Exploring Microbial Metabolite Receptors in Inflammatory Bowel Disease: An In Silico Analysis of Their Potential Role in Inflammation and Fibrosis
Abstract
1. Introduction
2. Results
2.1. Metabolite Receptor Genes Are Differentially Expressed in Crohn’s Disease and Ulcerative Colitis
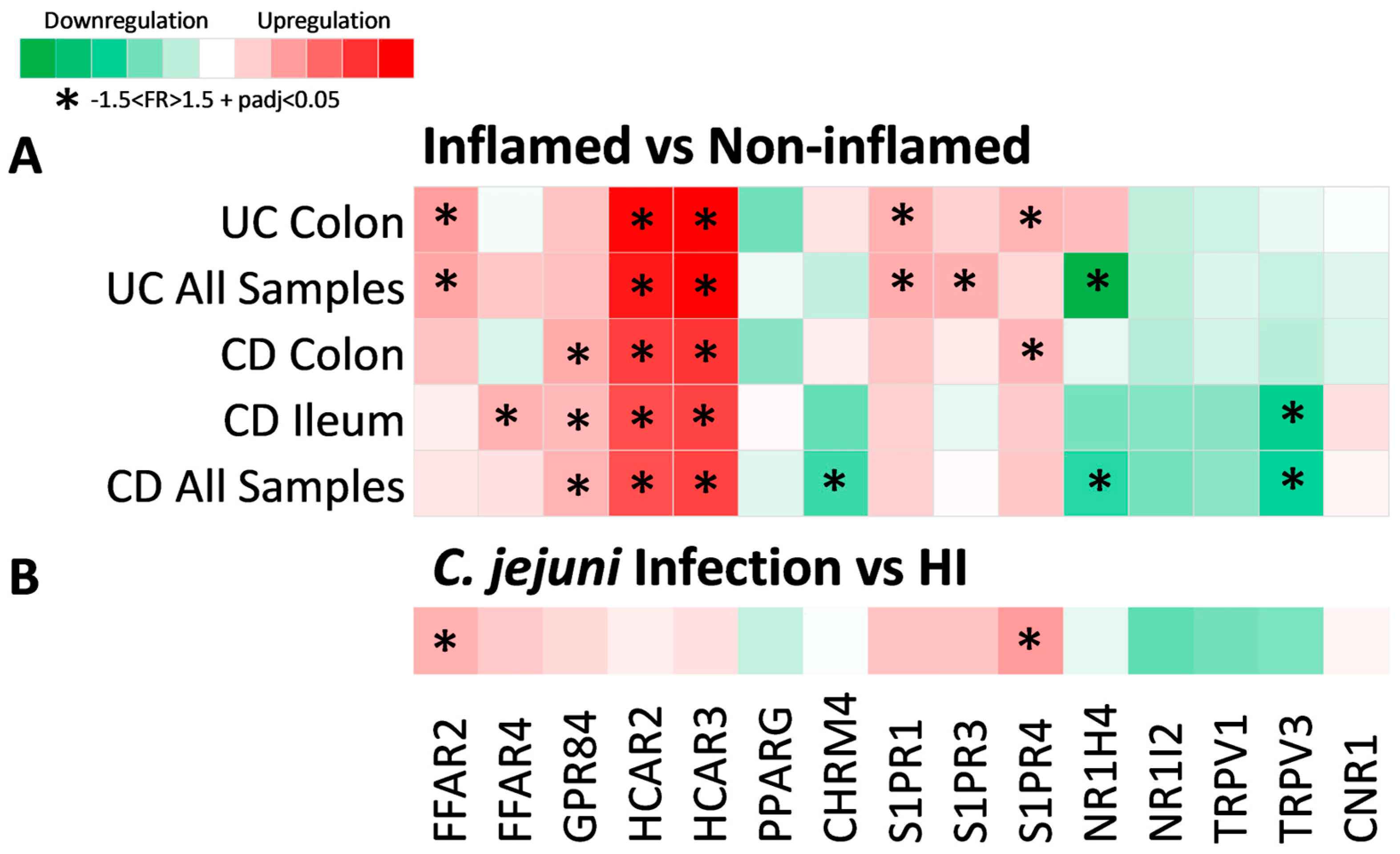
2.2. Expression of Metabolite Receptor Genes Is Associated with Inflammation in Inflammatory Bowel Disease
2.3. Expression of Metabolite Receptors Differs between Colon and Terminal Ileum
2.4. Metabolite Receptor Gene Expression Exhibits Differential Correlation with Up- and Downregulated Genes in Inflammatory Bowel Disease and Healthy Individuals
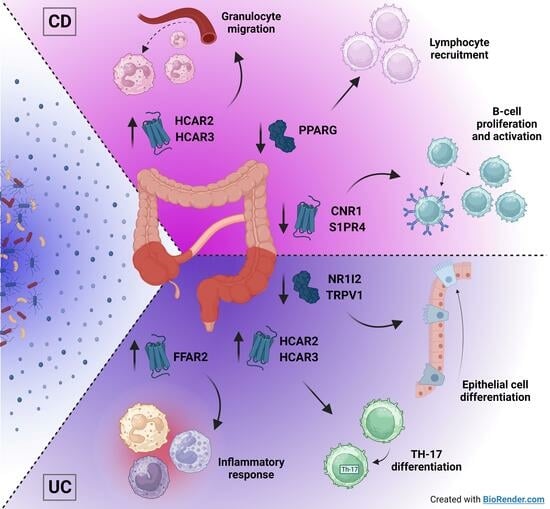
2.5. Metabolite Receptors Associate with the Processes of Inflammation and Fibrosis in Crohn’s Disease and Ulcerative Colitis
3. Discussion
4. Materials and Methods
4.1. Data Acquisition and Processing
4.2. Differential Expression Analysis
4.3. Correlation and Network Analysis
4.4. Functional Enrichment Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mak, W.Y.; Zhao, M.; Ng, S.C.; Burisch, J. The Epidemiology of Inflammatory Bowel Disease: East Meets West. J. Gastroenterol. Hepatol. 2020, 35, 380–389. [Google Scholar] [CrossRef]
- Guan, Q. A Comprehensive Review and Update on the Pathogenesis of Inflammatory Bowel Disease. J. Immunol. Res. 2019, 2019, 7247238. [Google Scholar] [CrossRef]
- Glassner, K.L.; Abraham, B.P.; Quigley, E.M.M. The Microbiome and Inflammatory Bowel Disease. J. Allergy Clin. Immunol. 2020, 145, 16–27. [Google Scholar] [CrossRef]
- Ni, J.; Wu, G.D.; Albenberg, L.; Tomov, V.T. Gut Microbiota and IBD: Causation or Correlation? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Huttenhower, C.; Gevers, D.; Knight, R.; Abubucker, S.; Badger, J.H.; Chinwalla, A.T.; Creasy, H.H.; Earl, A.M.; Fitzgerald, M.G.; Fulton, R.S.; et al. Structure, Function and Diversity of the Healthy Human Microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef]
- Bäckhed, F.; Fraser, C.M.; Ringel, Y.; Sanders, M.E.; Sartor, R.B.; Sherman, P.M.; Versalovic, J.; Young, V.; Finlay, B.B. Defining a Healthy Human Gut Microbiome: Current Concepts, Future Directions, and Clinical Applications. Cell Host Microbe 2012, 12, 611–622. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A Core Gut Microbiome in Obese and Lean Twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Dorrestein, P.C.; Mazmanian, S.K.; Knight, R. Finding the Missing Links among Metabolites, Microbes, and the Host. Immunity 2014, 40, 824–832. [Google Scholar] [CrossRef]
- Franzosa, E.A.; Sirota-Madi, A.; Avila-Pacheco, J.; Fornelos, N.; Haiser, H.J.; Reinker, S.; Vatanen, T.; Hall, A.B.; Mallick, H.; McIver, L.J.; et al. Gut Microbiome Structure and Metabolic Activity in Inflammatory Bowel Disease. Nat. Microbiol. 2019, 4, 293–305. [Google Scholar] [CrossRef]
- De Preter, V.; Arijs, I.; Windey, K.; Vanhove, W.; Vermeire, S.; Schuit, F.; Rutgeerts, P.; Verbeke, K. Impaired Butyrate Oxidation in Ulcerative Colitis Is Due to Decreased Butyrate Uptake and a Defect in the Oxidation Pathway. Inflamm. Bowel Dis. 2012, 18, 1127–1136. [Google Scholar] [CrossRef]
- Arifuzzaman, M.; Won, T.H.; Li, T.T.; Yano, H.; Digumarthi, S.; Heras, A.F.; Zhang, W.; Parkhurst, C.N.; Kashyap, S.; Jin, W.B.; et al. Inulin Fibre Promotes Microbiota-Derived Bile Acids and Type 2 Inflammation. Nature 2022, 611, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Colosimo, D.A.; Kohn, J.A.; Luo, P.M.; Piscotta, F.J.; Han, S.M.; Pickard, A.J.; Rao, A.; Cross, J.R.; Cohen, L.J.; Brady, S.F. Mapping Interactions of Microbial Metabolites with Human G-Protein-Coupled Receptors. Cell Host Microbe 2019, 26, 273–282.e7. [Google Scholar] [CrossRef] [PubMed]
- Nuzzo, A.; Saha, S.; Berg, E.; Jayawickreme, C.; Tocker, J.; Brown, J.R. Expanding the Drug Discovery Space with Predicted Metabolite–Target Interactions. Commun. Biol. 2021, 4, 288. [Google Scholar] [CrossRef] [PubMed]
- Wnorowski, A.; Wnorowska, S.; Kurzepa, J.; Parada-Turska, J. Alterations in Kynurenine and Nad+ Salvage Pathways during the Successful Treatment of Inflammatory Bowel Disease Suggest Hcar3 and Nnmt as Potential Drug Targets. Int. J. Mol. Sci. 2021, 22, 13497. [Google Scholar] [CrossRef] [PubMed]
- Kaddoura, R.; Ghelani, H.; Alqutami, F.; Altaher, H.; Hachim, M.; Jan, R.K. Identification of Specific Biomarkers and Pathways in the Treatment Response of Infliximab for Inflammatory Bowel Disease: In-Silico Analysis. Life 2023, 13, 680. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K. Biological Roles and Therapeutic Potential of Hydroxy-Carboxylic Acid Receptors. Front. Endocrinol. 2011, 2, 51. [Google Scholar] [CrossRef] [PubMed]
- Carretta, M.D.; Quiroga, J.; López, R.; Hidalgo, M.A.; Burgos, R.A. Participation of Short-Chain Fatty Acids and Their Receptors in Gut Inflammation and Colon Cancer. Front. Physiol. 2021, 12, 662739. [Google Scholar] [CrossRef] [PubMed]
- Kapolka, N.J.; Isom, D.G. HCAR3: An Underexplored Metabolite Sensor. Nat. Rev. Drug Discov. 2020, 19, 745. [Google Scholar] [CrossRef]
- Chen, G.; Ran, X.; Li, B.; Li, Y.; He, D.; Huang, B.; Fu, S.; Liu, J.; Wang, W. Sodium Butyrate Inhibits Inflammation and Maintains Epithelium Barrier Integrity in a TNBS-Induced Inflammatory Bowel Disease Mice Model. EBioMedicine 2018, 30, 317–325. [Google Scholar] [CrossRef]
- Peters, A.; Krumbholz, P.; Jäger, E.; Heintz-Buschart, A.; Çakir, M.V.; Rothemund, S.; Gaudl, A.; Ceglarek, U.; Schöneberg, T.; Stäubert, C. Metabolites of Lactic Acid Bacteria Present in Fermented Foods Are Highly Potent Agonists of Human Hydroxycarboxylic Acid Receptor 3. PLoS Genet. 2019, 15, e1008145. [Google Scholar] [CrossRef]
- Mandrika, I.; Tilgase, A.; Petrovska, R.; Klovins, J. Hydroxycarboxylic Acid Receptor Ligands Modulate Proinflammatory Cytokine Expression in Human Macrophages and Adipocytes without Affecting Adipose Differentiation. Biol. Pharm. Bull. 2018, 41, 1574–1580. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.; Tunaru, S.; Langhans, C.D.; Hanson, J.; Michalski, C.W.; Kölker, S.; Jones, P.M.; Okun, J.G.; Offermanns, S. Deorphanization of GPR109B as a Receptor for the β-Oxidation Intermediate 3-OH-Octanoic Acid and Its Role in the Regulation of Lipolysis. J. Biol. Chem. 2009, 284, 21928–21933. [Google Scholar] [CrossRef] [PubMed]
- Taggart, A.K.P.; Kero, J.; Gan, X.; Cai, T.Q.; Cheng, K.; Ippolito, M.; Ren, N.; Kaplan, R.; Wu, K.; Wu, T.J.; et al. (D)-β-Hydroxybutyrate Inhibits Adipocyte Lipolysis via the Nicotinic Acid Receptor PUMA-G. J. Biol. Chem. 2005, 280, 26649–26652. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Picón, E.; Dotti, I.; Corraliza, A.M.; Mayorgas, A.; Esteller, M.; Perales, J.C.; Ricart, E.; Masamunt, M.C.; Carrasco, A.; Tristán, E.; et al. Intestinal Inflammation Modulates the Epithelial Response to Butyrate in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2020, 26, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, T.; Watanabe, K.; Watanabe, T.; Yamagami, H.; Sogawa, M.; Tanigawa, T.; Shiba, M.; Tominaga, K.; Fujiwara, Y.; Maeda, K.; et al. Tumor Necrosis Factor α Decreases Glucagon-like Peptide-2 Expression by up-Regulating G-Protein-Coupled Receptor 120 in Crohn Disease. Am. J. Pathol. 2015, 185, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, L.H.; Yang, H.; Fang, Y.C.; Wang, S.W.; Wang, M.; Yuan, Q.T.; Wu, W.; Zhang, Y.M.; Liu, Z.J.; et al. GPR84 Signaling Promotes Intestinal Mucosal Inflammation via Enhancing NLRP3 Inflammasome Activation in Macrophages. Acta Pharmacol. Sin. 2022, 43, 2042–2054. [Google Scholar] [CrossRef]
- Bajic, D.; Niemann, A.; Hillmer, A.K.; Mejias-Luque, R.; Bluemel, S.; Docampo, M.; Funk, M.C.; Tonin, E.; Boutros, M.; Schnabl, B.; et al. Gut Microbiota-Derived Propionate Regulates the Expression of Reg3 Mucosal Lectins and Ameliorates Experimental Colitis in Mice. J. Crohns Colitis 2020, 14, 1462–1472. [Google Scholar] [CrossRef]
- Zhao, M.; Zhu, W.; Gong, J.; Zuo, L.; Zhao, J.; Sun, J.; Li, N.; Li, J. Dietary Fiber Intake Is Associated with Increased Colonic Mucosal GPR43+ Polymorphonuclear Infiltration in Active Crohn’s Disease. Nutrients 2015, 7, 5327–5346. [Google Scholar] [CrossRef]
- Oh, D.Y.; Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.Q.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 Is an Omega-3 Fatty Acid Receptor Mediating Potent Anti-Inflammatory and Insulin-Sensitizing Effects. Cell 2010, 142, 687–698. [Google Scholar] [CrossRef]
- Djuricic, I.; Calder, P.C. Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [CrossRef]
- Hagihara, M.; Yamashita, M.; Ariyoshi, T.; Eguchi, S.; Minemura, A.; Miura, D.; Higashi, S.; Oka, K.; Nonogaki, T.; Mori, T.; et al. Clostridium Butyricum-Induced ω-3 Fatty Acid 18-HEPE Elicits Anti-Influenza Virus Pneumonia Effects through Interferon-λ Upregulation. Cell Rep. 2022, 41, 111755. [Google Scholar] [CrossRef] [PubMed]
- Salaga, M.; Bartoszek, A.; Binienda, A.; Krajewska, J.B.; Fabisiak, A.; Mosińska, P.; Dziedziczak, K.; Niewinna, K.; Talar, M.; Tarasiuk, A.; et al. Activation of Free Fatty Acid Receptor 4 Affects Intestinal Inflammation and Improves Colon Permeability in Mice. Nutrients 2021, 13, 2716. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Liu, H.; Xu, L.; Yu, T.; Zhao, X.; Yao, S.; Zhao, Q.; Barnes, S.; Cohn, S.M.; Dann, S.M.; et al. GPR120 Inhibits Colitis Through Regulation of CD4+ T Cell Interleukin 10 Production. Gastroenterology 2022, 162, 150–165. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, D.; Zong, Y.; Yang, X. Dha Protects Hepatocytes from Oxidative Injury through Gpr120/Erk-Mediated Mitophagy. Int. J. Mol. Sci. 2021, 22, 5675. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.H.; Zhang, Q.; Xie, X.; Nan, F.J. Modulation of the G-Protein-Coupled Receptor 84 (GPR84) by Agonists and Antagonists. J. Med. Chem. 2020, 63, 15399–15409. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.K.; Su, S.C.; Chang, L.C.; Yang, K.J.; Lee, C.C.; Hsu, H.J.; Chen, Y.T.; Sun, C.Y.; Wu, I.W. Oral Absorbent AST-120 Is Associated with Compositional and Functional Adaptations of Gut Microbiota and Modification of Serum Short and Medium-Chain Fatty Acids in Advanced CKD Patients. Biomedicines 2022, 10, 2234. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Sweeney, T.R.; Shigenaga, J.K.; Chui, L.G.; Moser, A.; Grunfeld, C.; Feingold, K.R. Tumor Necrosis Factor and Interleukin 1 Decrease RXRα, PPARα, PPARγ, LXRα, and the Coactivators SRC-1, PGC-1α, and PGC-1β in Liver Cells. Metabolism 2007, 56, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Dubuquoy, L.; Å Jansson, E.; Deeb, S.; Rakotobe, S.; Karoui, M.; Colombel, J.F.; Auwerx, J.; Pettersson, S.; Desreumaux, P. Impaired Expression of Peroxisome Proliferator-Activated Receptor Γin Ulcerative Colitis. Gastroenterology 2003, 124, 1265–1276. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto-Furusho, J.K.; Jacintez-Cazares, M.; Furuzawa-Carballeda, J.; Fonseca-Camarillo, G. Peroxisome Proliferator-Activated Receptors Family Is Involved in the Response to Treatment and Mild Clinical Course in Patients with Ulcerative Colitis. Dis. Markers 2014, 2014, 932530. [Google Scholar] [CrossRef]
- Sugawara, K.; Olson, T.S.; Moskaluk, C.A.; Stevens, B.K.; Hoang, S.; Kozaiwa, K.; Cominelli, F.; Ley, K.F.; McDuffie, M. Linkage to Peroxisome Proliferator-Activated Receptor-γ in SAMP1/YitFc Mice and in Human Crohn’s Disease. Gastroenterology 2005, 128, 351–360. [Google Scholar] [CrossRef]
- Dubuquoy, L.; Rousseaux, C.; Thuru, X.; Peyrin-Biroulet, L.; Romano, O.; Chavatte, P.; Chamaillard, M.; Desreumaux, P. PPARγ as a New Therapeutic Target in Inflammatory Bowel Diseases. Gut 2006, 55, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Cevallos, S.A.; Lee, J.Y.; Velazquez, E.M.; Foegeding, N.J.; Shelton, C.D.; Tiffany, C.R.; Parry, B.H.; Stull-Lane, A.R.; Olsan, E.E.; Savage, H.P.; et al. 5-Aminosalicylic Acid Ameliorates Colitis and Checks Dysbiotic Escherichia Coli Expansion by Activating PPAR-G Signaling in the Intestinal Epithelium. mBio 2021, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sæterstad, S.; Østvik, A.E.; Røyset, E.S.; Bakke, I.; Sandvik, A.K.; Granlund, A.v.B. Profound Gene Expression Changes in the Epithelial Monolayer of Active Ulcerative Colitis and Crohn’s Disease. PLoS ONE 2022, 17, e0265189. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Yang, L.; Wang, Z.; Huang, W. Bile Acid Nuclear Receptor FXR and Digestive System Diseases. Acta Pharm. Sin. B 2015, 5, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, T.; Moschetta, A.; Lee, Y.-K.; Peng, L.; Zhao, G.; Downes, M.; Yu, R.T.; Shelton, J.M.; Richardson, J.A.; Repa, J.J.; et al. Regulation of Antibacterial Defense in the Small Intestine by the Nuclear Bile Acid Receptor. Proc. Natl. Acad. Sci. USA 2006, 103, 3920–3925. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Shigenaga, J.; Moser, A.; Feingold, K.; Grunfeld, C. Repression of Farnesoid X Receptor during the Acute Phase Response. J. Biol. Chem. 2003, 278, 8988–8995. [Google Scholar] [CrossRef] [PubMed]
- Shakhnovich, V.; Vyhlidal, C.; Friesen, C.; Hildreth, A.; Singh, V.; Daniel, J.; Kearns, G.L.; Leeder, J.S. Decreased Pregnane X Receptor Expression in Children with Active Crohn’s Disease. Drug Metab. Dispos. 2016, 44, 1066–1069. [Google Scholar] [CrossRef]
- Haskell, H.; Andrews, C.W.; Reddy, S.I.; Dendrinos, K.; Farraye, F.A.; Stucchi, A.F.; Becker, J.M.; Odze, R.D. Pathologic Features and Clinical Significance of “‘Backwash’” Ileitis in Ulcerative Colitis. Am. J. Surg. Pathol. 2005, 29, 1472–1481. [Google Scholar] [CrossRef]
- Fiorucci, S.; Carino, A.; Baldoni, M.; Santucci, L.; Costanzi, E.; Graziosi, L.; Distrutti, E.; Biagioli, M. Bile Acid Signaling in Inflammatory Bowel Diseases. Dig. Dis. Sci. 2021, 66, 674–693. [Google Scholar] [CrossRef]
- Flannigan, K.L.; Nieves, K.M.; Szczepanski, H.E.; Serra, A.; Lee, J.W.; Alston, L.A.; Ramay, H.; Mani, S.; Hirota, S.A. The Pregnane X Receptor and Indole-3-Propionic Acid Shape the Intestinal Mesenchyme to Restrain Inflammation and Fibrosis. CMGH 2023, 15, 765–795. [Google Scholar] [CrossRef]
- Camilleri, M.; Madsen, K.; Spiller, R.; Van Meerveld, B.G.; Verne, G.N. Intestinal Barrier Function in Health and Gastrointestinal Disease. Neurogastroenterol. Motil. 2012, 24, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, C.; Di Marzo, V. The Gut Microbiome-Endocannabinoidome Axis: A New Way of Controlling Metabolism, Inflammation, and Behavior. Function 2023, 4, zqad003. [Google Scholar] [CrossRef] [PubMed]
- Hryhorowicz, S.; Kaczmarek-Ryś, M.; Zielińska, A.; Scott, R.J.; Słomski, R.; Pławski, A. Endocannabinoid System as a Promising Therapeutic Target in Inflammatory Bowel Disease—A Systematic Review. Front. Immunol. 2021, 12, 790803. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Wang, Z.; Mu, J.; Zhu, M.; Zhen, Y.; Zhang, H. Upregulation of the Transient Receptor Potential Vanilloid 1 in Colonic Epithelium of Patients with Active Inflammatory Bowel Disease. Int. J. Clin. Exp. Pathol. 2017, 10, 11335–11344. [Google Scholar] [PubMed]
- Toledo-Mauriño, J.J.; Furuzawa-Carballeda, J.; Villeda-Ramírez, M.A.; Fonseca-Camarillo, G.; Meza-Guillen, D.; Barreto-Zúñiga, R.; Yamamoto-Furusho, J.K. The Transient Receptor Potential Vanilloid 1 Is Associated with Active Inflammation in Ulcerative Colitis. Mediat. Inflamm. 2018, 2018, 6570371. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lyu, M.; Bessman, N.J.; Xie, Z.; Arifuzzaman, M.; Yano, H.; Parkhurst, C.N.; Chu, C.; Zhou, L.; Putzel, G.G.; et al. Gut-Innervating Nociceptors Regulate the Intestinal Microbiota to Promote Tissue Protection. Cell 2022, 185, 4170–4189.e20. [Google Scholar] [CrossRef] [PubMed]
- Rizopoulos, T.; Papadaki-Petrou, H.; Assimakopoulou, M. Expression Profiling of the Transient Receptor Potential Vanilloid (TRPV) Channels 1, 2, 3 and 4 in Mucosal Epithelium of Human Ulcerative Colitis. Cells 2018, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- McVey, D.C.; Schmid, P.C.; Schmid, H.H.O.; Vigna, S.R. Endocannabinoids Induce Ileitis in Rats via the Capsaicin Receptor (VR1). J. Pharmacol. Exp. Ther. 2003, 304, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Duo, L.; Wu, T.; Ke, Z.; Hu, L.; Wang, C.; Teng, G.; Zhang, W.; Wang, W.; Ge, Q.; Yang, Y.; et al. Gain of Function of Ion Channel TRPV1 Exacerbates Experimental Colitis by Promoting Dendritic Cell Activation. Mol. Ther. Nucleic Acids 2020, 22, 924–936. [Google Scholar] [CrossRef]
- Morita, T.; Mitsuyama, K.; Yamasaki, H.; Mori, A.; Yoshimura, T.; Araki, T.; Morita, M.; Tsuruta, K.; Yamasaki, S.; Kuwaki, K.; et al. Gene Expression of Transient Receptor Potential Channels in Peripheral Blood Mononuclear Cells of Inflammatory Bowel Disease Patients. J. Clin. Med. 2020, 9, 2643. [Google Scholar] [CrossRef]
- Toledo Mauriño, J.J.; Fonseca-Camarillo, G.; Furuzawa-Carballeda, J.; Barreto-Zuñiga, R.; Martínez Benítez, B.; Granados, J.; Yamamoto-Furusho, J.K. TRPV Subfamily (TRPV2, TRPV3, TRPV4, TRPV5, and TRPV6) Gene and Protein Expression in Patients with Ulcerative Colitis. J. Immunol. Res. 2020, 2020, 2906845. [Google Scholar] [CrossRef]
- Ghishan, F.K.; Kiela, P.R. Vitamins and Minerals in Inflammatory Bowel Disease. Gastroenterol. Clin. N. Am. 2017, 46, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Grill, M.; Högenauer, C.; Blesl, A.; Haybaeck, J.; Golob-Schwarzl, N.; Ferreirós, N.; Thomas, D.; Gurke, R.; Trötzmüller, M.; Köfeler, H.C.; et al. Members of the Endocannabinoid System Are Distinctly Regulated in Inflammatory Bowel Disease and Colorectal Cancer. Sci. Rep. 2019, 9, 2358. [Google Scholar] [CrossRef] [PubMed]
- Massa, F.; Marsicano, G.; Hermana, H.; Cannich, A.; Monory, K.; Cravatt, B.F.; Ferri, G.L.; Sibaev, A.; Storr, M.; Lutz, B. The Endogenous Cannabinoid System Protects against Colonic Inflammation. J. Clin. Investig. 2004, 113, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Kimball, E.S.; Schneider, C.R.; Wallace, N.H.; Hornby, P.J. Agonists of Cannabinoid Receptor 1 and 2 Inhibit Experimental Colitis Induced by Oil of Mustard and by Dextran Sulfate Sodium. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 291, G364–G371. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, B.L.F. The Role of CB1 in Immune Modulation by Cannabinoids. Pharmacol. Ther. 2013, 137, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Knoop, K.A.; McDonald, K.G.; McCrate, S.; McDole, J.R.; Newberry, R.D. Microbial Sensing by Goblet Cells Controls Immune Surveillance of Luminal Antigens in the Colon. Mucosal Immunol. 2015, 8, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Dal Buono, A.; Gabbiadini, R.; Alfarone, L.; Solitano, V.; Repici, A.; Vetrano, S.; Spinelli, A.; Armuzzi, A. Sphingosine 1-Phosphate Modulation in Inflammatory Bowel Diseases: Keeping Lymphocytes Out of the Intestine. Biomedicines 2022, 10, 1735. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.H.; Degagné, É.; Gleghorn, E.E.; Setty, M.; Rodriguez, A.; Park, K.T.; Verstraete, S.G.; Heyman, M.B.; Patel, A.S.; Irek, M.; et al. Sphingosine-1-Phosphate Signaling and Metabolism Gene Signature in Pediatric Inflammatory Bowel Disease: A Matched-Case Control Pilot Study. Inflamm. Bowel Dis. 2018, 24, 1321–1334. [Google Scholar] [CrossRef]
- Abarca-Zabalía, J.; García, M.I.; Ros, A.L.; Marín-Jiménez, I.; Martínez-Ginés, M.L.; López-Cauce, B.; Martín-Barbero, M.L.; Salvador-Martín, S.; Sanjurjo-Saez, M.; García-Domínguez, J.M.; et al. Differential Expression of SMAD Genes and S1PR1 on Circulating Cd4+ t Cells in Multiple Sclerosis and Crohn’s Disease. Int. J. Mol. Sci. 2020, 21, 676. [Google Scholar] [CrossRef]
- Karuppuchamy, T.; Behrens, E.H.; González-Cabrera, P.; Sarkisyan, G.; Gima, L.; Boyer, J.D.; Bamias, G.; Jedlicka, P.; Veny, M.; Clark, D.; et al. Sphingosine-1-Phosphate Receptor-1 (S1P 1) Is Expressed by Lymphocytes, Dendritic Cells, and Endothelium and Modulated during Inflammatory Bowel Disease. Mucosal Immunol. 2017, 10, 162–171. [Google Scholar] [CrossRef]
- Aoki, M.; Aoki, H.; Ramanathan, R.; Hait, N.C.; Takabe, K. Sphingosine-1-Phosphate Signaling in Immune Cells and Inflammation: Roles and Therapeutic Potential. Mediat. Inflamm. 2016, 2016, 8606878. [Google Scholar] [CrossRef]
- Ning, L.; Lou, X.; Zhang, F.; Xu, G. Nuclear Receptors in the Pathogenesis and Management of Inflammatory Bowel Disease. Mediators Inflamm. 2019, 2019, 2624941. [Google Scholar] [CrossRef]
- Edgar, R.; Domrachev, M.; Lash, A.E. Gene Expression Omnibus: NCBI Gene Expression and Hybridization Array Data Repository. Nucleic Acids Res. 2002; 30, 207–210. [Google Scholar] [CrossRef]
- Argmann, C.; Hou, R.; Ungaro, R.C.; Irizar, H.; Al-Taie, Z.; Huang, R.; Kosoy, R.; Venkat, S.; Song, W.M.; Di’narzo, A.F.; et al. Biopsy and Blood-Based Molecular Biomarker of Inflammation in IBD. Gut 2022. [Google Scholar] [CrossRef] [PubMed]
- Mo, A.; Krishnakumar, C.; Arafat, D.; Dhere, T.; Iskandar, H.; Dodd, A.; Prince, J.; Kugathasan, S.; Gibson, G. African Ancestry Proportion Influences Ileal Gene Expression in Inflammatory Bowel Disease. CMGH 2020, 10, 203–205. [Google Scholar] [CrossRef]
- Peck, B.C.E.; Weiser, M.; Lee, S.E.; Gipson, G.R.; Iyer, V.B.; Sartor, R.B.; Herfarth, H.H.; Long, M.D.; Hansen, J.J.; Isaacs, K.L.; et al. MicroRNAs Classify Different Disease Behavior Phenotypes of Crohn’s Disease and May Have Prognostic Utility. Inflamm. Bowel Dis. 2015, 21, 2178–2187. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Zhu, S.; Tong, J.; Wang, T.; Feng, Q.; Qiao, Y.; Shen, J. Relating the Transcriptome and Microbiome by Paired Terminal Ileal Crohn Disease. iScience 2021, 24, 102516. [Google Scholar] [CrossRef] [PubMed]
- Lobo de Sá, F.D.; Backert, S.; Nattramilarasu, P.K.; Mousavi, S.; Sandle, G.I.; Bereswill, S.; Heimesaat, M.M.; Schulzke, J.D.; Bücker, R. Vitamin d Reverses Disruption of Gut Epithelial Barrier Function Caused by Campylobacter Jejuni. Int. J. Mol. Sci. 2021, 22, 8872. [Google Scholar] [CrossRef]
- Prieto, C.; Barrios, D. RaNA-Seq: Interactive RNA-Seq Analysis from FASTQ Files to Functional Analysis. Bioinformatics 2020, 36, 1955–1956. [Google Scholar] [CrossRef]
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon Provides Fast and Bias-Aware Quantification of Transcript Expression. Nat. Methods 2017, 14, 417–419. [Google Scholar] [CrossRef]
- Barrios, D.; Prieto, C. RJSplot: Interactive Graphs with R. Mol. Inform. 2018, 37, 1700090. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spathakis, M.; Dovrolis, N.; Filidou, E.; Kandilogiannakis, L.; Tarapatzi, G.; Valatas, V.; Drygiannakis, I.; Paspaliaris, V.; Arvanitidis, K.; Manolopoulos, V.G.; et al. Exploring Microbial Metabolite Receptors in Inflammatory Bowel Disease: An In Silico Analysis of Their Potential Role in Inflammation and Fibrosis. Pharmaceuticals 2024, 17, 492. https://doi.org/10.3390/ph17040492
Spathakis M, Dovrolis N, Filidou E, Kandilogiannakis L, Tarapatzi G, Valatas V, Drygiannakis I, Paspaliaris V, Arvanitidis K, Manolopoulos VG, et al. Exploring Microbial Metabolite Receptors in Inflammatory Bowel Disease: An In Silico Analysis of Their Potential Role in Inflammation and Fibrosis. Pharmaceuticals. 2024; 17(4):492. https://doi.org/10.3390/ph17040492
Chicago/Turabian StyleSpathakis, Michail, Nikolas Dovrolis, Eirini Filidou, Leonidas Kandilogiannakis, Gesthimani Tarapatzi, Vassilis Valatas, Ioannis Drygiannakis, Vasilis Paspaliaris, Konstantinos Arvanitidis, Vangelis G. Manolopoulos, and et al. 2024. "Exploring Microbial Metabolite Receptors in Inflammatory Bowel Disease: An In Silico Analysis of Their Potential Role in Inflammation and Fibrosis" Pharmaceuticals 17, no. 4: 492. https://doi.org/10.3390/ph17040492
APA StyleSpathakis, M., Dovrolis, N., Filidou, E., Kandilogiannakis, L., Tarapatzi, G., Valatas, V., Drygiannakis, I., Paspaliaris, V., Arvanitidis, K., Manolopoulos, V. G., Kolios, G., & Vradelis, S. (2024). Exploring Microbial Metabolite Receptors in Inflammatory Bowel Disease: An In Silico Analysis of Their Potential Role in Inflammation and Fibrosis. Pharmaceuticals, 17(4), 492. https://doi.org/10.3390/ph17040492