Prunus yedoensis Bark Downregulates the Expression of Cell Adhesion Molecules in Human Endothelial Cell Lines and Relaxes Blood Vessels in Rat Aortic Rings
Abstract
:1. Introduction
2. Results
2.1. Effects of Prunus yedoensis Bark Extracts (PYB) and Prunetin on HUVECs Cell Viability
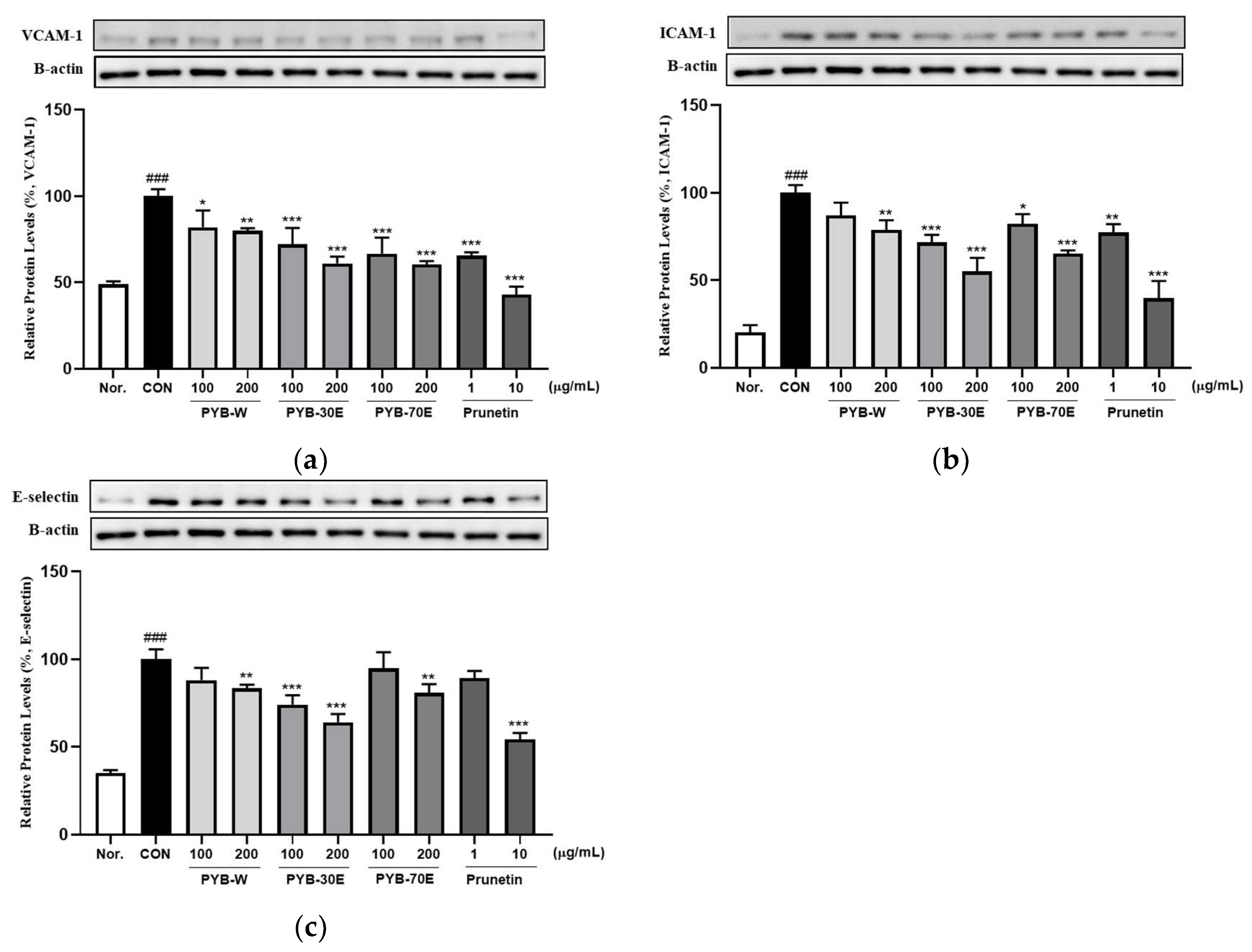
2.2. Effects of PYB Extracts and Prunetin on TNF-α-Induced Human Vascular Endothelial Cell Activation
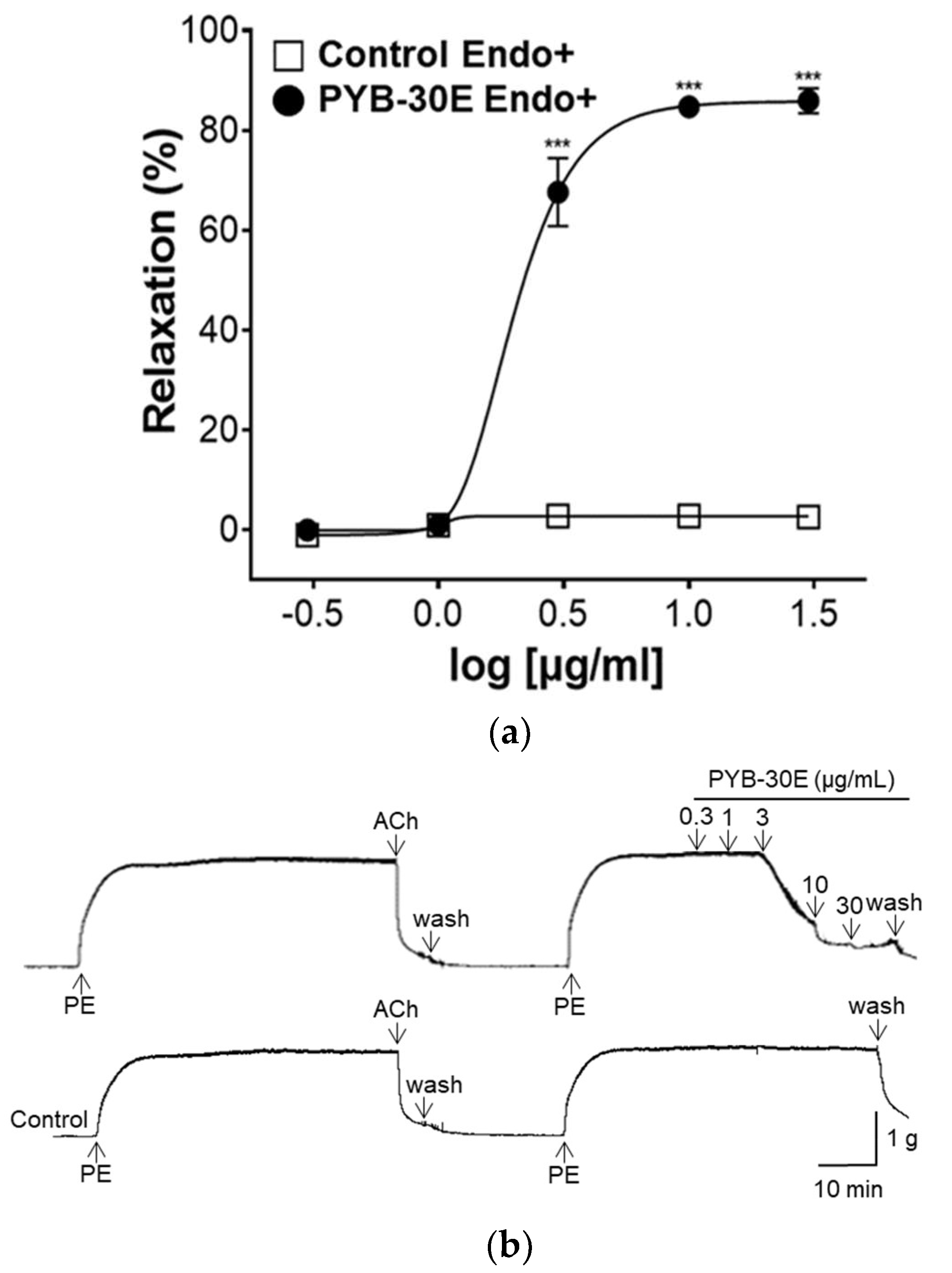
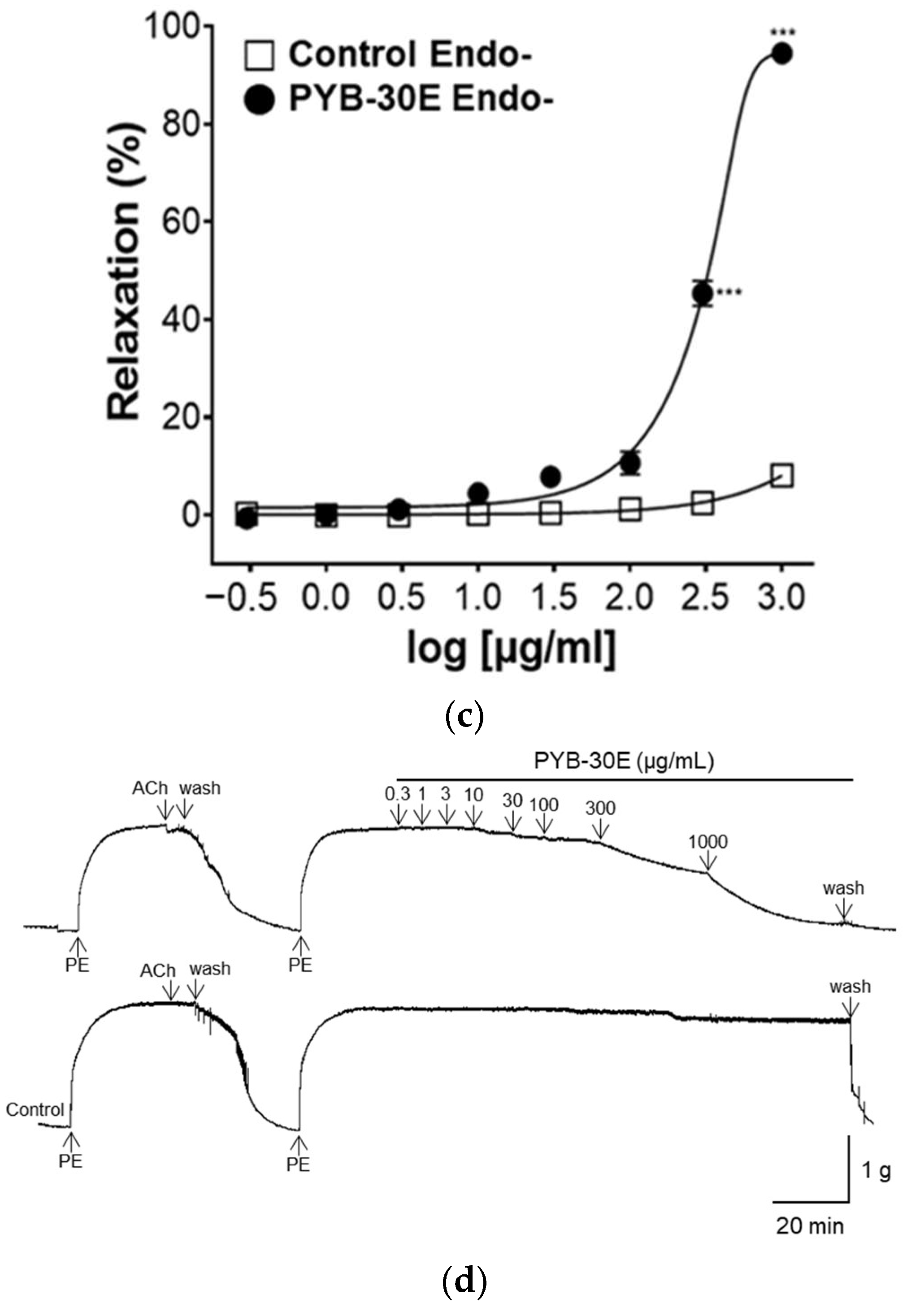
2.3. Effect of PYB-30E on Vascular Relaxation Depending on the Presence or Absence of Vascular Endothelial Cells
2.4. Effect of PYB-30E on the Vasorelaxant Effect of Endothelium-Intact Aortic Rings Precultured with N ω-Nitro-L-arginine Methyl Ester (L-NAME), Indomethacin, 1-H-[1,2,4]-Oxadiazolo-[4,3-α]-quinoxalin-1-one (ODQ), and Methylene Blue (MB)
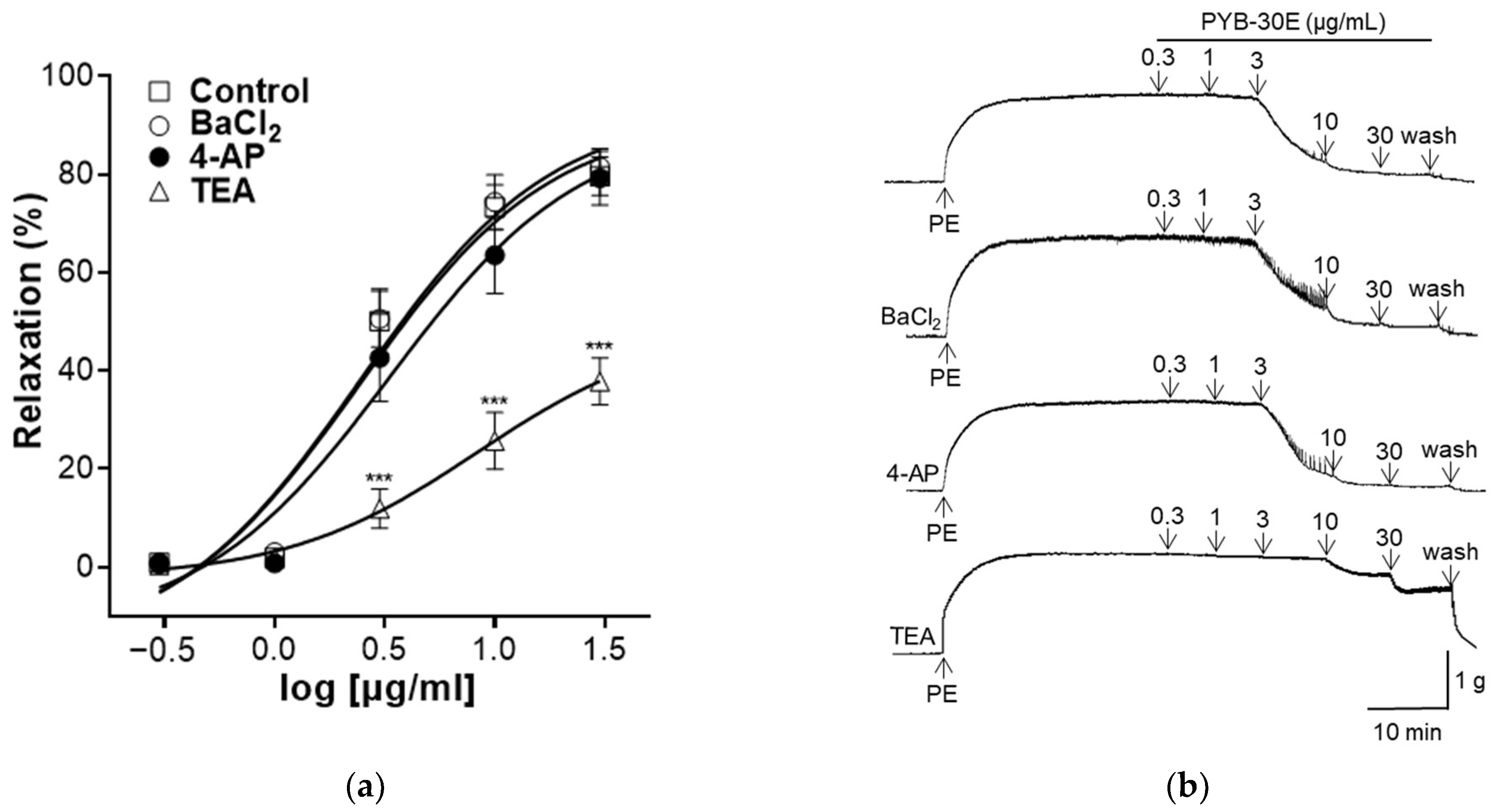
2.5. Effect of PYB-30E on Vasorelaxant Effect of Intact Endothelium Aortic Rings Pre-Incubated with Barium Chloride (BaCl2), 4-Aminopyridine (4-AP) and Tetraethylammonium Chloride (TEA)
2.6. Effect of PYB-30E on Vasorelaxant Effect on Extracellular Ca2+-Induced Contraction
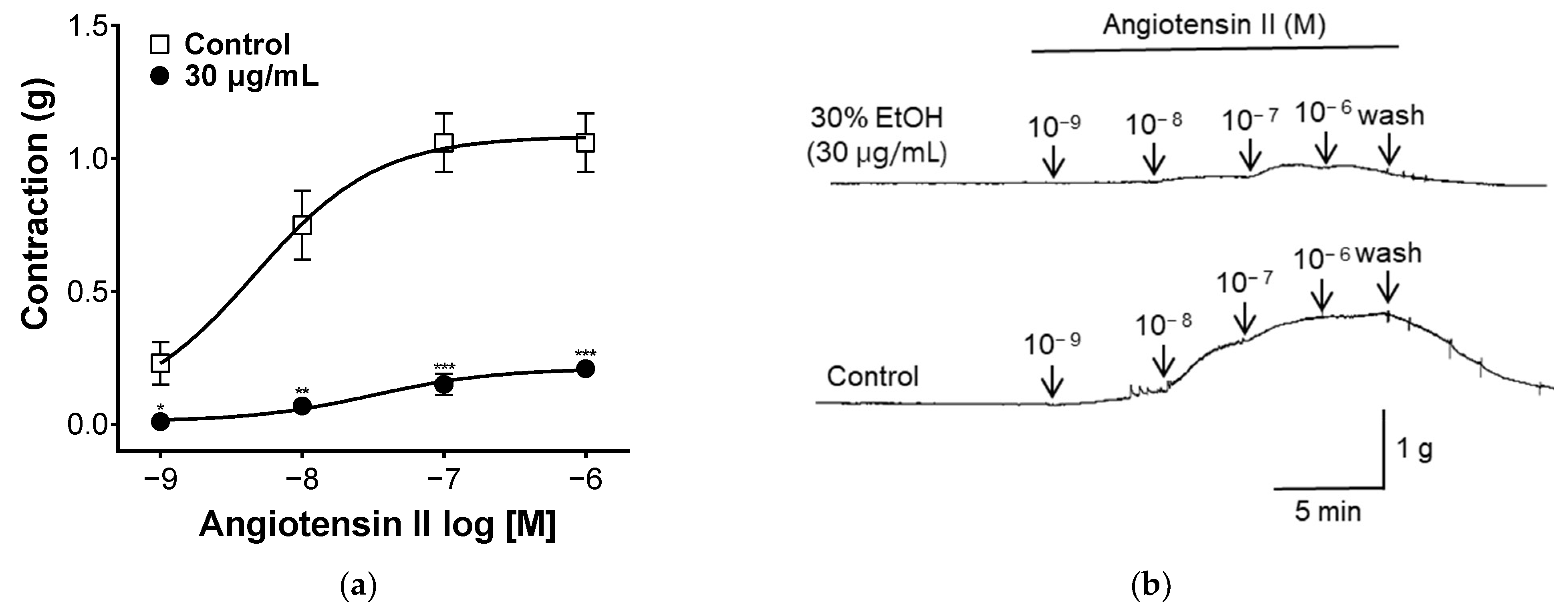
2.7. Effect of PYB-30E on Angiotensin II-Induced Vasoconstriction
2.8. Effect of PYB-30E on Lowering Blood Pressure in Spontaneously Hypertensive Rat (SHR)
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Sample Preparation
4.3. Cell Culture
4.4. Cell Viability
4.5. Western Blot
4.6. Animals and Experimental Design
4.7. Fabrication and Preparation of Rat Thoracic Aortic Rings
4.8. Measurement of Vascular Relaxation for Endothelium-Intact and -Denuded Aortic Rings
4.9. Confirmation of Vascular Relaxation Mechanism of Vascular Endothelium
4.10. Measurements of Vascular Relaxation in Aortic Rings Treated with K+ Channel Blockers
4.11. Measurement of Vasoconstriction by Extracellular Ca2+-Induced Contraction
4.12. Measurement of Vascular Relaxation Caused by Ang II Treatment
4.13. Measurement of Blood Pressure Drop in SHR
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Intengan, H.D.; Schiffrin, E.L. Structure and Mechanical Properties of Resistance Arteries in Hypertension: Role of Adhesion Molecules and Extracellular Matrix Determinants. Hypertension 2000, 36, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Endemann, D.H.; Schiffrin, E.L. Endothelial Dysfunction. J. Am. Soc. Nephrol. 2004, 15, 1983–1992. [Google Scholar] [CrossRef]
- Kearney, P.M.; Whelton, M.; Reynolds, K.; Muntner, P.; Whelton, P.K.; He, J. Global Burden of Hypertension: Analysis of Worldwide Data. Lancet 2005, 365, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, Z.; Zhang, L.; Wang, X.; Hao, G.; Zhang, Z.; Shao, L.; Tian, Y.; Dong, Y.; Zheng, C. Status of Hypertension in China: Results from the China Hypertension Survey, 2012–2015. Circulation 2018, 137, 2344–2356. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yuan, Y.; Zheng, M.; Pan, A.N.; Wang, M.; Zhao, M.; Li, Y.; Yao, S.; Chen, S.; Wu, S. Association of Age of Onset of Hypertension with Cardiovascular Diseases and Mortality. J. Am. Coll. Cardiol. 2020, 75, 2921–2930. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, R.; Dave, J.M.; Chandran, R.R.; Misra, A.; Sheikh, A.Q.; Greif, D.M. Vascular Cells in Blood Vessel Wall Development and Disease. Adv. Pharmacol. 2017, 78, 323–350. [Google Scholar] [PubMed]
- Cines, D.B.; Pollak, E.S.; Buck, C.A.; Loscalzo, J.; Zimmerman, G.A.; McEver, R.P.; Pober, J.S.; Wick, T.M.; Konkle, B.A.; Schwartz, B.S. Endothelial Cells in Physiology and in the Pathophysiology of Vascular Disorders. Blood. J. Am. Soc. Hematol. 1998, 91, 3527–3561. [Google Scholar]
- Verwoert, G.C.; Franco, O.H.; Hoeks, A.P.; Reneman, R.S.; Hofman, A.; Duijn, C.M.V.; Sijbrands, E.J.; Witteman, J.C.; Mattace-Raso, F.U. Arterial Stiffness and Hypertension in a Large Population of Untreated Individuals: The Rotterdam Study. J. Hypertens 2014, 32, 1606–1612. [Google Scholar] [CrossRef]
- Gebreyohannes, E.A.; Bhagavathula, A.S.; Abebe, T.B.; Tefera, Y.G.; Abegaz, T.M. Adverse Effects and Non-Adherence to Antihypertensive Medications in University of Gondar Comprehensive Specialized Hospital. Clin. Hypertens. 2019, 25, 1–9. [Google Scholar] [CrossRef]
- Albasri, A.; Hattle, M.; Koshiaris, C.; Dunnigan, A.; Paxton, B.; Fox, S.E.; Smith, M.; Archer, L.; Levis, B.; Payne, R.A. Association between Antihypertensive Treatment and Adverse Events: Systematic Review and Meta-Analysis. BMJ 2021, 372, n189. [Google Scholar] [CrossRef]
- Cheng, S.; McBride, J.R.; Fukunari, K. The Urban Forest of Tokyo. Arboric. J. 1999, 23, 379–392. [Google Scholar] [CrossRef]
- Roh, M.S.; Cheong, E.J.; Choi, I.; Joung, Y.H. Characterization of Wild Prunus Yedoensis Analyzed by Inter-Simple Sequence Repeat and Chloroplast DNA. Sci. Hortic. 2007, 114, 121–128. [Google Scholar] [CrossRef]
- Innan, H.; Terauchi, R.; Miyashita, N.T.; Tsunewaki, K. DNA Fingerprinting Study on the Intraspecific Variation and the Origin of Prunus Yedoensis (Someiyoshino). Jpn. J. Genet. 1995, 70, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Cho, J.; Pyo, B.; Kim, S.; Lee, K. Comparison of the Physiological Activities of Extracts from Different Parts of Prunus Sargentii. Korean J. Med. Crop Sci. 2012, 20, 159–164. [Google Scholar] [CrossRef]
- Ahn, D.K. Illustrated Book of Korean Medicinal Herbs; Kyo-hak Publishing Co: Seoul, Republic of Korea, 1998; p. 107. [Google Scholar]
- Yang, G.; Ham, I.; Choi, H. Anti-Inflammatory Effect of Prunetin Via the Suppression of NF-κB Pathway. Food Chem. Toxicol. 2013, 58, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.; Chang, H.; Wang, G.; Chiang, M.Y.; Huang, H.; Chen, C.; Tsai, S.; Lin, C.; Chen, I. Secondary Metabolites from the Roots of Neolitsea Daibuensis and their Anti-Inflammatory Activity. J. Nat. Prod. 2011, 74, 2489–2496. [Google Scholar] [CrossRef]
- Ahn, T.; Yang, G.; Lee, H.; Kim, M.; Choi, H.; Park, K.; Lee, S.; Kook, Y.; An, H. Molecular Mechanisms Underlying the Anti-Obesity Potential of Prunetin, an O-Methylated Isoflavone. Biochem. Pharmacol. 2013, 85, 1525–1533. [Google Scholar] [CrossRef]
- Khan, K.; Pal, S.; Yadav, M.; Maurya, R.; Trivedi, A.K.; Sanyal, S.; Chattopadhyay, N. Prunetin Signals Via G-Protein-Coupled Receptor, GPR30 (GPER1): Stimulation of Adenylyl Cyclase and cAMP-Mediated Activation of MAPK Signaling Induces Runx2 Expression in Osteoblasts to Promote Bone Regeneration. J. Nutr. Biochem. 2015, 26, 1491–1501. [Google Scholar] [CrossRef]
- Ibarra-Alvarado, C.; Rojas, A.; Luna, F.; Rojas, J.I.; Rivero-Cruz, B.; Rivero-Cruz, J.F. Vasorelaxant Constituents of the Leaves of Prunus Serotina “capulín”. Rev. Latinoam. Quim 2009, 37, 164–173. [Google Scholar]
- Luna-Vázquez, F.J.; Ibarra-Alvarado, C.; Rojas-Molina, A.; Romo-Mancillas, A.; López-Vallejo, F.H.; Solís-Gutiérrez, M.; Rojas-Molina, J.I.; Rivero-Cruz, F. Role of Nitric Oxide and Hydrogen Sulfide in the Vasodilator Effect of Ursolic Acid and Uvaol from Black Cherry Prunus Serotina Fruits. Molecules 2016, 21, 78. [Google Scholar] [CrossRef]
- Jo, C.; Kim, B.; Lee, S.; Ham, I.; Lee, K.; Choi, H. Vasorelaxant Effect of Prunus Mume (Siebold) Siebold & Zucc. Branch through the Endothelium-Dependent Pathway. Molecules 2019, 24, 3340. [Google Scholar] [CrossRef] [PubMed]
- Jo, C.; Kim, B.; Lee, K.; Choi, H. Vascular Relaxation and Blood Pressure Lowering Effects of Prunus Mume in Rats. Bioengineering 2023, 10, 74. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Jo, C.; Choi, H.; Lee, K. Prunetin Relaxed Isolated Rat Aortic Rings by Blocking Calcium Channels. Molecules 2018, 23, 2372. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Ham, I.; Yang, G.; Lee, M.; Bu, Y.; Kim, H.; Choi, H. Vasorelaxant Effect of Prunus yedoensis Bark. BMC Complement. Altern. Med. 2013, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Steyers III, C.M.; Miller Jr, F.J. Endothelial Dysfunction in Chronic Inflammatory Diseases. Int. J. Mol. Sci. 2014, 15, 11324–11349. [Google Scholar] [CrossRef] [PubMed]
- Landmesser, U.; Hornig, B.; Drexler, H. Endothelial Function: A Critical Determinant in Atherosclerosis? Circulation 2004, 109, II–33. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, I. Inherited Bleeding Disorders: Disorders of Platelet Adhesion and Aggregation. Crit. Revies Oncol./Hematol. 2004, 49, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Mackman, N. New Insights into the Mechanisms of Venous Thrombosis. J. Clin. Investig. 2012, 122, 2331–2336. [Google Scholar] [CrossRef] [PubMed]
- Lüscher, T.F.; Barton, M. Biology of the Endothelium. Clin. Cardiol. 1997, 20, II–10. [Google Scholar] [CrossRef] [PubMed]
- Lincoln, T.M.; Cornwell, T.L. Intracellular Cyclic GMP Receptor Proteins. FASEB J. 1993, 7, 328–338. [Google Scholar] [CrossRef]
- Zhao, Y.; Brandish, P.E.; Ballou, D.P.; Marletta, M.A. A Molecular Basis for Nitric Oxide Sensing by Soluble Guanylate Cyclase. Proc. Natl. Acad. Sci. USA 1999, 96, 14753–14758. [Google Scholar] [CrossRef] [PubMed]
- Wise, H. Multiple Signalling Options for Prostacyclin. Acta Pharmacol. Sin. 2003, 24, 625–630. [Google Scholar]
- Jackson, W.F. Potassium Channels in the Peripheral Microcirculation. Microcirculation 2005, 12, 113–127. [Google Scholar] [CrossRef]
- Jackson, W.F. Ion Channels and Vascular Tone. Hypertension 2000, 35, 173–178. [Google Scholar] [CrossRef]
- Nelson, M.T.; Quayle, J.M. Physiological Roles and Properties of Potassium Channels in Arterial Smooth Muscle. American J. Physiol.-Cell Physiol. 1995, 268, C799–C822. [Google Scholar] [CrossRef] [PubMed]
- Lipskaia, L.; del Monte, F.; Capiod, T.; Yacoubi, S.; Hadri, L.; Hours, M.; Hajjar, R.J.; Lompré, A. Sarco/Endoplasmic Reticulum Ca2-ATPase Gene Transfer Reduces Vascular Smooth Muscle Cell Proliferation and Neointima Formation in the Rat. Circ. Res. 2005, 97, 488–495. [Google Scholar] [CrossRef]
- Murphy, T.J.; Alexander, R.W.; Griendling, K.K.; Runge, M.S.; Bernstein, K.E. Isolation of a cDNA Encoding the Vascular Type-1 Angiotensin II Receptor. Nature 1991, 351, 233–236. [Google Scholar] [CrossRef]
- Sokolova, I.A.; Manukhina, E.B.; Blinkov, S.M.; Koshelev, V.B.; Pinelis, V.G.; Rodionov, I.M. Rarefication of the Arterioles and Capillary Network in the Brain of Rats with Different Forms of Hypertension. Microvasc. Res. 1985, 30, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Labat, C.; Cunha, R.S.; Challande, P.; Safar, M.E.; Lacolley, P. Respective Contribution of Age, Mean Arterial Pressure, and Body Weight on Central Arterial Distensibility in SHR. Am. J. Physiol.-Heart Circ. Physiol. 2006, 290, H1534–H1539. [Google Scholar] [CrossRef]
- Yun, J.; Im, S.; Roh, M.; Park, S.; Kwon, H.; Lee, J.; Choi, H.; Ham, I.; Kim, Y.B.; Lee, J. Prunus Yedoensis Bark Inhibits Lipopolysaccharide-Induced Inflammatory Cytokine Synthesis by IκBα Degradation and MAPK Activation in Macrophages. J. Med. Food 2014, 17, 407–413. [Google Scholar] [CrossRef]
- Kang, H.; Kwak, T.; Kim, B.; Lee, K. The Anti-Inflammatory Effect of Prunus Yedoensis Bark Extract on Adipose Tissue in Diet-Induced Obese Mice. Evid.-Based Complement. Altern. Med. 2015, 2015, 937904. [Google Scholar] [CrossRef]
- Berman, J.W.; Calderon, T.M. The Role of Endothelial Cell Adhesion Molecules in the Development of Atherosclerosis. Cardiovasc. Pathol. 1992, 1, 17–28. [Google Scholar] [CrossRef]
- Sun, H.; Wu, Z.; Nie, X.; Bian, J. Role of Endothelial Dysfunction in Cardiovascular Diseases: The Link between Inflammation and Hydrogen Sulfide. Front. Pharmacol. 2020, 10, 493254. [Google Scholar] [CrossRef]
- Sans, M.; Panés, J.; Ardite, E.; Elizalde, J.I.; Arce, Y.; Elena, M.; Palacín, A.; Fernández–Checa, J.C.; Anderson, D.C.; Lobb, R. VCAM-1 and ICAM-1 Mediate Leukocyte-Endothelial Cell Adhesion in Rat Experimental Colitis. Gastroenterology 1999, 116, 874–883. [Google Scholar] [CrossRef]
- Woollard, K.J.; Geissmann, F. Monocytes in Atherosclerosis: Subsets and Functions. Nat. Rev. Cardiol. 2010, 7, 77–86. [Google Scholar] [CrossRef]
- Lino, D.; Freitas, I.A.; Meneses, G.C.; Martins, A.; Daher, E.F.; Rocha, J.; Silva Junior, G.B. Interleukin-6 and Adhesion Molecules VCAM-1 and ICAM-1 as Biomarkers of Post-Acute Myocardial Infarction Heart Failure. Braz. J. Med. Biol. Res. 2019, 52, e8658. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, D.M.; Mircea, P.A.; Bala, C.; Rusu, A.; Vesa, Ş.; Roman, G. Intercellular Adhesion Molecule-1 (ICAM-1) Associates with 24-Hour Ambulatory Blood Pressure Variability in Type 2 Diabetes and Controls. Cytokine 2019, 116, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Sultana, B.; Anwar, F.; Ashraf, M. Effect of Extraction Solvent/Technique on the Antioxidant Activity of Selected Medicinal Plant Extracts. Molecules 2009, 14, 2167–2180. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, T.; Choi, Y.; Kim, Y. Impact of Different Extraction Solvents on Phenolic Content and Antioxidant Potential of Pinus Densiflora Bark Extract. BioMed Res. Int. 2019, 2019, 3520675. [Google Scholar] [CrossRef]
- Bernátová, I.; Pechánová, O.; Babál, P.; Kyselá, S.; Stvrtina, S.; Andriantsitohaina, R. Wine Polyphenols Improve Cardiovascular Remodeling and Vascular Function in NO-Deficient Hypertension. Am. J. Physiol.-Heart Circ. Physiol. 2002, 282, H942–H948. [Google Scholar] [CrossRef]
- Schini-Kerth, V.B.; Auger, C.; Kim, J.; Étienne-Selloum, N.; Chataigneau, T. Nutritional Improvement of the Endothelial Control of Vascular Tone by Polyphenols: Role of NO and EDHF. Pflügers Arch.-Eur. J. Physiol. 2010, 459, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Nicosia, R.F. The Aortic Ring Model of Angiogenesis: A Quarter Century of Search and Discovery. J. Cell. Mol. Med. 2009, 13, 4113–4136. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, A.; Chen, C.G.; Iozzo, R.V. A Simplified Aortic Ring Assay: A Useful Ex Vivo Method to Assess Biochemical and Functional Parameters of Angiogenesis. Matrix Biol. Plus 2020, 6, 100025. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Fukuda, N.; Yao, E.; Matsumoto, T.; Kobayashi, N.; Suzuki, R.; Tahira, Y.; Ueno, T.; Matsumoto, K. Effects of an ARB on Endothelial Progenitor Cell Function and Cardiovascular Oxidation in Hypertension. Am. J. Hypertens. 2008, 21, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Durier, S.; Fassot, C.; Laurent, S.; Boutouyrie, P.; Couetil, J.; Fine, E.; Lacolley, P.; Dzau, V.J.; Pratt, R.E. Physiological Genomics of Human Arteries: Quantitative Relationship between Gene Expression and Arterial Stiffness. Circulation 2003, 108, 1845–1851. [Google Scholar] [CrossRef] [PubMed]
- Feletou, M.; Tang, E.H.; Vanhoutte, P.M. Nitric Oxide the Gatekeeper of Endothelial Vasomotor Control. Front. Biosci. 2008, 13, 4198–4217. [Google Scholar] [CrossRef] [PubMed]
- Kang, K. Endothelium-Derived Relaxing Factors of Small Resistance Arteries in Hypertension. Toxicol. Res. 2014, 30, 141–148. [Google Scholar] [CrossRef]
- Tang, E.H.; Vanhoutte, P.M. Prostanoids and Reactive Oxygen Species: Team Players in Endothelium-Dependent Contractions. Pharmacol. Ther. 2009, 122, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.; Garcia, T.; Aniqa, M.; Ali, S.; Ally, A.; Nauli, S.M. Endothelial Nitric Oxide Synthase (eNOS) and the Cardiovascular System: In Physiology and in Disease States. Am. J. Biomed. Sci. Res. 2022, 15, 153. [Google Scholar]
- Motley, E.D.; Eguchi, K.; Patterson, M.M.; Palmer, P.D.; Suzuki, H.; Eguchi, S. Mechanism of Endothelial Nitric Oxide Synthase Phosphorylation and Activation by Thrombin. Hypertensin 2007, 49, 577–583. [Google Scholar] [CrossRef]
- Kukovetz, W.R.; Holzmann, S.; Wurm, A.; Pöch, G. Prostacyclin Increases cAMP in Coronary Arteries. J. Cycl. Nucleotide Res. 1979, 5, 469–476. [Google Scholar]
- Vanhoutte, P. Endothelium-Derived Relaxing Factors. Eur. J. Med. Chem. 1995, 30, 361s–370s. [Google Scholar] [CrossRef]
- Dorris, S.L.; Peebles, R.S., Jr. PGI2 as a Regulator of Inflammatory Diseases. Mediat. Inflamm. 2012, 2012, 926968. [Google Scholar] [CrossRef]
- Lincoln, T.M.; Cornwell, T.L. Towards an Understanding of the Mechanism of Action of Cyclic AMP and Cyclic GMP in Smooth Muscle Relaxation. J. Vasc. Res. 1991, 28, 129–137. [Google Scholar] [CrossRef]
- Glorian, M.; Limon, I. The Role of Cyclic 3′-5′adenosine Monophosphate (Camp) in Differentiated and Trans-Differentiated Vascular Smooth Muscle Cells. In Current Trends in Atherogenesis; BoD—Books on Demand GmbH: Norderstedt, Germany, 2013; Volume 121. [Google Scholar]
- Sorensen, C.M.; Braunstein, T.H.; Holstein-Rathlou, N.; Salomonsson, M. Role of Vascular Potassium Channels in the Regulation of Renal Hemodynamics. Am. J. Physiol.-Ren. Physiol. 2012, 302, F505–F518. [Google Scholar] [CrossRef]
- Jackson, W.F. Potassium Channels in Regulation of Vascular Smooth Muscle Contraction and Growth. Adv. Pharmacol. 2017, 78, 89–144. [Google Scholar]
- Jackson, W.F. KV Channels and the Regulation of Vascular Smooth Muscle Tone. Microcirculation 2018, 25, e12421. [Google Scholar] [CrossRef]
- McFadzean, I.; Gibson, A. The Developing Relationship between Receptor-operated and Store-operated Calcium Channels in Smooth Muscle. Br. J. Pharmacol. 2002, 135, 1–13. [Google Scholar] [CrossRef]
- Wynne, B.M.; Chiao, C.; Webb, R.C. Vascular Smooth Muscle Cell Signaling Mechanisms for Contraction to Angiotensin II and Endothelin-1. J. Am. Soc. Hypertens. 2009, 3, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Cohn, J.N. Arterial Compliance to Stratify Cardiovascular Risk: More Precision in Therapeutic Decision Making. Am. J. Hypertens. 2001, 14, 258S–263S. [Google Scholar] [CrossRef]
- MacGregor, G.A.; He, F.J. Importance of controlling blood pressure. Climacteric 2005, 8 (Suppl. S3), 13–18. [Google Scholar] [CrossRef] [PubMed]
- Basile, J. The importance of prompt blood pressure control. J. Clin. Hypertens. 2008, 10, 13–19. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.E.; Yang, J.M.; Jeong, C.W.; Shin, S.; Park, J.; Lee, K.; Cho, J.H. Prunus yedoensis Bark Downregulates the Expression of Cell Adhesion Molecules in Human Endothelial Cell Lines and Relaxes Blood Vessels in Rat Aortic Rings. Pharmaceuticals 2024, 17, 926. https://doi.org/10.3390/ph17070926
Choi YE, Yang JM, Jeong CW, Shin S, Park J, Lee K, Cho JH. Prunus yedoensis Bark Downregulates the Expression of Cell Adhesion Molecules in Human Endothelial Cell Lines and Relaxes Blood Vessels in Rat Aortic Rings. Pharmaceuticals. 2024; 17(7):926. https://doi.org/10.3390/ph17070926
Chicago/Turabian StyleChoi, Ye Eun, Jung Mo Yang, Chae Won Jeong, Sujin Shin, Junkyu Park, Kyungjin Lee, and Ju Hyun Cho. 2024. "Prunus yedoensis Bark Downregulates the Expression of Cell Adhesion Molecules in Human Endothelial Cell Lines and Relaxes Blood Vessels in Rat Aortic Rings" Pharmaceuticals 17, no. 7: 926. https://doi.org/10.3390/ph17070926
APA StyleChoi, Y. E., Yang, J. M., Jeong, C. W., Shin, S., Park, J., Lee, K., & Cho, J. H. (2024). Prunus yedoensis Bark Downregulates the Expression of Cell Adhesion Molecules in Human Endothelial Cell Lines and Relaxes Blood Vessels in Rat Aortic Rings. Pharmaceuticals, 17(7), 926. https://doi.org/10.3390/ph17070926





