Pregnancy Recommendations Solely Based on Preclinical Evidence Should Be Integrated with Real-World Evidence: A Disproportionality Analysis of Certolizumab and Other TNF-Alpha Inhibitors Used in Pregnant Patients with Psoriasis
Abstract
1. Introduction
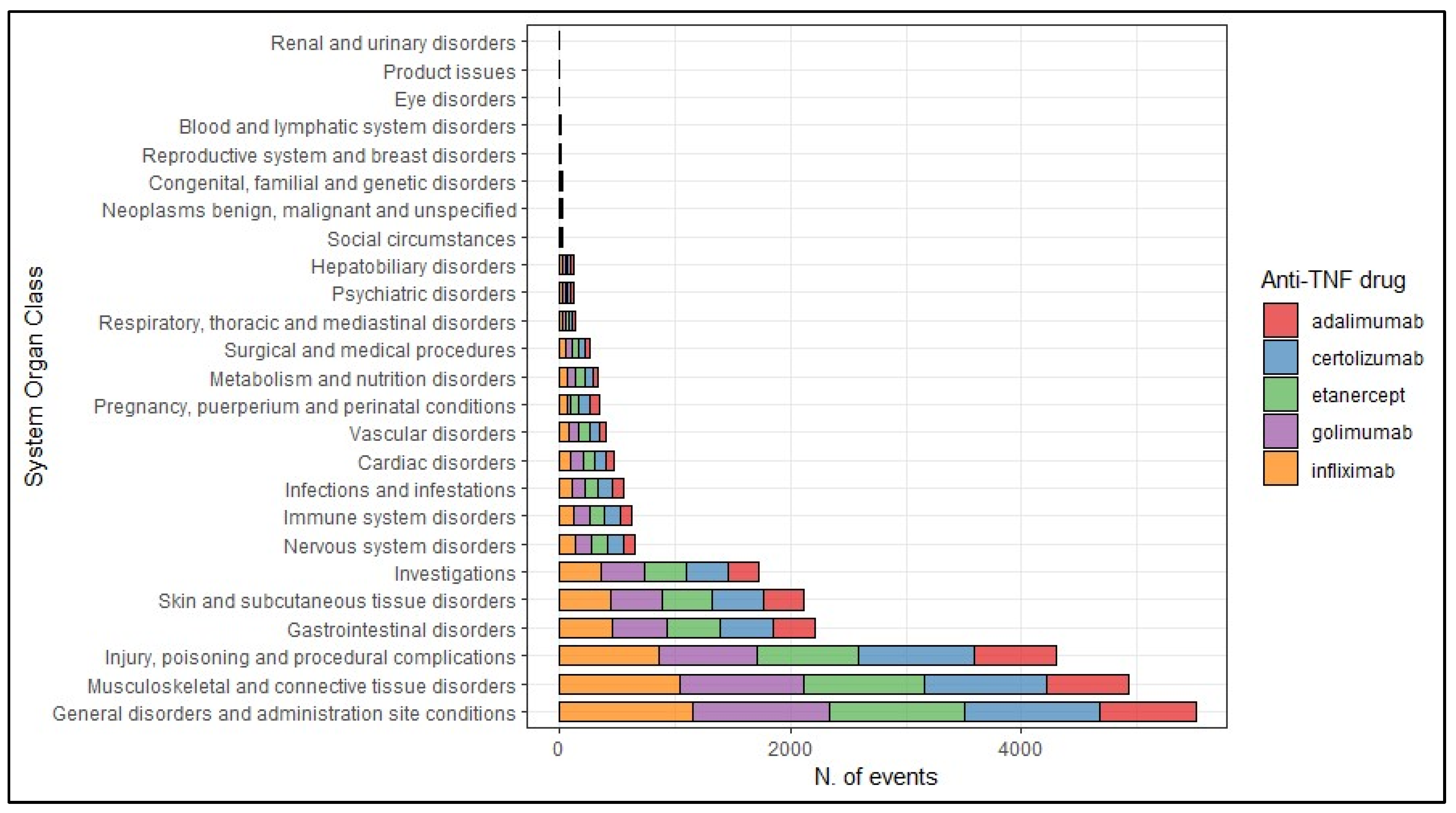
2. Results
3. Discussion
Strengths and Limitations
4. Materials and Methods
4.1. Data Source
4.2. Study Population
4.3. Descriptive Analysis
4.4. Disproportionality Analysis
4.5. Sensitivity Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Griffiths, C.E.; Barker, J.N. Pathogenesis and Clinical Features of Psoriasis. Lancet Lond. Engl. 2007, 370, 263–271. [Google Scholar] [CrossRef]
- Parisi, R.; Iskandar, I.Y.K.; Kontopantelis, E.; Augustin, M.; Griffiths, C.E.M.; Ashcroft, D.M. National, Regional, and Worldwide Epidemiology of Psoriasis: Systematic Analysis and Modelling Study. BMJ 2020, 369, m1590. [Google Scholar] [CrossRef]
- Kavanaugh, A.; Cush, J.J.; Ahmed, M.S.; Bermas, B.L.; Chakravarty, E.; Chambers, C.; Clowse, M.; Curtis, J.R.; Dao, K.; Hankins, G.D.V.; et al. Proceedings From the American College of Rheumatology Reproductive Health Summit: The Management of Fertility, Pregnancy, and Lactation in Women With Autoimmune and Systemic Inflammatory Diseases. Arthritis Care Res. 2015, 67, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Iskandar, I.Y.K.; Parisi, R.; Griffiths, C.E.M.; Ashcroft, D.M.; on behalf of the Global Psoriasis Atlas. Systematic Review Examining Changes over Time and Variation in the Incidence and Prevalence of Psoriasis by Age and Gender*. Br. J. Dermatol. 2021, 184, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Colombo, D.; Cassano, N.; Bellia, G.; Vena, G.A. Gender Medicine and Psoriasis. World J. Dermatol. 2014, 3, 36–44. [Google Scholar] [CrossRef]
- Tauscher, A.E.; Fleischer, A.B.; Phelps, K.C.; Feldman, S.R. Psoriasis and Pregnancy. J. Cutan. Med. Surg. 2002, 6, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Campa, M.; Ryan, C.; Menter, A. An Overview of Developing TNF-α Targeted Therapy for the Treatment of Psoriasis. Expert Opin. Investig. Drugs 2015, 24, 1343–1354. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Remicade. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/remicade (accessed on 5 February 2024).
- European Medicines Agency. Enbrel. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/enbrel (accessed on 5 February 2024).
- European Medicines Agency. Humira. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/humira (accessed on 5 February 2024).
- European Medicines Agency. Cimzia. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/cimzia (accessed on 5 February 2024).
- European Medicines Agency. Simponi. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/simponi (accessed on 5 February 2024).
- Skorpen, C.G.; Hoeltzenbein, M.; Tincani, A.; Fischer-Betz, R.; Elefant, E.; Chambers, C.; Da Silva, J.; Nelson-Piercy, C.; Cetin, I.; Costedoat-Chalumeau, N.; et al. The EULAR Points to Consider for Use of Antirheumatic Drugs before Pregnancy, and during Pregnancy and Lactation. Ann. Rheum. Dis. 2016, 75, 795–810. [Google Scholar] [CrossRef]
- Flint, J.; Panchal, S.; Hurrell, A.; van de Venne, M.; Gayed, M.; Schreiber, K.; Arthanari, S.; Cunningham, J.; Flanders, L.; Moore, L.; et al. BSR and BHPR Guideline on Prescribing Drugs in Pregnancy and Breastfeeding—Part I: Standard and Biologic Disease Modifying Anti-Rheumatic Drugs and Corticosteroids. Rheumatology 2016, 55, 1693–1697. [Google Scholar] [CrossRef]
- Porter, M.L.; Lockwood, S.J.; Kimball, A.B. Update on Biologic Safety for Patients with Psoriasis during Pregnancy. Int. J. Womens Dermatol. 2017, 3, 21–25. [Google Scholar] [CrossRef]
- Leach, J.L.; Sedmak, D.D.; Osborne, J.M.; Rahill, B.; Lairmore, M.D.; Anderson, C.L. Isolation from Human Placenta of the IgG Transporter, FcRn, and Localization to the Syncytiotrophoblast: Implications for Maternal-Fetal Antibody Transport. J. Immunol. 1996, 157, 3317–3322. [Google Scholar] [CrossRef] [PubMed]
- Porter, C.; Armstrong-Fisher, S.; Kopotsha, T.; Smith, B.; Baker, T.; Kevorkian, L.; Nesbitt, A. Certolizumab Pegol Does Not Bind the Neonatal Fc Receptor (FcRn): Consequences for FcRn-Mediated in Vitro Transcytosis and Ex Vivo Human Placental Transfer. J. Reprod. Immunol. 2016, 116, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.; Nesbitt, A.; Stephens, S.; Foulkes, R. Lack of Placental Transfer and Accumulation in Milk of an Anti-TNF PEGylated Fab’Fragment in Rats: P-0030. Inflamm. Bowel Dis. 2007, 13, 656. [Google Scholar] [CrossRef]
- Förger, F.; Zbinden, A.; Villiger, P.M. Certolizumab Treatment during Late Pregnancy in Patients with Rheumatic Diseases: Low Drug Levels in Cord Blood but Possible Risk for Maternal Infections. A Case Series of 13 Patients. Jt. Bone Spine 2016, 83, 341–343. [Google Scholar] [CrossRef] [PubMed]
- Mariette, X.; Förger, F.; Abraham, B.; Flynn, A.; Moltó, A.; Flipo, R.-M.; Tubergen, A.; Shaughnessy, L.; Simpson, J.; Teil, M.; et al. Lack of Placental Transfer of Certolizumab Pegol during Pregnancy: Results from CRIB, a Prospective, Postmarketing, Pharmacokinetic Study. Ann. Rheum. Dis. 2017, 77, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Puig, L.; Barco, D.; Alomar, A. Treatment of Psoriasis with Anti-TNF Drugs during Pregnancy: Case Report and Review of the Literature. Dermatol. Basel Switz. 2010, 220, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Echeverría-García, B.; Nuño-González, A.; Dauden, E.; Vanaclocha, F.; Torrado, R.; Belinchón, I.; Pérez-Zafrilla, B.; Grupo de estudio BIOBADADERM. A Case Series of Patients With Psoriasis Exposed to Biologic Therapy During Pregnancy: The BIOBADADERM Register and a Review of the Literature. Actas Dermosifiliogr. 2017, 108, 168–170. [Google Scholar] [CrossRef] [PubMed]
- Odorici, G.; Di Lernia, V.; Bardazzi, F.; Magnano, M.; Di Nuzzo, S.; Cortelazzi, C.; Lasagni, C.; Bigi, L.; Corazza, M.; Pellacani, G.; et al. Psoriasis and Pregnancy Outcomes in Biological Therapies: A Real-Life, Multi-Centre Experience. J. Eur. Acad. Dermatol. Venereol. 2019, 33, e374–e377. [Google Scholar] [CrossRef]
- Rahmati, S.; Moameri, H.; Mohammadi, N.M.; Norouzi, M.; Ghalekhani, N.; Beigzadeh, A.; Changizi, N.; Sharifi, H. Impact of Maternal Psoriasis on Adverse Maternal and Neonatal Outcomes: A Systematic Review and Meta-Analysis. BMC Pregnancy Childbirth 2023, 23, 703. [Google Scholar] [CrossRef]
- Burmester, G.R.; Panaccione, R.; Gordon, K.B.; McIlraith, M.J.; Lacerda, A.P.M. Adalimumab: Long-Term Safety in 23,458 Patients from Global Clinical Trials in Rheumatoid Arthritis, Juvenile Idiopathic Arthritis, Ankylosing Spondylitis, Psoriatic Arthritis, Psoriasis and Crohn’s Disease. Ann. Rheum. Dis. 2013, 72, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Gubatan, J.; Nielsen, O.H.; Levitte, S.; Juhl, C.B.; Maxwell, C.; Streett, S.E.; Habtezion, A. Biologics During Pregnancy in Women With Inflammatory Bowel Disease and Risk of Infantile Infections: A Systematic Review and Meta-Analysis. Off. J. Am. Coll. Gastroenterol. ACG 2021, 116, 243. [Google Scholar] [CrossRef]
- Mahadevan, U.; Long, M.D.; Kane, S.V.; Roy, A.; Dubinsky, M.C.; Sands, B.E.; Cohen, R.D.; Chambers, C.D.; Sandborn, W.J. Pregnancy and Neonatal Outcomes After Fetal Exposure to Biologics and Thiopurines Among Women With Inflammatory Bowel Disease. Gastroenterology 2021, 160, 1131–1139. [Google Scholar] [CrossRef]
- Long, M.D.; Siegel, C.A.; Abraham, B.P.; Chiorean, M.; Mahadevan, U. Day Care Attendance and Infectious Complications in Children Born to Mothers with Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2022, 20, 706–708.e1. [Google Scholar] [CrossRef]
- Sosinsky, A.Z.; Rich-Edwards, J.W.; Wiley, A.; Wright, K.; Spagnolo, P.A.; Joffe, H. Enrollment of Female Participants in United States Drug and Device Phase 1–3 Clinical Trials between 2016 and 2019. Contemp. Clin. Trials 2022, 115, 106718. [Google Scholar] [CrossRef] [PubMed]
- Ingrasciotta, Y.; Spini, A.; L’Abbate, L.; Fiore, E.S.; Carollo, M.; Ientile, V.; Isgrò, V.; Cavazzana, A.; Biasi, V.; Rossi, P.; et al. Comparing Clinical Trial Population Representativeness to Real-World Users of 17 Biologics Approved for Immune-Mediated Inflammatory Diseases: An External Validity Analysis of 66,639 Biologic Users from the Italian VALORE Project. Pharmacol. Res. 2024, 200, 107074. [Google Scholar] [CrossRef]
- Zur, R.L. Protected from Harm, Harmed by Protection: Ethical Consequences of the Exclusion of Pregnant Participants from Clinical Trials. Res. Ethics 2023, 19, 536–545. [Google Scholar] [CrossRef]
- Blehar, M.C.; Spong, C.; Grady, C.; Goldkind, S.F.; Sahin, L.; Clayton, J.A. Enrolling Pregnant Women: Issues in Clinical Research. Womens Health Issues 2013, 23, e39–e45. [Google Scholar] [CrossRef]
- Carter, J.D.; Valeriano, J.; Vasey, F.B. Tumor Necrosis Factor-Alpha Inhibition and VATER Association: A Causal Relationship. J. Rheumatol. 2006, 33, 1014–1017. [Google Scholar] [PubMed]
- Berthelot, J.-M.; De Bandt, M.; Goupille, P.; Solau-Gervais, E.; Lioté, F.; Goeb, V.; Azaïs, I.; Martin, A.; Pallot-Prades, B.; Maugars, Y.; et al. Exposition to Anti-TNF Drugs during Pregnancy: Outcome of 15 Cases and Review of the Literature. Jt. Bone Spine 2009, 76, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Orhon, P.; Robert, M.; Morand, T.; Cracowski, J.-L.; Khouri, C. Investigating the Link between Drug Consumption and Adverse Events Reporting in France. Fundam. Clin. Pharmacol. 2023, 37, 879–882. [Google Scholar] [CrossRef] [PubMed]
- Banovac, M.; Candore, G.; Slattery, J.; Houÿez, F.; Haerry, D.; Genov, G.; Arlett, P. Patient Reporting in the EU: Analysis of EudraVigilance Data. Drug Saf. 2017, 40, 629–645. [Google Scholar] [CrossRef]
- European Medicines Agency. EudraVigilance. Available online: https://www.ema.europa.eu/en/human-regulatory-overview/research-and-development/pharmacovigilance-research-and-development/eudravigilance (accessed on 7 February 2024).
- Lindquist, M. Data Quality Management in Pharmacovigilance. Drug Saf. 2004, 27, 857–870. [Google Scholar] [CrossRef]
- Blenkinsopp, A.; Wilkie, P.; Wang, M.; Routledge, P.A. Patient Reporting of Suspected Adverse Drug Reactions: A Review of Published Literature and International Experience. Br. J. Clin. Pharmacol. 2007, 63, 148–156. [Google Scholar] [CrossRef]
- Aagaard, L.; Nielsen, L.H.; Hansen, E.H. Consumer Reporting of Adverse Drug Reactions: A Retrospective Analysis of the Danish Adverse Drug Reaction Database from 2004 to 2006. Drug Saf. 2009, 32, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Tarahomi, T.; Singh, L.; Bollampally, M.; Heydari-Kamjani, M.; Kesselman, M.M. Cardiovascular Risk Associated With TNF Alpha Inhibitor Use in Patients with Rheumatoid Arthritis. Cureus 2021, 13, e17938. [Google Scholar] [CrossRef] [PubMed]
- Maruotti, N.; d’Onofrio, F.; Cantatore, F.P. Metabolic Syndrome and Chronic Arthritis: Effects of Anti-TNF-α Therapy. Clin. Exp. Med. 2015, 15, 433–438. [Google Scholar] [CrossRef]
- French, J.B.; Bonacini, M.; Ghabril, M.; Foureau, D.; Bonkovsky, H.L. Hepatotoxicity Associated with the Use of Anti-TNF-α Agents. Drug Saf. 2016, 39, 199–208. [Google Scholar] [CrossRef]
- Blanchard, E.; Truchetet, M.-E.; Machelart, I.; Séneschal, J.; Raherison-Semjen, C. Respiratory Infections Associated with Anti-TNFα Agents. Med. Mal. Infect. 2017, 47, 375–381. [Google Scholar] [CrossRef]
- Wakabayashi, T.; Hosohata, K.; Oyama, S.; Inada, A.; Ueno, S.; Kambara, H.; Iida, T.; Nakatsuji, T.; Uchida, M.; Iwanaga, K. Comparison of Adverse Event Profiles of Tumor Necrosis Factor-Alfa Inhibitors: Analysis of a Spontaneous Reporting Database. Ther. Clin. Risk Manag. 2020, 16, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Bröms, G.; Haerskjold, A.; Granath, F.; Kieler, H.; Pedersen, L.; Berglind, I.A. Effect of Maternal Psoriasis on Pregnancy and Birth Outcomes: A Population-Based Cohort Study from Denmark and Sweden. Acta Derm. Venereol. 2018, 98, 728–734. [Google Scholar] [CrossRef]
- Clowse, M.E.B.; Scheuerle, A.E.; Chambers, C.; Afzali, A.; Kimball, A.B.; Cush, J.J.; Cooney, M.; Shaughnessy, L.; Vanderkelen, M.; Förger, F. Pregnancy Outcomes After Exposure to Certolizumab Pegol: Updated Results From a Pharmacovigilance Safety Database. Arthritis Rheumatol. 2018, 70, 1399–1407. [Google Scholar] [CrossRef] [PubMed]
- Bandoli, G.; Chambers, C.D. Autoimmune Conditions and Comorbid Depression in Pregnancy: Examining the Risk of Preterm Birth and Preeclampsia. J. Perinatol. 2017, 37, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- Polachek, A.; Polachek Shlomi, I.; Spitzer, K.; Pereira, D.; Ye, J.Y.; Chandran, V.; Laskin, C.A.; Gladman, D.D. Outcome of Pregnancy in Women with Psoriatic Arthritis Compared to Healthy Controls. Clin. Rheumatol. 2019, 38, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Remaeus, K.; Stephansson, O.; Johansson, K.; Granath, F.; Hellgren, K. Maternal and Infant Pregnancy Outcomes in Women with Psoriatic Arthritis: A Swedish Nationwide Cohort Study. BJOG Int. J. Obstet. Gynaecol. 2019, 126, 1213–1222. [Google Scholar] [CrossRef]
- Smith, C.J.F.; Bandoli, G.; Kavanaugh, A.; Chambers, C.D. Birth Outcomes and Disease Activity During Pregnancy in a Prospective Cohort of Women with Psoriatic Arthritis and Ankylosing Spondylitis. Arthritis Care Res. 2020, 72, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Strouse, J.; Donovan, B.M.; Fatima, M.; Fernandez-Ruiz, R.; Baer, R.J.; Nidey, N.; Forbess, C.; Bandoli, G.; Paynter, R.; Parikh, N.; et al. Impact of Autoimmune Rheumatic Diseases on Birth Outcomes: A Population-Based Study. RMD Open 2019, 5, e000878. [Google Scholar] [CrossRef] [PubMed]
- Bandoli, G.; Singh, N.; Strouse, J.; Baer, R.J.; Donovan, B.M.; Feuer, S.K.; Nidey, N.; Ryckman, K.K.; Jelliffe-Pawlowski, L.L.; Chambers, C.D. Mediation of Adverse Pregnancy Outcomes in Autoimmune Conditions by Pregnancy Complications: A Mediation Analysis of Autoimmune Conditions and Adverse Pregnancy Outcomes. Arthritis Care Res. 2020, 72, 256–264. [Google Scholar] [CrossRef]
- Lambe, M.; Bergstrom, A.V.; Johansson, A.L.V.; Weibull, C.E. Reproductive Patterns and Maternal and Pregnancy Outcomes in Women with Psoriasis-A Population-Based Study. J. Am. Acad. Dermatol. 2020, 82, 1109–1116. [Google Scholar] [CrossRef]
- Xie, W.; Huang, H.; Ji, L.; Zhang, Z. Maternal and Neonatal Outcomes in Pregnant Women with Psoriasis and Psoriatic Arthritis: A Systematic Review and Meta-Analysis. Rheumatol. Oxf. Engl. 2021, 60, 4018–4028. [Google Scholar] [CrossRef]
- Zinzi, A.; Gaio, M.; Liguori, V.; Ruggiero, R.; Tesorone, M.; Rossi, F.; Rafaniello, C.; Capuano, A. Safety Monitoring of mRNA COVID-19 Vaccines in Children Aged 5 to 11 Years by Using EudraVigilance Pharmacovigilance Database: The CoVaxChild Study. Vaccines 2023, 11, 401. [Google Scholar] [CrossRef]
- Rafaniello, C.; Ferrajolo, C.; Gaio, M.; Zinzi, A.; Scavone, C.; Sullo, M.G.; Rossi, F.; Berrino, L.; Capuano, A. Tisagenlecleucel in Children and Young Adults: Reverse Translational Research by Using Real-World Safety Data. Pharm. Basel Switz. 2020, 13, 258. [Google Scholar] [CrossRef]
- Brewer, T.; Colditz, G.A. Postmarketing Surveillance and Adverse Drug Reactions: Current Perspectives and Future Needs. JAMA 1999, 281, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Montastruc, J.-L.; Sommet, A.; Lacroix, I.; Olivier, P.; Durrieu, G.; Damase-Michel, C.; Lapeyre-Mestre, M.; Bagheri, H. Pharmacovigilance for Evaluating Adverse Drug Reactions: Value, Organization, and Methods. Jt. Bone Spine 2006, 73, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Mori, C.; Ohtsu, F. Potential Safety Signal of Pregnancy Loss with Vascular Endothelial Growth Factor Inhibitor Intraocular Injection: A Disproportionality Analysis Using the Food and Drug Administration Adverse Event Reporting System. Front. Pharmacol. 2022, 13, 1063625. [Google Scholar] [CrossRef]
- Pariente, A.; Gregoire, F.; Fourrier-Reglat, A.; Haramburu, F.; Moore, N. Impact of Safety Alerts on Measures of Disproportionality in Spontaneous Reporting Databases: The Notoriety Bias. Drug Saf. 2007, 30, 891–898. [Google Scholar] [CrossRef]
- MedDRA. Help to Shape the MedDRA Terminology. Available online: https://www.meddra.org/ (accessed on 12 April 2023).




| Anti-TNF Drugs | |||||
|---|---|---|---|---|---|
| Adalimumab | Certolizumab | Etanercept | Golimumab | Infliximab | |
| Number of ICSRs | 183 | 259 | 232 | 178 | 198 |
| Age range (%) | |||||
| 0–1 month | 1 (0.5) | 3 (1.2) | 1 (0.4) | 0 (0.0) | 0 (0.0) |
| 2 months–2 years | 0 (0.0) | 1 (0.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 3–11 years | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 12–17 years | 0 (0.0) | 0 (0.0) | 1 (0.4) | 0 (0.0) | 0 (0.0) |
| 18–64 years | 90 (49.2) | 105 (40.5) | 97 (41.8) | 62 (34.8) | 77 (38.9) |
| Not available | 92 (50.3) | 150 (57.9) | 133 (57.3) | 116 (65.2) | 121 (61.1) |
| Type of report (%) | |||||
| Spontaneous | 92 (50.3) | 150 (57.9) | 133 (57.3) | 116 (65.2) | 121 (61.1) |
| Non-spontaneous | 91 (49.7) | 109 (42.1) | 99 (42.7) | 62 (34.8) | 77 (38.9) |
| Source qualification = Patient (%) | 155 (84.7) | 217 (83.8) | 207 (89.2) | 176 (98.9) | 191 (96.5) |
| Certolizumab vs. Other TNF-Alpha Drugs (ROR (95% CI)) | ||
|---|---|---|
| System Organ Class | Pregnant Population | Non-Pregnant Population |
| Blood and lymphatic system disorders | (less than 3 cases) | 1.33 (0.75–2.37) |
| Cardiac disorders | 1.00 (0.80–1.25) | 1.13 (0.69–1.86) |
| Congenital, familial, and genetic disorders | 2.07 (0.87–4.94) | (less than 3 cases) |
| Ear and labyrinth disorders | (less than 3 cases) | 1.91 (0.98–3.73) |
| Endocrine disorders | (less than 3 cases) | 3.27 (1.51–7.08) |
| Eye disorders | (less than 3 cases) | 2.05 (1.41–2.98) |
| Gastrointestinal disorders | 0.94 (0.84–1.05) | 2.30 (1.93–2.74) |
| General disorders | 0.97 (0.90–1.04) | 2.88 (2.60–3.19) |
| Hepatobiliary disorders | 0.82 (0.52–1.30) | 0.76 (0.39–1.48) |
| Immune system disorders | 1.01 (0.83–1.22) | 3.53 (2.66–4.68) |
| Infections and infestations | 1.00 (0.82–1.23) | 2.05 (1.75–2.40) |
| Injury, poisoning, and procedural complications | 1.12 (1.03–1.21) | 5.40 (4.72–6.18) |
| Investigations | 0.94 (0.83–1.06) | 1.25 (1.01–1.56) |
| Metabolism and nutrition disorders | 0.99 (0.77–1.29) | 1.28 (0.80–2.04) |
| Musculoskeletal and connective tissue disorders | 0.97 (0.90–1.05) | 2.94 (2.60–3.34) |
| Neoplasms benign, malignant, and unspecified | 1.03 (0.42–2.56) | 0.52 (0.34–0.78) |
| Nervous system disorders | 0.95 (0.79–1.15) | 1.89 (1.57–2.28) |
| Pregnancy, puerperium, and perinatal conditions | 1.30 (1.02–1.65) | 2.08 (0.98–4.42) |
| Product issues | (less than 3 cases) | 3.29 (2.18–4.96) |
| Psychiatric disorders | 0.96 (0.63–1.47) | 1.87 (1.41–2.48) |
| Renal and urinary disorders | (less than 3 cases) | 2.49 (1.59–3.92) |
| Reproductive system and breast disorders | 2.41 (0.68–8.56) | 1.38 (0.71–2.69) |
| Respiratory, thoracic, and mediastinal disorders | 1.05 (0.70–1.59) | 1.26 (0.94–1.69) |
| Skin and subcutaneous tissue disorders | 0.98 (0.88–1.09) | 3.25 (2.90–3.63) |
| Social circumstances | 1.45 (0.64–3.29) | 4.56 (2.85–7.30) |
| Surgical and medical procedures | 1.15 (0.87–1.52) | 8.25 (6.39–10.65) |
| Vascular disorders | 0.99 (0.78–1.27) | 1.50 (0.99–2.27) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaio, M.; Vastarella, M.G.; Sullo, M.G.; Scavone, C.; Riccardi, C.; Campitiello, M.R.; Sportiello, L.; Rafaniello, C. Pregnancy Recommendations Solely Based on Preclinical Evidence Should Be Integrated with Real-World Evidence: A Disproportionality Analysis of Certolizumab and Other TNF-Alpha Inhibitors Used in Pregnant Patients with Psoriasis. Pharmaceuticals 2024, 17, 904. https://doi.org/10.3390/ph17070904
Gaio M, Vastarella MG, Sullo MG, Scavone C, Riccardi C, Campitiello MR, Sportiello L, Rafaniello C. Pregnancy Recommendations Solely Based on Preclinical Evidence Should Be Integrated with Real-World Evidence: A Disproportionality Analysis of Certolizumab and Other TNF-Alpha Inhibitors Used in Pregnant Patients with Psoriasis. Pharmaceuticals. 2024; 17(7):904. https://doi.org/10.3390/ph17070904
Chicago/Turabian StyleGaio, Mario, Maria Giovanna Vastarella, Maria Giuseppa Sullo, Cristina Scavone, Consiglia Riccardi, Maria Rosaria Campitiello, Liberata Sportiello, and Concetta Rafaniello. 2024. "Pregnancy Recommendations Solely Based on Preclinical Evidence Should Be Integrated with Real-World Evidence: A Disproportionality Analysis of Certolizumab and Other TNF-Alpha Inhibitors Used in Pregnant Patients with Psoriasis" Pharmaceuticals 17, no. 7: 904. https://doi.org/10.3390/ph17070904
APA StyleGaio, M., Vastarella, M. G., Sullo, M. G., Scavone, C., Riccardi, C., Campitiello, M. R., Sportiello, L., & Rafaniello, C. (2024). Pregnancy Recommendations Solely Based on Preclinical Evidence Should Be Integrated with Real-World Evidence: A Disproportionality Analysis of Certolizumab and Other TNF-Alpha Inhibitors Used in Pregnant Patients with Psoriasis. Pharmaceuticals, 17(7), 904. https://doi.org/10.3390/ph17070904





