Impact of Microbiota and Metabolites on Intestinal Integrity and Inflammation in Severe Obesity
Abstract
1. Introduction
2. Results
2.1. Descriptive Statistics
2.2. Intestinal Inflammation and Integrity
2.3. Intestinal Microbiota
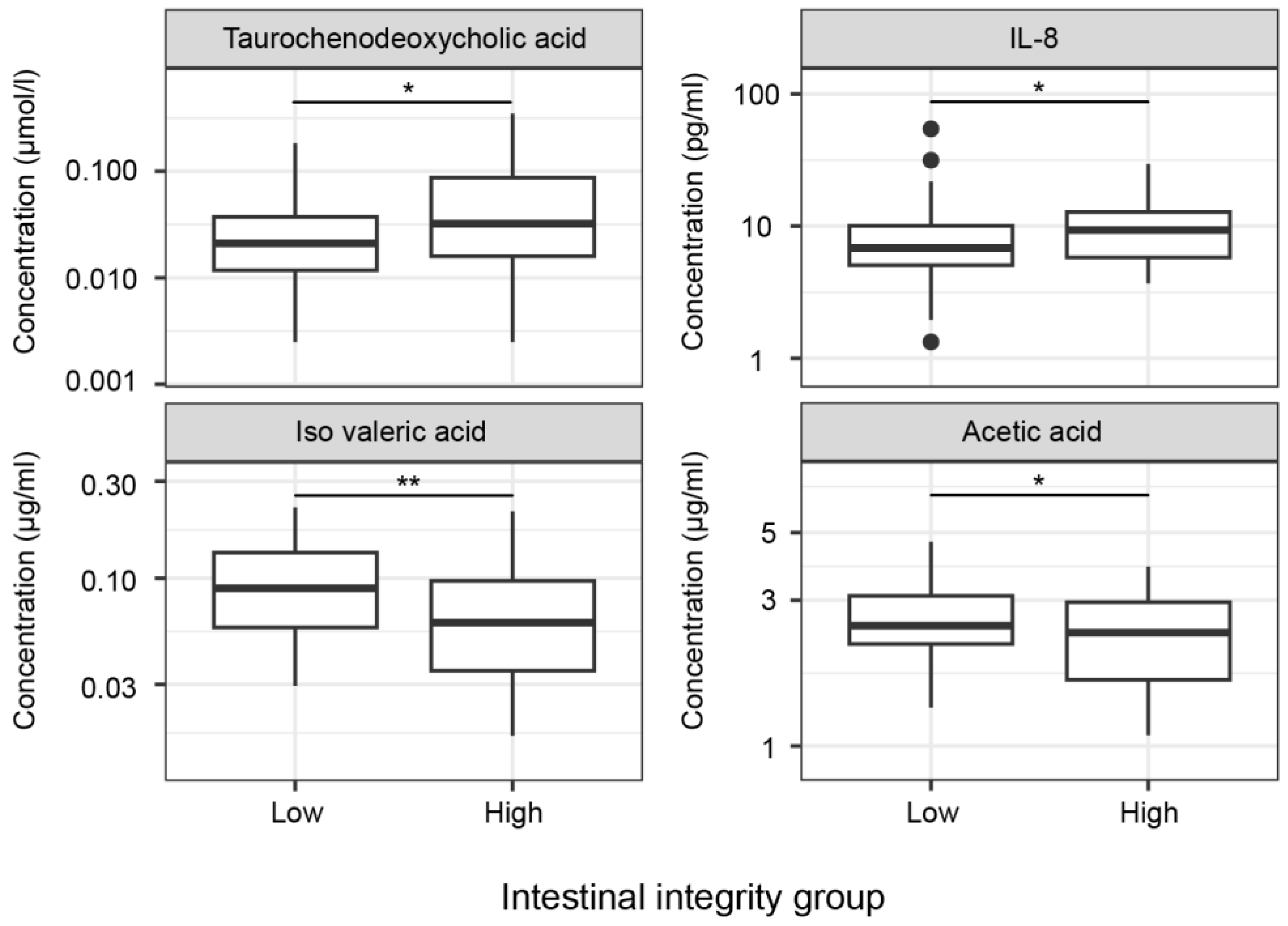
2.4. Metabolites
2.5. Cytokines
3. Discussion
3.1. Limitations
3.2. Conclusions
4. Materials and Methods
4.1. Description of Study Population
4.2. Standard Protocol Approvals, Registration and Patient Consents
4.3. Medical Examination
4.4. Biochemical Analysis in Plasma
4.5. DNA Isolation
4.6. Amplicon Sequencing
4.7. Jejunal Histopathology
4.8. Immunohistochemistry
4.9. Zonula Occludens-1 Immunofluorescence
4.10. Post-Processing of Sections
4.11. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teixeira, T.F.S.; Collado, M.C.; Ferreira, C.L.L.F.; Bressan, J.; Peluzio, M.D.G. Potential mechanisms for the emerging link between obesity and increased intestinal permeability. Nutr. Res. 2012, 32, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Lobionda, S.; Sittipo, P.; Kwon, H.Y.; Lee, Y.K. The Role of Gut Microbiota in Intestinal Inflammation with Respect to Diet and Extrinsic Stressors. Microorganisms 2019, 7, 271. [Google Scholar] [CrossRef] [PubMed]
- Mullin, G.E.; Delzenne, N.M. The Human Gut Microbiome and Its Role in Obesity and the Metabolic Syndrome. In Integrative Weight Management; Nutrition and Health Series; Humana Press: New York, NY, USA, 2014; pp. 71–105. [Google Scholar]
- Schirmer, M.; Smeekens, S.P.; Vlamakis, H.; Jaeger, M.; Oosting, M.; Franzosa, E.A.; ter Horst, R.; Jansen, T.; Jacobs, L.; Bonder, M.J.; et al. Linking the Human Gut Microbiome to Inflammatory Cytokine Production Capacity. Cell 2016, 167, 1897. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.; Neurath, M.F.; Wirtz, S. The Intestinal Microbiota in Inflammatory Bowel Disease. Ilar J. 2015, 56, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Nagao-Kitamoto, H.; Kamada, N. Host-microbial Cross-talk in Inflammatory Bowel Disease. Immune Netw. 2017, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Machiels, K.; Joossens, M.; Sabino, J.; De Preter, V.; Arijs, I.; Eeckhaut, V.; Ballet, V.; Claes, K.; Van Immerseel, F.; Verbeke, K.; et al. A decrease of the butyrate-producing species and defines dysbiosis in patients with ulcerative colitis. Gut 2014, 63, 1275–1283. [Google Scholar] [CrossRef]
- Genser, L.; Aguanno, D.; Soula, H.A.; Dong, L.P.; Trystram, L.; Assmann, K.; Salem, J.E.; Vaillant, J.C.; Oppert, J.M.; Laugerette, F.; et al. Increased jejunal permeability in human obesity is revealed by a lipid challenge and is linked to inflammation and type 2 diabetes. J. Pathol. 2018, 246, 217–230. [Google Scholar] [CrossRef]
- Di Vincenzo, F.; Del Gaudio, A.; Petito, V.; Lopetuso, L.; Scaldaferri, F. Gut microbiota, intestinal permeability, and systemic inflammation: A narrative review. Intern. Emerg. Med. 2023, 19, 275–293. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef]
- Venegas, D.P.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar]
- Perez-Reytor, D.; Puebla, C.; Karahanian, E.; Garcia, K. Use of Short-Chain Fatty Acids for the Recovery of the Intestinal Epithelial Barrier Affected by Bacterial Toxins. Front. Physiol. 2021, 12, 650313. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, M.; Wang, Y.; Yu, T.; Chen, X.; Tang, D.; Xu, L.; Yin, Y.; Pan, Y.; Zhou, Q.; et al. Faecalibacterium prausnitzii produces butyrate to maintain Th17/Treg balance and to ameliorate colorectal colitis by inhibiting histone deacetylase 1. J. Crohns Colitis 2018, 12, S110–S111. [Google Scholar] [CrossRef][Green Version]
- Cao, Y.; Shen, J.; Ran, Z.H. Association between Reduction and Inflammatory Bowel Disease: A Meta-Analysis and Systematic Review of the Literature. Gastroent Res. Pract. 2014, 2014, 872725. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Agus, A.; Clement, K.; Sokol, H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut 2021, 70, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Nighot, M.; Al-Sadi, R.; Guo, S.H.; Rawat, M.; Nighot, P.; Watterson, M.D.; Ma, T.Y. Lipopolysaccharide-Induced Increase in Intestinal Epithelial Tight Permeability Is Mediated by Toll-Like Receptor 4/Myeloid Differentiation Primary Response 88 (MyD88) Activation of Myosin Light Chain Kinase Expression. Am. J. Pathol. 2017, 187, 2698–2710. [Google Scholar] [CrossRef] [PubMed]
- Acciarino, A.; Diwakarla, S.; Handreck, J.; Bergola, C.; Sahakian, L.; Mcquade, R.M. The role of the gastrointestinal barrier in obesity-associated systemic inflammation. Obes. Rev. 2024, 25, e13673. [Google Scholar] [CrossRef] [PubMed]
- Vreeken, D.; Wiesmann, M.; Deden, L.N.; Arnoldussen, I.A.C.; Aarts, E.; Kessels, R.P.C.; Kleemann, R.; Hazebroek, E.J.; Aarts, E.O.; Kiliaan, A.J. Study rationale and protocol of the BARICO study: A longitudinal, prospective, observational study to evaluate the effects of weight loss on brain function and structure after bariatric surgery. BMJ Open 2019, 9, e025464. [Google Scholar] [CrossRef] [PubMed]
- Monteiro-Sepulveda, M.; Touch, S.; Mendes-Sá, C.; André, S.; Poitou, C.; Allatif, O.; Cotillard, A.; Fohrer-Ting, H.; Hubert, E.L.; Remark, R.; et al. Jejunal T Cell Inflammation in Human Obesity Correlates with Decreased Enterocyte Insulin Signaling. Cell Metab. 2015, 22, 113–124. [Google Scholar] [CrossRef]
- Liu, S.; Russo, P.A.; Baldassano, R.N.; Sullivan, K.E. CD68 Expression Is Markedly Different in Crohn’s Disease and the Colitis Associated with Chronic Granulomatous Disease. Inflamm. Bowel Dis. 2009, 15, 1213–1217. [Google Scholar] [CrossRef]
- Yao, K.; Iwashita, A.; Yao, T.; Takemura, S.; Furukawa, K.; Matsui, T.; Aoyagi, K. Increased numbers of macrophages in noninflamed gastroduodenal mucosa of patients with Crohn’s disease. Digest Dis. Sci. 1996, 41, 2260–2267. [Google Scholar] [CrossRef] [PubMed]
- Bottois, H.; Ngollo, M.; Hammoudi, N.; Courau, T.; Bonnereau, J.; Chardiny, V.; Grand, C.; Gergaud, B.; Allez, M.; Le Bourhis, L. KLRG1 and CD103 Expressions Define Distinct Intestinal Tissue-Resident Memory CD8 T Cell Subsets Modulated in Crohn’s Disease. Front. Immunol. 2020, 11, 896. [Google Scholar] [CrossRef] [PubMed]
- Araújo, J.R.; Tomas, J.; Brenner, C.; Sansonetti, P.J. Impact of high-fat diet on the intestinal microbiota and small intestinal physiology before and after the onset of obesity. Biochimie 2017, 141, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Pan, L.B.; Yu, H.; Han, P.; Fu, J.; Zhang, Z.W.; Hu, J.C.; Yang, X.Y.; Keranmu, A.; Zhang, H.J.; et al. Gut microbiota-derived metabolites in inflammatory diseases based on targeted metabolomics. Front. Pharmacol. 2022, 13, 919181. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, K.; Yang, D.; Oppenheim, J.J. Interleukin-8: An evolving chemokine. Cytokine 2022, 153, 155828. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Bai, C. Elevated Serum Interleukin-8 Level as a Preferable Biomarker for Identifying Uncontrolled Asthma and Glucocorticosteroid Responsiveness. Tanaffos 2017, 16, 260–269. [Google Scholar] [PubMed]
- Deleu, S.; Arnauts, K.; Deprez, L.; Machiels, K.; Ferrante, M.; Huys, G.R.B.; Thevelein, J.M.; Raes, J.; Vermeire, S. High Acetate Concentration Protects Intestinal Barrier and Exerts Anti-Inflammatory Effects in Organoid-Derived Epithelial Monolayer Cultures from Patients with Ulcerative Colitis. Int. J. Mol. Sci. 2023, 24, 768. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.Y.; Wang, Y.B.; Yang, G.; Zhang, Q.H.; Meng, L.B.; Xin, Y.; Jiang, X. The role of short-chain fatty acids in intestinal barrier function, inflammation, oxidative stress, and colonic carcinogenesis. Pharmacol. Res. 2021, 165, 105420. [Google Scholar] [CrossRef] [PubMed]
- Pravasia, S.D. Acetic Acid. In Encyclopedia of Toxicology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Niccolai, E.; Baldi, S.; Ricci, F.; Russo, E.; Nannini, G.; Menicatti, M.; Poli, G.; Taddei, A.; Bartolucci, G.; Calabrò, A.S.; et al. Evaluation and comparison of short chain fatty acids composition in gut diseases. World J. Gastroentero 2019, 25, 5543–5558. [Google Scholar] [CrossRef]
- Calzadilla, N.; Comiskey, S.M.; Dudeja, P.K.; Saksena, S.; Gill, R.K.; Alrefai, W.A. Bile acids as inflammatory mediators and modulators of intestinal permeability. Front. Immunol. 2022, 13, 1021924. [Google Scholar] [CrossRef]
- Tian, Q.J.; Yang, R.Y.; Wang, Y.; Liu, J.M.; Wee, A.; Saxena, R.; Wang, L.; Li, M.; Liu, L.W.; Shan, S.; et al. A High Serum Level of Taurocholic Acid Is Correlated With the Severity and Resolution of Drug-induced Liver Injury. Clin. Gastroenterol. Hepatol. 2021, 19, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Young, M.T.; Phelan, M.J.; Nguyen, N.T. A Decade Analysis of Trends and Outcomes of Male vs Female Patients Who Underwent Bariatric Surgery. J. Am. Coll. Surg. 2016, 222, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Brettle, H.; Tran, V.; Drummond, G.R.; Franks, A.E.; Petrovski, S.; Vinh, A.; Jelinic, M. Sex hormones, intestinal inflammation, and the gut microbiome: Major influencers of the sexual dimorphisms in obesity. Front. Immunol. 2022, 13, 971048. [Google Scholar] [CrossRef] [PubMed]
- Fried, M.; Hainer, V.; Basdevant, A.; Buchwald, H.; Deitel, M.; Finer, N.; Greve, J.W.M.; Horber, F.; Mathus-Vliegen, E.; Scopinaro, N.; et al. Interdisciplinary European Guidelines on Surgery of Severe Obesity. Obes. Facts 2008, 1, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Morrison, M.C.; Gart, E.; van Duyvenvoorde, W.; Snabel, J.; Nielsen, M.J.; Leeming, D.J.; Menke, A.; Kleemann, R. Heat-Inactivated Improves Gut Permeability but Does Not Prevent Development of Non-Alcoholic Steatohepatitis in Diet-Induced Obese Ldlr−/−. Leiden Mice. Int. J. Mol. Sci. 2022, 23, 2325. [Google Scholar] [CrossRef]
- Schutte, M.H.; Kleemann, R.; Nota, N.M.; Wiepjes, C.M.; Snabel, J.M.; T’Sjoen, G.; Thijs, A.; den Heijer, M. The effect of transdermal gender-affirming hormone therapy on markers of inflammation and hemostasis. PLoS ONE 2022, 17, e0261312. [Google Scholar] [CrossRef]
- Vreeken, D.; Seidel, F.; de la Roij, G.; Vening, W.; den Hengst, W.A.; Verschuren, L.; Oezsezen, S.; Kessels, R.P.C.; Duering, M.; Mutsaerts, H.J.M.M.; et al. Impact of White Adipose Tissue on Brain Structure, Perfusion, and Cognitive Function in Patients With Severe Obesity The BARICO Study. Neurology 2023, 100, E703–E718. [Google Scholar] [CrossRef]
- Kozich, J.J.; Westcott, S.L.; Baxter, N.T.; Highlander, S.K.; Schloss, P.D. Development of a Dual-Index Sequencing Strategy and Curation Pipeline for Analyzing Amplicon Sequence Data on the MiSeq Illumina Sequencing Platform. Appl. Environ. Microb. 2013, 79, 5112–5120. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- MathWorks. Approaches to Registering Images—MATLAB & Simulink. 2020. Available online: https://nl.mathworks.com/help/images/approaches-to-registering-images.html (accessed on 9 September 2020).
- MathWorks. Polygon—MATLAB & Simulink. Available online: https://www.mathworks.com/help/images/ref/images.roi.polygon.html (accessed on 15 September 2020).
- Ruifrok, A.C.; Johnston, D.A. Quantification of histochemical staining by color deconvolution. Anal. Quant. Cytol. Histol. 2001, 23, 291–299. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer-Verlag: New York, NY, USA, 2016. [Google Scholar]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Wiese, M.; Schuren, F.H.J.; Smits, W.K.; Kuijper, E.J.; Ouwens, A.; Heerikhuisen, M.; Vigsnaes, L.; van den Broek, T.J.; de Boer, P.; Montijn, R.C.; et al. 2′-Fucosyllactose inhibits proliferation of ATCC 43599 in the CDi-screen, an model simulating infection. Front. Cell. Infect. Microbiol. 2022, 12, 991150. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 1–21. [Google Scholar] [CrossRef] [PubMed]

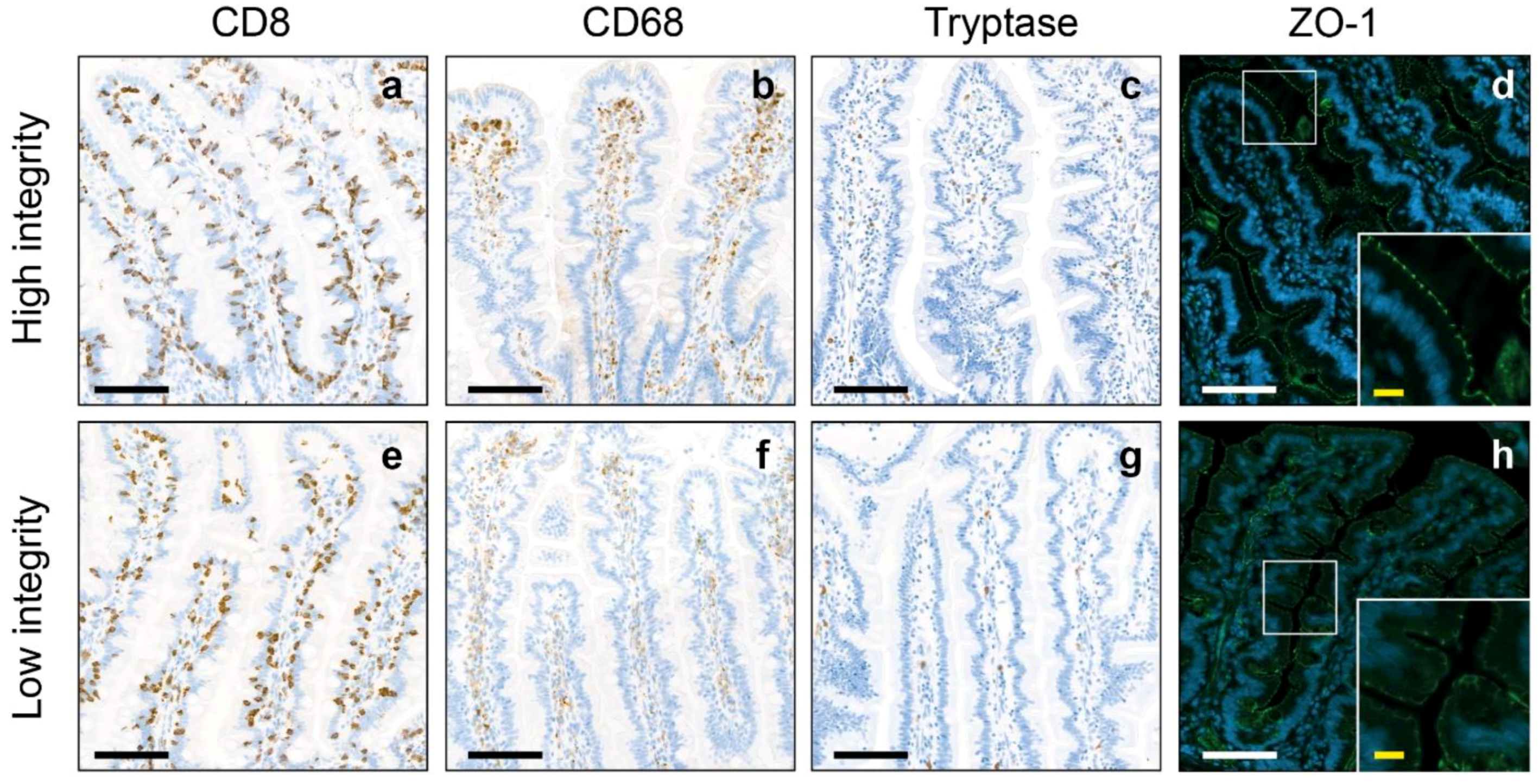

| Intestinal Inflammation | Intestinal Integrity | ||||||
|---|---|---|---|---|---|---|---|
| All (n = 100) | Low (n = 52) | High (n = 48) | p-Value | Low (n = 62) | High (n = 38) | p-Value | |
| Age, mean ± SD, y | 46.48 ± 5.73 | 46.48 ± 5.68 | 46.47 ± 5.85 | 0.993 | 46.74 ± 5.32 | 46.05 ± 6.40 | 0.564 |
| Sex, women, n (%) | 82 (82.0) | 43 (82.69) | 39 (81.25) | 0.528 | 46 (74.19) | 36 (94.74) | 0.007 |
| Body length, mean ± SD, m | 1.71 ± 0.07 | 1.71 ± 0.08 | 1.71 ± 0.07 | 0.997 | 1.73 ± 0.08 | 1.69 ± 0.06 | 0.013 |
| Body weight, mean ± SD, kg | 122.69 ± 15.69 | 120.96 ± 16.08 | 124.56 ± 15.20 | 0.253 | 124.21 ± 17.0 | 120.19 ± 13.20 | 0.216 |
| BMI, mean ± SD, kg/m2 | 41.61 ± 3.92 | 40.98 ± 3.60 | 42.28 ± 4.17 | 0.097 | 41.39 ± 3.63 | 41.97 ± 4.37 | 0.476 |
| WC a, mean ± SD, cm | 124.75 ± 11.14 | 124.16 ± 11.27 | 125.36 ± 11.11 | 0.613 | 125.03 ± 11.57 | 124.34 ± 10.64 | 0.777 |
| Comorbidities, n (%) | |||||||
| Pre-diabetes | 23 (23.0) | 12 (23.08) | 11 (22.92) | 0.587 | 16 (25.81) | 7 (18.42) | 0.275 |
| Diabetes | 14 (14.0) | 5 (9.62) | 9 (18.75) | 0.152 | 10 (16.13) | 4 (10.53) | 0.319 |
| Hypertension | 71 (71.0) | 37 (71.15) | 34 (70.83) | 0.573 | 45 (72.58) | 26 (68.42) | 0.411 |
| Irritable bowel syndrome | 5 (5.0) | 3 (5.77) | 2 (4.17) | 0.538 | 4 (6.45) | 1 (2.63) | 0.368 |
| Ulcerative colitis | 1 (1.0) | 1 (1.92) | 0 (0) | 0.520 | 1 (1.61) | 0 (0) | 0.620 |
| Smoking current, n (%) | 6 (6.0) | 3 (5.77) | 3 (6.25) | 0.622 | 3 (4.84) | 3 (7.89) | 0.413 |
| Consuming alcohol, n (%) | 40 (40.0) | 18 (34.62) | 22 (45.83) | 0.174 | 25 (40.32) | 15 (39.47) | 0.551 |
| Alcohol consumption, median (IQR), units per week | 1.0 (3.0) | 1.0 (3.25) | 1.5 (3.0) | 0.965 | 2.0 (4.0) | 1.0 (2.0) | 0.188 |
| Blood pressure, mean ± SD | |||||||
| Systolic (mm Hg) | 136.72 ± 16.30 | 136.17 ± 18.06 | 137.31 ± 14.31 | 0.729 | 136.90 ± 16.49 | 136.42 ± 16.21 | 0.887 |
| Diastolic (mm Hg) | 84.78 ± 7.96 | 81.29 ± 8.03 | 85.42 ± 7.92 | 0.445 | 84.84 ± 8.25 | 84.68 ± 7.57 | 0.926 |
| Gut pathology, mean ± SD | |||||||
| Cytotoxic T cells, number/mm2 | 1040.16 ± 285.17 | 1021.97 ± 269.94 | 1059.87 ± 302.42 | 0.509 | 1007.72 ± 297.37 | 1093.10 ± 259.15 | 0.147 |
| Macrophages, number/mm2 | 628.36 ± 308.72 | 482.25 ± 236.27 | 786.64 ± 298.28 | <0.001 | 555.63 ± 268.25 | 747.02 ± 331.30 | 0.002 |
| Mast cells, number/mm2 | 103.18 ± 66.19 | 58.05 ± 40.22 | 152.08 ± 52.68 | <0.001 | 104.12 ± 72.00 | 101.65 ± 56.30 | 0.857 |
| ZO-1 intensity (MGV) | 76.34 ± 6.48 | 75.55 ± 6.81 | 77.19 ± 6.05 | 0.205 | 72.43 ± 2.57 | 82.72 ± 5.83 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Custers, E.; Vreeken, D.; Schuren, F.; van den Broek, T.J.; van Dongen, L.; Geenen, B.; de Blaauw, I.; Wiesmann, M.; Hazebroek, E.J.; Kleemann, R.; et al. Impact of Microbiota and Metabolites on Intestinal Integrity and Inflammation in Severe Obesity. Pharmaceuticals 2024, 17, 918. https://doi.org/10.3390/ph17070918
Custers E, Vreeken D, Schuren F, van den Broek TJ, van Dongen L, Geenen B, de Blaauw I, Wiesmann M, Hazebroek EJ, Kleemann R, et al. Impact of Microbiota and Metabolites on Intestinal Integrity and Inflammation in Severe Obesity. Pharmaceuticals. 2024; 17(7):918. https://doi.org/10.3390/ph17070918
Chicago/Turabian StyleCusters, Emma, Debby Vreeken, Frank Schuren, Tim J. van den Broek, Lieke van Dongen, Bram Geenen, Ivo de Blaauw, Maximilian Wiesmann, Eric J. Hazebroek, Robert Kleemann, and et al. 2024. "Impact of Microbiota and Metabolites on Intestinal Integrity and Inflammation in Severe Obesity" Pharmaceuticals 17, no. 7: 918. https://doi.org/10.3390/ph17070918
APA StyleCusters, E., Vreeken, D., Schuren, F., van den Broek, T. J., van Dongen, L., Geenen, B., de Blaauw, I., Wiesmann, M., Hazebroek, E. J., Kleemann, R., & Kiliaan, A. J. (2024). Impact of Microbiota and Metabolites on Intestinal Integrity and Inflammation in Severe Obesity. Pharmaceuticals, 17(7), 918. https://doi.org/10.3390/ph17070918







