Physicochemical Compatibility of Ceftolozane-Tazobactam with Parenteral Nutrition
Abstract
1. Introduction
2. Results
2.1. Physical Stability
2.2. Chemical Stability
3. Discussion
4. Materials and Methods
4.1. General Procedures
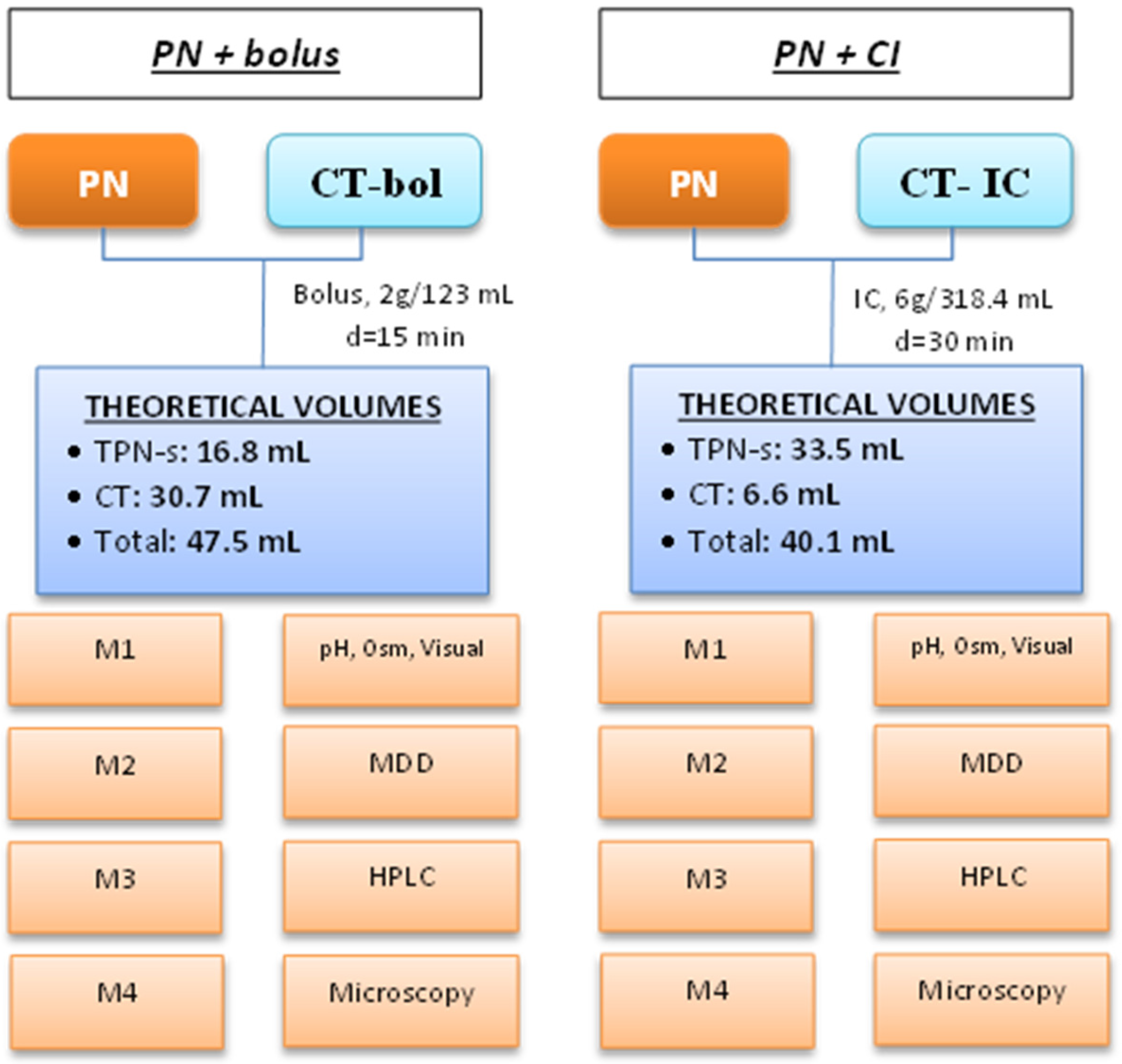
4.2. Composition of PN Emulsions and CT Solutions
4.3. Simulation of Y-Site Administration
4.4. Sample Collection, Storage, and Analysis
4.5. Stability Assessment
- The presence of macroscopic precipitates, changes in color, and phase separation were assessed according to the European Pharmacopeia [35]. Visual inspection was conducted against a black-and-white contrast background by two pharmacists. Visual inspection was also performed with PN emulsions, without the drug.
- Microscopy was assessed using a LEICA DM2500 LED microscope (Leica Microsistemas S.L.U. L’Hospitalet de Llobregat, Spain) by two observers at t = 0 h and t = 24 h. Ten µL of the mixture were assessed at 400× magnification (10× ocular lens and 40× objective lens). Admixtures with CT as a bolus and as continuous infusion concentrations were used as negative control solutions. We also assessed PN with no drug. PN with final unstable calcium and phosphate concentrations was used as a positive control. Each combination of drug and PN was prepared in triplicate.
- pH measurement was made using potentiometry (senIONTM+ PH 1, Hach, Spain) at room temperature.
- Osmolality was measured at room temperature (Osmo1, Advanced Instruments, Tecil, Spain).
- The particle size of the lipid emulsion was measured at 25 °C using dynamic light scattering (Zetasizer NanoZS90, Malvern Instruments Ltd., Malvern, UK) 6 h after the simulation. Samples for DLS were prepared by diluting the solutions into PBS at a final concentration of approximately 30 μg/mL. The results of particle diameter are presented as MDD. (Figure 2 and Supplementary Materials Figure S1)

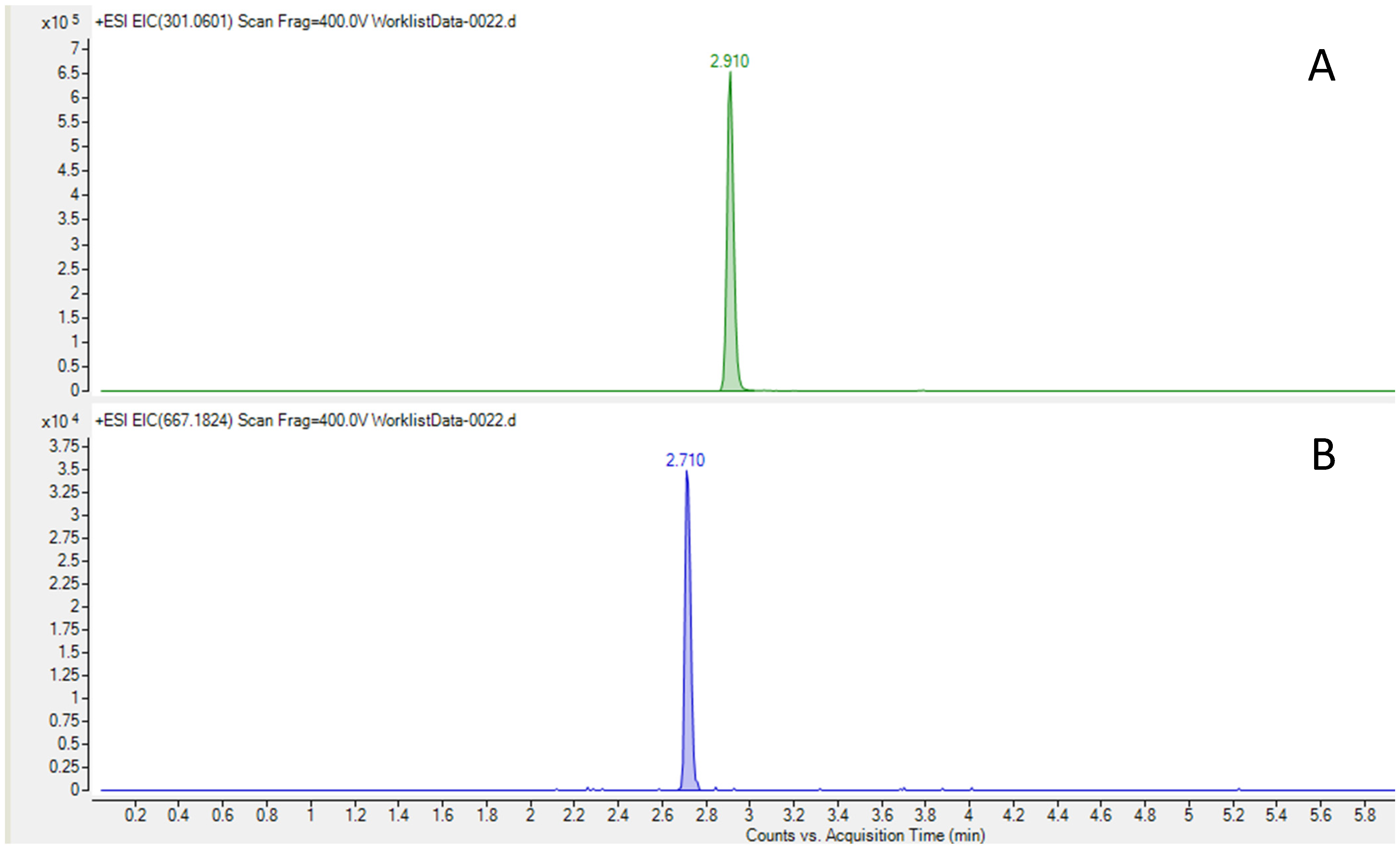
- HPLC–HRMS was used to quantify CT concentration in the admixtures. Samples were measured in triplicate. An Infinity II LC system coupled to a 6560-ion mobility QTOF mass spectrometer, both from Agilent Technologies (Santa Clara, CA, USA), was used in positive ionization mode from m/z 100–1700. A Kinetex F5 150 × 2.1 mm, 2.6 µm column from Phenomenex (Torrance, CA, USA) was used with mobile phase A (water and 0.1% formic acid and mobile phase B (Acetonitrile and 0.1% formic acid) at 0.4 mL/min, following the gradient (t(min), %B): (0, 2), (1, 2), (6, 95), (7.5, 95), (7.8, 2), and (10, 2). Samples were diluted 1/10,000 with ultrapure water in three steps and centrifuged at 10,000 rpm at 4 °C for 5 min. The injection volume was 2 µL. Trace chromatograms were extracted with a 3 ppm error at m/z 667.1824 for ceftolozane and m/z 301.0601 for tazobactam. (Figure 3) Calibration standards were prepared in a blank matrix diluted 1/10,000, and the weighting of calibration curves was adjusted (1/x or 1/x2) to obtain accuracies between 80 and 120%. The matrix-matched calibration curve was prepared in a blank sample diluted 1/10,000, which was spiked with standards at five concentrations between 0.1 and 4.5 mg/L. The calibration curve was prepared daily and was injected in triplicate each day of analysis. Regarding selectivity, the ability to distinguish the analyte from other substances was indicated by an absence of the respective peaks at the same retention time as the corresponding standards in trace chromatograms extracted with a 5 ppm error. Accuracy was calculated at the five spiked levels (based on the ratio between the calculated concentration and the spiked concentration) between 90.5 and 101.2% for tazobactam and between 82.6 and 111.1% for ceftolozane. Intra-day repeatability expressed as relative standard deviation (%) between 0.4 and 3.4% for ceftolozane and between 0.1 and 3.3% for tazobactam was obtained. Inter-day repeatability was between 2.1 and 12.8% for ceftolozane and between 0.8 and 15.9% for tazobactam. The limit of detection LOD, defined as S/N = 3, was 10 ng/mL for ceftolozane and 1 ng/mL for tazobactam.
- No changes in visual inspection, defined as a homogeneous admixture with no changes in color, no phase separation, and lack of macroscopic precipitates or gas formation altering the admixture, as assessed by two independent observers against a black-and-white contrast background at t = 0 h and t = 6 h [35].
- No signs of precipitation, emulsion disruption, or presence of particles >5 µm at microscopic inspection.
- No relevant CT concentration changes in the mixture, defined as <20% change at t = 24 h (measured using HPLC–HRMS) [15].
- No relevant particle size changes, defined as MDD ≤ 500 nm in all samples (United States Pharmacopeia), and percentage of particles greater than 5 µm < 0.05% according to the US pharmacopeia method I [38].
4.6. Data Analysis and Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Medicines Agency. Zerbaxa (Ceftolozane/Tazobactam) Summary of Product Characteristics [Internet]. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/zerbaxa (accessed on 1 February 2024).
- Puzniak, L.; Dillon, R.; Palmer, T.; Collings, H.; Enstone, A. Real-world use of ceftolozane/tazobactam: A systematic literature review. Antimicrob. Resist. Infect. Control 2021, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; van Duin, D.; Clancy, C.J. Infectious Diseases Society of America 2023 Guidance on the Treatment of Antimicrobial Resistant Gram-Negative Infections. Clin. Infect. Dis. 2023, 1–53. [Google Scholar] [CrossRef]
- Montravers, P.; Bassetti, M. The ideal patient profile for new beta-lactam/betalactamase inhibitors. Curr. Opin. Infect. Dis. 2018, 31, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Vardakas, K.Z.; Voulgaris, G.L.; Maliaros, A.; Samonis, G.; Falagas, M.E. Prolonged versus short-term intravenous infusion of antipseudomonal β-lactams for patients with sepsis: A systematic review and meta-analysis of randomised trials. Lancet Infect. Dis. 2018, 18, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Pilmis, B.; Petitjean, G.; Lesprit, P.; Lafaurie, M.; El Helali, N.; Le Monnier, A.; Dinh, A.; de Laroche, M.; Parquin, F.; Grenet, D.; et al. Continuous infusion of ceftolozane/tazobactam is associated with a higher probability of target attainment in patients infected with Pseudomonas aeruginosa. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1457–1461. [Google Scholar] [CrossRef] [PubMed]
- Veiga, R.P.; Paiva, J.-A. Pharmacokinetics-pharmacodynamics issues relevant for the clinical use of beta-lactam antibiotics in critically ill patients. Crit. Care 2018, 22, 233. [Google Scholar] [CrossRef] [PubMed]
- Tamma, P.D.; Beisken, S.; Bergman, Y.; Posch, A.E.; Avdic, E.; Sharara, S.L.; Cosgrove, S.E.; Simner, P.J. Modifiable Risk Factors for the Emergence of Ceftolozane-tazobactam Resistance. Clin. Infect. Dis 2021, 73, e4599–e4606. [Google Scholar] [CrossRef] [PubMed]
- Cober, M.P.; Stout, S.M. Cyclic Parenteral Nutrition Infusion: Considerations for the Clinician. Practical Gastroenterology, July 2011, pp. 11–24. Available online: https://med.virginia.edu/ginutrition/wp-content/uploads/sites/199/2014/06/StoutArticle.pdf (accessed on 15 January 2024).
- Singer, P.; Reintam, A.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P.; Hiesmayr, M.; Mayer, K.; Carlos, J.; Pichard, C.; et al. ESPEN Guideline ESPEN guideline on clinical nutrition in the intensive care unit. Clin. Nutr. 2019, 38, 48–79. [Google Scholar] [CrossRef] [PubMed]
- Compher, C.; Bingham, A.L.; McCall, M.; Patel, J.; Rice, T.W.; Braunschweig, C.; McKeever, L. Guidelines for the provision of nutrition support therapy in the adult critically ill patient: The American Society for Parenteral and Enteral Nutrition. J. Parenter. Enter. Nutr. 2022, 46, 12–41. [Google Scholar] [CrossRef]
- Hill, A.; Elke, G.; Weimann, A. Nutrition in the intensive care unit—A narrative review. Nutrients 2021, 13, 2851. [Google Scholar] [CrossRef]
- Boullata, J.I.; Mirtallo, J.M.; Sacks, G.S.; Salman, G.; Gura, K.; Canada, T.; Maguire, A. Parenteral nutrition compatibility and stability: A comprehensive review. J. Parenter. Enter. Nutr. 2022, 46, 273–299. [Google Scholar] [CrossRef] [PubMed]
- Farhan, M.; McCallion, N.; Bennett, J.; Cram, A.; O’Brien, F. Stability and compatibility of parenteral nutrition solutions; a review of influencing factors. Eur. J. Pharm. Biopharm. 2023, 187, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Aeberhard, C.; Steuer, C.; Saxer, C.; Huber, A.; Stanga, Z.; Mühlebach, S. Physicochemical stability and compatibility testing of levetiracetam in all-in-one parenteral nutrition admixtures in daily practice. Eur. J. Pharm. Sci. 2017, 96, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Bouchoud, L.; Fonzo-christe, C.; Klingmüller, M.; Bonnabry, P. Compatibility of Intravenous Medications With Parenteral Nutrition: In Vitro Evaluation. J. Parenter. Enter. Nutr. 2013, 37, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Campos-Baeta, Y.; Saavedra-Mitjans, M.; Garin, N.; Cardenete, J.; Cardona, D.; Riera, P. Physicochemical Compatibility of Dexmedetomidine With Parenteral Nutrition. Nutr. Clin. Pract. 2020, 35, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Stawny, M.; Gostyńska, A.; Dettlaff, K.; Jelińska, A.; Główka, E.; Ogrodowczyk, M. Effect of lipid emulsion on stability of ampicillin in total parenteral nutrition. Nutrients 2019, 11, 559. [Google Scholar] [CrossRef] [PubMed]
- Stawny, M.; Gostyńska, A.; Nadolna, M.; Jelińska, A. Safe practice of Y-site drug administration: The case of colistin and parenteral nutrition. Pharmaceutics 2020, 12, 292. [Google Scholar] [CrossRef] [PubMed]
- Stawny, M.; Nadolna, M.; Jeli, A. In vitro compatibility studies of vancomycin with ready-to-use parenteral nutrition admixtures for safer clinical practice. Clin. Nutr. 2020, 39, 2539–2546. [Google Scholar] [CrossRef] [PubMed]
- Gostyńska, A.; Stawny, M.; Dettlaff, K.; Jelińska, A. The interactions between ciprofloxacin and parenteral nutrition admixtures. Pharmaceutics 2020, 12, 27. [Google Scholar] [CrossRef]
- Tomczak, S.; Stawny, M.; Dettlaff, K.; Kieliszek, M.; Słomińska, D.; Jelińska, A. Physicochemical compatibility and stability of linezolid with parenteral nutrition. Molecules 2019, 24, 1242. [Google Scholar] [CrossRef]
- Gostyńska, A.; Piwowarczyk, L.; Nadolna, M.; Jelińska, A.; Dettlaff, K.; Ogrodowczyk, M.; Popielarz-Brzezińska, M.; Stawny, M. Toward safe pharmacotherapy: The interplay between meropenem and parenteral nutrition admixtures. Antibiotics 2021, 10, 217. [Google Scholar] [CrossRef] [PubMed]
- Dettlaff, K.; Stawny, M.; Gostyńska, A.; Popielarz-Brzezińska, M.; Ogrodowczyk, M. Compatibility of intravenous metronidazole with some all-in-one parenteral nutrition regimens. Nutrition 2021, 84, 20. [Google Scholar] [CrossRef] [PubMed]
- Tomczak, S.; Gostyńska, A.; Nadolna, M.; Reisner, K.; Orlando, M.; Jelińska, A.; Stawny, M. Stability and Compatibility Aspects of Drugs: The Case of Selected Cephalosporins. Antibiotics 2021, 10, 549. [Google Scholar] [CrossRef] [PubMed]
- Négrier, L.; Martin Mena, A.; Lebuffe, G.; Odou, P.; Genay, S.; Décaudin, B. Strategies to prevent drug incompatibility during simultaneous multi-drug infusion in intensive care units: A literature review. Eur. J. Clin. Pharmacol. 2021, 77, 1309–1321. [Google Scholar] [CrossRef] [PubMed]
- Gostyńska, A.; Przybylski, T.; Ogrodowczyk, M. Y-Site Compatibility Studies of Parenteral Nutrition and Other Intravenous Medications in Neonatal and Pediatric Patients: A Review of the Literature Evidence. Pharmaceutics 2024, 16, 264. [Google Scholar] [CrossRef] [PubMed]
- Berlana, D. Parenteral Nutrition Overview. Nutrients 2022, 14, 4480. [Google Scholar] [CrossRef]
- McClave, S.A.; Taylor, B.E.; Martindale, R.G.; Warren, M.M.; Johnson, D.R.; Braunschweig, C.; McCarthy, M.S.; Davanos, E.; Rice, T.W.; Cresci, G.A.; et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN). J. Parenter. Enter. Nutr. 2016, 40, 159–211. [Google Scholar] [CrossRef]
- Pradelli, L.; Klek, S.; Mayer, K.; Omar Alsaleh, A.J.; Rosenthal, M.D.; Heller, A.R.; Muscaritoli, M. Omega-3 fatty acid-containing parenteral nutrition in ICU patients: Systematic review with meta-analysis and cost-effectiveness analysis. Crit. Care 2020, 24, 634. [Google Scholar] [CrossRef] [PubMed]
- Pradelli, L.; Mayer, K.; Klek, S.; Rosenthal, M.D.; Povero, M.; Heller, A.R.; Muscaritoli, M. Omega-3 fatty acids in parenteral nutrition—A systematic review with network meta-analysis on clinical outcomes. Clin. Nutr. 2023, 42, 590–599. [Google Scholar] [CrossRef]
- Thabit, A.K.; Hamada, Y.; Nicolau, D.P. Physical compatibility of ceftolozane–tazobactam with selected i.v. drugs during simulated Y-site. Am. J. Health-Syst. Pharm. 2017, 74, 47–54. [Google Scholar] [CrossRef]
- Jones, B.M.; Huelfer, K.; Bland, C.M. Clinical and safety evaluation of continuously infused ceftolozane/ tazobactam in the outpatient setting. Open Forum Infect. Dis. 2020, 7, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Kratzer, A.; Rothe, U.; Dorn, C. NP-008 Stability of ceftolozane/tazobactam in solution as infusion for prolonged or continuous application. Eur. J. Hosp. Pharm. 2019, 26 (Suppl 1), A294. [Google Scholar] [CrossRef]
- Mediavilla, M.M.; Molina, A.; Navarro, L.; Grau, L.; Pujol, M.D.; Cardenete, J.; Cardona, D.; Riera, P. Physicochemical Compatibility of Amiodarone with Parenteral Nutrition. J. Parenter. Enter. Nutr. 2019, 43, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Riera, P.; Garrido-Alejos, G.; Cardenete, J.; Moliner, E.; Zapico-Muñiz, E.; Cardona, D.; Garin, N. Physicochemical Stability and Sterility of Standard Parenteral Nutrition Solutions and Simulated Y-Site Admixtures for Neonates. Nutr. Clin. Pract. 2018, 33, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.M.; Villareal, C.L.; Meyer, L.M. Y-Site Compatibility of Intravenous Levetiracetam With Commonly Used Critical Care Medications. Hosp. Pharm. 2021, 56, 282–286. [Google Scholar] [CrossRef] [PubMed]
- European Council. Monograph 04/2015:0520. In European Council, European Pharmacopoeia 9.0, 9th ed.; European Council: Strasbourg, France, 2017; pp. 2841–2843. [Google Scholar]
- Klang, M.G. PFAT5 and the Evolution of Lipid Admixture Stability. J. Parenter. Enter. Nutr. 2015, 39, 67S–71S. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, D.F.; Etzler, F.; Barber, T.A.; Nehne, J.; Niemann, W.; Bistrian, B.R. Physicochemical assessments of parenteral lipid emulsions: Light obscuration versus laser diffraction. Int. J. Pharm. 2001, 219, 21–37. [Google Scholar] [CrossRef] [PubMed]
- United States Pharmacopeial Convention. The United States Pharmacopeia and National Formulary, 38th ed.; United States Pharmacopeial Convention: Rockville, MD, USA, 2015. [Google Scholar]
- Tomczak, S.; Stawny, M.; Jelińska, A. Co-administration of drugs and parenteral nutrition: In vitro compatibility studies of loop diuretics for safer clinical practice. Pharmaceutics 2020, 12, 1092. [Google Scholar] [CrossRef] [PubMed]
- Otero-Millán, L.; Lago Rivero, N.; Blanco Rodicio, A.; García Beloso, N.; Legido Soto, J.L.; Piñeiro-Corrales, G. Stability of lipid emulsion in total parenteral nutrition: An overview of literature. Clin. Nutr. ESPEN 2021, 45, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Tomczak, S.; Chmielewski, M.; Szkudlarek, J.; Jelińska, A. Antiemetic Drugs Compatibility Evaluation with Paediatric Parenteral Nutrition Admixtures. Pharmaceutics 2023, 15, 2143. [Google Scholar] [CrossRef]
- Piwowarczyk, L.; Tomczak, S.; Antkowiak, P.; Jelińska, A.; Stawny, M. Sodium Valproate Incompatibility with Parenteral Nutrition Admixtures—A Risk to Patient Safety: An In Vitro Evaluation Study. Pharmaceutics 2022, 14, 371. [Google Scholar] [CrossRef] [PubMed]
- Worthington, P.; Gura, K.M.; Kraft, M.D.; Nishikawa, R.; Guenter, P.; Sacks, G.S. Update on the Use of Filters for Parenteral Nutrition: An ASPEN Position Paper. Nutr. Clin. Pract. 2021, 36, 29–39. [Google Scholar] [CrossRef] [PubMed]
| Sample | MDD |
|---|---|
| PN1 | 276.1 ± 7.7 nm |
| PN2 | 286.8 ± 4.7 nm |
| PN3 | 284.0 ± 6.5 nm |
| PN1-bolus1 | 286.5 ± 8.9 nm |
| PN2-bolus2 | 284.7 ± 4.6 nm |
| PN3-bolus3 | 282.3 ± 6.3 nm |
| PN1-IC1 | 279.2 ± 4.0 nm |
| PN2-IC2 | 285.4 ± 2.8 nm |
| PN3-IC3 | 294.2 ± 7.3 nm |
| Sample | Osmolality (mOsm/kg) t = 0 h | Osmolality (mOsm/kg) t = 6 h | Percentage of Initial Osmolality |
|---|---|---|---|
| PN1 | 1944.3 ± 31.6 | 1931.7 ± 21.1 | −0.7% |
| PN2 | 1940 ± 11.1 | 1922.7 ± 23 | −0.9% |
| PN3 | 1920.3 ± 17.1 | 1910.3 ± 12.1 | −0.5% |
| PN1-bolus1 | 1171 ± 6.6 | 1149 ± 2.6 | −1.9% |
| PN2-bolus2 | 1063.3 ± 7.1 | 1053.3 ± 4.6 | −0.9% |
| PN3-bolus3 | 1076.3 ± 21 | 1060.3 ± 8.6 | −1.5% |
| PN1-IC1 | 1741 ± 23.4 | 1714 ± 5.3 | −1.6% |
| PN2-IC2 | 1808 ± 6.6 | 1744.3 ± 11.2 | −3.5% |
| PN3-IC3 | 1695.3 ± 16.7 | 1709 ± 12.1 | +0.8% |
| Sample | Concentration (mg/mL) t = 0 h | Concentration (mg/mL) t = 24 h | Percentage of the Initial Concentration |
|---|---|---|---|
| PN1-bolus1 | 8.64 ± 0.32 | 9.08 ± 0.57 | +5% |
| PN2-bolus2 | 8.21 ± 0.53 | 9.58 ± 0.61 | +17% |
| PN3-bolus3 | 8.45 ± 0.06 | 9.44 ± 0.44 | +12% |
| PN1-IC1 | 1.46 ± 0.05 | 1.32 ± 0.01 | −10% |
| PN2-IC2 | 1.45 ± 0.04 | 1.35 ± 0.01 | −7% |
| PN3-IC3 | 1.81 ± 0.07 | 1.83 ± 0.04 | +1% |
| Sample | Concentration (mg/mL) t = 0 h | Concentration (mg/mL) t = 24 h | Percentage of the Initial Concentration |
|---|---|---|---|
| PN1-bolus1 | 4.25 ± 0.10 | 4.16 ± 0.04 | −2% |
| PN2-bolus2 | 4.33 ± 0.23 | 4.37 ± 0.01 | +1% |
| PN3-bolus3 | 4.32 ± 0.11 | 4.09 ± 0.28 | −5% |
| PN1-IC1 | 0.78 ± 0.01 | 0.78 ± 0.01 | 0% |
| PN2-IC2 | 0.80 ± 0.02 | 0.77 ± 0.04 | −4% |
| PN3-IC3 | 1.09 ± 0.05 | 1.07 ± 0.02 | −2% |
| Sample | Concentration Ratio t = 0 h | Concentration Ratio t = 24 h | Percentage of the Initial Concentration Ratio |
|---|---|---|---|
| PN1-bolus1 | 2.03 | 2.18 | +7% |
| PN2-bolus2 | 1.89 | 2.19 | +16% |
| PN3-bolus3 | 1.96 | 2.31 | +18% |
| PN1-IC1 | 1.87 | 1.70 | −9% |
| PN2-IC2 | 1.81 | 1.75 | −3% |
| PN3-IC3 | 1.66 | 1.71 | +3% |
| PN | |
|---|---|
| Volume (mL) | 1615 |
| Total calories (kcal) | 1850 |
| Non-protein calories (kcal) | 1500 |
| Non-protein calories/gN Ratio | 107 |
| Glucose (g) | 250 |
| Aminoacids (g) | 87.5 |
| Nitrogen (g) | 14 |
| Lipids (g) | 50 |
| Na+ (mEq) | 80 |
| K+ (mEq) | 60 |
| Ca2+ (mEq) | 9.2 |
| Mg2+ (mEq) | 10 |
| Phosphate (mEq) | 20 |
| Sulphate (mEq) | 5 |
| Chlorate (mEq) | 60 |
| Acetate (mM) | 50 |
| Multivitamin Cernevit® a | 5 mL |
| Trace elements Supliven® b | 10 mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Pourcq, J.T.; Riera, A.; Gras, L.; Garin, N.; Busquets, M.A.; Cardenete, J.; Cardona, D.; Riera, P. Physicochemical Compatibility of Ceftolozane-Tazobactam with Parenteral Nutrition. Pharmaceuticals 2024, 17, 896. https://doi.org/10.3390/ph17070896
De Pourcq JT, Riera A, Gras L, Garin N, Busquets MA, Cardenete J, Cardona D, Riera P. Physicochemical Compatibility of Ceftolozane-Tazobactam with Parenteral Nutrition. Pharmaceuticals. 2024; 17(7):896. https://doi.org/10.3390/ph17070896
Chicago/Turabian StyleDe Pourcq, Jan Thomas, Adria Riera, Laura Gras, Noe Garin, Maria Antònia Busquets, Joana Cardenete, Daniel Cardona, and Pau Riera. 2024. "Physicochemical Compatibility of Ceftolozane-Tazobactam with Parenteral Nutrition" Pharmaceuticals 17, no. 7: 896. https://doi.org/10.3390/ph17070896
APA StyleDe Pourcq, J. T., Riera, A., Gras, L., Garin, N., Busquets, M. A., Cardenete, J., Cardona, D., & Riera, P. (2024). Physicochemical Compatibility of Ceftolozane-Tazobactam with Parenteral Nutrition. Pharmaceuticals, 17(7), 896. https://doi.org/10.3390/ph17070896







