Biosimilars in the Era of Artificial Intelligence—International Regulations and the Use in Oncological Treatments
Abstract
1. Introduction
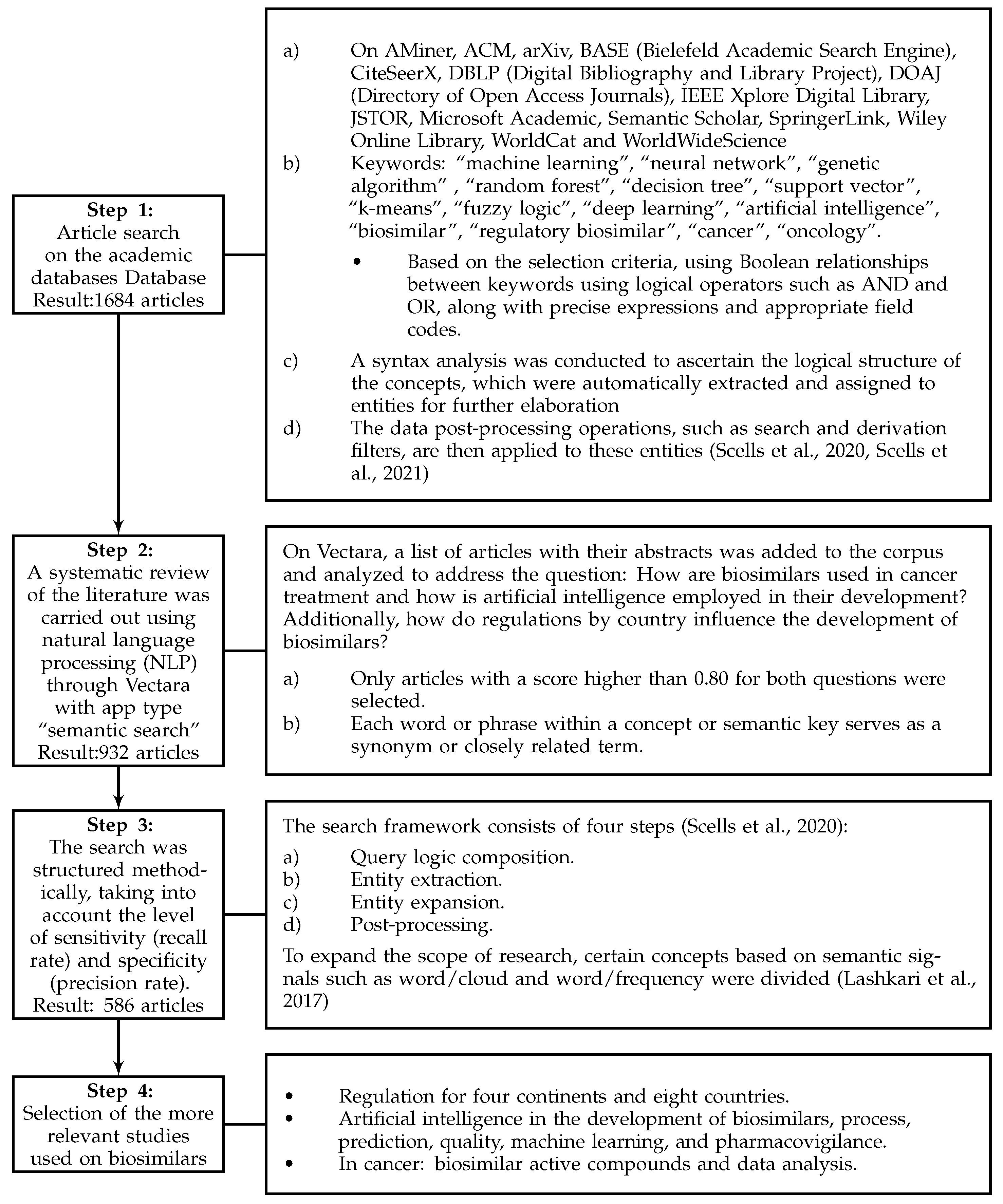
2. Methodology
3. Biosimilars: Characteristics and Perspectives
3.1. Patient and Physician Perspectives, Nocebo Effect, and Clinical Outcomes
3.2. Impact of the Cost-Saving Potential of Biosimilars on Health Systems
- United States
- Biosimilars can significantly reduce treatment costs for healthcare systems in the US due to their lower prices compared to reference biologic drugs [80]. This is crucial in a market with high drug costs, where biosimilars can relieve financial pressure on public health programs such as Medicare and Medicaid.
- The introduction of biosimilars could save the US healthcare system up to USD 100 billion over the next decade, depending on the adoption rate and market competition [81].
- Reducing biosimilar prices improves accessibility and adherence to oncological treatments, which could translate into better clinical outcomes and a decrease in disease progression [82].
- The availability of biosimilars could expand access to innovative treatments, especially in underserved communities that traditionally have less access to expensive therapies [83].
- Europe, meanwhile, significant savings and system sustainability:
- European healthcare systems have achieved significant savings with the introduction of biosimilars, particularly in countries with favorable pricing and reimbursement policies. These savings have been reinvested in the health system to improve the quality of care and access to new therapies [84].
- The adoption of biosimilars in Europe has enabled health systems to maintain financial sustainability in the face of increasing costs of innovative biological medicines [85]
- The competition generated by biosimilars has led to a reduction in the prices of original biological medicines on the European market, benefiting both patients and health systems [86].
- The implementation of incentive policies for the adoption of biosimilars in Europe has accelerated competition in the pharmaceutical market, promoting innovation and reducing long-term treatment costs [87]
- Strategies to maximize the benefits of biosimilars in the fight against cancer:
- Governments should implement policies that encourage the adoption of biosimilars, such as tax discounts and subsidies for institutions that choose to use these drugs instead of their reference counterparts [88]. These policies can facilitate a faster and more efficient transition toward the use of biosimilars.
- Establishing specific pricing and reimbursement agreements that favor biosimilars is important, ensuring that these are accessible and attractive to both providers and patients [89].
- Education and awareness:
- The importance of educational campaigns aimed at health professionals and patients to increase confidence in the safety and effectiveness of biosimilars. This is essential to promote its acceptance and widespread use in cancer treatment [90].
- The need for workshops and continuing education programs for doctors to ensure that they are well [91].
- Promotion of research and development:
- Investments in research and development should be encouraged to improve the production technology of biosimilars and optimize their regulatory processes, which can lead to cost reduction and greater availability in the market [92].
- The creation of public–private research consortia to develop new biosimilars, which can accelerate their arrival on the market and increase competition, benefiting patients and health systems with more therapeutic options and lower costs [93].
- Continuous evaluation and monitoring:
- The implementation of continuous monitoring and evaluation systems to track the impact of biosimilars on health costs and treatment effectiveness, which can help adjust adoption policies and strategies in real-time [93].
3.3. The Profitability of Biosimilars against Cancer According to the Perspective of the Actors Involved (Patients, Treating Physicians, Manufacturers)
- Biosimilars can offer more accessible treatment options due to their lower costs while maintaining the clinical effectiveness necessary to treat serious infections such as the respiratory synaptic virus [96].
- Biosimilars, due to their lower cost, can allow greater patient adherence to treatment, which can translate into better clinical outcomes and quality of life [97].
- The use of biosimilars can improve patients’ quality of life by making necessary ongoing treatments for chronic conditions—such as cancer—more accessible [98].
- Although some biosimilars may be less effective in certain clinical contexts, their lower costs can compensate for this difference, allowing broader and continued access to treatment [99].
- Health system perspective.
- Biosimilars represent a more economical option for health systems by negotiating lower prices for drugs that are equally effective as their brand-name counterparts [100].
- The implementation of biosimilar incentives and education programs can accelerate their adoption, resulting in long-term savings for healthcare systems in Europe [103].
- Manufacturer perspective.
- The competition generated by biosimilars can put downward pressure on the prices of original medicines, promoting a more competitive and accessible market [104].
- Manufacturers can benefit from economies of scale and lower long-term production costs, which allows them to offer more competitive prices without sacrificing profit margins [105].
- Biosimilar manufacturers face significant challenges, including high upfront development costs and strict regulations, but they also have the opportunity to capture a considerable share of the global cancer market [53].
- Investment in the research and development of biosimilars is crucial for manufacturers who want to remain competitive and comply with international regulatory standards [92].
4. Biosimilar Process and Regulation in Eight Countries on Four Continents
4.1. Regulatory Guidelines and Approvals for Each Selected Country by Continent
4.1.1. North American Continent (United States and Canada)
4.1.2. Southeast Asia Continent (Japan and South Korea)
4.1.3. Oceania Continent (Australia)
4.1.4. Latin American Continent (Argentina and Brazil)
4.1.5. African Continent (South Africa)
5. Artificial Intelligence Applied to R&D Processes in Biosimilars
5.1. Benefits of Artificial Intelligence in the Development of Biosimilars
| Application of AI in Biosimilar Development | Description |
|---|---|
| Machine learning in healthcare for biosimilars [142] |
|
| Support vector machine for classification tasks [156] |
|
| Artificial neural networks for biosimilar development [36] |
|
| Deep learning applications for advanced biosimilar analysis [37] |
|
5.2. Accelerated Discovery and Development Process
5.3. Improved Prediction of Biological Activity
5.4. Identification of Critical Quality Attribute
5.5. Machine Learning and Deep Learning Integration
5.6. Application of AI in Pharmacovigilance
5.7. Collaborative AI Platforms for the Development of Biosimilars
5.8. Natural Language Processing in Biosimilar Development
6. Application of Biosimilars in Cancer
6.1. Modeling Behaviors of Active Compounds
6.2. Spectroscopy Data Analysis
7. Discussion
- Gradual increase in the approval and use of biosimilars: In recent years, a significant increase in the approval and use of biosimilars in cancer treatment has been observed, especially in Europe and the United States. In this sense, the authorization of biosimilars has increased as regulatory agencies such as the EMA and the FDA have developed clearer and more precise regulatory frameworks for their evaluation and approval [225].
- Expansion of competition and cost reduction: The introduction of biosimilars has encouraged competition in the pharmaceutical market for biomolecules, leading to lower prices for both biosimilars and original biological medicines. This competition benefits the different health systems involved in funding programs and the patients by making treatments more accessible [101].
- Adoption in developing countries: Biosimilars are gaining ground in developing countries precisely because of their lower cost compared to reference biologics. The trend is particularly important in resource-limited regions, where biosimilars offer a viable option for the treatment of chronic diseases such as cancer [246].
- Expansion of the treatment portfolio: The number of biosimilars available on the market is expected to continue to increase. In the coming years, biosimilars will be here to stay, and more massive approvals are a matter of time, and therefore a broader range of oncological indications will be approved, thus expanding treatment options against an even greater variety of treatments against different types of cancer [247].
- Improvements in production technology through the use of more massive AI: Innovations in biosimilar production technology should continue to improve the efficiency and quality of these medicines, with the greater use of different AI tools, which could further reduce costs, improve quality and safety, and obviously, accessibility [248].
- Integration into standard treatment protocols: Biosimilars are likely to become increasingly integrated into standard treatment protocols for various types of cancer, allowing healthcare professionals to offer more accessible, flexible, and cost-effective treatment options. This integration will likely accelerate as more data on the long-term safety and efficacy of biosimilars become available [249].
- More favorable policies and refunds: Policies for biosimilars, both in Europe and other markets, would promote greater adoption of these medicines, making healthcare systems more financially sustainable and lowering payment premiums for all involved [85].
- Greater education and confidence of patients and treating physicians: Education and awareness of the benefits and safety of biosimilars as they become available will be crucial to increasing trust among healthcare professionals and patients, facilitating their adoption and regular use in clinical practice [235].
- Development of updated treatment protocols: It is important to generate and develop an update of the treatment guidelines to include specific recommendations on the use of biosimilars, ensuring that professionals have a clear framework for their prescription and monitoring [12].
- Promoting evidence-based adoption: It is critical to building trust between healthcare professionals and patients [250].
- Implementation of financial incentive policies: The implementation of financial incentives for the prescribing of biosimilars could encourage healthcare professionals to opt for these treatments, ensuring that economic benefits are passed on to patients and the health system, in general, [251].
- Monitoring and evaluation of results: The creation of robust monitoring and evaluation systems for patients who use biosimilars, allowing adjustments in health practices and policies over time and as necessary to optimize the effectiveness and efficiency of treatment, since being biomolecules, reactions can vary from patient to patient [252].
- Promotion of participation in clinical studies and information on patient associations: Participation in biosimilar clinical studies can help healthcare professionals stay up-to-date with the latest research improve the adoption of these treatments in their daily practice and inform respective patient associations [95,246].
8. Conclusions
- Global regulations and challenges: The regulatory framework for biosimilars worldwide is disorganized and without unifying criteria, where each country establishes the rules according to its own health needs and interests. Likewise, there is an interest in extremely strict regulations in all countries, most of the time there is a lot of disparity, fully impacting the quality of the medicine and, therefore, the safety of the patients involved, generating fear in their use.
- –
- Recommendation: Implement a harmonized international regulatory framework to facilitate the approval and adoption of biosimilars in different parts of the world.
- *
- Actions: Establish a global working group composed of representatives from major regulatory bodies such as the FDA (US), EMA (Europe), PMDA (Japan), patient representative groups, and other authorities responsible for the manufacturing and distribution of medicines. Develop unified guidelines for biosimilar approval processes, including clinical trial requirements, quality standards, and post-marketing surveillance. Create an international database to share regulatory data and best practices.
- *
- Justification: Currently, regulatory requirements for biosimilars vary significantly between countries, resulting in delays and increased costs for manufacturers, who must navigate multiple regulatory landscapes generating mistrust and uncertainty. A harmonized approach would streamline the approval process, reduce duplication of efforts, and facilitate faster access to biosimilars around the world.
- Use of artificial intelligence in the development of biosimilars: The use of artificial intelligence (AI) in the development of biosimilars for cancer treatments offers both positive and negative contributions. Among the positive contributions, can be said to be the acceleration of development and cost reduction, where AI can analyze large volumes of complex biological and clinical data quickly and efficiently, significantly accelerating the process of discovery and development of biosimilars. Similarly, artificial intelligence improves the efficiency, precision, and personalization of treatments, enabling the identification of new therapeutic targets and the design of molecules that precisely imitate the properties of original biological medicines. It allows a detailed analysis of the genomic and proteomic profiles of patients, helping to predict the behavior of biosimilars in different clinical settings, and optimizing the formulation and dose of these drugs. The potential negative impacts are data dependence and risk of bias since we are highly dependent on the quality and quantity of data available and therefore there is a risk that AI models reproduce or amplify biases existing in the data, which can result in the under-representation of certain patient groups or incorrect decision making. Ethical challenges are also faced regarding the transparency and interpretability of AI models used in decision-making clinics and the development of treatments.
- –
- Recommendation: Invest in artificial intelligence and machine learning technologies that help improve the efficiency and precision of the biosimilar development process.
- *
- Actions: Allocate funding for R&D initiatives focused on the application of artificial intelligence in the development of biosimilars. Foster partnerships between pharmaceutical companies and technology companies specializing in AI. Deploy AI-powered platforms to predict molecular structures, optimize cell culture conditions, and conduct virtual clinical trials. Integrate artificial intelligence tools into regulatory review processes to assess biosimilarity and predict clinical outcomes.
- *
- Justification: Artificial intelligence technologies can analyze large data sets more quickly and accurately than traditional methods, identifying optimal biosimilar candidates and predicting their clinical performance. This can significantly reduce the time and cost involved in bringing biosimilars to market, ensuring that patients receive safe and effective treatments sooner.
- Adoption of biosimilars in cancer treatment: At the molecular level, biosimilars are designed to be highly comparable to the original biologics in terms of structure, function, and biological activity. Through extensive characterization and comparability studies, biosimilars ensure that any molecular differences do not compromise the safety or efficacy of treatment. A strict evaluation of immunogenicity and stability ensures that these drugs can be used safely and effectively in the treatment of cancer, providing a viable and more accessible alternative to original biological medicines.
- –
- Recommendation: Increase comparative clinical studies and educational programs for patients and health professionals on the safety and efficacy of biosimilars in oncology compared to the reference ones. Focus on points such as structural and functional equivalence, glycosylation and post-translational modifications, the main mechanisms of action, further studies of comparability and immunogenicity, and finally, stability and purity.
- *
- Actions: Conduct large-scale, multicenter clinical trials that compare biosimilars with their reference biologics in cancer treatment. Develop comprehensive educational modules and certification programs for oncologists and other healthcare providers. Host international conferences and seminars to share trial results and real-world data on the efficacy and safety of biosimilars. Generate and communicate relevant information to patient associations to improve understanding of biosimilars. Collaborate with medical societies to update clinical guidelines that incorporate biosimilars.
- *
- Justification: Despite their potential, biosimilars have faced slow adoption in cancer due to more limited clinical evidence relative to reference molecules, and there are concerns about their efficacy and safety. Robust comparative studies and continuing education could build greater confidence among healthcare providers, leading to greater acceptance and use of biosimilars in cancer treatment.
- Accessibility and affordability (cost) of biosimilars: Biosimilars have had a transformative impact on cancer treatment in both the United States and Europe, providing significant benefits in terms of reducing costs, expanding access to treatments, and fostering competition and innovation in the pharmaceutical market. These benefits are essential to improve the sustainability and effectiveness of health systems, allowing better resource management and broader, more affordable care for cancer patients.
- –
- Recommendation: Establish pricing and reimbursement policies that encourage the adoption of biosimilars, especially in developing countries.
- *
- Actions: Implement government subsidies and financial incentives for biosimilar manufacturers to reduce production costs. Negotiate wholesale purchasing agreements with manufacturers to ensure lower prices for national healthcare systems. Develop reimbursement policies that favor the use of cost-effective biosimilars over more expensive reference biologics. Provide grants or low-interest loans to local companies in developing countries to develop biosimilar manufacturing capabilities.
- *
- Rationale: High treatment costs constitute a major barrier to access to advanced biological therapies, especially in low- and middle-income countries. By reducing the cost of biosimilars through supportive policies and incentives, more patients can benefit from high-quality, affordable treatments and ultimately improve public health outcomes.
- International collaboration and support for developing countries: International support and collaboration in the development and use of biosimilars are essential to improve access to biological medicines, mainly in developing countries. This is achieved through technology transfer, training, strategic alliances, regulatory support, and financing of research projects. These actions not only help reduce costs and improve the availability of treatments for serious diseases such as cancer but also strengthen the capacity of these countries to produce and regulate high-quality biosimilars in a sustainable manner.
- –
- Recommendation: Strengthen international cooperation to support infrastructure and regulatory capacity in developing countries, allowing them to fully benefit from biosimilars.
- *
- Actions: Establish international training programs to develop regulatory expertise in developing countries. Create twinning agreements in which regulatory agencies in developed countries advise their counterparts in developing regions. Provide technical assistance and funding to improve regulatory infrastructure and laboratory facilities. Facilitate the exchange of knowledge and best practices through international forums and collaborative networks.
- *
- Justification: Developing countries often lack the resources and expertise to effectively regulate and monitor the introduction of biosimilars. International support can help these countries establish strong regulatory frameworks, ensuring that biosimilars are safe, effective, and accessible to those who need them. Improving regulatory capacity will also attract investment in local production of biosimilars, fostering economic growth and improving healthcare outcomes.
- Patient and physician opinion: Patient and physician opinion on the use of biosimilars in cancer treatment is generally positive once initial mistrust is overcome through education and clinical experience. The different patient associations do a remarkable job informing patients. On the other hand, patients and health insurance associations value the reduction in costs and greater access to treatments, while physicians appreciate the comparable efficacy and economic benefits of biosimilars. However, both patients and physicians highlight the importance of clear regulation and continued education to maximize the adoption and success of biosimilars in clinical practice.
- –
- Recommendation: Develop comprehensive educational programs for physicians and patients that clarify pharmacist substitution policies. Incorporate the different associations into the discussion.
- *
- Actions: Implement targeted educational initiatives focused on the safety, efficacy, and immunogenicity of biosimilars, supported by current clinical trial data and real-world evidence. Develop clear policies on the pharmaceutical substitution of biologicals with biosimilars, guaranteeing transparency and communication.
- *
- Justification: Better education will close knowledge gaps, build clinician confidence, and address concerns about the use of biosimilars, thereby promoting their broader prescription and integration into clinical practice. Clear communication can alleviate concerns and build trust, supporting the wider adoption of biosimilars.
9. Challenges and Future Considerations
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Study | Main Contribution | Methodology | Significance |
|---|---|---|---|
| M-FLAG: medical vision–language pre-training with frozen language models and latent space geometry optimization [205]. | Introduces M-FLAG, a model that combines frozen language models with vision–language pre-training for medical applications. |
|
|
| Frozen language model helps ECG zero-shot learning [206]. | Demonstrates the effectiveness of frozen language models in performing zero-shot learning on ECG data. |
|
|
| Med-UniC: unifying cross-lingual medical vision–language pre-training by diminishing bias [207]. | Proposes Med-UniC, a model that addresses cross-lingual and cross-modal biases in medical vision–language pre-training. |
|
|
References
- Aggarwal, G.; Nagpal, M.; Sharma, A.; Puri, V.; Dhingra, G.A. Upcoming Drifts in Bio-similars. Curr. Rev. Clin. Exp. Pharmacol. 2021, 16, 39–51. [Google Scholar] [CrossRef]
- Conlé, M. Recent developments in China’s biopharmaceutical industry (2012–2017): Patterns of product innovation and firm scope. J. Sci. Technol. Policy Manag. 2019, 10, 686–707. [Google Scholar] [CrossRef]
- González-Ramírez, R.; Castañeda-Hernández, G. The challenges of developing and commercializing biosimilars in Latin America. Pharm. Pat. Anal. 2019, 8, 221–224. [Google Scholar] [CrossRef]
- Rose, S.A.; Rice, T. The Biosimilar Action Plan: An Effective Mechanism for Balancing Biologic Innovation and Competition in the United States? (18 November 2019). McGeorge Law Review, Forthcoming, Wake Forest Univ. Legal Studies Paper. Available online: https://ssrn.com/abstract=3489444 (accessed on 15 May 2024). [CrossRef]
- Akram, M.S.; Pery, N.; Butler, L.; Shafiq, M.I.; Batool, N.; Rehman, M.F.U.; Grahame-Dunn, L.G.; Yetisen, A.K. Challenges for biosimilars: Focus on rheumatoid arthritis. Crit. Rev. Biotechnol. 2021, 41, 121–153. [Google Scholar] [CrossRef]
- Eniu, A.; Cherny, N.I.; Bertram, M.; Thongprasert, S.; Douillard, J.Y.; Bricalli, G.; Vyas, M.; Trapani, D. Cancer medicines in Asia and Asia-Pacific: What is available, and is it effective enough? ESMO Open 2019, 4, e000483. [Google Scholar] [CrossRef]
- Santos, S.B.; Sousa Lobo, J.M.; Silva, A.C. Biosimilar medicines used for cancer therapy in Europe: A review. Drug Discov. Today 2019, 24, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Annett, S. Pharmaceutical drug development: High drug prices and the hidden role of public funding. Biol. Futur. 2021, 72, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Bourgeron, T.; Geiger, S. (De-)assetizing pharmaceutical patents: Patent contestations behind a blockbuster drug. Econ. Soc. 2022, 51, 23–45. [Google Scholar] [CrossRef]
- Nicholson Price, W., II. The Cost of Novelty (March 11, 2019). 120 Colum. L. Rev. 769 (2020), U of Michigan Public Law Research Paper No. 633, U of Michigan Law & Econ Research Paper No. 19-004. Available online: https://ssrn.com/abstract=3350477 (accessed on 15 May 2024).
- Kabir, E.R.; Moreino, S.S.; Sharif Siam, M.K. The Breakthrough of Biosimilars: A Twist in the Narrative of Biological Therapy. Biomolecules 2019, 9, 410. [Google Scholar] [CrossRef]
- Bas, T.G. Biosimilars for the next decade in Latin America: A window of opportunity. Expert Opin. Biol. Ther. 2023, 23, 659–669. [Google Scholar] [CrossRef]
- Li, P.; Zheng, Y.; Chen, X. Drugs for Autoimmune Inflammatory Diseases: From Small Molecule Compounds to Anti-TNF Biologics. Front. Pharmacol. 2017, 8, 460. [Google Scholar] [CrossRef] [PubMed]
- Koźmiński, P.; Halik, P.K.; Chesori, R.; Gniazdowska, E. Overview of Dual-Acting Drug Methotrexate in Different Neurological Diseases, Autoimmune Pathologies and Cancers. Int. J. Mol. Sci. 2020, 21, 3483. [Google Scholar] [CrossRef] [PubMed]
- Najeeb, H.; Yasmin, F.; Surani, S. Emerging role of biosimilars in the clinical care of inflammatory bowel disease patients. World J. Clin. Cases 2022, 10, 4327–4333. [Google Scholar] [CrossRef] [PubMed]
- Leufkens, H.G.; Kusynová, Z.; Aitken, M.; Hoekman, J.; Stolk, P.; Klein, K.; Mantel-Teeuwisse, A.K. Four scenarios for the future of medicines and social policy in 2030. Drug Discov. Today 2022, 27, 2252–2260. [Google Scholar] [CrossRef] [PubMed]
- Wasan, H.; Singh, D.; Reeta, K.H.; Gupta, P.; Gupta, Y.K. Drug development process and COVID-19 pandemic: Flourishing era of outsourcing. Indian J. Pharmacol. 2022, 54, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Oriama, R.; Mudida, R.; Burger-Helmchen, T. A Multi-level Perspective to Biosimilars Development: Pathways Towards Incremental Innovation in the Health Bioeconomy. In Transdisciplinarity; Rezaei, N., Ed.; Series Title: Integrated Science; Springer International Publishing: Cham, Switzerland, 2022; Volume 5, pp. 249–266. [Google Scholar] [CrossRef]
- Agbogbo, F.K.; Ecker, D.M.; Farrand, A.; Han, K.; Khoury, A.; Martin, A.; McCool, J.; Rasche, U.; Rau, T.D.; Schmidt, D.; et al. Current perspectives on biosimilars. J. Ind. Microbiol. Biotechnol. 2019, 46, 1297–1311. [Google Scholar] [CrossRef] [PubMed]
- Sayah, R.; Awada, S.; Ismaiil, L.; Hatem, G. Assessment of the differences between generic and biosimilar drugs: A brief literature review. J. Generic Med. 2023, 19, 124–129. [Google Scholar] [CrossRef]
- Abraham, I. Preparing for the third decade of biosimilars. Expert Opin. Biol. Ther. 2023, 23, 651–652. [Google Scholar] [CrossRef]
- Hobbs, A.L.; Crawford, J.P. Biosimilars and implications for pharmacy practice: Ready or not, here they come! Pharm. Pract. 2019, 17, 1659. [Google Scholar] [CrossRef]
- Ratih, R.; Asmari, M.; Abdel-Megied, A.M.; Elbarbry, F.; El Deeb, S. Biosimilars: Review of regulatory, manufacturing, analytical aspects and beyond. Microchem. J. 2021, 165, 106143. [Google Scholar] [CrossRef]
- Kang, H.N.; Thorpe, R.; Knezevic, I.; Casas Levano, M.; Chilufya, M.B.; Chirachanakul, P.; Chua, H.M.; Dalili, D.; Foo, F.; Gao, K.; et al. Regulatory challenges with biosimilars: An update from 20 countries. Ann. N. Y. Acad. Sci. 2021, 1491, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Rathore, A.S.; Bhargava, A. Regulatory considerations in biosimilars: Latin America region. Prep. Biochem. Biotechnol. 2021, 51, 201–206. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, J.; Barry, S.P.; Bermingham, M.; Morris, J.M.; Griffin, B.T. Regulation of biosimilar medicines and current perspectives on interchangeability and policy. Eur. J. Clin. Pharmacol. 2019, 75, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rahalkar, H.; Sheppard, A.; Salek, S. Biosimilar development and review process in the BRICS-TM countries: Proposal for a standardized model to improve regulatory performance. Expert Rev. Clin. Pharmacol. 2022, 15, 215–236. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, S.K.; Dureja, H. Comparative analysis of evolution of regulatory environment in USA, Europe and Japan. Pharma Innov. 2021, 10, 6–14. [Google Scholar] [CrossRef]
- Chhabra, H.; Mouslim, M.C.; Kashiramka, S.; Rathore, A.S. Dynamics of biosimilar uptake in emerging markets. Expert Opin. Biol. Ther. 2022, 22, 679–688. [Google Scholar] [CrossRef]
- Sadek, M.A.Z. Global Status of Biosimilars and Its Influential Factors. Eur. J. Bus. Manag. Res. 2020, 5. [Google Scholar] [CrossRef]
- Wadhwa, M.; Kang, H.N.; Jivapaisarnpong, T.; Andalucia, L.R.; Blades, C.D.R.Z.; Casas Levano, M.; Chang, W.; Chew, J.Y.; Chilufya, M.B.; Chirachanakul, P.; et al. WHO implementation workshop on guidelines on procedures and data requirements for changes to approved biotherapeutic products, Seoul, Republic of Korea, 25–26 June 2019. Biologicals 2020, 65, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Prado, E.; Ponce-Zea, J.; Vasconez, J.E.; Castillo, D.; Checa-Jaramilloz, D.C.; Rodríguez-Burneo, N.; Andrade, F.; Intriago-Baldeón, D.P.; Galarza-Maldonado, C. Current trends for biosimilars in the Latin American market. Generics Biosimilars Initiat. J. 2020, 9, 64–74. [Google Scholar] [CrossRef]
- Araujo, R.L.; Mosegui, G.B.G.; Vianna, C.M.D.E.M.; Villar, F.A.; Catao, T.P. Reflections and Perspectives on Biosimilars in Brazil. Int. J. Pharm. Pharm. Sci. 2020, 12, 26–31. [Google Scholar] [CrossRef]
- Moeti, L.; Litedu, M.; Joubert, J. The Implementation of a Risk-Based Assessment Approach by the South African Health Products Regulatory Authority (SAHPRA). Pharm. Med. 2023, 37, 71–91. [Google Scholar] [CrossRef] [PubMed]
- Kolluri, S.; Lin, J.; Liu, R.; Zhang, Y.; Zhang, W. Machine Learning and Artificial Intelligence in Pharmaceutical Research and Development: A Review. AAPS J. 2022, 24, 19. [Google Scholar] [CrossRef] [PubMed]
- Puranik, A.; Dandekar, P.; Jain, R. Exploring the potential of machine learning for more efficient development and production of biopharmaceuticals. Biotechnol. Prog. 2022, 38, e3291. [Google Scholar] [CrossRef]
- Niazi, S.K. Molecular Biosimilarity—An AI-Driven Paradigm Shift. Int. J. Mol. Sci. 2022, 23, 10690. [Google Scholar] [CrossRef] [PubMed]
- Lamanna, W.C.; Holzmann, J.; Cohen, H.P.; Guo, X.; Schweigler, M.; Stangler, T.; Seidl, A.; Schiestl, M. Maintaining consistent quality and clinical performance of biopharmaceuticals. Expert Opin. Biol. Ther. 2018, 18, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Huggins, D.J.; Biggin, P.C.; Dämgen, M.A.; Essex, J.W.; Harris, S.A.; Henchman, R.H.; Khalid, S.; Kuzmanic, A.; Laughton, C.A.; Michel, J.; et al. Biomolecular simulations: From dynamics and mechanisms to computational assays of biological activity. WIREs Comput. Mol. Sci. 2019, 9, e1393. [Google Scholar] [CrossRef]
- Vamathevan, J.; Clark, D.; Czodrowski, P.; Dunham, I.; Ferran, E.; Lee, G.; Li, B.; Madabhushi, A.; Shah, P.; Spitzer, M.; et al. Applications of machine learning in drug discovery and development. Nat. Rev. Drug Discov. 2019, 18, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Deng, H.; Liu, B.; Hu, A.; Liang, J.; Fan, L.; Zheng, X.; Wang, T.; Lei, J. Systematic Evaluation of Research Progress on Natural Language Processing in Medicine Over the Past 20 Years: Bibliometric Study on PubMed. J. Med. Internet Res. 2020, 22, e16816. [Google Scholar] [CrossRef] [PubMed]
- Galo, E.B.; Patricio, G.B.; Andres, D.P.; Silvana, L.G.; María, C.M.-C. Machine Learning Approaches to Improve Prediction of Target-Drug Interactions. In Drug Design Using Machine Learning, 1st ed.; Inamuddin, Altalhi, T., Cruz, J.N., Refat, M.S.E., Eds.; Wiley: Hoboken, NJ, USA, 2022; pp. 21–96. [Google Scholar] [CrossRef]
- Malakar, S.; Gontor, E.N.; Dugbaye, M.Y.; Shah, K.; Sinha, S.; Sutaoney, P.; Chauhan, N.S. Cancer treatment with biosimilar drugs: A review. Cancer Innov. 2024, 3, e115. [Google Scholar] [CrossRef]
- Bachu, R.D.; Abou-Dahech, M.; Balaji, S.; Boddu, S.H.S.; Amos, S.; Singh, V.; Babu, R.J.; Tiwari, A.K. Oncology biosimilars: New developments and future directions. Cancer Rep. 2022, 5, e1720. [Google Scholar] [CrossRef]
- Ditani, A.S.; Mallick, P.P.; Anup, N.; Tambe, V.; Polaka, S.; Sengupta, P.; Rajpoot, K.; Tekade, R.K. Biosimilars accessible in the market for the treatment of cancer. J. Control. Release 2021, 336, 112–129. [Google Scholar] [CrossRef] [PubMed]
- Peña, A.N.; Rodríguez, C.D.C. Requirements for Biosimilar Authorization: A Legal and Comparative Perspective—FDA Versus EMA. Curr. Sci. 2021, 120, 56. [Google Scholar] [CrossRef]
- Bas, T.G.; Sáez, M.L.; Sáez, N. Sustainable Development versus Extractivist Deforestation in Tropical, Subtropical, and Boreal Forest Ecosystems: Repercussions and Controversies about the Mother Tree and the Mycorrhizal Network Hypothesis. Plants 2024, 13, 1231. [Google Scholar] [CrossRef] [PubMed]
- Barros, M.V.; Salvador, R.; Do Prado, G.F.; De Francisco, A.C.; Piekarski, C.M. Circular economy as a driver to sustainable businesses. Clean. Environ. Syst. 2021, 2, 100006. [Google Scholar] [CrossRef]
- Gusenbauer, M.; Haddaway, N.R. Which academic search systems are suitable for systematic reviews or meta-analyses? Evaluating retrieval qualities of Google Scholar, PubMed, and 26 other resources. Res. Synth. Methods 2020, 11, 181–217. [Google Scholar] [CrossRef] [PubMed]
- Scells, H.; Zuccon, G.; Koopman, B.; Clark, J. Automatic Boolean Query Formulation for Systematic Review Literature Search. In Proceedings of the Web Conference 2020, New York, NY, USA, 20–24 April 2020; pp. 1071–1081. [Google Scholar] [CrossRef]
- Scells, H.; Zuccon, G.; Koopman, B. A comparison of automatic Boolean query formulation for systematic reviews. Inf. Retr. J. 2021, 24, 3–28. [Google Scholar] [CrossRef]
- Lashkari, F.; Ensan, F.; Bagheri, E.; Ghorbani, A.A. Efficient indexing for semantic search. Expert Syst. Appl. 2017, 73, 92–114. [Google Scholar] [CrossRef]
- Rugo, H.S.; Rifkin, R.M.; Declerck, P.; Bair, A.H.; Morgan, G. Demystifying biosimilars: Development, regulation and clinical use. Future Oncol. 2019, 15, 777–790. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Rathore, A.S. Assessment of Structural and Functional Comparability of Biosimilar Products: Trastuzumab as a Case Study. Biodrugs Clin. Immunother. Biopharm. Gene Ther. 2020, 34, 209–223. [Google Scholar] [CrossRef]
- Genovese, M.C.; Sanchez-Burson, J.; Oh, M.; Balazs, E.; Neal, J.; Everding, A.; Hala, T.; Wojciechowski, R.; Fanjiang, G.; Cohen, S. Comparative clinical efficacy and safety of the proposed biosimilar ABP 710 with infliximab reference product in patients with rheumatoid arthritis. Arthritis Res. Ther. 2020, 22, 60. [Google Scholar] [CrossRef]
- Rathore, A.S.; Stevenson, J.G.; Chhabra, H. Considerations related to comparative clinical studies for biosimilars. Expert Opin. Drug Saf. 2021, 20, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Bielsky, M.C.; Cook, A.; Wallington, A.; Exley, A.; Kauser, S.; Hay, J.L.; Both, L.; Brown, D. Streamlined approval of biosimilars: Moving on from the confirmatory efficacy trial. Drug Discov. Today 2020, 25, 1910–1918. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.J.; Mouslim, M.C.; Blunt, J.L.; Alexander, G.C.; Shermock, K.M. Assessment of Availability, Clinical Testing, and US Food and Drug Administration Review of Biosimilar Biologic Products. JAMA Intern. Med. 2021, 181, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Guttman, A.; Rathore, A.S. Applications of capillary electrophoresis for biopharmaceutical product characterization. Electrophoresis 2022, 43, 143–166. [Google Scholar] [CrossRef] [PubMed]
- Reardon, G. Pharmacoeconomics of Biologic Medicines and Biosimilars. In Biologics, Biosimilars, and Biobetters; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020; pp. 195–212. [Google Scholar] [CrossRef]
- Rezk, M.F.; Pieper, B. To See or NOsee: The Debate on the Nocebo Effect and Optimizing the Use of Biosimilars. Adv. Ther. 2018, 35, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Colloca, L.; Panaccione, R.; Murphy, T.K. The Clinical Implications of Nocebo Effects for Biosimilar Therapy. Front. Pharmacol. 2019, 10, 1372. [Google Scholar] [CrossRef] [PubMed]
- Grosso, F.; Barbiani, D.; Cavalera, C.; Volpato, E.; Pagnini, F. Risk factors associated with nocebo effects: A review of reviews. Brain Behav. Immun. Health 2024, 38, 100800. [Google Scholar] [CrossRef]
- Colloca, L. The Nocebo Effect. Annu. Rev. Pharmacol. Toxicol. 2024, 64, 171–190. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wang, Z.; Wang, X.; Yu, H.; Sun, J. Patients’ Perceptions of Biosimilars: A Systematic Review. BioDrugs 2023, 37, 829–841. [Google Scholar] [CrossRef]
- Gibofsky, A.; Jacobson, G.; Franklin, A.; O’Hara-Levi, S.; Peyrin-Biroulet, L.; McGrath, M.; McCabe, D. An online survey among US patients with immune-mediated conditions: Attitudes about biosimilars. J. Manag. Care Spec. Pharm. 2023, 29, 343–349. [Google Scholar] [CrossRef]
- Wojtukiewicz, M.Z.; Politynska, B.; Skalij, P.; Tokajuk, P.; Wojtukiewicz, A.M.; Honn, K.V. It is not just the drugs that matter: The nocebo effect. Cancer Metastasis Rev. 2019, 38, 315–326. [Google Scholar] [CrossRef] [PubMed]
- McGarvey, N.; Gitlin, M.; Fadli, E.; Chung, K.C. Increased healthcare costs by later stage cancer diagnosis. BMC Health Serv. Res. 2022, 22, 1155. [Google Scholar] [CrossRef] [PubMed]
- Seidman, J.; Masi, D.; Gomez-Rexrode, A.E. Personalizing Value in Cancer Care: The Case for Incorporating Patient Preferences Into Routine Clinical Decision Making. J. Particip. Med. 2019, 11, e13800. [Google Scholar] [CrossRef] [PubMed]
- Oehrlein, E.M.; Harris, J.; Balch, A.; Furlong, P.; Hargis, E.; Woolley, M.; Perfetto, E. Improving Access and Quality of Health Care in the United States: Shared Goals Among Patient Advocates. Patient Patient-Centered Outcomes Res. 2021, 14, 687–690. [Google Scholar] [CrossRef] [PubMed]
- Feldman, D.; Kruger, P.; Delbecque, L.; Duenas, A.; Bernard-Poenaru, O.; Wollenschneider, S.; Hicks, N.; Reed, J.A.; Sargeant, I.; Pakarinen, C.; et al. Co-creation of practical “how-to guides” for patient engagement in key phases of medicines development—From theory to implementation. Res. Involv. Engagem. 2021, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Coylewright, M.; Otero, D.; Lindman, B.R.; Levack, M.M.; Horne, A.; Ngo, L.H.; Beaudry, M.; Col, H.V.; Col, N.F. An interactive, online decision aid assessing patient goals and preferences for treatment of aortic stenosis to support physician-led shared decision-making: Early feasibility pilot study. PLoS ONE 2024, 19, e0302378. [Google Scholar] [CrossRef] [PubMed]
- Rosembert, D.C.; Twigg, M.J.; Wright, D.J. Patient’s and Consultant’s Views and Perceptions on Switching from an Originator Biologic to Biosimilar Medication: A Qualitative Study. Pharmacy 2024, 12, 65. [Google Scholar] [CrossRef] [PubMed]
- Varma, M.; Almarsdóttir, A.B.; Druedahl, L.C. “Biosimilar, so it looks alike, but what does it mean?” A qualitative study of Danish patients’ perceptions of biosimilars. Basic Clin. Pharmacol. Toxicol. 2022, 130, 581–591. [Google Scholar] [CrossRef]
- Sarnola, K.; Merikoski, M.; Jyrkkä, J.; Hämeen-Anttila, K. Physicians’ perceptions of the uptake of biosimilars: A systematic review. BMJ Open 2020, 10, e034183. [Google Scholar] [CrossRef]
- Druedahl, L.C.; Kälvemark Sporrong, S.; Van De Weert, M.; De Bruin, M.L.; Hoogland, H.; Minssen, T.; Almarsdóttir, A.B. Evolving Biosimilar Clinical Requirements: A Qualitative Interview Study with Industry Experts and European National Medicines Agency Regulators. BioDrugs 2021, 35, 351–361. [Google Scholar] [CrossRef]
- Barbier, L.; Mbuaki, A.; Simoens, S.; Declerck, P.; Vulto, A.G.; Huys, I. Regulatory Information and Guidance on Biosimilars and Their Use Across Europe: A Call for Strengthened One Voice Messaging. Front. Med. 2022, 9, 820755. [Google Scholar] [CrossRef] [PubMed]
- Foreman, E.; Patel, H.; Siderov, J.; Harchowal, J.; Bubalo, J.; Chan, A. A survey of global biosimilar implementation practice conducted by the International Society of Oncology Pharmacy Practitioners. J. Oncol. Pharm. Pract. 2020, 26, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, A.W.; Hlavka, J.P.; Case, S.R. Biosimilar Cost Savings in the United States: Initial Experience and Future Potential. Rand Health Q. 2018, 7, 3. [Google Scholar] [PubMed]
- Yang, J.; Liu, R.; Ektare, V.; Stephens, J.; Shelbaya, A. Does Biosimilar Bevacizumab Offer Affordable Treatment Options for Cancer Patients in the USA? A Budget Impact Analysis from US Commercial and Medicare Payer Perspectives. Appl. Health Econ. Health Policy 2021, 19, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Van De Wiele, V.L.; Kesselheim, A.S.; Sarpatwari, A. Barriers To US Biosimilar Market Growth: Lessons From Biosimilar Patent Litigation: Article examines barriers to US biosimilar market growth. Health Aff. 2021, 40, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Nabhan, C.; Parsad, S.; Mato, A.R.; Feinberg, B.A. Biosimilars in Oncology in the United States: A Review. JAMA Oncol. 2018, 4, 241. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, D.C.; Misery, L.; Naeyaert, S.; Taylor, P.C. Biological Therapies in Immune-Mediated Inflammatory Diseases: Can Biosimilars Reduce Access Inequities? Front. Pharmacol. 2019, 10, 279. [Google Scholar] [CrossRef] [PubMed]
- Dutta, B.; Huys, I.; Vulto, A.G.; Simoens, S. Identifying Key Benefits in European Off-Patent Biologics and Biosimilar Markets: It is Not Only About Price! BioDrugs 2020, 34, 159–170. [Google Scholar] [CrossRef]
- Alnaqbi, K.A.; Bellanger, A.; Brill, A.; Castañeda-Hernández, G.; Clopés Estela, A.; Delgado Sánchez, O.; García-Alfonso, P.; Gyger, P.; Heinrich, D.; Hezard, G.; et al. An international comparative analysis and roadmap to sustainable biosimilar markets. Front. Pharmacol. 2023, 14, 1188368. [Google Scholar] [CrossRef]
- Moorkens, E.; Vulto, A.G.; Huys, I. Biosimilars in Belgium: A proposal for a more competitive market. Acta Clin. Belg. 2021, 76, 441–452. [Google Scholar] [CrossRef]
- Simoens, S.; Lacosta, T.B.; Inotai, A. Learnings from cross-border biosimilar pricing policies in Europe. Expert Rev. Pharmacoecon. Outcomes Res. 2024, 24, 585–588. [Google Scholar] [CrossRef]
- Tachkov, K.; Savova, A.; Manova, M.; Petrova, G. Tackling reimbursement challenges to fair access to medicines – introduction to the topic. Expert Rev. Pharmacoecon. Outcomes Res. 2023, 23, 597–606. [Google Scholar] [CrossRef]
- Simoens, S.; Cheung, R. Tendering and biosimilars: What role for value-added services? J. Mark. Access Health Policy 2020, 8, 1705120. [Google Scholar] [CrossRef] [PubMed]
- Oskouei, S.T.; Kusmierczyk, A.R. Biosimilar Uptake: The Importance of Healthcare Provider Education. Pharm. Med. 2021, 35, 215–224. [Google Scholar] [CrossRef]
- Leonard, E.; Wascovich, M.; Oskouei, S.; Gurz, P.; Carpenter, D. Factors Affecting Health Care Provider Knowledge and Acceptance of Biosimilar Medicines: A Systematic Review. J. Manag. Care Spec. Pharm. 2019, 25, 102–112. [Google Scholar] [CrossRef]
- Dalpoas, S.E.; Socal, M.; Proctor, C.; Shermock, K.M. Barriers to biosimilar utilization in the United States. Am. J. Health-Syst. Pharm. 2020, 77, 2006–2014. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Singh, P.K.; Mishra, S.; Mitra, S.; Pattnaik, P.; Adhikary, S.D.; Mohapatra, R.K. Indian Biosimilars and Vaccines at Crossroads–Replicating the Success of Pharmagenerics. Vaccines 2023, 11, 110. [Google Scholar] [CrossRef]
- Perpoil, A.; Grimandi, G.; Birklé, S.; Simonet, J.F.; Chiffoleau, A.; Bocquet, F. Public Health Impact of Using Biosimilars, Is Automated Follow up Relevant? Int. J. Environ. Res. Public Health 2020, 18, 186. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Simoens, S.; Van Wilder, P.; Vulto, A.G.; Huys, I. Informing Patients about Biosimilar Medicines: The Role of European Patient Associations. Pharmaceuticals 2021, 14, 117. [Google Scholar] [CrossRef]
- Blanken, M.O.; Frederix, G.W.; Nibbelke, E.E.; Koffijberg, H.; Sanders, E.A.M.; Rovers, M.M.; Bont, L. Cost-effectiveness of rule-based immunoprophylaxis against respiratory syncytial virus infections in preterm infants. Eur. J. Pediatr. 2018, 177, 133–144. [Google Scholar] [CrossRef]
- Kim, H.; Alten, R.; Avedano, L.; Dignass, A.; Gomollón, F.; Greveson, K.; Halfvarson, J.; Irving, P.M.; Jahnsen, J.; Lakatos, P.L.; et al. The Future of Biosimilars: Maximizing Benefits Across Immune-Mediated Inflammatory Diseases. Drugs 2020, 80, 99–113. [Google Scholar] [CrossRef]
- Goeree, R.; Chiva-Razavi, S.; Gunda, P.; Jain, M.; Jugl, S. Cost-effectiveness analysis of secukinumab in ankylosing spondylitis from the Canadian perspective. J. Med. Econ. 2019, 22, 45–52. [Google Scholar] [CrossRef]
- Olfatifar, M.; Aghdaei, H.A.; Dehkordi, A.H.; Shahrokh, S.; Pourhoseingholi, M.A.; Balaii, H.; Rajabnia, M.; Ivanchuk, M.; Ivanchuk, P.; Nazari, S.H.; et al. Cost-effectiveness analysis of infliximab versus CinnoRA in the treatment of moderate to severe ulcerative colitis in Iranian patients. Immunopathol. Persa 2022, 9, 29293. [Google Scholar] [CrossRef]
- Dilokthornsakul, P.; Sawangjit, R.; Osiri, M.; Chiowchanwisawakit, P.; Louthrenoo, W.; Permsuwan, U. Cost-Utility Analysis of Biologic Disease-Modifying Antirheumatic Drugs for Patients With Psoriatic Arthritis in Thailand. Value Health Reg. Issues 2023, 34, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Vogler, S.; Schneider, P.; Zuba, M.; Busse, R.; Panteli, D. Policies to Encourage the Use of Biosimilars in European Countries and Their Potential Impact on Pharmaceutical Expenditure. Front. Pharmacol. 2021, 12, 625296. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, S.Z. Real-World Evidence of a Successful Biosimilar Adoption Program. Future Oncol. 2022, 18, 1997–2006. [Google Scholar] [CrossRef] [PubMed]
- Mestre-Ferrandiz, J.; Czech, M.; Smolen, J.S.; Cornes, P.; Aapro, M.S.; Danese, S.; Deitch, S.; Tyldsley, H.; Foster, W.; Shah, P.; et al. Capturing the holistic value of biosimilars in Europe—Part 1: A historical perspective. Expert Rev. Pharmacoecon. Outcomes Res. 2024, 24, 237–250. [Google Scholar] [CrossRef]
- Yousefi, N.; Ahmadi, R.; Tayeba, H.; Taheri, S.; Mahboudi, F.; Peiravian, F. Biosimilar Medicines in the Iranian Market: A Way to More Affordable Medicines. Indian J. Pharm. Sci. 2020, 82, 483–490. [Google Scholar] [CrossRef]
- Brian, G.; Biljana, T.; Eleonora, A.; Magdalene, W.; Stuart, M.; Amanj, K.; Mainul, H.; Sean, M.S.; Francis, K.; Amos, M.; et al. Biosimilars are essential for sustainable healthcare systems; however, key challenges remain as seen with long-acting insulin analogues. J. Appl. Pharm. Sci. 2022, 12, 55–72. [Google Scholar] [CrossRef]
- Bas, T.G.; Oliu, C.A. Innovation strategy management survey of the Chilean biomedical industry. Assessment of windows of opportunities to reduce technological gaps. Int. J. Health Plan. Manag. 2018, 33, e512–e530. [Google Scholar] [CrossRef]
- Esteban, E.; Bustos, R.H.; García, J.C.; Jáuregui, E. Biosimilars: An Approach to some Current Worldwide Regulation Frameworks. Curr. Clin. Pharmacol. 2019, 14, 16–40. [Google Scholar] [CrossRef] [PubMed]
- Klein, K.; Gencoglu, M.; Heisterberg, J.; Acha, V.; Stolk, P. The Global Landscape of Manufacturers of Follow-on Biologics: An Overview of Five Major Biosimilar Markets and 15 Countries. BioDrugs 2023, 37, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Sheth, S.; Vashishta, L.; Goyal, R.; Rathore, A.; Chirmule, N. On the Manufacturers of Biosimilars in Asia. Clin. Pharmacol. Ther. 2023, 113, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Darrow, J.J.; Avorn, J.; Kesselheim, A.S. FDA Approval and Regulation of Pharmaceuticals, 1983–2018. JAMA 2020, 323, 164. [Google Scholar] [CrossRef] [PubMed]
- Goode, R.; Chao, B. Biological patent thickets and delayed access to biosimilars, an American problem. J. Law Biosci. 2022, 9, lsac022. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.N.; Thorpe, R.; Knezevic, I.; Survey Participants from 19 Countries. The regulatory landscape of biosimilars: WHO efforts and progress made from 2009 to 2019. Biol. J. Int. Assoc. Biol. Stand. 2020, 65, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yazdany, J. Failure to Launch: Biosimilar Sales Continue to Fall Flat in the United States. Arthritis Rheumatol. 2020, 72, 870–873. [Google Scholar] [CrossRef] [PubMed]
- Halimi, V.; Daci, A.; Ancevska Netkovska, K.; Suturkova, L.; Babar, Z.U.D.; Grozdanova, A. Clinical and Regulatory Concerns of Biosimilars: A Review of Literature. Int. J. Environ. Res. Public Health 2020, 17, 5800. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.G.; Wobst, H.J. A Decade of FDA-Approved Drugs (2010–2019): Trends and Future Directions. J. Med. Chem. 2021, 64, 2312–2338. [Google Scholar] [CrossRef]
- Carrier, M.A.; Tu, S. Why Pharmaceutical Patent Thickets Are Unique (August 1, 2023). Texas Intellectual Property Law Journal, Forthcoming, Rutgers Law School Research Paper, WVU College of Law Research Paper No. 2023-25. Available online: https://ssrn.com/abstract=4571486 (accessed on 15 May 2024). [CrossRef]
- Reilly, M.S.; Schneider, P.J. A critical review of substitution policy for biosimilars in Canada. Generics Biosimilars Initiat. J. 2021, 10, 123–129. [Google Scholar] [CrossRef]
- Rathore, A.S.; Bhargava, A. Biosimilars in Developed Economies: Overview, Status, and Regulatory Considerations. Regul. Toxicol. Pharmacol. 2020, 110, 104525. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, B.; Caulfield, T. The Law and Ethics of Switching from Biologic to Biosimilar in Canada. J. Can. Assoc. Gastroenterol. 2020, 3, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.L.; Schoen, M.W.; Hoque, S.; Witherspoon, B.J.; Aboulafia, D.M.; Hwang, C.S.; Ray, P.; Yarnold, P.R.; Chen, B.K.; Schooley, B.; et al. Improving oncology biosimilar launches in the EU, the USA, and Japan: An updated Policy Review from the Southern Network on Adverse Reactions. Lancet. Oncol. 2020, 21, e575–e588. [Google Scholar] [CrossRef] [PubMed]
- Punia, A.; Malhotra, H. International Regulatory Processes and Policies for Innovator Biologics, Biosimilars, and Biobetters. In Biologics, Biosimilars, and Biobetters, 1st ed.; Ramzan, I., Ed.; Wiley: Hoboken, NJ, USA, 2020; pp. 159–176. [Google Scholar] [CrossRef]
- Na, S.Y.; Choi, C.H.; Song, E.M.; Bang, K.B.; Park, S.H.; Kim, E.S.; Park, J.J.; Keum, B.; Lee, C.K.; Lee, B.I.; et al. Korean clinical practice guidelines on biologics and small molecules for moderate-to-severe ulcerative colitis. Intest. Res. 2023, 21, 61–87. [Google Scholar] [CrossRef] [PubMed]
- Son, K.B. Market Exclusivity of the Originator Drugs in South Korea: A Retrospective Cohort Study. Front. Public Health 2021, 9, 654952. [Google Scholar] [CrossRef] [PubMed]
- Babu, D.N.A. Biosimilars The Emerging Economical Oncotherapy; BFC Publications: Lucknow, India, 2023. [Google Scholar]
- Gregory, G.P.; Carrington, C.; Cheah, C.Y.; Hawkes, E.A.; Irving, I.M.; Siderov, J.; Opat, S. A consensus statement on the use of biosimilar medicines in hematology in Australia. Asia-Pac. J. Clin. Oncol. 2020, 16, 211–221. [Google Scholar] [CrossRef]
- Da Silva Machado, F.L.; Cañás, M.; Doubova, S.V.; Urtasun, M.A.; Marín, G.H.; Osorio-de Castro, C.G.S.; Albuquerque, F.C.; Ribeiro, T.B.; Pont, L.; Crisóstomo Landeros, J.; et al. Biosimilars approvals by thirteen regulatory authorities: A cross-national comparison. Regul. Toxicol. Pharmacol. 2023, 144, 105485. [Google Scholar] [CrossRef]
- Deeksha, K.S.; Veeranna, B.; Ravindra, G.K. Analyzing Biosimilars in Brazil: Comprehensive Specifications of the Regulatory System. Indian J. Pharm. Educ. Res. 2023, 57, s499–s506. [Google Scholar] [CrossRef]
- Prasanthi Nori, L.; Ravi, P. Comparability Pathway for the Approval of Similar Biologics with Respect to Reference Biologics in Europe and Brazil. Indian J. Pharm. Educ. Res. 2020, 54, s19–s31. [Google Scholar] [CrossRef]
- Abu-Zaid, M.H.; Adebajo, A.; Miedany, Y.E. Potential for biosimilars in rheumatology in Africa. Ann. Rheum. Dis. 2023, 82, 1508–1510. [Google Scholar] [CrossRef]
- Pategou, J. Africa’s Biosimilar Landscape: Outlook & Current Challenges. 2020. Available online: https://www.biosimilardevelopment.com/doc/africa-s-biosimilar-landscape-outlook-current-challenges-0001 (accessed on 10 March 2024).
- Kale, D. From small molecule generics to biosimilars: Technological upgrading and patterns of distinctive learning processes in the Indian pharmaceutical industry. Technol. Forecast. Soc. Chang. 2019, 145, 370–383. [Google Scholar] [CrossRef]
- Dureja, H.; Dhiman, S. SAHPRA—Relevance of the New South African Health Products Regulatory Authority and Opportunities Ahead. J. Regul. Sci. 2021, 9, 1–14. [Google Scholar] [CrossRef]
- Ncube, B.M.; Dube, A.; Ward, K. Establishment of the African Medicines Agency: Progress, challenges and regulatory readiness. J. Pharm. Policy Pract. 2021, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Smart & Biggar. Biosimilars Approved in Canada. 2022. Available online: https://www.smartbiggar.ca/insights/biosimilars (accessed on 15 March 2024).
- Cestari de Oliveira, S.H. Follow-on biologicals/biosimilars approved in Brazil: May 2023 update—GaBI Journal. Generics Biosimilars Initiat. J. 2023, 12, 67–72. [Google Scholar] [CrossRef]
- Stewart, J. What Biosimilars Have Been Approved in the United States? 2024. Available online: https://www.drugs.com/medical-answers/many-biosimilars-approved-united-states-3463281/ (accessed on 15 March 2024).
- Jeremias, S. Regulatory Updates from around the Globe Provide Hope for Biosimilars. 2023. Available online: https://www.centerforbiosimilars.com/view/regulatory-updates-from-around-the-globe-provide-hope-for-biosimilars (accessed on 15 March 2024).
- Kuribayashi, R.; Nakano, A.; Hariu, A.; Kishioka, Y.; Honda, F. Historical Overview of Regulatory Approvals and PMDA Assessments for Biosimilar Products in Japan During 2009–2022. BioDrugs 2023, 37, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Jeremias, S. Alvotech and Cipla Partner on 5 Biosimilars for South Africa. 2020. Available online: https://www.centerforbiosimilars.com/view/alvotech-and-cipla-partner-on-5-biosimilars-for-south-africa (accessed on 10 March 2024).
- Opderbeck, D. Artificial Intelligence in Pharmaceuticals, Biologics, and Medical Devices: Present and Future Regulatory Models. Fordham Law Rev. 2019, 88, 553. [Google Scholar]
- Zohuri, B.; Behgounia, F. Application of artificial intelligence driving nano-based drug delivery system. In A Handbook of Artificial Intelligence in Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2023; pp. 145–212. [Google Scholar] [CrossRef]
- Askari, S.; Ghofrani, A.; Taherdoost, H. Transforming Drug Design: Innovations in Computer-Aided Discovery for Biosimilar Agents. BioMedInformatics 2023, 3, 1178–1196. [Google Scholar] [CrossRef]
- Pandya, S.; Thakur, A.; Saxena, S.; Jassal, N.; Patel, C.; Modi, K.; Shah, P.; Joshi, R.; Gonge, S.; Kadam, K.; et al. A Study of the Recent Trends of Immunology: Key Challenges, Domains, Applications, Datasets, and Future Directions. Sensors 2021, 21, 7786. [Google Scholar] [CrossRef] [PubMed]
- Walsh, I.; Myint, M.; Nguyen-Khuong, T.; Ho, Y.S.; Ng, S.K.; Lakshmanan, M. Harnessing the potential of machine learning for advancing “Quality by Design” in biomanufacturing. mAbs 2022, 14, 2013593. [Google Scholar] [CrossRef]
- Sharma, D.K.; Bhargava, S.; Singhal, K. Internet of Things applications in the pharmaceutical industry. In An Industrial IoT Approach for Pharmaceutical Industry Growth; Elsevier: Amsterdam, The Netherlands, 2020; pp. 153–190. [Google Scholar] [CrossRef]
- Agamah, F.E.; Mazandu, G.K.; Hassan, R.; Bope, C.D.; Thomford, N.E.; Ghansah, A.; Chimusa, E.R. Computational/in silico methods in drug target and lead prediction. Briefings Bioinform. 2020, 21, 1663–1675. [Google Scholar] [CrossRef]
- Viceconti, M.; Pappalardo, F.; Rodriguez, B.; Horner, M.; Bischoff, J.; Musuamba Tshinanu, F. In silico trials: Verification, validation and uncertainty quantification of predictive models used in the regulatory evaluation of biomedical products. Methods 2021, 185, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Rathore, A.S.; Nikita, S.; Thakur, G.; Mishra, S. Artificial intelligence and machine learning applications in biopharmaceutical manufacturing. Trends Biotechnol. 2023, 41, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Nupur, N.; Joshi, S.; Gulliarme, D.; Rathore, A.S. Analytical Similarity Assessment of Biosimilars: Global Regulatory Landscape, Recent Studies and Major Advancements in Orthogonal Platforms. Front. Bioeng. Biotechnol. 2022, 10, 832059. [Google Scholar] [CrossRef] [PubMed]
- Alarcón-Zendejas, A.P.; Scavuzzo, A.; Jiménez-Ríos, M.A.; Álvarez Gómez, R.M.; Montiel-Manríquez, R.; Castro-Hernández, C.; Jiménez-Dávila, M.A.; Pérez-Montiel, D.; González-Barrios, R.; Jiménez-Trejo, F.; et al. The promising role of new molecular biomarkers in prostate cancer: From coding and non-coding genes to artificial intelligence approaches. Prostate Cancer Prostatic Dis. 2022, 25, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari Laleh, N.; Ligero, M.; Perez-Lopez, R.; Kather, J.N. Facts and Hopes on the Use of Artificial Intelligence for Predictive Immunotherapy Biomarkers in Cancer. Clin. Cancer Res. 2023, 29, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Volovat, S.R.; Augustin, I.; Zob, D.; Boboc, D.; Amurariti, F.; Volovat, C.; Stefanescu, C.; Stolniceanu, C.R.; Ciocoiu, M.; Dumitras, E.A.; et al. Use of Personalized Biomarkers in Metastatic Colorectal Cancer and the Impact of AI. Cancers 2022, 14, 4834. [Google Scholar] [CrossRef] [PubMed]
- Kyriazakos, S.; Pnevmatikakis, A.; Cesario, A.; Kostopoulou, K.; Boldrini, L.; Valentini, V.; Scambia, G. Discovering Composite Lifestyle Biomarkers With Artificial Intelligence From Clinical Studies to Enable Smart eHealth and Digital Therapeutic Services. Front. Digit. Health 2021, 3, 648190. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Zhang, F.; Xu, L.; Yue, L.; Kon, O.L.; Zhu, Y.; Guo, T. High-throughput proteomics and AI for cancer biomarker discovery. Adv. Drug Deliv. Rev. 2021, 176, 113844. [Google Scholar] [CrossRef] [PubMed]
- Olivera, P.; Danese, S.; Jay, N.; Natoli, G.; Peyrin-Biroulet, L. Big data in IBD: A look into the future. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 312–321. [Google Scholar] [CrossRef]
- Castro Corredor, D.; Calvo Pascual, L.A. Imbalanced machine learning classification models for removal biosimilar drugs and increased activity in patients with rheumatic diseases. PLoS ONE 2023, 18, e0291891. [Google Scholar] [CrossRef]
- Sarkar, C.; Das, B.; Rawat, V.S.; Wahlang, J.B.; Nongpiur, A.; Tiewsoh, I.; Lyngdoh, N.M.; Das, D.; Bidarolli, M.; Sony, H.T. Artificial Intelligence and Machine Learning Technology Driven Modern Drug Discovery and Development. Int. J. Mol. Sci. 2023, 24, 2026. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.V.; Ansari, M.H.D.; Rosenkranz, D.; Maharjan, R.S.; Kriegel, F.L.; Gandhi, K.; Kanase, A.; Singh, R.; Laux, P.; Luch, A. Artificial Intelligence and Machine Learning in Computational Nanotoxicology: Unlocking and Empowering Nanomedicine. Adv. Healthc. Mater. 2020, 9, 1901862. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Srivastava, D.; Sahu, M.; Tiwari, S.; Ambasta, R.K.; Kumar, P. Artificial intelligence to deep learning: Machine intelligence approach for drug discovery. Mol. Divers. 2021, 25, 1315–1360. [Google Scholar] [CrossRef] [PubMed]
- Krenn, M.; Ai, Q.; Barthel, S.; Carson, N.; Frei, A.; Frey, N.C.; Friederich, P.; Gaudin, T.; Gayle, A.A.; Jablonka, K.M.; et al. SELFIES and the future of molecular string representations. Patterns 2022, 3, 100588. [Google Scholar] [CrossRef] [PubMed]
- Brogi, S.; Ramalho, T.C.; Kuca, K.; Medina-Franco, J.L.; Valko, M. Editorial: In silico Methods for Drug Design and Discovery. Front. Chem. 2020, 8, 612. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Luna, J.; Grisoni, F.; Weskamp, N.; Schneider, G. Artificial intelligence in drug discovery: Recent advances and future perspectives. Expert Opin. Drug Discov. 2021, 16, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Greener, J.G.; Kandathil, S.M.; Moffat, L.; Jones, D.T. A guide to machine learning for biologists. Nat. Rev. Mol. Cell Biol. 2022, 23, 40–55. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.; Chen, X.; Zhang, H.; Wei, Q.; Luo, K. Artificial intelligence aids in development of nanomedicines for cancer management. Semin. Cancer Biol. 2023, 89, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Noé, F.; Tkatchenko, A.; Müller, K.R.; Clementi, C. Machine Learning for Molecular Simulation. Annu. Rev. Phys. Chem. 2020, 71, 361–390. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, D.; Ren, B.; Gong, X.; Xu, L.; Feng, Z.Q.; Chang, Y.; He, Y.; Zheng, J. Molecular simulations and understanding of antifouling zwitterionic polymer brushes. J. Mater. Chem. B 2020, 8, 3814–3828. [Google Scholar] [CrossRef]
- Temml, V.; Kutil, Z. Structure-based molecular modeling in SAR analysis and lead optimization. Comput. Struct. Biotechnol. J. 2021, 19, 1431–1444. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.I.; Rao, S.; Sansom, M.S.P. Water in Nanopores and Biological Channels: A Molecular Simulation Perspective. Chem. Rev. 2020, 120, 10298–10335. [Google Scholar] [CrossRef] [PubMed]
- Kurki, P.; Kang, H.N.; Ekman, N.; Knezevic, I.; Weise, M.; Wolff-Holz, E. Regulatory Evaluation of Biosimilars: Refinement of Principles Based on the Scientific Evidence and Clinical Experience. BioDrugs 2022, 36, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Woollett, G.R.; Park, J.P.; Han, J.; Jung, B. The Role of PD Biomarkers in Biosimilar Development—To Get the Right Answer One Must First Ask the Right Question. Clin. Pharmacol. Ther. 2023, 113, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Terranova, N.; Renard, D.; Shahin, M.H.; Menon, S.; Cao, Y.; Hop, C.E.; Hayes, S.; Madrasi, K.; Stodtmann, S.; Tensfeldt, T.; et al. Artificial Intelligence for Quantitative Modeling in Drug Discovery and Development: An Innovation and Quality Consortium Perspective on Use Cases and Best Practices. Clin. Pharmacol. Ther. 2024, 115, 658–672. [Google Scholar] [CrossRef]
- Staszak, M.; Staszak, K.; Wieszczycka, K.; Bajek, A.; Roszkowski, K.; Tylkowski, B. Machine learning in drug design: Use of artificial intelligence to explore the chemical structure–biological activity relationship. WIREs Comput. Mol. Sci. 2022, 12, e1568. [Google Scholar] [CrossRef]
- Selvaraj, C.; Chandra, I.; Singh, S.K. Artificial intelligence and machine learning approaches for drug design: Challenges and opportunities for the pharmaceutical industries. Mol. Divers. 2022, 26, 1893–1913. [Google Scholar] [CrossRef]
- Spitz, S.; Zhang, Y.; Fischer, S.; McGuire, K.; Sommer, U.; Amaravadi, L.; Bandukwala, A.; Eck, S.; Garofolo, F.; Islam, R.; et al. 2020 White Paper on Recent Issues in Bioanalysis: BAV Guidance, CLSI H62, Biotherapeutics Stability, Parallelism Testing, CyTOF and Regulatory Feedback (Part 2A—Recommendations on Biotherapeutics Stability, PK LBA Regulated Bioanalysis, Biomarkers Assays, Cytometry Validation & Innovation Part 2B—Regulatory Agencies’ Inputs on Bioanalysis, Biomarkers, Immunogenicity, Gene & Cell Therapy and Vaccine). Bioanalysis 2021, 13, 295–361. [Google Scholar] [CrossRef] [PubMed]
- Jose, J.; Shifali, S.; Mathew, B.; Parambi, D.G.T. In Silico Trial Approach for Biomedical Products: A Regulatory Perspective. Comb. Chem. High Throughput Screen. 2022, 25, 1991–2000. [Google Scholar] [CrossRef]
- Deshmukh, A.; Goyal, R.; Sundaram, K.; Dange, K.; Lakhote, T.; Niranjan, S.; Bharucha, J.; Mishra, A.; Vats, B.; Tiwari, S. Analytical sameness methodology for the evaluation of structural, physicochemical, and biological characteristics of Armlupeg: A pegfilgrastim biosimilar case study. PLoS ONE 2023, 18, e0289745. [Google Scholar] [CrossRef]
- Rahalkar, H.; Cetintas, H.C.; Salek, S. Quality, Non-clinical and Clinical Considerations for Biosimilar Monoclonal Antibody Development: EU, WHO, USA, Canada, and BRICS-TM Regulatory Guidelines. Front. Pharmacol. 2018, 9, 1079. [Google Scholar] [CrossRef] [PubMed]
- Zagalo, D.M.; Sousa, J.; Simões, S. Quality by design (QbD) approach in marketing authorization procedures of Non-Biological Complex Drugs: A critical evaluation. Eur. J. Pharm. Biopharm. 2022, 178, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Wolff-Holz, E.; Tiitso, K.; Vleminckx, C.; Weise, M. Evolution of the EU Biosimilar Framework: Past and Future. BioDrugs 2019, 33, 621–634. [Google Scholar] [CrossRef]
- Zhang, R.; Li, X.; Zhang, X.; Qin, H.; Xiao, W. Machine learning approaches for elucidating the biological effects of natural products. Nat. Prod. Rep. 2021, 38, 346–361. [Google Scholar] [CrossRef]
- Kovács, B.; Péterfi, O.; Kovács-Deák, B.; Székely-Szentmiklósi, I.; Fülöp, I.; Bába, L.I.; Boda, F. Quality-by-design in pharmaceutical development: From current perspectives to practical applications. Acta Pharm. 2021, 71, 497–526. [Google Scholar] [CrossRef]
- Mohseni-Motlagh, S.F.; Dolatabadi, R.; Baniassadi, M.; Baghani, M. Application of the Quality by Design Concept (QbD) in the Development of Hydrogel-Based Drug Delivery Systems. Polymers 2023, 15, 4407. [Google Scholar] [CrossRef]
- Pardeshi, S.R.; Deshmukh, N.S.; Telange, D.R.; Nangare, S.N.; Sonar, Y.Y.; Lakade, S.H.; Harde, M.T.; Pardeshi, C.V.; Gholap, A.; Deshmukh, P.K.; et al. Process development and quality attributes for the freeze-drying process in pharmaceuticals, biopharmaceuticals and nanomedicine delivery: A state-of-the-art review. Future J. Pharm. Sci. 2023, 9, 99. [Google Scholar] [CrossRef]
- Forbes, T.P.; Gillen, J.G.; Feeney, W.; Ho, J. Quality by Design Considerations for Drop-on-Demand Point-of-Care Pharmaceutical Manufacturing of Precision Medicine. Mol. Pharm. 2024, 21, 3268–3280. [Google Scholar] [CrossRef]
- Castel, J.; Delaux, S.; Hernandez-Alba, O.; Cianférani, S. Recent advances in structural mass spectrometry methods in the context of biosimilarity assessment: From sequence heterogeneities to higher order structures. J. Pharm. Biomed. Anal. 2023, 236, 115696. [Google Scholar] [CrossRef] [PubMed]
- Biehn, S.E.; Lindert, S. Protein Structure Prediction with Mass Spectrometry Data. Annu. Rev. Phys. Chem. 2022, 73, 1–19. [Google Scholar] [CrossRef]
- Fukushima, Y.; Yoshidome, T. A deep-learning model for grid-based solvation free energy. Biophys. J. 2023, 122, 141a–142a. [Google Scholar] [CrossRef]
- Muzio, G.; O’Bray, L.; Borgwardt, K. Biological network analysis with deep learning. Briefings Bioinform. 2021, 22, 1515–1530. [Google Scholar] [CrossRef] [PubMed]
- Vora, L.K.; Gholap, A.D.; Jetha, K.; Thakur, R.R.S.; Solanki, H.K.; Chavda, V.P. Artificial Intelligence in Pharmaceutical Technology and Drug Delivery Design. Pharmaceutics 2023, 15, 1916. [Google Scholar] [CrossRef] [PubMed]
- Borjali, A.; Magnéli, M.; Shin, D.; Malchau, H.; Muratoglu, O.K.; Varadarajan, K.M. Natural language processing with deep learning for medical adverse event detection from free-text medical narratives: A case study of detecting total hip replacement dislocation. Comput. Biol. Med. 2021, 129, 104140. [Google Scholar] [CrossRef] [PubMed]
- McComb, M.; Bies, R.; Ramanathan, M. Machine learning in pharmacometrics: Opportunities and challenges. Br. J. Clin. Pharmacol. 2022, 88, 1482–1499. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Qian, J. Safety of marketed biosimilar monoclonal antibody cancer treatments in the US: A disproportionality analysis using the food and drug administration adverse event reporting system (FAERS) database. Expert Opin. Drug Saf. 2024, 1–10. [Google Scholar] [CrossRef]
- Xue, X.; Truong, B.; Qian, J. Adverse event reporting of marketed biosimilar and biological monoclonal antibody cancer treatments in the United States. Expert Opin. Biol. Ther. 2023, 23, 841–849. [Google Scholar] [CrossRef]
- Batko, K.; Ślęzak, A. The use of Big Data Analytics in healthcare. J. Big Data 2022, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Lindroth, H.; Nalaie, K.; Raghu, R.; Ayala, I.N.; Busch, C.; Bhattacharyya, A.; Moreno Franco, P.; Diedrich, D.A.; Pickering, B.W.; Herasevich, V. Applied Artificial Intelligence in Healthcare: A Review of Computer Vision Technology Application in Hospital Settings. J. Imaging 2024, 10, 81. [Google Scholar] [CrossRef]
- Mascarenhas-Melo, F.; Diaz, M.; Gonçalves, M.B.S.; Vieira, P.; Bell, V.; Viana, S.; Nunes, S.; Paiva-Santos, A.C.; Veiga, F. An Overview of Biosimilars—Development, Quality, Regulatory Issues, and Management in Healthcare. Pharmaceuticals 2024, 17, 235. [Google Scholar] [CrossRef]
- Kwon, M.; Joung, C.I.; Shin, H.; Lee, C.C.; Song, Y.S.; Lee, Y.J.; Kang, S.; Kim, J.Y.; Lee, S. Detection of novel drug-adverse drug reaction signals in rheumatoid arthritis and ankylosing spondylitis: Analysis of Korean real-world biologics registry data. Sci. Rep. 2024, 14, 2660. [Google Scholar] [CrossRef] [PubMed]
- Malikova, M.A. Practical applications of regulatory requirements for signal detection and communications in pharmacovigilance. Ther. Adv. Drug Saf. 2020, 11, 204209862090961. [Google Scholar] [CrossRef] [PubMed]
- Alowais, S.A.; Alghamdi, S.S.; Alsuhebany, N.; Alqahtani, T.; Alshaya, A.I.; Almohareb, S.N.; Aldairem, A.; Alrashed, M.; Bin Saleh, K.; Badreldin, H.A.; et al. Revolutionizing healthcare: The role of artificial intelligence in clinical practice. BMC Med. Educ. 2023, 23, 689. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, R. How Can Artificial Intelligence Be Implemented Effectively in Diabetic Retinopathy Screening in Japan? Medicina 2024, 60, 243. [Google Scholar] [CrossRef] [PubMed]
- Mariam, Z.; Niazi, S.K.; Magoola, M. Unlocking the Future of Drug Development: Generative AI, Digital Twins, and Beyond. BioMedInformatics 2024, 4, 1441–1456. [Google Scholar] [CrossRef]
- Ang, D.; Rakovski, C.; Atamian, H.S. De Novo Drug Design Using Transformer-Based Machine Translation and Reinforcement Learning of an Adaptive Monte Carlo Tree Search. Pharmaceuticals 2024, 17, 161. [Google Scholar] [CrossRef] [PubMed]
- Grechishnikova, D. Transformer neural network for protein-specific de novo drug generation as a machine translation problem. Sci. Rep. 2021, 11, 321. [Google Scholar] [CrossRef] [PubMed]
- Chiu, K.; Racz, R.; Burkhart, K.; Florian, J.; Ford, K.; Iveth Garcia, M.; Geiger, R.M.; Howard, K.E.; Hyland, P.L.; Ismaiel, O.A.; et al. New science, drug regulation, and emergent public health issues: The work of FDA’s division of applied regulatory science. Front. Med. 2023, 9, 1109541. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Cheng, S.; Chen, C.; Qiao, M.; Zhang, W.; Shah, A.; Bai, W.; Arcucci, R. M-FLAG: Medical Vision-Language Pre-training with Frozen Language Models and Latent Space Geometry Optimization. In Proceedings of the Medical Image Computing and Computer Assisted Intervention—MICCAI, Vancouver, BC, Canada, 8–12 October 2023; Springer: Cham, Switzerland, 2023; pp. 637–647, ISSN 1611-3349. [Google Scholar] [CrossRef]
- Li, J.; Liu, C.; Cheng, S.; Arcucci, R.; Hong, S. Frozen Language Model Helps ECG Zero-Shot Learning. In Proceedings of the Medical Imaging with Deep Learning, Paris, France, 3–5 July 2024; pp. 402–415, ISSN 2640-3498. [Google Scholar]
- Wan, Z.; Liu, C.; Zhang, M.; Fu, J.; Wang, B.; Cheng, S.; Ma, L.; Quilodrán-Casas, C.; Arcucci, R. Med-UniC: Unifying Cross-Lingual Medical Vision-Language Pre-Training by Diminishing Bias. arXiv 2023, arXiv:2305.19894. [Google Scholar] [CrossRef]
- Hair, J.; Maryon, T.; Lieneck, C. Identification of Barriers Preventing Biosimiliar Oncology Medication Adoption. Medicina 2022, 58, 1533. [Google Scholar] [CrossRef]
- Joshi, D.; Khursheed, R.; Gupta, S.; Wadhwa, D.; Singh, T.G.; Sharma, S.; Porwal, S.; Gauniyal, S.; Vishwas, S.; Goyal, S.; et al. Biosimilars in Oncology: Latest Trends and Regulatory Status. Pharmaceutics 2022, 14, 2721. [Google Scholar] [CrossRef] [PubMed]
- Teng, F.; Cui, T.; Zhou, L.; Gao, Q.; Zhou, Q.; Li, W. Programmable synthetic receptors: The next-generation of cell and gene therapies. Signal Transduct. Target. Ther. 2024, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Arman, S.; Tilley, R.D.; Gooding, J.J. A review of electrochemical impedance as a tool for examining cell biology and subcellular mechanisms: Merits, limits, and future prospects. Analyst 2024, 149, 269–289. [Google Scholar] [CrossRef]
- Foglizzo, V.; Marchiò, S. Nanoparticles as Physically- and Biochemically-Tuned Drug Formulations for Cancers Therapy. Cancers 2022, 14, 2473. [Google Scholar] [CrossRef] [PubMed]
- Fong, E.J.; Strelez, C.; Mumenthaler, S.M. A Perspective on Expanding Our Understanding of Cancer Treatments by Integrating Approaches from the Biological and Physical Sciences. SLAS Discov. 2020, 25, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, G.; Ahmed, S.R.; Hormuth, D.A., II; Vaughn, B.; Kalpathy-Cramer, J.; Solorio, L.; Yankeelov, T.E.; Gomez, H. Patient-specific, mechanistic models of tumor growth incorporating artificial intelligence and big data. arXiv 2023, arXiv:2308.14925. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.i.; Matsumoto, N.; Ishida, H.; Kubota, Y.; Iwai, S.; Shibanuma, M.; Kato, Y. Decreased Disposition of Anticancer Drugs Predominantly Eliminated via the Liver in Patients with Renal Failure. Curr. Drug Metab. 2019, 20, 361–376. [Google Scholar] [CrossRef]
- Malinzi, J.; Basita, K.B.; Padidar, S.; Adeola, H.A. Prospect for application of mathematical models in combination cancer treatments. Inform. Med. Unlocked 2021, 23, 100534. [Google Scholar] [CrossRef]
- Delrue, C.; Speeckaert, M.M. The Potential Applications of Raman Spectroscopy in Kidney Diseases. J. Pers. Med. 2022, 12, 1644. [Google Scholar] [CrossRef]
- Amasawa, E.; Kuroda, H.; Okamura, K.; Badr, S.; Sugiyama, H. Cost–Benefit Analysis of Monoclonal Antibody Cultivation Scenarios in Terms of Life Cycle Environmental Impact and Operating Cost. ACS Sustain. Chem. Eng. 2021, 9, 14012–14021. [Google Scholar] [CrossRef]
- Bittner, B. Customer-centric product presentations for monoclonal antibodies. AAPS Open 2023, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Krzyzanski, W.; Wong, R.S.M.; Liu, D.; Yan, X. Novel Combination of Erythropoietin and Romiplostim to Treat Chemotherapy-Induced Anemia and Thrombocytopenia via Pharmacodynamic Interaction on Hematopoietic Stem and Progenitor Cells. ACS Pharmacol. Transl. Sci. 2023, 6, 1884–1897. [Google Scholar] [CrossRef] [PubMed]
- Gilaberte Reyzabal, S.; Isenberg, D. Differences in the Development of Adverse Infusion Reactions to Rituximab in Patients With Systemic Lupus Erythematosus, Rheumatoid Arthritis and Non-Hodgkin’s Lymphoma-Enigma Variations. Front. Med. 2022, 9, 882891. [Google Scholar] [CrossRef] [PubMed]
- Peipert, J.D.; Kaiser, K.; Kircher, S.; Greene, G.J.; Shaunfield, S.; Hauner, K.; Cella, D.; Mroczek, D.K. Medical Oncologists’ Knowledge and Perspectives on the Use of Biosimilars in the United States. JCO Oncol. Pract. 2023, 19, e457–e464. [Google Scholar] [CrossRef] [PubMed]
- Evangelatos, G.; Bamias, G.; Kitas, G.D.; Kollias, G.; Sfikakis, P.P. The second decade of anti-TNF-a therapy in clinical practice: New lessons and future directions in the COVID-19 era. Rheumatol. Int. 2022, 42, 1493–1511. [Google Scholar] [CrossRef] [PubMed]
- Cliff, E.R.S.; Rome, R.S.; Kesselheim, A.S.; Rome, B.N. National Comprehensive Cancer Network Guideline Recommendations of Cancer Drugs With Accelerated Approval. JAMA Netw. Open 2023, 6, e2343285. [Google Scholar] [CrossRef] [PubMed]
- Gherghescu, I.; Delgado-Charro, M.B. The Biosimilar Landscape: An Overview of Regulatory Approvals by the EMA and FDA. Pharmaceutics 2020, 13, 48. [Google Scholar] [CrossRef] [PubMed]
- Kvien, T.K.; Patel, K.; Strand, V. The cost savings of biosimilars can help increase patient access and lift the financial burden of health care systems. Semin. Arthritis Rheum. 2022, 52, 151939. [Google Scholar] [CrossRef]
- Greene, L.; Singh, R.M.; Carden, M.J.; Pardo, C.O.; Lichtenstein, G.R. Strategies for Overcoming Barriers to Adopting Biosimilars and Achieving Goals of the Biologics Price Competition and Innovation Act: A Survey of Managed Care and Specialty Pharmacy Professionals. J. Manag. Care Spec. Pharm. 2019, 25, 904–912. [Google Scholar] [CrossRef]
- Lengyel, C.G.; Habeeb, B.S.; Altuna, S.C.; Trapani, D.; Khan, S.Z.; Hussain, S. The Global Landscape on the Access to Cancer Medicines for Breast Cancer: The ONCOLLEGE Experience. In Breast Cancer Research and Treatment; Al Jarroudi, O., El Bairi, K., Curigliano, G., Eds.; Series Title: Cancer Treatment and Research; Springer International Publishing: Cham, Switzerland, 2023; Volume 188, pp. 353–368. [Google Scholar] [CrossRef]
- Wang, L.; Chen, X.; Zhang, L.; Li, L.; Huang, Y.; Sun, Y.; Yuan, X. Artificial intelligence in clinical decision support systems for oncology. Int. J. Med. Sci. 2023, 20, 79–86. [Google Scholar] [CrossRef]
- Mysler, E.; Azevedo, V.F.; Danese, S.; Alvarez, D.; Iikuni, N.; Ingram, B.; Mueller, M.; Peyrin-Biroulet, L. Biosimilar-to-Biosimilar Switching: What is the Rationale and Current Experience? Drugs 2021, 81, 1859–1879. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, M.M.; Crisci, E.; Pentella, C.; Cantone, A.; Ruggiero, D.; Anatriello, A.; Scavone, C. Switching between Originators and Biosimilars in Dermatology: A Systematic Review of Real-World Clinical Studies. Biologics 2023, 3, 95–115. [Google Scholar] [CrossRef]
- Wirth, K.; Boes, S.; Näpflin, M.; Huber, C.; Blozik, E. Initial prescriptions and medication switches of biological products: An analysis of prescription pathways and determinants in the Swiss healthcare setting. BMJ Open 2023, 13, e077454. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wang, L.; Rizzo, S.; Garmhausen, M.R.; Pal, N.; Waliany, S.; McGough, S.; Lin, Y.G.; Huang, Z.; Neal, J.; et al. Systematic analysis of off-label and off-guideline cancer therapy usage in a real-world cohort of 165,912 US patients. Cell Rep. Med. 2024, 5, 101444. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, Z.; Sadaf, S. Biosimilars: A Comparative Study of Regulatory, Safety and Pharmacovigilance Monograph in the Developed and Developing Economies. J. Pharm. Pharm. Sci. 2022, 25, 149–182. [Google Scholar] [CrossRef] [PubMed]
- Barbier, L.; Simoens, S.; Vulto, A.G.; Huys, I. European Stakeholder Learnings Regarding Biosimilars: Part I—Improving Biosimilar Understanding and Adoption. BioDrugs 2020, 34, 783–796. [Google Scholar] [CrossRef] [PubMed]
- Florio, M.; Gamba, S. Biomed Europa: After the coronavirus, a public infrastructure to overcome the pharmaceutical oligopoly. Ann. Public Coop. Econ. 2021, 92, 387–409. [Google Scholar] [CrossRef]
- Feldman, R. Trade Secrets in Biologic Medicine: The Boundary with Patents. Sci. Technol. Law Rev. 2023, 24, 1–54. [Google Scholar] [CrossRef]
- Rahalkar, H.; Sheppard, A.; Santos, G.M.L.; Dasgupta, C.; Perez-Tapia, S.M.; Lopez-Morales, C.A.; Salek, S. Current Regulatory Requirements for Biosimilars in Six Member Countries of BRICS-TM: Challenges and Opportunities. Front. Med. 2021, 8, 726660. [Google Scholar] [CrossRef]
- Mercurio, B.; Upreti, P.N. Patent term extension and test data protection obligations: Identifying the gap in policy, research, and practice of implementing free trade agreements. J. Law Biosci. 2023, 10, lsad017. [Google Scholar] [CrossRef]
- Simoens, S. How do biosimilars sustain value, affordability, and access to oncology care? Expert Rev. Pharmacoecon. Outcomes Res. 2021, 21, 327–329. [Google Scholar] [CrossRef] [PubMed]
- Hübel, K.; Kron, F.; Lux, M.P. Biosimilars in oncology: Effects on economy and therapeutic innovations. Eur. J. Cancer 2020, 139, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Nahleh, Z.; Lyman, G.H.; Schilsky, R.L.; Peterson, D.E.; Tagawa, S.T.; Chavez-MacGregor, M.; Rumble, R.B.; Gupta, S. Use of Biosimilar Medications in Oncology. JCO Oncol. Pract. 2022, 18, 177–186. [Google Scholar] [CrossRef]
- García, J.J.; Raez, L.E.; Rosas, D. A narrative review of biosimilars: A continued journey from the scientific evidence to practice implementation. Transl. Lung Cancer Res. 2020, 9, 2113–2119. [Google Scholar] [CrossRef] [PubMed]
- Kurki, P.; Barry, S.; Bourges, I.; Tsantili, P.; Wolff-Holz, E. Safety, Immunogenicity and Interchangeability of Biosimilar Monoclonal Antibodies and Fusion Proteins: A Regulatory Perspective. Drugs 2021, 81, 1881–1896. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.A.K.; Turjya, R.R.; Islam, A.B.M.M.K. Computational engineering the binding affinity of Adalimumab monoclonal antibody for designing potential biosimilar candidate. J. Mol. Graph. Model. 2021, 102, 107774. [Google Scholar] [CrossRef] [PubMed]
- Kute, N.; Mankar, S.D.; Bhawar, S.B. Biosimilar and it’s Current Perspective—A Review. Res. J. Pharmacol. Pharmacodyn. 2022, 14, 84–88. [Google Scholar] [CrossRef]
- Blackstone, E.A.; Joseph, P.F. The Economics of Biosimilars. Am. Health Drug Benefits 2013, 6, 469–478. [Google Scholar]
- Da Silva, R.G.L. The advancement of artificial intelligence in biomedical research and health innovation: Challenges and opportunities in emerging economies. Glob. Health 2024, 20, 44. [Google Scholar] [CrossRef]
- Cortes, J.; Perez-García, J.M.; Llombart-Cussac, A.; Curigliano, G.; El Saghir, N.S.; Cardoso, F.; Barrios, C.H.; Wagle, S.; Roman, J.; Harbeck, N.; et al. Enhancing global access to cancer medicines. CA Cancer J. Clin. 2020, 70, 105–124. [Google Scholar] [CrossRef]
- Kar, I.; Kronz, M.; Kolychev, E.; Silverman, P.; Mendiratta, P.; Tomlinson, B.K.N.; Prunty, J.; Copley, M.; Patel, S.; Caudill, S.; et al. Biosimilar strategic implementation at a large health system. Am. J. Health-Syst. Pharm. 2022, 79, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Bond, A.M.; Dean, E.B.; Desai, S.M. The Role of Financial Incentives in Biosimilar Uptake in Medicare: Evidence from the 340B Program: Study examines the role of financial incentives in the uptake of biosimilar drugs in Medicare. Health Aff. 2023, 42, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.W.; Amoline, K.; Marcum, C.; Leonard, M. Healthcare system conversion to a biosimilar: Trials and tribulations. Am. J. Health-Syst. Pharm. 2021, 78, 2159–2163. [Google Scholar] [CrossRef] [PubMed]
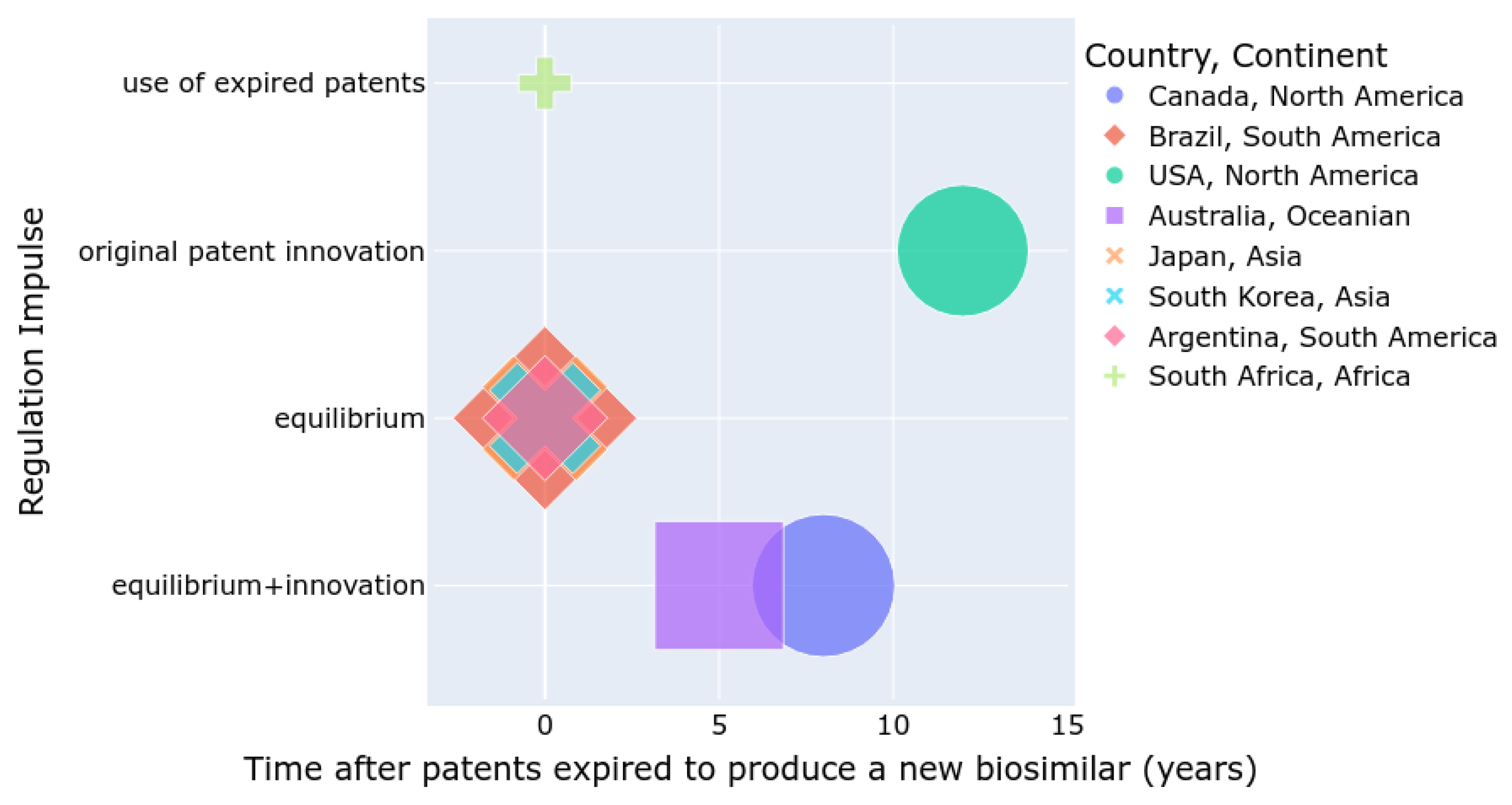

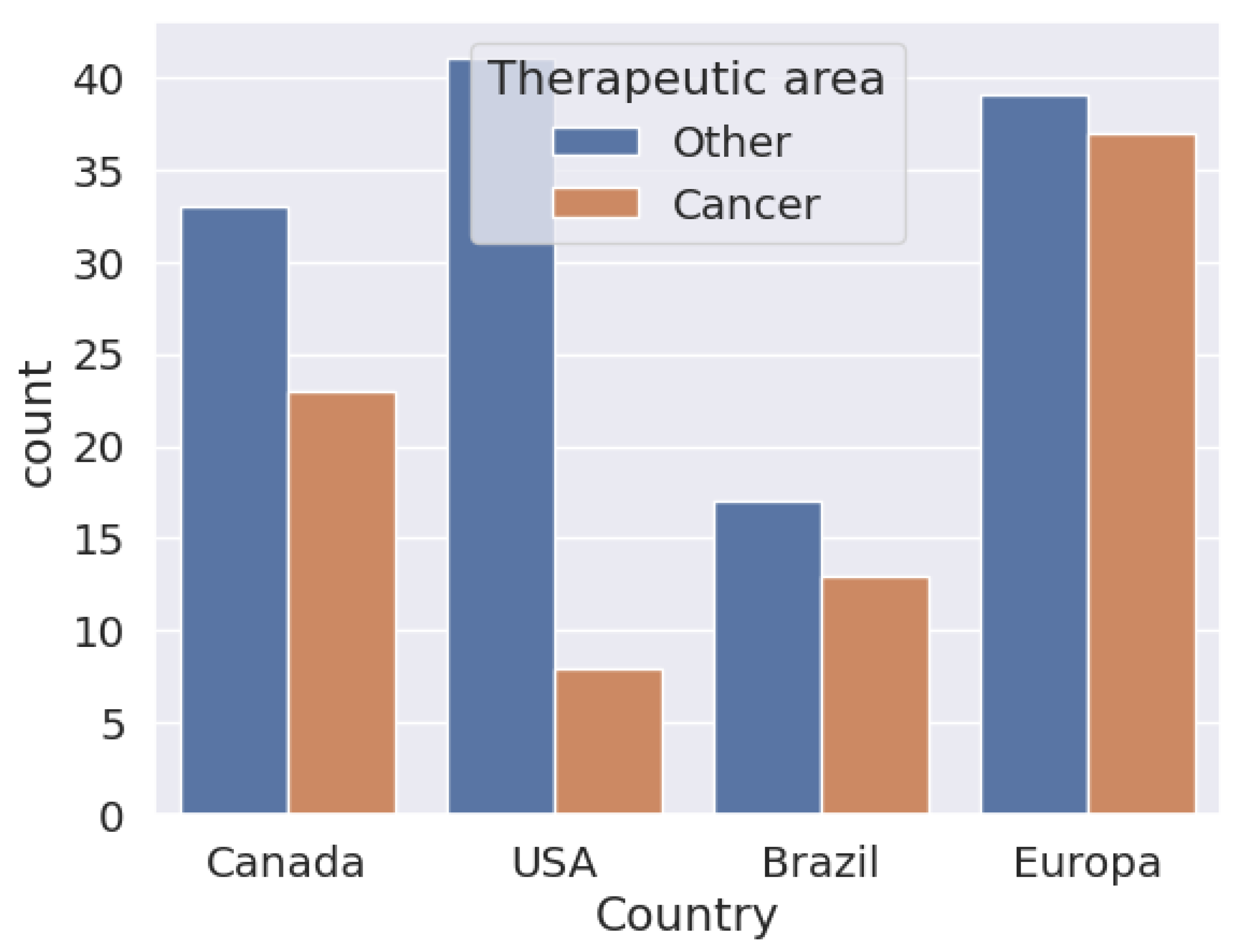
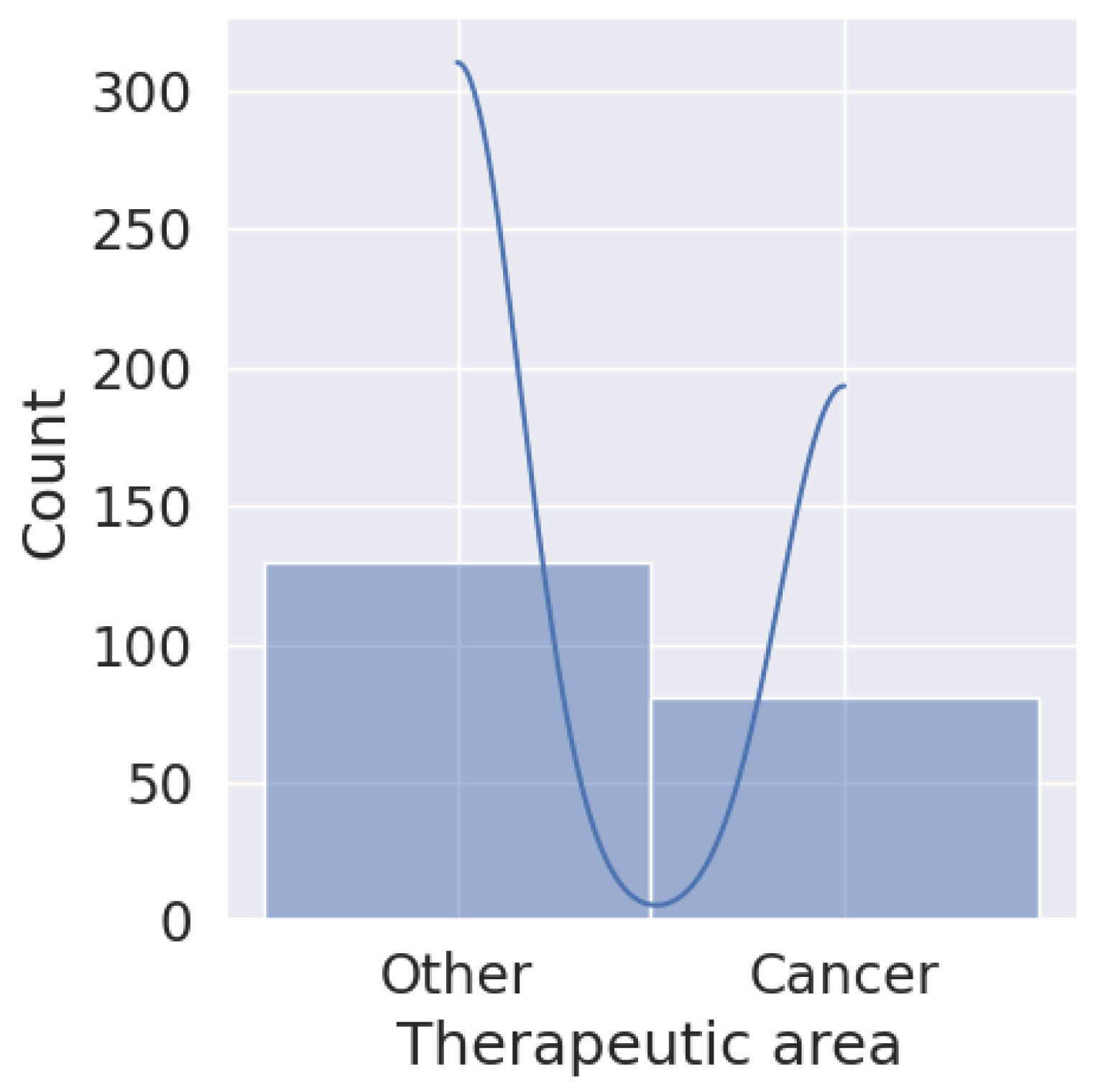
| Countries and Sources | Total Number Approved | Regulatory Framework for Biosimilars |
|---|---|---|
| Canada [134] | 53 (September 2023) | CMA |
| Brazil [135] | 52 (May 2023) | ANVISA |
| United States [136] | 45 (December 2023) | FDA |
| Australia [137] | 43 (September 2023) | TGA |
| Japan [138] | 32 (December 2022) | PMDA |
| South Korea [108] | 25 (December 2022) | MFDS |
| Argentina [126] | 24 (December 2022) | ANMAT |
| South Africa [139] | 5 (November 2020) | SAHPRA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bas, T.G.; Duarte, V. Biosimilars in the Era of Artificial Intelligence—International Regulations and the Use in Oncological Treatments. Pharmaceuticals 2024, 17, 925. https://doi.org/10.3390/ph17070925
Bas TG, Duarte V. Biosimilars in the Era of Artificial Intelligence—International Regulations and the Use in Oncological Treatments. Pharmaceuticals. 2024; 17(7):925. https://doi.org/10.3390/ph17070925
Chicago/Turabian StyleBas, Tomas Gabriel, and Vannessa Duarte. 2024. "Biosimilars in the Era of Artificial Intelligence—International Regulations and the Use in Oncological Treatments" Pharmaceuticals 17, no. 7: 925. https://doi.org/10.3390/ph17070925
APA StyleBas, T. G., & Duarte, V. (2024). Biosimilars in the Era of Artificial Intelligence—International Regulations and the Use in Oncological Treatments. Pharmaceuticals, 17(7), 925. https://doi.org/10.3390/ph17070925