Phenolic Composition of Crataegus monogyna Jacq. Extract and Its Anti-Inflammatory, Hepatoprotective, and Antileukemia Effects
Abstract
1. Introduction
2. Results
2.1. Phytochemical Analysis of C. monogyna
2.2. Cytotoxic Activity of C. monogyna
2.3. Anti-Inflammatory Activity of C. monogyna
2.4. Hepatoprotective Effect of C. monogyna
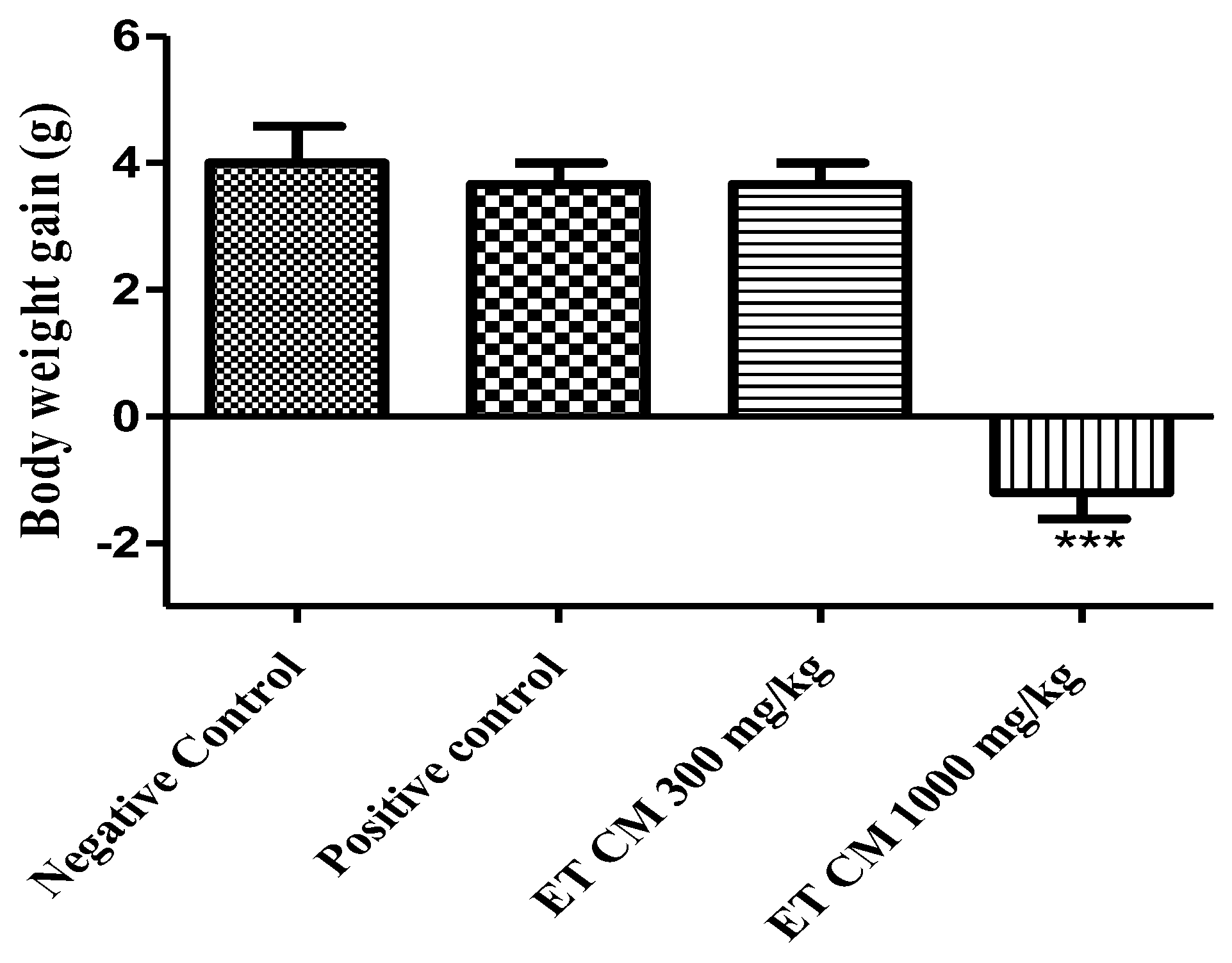
2.4.1. General Signs and Body Weight
2.4.2. Relative Organ Weight
2.4.3. Biochemical Parameters
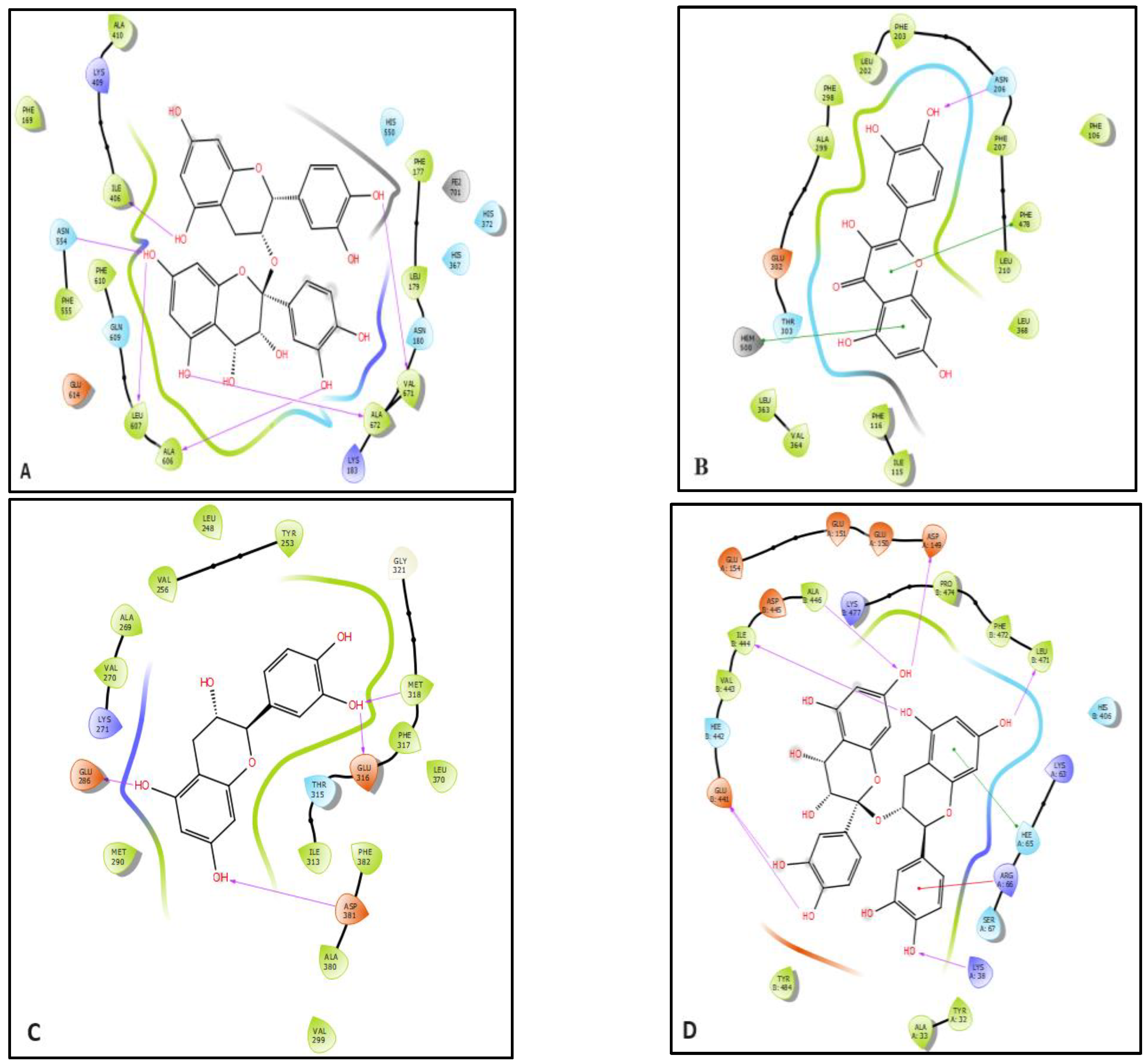
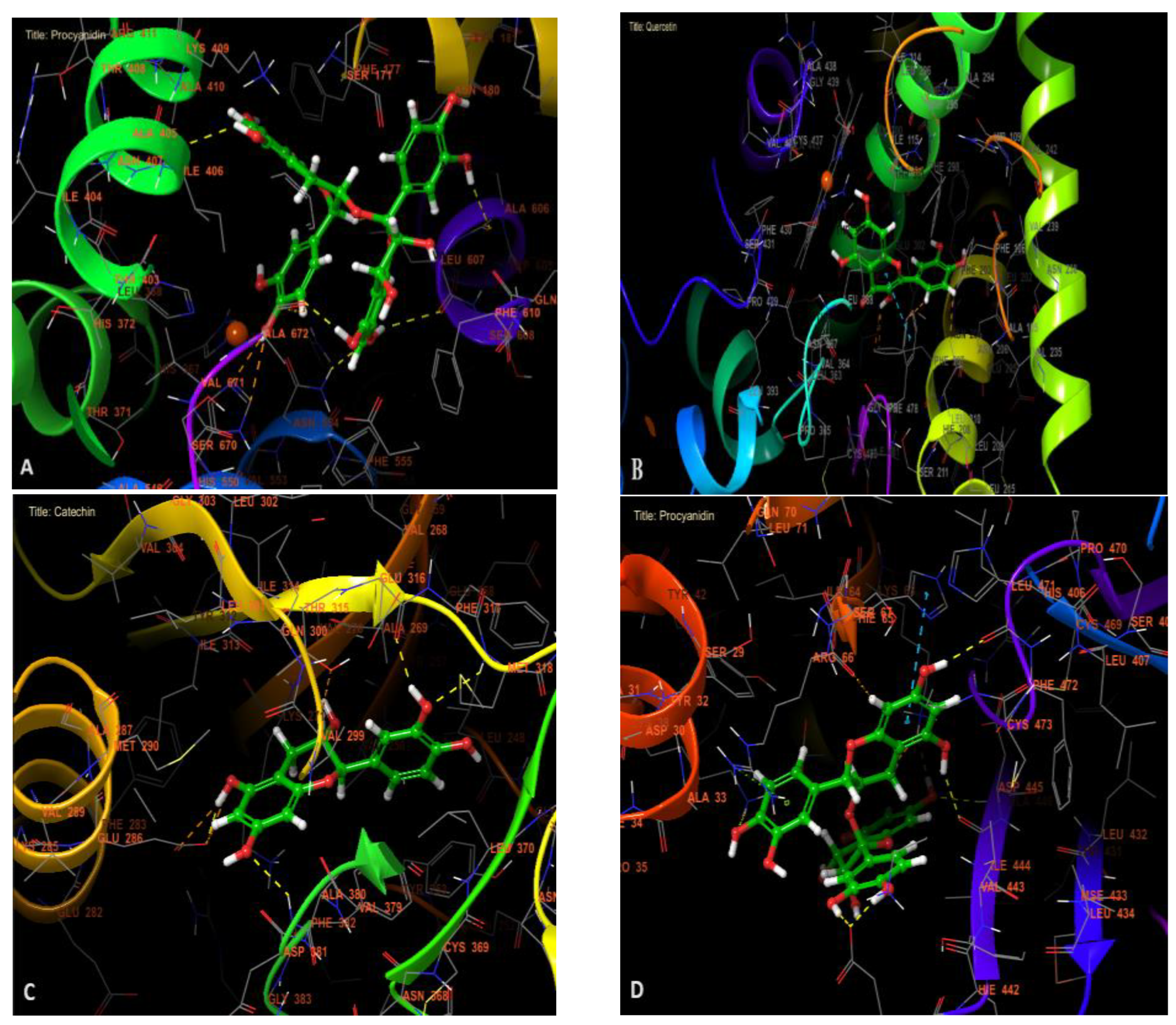
2.5. In Silico Study
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Animal Material
4.3. Preparation of the Extract
4.4. Chemical Composition
4.5. Cytotoxicity Effect of C. monogyna Extract
4.6. Anti-Inflammatory Test of C. monogyna
4.7. Hepatoprotective Effect of C. monogyna Extract
4.7.1. Experimental Protocol
- Group 1: Negative control (NaCl (0.9%)).
- Group 2: Positive control, acetaminophen (1000 mg/kg).
- Group 3: Hydroethanolic extract (300 mg/kg) + acetaminophen (1000 mg/kg).
- Group 4: Hydroethanolic extract (1000 mg/kg) + acetaminophen (1000 mg/kg).
4.7.2. Biochemical Evaluation
4.7.3. Relative Organ Weight
4.8. In Silico Molecular Prediction
4.8.1. Preparation of Ligands
4.8.2. Protein Preparation
4.8.3. Glide Standard Precision (SP) Ligand Docking
4.9. Statistical Approaches
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agour, A.; Mssillou, I.; Es-safi, I.; Conte, R.; Mechchate, H.; Slighoua, M.; Amrati, F.E.-Z.; Parvez, M.K.; Numan, O.; Bari, A.; et al. The Antioxidant, Analgesic, Anti-Inflammatory, and Wound Healing Activities of Haplophyllum Tuberculatum (Forsskal) A. Juss Aqueous and Ethanolic Extract. Life 2022, 12, 1553. [Google Scholar] [CrossRef] [PubMed]
- Amrati, F.E.-Z.; Chebaibi, M.; Galvão de Azevedo, R.; Conte, R.; Slighoua, M.; Mssillou, I.; Kiokias, S.; de Freitas Gomes, A.; Soares Pontes, G.; Bousta, D. Phenolic Composition, Wound Healing, Antinociceptive, and Anticancer Effects of Caralluma Europaea Extracts. Molecules 2023, 28, 1780. [Google Scholar] [CrossRef] [PubMed]
- Radi, F.Z.; Bencheikh, N.; Bouhrim, M.; Saleh, A.; Al kamaly, O.; Parvez, M.K.; Elbouzidi, A.; Bnouham, M.; Zair, T. Phytochemical Analysis, Antioxidant, and Antihyperglycemic Activities of Crataegus Monogyna Jacq Aqueous Extract. Nat. Prod. Commun. 2023, 18, 1934578X231195157. [Google Scholar] [CrossRef]
- Fakchich, J.; Elachouri, M. An Overview on Ethnobotanico-Pharmacological Studies Carried out in Morocco, from 1991 to 2015: Systematic Review (Part 1). J. Ethnopharmacol. 2021, 267, 113200. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Liu, P. Composition and Health Effects of Phenolic Compounds in Hawthorn (Crataegus Spp.) of Different Origins. J. Sci. Food Agric. 2012, 92, 1578–1590. [Google Scholar] [CrossRef] [PubMed]
- Cui, T.; Li, J.-Z.; Kayahara, H.; Ma, L.; Wu, L.-X.; Nakamura, K. Quantification of the Polyphenols and Triterpene Acids in Chinese Hawthorn Fruit by High-Performance Liquid Chromatography. J. Agric. Food Chem. 2006, 54, 4574–4581. [Google Scholar] [CrossRef] [PubMed]
- Zahrae Radi, F.; Bencheikh, N.; Anarghou, H.; Bouhrim, M.; Alqahtani, A.S.; Hawwal, M.F.; Noman, O.M.; Bnouham, M.; Zair, T. Quality Control, Phytochemical Profile, and Biological Activities of Crataegus Monogyna Jacq. and Crataegus Laciniata Ucria Fruits Aqueous Extracts. Saudi Pharm. J. 2023, 31, 101753. [Google Scholar] [CrossRef] [PubMed]
- Abuashwashi, M.A.; Palomino, O.M.; Gómez-Serranillos, M.P. Geographic Origin Influences the Phenolic Composition and Antioxidant Potential of Wild Crataegus Monogyna from Spain. Pharm. Biol. 2016, 54, 2708–2713. [Google Scholar] [CrossRef]
- Shakya, A.K. Medicinal Plants: Future Source of New Drugs. Int. J. Herb. Med. 2016, 4, 59–64. [Google Scholar]
- Mssillou, I.; Agour, A.; Hamamouch, N.; Badiaa, L.; Derwich, E. Chemical Composition and In Vitro Antioxidant and Antimicrobial Activities of Marrubium Vulgare L. Sci. World J. 2021, 2021, 7011493. [Google Scholar] [CrossRef]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.S.; Viera, A.J.; Mead, M.D. Leukemia: An Overview for Primary Care. afp 2014, 89, 731–738. [Google Scholar]
- Deschler, B.; Lübbert, M. Acute Myeloid Leukemia: Epidemiology and Etiology. Cancer 2006, 107, 2099–2107. [Google Scholar] [CrossRef] [PubMed]
- Maher, T.; Ahmad Raus, R.; Daddiouaissa, D.; Ahmad, F.; Adzhar, N.S.; Latif, E.S.; Abdulhafiz, F.; Mohammed, A. Medicinal Plants with Anti-Leukemic Effects: A Review. Molecules 2021, 26, 2741. [Google Scholar] [CrossRef] [PubMed]
- Chander, V.; Gangenahalli, G. Emerging Strategies for Enhancing the Homing of Hematopoietic Stem Cells to the Bone Marrow after Transplantation. Exp. Cell Res. 2020, 390, 111954. [Google Scholar] [CrossRef] [PubMed]
- Woll, P.S.; Yoshizato, T.; Hellström-Lindberg, E.; Fioretos, T.; Ebert, B.L.; Jacobsen, S.E.W. Targeting Stem Cells in Myelodysplastic Syndromes and Acute Myeloid Leukemia. J. Intern. Med. 2022, 292, 262–277. [Google Scholar] [CrossRef]
- Herrmann, J. Adverse Cardiac Effects of Cancer Therapies: Cardiotoxicity and Arrhythmia. Nat. Rev. Cardiol. 2020, 17, 474–502. [Google Scholar] [CrossRef] [PubMed]
- Was, H.; Borkowska, A.; Bagues, A.; Tu, L.; Liu, J.Y.H.; Lu, Z.; Rudd, J.A.; Nurgali, K.; Abalo, R. Mechanisms of Chemotherapy-Induced Neurotoxicity. Front. Pharmacol. 2022, 13, 750507. [Google Scholar] [CrossRef] [PubMed]
- Tao, G.; Huang, J.; Moorthy, B.; Wang, C.; Hu, M.; Gao, S.; Ghose, R. Potential Role of Drug Metabolizing Enzymes in Chemotherapy-Induced Gastrointestinal Toxicity and Hepatotoxicity. Expert. Opin. Drug Metab. Toxicol. 2020, 16, 1109–1124. [Google Scholar] [CrossRef]
- Akin, A.T.; Öztürk, E.; Kaymak, E.; Karabulut, D.; Yakan, B. Therapeutic Effects of Thymoquinone in Doxorubicin-Induced Hepatotoxicity via Oxidative Stress, Inflammation and Apoptosis. Anat. Histol. Embryol. 2021, 50, 908–917. [Google Scholar] [CrossRef]
- Bhushan, B.; Apte, U. Liver Regeneration after Acetaminophen Hepatotoxicity: Mechanisms and Therapeutic Opportunities. Am. J. Pathol. 2019, 189, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Kurek, M.; Benaida-Debbache, N.; Elez Garofulić, I.; Galić, K.; Avallone, S.; Voilley, A.; Waché, Y. Antioxidants and Bioactive Compounds in Food: Critical Review of Issues and Prospects. Antioxidants 2022, 11, 742. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, F.; Perrone, A.; Yousefi, S.; Papini, A.; Castiglione, S.; Guarino, F.; Cicatelli, A.; Aelaei, M.; Arad, N.; Gholami, M.; et al. Botanical, Phytochemical, Anti-Microbial and Pharmaceutical Characteristics of Hawthorn (Crataegus Monogyna Jacq.), Rosaceae. Molecules 2021, 26, 7266. [Google Scholar] [CrossRef] [PubMed]
- Basavarajappa, G.M.; Rehman, A.; Shiroorkar, P.N.; Sreeharsha, N.; Anwer, M.K.; Aloufi, B. Therapeutic Effects of Crataegus Monogyna Inhibitors against Breast Cancer. Front. Pharmacol. 2023, 14, 1187079. [Google Scholar] [CrossRef] [PubMed]
- Sokół-Łętowska, A.; Oszmiański, J.; Wojdyło, A. Antioxidant Activity of the Phenolic Compounds of Hawthorn, Pine and Skullcap. Food Chem. 2007, 103, 853–859. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Habtemariam, S.; Ahmed, T.; Sureda, A.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.M. Polyphenolic Composition of Crataegus Monogyna Jacq.: From Chemistry to Medical Applications. Nutrients 2015, 7, 7708–7728. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Adachi, S.; Masuda, M.; Kozawa, O.; Moriwaki, H. Cancer Chemoprevention with Green Tea Catechins by Targeting Receptor Tyrosine Kinases. Mol. Nutr. Food Res. 2011, 55, 832–843. [Google Scholar] [CrossRef] [PubMed]
- Larsen, C.A.; Dashwood, R.H.; Bisson, W.H. Tea Catechins as Inhibitors of Receptor Tyrosine Kinases: Mechanistic Insights and Human Relevance. Pharmacol. Res. 2010, 62, 457–464. [Google Scholar] [CrossRef]
- Huang, X.; Li, X.; Xie, M.; Huang, Z.; Huang, Y.; Wu, G.; Peng, Z.; Sun, Y.; Ming, Q.; Liu, Y.; et al. Resveratrol: Review on Its Discovery, Anti-Leukemia Effects and Pharmacokinetics. Chem. Biol. Interact. 2019, 306, 29–38. [Google Scholar] [CrossRef]
- Cheng, S.; Gao, N.; Zhang, Z.; Chen, G.; Budhraja, A.; Ke, Z.; Son, Y.; Wang, X.; Luo, J.; Shi, X. Quercetin Induces Tumor-Selective Apoptosis through Downregulation of Mcl-1 and Activation of Bax. Clin. Cancer Res. 2010, 16, 5679–5691. [Google Scholar] [CrossRef]
- Zhou, J.; Li, L.; Fang, L.; Xie, H.; Yao, W.; Zhou, X.; Xiong, Z.; Wang, L.; Li, Z.; Luo, F. Quercetin Reduces Cyclin D1 Activity and Induces G1 Phase Arrest in HepG2 Cells. Oncol. Lett. 2016, 12, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Imran, M.; Khan, I.A.; ur-Rehman, M.-; Gilani, S.A.; Mehmood, Z.; Mubarak, M.S. Anticancer Potential of Quercetin: A Comprehensive Review. Phytother. Res. 2018, 32, 2109–2130. [Google Scholar] [CrossRef] [PubMed]
- Wyspiańska, D.; Kucharska, A.Z.; Sokół-Łętowska, A.; Kolniak-Ostek, J. Physico-Chemical, Antioxidant, and Anti-Inflammatory Properties and Stability of Hawthorn (Crataegus monogyna Jacq.) Procyanidins Microcapsules with Inulin and Maltodextrin. J. Sci. Food Agric. 2017, 97, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Ahumada, C.; Sáenz, T.; García, D.; De La Puerta, R.; Fernandez, A.; Martinez, E. The Effects of a Triterpene Fraction Isolated from Crataegus Monogyna Jacq. on Different Acute Inflammation Models in Rats and Mice. Leucocyte Migration and Phospholipase A2 Inhibition. J. Pharm. Pharmacol. 1997, 49, 329–331. [Google Scholar] [CrossRef] [PubMed]
- Nigro, O.; Tuzi, A.; Tartaro, T.; Giaquinto, A.; Vallini, I.; Pinotti, G. Biological Effects of Verbascoside and Its Anti-Inflammatory Activity on Oral Mucositis: A Review of the Literature. Anti-Cancer Drugs 2020, 31, 1. [Google Scholar] [CrossRef] [PubMed]
- Speranza, L.; Franceschelli, S.; Pesce, M.; Reale, M.; Menghini, L.; Vinciguerra, I.; De Lutiis, M.A.; Felaco, M.; Grilli, A. Antiinflammatory Effects in THP-1 Cells Treated with Verbascoside. Phytother. Res. 2010, 24, 1398–1404. [Google Scholar] [CrossRef] [PubMed]
- Alipieva, K.; Korkina, L.; Orhan, I.E.; Georgiev, M.I. Verbascoside—A Review of Its Occurrence, (Bio)Synthesis and Pharmacological Significance. Biotechnol. Adv. 2014, 32, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Jang, E.; Lee, J.-H. Potential Roles and Key Mechanisms of Hawthorn Extract against Various Liver Diseases. Nutrients 2022, 14, 867. [Google Scholar] [CrossRef] [PubMed]
- Rezaei-Golmisheh, A.; Malekinejad, H.; Asri-Rezaei, S.; Farshid, A.A.; Akbari, P. Hawthorn Ethanolic Extracts with Triterpenoids and Flavonoids Exert Hepatoprotective Effects and Suppress the Hypercholesterolemia-Induced Oxidative Stress in Rats. Iran. J. Basic. Med. Sci. 2015, 18, 691–699. [Google Scholar] [PubMed]
- Remita, F.; Abdennour, C.; Talbi, A.; Khelili, K. The Aqueous Extract of Fruits and Leaves of Crateagus Monogyna Jacq. in Mitigating Copper Sulphate-Induced Hepatotoxicity and Nephrotoxicity of Wistar Rats. Int. J. Minor. Fruits Med. Aromat. Plants 2020, 6, 20–27. [Google Scholar]
- Kiokias, S.; Proestos, C.; Oreopoulou, V. Effect of Natural Food Antioxidants against LDL and DNA Oxidative Changes. Antioxidants 2018, 7, 133. [Google Scholar] [CrossRef] [PubMed]
- Miltonprabu, S.; Tomczyk, M.; Skalicka-Woźniak, K.; Rastrelli, L.; Daglia, M.; Nabavi, S.F.; Alavian, S.M.; Nabavi, S.M. Hepatoprotective Effect of Quercetin: From Chemistry to Medicine. Food Chem. Toxicol. 2017, 108, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Pingili, R.B.; Challa, S.R.; Pawar, A.K.; Toleti, V.; Kodali, T.; Koppula, S. A Systematic Review on Hepatoprotective Activity of Quercetin against Various Drugs and Toxic Agents: Evidence from Preclinical Studies. Phytother. Res. 2020, 34, 5–32. [Google Scholar] [CrossRef] [PubMed]
- National Research Council Committee. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Collection: Reports funded by National Institutes of Health; National Academies Press (US): Washington, DC, USA, 2011; ISBN 978-0-309-15400-0. [Google Scholar]
- Mssillou, I.; Agour, A.; Slighoua, M.; Tourabi, M.; Nouioura, G.; Lyoussi, B.; Derwich, E. Phytochemical Characterization, Antioxidant Activity, and in Vitro Investigation of Antimicrobial Potential of Dittrichia viscosa L. Leaf Extracts against Nosocomial Infections. Acta Ecol. Sin. 2022, 42, 661–669. [Google Scholar] [CrossRef]
- Amrati, F.E.-Z.; Elmadbouh, O.H.M.; Chebaibi, M.; Soufi, B.; Conte, R.; Slighoua, M.; Saleh, A.; Al Kamaly, O.; Drioiche, A.; Zair, T.; et al. Evaluation of the Toxicity of Caralluma Europaea (C.E) Extracts and Their Effects on Apoptosis and Chemoresistance in Pancreatic Cancer Cells. J. Biomol. Struct. Dyn. 2023, 41, 8517–8534. [Google Scholar] [CrossRef] [PubMed]
- De Castro Alves, C.E.; Koidan, G.; Hurieva, A.N.; de Freitas Gomes, A.; Costa de Oliveira, R.; Guimarães Costa, A.; Ribeiro Boechat, A.L.; Correa de Oliveira, A.; Zahorulko, S.; Kostyuk, A.; et al. Cytotoxic and Immunomodulatory Potential of a Novel [2-(4-(2,5-Dimethyl-1H-Pyrrol-1-Yl)−1H-Pyrazol-3-Yl)Pyridine] in Myeloid Leukemia. Biomed. Pharmacother. 2023, 162, 114701. [Google Scholar] [CrossRef] [PubMed]
- Amrati, F.E.-Z.; Slighoua, M.; Mssillou, I.; Chebaibi, M.; Galvão de Azevedo, R.; Boukhira, S.; Moslova, K.; Al Kamaly, O.; Saleh, A.; Correa de Oliveira, A.; et al. Lipids Fraction from Caralluma Europaea (Guss.): MicroTOF and HPLC Analyses and Exploration of Its Antioxidant, Cytotoxic, Anti-Inflammatory, and Wound Healing Effects. Separations 2023, 10, 172. [Google Scholar] [CrossRef]
- Amrati, F.E.-Z.; Bourhia, M.; Saghrouchni, H.; Slighoua, M.; Grafov, A.; Ullah, R.; Ezzeldin, E.; Mostafa, G.A.; Bari, A.; Ibenmoussa, S.; et al. Caralluma Europaea (Guss.) N.E.Br.: Anti-Inflammatory, Antifungal, and Antibacterial Activities against Nosocomial Antibiotic-Resistant Microbes of Chemically Characterized Fractions. Molecules 2021, 26, 636. [Google Scholar] [CrossRef] [PubMed]
- Napagoda, M.; Jayasinghe, L. Chemistry of Natural Products: Phytochemistry and Pharmacognosy of Medicinal Plants; Napagoda, M., Jayasinghe, L., Eds.; De Gruyter: Berlin, Germany, 2022; ISBN 978-3-11-059594-9. [Google Scholar]
- Autissier, C. Réglementation éthique de l’expérimentation animale en recherche biomédicale. Med. Sci. 2008, 24, 437–442. [Google Scholar] [CrossRef]
- Amrati, F.E.-Z.; Bourhia, M.; Slighoua, M.; Boukhira, S.; Ullah, R.; Ezzeldin, E.; Mostafa, G.A.E.; Grafov, A.; Bousta, D. Protective Effect of Chemically Characterized Polyphenol-Rich Fraction from Apteranthes Europaea (Guss.) Murb. Subsp. Maroccana (Hook.f.) Plowes on Carbon Tetrachloride-Induced Liver Injury in Mice. Appl. Sci. 2021, 11, 554. [Google Scholar] [CrossRef]
- Aboul-Soud, M.A.M.; Ennaji, H.; Kumar, A.; Alfhili, M.A.; Bari, A.; Ahamed, M.; Chebaibi, M.; Bourhia, M.; Khallouki, F.; Alghamdi, K.M.; et al. Antioxidant, Anti-Proliferative Activity and Chemical Fingerprinting of Centaurea Calcitrapa against Breast Cancer Cells and Molecular Docking of Caspase-3. Antioxidants 2022, 11, 1514. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.; jawaid, T.; Alam, P. In Vitro Antioxidant and Anti-Inflammatory Activities of Green Cardamom Essential Oil and in Silico Molecular Docking of Its Major Bioactives. J. Taibah Univ. Sci. 2021, 15, 757–768. [Google Scholar] [CrossRef]
- Costea, L.; Chițescu, C.L.; Boscencu, R.; Ghica, M.; Lupuliasa, D.; Mihai, D.P.; Deculescu-Ioniță, T.; Duțu, L.E.; Popescu, M.L.; Luță, E.-A.; et al. The Polyphenolic Profile and Antioxidant Activity of Five Vegetal Extracts with Hepatoprotective Potential. Plants 2022, 11, 1680. [Google Scholar] [CrossRef] [PubMed]
- Ouahabi, S.; Loukili, E.H.; Daoudi, N.E.; Chebaibi, M.; Ramdani, M.; Rahhou, I.; Bnouham, M.; Fauconnier, M.-L.; Hammouti, B.; Rhazi, L.; et al. Study of the Phytochemical Composition, Antioxidant Properties, and In Vitro Anti-Diabetic Efficacy of Gracilaria Bursa-Pastoris Extracts. Mar. Drugs 2023, 21, 372. [Google Scholar] [CrossRef]
- Kumar, A.; Alfhili, M.A.; Bari, A.; Ennaji, H.; Ahamed, M.; Bourhia, M.; Chebaibi, M.; Benbacer, L.; Ghneim, H.K.; Abudawood, M.; et al. Apoptosis-Mediated Anti-Proliferative Activity of Calligonum Comosum against Human Breast Cancer Cells, and Molecular Docking of Its Major Polyphenolics to Caspase-3. Front. Cell Dev. Biol. 2022, 10, 972111. [Google Scholar] [CrossRef]
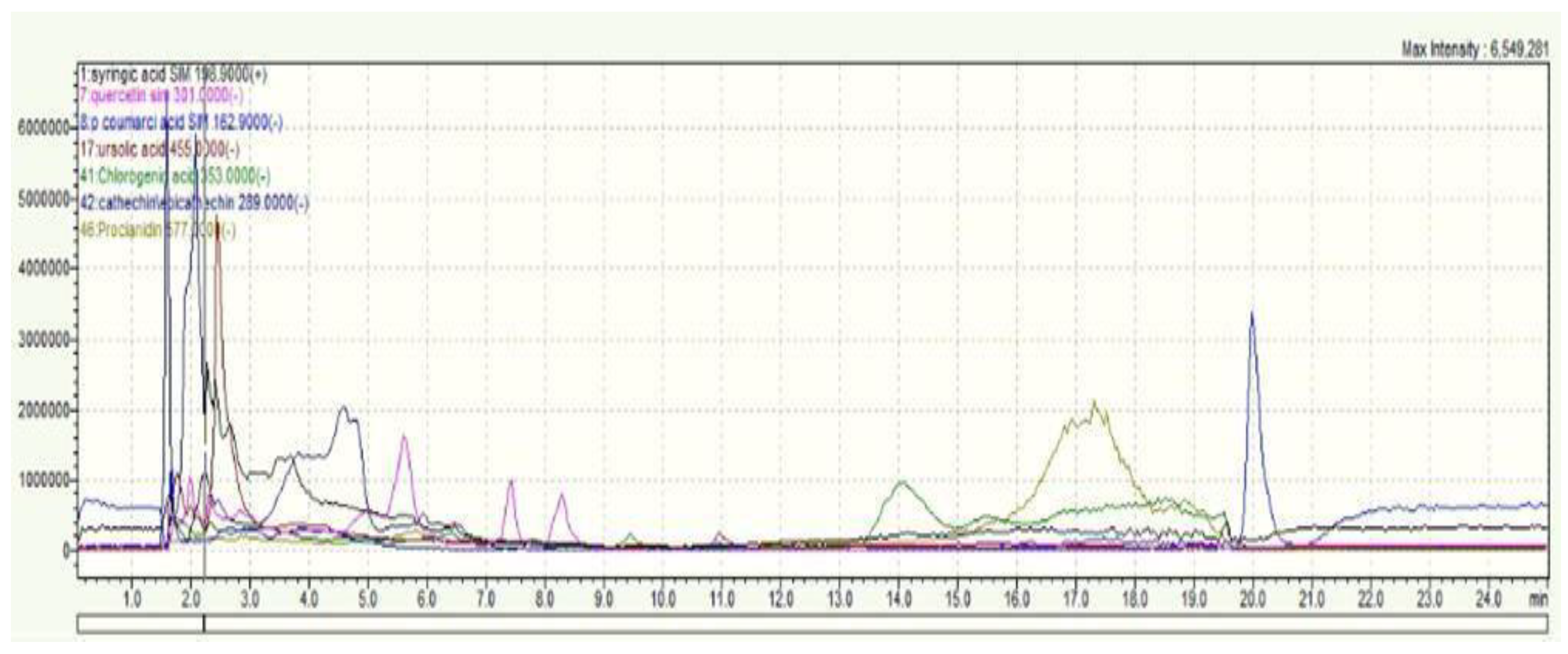


| Compounds | m/z | mg/100 g |
|---|---|---|
| Syringic acid | 197.000 | 123 |
| Quercetin | 302.236 | 171 |
| p-coumaric acid | 164.047 | 35.2 |
| Resveratrol | 228.250 | 353 |
| Verbascoside | 624.590 | 129 |
| Catechin\Epicatehcin | 289.00 | 458 |
| Chlorogenic acid | 353.00 | 415 |
| Ursolic acid | 455.00 | 329 |
| Procyanidin | 577.00 | 1356 |
| Treatment | Diameter (cm) and % of Inhibition | ||||
|---|---|---|---|---|---|
| 0 h | 3 h | 4 h | 5 h | 6 h | |
| Control NaCl (0.9%) | 2.22 ± 0.07 | 2.48 ± 0.09 | 2.51 ± 0.06 | 2.59 ± 0.06 | 2.59 ± 0.05 |
| Indomethacin®10 mg/kg | 2.25 ± 0.02 | 2.45 ± 0.02 * 23.08% | 2.39 ± 0.02 * 50.86% | 2.32 ± 0.01 *** 79.73% | 2.29 ± 0.02 *** 89.19% |
| ET CM 300 mg/kg | 2.30 ± 0.05 | 2.56 ± 0.08 * 21.79% | 2.40 ± 0.07 * 42.53% | 2.39 ± 0.07 ** 57.66% | 2.30 ± 0.05 *** 76.58% |
| ET CM 1000 mg/kg | 2.43 ± 0.07 | 2.60 ± 0.07 ** 32.21% | 2.53 ± 0.07 ** 64.22% | 2.50 ± 0.07 *** 79.39% | 2.49 ± 0.07 *** 84.12% |
| Organ | Negative Control | Positive Control | ET CM 300 mg/kg | ET CM 1000 mg/kg |
|---|---|---|---|---|
| Liver | 4.46 ± 0.18 | 5.85 ± 0.08 ** | 4.64 ± 0.15 | 3.67 ± 0.20 * |
| Kidney | 1.11 ± 0.01 | 1.53 ± 0.13 * | 1.45 ± 0.11 | 1.46 ± 0.02 |
| Spleen | 0.44 ± 0.03 | 0.62 ± 0.10 | 0.51 ± 0.05 | 0.46 ± 0.01 |
| Glide (Kcal/mol) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gscore | Emodel | Energy | Gscore | Emodel | Energy | Gscore | Emodel | Energy | Gscore | Emodel | Energy | |
| Lipoxygenase (PDB ID: 3V99) | Human Cytochrome P450 2E1 (PDB ID: 3LC4) | Tyrosine Kinase (PDB ID: 1IEP) | TRADD (PDB ID: 1F3V) | |||||||||
| Verbascoside | −6.84 | −99.65 | −66.227 | - | - | - | −7.588 | −96.653 | −69.125 | −6.57 | −94.159 | −68.309 |
| p-coumaric acid | −6.394 | −41.106 | −19.167 | −5.111 | −30.176 | −21.796 | −7.154 | −38.772 | −25.712 | −3.992 | −27.567 | −20.825 |
| Syringic acid | −6.333 | −43.066 | −18.439 | −5.335 | −15.566 | −14.302 | −6.075 | −28.892 | −24.675 | −4.794 | −32.686 | −24.841 |
| Resveratrol | −5.471 | −45.402 | −33.758 | −8.01 | −22.454 | −22.384 | −8.781 | −60.805 | −39.999 | −5.411 | −42.269 | −32.426 |
| Quercetin | −6.707 | −51.068 | −27.781 | −8.102 | −55.336 | −22.108 | −8.687 | −71.345 | −50.224 | −6.312 | −60.618 | −45.675 |
| Catechin | −5.681 | −53.357 | −36.181 | - | - | - | −9.037 | −72.876 | −48.08 | −6.6 | −58.723 | −42.595 |
| Chlorogenic acid | −6.132 | −56.833 | −32.473 | −6.292 | −20.511 | −19.19 | −5.188 | −57.195 | −47.258 | −4.656 | −54.38 | −43.533 |
| Ursolic acid | - | - | - | - | - | - | - | - | - | - | - | - |
| Procyanidin | −7.27 | −86.349 | −59.31 | - | - | - | −7.459 | −96.968 | −66.703 | −8.026 | −95.471 | −69.345 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ez-Zahra Amrati, F.; Mssillou, I.; Boukhira, S.; Djiddi Bichara, M.; El Abdali, Y.; Galvão de Azevedo, R.; Mohamed, C.; Slighoua, M.; Conte, R.; Kiokias, S.; et al. Phenolic Composition of Crataegus monogyna Jacq. Extract and Its Anti-Inflammatory, Hepatoprotective, and Antileukemia Effects. Pharmaceuticals 2024, 17, 786. https://doi.org/10.3390/ph17060786
Ez-Zahra Amrati F, Mssillou I, Boukhira S, Djiddi Bichara M, El Abdali Y, Galvão de Azevedo R, Mohamed C, Slighoua M, Conte R, Kiokias S, et al. Phenolic Composition of Crataegus monogyna Jacq. Extract and Its Anti-Inflammatory, Hepatoprotective, and Antileukemia Effects. Pharmaceuticals. 2024; 17(6):786. https://doi.org/10.3390/ph17060786
Chicago/Turabian StyleEz-Zahra Amrati, Fatima, Ibrahim Mssillou, Smahane Boukhira, Mehdi Djiddi Bichara, Youness El Abdali, Renata Galvão de Azevedo, Chebaibi Mohamed, Meryem Slighoua, Raffaele Conte, Sotirios Kiokias, and et al. 2024. "Phenolic Composition of Crataegus monogyna Jacq. Extract and Its Anti-Inflammatory, Hepatoprotective, and Antileukemia Effects" Pharmaceuticals 17, no. 6: 786. https://doi.org/10.3390/ph17060786
APA StyleEz-Zahra Amrati, F., Mssillou, I., Boukhira, S., Djiddi Bichara, M., El Abdali, Y., Galvão de Azevedo, R., Mohamed, C., Slighoua, M., Conte, R., Kiokias, S., Soares Pontes, G., & Bousta, D. (2024). Phenolic Composition of Crataegus monogyna Jacq. Extract and Its Anti-Inflammatory, Hepatoprotective, and Antileukemia Effects. Pharmaceuticals, 17(6), 786. https://doi.org/10.3390/ph17060786






