Synthesis and Characterization of Chemically and Green-Synthesized Silver Oxide Particles for Evaluation of Antiviral and Anticancer Activity
Abstract
:1. Introduction
2. Results and Discussions
2.1. X-ray Diffraction Analysis
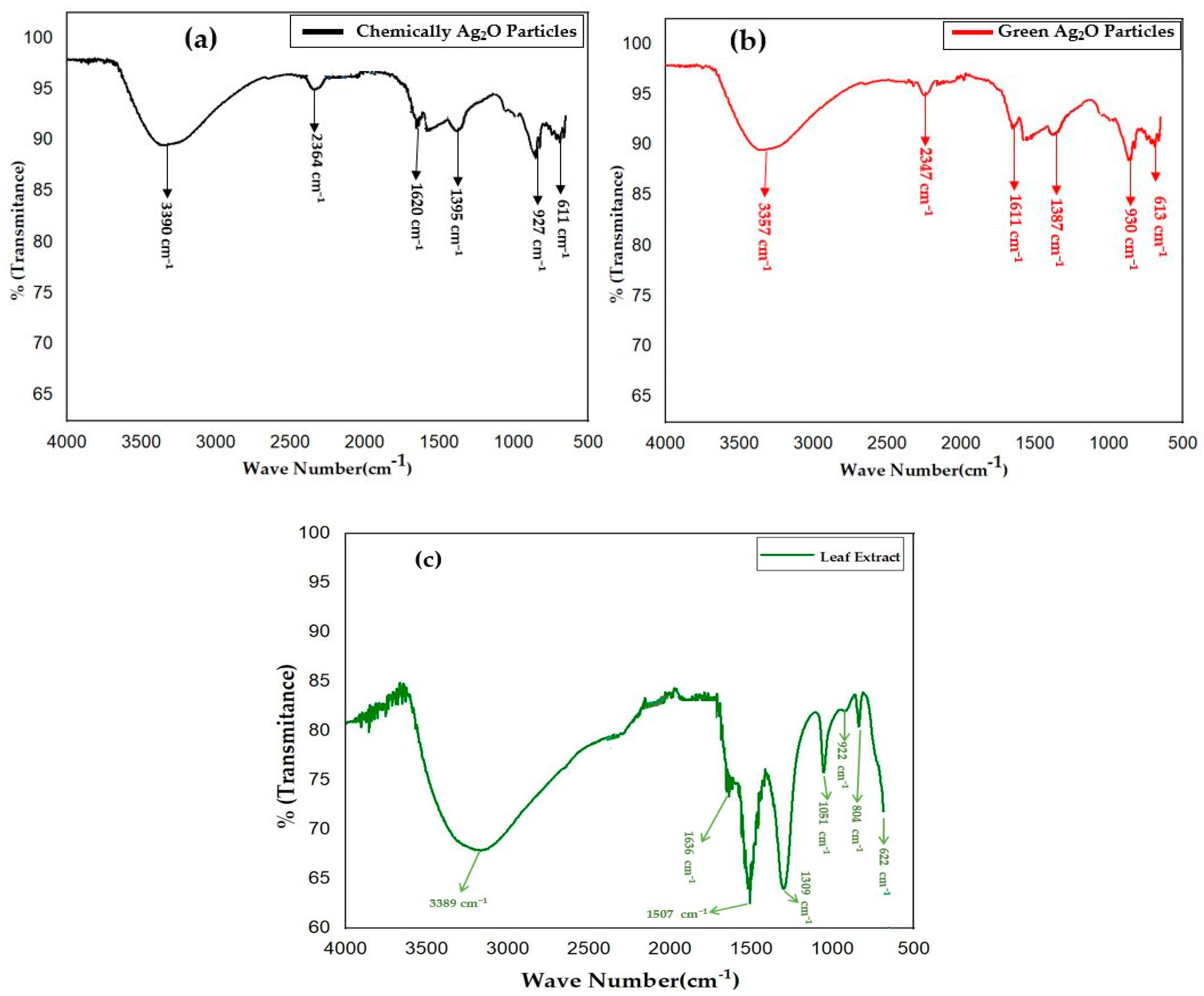
2.2. FTIR Analysis
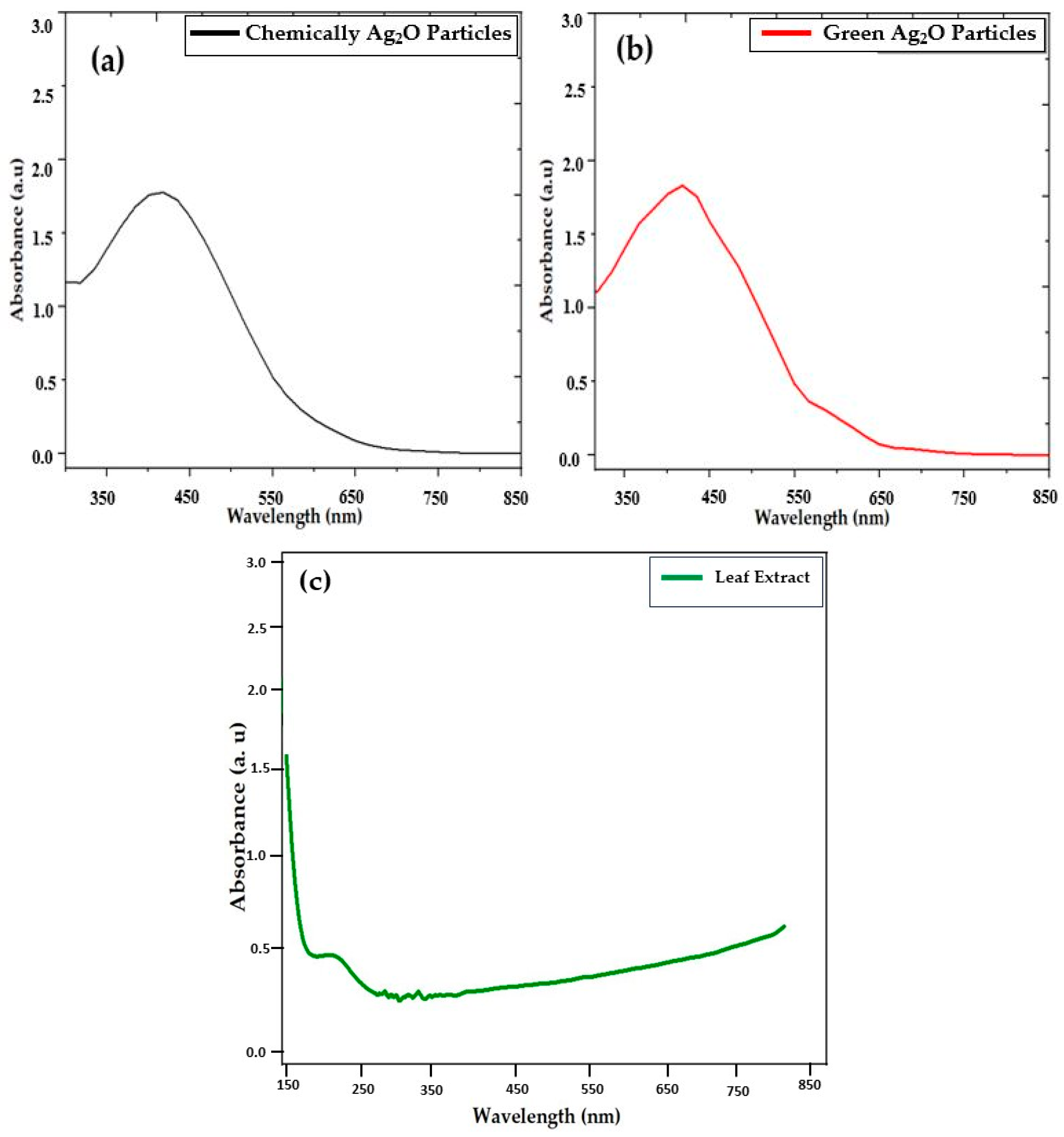
2.3. UV–Visible Analysis
2.4. SEM and EDX Analysis
2.5. Anticancer Activity against MCF-7 Cells
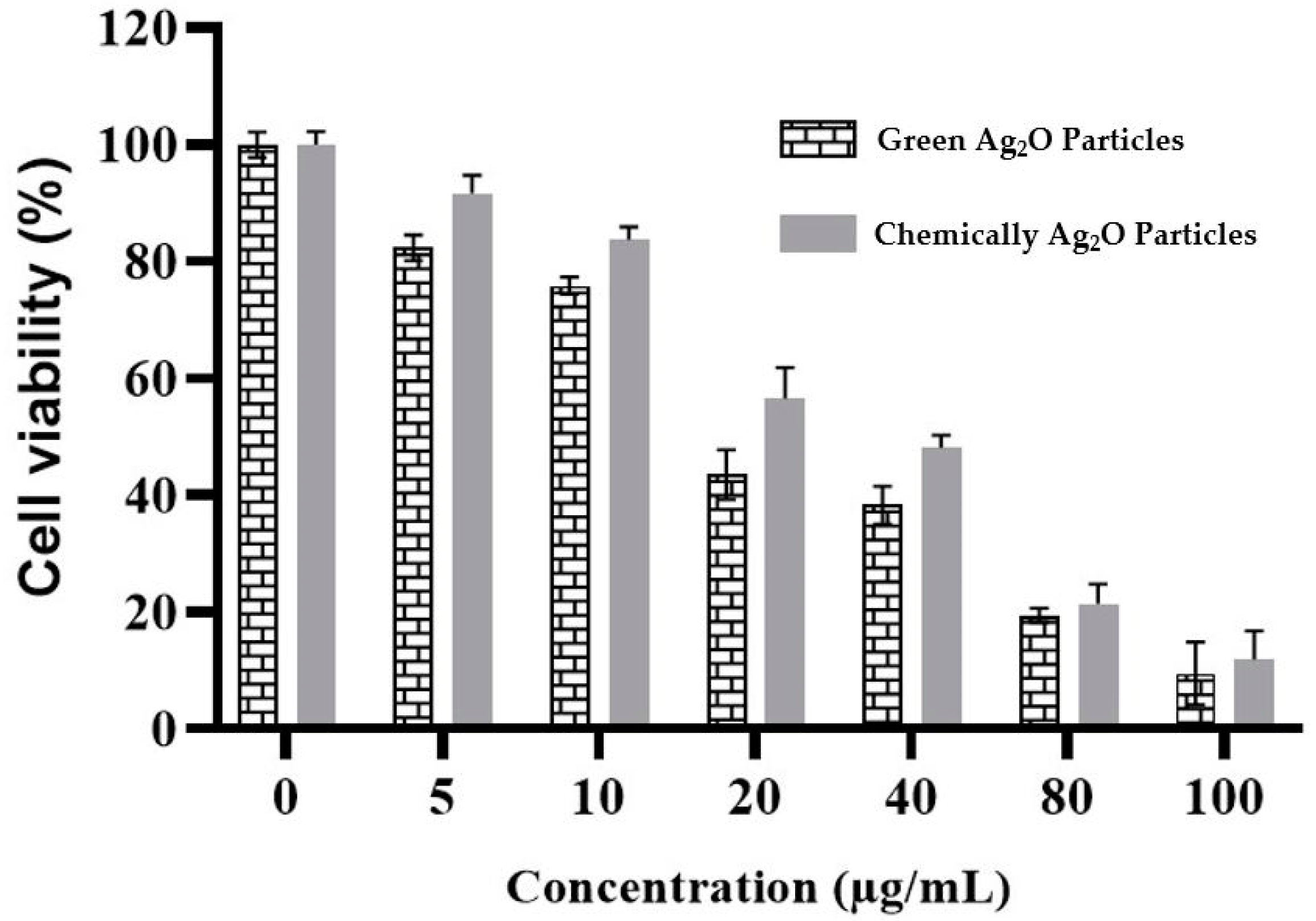
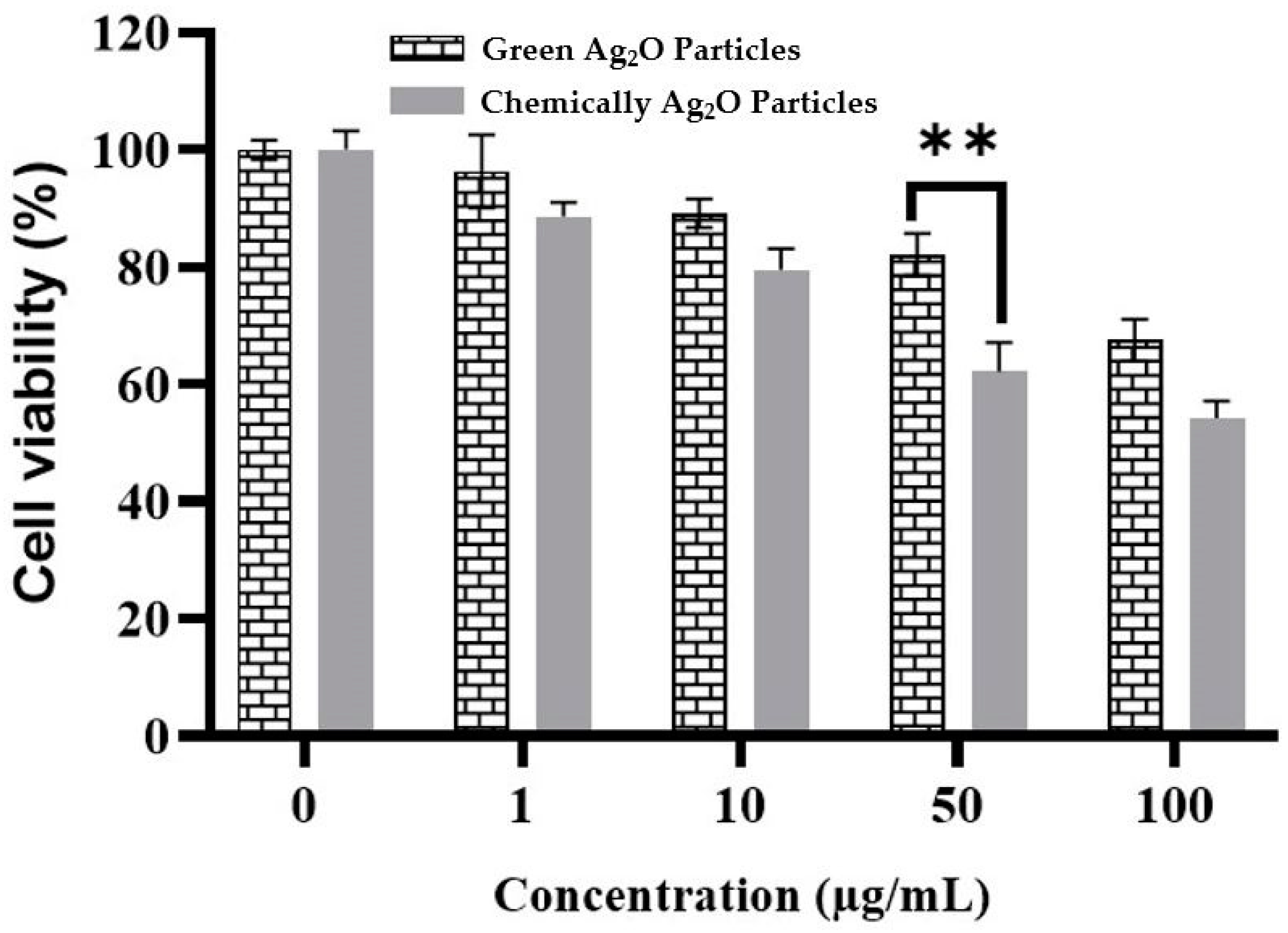
2.6. Cytotoxicity Activity against Vero Cells
2.7. Antiviral Activity
2.8. Properties of Green-Synthesized Ag2O Particles
3. Materials and Methods
3.1. Materials
3.2. Preparation of Extract
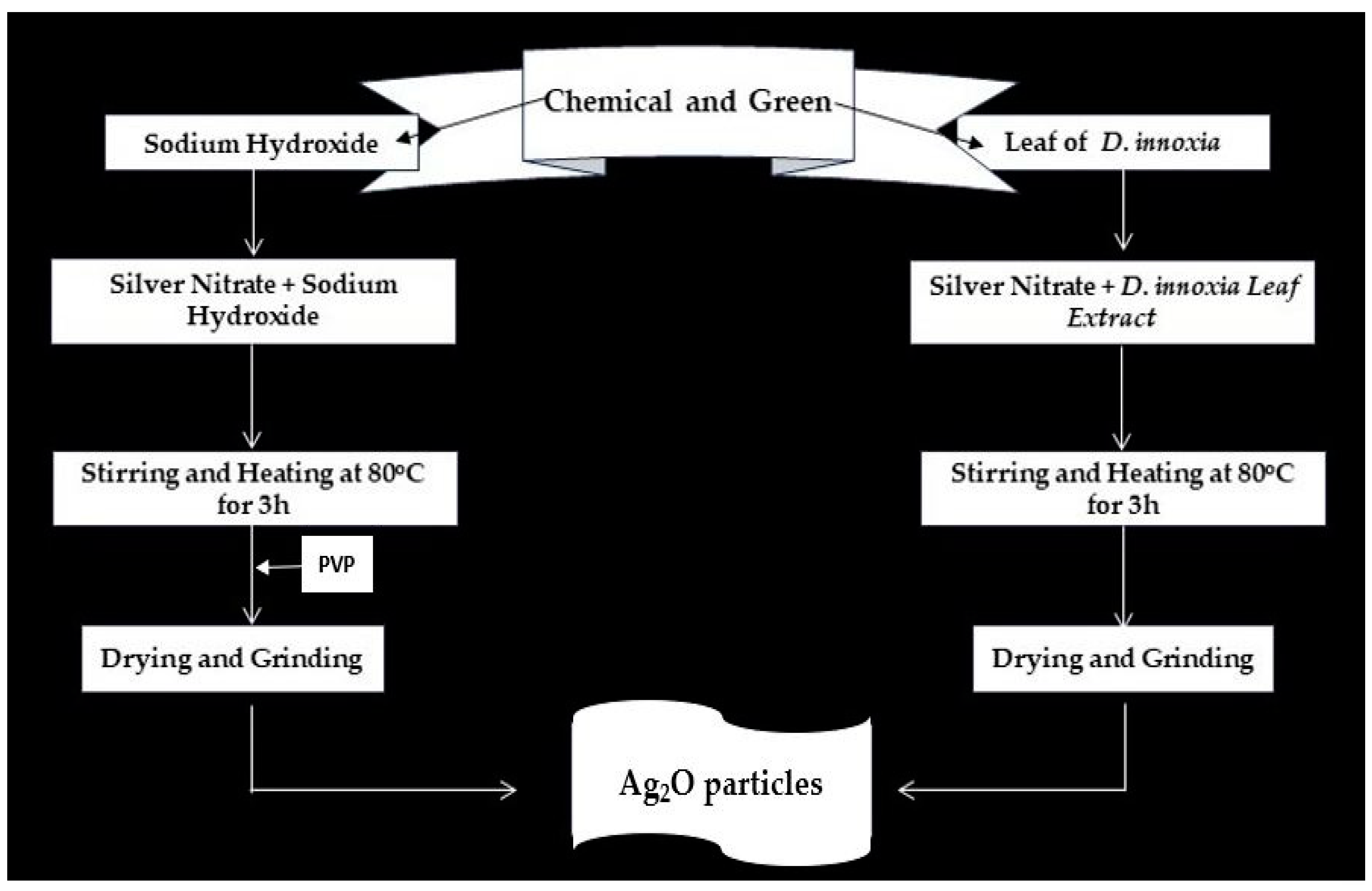
3.3. Green Synthesis Method
3.4. Chemical Synthesis Method
3.5. Characterization of the Synthesized Nanomaterial
3.6. Bioassay
3.6.1. Cell Lines and Viruses
3.6.2. Cytotoxicity Assay
3.6.3. Antiviral Activity
3.7. Statistical Analysis (S.A)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Parkin, D.M. Cancer in sub-Saharan Africa in 2020: A review of current estimates of the national burden, data gaps, and future needs. Lancet Oncol. 2022, 23, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Mettlin, C. Global breast cancer mortality statistics. CA Cancer J. Clin. 1999, 49, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.H.; Duan, S.F.; Wu, D.D.; Ji, X.Y. Better reporting and awareness campaigns needed for breast cancer in Pakistani women. Cancer Manag. Res. 2021, 13, 2125–2129. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Fakhar-e-Alam, M.; Akbar, F.; Shafiq, M.; Atif, M.; Amin, N.; Ismail, M.; Hanif, A.; Farooq, W.A. Application of silver oxide nanoparticles for the treatment of cancer. J. Mol. Struct. 2019, 1189, 203–209. [Google Scholar] [CrossRef]
- Bonam, S.R.; Wu, Y.S.; Tunki, L.; Chellian, R.; Halmuthur, M.S.K.; Muller, S.; Pandy, V. What has come out from phytomedicines and herbal edibles for the treatment of cancer? ChemMedChem 2018, 13, 1854–1872. [Google Scholar] [CrossRef]
- Zargar, M.; Shameli, K.; Najafi, G.R.; Farahani, F. Plant mediated green biosynthesis of silver nanoparticles using Vitex negundo L. extract. J. Ind. Eng. Chem. 2014, 20, 4169–4175. [Google Scholar] [CrossRef]
- Wiley, B.J.; Im, S.H.; McLellan, J.; Siekkinen, A.; Xia, Y. Maneuvering the surface plasmon resonance of silver nanostructures through shape-controlled synthesis. J. Phys. Chem. B 2006, 110, 15666–15675. [Google Scholar] [CrossRef]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef]
- Yao, J.; Yang, M.; Duan, Y. Chemistry, biology, and medicine of fluorescent nanomaterials and related systems: New insights into biosensing, bioimaging, genomics, diagnostics, and therapy. Chem. Rev. 2014, 114, 6130–6178. [Google Scholar] [CrossRef]
- Pryshchepa, O.; Pomastowski, P.; Buszewski, B. Silver nanoparticles: Synthesis, investigation techniques, and properties. Adv. Colloid Interface Sci. 2020, 284, 102246. [Google Scholar] [CrossRef]
- Danish, M.S.S.; Estrella-Pajulas, L.L.; Alemaida, I.M.; Grilli, M.L.; Mikhaylov, A.; Senjyu, T. Green synthesis of silver oxide nanoparticles for photocatalytic environmental remediation and biomedical applications. Metals 2022, 12, 769. [Google Scholar] [CrossRef]
- Ismail, G.A.; El-Sheekh, M.M.; Samy, R.M.; Gheda, S.F. Antimicrobial, antioxidant, and antiviral activities of biosynthesized silver nanoparticles by phycobiliprotein crude extract of the cyanobacteria Spirulina platensis and Nostoc linckia. Bionanoscience 2021, 11, 355–370. [Google Scholar] [CrossRef]
- Islam, T.; Ara, I.; Islam, T.; Sah, P.K.; de Almeida, R.S.; Matias, E.F.F.; Ramalho, C.L.G.; Coutinho, H.D.M.; Islam, M.T. Ethnobotanical uses and phytochemical, biological, and toxicological profiles of Datura metel L.: A review. Curr. Res. Toxicol. 2023, 4, 100106. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Al-Thabaiti, S.A.; Obaid, A.Y.; Al-Youbi, A. Preparation and characterization of silver nanoparticles by chemical reduction method. Colloids Surf. B Biointerfaces 2011, 82, 513–517. [Google Scholar] [CrossRef]
- Moond, M.; Singh, S.; Sangwan, S.; Devi, R.; Beniwal, R. Green synthesis and applications of silver nanoparticles: A systematic review. AATCC J. Res. 2022, 9, 272–285. [Google Scholar] [CrossRef]
- Mousavi-Khattat, M.; Keyhanfar, M.; Razmjou, A. A comparative study of stability, antioxidant, DNA cleavage and antibacterial activities of green and chemically synthesized silver nanoparticles. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. 3), 1022–1031. [Google Scholar] [CrossRef]
- Flieger, J.; Franus, W.; Panek, R.; Szymańska-Chargot, M.; Flieger, W.; Flieger, M.; Kołodziej, P. Green synthesis of silver nanoparticles using natural extracts with proven antioxidant activity. Molecules 2021, 26, 4986. [Google Scholar] [CrossRef] [PubMed]
- Martina, B.E.E.; Koraka, P.; Osterhaus, A.D.M.E. Dengue virus pathogenesis: An integrated view. Clin. Microbiol. Rev. 2009, 22, 564–581. [Google Scholar] [CrossRef]
- Gaikwad, S.; Ingle, A.; Gade, A.; Rai, M.; Falanga, A.; Incoronato, N.; Russo, L.; Galdiero, S.; Galdiero, M. Antiviral activity of mycosynthesized silver nanoparticles against herpes simplex virus and human parainfluenza virus type 3. Int. J. Nanomed. 2013, 8, 4303–4314. [Google Scholar]
- Scaturro, P.; Cortese, M.; Chatel-Chaix, L.; Fischl, W.; Bartenschlager, R. Dengue virus non-structural protein 1 modulates infectious particle production via interaction with the structural proteins. PLoS Pathog. 2015, 11, e1005277. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Nemunaitis, J. Herpes simplex virus 1 (HSV-1) for cancer treatment. Cancer Gene Ther. 2006, 13, 975–992. [Google Scholar] [CrossRef] [PubMed]
- Pierson, T.C.; Diamond, M.S. The continued threat of emerging flaviviruses. Nat. Microbiol. 2020, 5, 796–812. [Google Scholar] [CrossRef] [PubMed]
- Wasik, B.R.; de Wit, E.; Munster, V.; Lloyd-Smith, J.O.; Martinez-Sobrido, L.; Parrish, C.R. Onward transmission of viruses: How do viruses emerge to cause epidemics after spillover? Philos. Trans. R. Soc. B 2019, 374, 20190017. [Google Scholar] [CrossRef] [PubMed]
- Panáček, A.; Kolář, M.; Večeřová, R.; Prucek, R.; Soukupová, J.; Kryštof, V.; Hamal, P.; Zbořil, R.; Kvítek, L. Antifungal activity of silver nanoparticles against Candida spp. Biomaterials 2009, 30, 6333–6340. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Rhim, J.W. Amino acid mediated synthesis of silver nanoparticles and preparation of antimicrobial agar/silver nanoparticles composite films. Carbohydr. Polym. 2015, 130, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Mani, M.; Harikrishnan, R.; Purushothaman, P.; Pavithra, S.; Rajkumar, P.; Kumaresan, S.; Al Farraj, D.A.; Elshikh, M.S.; Balasubramanian, B.; Kaviyarasu, K. Systematic green synthesis of silver oxide nanoparticles for antimicrobial activity. Environ. Res. 2021, 202, 111627. [Google Scholar] [CrossRef] [PubMed]
- Gajendran, B.; Chinnasamy, A.; Durai, P.; Raman, J.; Ramar, M. Biosynthesis and characterization of silver nanoparticles from Datura inoxia and its apoptotic effect on human breast cancer cell line MCF7. Mater. Lett. 2014, 122, 98–102. [Google Scholar] [CrossRef]
- Al-Khedhairy, A.A.; Wahab, R. Silver nanoparticles: An instantaneous solution for anticancer activity against human liver (HepG2) and breast (MCF-7) cancer cells. Metals 2022, 12, 148. [Google Scholar] [CrossRef]
- Allahverdiyev, A.M.; Abamor, E.S.; Bagirova, M.; Rafailovich, M. Antimicrobial effects of TiO2 and Ag2O nanoparticles against drug-resistant bacteria and leishmania parasites. Future Microbiol. 2011, 6, 933–940. [Google Scholar] [CrossRef]
- Fayyadh, A.A.; Jaduaa Alzubaidy, M.H. Green-synthesis of Ag2O nanoparticles for antimicrobial assays. J. Mech. Behav. Mater. 2021, 30, 228–236. [Google Scholar] [CrossRef]
- Stoimenov, P.K.; Klinger, R.L.; Marchin, G.L.; Klabunde, K.J. Metal oxide nanoparticles as bactericidal agents. Langmuir 2002, 18, 6679–6686. [Google Scholar] [CrossRef]
- Chandirika, J.U.; Annadurai, G. Biosynthesis and characterization of silver nanoparticles using leaf extract Abutilon indicum. Glob. J. Biotechnol. Biochem 2018, 13, 7–11. [Google Scholar]
- Mardare, G.; Lazar, L.; Malutan, T. Potential Application of Invasive Plant Species Datura innoxia for the Scopolamine Extracts of the Plant Organs and Analysis Using UV–VIS Spectrophotometry. Forests 2022, 13, 1555. [Google Scholar] [CrossRef]
- Kasthuri, J.; Veerapandian, S.; Rajendiran, N. Biological synthesis of silver and gold nanoparticles using apiin as reducing agent. Colloids Surf. B Biointerfaces 2009, 68, 55–60. [Google Scholar] [CrossRef]
- Rogers, J.V.; Parkinson, C.V.; Choi, Y.W.; Speshock, J.L.; Hussain, S.M. A preliminary assessment of silver nanoparticle inhibition of monkeypox virus plaque formation. Nanoscale Res. Lett. 2008, 3, 129–133. [Google Scholar] [CrossRef]
- Patil, S.; Chandrasekaran, R. Biogenic nanoparticles: A comprehensive perspective in synthesis, characterization, application and its challenges. J. Genet. Eng. Biotechnol. 2020, 18, 67. [Google Scholar] [CrossRef]
- Banerjee, S.; Islam, S.; Chattopadhyay, A.; Sen, A.; Kar, P. Synthesis of silver nanoparticles using underutilized fruit Baccaurea ramiflora (Latka) juice and its biological and cytotoxic efficacy against MCF-7 and MDA-MB 231 cancer cell lines. S. Afr. J. Bot. 2022, 145, 228–235. [Google Scholar] [CrossRef]
- Venugopal, K.; Rather, H.A.; Rajagopal, K.; Shanthi, M.P.; Sheriff, K.; Illiyas, M.; Rather, R.A.; Manikandan, E.; Uvarajan, S.; Bhaskar, M.; et al. Synthesis of silver nanoparticles (Ag NPs) for anticancer activities (MCF 7 breast and A549 lung cell lines) of the crude extract of Syzygium aromaticum. J. Photochem. Photobiol. B Biol. 2017, 167, 282–289. [Google Scholar] [CrossRef]
- Narayanan, M.; Divya, S.; Natarajan, D.; Senthil-Nathan, S.; Kandasamy, S.; Chinnathambi, A.; Alahmadi, T.A.; Pugazhendhi, A. Green synthesis of silver nanoparticles from aqueous extract of Ctenolepis garcini L. and assess their possible biological applications. Process Biochem. 2021, 107, 91–99. [Google Scholar] [CrossRef]
- Gupta, A.; Pandey, S.; Variya, B.; Shah, S.; Yadav, J.S. Green synthesis of gold nanoparticles using different leaf extracts of Ocimum gratissimum Linn for anti-tubercular activity. Curr. Nanomed. 2019, 9, 146–157. [Google Scholar] [CrossRef]
- Akintelu, S.A.; Oyebamiji, A.K.; Olugbeko, S.C.; Latona, D.F. Green chemistry approach towards the synthesis of copper nanoparticles and its potential applications as therapeutic agents and environmental control. Curr. Res. Green Sustain. Chem. 2021, 4, 100176. [Google Scholar] [CrossRef]
- Lara, H.H.; Ayala-Nuñez, N.V.; Ixtepan-Turrent, L.; Rodriguez-Padilla, C. Mode of antiviral action of silver nanoparticles against HIV-1. J. Nanobiotechnology 2010, 8, 1. [Google Scholar] [CrossRef]
- Chamani, E.; Ebrahimi, R.; Khorsandi, K.; Meshkini, A.; Zarban, A.; Sharifzadeh, G. In vitro cytotoxicity of polyphenols from Datura innoxia aqueous leaf-extract on human leukemia K562 cells: DNA and nuclear proteins as targets. Drug Chem. Toxicol. 2020, 43, 138–148. [Google Scholar] [CrossRef]
- Tahir, M.; Fakhar-e-Alam, M.; Asif, M.; Iqbal, M.J.; Abbas, A.; Hassan, M.; Rehman, J.; Bhatti, Q.A.; Mustafa, G.; Alothman, A.A.; et al. Investigation of gadolinium doped manganese nano spinel ferrites via magnetic hypothermia therapy effect towards MCF-7 breast cancer. Heliyon 2024, 10, E24792. [Google Scholar] [CrossRef]
- Rhim, J.W.; Wang, L.F.; Hong, S.I. Preparation and characterization of agar/silver nanoparticles composite films with antimicrobial activity. Food Hydrocoll. 2013, 33, 327–335. [Google Scholar] [CrossRef]
- Sadiqa, A.; Rasul, A.; Hassan, M.; Sultana, S.; Jabeen, F. Identification of Novel Natural Inhibitors to Human 3-Phosphoglycerate Dehydrogenase (PHGDH) for Cancer Treatment. Molecules 2022, 27, 6108. [Google Scholar] [CrossRef]
- Fakhar-e-Alam, M.; Amjad, I.; Saadullah, M.; Tahir, M.; Jawad, M.; Asif, M.; Atif, M.; Zara, S.; Rashad, M. Antitumor activity of Zinc oxide nanoparticles fused with Green Extract of Nigella Sativa. J. Saudi Chem. Soc. 2024, 28, 101814. [Google Scholar] [CrossRef]
- Shetti, N.P.; Mishra, A.; Bukkitgar, S.D.; Basu, S.; Narang, J.; Raghava Reddy, K.; Aminabhavi, T.M. Conventional and nanotechnology-based sensing methods for SARS coronavirus (2019-nCoV). ACS Appl. Bio Mater. 2021, 4, 1178–1190. [Google Scholar] [CrossRef] [PubMed]
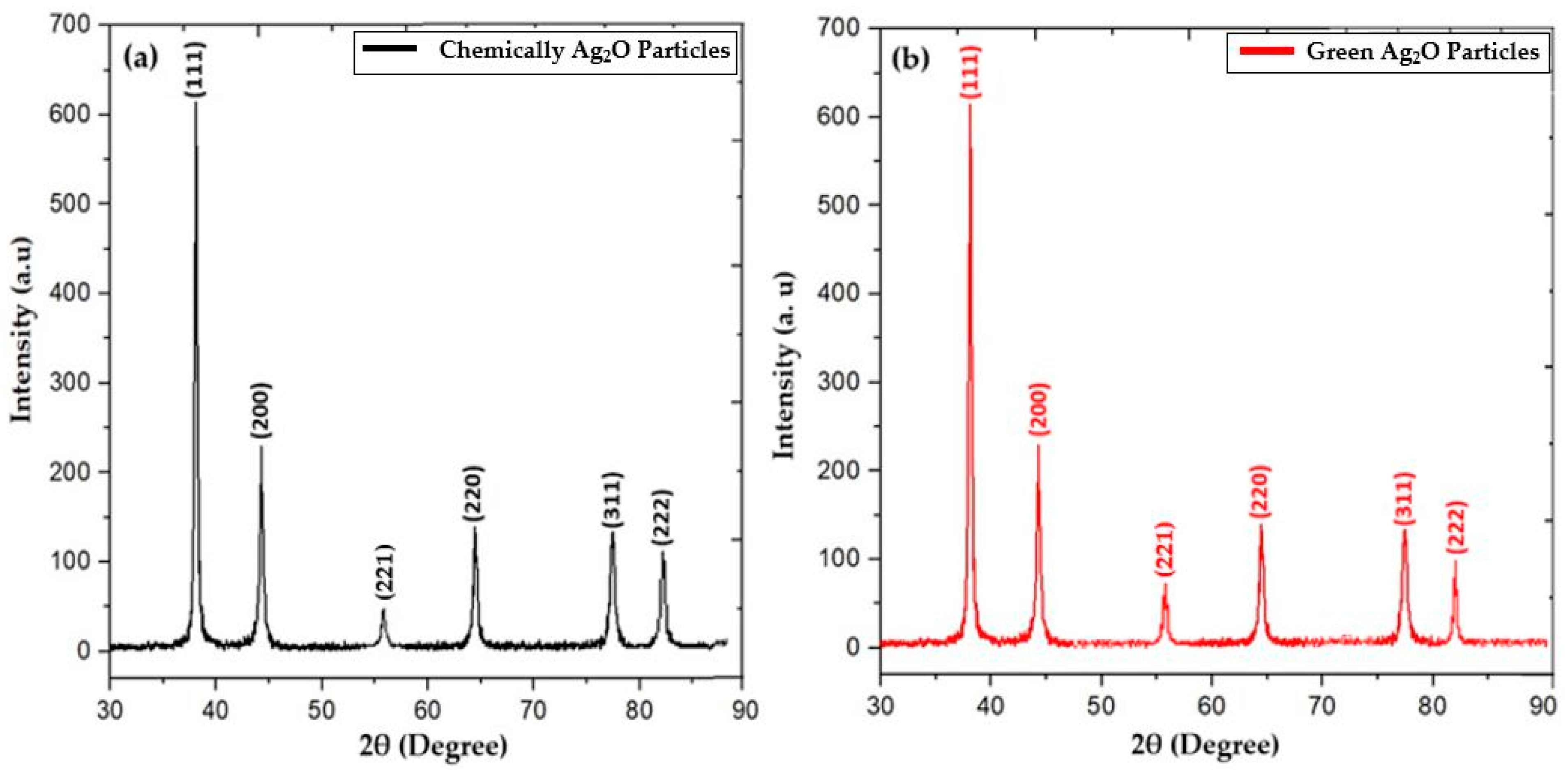


| Miller Indices (h,k,l) | Diffraction Angle 2θ (Theta) | Crystallite Size “C.S” (nm) |
|---|---|---|
| (a) | ||
| (111) | 38.1 | 41.3 |
| (200) | 44.3 | 55.7 |
| (220) | 64.5 | 75.6 |
| (311) | 77.4 | 81.2 |
| (b) | ||
| (111) | 38.1 | 32.2 |
| (200) | 44.3 | 46.6 |
| (220) | 64.8 | 66.6 |
| (311) | 77.7 | 69.9 |
| Sr# | Materials and Plants Name | Applications | IC50 (µg/mL) | References |
|---|---|---|---|---|
| 1 | Green-synthesized AgNPs (Datura innoxia leaf extract) | anticancer | 15.2 and 8.5 | [37] |
| 2 | Biosynthesis of AgNPs with Datura innoxia | anticancer | 20 | [28] |
| 3 | Green-synthesized AgNPs (Baccaurea ramiflora) | anticancer | 110 and 140 | [38] |
| 4 | AgNPs with Syzygium aromaicum | anticancer | 60 and 50 | [39] |
| 5 | Green-synthesized AgNPs (Ctenolepis garcini) | anticancer | 33.78 | [40] |
| 6 | Green-synthesized gold nanoparticles (AuNPs) with (leaf extracts of Ocimum gratissimum Linn) | anticancer | 10 and 25 | [41] |
| 7 | Green copper nanoparticles (CuNPs) with Eclipta prostrata | anticancer | 1.71 and 1.81 | [42] |
| 8 | PVP-coated AgNPs | antiviral | 0.44 | [43] |
| 9 | Datura innoxia leaf extract | anticancer | 0.6 | [44] |
| 10 | Green (Datura innoxia extract) and chemically synthesized Ag NPs | Anticancer, antibacterial and antioxidant | -- | [17] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asif, M.; Iqbal, W.; Fakhar-e-Alam, M.; Hussain, Z.; Saadullah, M.; Hassan, M.; Rehman, J.; Dahlous, K.A.; Al-Qahtani, N.H. Synthesis and Characterization of Chemically and Green-Synthesized Silver Oxide Particles for Evaluation of Antiviral and Anticancer Activity. Pharmaceuticals 2024, 17, 908. https://doi.org/10.3390/ph17070908
Asif M, Iqbal W, Fakhar-e-Alam M, Hussain Z, Saadullah M, Hassan M, Rehman J, Dahlous KA, Al-Qahtani NH. Synthesis and Characterization of Chemically and Green-Synthesized Silver Oxide Particles for Evaluation of Antiviral and Anticancer Activity. Pharmaceuticals. 2024; 17(7):908. https://doi.org/10.3390/ph17070908
Chicago/Turabian StyleAsif, Muhammad, Wajeeha Iqbal, Muhammad Fakhar-e-Alam, Zahid Hussain, Malik Saadullah, Mudassir Hassan, Javed Rehman, Kholood A. Dahlous, and Noora H. Al-Qahtani. 2024. "Synthesis and Characterization of Chemically and Green-Synthesized Silver Oxide Particles for Evaluation of Antiviral and Anticancer Activity" Pharmaceuticals 17, no. 7: 908. https://doi.org/10.3390/ph17070908
APA StyleAsif, M., Iqbal, W., Fakhar-e-Alam, M., Hussain, Z., Saadullah, M., Hassan, M., Rehman, J., Dahlous, K. A., & Al-Qahtani, N. H. (2024). Synthesis and Characterization of Chemically and Green-Synthesized Silver Oxide Particles for Evaluation of Antiviral and Anticancer Activity. Pharmaceuticals, 17(7), 908. https://doi.org/10.3390/ph17070908







