of LMWH. Along them, point-of-care methods for an assessment of the coagulation status of pregnant patients might also be used (Table 9) [7,77].

Table 9.
Advantages and disadvantages of the use of various laboratory methods in pregnancy [7,77].
According to the RCOG (2015), detection of the peak anti-Xa activity for LMWH administered for treatment of acute VTE during pregnancy or postpartum period is not routinely recommended. Exceptions include patients with extreme body weight (<50 and ≥90 kg) or with other risk factors (renal impairment, recurrency of VTE) (grade of evidence C). The Society of Obstetricians and Gynecologists of Canada (SOGC) (2014) guidelines indicate that its measurement could be considered during the first month of treatment to target levels between 0.6 and 1.0 IU/mL achieved 4 h after the administration of LMWH [77].
7.2. How to Manage Cesarean Section in Patients with Anticoagulant Thromboprophylaxis
An overview of the current guidelines providing recommendations about further steps after cesarean delivery is available in Table 10 [77].

Table 10.
Recommendations regarding thromboprophylaxis with LMWH after cesarean delivery [77].
7.3. How to Manage Neuraxial Anesthesia in Patients Using Anticoagulants
The most dangerous complication of neuraxial anesthesia in patients administered anticoagulation is the development of vertebral canal hematoma. This clinical condition can lead to paralysis when not treated within 12 h [65]. The incidence of spinal epidural hematoma under the setting of neuraxial anesthesia in the obstetric population is rare (1:200,000–1:250,000). In addition to labor pain relief, such an intervention might help in minimizing the need to bear down before complete cervical dilation and contribute to decreasing the levels of circulating catecholamines, which are essential in facilitating vaginal delivery in high-risk settings [7]. Table 11 contains the recommendations associated with the use of NB in the case of anticoagulant treatment [7].

Table 11.
Management of NB in pregnant patients using anticoagulants according to the Society for Obstetric Anesthesia and Perinatology [7].
A comparison of the management of NB according to the obstetric and hematologic societies is outlined in Table 12 [67,68,72,77].

Table 12.
NB in relation to anticoagulant thromboprophylaxis in pregnancy according to further international guidelines [67,68,72,77].
Additionally, according to the RCOG, normal risk associated with the insertion of a neuraxial block in pregnant women receiving warfarin occurs in the case of INR ≤ 1.4 [65].
To summarize, the strongest risk factors favoring antenatal thromboprophylaxis are previous recurrent VTE, especially when unprovoked, developed during pregnancy or not associated with surgical intervention, and high-risk thrombophilia (antiphospholipid syndrome, antithrombin, PC or PS deficiency, homozygous form of factor V Leiden mutation, prothrombin mutation or their combination).
However, as outlined above, even in these high-risk clinical situations, different guidelines recommend different time intervals for the beginning of thromboprophylaxis. The SFOG suggests antepartum thromboprophylaxis from the time of detection of pregnancy up to at least six weeks postpartum; the QCG recommends its initiation from the first trimester with similar continuation up to postpartum; the ACCP recommends extension of prophylaxis up to 6 weeks following delivery in the presence of significant prothrombotic risk factors, and the RCOG suggests antenatal thromboprophylaxis in the case of previous VTE, hospital admission, surgery or OHSS.
OHSS as the prothrombotic risk factor is thus the subject of various approaches. According to the QCG, it might be the cause of transient thromboprophylaxis until this state resolves. The SFOG recommends thromboprophylaxis from the time of OHSS development up to the end of postpartum period.
According to the QCG and RCOG, comorbidities including cancer, diabetes mellitus or inflammatory diseases also represent a risk for antenatal thromboprophylaxis. The QCG and SFOG stratify further prenatal and postnatal risk factors and recommend their calculation. Based on the synthesis of these documents, the Asian venous thromboembolism guidelines recommend to patients with four or more risk factors thromboprophylaxis throughout the pregnancy and for six weeks postpartum (for more details, see Table 1) [65,66,67,68].
Regarding the dose of LMWH in various clinical situations, reviewed guidelines recommend different approaches, as well. The ASH and ACCP do not consider an adjusted dose of LMWH. In women receiving anticoagulants before pregnancy, the RANZOG, ASH and ACCP guidelines suggest therapeutic anticoagulation during pregnancy. The ACOG and ACCP guidelines recommend its continuation to the postpartum period, and the RANZOG document prefers the use of pre-pregnancy anticoagulation (Table 2) [70].
The RCOG, ACOG and GTH guidelines consider the heterozygous form of factor V Leiden or prothrombin mutation as the reason for antepartum thromboprophylaxis. Postpartum anticoagulant prophylaxis in these patients is recommended by all guidelines except ASH. In the co-existence of these inherited thrombophilic mutations and in patients with antithrombin deficiency, all guidelines except the ACCP recommend thromboprophylaxis before delivery. In pregnant women with the homozygous form of factor V Leiden mutation and prothrombin variant G20210A, all guidelines suggest anticoagulant prophylaxis throughout the pregnancy and postpartum period, with consideration of further various aspects. In high-risk pregnant patients with antithrombin deficiency, thromboprophylaxis up to six weeks postpartum is recommended.
In patients with PC and PS deficiency, RCOG, ACOG and GTH guidelines suggest prophylaxis with LMWH during pregnancy, and all guidelines recommend thromboprophylaxis in the postpartum period (Table 3) [32,71].
Differences in the recommendations for thromboprophylaxis in patients undergoing cesarean section can also be found. In the case of emergent cesarean delivery, ANZJOG guidelines suggest thromboprophylaxis lasting for 5 days, and RCOG guidelines 10 days, after the delivery. However, further risk factors are considered, as well (Table 10) [77].
Conclusively, most studies consider a previous VTE episode and selected thrombophilia, such as the homozygous form of factor V Leiden mutation and prothrombin variant G20210A, as significant prothrombotic risk factors. Therefore, in these situations, they recommend prolonged thromboprophylaxis during pregnancy and six weeks following delivery. Moreover, there is still general agreement that LMWH is the drug of choice during the pregnancy and postpartum period.
On the other hand, despite the current amount of data on risk factors for prevention of VTE during pregnancy and the postpartum period [70], the available international recommendations are inconclusive regarding antepartum thromboprophylaxis in special situations in patients with thrombophilia. They also differ in the postpartum period, but to a lesser extent [32].
The recommendations for clinical practice are as follows:
- -
- To evaluate the need for thromboprophylaxis as soon as the pregnancy is confirmed, consider the presence of the prothrombotic risk factors related to the comorbidities and circumstances of a previous VTE episode;
- -
- In patients with previous idiopathic, recurrent or hormone-related VTE, use ante- and also postpartum prophylaxis;
- -
- In patients with VTE associated with a major reversible risk factor/without thrombophilic state, use postpartum thromboprophylaxis;
- -
- In pregnant patients with prothrombotic risk factors undergoing cesarean section, consider postpartum thromboprophylaxis;
- -
- Control changes in the health condition of the patient and modify the actual thromboprophylaxis on an individual basis;
- -
- Evaluate the presence of bleeding symptoms and allergic reaction, regularly assess platelet count, function of antithrombin, renal parameters and liver function tests;
- -
- In pregnant women with extreme body weight, renal impairment, recurrence of VTE or in the suspicion of noncompliance, evaluate anti-Xa activity of LMWH;
- -
- To take control over prothrombotic comorbidities and complications developed during pregnancy, the multidisciplinary approach is preferred;
- -
- Along with anticoagulant thromboprophylaxis, use nonpharmacologic prophylaxis, such as intermittent pneumatic compression or elastic stockings.
Limitations of the presented review are the inclusion of studies with different designs—prospective and retrospective studies, randomized controlled trials and also systematic reviews. Moreover, we admit that the review contains data representing a selection bias caused by the inclusion of studies with the absence of a control group. However, these studies confirm the conclusions of the randomized controlled trials and systematic reviews used in this review, as well.
Areas for future research include the relevance of monitoring anti-Xa activity, as well as the impact of the application of risk scores to balance the absolute risk of thromboembolic episodes and bleeding complications. However, currently, when determining the factors increasing the risk of VTE, data are extrapolated mostly from retrospective studies [70,72].
8. Conclusions
A previous VTE episode and selected thrombophilia (e.g., the homozygous form of factor V Leiden mutation and prothrombin variant G20210A) are the most significant prothrombotic risk factors. Therefore, in these circumstances, the international guidelines recommend thromboprophylaxis during the pregnancy and postpartum period. LMWH still represents the drug of choice during this period.
The practical implication of this review might be the information about the most appropriate dosing regimens and the summary of the risk of VTE in the course of pregnancy and puerperium in the context of the risk factors discussed.
Data from large prospective registries might provide more information on the absolute risk factors warranting thromboprophylaxis in this setting [70,72].
Author Contributions
Conceptualization, L.S., M.S. and T.B.; methodology, K.B. (Kristína Brisudová) and I.Š.; formal analysis, L.S.; resources, L.S.; writing—original draft preparation, L.S.; writing—review and editing, L.S.; supervision, J.S. (Ján Staško), J.S. (Juraj Sokol), M.S. and K.B. (Kamil Biringer); funding acquisition, L.S. and J.S. (Ján Staško). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. It was funded by projects of the Scientific Grant Agency (Vega) 1/0549/19 and Vega 1/0479/21 received by our faculty.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| ACCP | American College of Chest Physicians |
| ACOG | American College of Obstetricians and Gynecologists |
| AHA/ASA | American Heart Association and American Stroke Association |
| AOR | Adjusted odds ratio |
| aPTT | Activated partial thromboplastin time |
| ASA | Acetylsalicylic acid |
| ASH | American Society of Hematology |
| ASMBS | American Society for Metabolic and Bariatric Surgery |
| CI | Confidence interval |
| Dll4 | Delta-like ligand 4 |
| DOACs | Direct oral anticoagulants |
| DVT | Deep vein thrombosis |
| EG | Endothelial glycocalyx |
| ELAM-1 | Endothelial cell-leukocyte adhesion molecule 1 |
| ESMO | European Society for Medical Oncology |
| FGR | Fetal growth restriction |
| FII | Coagulation factor II |
| FV | Coagulation factor V |
| FVII | Coagulation factor VII |
| FVIII | Coagulation factor VIII |
| FIX | Coagulation factor IX |
| FX | Coagulation factor X |
| FXI | Coagulation factor XI |
| FXII | Coagulation factor XII |
| FXIII | Coagulation factor XIII |
| GARFIELD | Global Anticoagulant Registry in the FIELD |
| GTH | Society of Thrombosis and Haemostasis Research/Gesellschaft für Thrombose und Hämostaseforschung |
| hCG | Human chorionic gonadotropin |
| HIT | Heparin-induced thrombocytopenia |
| HR | Hazard ratio |
| ICAM-1 | Intercellular adhesion molecule 1 |
| INR | International Normalized Ratio |
| IRR | Incidence rate ratio |
| IVF | In vitro fertilization |
| LMWH | Low-molecular-weight heparin |
| NB | Neuraxial blockade |
| NETs | Neutrophil extracellular traps |
| OHSS | Ovarian hyperstimulation syndrome |
| PAI-1 | Plasminogen activator inhibitor 1 |
| PC | Protein C |
| PCOS | Polycystic ovarian syndrome |
| PE | Pulmonary embolism |
| PIC | Plasmin–α2 plasmin inhibitor complex |
| PS | Protein S |
| RANZOG | The Royal Australian and New Zealand College of Obstetricians and Gynaecologists |
| RCOG | Royal College of Obstetricians and Gynaecologists |
| ROTEM | Rotational thromboelastometry |
| QCG | Queensland Clinical Guidelines |
| SC | Subcutaneously |
| SFOG | Swedish Society of Obstetrics and Gynecology |
| SLE | Systemic lupus erythematosus |
| SOGC | Society of Obstetricians and Gynecologists of Canada |
| TAT | Thrombin–antithrombin complex |
| TEG | Thromboelastography |
| TM | Thrombomodulin |
| tPA | Tissue plasminogen activator |
| tPAI-C | Tissue plasminogen activator/plasminogen activator inhibitor compound |
| UFH | Unfractionated heparin |
| VCAM-1 | Vascular cell adhesion molecule 1 |
| VEGF | Vascular endothelial growth factor |
| VKA | Vitamin K antagonists |
| VTE | Venous thromboembolism |
| vWF | Von Willebrand factor |
References
- Galanaud, J.-P.; Laroche, J.-P.; Righini, M. The history and historical treatments of deep vein thrombosis. J. Thromb. Haemost. 2013, 11, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Linnemann, B.; Rott, H.; Zotz, R.; Hart, C. Venous Thromboembolism Issues in Women. Hamostaseologie 2022, 42, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Felis, S.; Marchese, B.; Gavini, I. The Latest Evidence of Risks and Management of Venous Thromboembolism in Pregnancy: A Comprehensive Review. J. Blood Disord. Transfus. 2023, S2, 006. [Google Scholar]
- Eke, A.C.; Gebreyohannes, R.D.; Scantamburlo Fernandes, M.F.; Pillai, V.C. Physiologic Changes During Pregnancy and Impact on Small-Molecule Drugs, Biologic (Monoclonal Antibody) Disposition, and Response. J. Clin. Pharmacol. 2023, 63 (Suppl. S1), S34–S50. [Google Scholar] [CrossRef] [PubMed]
- Tlamcani, I.; Mouh, N.E.; Amrani, K.E.; Hassani, M.A. Pregnancy and Hemostasis: From Physiology to Pathological States. Clin. Res. Hematol. 2018, 1, 1–7. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, Y.; Wu, M.; Xiang, Z. Changes of new coagulation markers in healthy pregnant women and establishment of reference intervals in Changsha. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2022, 47, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Leffert, L.; Butwick, A.; Carvalho, B.; Arendt, K.; Bates, S.M.; Friedman, A.; Horlocker, T.; Houle, T.; Landau, R.; Dubois, H.; et al. The Society for Obstetric Anesthesia and Perinatology Consensus Statement on the Anesthetic Management of Pregnant and Postpartum Women Receiving Thromboprophylaxis or Higher Dose Anticoagulants. Anesth. Analg. 2018, 126, 928–944. [Google Scholar] [CrossRef] [PubMed]
- Solomon, C.; Collis, R.E.; Collins, P.W. Haemostatic monitoring during postpartum haemorrhage and implications for management. Br. J. Anaesth. 2012, 109, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Kornacki, J.; Gutaj, P.; Kalantarova, A.; Sibiak, R.; Jankowski, M.; Wender-Ozegowska, E. Endothelial Dysfunction in Pregnancy Complications. Biomedicines 2021, 9, 1756. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, L. Uteroplacental Circulation in Normal Pregnancy and Preeclampsia: Functional Adaptation and Maladap-tation. Int. J. Mol. Sci. 2021, 22, 8622. [Google Scholar] [CrossRef]
- Kevane, B.; Ní Áinle, F.N. Prevention, diagnosis, and management of PE and DVT in pregnant women. Hematol. Am. Soc. Hematol. Educ. Program. 2023, 2023, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Meng, K.; Hu, X.; Peng, X.; Zhang, Z. Incidence of venous thromboembolism during pregnancy and the puerperium: A systematic review and meta-analysis. J. Matern.-Fetal Neonatal Med. 2015, 28, 245–253. [Google Scholar] [CrossRef]
- Maughan, B.C.; Marin, M.; Han, J.; Gibbins, K.J.; Brixey, A.G.; Caughey, A.B.; Kline, J.A.; Jarman, A.F. Venous Thromboem-bolism During Pregnancy and the Postpartum Period: Risk Factors, Diagnostic Testing and Treatment. Obstet. Gynecol. Surv. 2022, 77, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Montinari, M.R.; Minelli, S.; De Caterina, R. Eighty years of oral anticoagulation: Learning from history. Vascul. Pharmacol. 2021, 141, 106918. [Google Scholar] [CrossRef]
- Rath, W.; von Tempelhoff, G.-F.; Tsikouras, P. How to Reduce Maternal Mortality from Venous Thromboembolism. Clin. Appl. Thromb. Hemost. 2018, 24, 6S–7S. [Google Scholar] [CrossRef] [PubMed]
- De Stefano, V.; Martinelli, I.; Rossi, E.; Battaglioli, T.; Za, T.; Mannucci, P.M.; Leone, G. The risk of recurrent venous thromboembolism in pregnancy and puerperium without antithrombotic prophylaxis. Br. J. Haematol. 2006, 135, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Galambosi, P.J.; Ulander, V.M.; Kaaja, R.J. The incidence and risk factors of recurrent venous thromboembolism during pregnancy. Thromb. Res. 2014, 134, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Novak, P.; Novak, A.; Šabović, M.; Kozak, M. Prophylactic Dose of Dalteparin in Pregnant Women with History of Venous Thromboembolisms and/or Thrombophilia: Real-World Data. Angiology 2023, 74, 783–789. [Google Scholar] [CrossRef]
- Pabinger, I.; Grafenhofer, H.; Kyrle, P.A.; Quehenberger, P.; Mannhalter, C.; Lechner, K.; Kaider, A. Temporary increase in the risk for recurrence during pregnancy in women with a history of venous Thromboembolism. Blood 2002, 100, 1060–1062. [Google Scholar] [CrossRef]
- Alsheef, M.A.; Alabbad, A.M.; Albassam, R.A.; Alarfaj, R.M.; Zia Zaidi, A.R.; Alarfaj, O.A.; Ayyash, M.; Abu-Shaheen, A. Predictors of pregnancy-associated venous thromboembolism: A case-control study. Front. Cardiovasc. Med. 2022, 9, 920089. [Google Scholar] [CrossRef]
- Jerjes-Sánchez, C.; Rodriguez, D.; Farjat, A.E.; Kayani, G.; MacCallum, P.; Lopes, R.D.; Turpie, A.G.G.; Weitz, J.I.; Haas, S.; Ageno, W.; et al. Pregnancy-Associated Venous Thromboembolism: Insights from GARFIELD-VTE. TH Open 2021, 5, e24–e34. [Google Scholar] [CrossRef] [PubMed]
- Grouzi, E.; Pouliakis, A.; Aktypi, A.; Christoforidou, A.; Kotsi, P.; Anagnostou, G.; Foifa, A.; Papadakis, E. Pregnancy and thrombosis risk for women without a history of thrombotic events: A retrospective study of the real risks. Thromb. J. 2022, 20, 60. [Google Scholar] [CrossRef] [PubMed]
- Nanne Croles, F.; Nasserinejad, K.; Duvekot, J.J.; Kruip, M.J.; Meijer, K.; Wg Leebeek, F. Pregnancy, thrombophilia, and the risk of a first venous thrombosis: Systematic review and bayesian meta-analysis. BMJ 2017, 359, j4452. [Google Scholar] [CrossRef] [PubMed]
- Al-Nasser, B. Influence of Tobacco Smoking on Perioperative Risk of Venous Thromboembolism. Turk. J. Anaesthesiol. Reanim. 2020, 48, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.M.; Bellone, J.M.; Hornsby, L.B.; Treadway, S.; Phillippe, H.M. Pregnancy-Related Venous Thromboembolism. J. Pharm. Pract. 2014, 27, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Hart, C.; Bauersachs, R.; Scholz, U.; Zotz, R.; Bergmann, F.; Rott, H.; Linnemann, B. Prevention of venous thromboembolism during pregnancy and the puerperium with a special focus on women with hereditary thrombophilia or prior VTE—Position Paper of the Working Group in Women’s Health of the Society of Thrombosis and Haemostasis (GTH). Hamostaseologie 2020, 40, 572–590. [Google Scholar] [CrossRef] [PubMed]
- Eichinger, S.; Hron, G.; Bialonczyk, C.; Hirschl, M.; Minar, E.; Wagner, O.; Heinze, G.; Kyrle, P.A. Overweight, obesity, and the risk of recurrent venous thromboembolism. Arch. Intern. Med. 2008, 168, 1678–1683. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.; Glise Sandblad, G.; Lundberg, C.E.; Hellsén, G.; Hansson, P.O.; Adiels, M.; Rosengren, A. Prepregnancy overweight and obesity and long-term risk of venous thromboembolism in women. Sci. Rep. 2023, 13, 14597. [Google Scholar] [CrossRef]
- Nurmohamed, M.T.; Büller, H.R.; ten Cate, J.W. Physiological changes due to age. Implications for the prevention and treatment of thrombosis in older patients. Drugs Aging 1994, 5, 20–33. [Google Scholar] [CrossRef]
- Varrias, D.; Spanos, M.; Kokkinidis, D.G.; Zoumpourlis, P.; Kalaitzopoulos, D.R. Venous Thromboembolism in Pregnancy: Challenges and Solutions. Vasc. Health Risk Manag. 2023, 19, 469–484. [Google Scholar] [CrossRef]
- De Barros, V.I.P.L.V.; Kondo Igai, A.M.; Spadotto Baptista, F.; de Figueiredo Lemos Bortolotto, M.R.; Verzinhasse Peres, S.; Pulcinelli Vieira Francisco, R. Venous thromboembolism risk score during hospitalization in pregnancy: Results of 10694 prospective evaluations in a clinical trial. Clinics 2023, 78, 100230. [Google Scholar] [CrossRef]
- Middeldorp, S.; Naue, C.; Köhler, C. Thrombophilia, Thrombosis and Thromboprophylaxis in Pregnancy: For What and in Whom? Hamostaseologie 2022, 42, 54–64. [Google Scholar] [CrossRef]
- Crowley, M.P.; Noone, C.; Higgins, J.R.; O’Shea, S. A Multicentre Study of Thromboprophylaxis in Pregnancy. Ir. Med. J. 2017, 110, 567. [Google Scholar] [PubMed]
- Mokhtari, M.; Nasri, K.; Tara, F.; Zarean, E.; Hantoushzadeh, S.; Radmehr, M.; Kashanian, M. A Survey of Venous Throm boembolism (VTE) Prophylaxis in Obstetrics Patients in Iran. J. Fam. Reprod. Health 2019, 13, 21–25. [Google Scholar]
- Wang, Z.L.; Geng, H.Z.; Zhao, X.L.; Zhu, Q.Y.; Lin, J.H.; Zou, L.; Mi, Y.; Hu, Y.L.; Fan, S.R.; Chen, X.; et al. Survey of related factors of maternal venous thromboembolism in nine hospitals of China. Zhonghua Fu Chan Ke Za Zhi 2020, 55, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.-G.; Lee, J.H.; Bang, S.-M. Incidence of Pregnancy-Associated Venous Thromboembolism: Second Nationwide Study. Thromb. Haemost. 2023, 123, 904–910. [Google Scholar] [CrossRef]
- Lok, W.Y.; Kong, C.W.; To, W.W.K. A local risk score model for venous thromboembolism prophylaxis for caesarean section in Chinese women and comparison with international guidelines. Taiwan J. Obstet. Gynecol. 2019, 58, 520–525. [Google Scholar] [CrossRef]
- Ezeh, E.; Katabi, A.; Khawaja, I. Iron Deficiency Anemia as a Rare Risk Factor for Recurrent Pulmonary Embolism and Deep Vein Thrombosis. Cureus 2021, 13, e13721. [Google Scholar] [CrossRef] [PubMed]
- Pathophysiology and Principles of Management of Varicose Veins. Available online: https://www.ncbi.nlm.nih.gov/books/NBK534256/ (accessed on 22 January 2024).
- Deep Vein Thrombosis (Clinical). Available online: https://www.lecturio.com/concepts/deep-vein-thrombosis-clinical/ (accessed on 22 January 2024).
- Jacobs, B.N.; Andraska, E.A.; Obi, A.T.; Wakefield, T.W. Pathophysiology of varicose veins. J. Vasc. Surg. Venous Lymphat. Disord. 2017, 5, 460–467. [Google Scholar] [CrossRef]
- Choy, K.; Emmett, S.; Wong, A. Venous thromboembolism prophylaxis in pregnancy: Are we adequately identifying and managing risks? Aust. N. Z. J. Obstet. Gynaecol. 2022, 62, 915–920. [Google Scholar] [CrossRef]
- Lussana, F.; Coppens, M.; Cattaneo, M.; Middeldorp, S. Pregnancy-related venous thromboembolism: Risk and the effect of thromboprophylaxis. Thromb. Res. 2012, 129, 673–680. [Google Scholar] [CrossRef]
- Sultan, A.A.; Tata, L.J.; West, J.; Fiaschi, L.; Fleming, K.M.; Nelson-Piercy, C.; Grainge, M.J. Risk factors for first venous thromboembolism around pregnancy: A population-based cohort study from the United Kingdom. Blood 2013, 121, 3953–3961. [Google Scholar] [CrossRef] [PubMed]
- Kelliher, S.; Maguire, P.B.; Szklanna, P.B.; Weiss, L.; Ewins, K.; O’Doherty, R.; Angelov, D.; Áinle, F.N.; Kevane, B. Pathophysiology of the Venous Thromboembolism Risk in Preeclampsia. Hamostaseologie 2020, 40, 594–604. [Google Scholar] [CrossRef]
- Egan, K.; Kevane, B.; Áinle, F.N. Elevated venous thromboembolism risk in preeclampsia: Molecular mechanisms and clinical impact. Biochem. Soc. Trans. 2015, 43, 696–701. [Google Scholar] [CrossRef]
- Hernández González, L.L.; Pérez-Campos Mayoral, L.; Hernández-Huerta, M.T.; Mayoral Andrade, G.; Martínez Cruz, M.; Ramos-Martínez, E.; Pérez-Campos Mayoral, E.; Cruz Hernández, V.; García, I.A.; Matias-Cervantes, C.A.; et al. Targeting Neutrophil Extracellular Trap Formation: Exploring Promising Pharmacological Strategies for the Treatment of Preeclampsia. Pharmaceuticals 2024, 17, 605. [Google Scholar] [CrossRef] [PubMed]
- Scheres, L.J.J.; Lijfering, W.M.; Groenewegen, N.F.M.; Koole, S.; de Groot, C.J.M.; Middeldorp, S.; Cannegieter, S.C. Hypertensive Complications of Pregnancy and Risk of Venous Thromboembolism. Hypertension 2020, 75, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Havers-Borgersen, E.; Butt, J.H.; Johansen, M.; Petersen, O.B.; Ekelund, C.K.; Rode, L.; Olesen, J.B.; Køber, L.; Fosbøl, E.L. Preeclampsia and Long-Term Risk of Venous Thromboembolism. JAMA Netw. Open 2023, 6, e2343804. [Google Scholar] [CrossRef] [PubMed]
- Hague, W.M.; Dekker, G.A. Risk factors for thrombosis in pregnancy. Best. Pract. Res. Clin. Haematol. 2003, 16, 197–210. [Google Scholar] [CrossRef]
- Wu, Y.; Pei, J.; Dong, L.; Zhou, Z.; Zhou, T.; Zhao, X.; Che, R.; Han, Z.; Hua, X. Association Between Maternal Weight Gain in Different Periods of Pregnancy and the Risk of Venous Thromboembolism: A Retrospective Case-Control Study. Front. Endocrinol. 2022, 13, 858868. [Google Scholar] [CrossRef]
- Rybstein, M.D.; DeSancho, M.T. Risk factors for and clinical management of venous thromboembolism during pregnancy. Clin. Adv. Hematol. Oncol. 2019, 17, 396–404. [Google Scholar]
- Raia-Barjat, T.; Edebiri, O.; Chauleur, C. Venous Thromboembolism Risk Score and Pregnancy. Front. Cardiovasc. Med. 2022, 9, 863612. [Google Scholar] [CrossRef] [PubMed]
- Stillbirth or Pre-Term Birth Outcomes Linked to Elevated Risk of Blood Clots after Pregnancy. Available online: https://www.prnewswire.com/news-releases/stillbirth-or-pre-term-birth-outcomes-linked-to-elevated-risk-of-blood-clots-after-pregnancy-201053771.html (accessed on 11 February 2024).
- Blondon, M.; Quon, B.S.; Harrington, L.B.; Bounameaux, H.; Smith, N.L. Association between newborn birth weight and the risk of postpartum maternal venous thromboembolism: A population-based case-control study. Circulation 2015, 131, 1471–1476, discussion 1476. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.; Fernández-Alonso, A.M.; Rodríguez, I.; Garrigosa, L.; Caño, A.; Carretero, P.; Vizcaíno, A.; Rocío Gonzalez-Ramirez, A. Postcesarean Thromboprophylaxis with Two Different Regimens of Bemiparin. Obstet. Gynecol. Int. 2011, 2011, 548327. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, C.; Zhong, M.; Yang, F.; Chen, H.; Kong, W.; Lv, P.; Chen, W.; Yao, Y.; Cao, Q.; et al. Changes in Coagulation and Fibrinolysis in Post-Cesarean Section Parturients Treated with Low Molecular Weight Heparin. Clin. Appl. Thromb. Hemost. 2020, 26, 1076029620978809. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, A.F.; Skjeldestad, F.E.; Sandset, P.M. Ante- and postnatal risk factors of venous thrombosis: A hospital-based case–control study. J. Thromb. Haemost. 2008, 6, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Sirilak, T.; Kanjanarat, P.; Nochaiwong, S.; Katip, W. Incidence of postpartum infections and outcomes associated with antibiotic prophylaxis after normal vaginal birth. Front. Med. 2022, 9, 939421. [Google Scholar] [CrossRef] [PubMed]
- Devis, P.; Knuttinen, M.G. Deep venous thrombosis in pregnancy: Incidence, pathogenesis and endovascular management. Cardiovasc. Diagn. Ther. 2017, 7 (Suppl. S3), S309–S319. [Google Scholar] [CrossRef] [PubMed]
- Gris, J.-C.; Guillotin, F.; Chéa, M.; Bourguignon, C.; Bouvier, S. The Risk of Thrombosis Around Pregnancy: Where Do We Stand? Front. Cardiovasc. Med. 2022, 9, 901869. [Google Scholar] [CrossRef]
- Gurunath, S.; Vinekar, S.; Biliangady, R. Assisted Reproductive Techniques in a Patient with History of Venous Thromboembolism: A Case Report and Review of Literature. J. Hum. Reprod. Sci. 2018, 11, 193–197. [Google Scholar] [CrossRef]
- Sayyadi, A.; Mahdavi, M.; Dalfardi, B.; Robati, F.K.; Shafiepour, M. Right atrial thrombus and pulmonary thromboembolism related to ovarian hyperstimulation syndrome: A case report and literature review. Clin. Case Rep. 2023, 11, e7018. [Google Scholar] [CrossRef]
- Mitchell-Jones, N.; McEwan, M.; Johnson, M. Management of venous thromboembolism secondary to ovarian hyperstimulation syndrome: A case report documenting the first use of a superior vena caval filter for upper limb venous thromboembolism in pregnancy, and the difficulties and complications relating to anticoagulation in antenatal and peri-partum periods. Obstet. Med. 2016, 9, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Kearsley, R.; Stocks, G. Venous thromboembolism in pregnancy diagnosis, management, and treatment. BJA Educ. 2021, 21, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, P.G.; Westerlund, E.; Hellgren, M. Swedish obstetric thromboprophylaxis guideline: Background and update. J. Obstet. Gynaecol. 2023, 43, 2241527. [Google Scholar] [CrossRef] [PubMed]
- Queensland Clinical Guidelines. Available online: https://www.health.qld.gov.au/__data/assets/pdf_file/0011/140024/g-vte.pdf (accessed on 11 February 2024).
- Bates, S.M.; Greer, I.A.; Middeldorp, S.; Veenstra, D.L.; Prabulos, A.-M.; Vandvik, P.O. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141, e691S–e736S. [Google Scholar] [CrossRef] [PubMed]
- Liew, N.C.; Alemany, G.V.; Angchaisuksiri, P.; Bang, S.-M.; Choi, G.; De Silva, D.A.; Hong, J.M.; Lee, L.; Li, Y.J.; Rajamoney, G.N.; et al. Asian venous thromboembolism guidelines: Updated recommendations for the prevention of venous thromboembolism. Int. Angiol. 2017, 36, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Sanches, S.M.V.; da Fonseca Cerqueira, M.M.B.; Junqueira, P.L.; Gomez, M.T. Thromboprophylaxis during the Pregnancy-Puerperal Cycle—Literature Review. Rev. Bras. Ginecol. Obstet. 2020, 42, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Bates, S.M.; Rajasekhar, A.; Middeldorp, S.; McLintock, C.; Rodger, M.A.; James, A.H.; Vazquez, S.R.; Greer, I.A.; Riva, J.J.; Bhatt, M.; et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: Venous thromboembolism in the context of pregnancy. Blood Adv. 2018, 2, 3317–3359. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.-S.; Rey, E.; Kent, N.E.; VTE in Pregnancy Guideline Working Group. Venous thromboembolism and antithrombotic therapy in pregnancy. J. Obstet. Gynaecol. Can. 2014, 36, 527–553. [Google Scholar] [CrossRef] [PubMed]
- Bates, S.M.; Middeldorp, S.; Rodger, M.; James, A.H.; Greer, I. Guidance for the treatment and prevention of obstetric-associated venous thromboembolism. J. Thromb. Thrombolysis 2016, 41, 92–128. [Google Scholar] [CrossRef]
- ASH VTE Guidelines: Pregnancy. Available online: https://www.hematology.org/education/clinicians/guidelines-and-quality-care/clinical-practice-guidelines/venous-thromboembolism-guidelines/pregnancy (accessed on 20 February 2024).
- ACOG Recommended Thromboprophylaxis for Pregnancies Complicated by Inherited Thrombophilias. Available online: https://www.ersebeszet.com/wp-content/uploads/2015/08/ACOG-thromboprophylaxis-recommendations.pdf (accessed on 20 February 2024).
- Wiegers, H.M.G.; Middeldorp, S. Contemporary best practice in the management of pulmonary embolism during pregnancy. Ther. Adv. Respir. Dis. 2020, 14, 1753466620914222. [Google Scholar] [CrossRef]
- Skeith, L. Prevention and management of venous thromboembolism in pregnancy: Cutting through the practice variation. Hematol. Am. Soc. Hematol. Educ. Program. 2021, 2021, 559–569. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).