Impact of Anti-CD38 Monoclonal Antibody Therapy on CD34+ Hematopoietic Stem Cell Mobilization, Collection, and Engraftment in Multiple Myeloma Patients—A Systematic Review
Abstract
:- ∘
- Lower peaks of circulating CD34+ cells after mobilization;
- ∘
- Higher use of plerixafor and longer mobilization procedures;
- ∘
- Lower CD34+ cell collection yields;
- ∘
- Slower hematopoietic recovery after autologous transplant.
1. Introduction
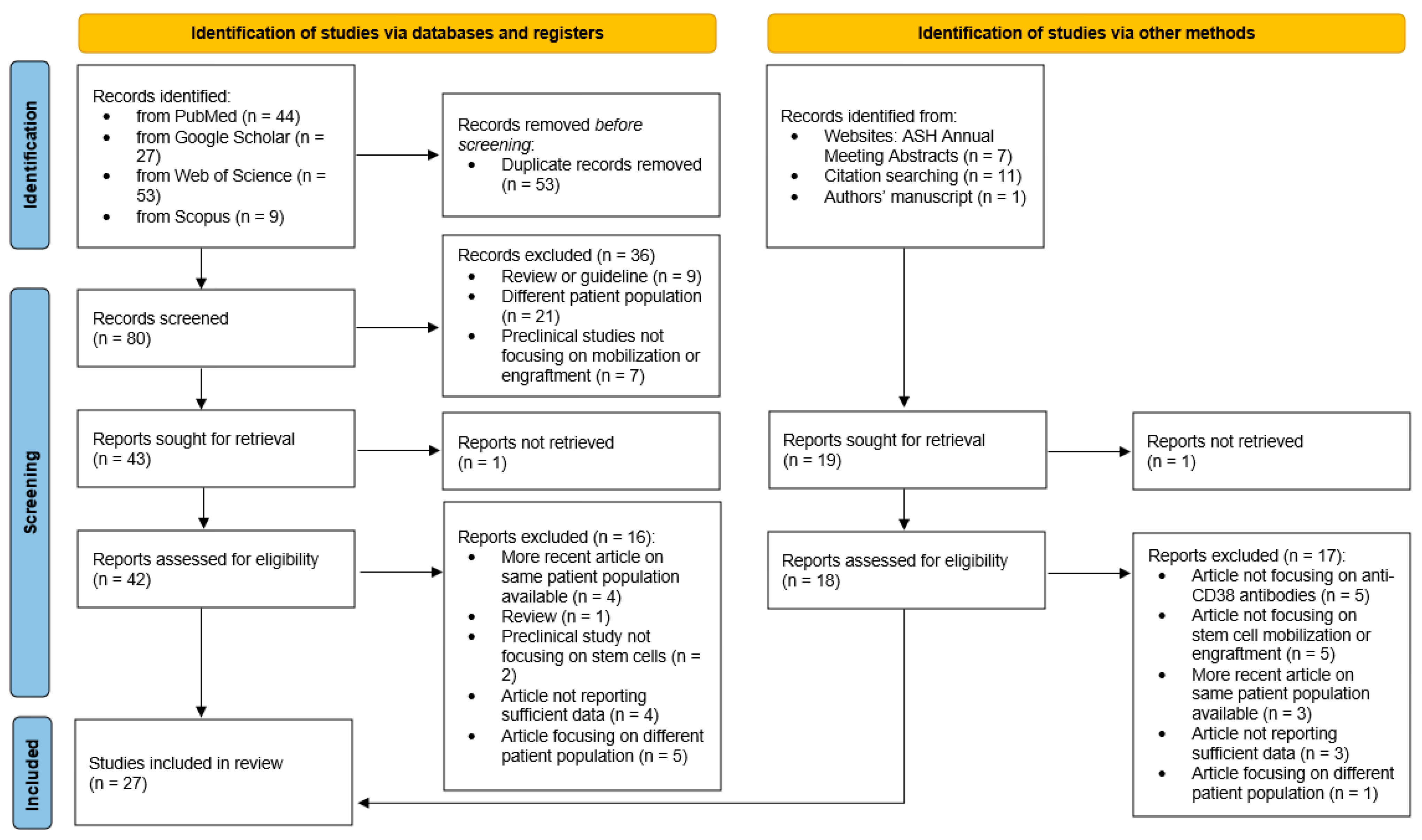
2. Methods
3. Results
3.1. Characteristics of the Studies
| First Author, EU/SA/US [Reference] | Anti-CD38 MoAb-Treated Patients (nr) | Induction Quadruplet | Mobilization Therapy | Plerixafor Strategy | Collection Goal (CD34+ Cells × 106/kg) |
|---|---|---|---|---|---|
| Studies with a non-anti-CD38 MoAb-treated control group | |||||
| Al Saleh, US [29] | 12 | DIRd or DVCd | G-CSF 10 μg/kg/d | ns | |
| Bigi, EU [18] | 44 | DVTd or DVCd | CY 2–3 g/sqm + G-CSF 10 μg/kg/d | rescue | 3–6 |
| Cavallaro, EU [31] | 109 | DVTd | CY 1–3 g/sqm + G-CSF 10 μg/kg/d | rescue | ns |
| Chaabra, US [23] (GRIFFIN) | 95 | DVRd | G-CSF | rescue or upfront | 2–5 |
| Edmisson, US [32] | 58 | DVRd | G-CSF | upfront | 5 |
| Fazio, EU [33] | 28 | DVTd | CY 2.4–3 g/sqm + G-CSF 10 μg/kg/d | rescue | ns |
| Hulin, EU [27] (CASSIOPEIA) | 506 | DVTd | CY 2–3 g/sqm + G-CSF 10 μg/kg/d | rescue | ns |
| Kauer, EU [40] | 35 | IVRd | CAD or CY 2 g/sqm + G-CSF 10 μg/kg/d | rescue | 6 |
| Lemonakis, EU [34] | 92 | DVTd or DVRd | CY + G-CSF | rescue | 4 |
| Luan, US [36] | 16 | ns | G-CSF | rescue | ns |
| Manjappa, US [21] | 16 | ns | G-CSF | ns | ns |
| Mina, EU [42] | 57 | DVTd | G-CSF 10 μg/kg/d | rescue | 4 |
| Oza, US [44] | 47 | ns | CdE + G-CSF or G-CSF | rescue or upfront | 8–12 |
| Papaiakovou, EU [37] | 40 | ns | CY 2.5 g/sqm + G-CSF 10 μg/kg/d | rescue | 5 |
| Sauer, EU [38] | 58 | DVTd | CAD or CY 2 g/sqm + G-CSF 10 μg/kg/d | rescue | 6 |
| Thurlapati, US [39] | 43 | DVRd | G-CSF 6 μg/kg/d | upfront | 2.5–5 |
| Unis, US [41] | 62 | ns | Ns | ns | ns |
| Venglar, EU [20] | 20 | DVCd or IKRd | CY 2.5 g/sqm + G-CSF 10 μg/kg/d | rescue | 5 |
| Zappaterra, EU [19] | 20 | DVTd, DVCd or DRd | CY (2–3) + G-CSF 5 μg/kg/d | rescue | 6 |
| Studies without a non-anti-CD38 MoAb-treated control group | |||||
| Bhutani, US [28] (LCI-HEM-MYE-KRdD-001) | 22 | DKRd | G-CSF 10 μg/kg/d | rescue | 8–12 |
| Bourlon, EU [30] | 95 | DVTd | CY 1.5 g/sqm + G-CSF 5 μg/kg/d | rescue | 2 |
| Chaabra, US [23] (MASTER) | 116 | DKRd | G-CSF 10 μg/kg/d | rescue or upfront | 2–12 |
| Crusoe, SA [43] | 21 | DCTd | G-CSF | rescue | 2.5 |
| Liberatore, EU [35] | 47 | DVTd | CY 4 g/sqm + G-CSF 5 μg/kg/d | rescue | 10 |
| First Author [Reference] | First Day Yield (Median, CD34+ Cells × 106/kg) | Total Yield (Median, CD34+ Cells × 106/kg) | Circulating CD34+ Cells (Median, /µL), (b) | Plerixafor Use (%) | Target Failure (%) | Days of Apheresis (Median) |
|---|---|---|---|---|---|---|
| Studies with a non-anti-CD38 MoAb-treated control group | ||||||
| vs. controls | vs. controls | vs. controls | vs. controls | vs. controls | vs. controls | |
| Al Saleh [29] | ns | ns | ns | ns | ns | ns |
| Bigi [18] | 3.5 vs. 5.92 | 6.7 vs. 8.03 | 21 vs. 81 | 52 vs. 20 | 16 vs. 16 | 1.9 vs. 1.7 |
| Cavallaro [31] | ns | 5.2 vs. 8.7 | 26 vs. 76 | 50 vs. 14 | ns | ns |
| Chaabra [23] (GRIFFIN) | ns | 8.3 vs. 9.4 | ns | 41 vs. 27 (d) | 2 vs. 6 | 2 vs. 1 |
| Edmisson [32] | 6.0 vs. 10.6 | ns | 57 vs. 96 (a) | ns | 14 vs. 3 | 1 vs. 1 |
| Fazio [33] | ns | 9 vs. 9 | 44 vs. 98 (b) | 29 vs. 13 | 32 vs. 6 | ns |
| Hulin [27] (CASSIOPEIA) | ns | 6.7 vs. 10.0 * | ns | 22 vs. 8 | ns | 1.9 vs. 1.4 * |
| Kauer [40] | 5.8 vs. 7.6 * | 8.8 vs. 9.7 * | 80 vs. 116 * | 34 vs. 16 | 0 vs. 5 | 2 vs. 1 |
| Lemonakis [34] | ns | 5.1 vs. 7.2 * | ns | 37 vs. 6 | 24 vs. 14 | 2 vs. 1 * |
| Luan [36] | ns | 8 vs. 10 | 17.2 vs. 35 | 94 vs. 69 | ns | 2.4 vs. 1.6 |
| Manjappa [21] | ns | 7.2 vs. 8.8 | ns | ns | ns | ns |
| Mina [42] | ns | 7.1 vs. 7.9 | 19 vs. 24 | 53 vs. 28 | ns | 2 vs. 1 |
| Oza [44] | ns | 9.3 vs. 11.8 | ns | ns | 55 vs. 27 | 3 vs. 2 |
| Papaiakovou [37] | 8 vs. 16 | 10.5 vs. 16.6 | ns | 42 vs. 8 | 12.5 vs. 3.8 | ns |
| Sauer [38] | 5.5 vs. 8.3 | 8.4 vs. 9.6 | 65 vs. 106 * | 33 vs. 21 | 21 vs. 3 | 2 vs. 1 |
| Thurlapati [39] | 4.9 vs. 6.1 | 6.5 vs. 6.8 | 43 vs. 63 (a) | 95 vs. 95 | ns | 1 vs. 1 |
| Unis [41] | ns | 5.3 vs. 6.7 | ns | ns | ns | 1.4 vs. 1.3 |
| Venglar [20] | ns | 10.6 vs. 13.2 | 63 vs. 128 (b) | 28 vs. 0 | 39 vs. 0 | ns |
| Zappaterra [19] | 3.9 vs. 6.9 | 4.0 vs. 6.9 | 39 vs. 64 | 20 vs. 5 | 0 vs. 0 | 2 vs. 1 |
| Studies without a non-anti-CD38 MoAb-treated control group | ||||||
| Bhutani [28] (LCI-HEM-MYE-KRdD-001) | ns | 7.7 | 4.2 | 82 | 77 | 1 |
| Bourlon [30] | ns | 4.5 | 29.2 | 22 | 15 | ns |
| Chaabra [23] (MASTER) | ns | 6 | ns | 88 | ns | 2 |
| Crusoe [43] | ns | 3.9 | ns | 42 (c) | ns | 1 |
| Liberatore [35] | 7 | 10.7 | 57 | 49 | 6 | 1.6 |
| Range of median values in anti-CD38 MoAb-treated patients | ||||||
| Min | 3.5 | 3.9 | 17.2 | 20 | 0 | 1 |
| Max | 8 | 10.7 | 80 * | 95 | 77 | 3 |
| First Author [Reference] | Time to Neutrophil Recovery in Anti-CD38 MoAb-Treated vs. Control Patients, Median (Days) | Time to Platelet Recovery in Anti-CD38 MoAb-Treated vs. Control Patients, Median (Days) |
|---|---|---|
| Al Saleh [29] | 19 vs. 16 | 18 vs. 17 |
| Bigi [18] | 12 vs. 11 | 14 vs. 12 |
| Cavallaro [31] | 13 vs. 11 | 13 vs. 11 |
| Chaabra [23] (GRIFFIN) | 12 vs. ns | 13 vs. ns |
| Crusoe [43] | 11 vs. 11 | 12 vs. 11 |
| Fazio [33] | 14 vs. 11 | 15 vs. 14 |
| Hulin [27] (CASSIOPEIA) | 14.4 vs. 13.7 | 14.9 vs. 13.6 |
| Luan [36] | 12.1 vs. 12.3 | 14.6 vs. 13.7 |
| Manjappa [21] | 12 vs. 12 | 13 vs. 12 |
| Mina [42] | 13 vs. 15 | 14 vs. 16 |
| Oza [44] | 11 vs. 11 | 14 vs. 13 |
| Papaiakovou [37] | 11 vs. 10 | 12 vs. 10 |
| Venglar [20] | 12 vs. 11 | 13 vs. 12 |
| Zappaterra [19] | 9.5 vs. 10 | 10.5 vs. 11 |
3.2. Study Populations
3.3. Mobilization Regimens and Apheresis Targets
3.4. CD34+ Cell Yield
3.5. Circulating CD34+ Cells
3.6. Plerixafor
3.7. Duration of Leukapheresis
3.8. Target Failure
3.9. Hematopoietic Reconstitution after ASCT
3.10. Reports on Isatuximab
3.11. Related Factors: Mobilization Strategies
3.12. Related Factors: Plerixafor Strategy
3.13. Related Factors: Daratumumab Cumulative Dose and Timing
3.14. Other Related Factors
3.15. Molecular Mechanisms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Cavo, M.; Gay, F.; Beksac, M.; Pantani, L.; Petrucci, M.T.; Dimopoulos, M.A.; Dozza, L.; van der Holt, B.; Zweegman, S.; Oliva, S.; et al. Autologous haematopoietic stem-cell transplantation versus bortezomib-melphalan-prednisone, with or without bortezomib-lenalidomide-dexamethasone consolidation therapy, and lenalidomide maintenance for newly diagnosed multiple myeloma (EMN02/HO95): A multicentre, randomised, open-label, phase 3 study. Lancet Haematol. 2020, 7, e456–e468. [Google Scholar] [CrossRef]
- Attal, M.; Lauwers-Cances, V.; Hulin, C.; Leleu, X.; Caillot, D.; Escoffre, M.; Arnulf, B.; Macro, M.; Belhadj, K.; Garderet, L.; et al. Lenalidomide, Bortezomib, and Dexamethasone with Transplantation for Myeloma. N. Engl. J. Med. 2017, 376, 1311–1320. [Google Scholar] [CrossRef]
- Gay, F.; Musto, P.; Rota-Scalabrini, D.; Bertamini, L.; Belotti, A.; Galli, M.; Offidani, M.; Zamagni, E.; Ledda, A.; Grasso, M.; et al. Carfilzomib with cyclophosphamide and dexamethasone or lenalidomide and dexamethasone plus autologous transplantation or carfilzomib plus lenalidomide and dexamethasone, followed by maintenance with carfilzomib plus lenalidomide or lenalidomide alone for patients with newly diagnosed multiple myeloma (FORTE): A randomised, open-label, phase 2 trial. Lancet Oncol. 2021, 22, 1705–1720. [Google Scholar] [CrossRef] [PubMed]
- Moreau, P.; Attal, M.; Hulin, C.; Arnulf, B.; Belhadj, K.; Benboubker, L.; Béné, M.C.; Broijl, A.; Caillon, H.; Caillot, D.; et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): A randomised, open-label, phase 3 study. Lancet 2019, 394, 29–38. [Google Scholar] [CrossRef]
- Costa, L.J.; Chhabra, S.; Medvedova, E.; Dholaria, B.R.; Schmidt, T.M.; Godby, K.N.; Silbermann, R.; Dhakal, B.; Bal, S.; Giri, S.; et al. Minimal residual disease response-adapted therapy in newly diagnosed multiple myeloma (MASTER): Final report of the multicentre, single-arm, phase 2 trial. Lancet Haematol. 2023, 10, e890–e901. [Google Scholar] [CrossRef] [PubMed]
- Voorhees, P.M.; Kaufman, J.L.; Laubach, J.; Sborov, D.W.; Reeves, B.; Rodriguez, C.; Chari, A.; Silbermann, R.; Costa, L.J.; Anderson, L.D., Jr.; et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: The GRIFFIN trial. Blood 2020, 136, 936–945. [Google Scholar] [CrossRef] [PubMed]
- Gay, F.; Roeloffzen, W.; Dimopoulos, M.A.; Rosiñol, L.; van der Klift, M.; Mina, R.; Rocafiguera, A.O.; Katodritou, E.; Wu, K.L.; Otero, P.R.; et al. Results of the Phase III Randomized Iskia Trial: Isatuximab-Carfilzomib-Lenalidomide-Dexamethasone vs Carfilzomib-Lenalidomide-Dexamethasone as Pre-Transplant Induction and Post-Transplant Consolidation in Newly Diagnosed Multiple Myeloma Patients. Blood 2023, 142 (Suppl. S1), 4. [Google Scholar] [CrossRef]
- Pieter, S.; Meletios, A.D.; Mario, B.; Quach, H.; Ho, P.J.; Beksac, M.; Hulin, C.; Antonioli, E.; Leleu, X.; Mangiacavalli, S.; et al. Daratumumab, Bortezomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2024, 390, 301–313. [Google Scholar] [CrossRef]
- Leypoldt, L.B.; Tichy, D.; Besemer, B.; Hänel, M.; Raab, M.S.; Mann, C.; Munder, M.; Reinhardt, H.C.; Nogai, A.; Görner, M.; et al. Isatuximab, Carfilzomib, Lenalidomide, and Dexamethasone for the Treatment of High-Risk Newly Diagnosed Multiple Myeloma. JCO 2024, 42, 26–37. [Google Scholar] [CrossRef]
- Mohty, M.; Avet-Loiseau, H.; Malard, F.; Harousseau, J.L. Potential future direction of measurable residual disease evaluation in multiple myeloma. Blood 2023, 142, 1509–1517. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Moreau, P.; Terpos, E.; Mateos, M.V.; Zweegman, S.; Cook, G.; Delforge, M.; Hájek, R.; Schjesvold, F.; Cavo, M.; et al. Multiple myeloma: EHA-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. J. Clin. Oncol. 2021, 17, 309–322. [Google Scholar] [CrossRef]
- NCCN Guidelines Versione 1.2022 Mieloma multiplo NCCN Guidelines® e Questa Illustrazione non Possono Essere Riprodotte in Nessuna Forma Senza L’espresso Permesso Scritto di NCCN. NCCN Guidelines Index Indice Discussione Divulgazioni del Gruppo di Esperti. 2022. Available online: https://www.nccn.org/ (accessed on 30 November 2021).
- Fitoussi, O.; Perreau, V.; Boiron, J.M.; Bouzigon, E.; Cony-Makhoul, P.; Pigneux, A.; Agape, P.; Nicolini, F.; Dazey, B.; Reiffers, J.; et al. A comparison of toxicity following two different doses of cyclophosphamide for mobilization of peripheral blood progenitor cells in 116 multiple myeloma patients. Bone Marrow Transpl. 2001, 27, 837–842. [Google Scholar] [CrossRef]
- Jantunen, E.; Putkonen, M.; Nousiainen, T.; Pelliniemi, T.T.; Mahlamäki, E.; Remes, K. Low-dose or intermediate-dose cyclophosphamide plus granulocyte colony-stimulating factor for progenitor cell mobilisation in patients with multiple myeloma. Bone Marrow Transpl. 2003, 31, 347–351. [Google Scholar] [CrossRef]
- Hopman, R.K.; DiPersio, J.F. Advances in stem cell mobilization. Blood Rev. 2014, 28, 31–40. [Google Scholar] [CrossRef]
- Lazzaro, C.; Castagna, L.; Lanza, F.; Laszlo, D.; Milone, G.; Pierelli, L.; Saccardi, R. Chemotherapy-based versus chemotherapy-free stem cell mobilization (±plerixafor) in multiple myeloma patients: An Italian cost-effectiveness analysis. Bone Marrow Transpl. 2021, 56, 1876–1887. [Google Scholar] [CrossRef]
- Chandler, T.; Parrish, C.; Karakantza, M.; Carmichael, J.; Pawson, D.; Cook, G.; Seymour, F. A comparison of peripheral blood stem cell collection outcomes for multiple myeloma; mobilization matters in the era of IMiD induction. EJHaem 2023, 4, 625–630. [Google Scholar] [CrossRef]
- Bigi, F.; Tacchetti, P.; Giorgi, A.; Mazzocchetti, G.; Solli, V.; Barbato, S.; Sinigaglia, B.; Campanini, E.; Favero, E.; Talarico, M.; et al. Interference of daratumumab and efficacy of plerixafor on haematopoietic stem cells in Multiple Myeloma patients. Front. Hematol. 2024, 3, 1386973. [Google Scholar] [CrossRef]
- Zappaterra, A.; Civettini, I.; Cafro, A.M.; Pezzetti, L.; Pierini, S.; Anghilieri, M.; Bellio, L.; Bertazzoni, P.; Grillo, G.; Minga, P.; et al. Anti-CD38 monoclonal antibody impairs CD34+ mobilization and affects clonogenic potential in multiple myeloma patients: CD38 antibody impacts on HSC mobilization and clonogenicity. Blood Transfus. 2024; online ahead of print. [Google Scholar] [CrossRef]
- Venglar, O.; Kapustova, V.; Anilkumar Sithara, A.; Zihala, D.; Muronova, L.; Sevcikova, T.; Vrana, J.; Vdovin, A.; Radocha, J.; Krhovska, P.; et al. Insight into the mechanism of CD34+ cell mobilisation impairment in multiple myeloma patients treated with anti-CD38 therapy. Br. J. Haematol. 2023, 204, 1439–1449. [Google Scholar] [CrossRef] [PubMed]
- Manjappa, S.; Fox, R.; Reese, J.; Firoozamand, A.; Schmikla, H.; Nall, S.; Kolk, M.; Caimi, P.F.; Driscoll, J.J.; de Lima, M.; et al. Impact of Daratumumab on Stem Cell Collection, Graft Composition and Engraftment among Multiple Myeloma Patients Undergoing Autologous Stem Cell Transplant. Blood 2020, 136, 35–37. [Google Scholar] [CrossRef]
- Ma, X.; Wong, S.W.; Zhou, P.; Chaulagain, C.P.; Doshi, P.; Klein, A.K.; Sprague, K.; Kugelmass, A.; Toskic, D.; Warner, M.; et al. Daratumumab binds to mobilized CD34+ cells of myeloma patients in vitro without cytotoxicity or impaired progenitor cell growth. Exp. Hematol. Oncol. 2018, 7, 27. [Google Scholar] [CrossRef]
- Chhabra, S.; Callander, N.; Watts, N.L.; Costa, L.J.; Thapa, B.; Kaufman, J.L.; Laubach, J.; Sborov, D.W.; Reeves, B.; Rodriguez, C.; et al. Stem cell mobilization yields with daratumumab and lenalidomide–containing quadruplet induction therapy in newly diagnosed multiple myeloma: Findings from the MASTER and GRIFFIN trials. Transplant. Cell. Ther. 2022, 29, 174.e1–174.e10. [Google Scholar] [CrossRef]
- Mishra, K.; Jandial, A.; Sandal, R.; Lad, D.; Prakash, G.; Khadwal, A.; Malhotra, P. Poor Mobilisation after Daratumumab Based Combination Chemotherapy in Patients of Newly Diagnosed Multiple Myeloma. Indian. J. Hematol. Blood Transfus. 2019, 35, 584–586. [Google Scholar] [CrossRef]
- Seth, A.; Murray, D.; Buadi, F.K.; Gertz, M.A.; Yadav, U.; Kumar, S.K.; Gonsalves, W.I. Failure of mobilization of hematopoietic stem cells associated with elevated serum levels of anti-CD38 monoclonal antibody. Eur. J. Haematol. 2023, 111, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Jain, A. Peri-transplant Stem Cell Kinetics After Daratumumab-Based Induction in Patients with Multiple Myeloma: Response to Mishra et al. Indian J. Hematol. Blood Transfus. 2020, 36, 763–765. [Google Scholar] [CrossRef] [PubMed]
- Hulin, C.; Offner, F.; Moreau, P.; Roussel, M.; Belhadj, K.; Benboubker, L.; Caillot, D.; Facon, T.; Garderet, L.; Kuhnowski, F.; et al. Stem cell yield and transplantation in transplant-eligible newly diagnosed multiple myeloma patients receiving daratumumab + bortezomib/thalidomide/dexamethasone in the phase 3 CASSIOPEIA study. Haematologica 2021, 106, 2257–2260. [Google Scholar] [CrossRef]
- Manisha, B. Stem Cell Mobilization Characteristics for Transplant Eligible Patients with Newly Diagnosed Multiple Myeloma (NDMM) Treated with Carfilzomib, Lenalidomide, Dexamethasone, and Daratumumab (KRd-Dara) | Elsevier Enhanced Reader. Blood 2022, 140 (Suppl. S1), 4393–4395. [Google Scholar] [CrossRef]
- Al Saleh, A.S.; Sidiqi, M.H.; Gertz, M.A.; Muchtar, E.; Lacy, M.Q.; Warsame, R.M.; Gonsalves, W.I.; Kourelis, T.V.; Hogan, W.J.; Hayman, S.R.; et al. Delayed neutrophil engraftment in patients receiving Daratumumab as part of their first induction regimen for multiple myeloma. Am. J. Hematol. 2020, 95, E8–E10. [Google Scholar] [CrossRef] [PubMed]
- Bourlon, C.; Bailey, K.E.; Benjamin, R.; Cuthill, K.; Gunawan, A.; Kazmi, M.; Krishnamurthy, P.; Potter, V.; Sanderson, R.; Kenyon, M.; et al. Real-World Mobilization, Harvest, and Transplant Outcomes in Newly Diagnosed Multiple Myeloma Patients Receiving D-VTd: A UK Centre Experience. Blood 2023, 142, 4971. [Google Scholar] [CrossRef]
- Cavallaro, G.; Galli, M.; Paris, L.; Stefanoni, P.; Pavoni, C.; Mangiacavalli, S.; Masoni, V.; Palumbo, M.; Pompa, A.; Cafro, A.M.; et al. Impact of the Addition of Daratumumab to the Standard Bortezomib-Thalidomide-Dexamethasone Regimen on Hematopoietic Stem Cell Mobilization and Collection, Post-Transplant Engraftment and Infectious Complications: A Case-Control Multicentre Real-Life Analysis. Blood 2023, 142, 4706. [Google Scholar] [CrossRef]
- Jacob, E. Despite Use of Upfront Plerixafor and G-CSF, Daratumumab Exposure Reduces Stem Cell Mobilization in Patients with Multiple Myeloma. Blood 2022, 140 (Suppl. S1), 4295–4296. [Google Scholar] [CrossRef]
- Fazio, F.; Passucci, M.; Micozzi, J.; Sorella, S.; Lisi, C.; Fanciullo, D.; Piciocchi, A.; Bafti, M.S.; Martelli, M.; Gozzer, M.; et al. Autologous STEM Cell Collection after Daratumumab, Bortezomib, Thalidomide and Dexamethasone versus Bortezomib, Thalidomide and Dexamethasone in NEWLY Diagnosed Multiple Myeloma: A Real-Life Monocentric Italian Experience. Blood 2023, 142, 6651. [Google Scholar] [CrossRef]
- Lemonakis, K.; Tatting, L.; Lisak, M.; Carlson, K.; Crafoord, J.; Blimark, C.H.; I Santamaria, A.; Wichert, S.; Lenhoff, S.; Hansson, M. Impact of daratumumab based induction on stem cell collection parameters in Swedish myeloma patients. Haematol 2022, 108, 610–614. [Google Scholar] [CrossRef]
- Liberatore, C.; Perini, T.; Passeri, C.; Ferla, V.; Fioritoni, F.; Girlando, V.; Iuliani, O.; Orsini, A.; Montanaro, G.; Farina, F.; et al. Higher cyclophosphamide dose grants optimal stem-cell collection after daratumumab-based induction in multiple myeloma. Haematologica 2023, 108, 3502–3505. [Google Scholar] [CrossRef] [PubMed]
- Luan, D.; Christos, P.J.; Ancharski, M.; Guarneri, D.; Pearse, R.; Rossi, A.C.; Shore, T.B.; Mayer, S.; Phillips, A.A.; Hsu, J.; et al. Timing of Daratumumab Administered Pre-Mobilization in Multiple Myeloma Impacts Pre-Harvest Peripheral Blood CD34+ Cell Counts and Plerixafor Use. Blood 2020, 136, 15–16. [Google Scholar] [CrossRef]
- Eleutherakis Papaiakovou, E.; Terpos, E.; Kanellias, N.; Migkou, M.; Gavriatopoulou, M.; Ntanasis-Stathopoulos, I.; Fotiou, D.; Malandrakis, P.; Theodorakakou, F.; Spiliopoulou, V.; et al. Impact of daratumumab on stem cell mobilization and collection, engraftment and early post-transplant complications among multiple myeloma patients undergoing autologous stem cell transplantation. Leuk. Lymphoma 2023, 64, 2140–2147. [Google Scholar] [CrossRef] [PubMed]
- Sauer, S.; Kriegsmann, K.; Nientiedt, C.; Schmitt, A.; Müller-Tidow, C.; Raab, M.-S.; Kauer, J. Autologous Stem Cell Collection after Daratumumab, Bortezomib, Thalidomide, and Dexamethasone versus Bortezomib, Cyclophosphamide, and Dexamethasone in Newly Diagnosed Multiple Myeloma. Transfus. Med. Hemoth. 2023, 50, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Thurlapati, A.; Roubal, K.; Davis, J.A.; Shah, S.Z.; Smith, D.; McGann, M.; Gaffney, K.; Cendagorta, A.; Maldonado, A.; Weeda, E.; et al. Stem Cell Mobilization for Multiple Myeloma Patients Receiving Daratumumab Based Induction Therapy—A Real World Experience. Transplant. Cell. Ther. 2023, 29, 340.e1–340.e4. [Google Scholar] [CrossRef]
- Kauer, J.; Freundt, E.P.; Schmitt, A.; Weinhold, N.; Mai, E.K.; Müller-Tidow, C.; Goldschmidt, H.; Raab, M.S.; Kriegsmann, K.; Sauer, S. Stem cell collection after lenalidomide, bortezomib and dexamethasone plus elotuzumab or isatuximab in newly diagnosed multiple myeloma patients: A single centre experience from the GMMG-HD6 and -HD7 trials. BMC Cancer 2023, 23, 1132. [Google Scholar] [CrossRef] [PubMed]
- Unis, G.D.; Gudiel, C.; Philon, E.; Finn, L.E. Prior Daratumumab Exposure and It’s Correlation to Parameters Related to Apheresis Prior to Hematopoietic Stem Cell Transplantation in Multiple Myeloma. Blood 2023, 142 (Suppl. S1), 2145. [Google Scholar] [CrossRef]
- Mina, R.; Garibaldi, B.; Bertuglia, G.; Casson, A.; Sarina, B.; Gay, F.; Mercadante, S.; Mariotti, J.; D’Agostino, M.; Taurino, D.; et al. Impact of Daratumumab on Hematopoietic Stem Cell Mobilization with G-CSF and on-Demand Plerixafor in Newly-Diagnosed Multiple Myeloma Patients. Blood 2023, 142, 6633. [Google Scholar] [CrossRef]
- Crusoe, E.Q.; Moura, A.; Chaves, M.; Salvino, M.; Santos, J.; Santos, H.; Santos, A.; Lucas, L.; Leal, J.; Arruda, M. Impact of daratumumab (DARA) administration during transplant-eligible newly diagnosed multiple myeloma (TE NDMM) induction on stem cell (SC) mobilization count and post-transplant engraftment. Rev. Bras. Hematol. Hemoter. 2021, 43, S261–S262. [Google Scholar] [CrossRef]
- Oza, S.; Slotky, R.; Vissa, P.; Phull, P.; Kaur, S.; Suh, H.C.; Donato, M.L.; Rowley, S.D.; Biran, N.; Vesole, D.H.; et al. Effect of Daratumumab on Stem Cell Mobilization and Engraftment Kinetics Post Autologous Stem Cell Transplantation in Patients with Newly Diagnosed Multiple Myeloma. Blood 2022, 140 (Suppl. S1), 10441–10442. [Google Scholar] [CrossRef]
- Wuchter, P.; Ran, D.; Bruckner, T.; Schmitt, T.; Witzens-Harig, M.; Neben, K.; Goldschmidt, H.; Ho, A.D. Poor Mobilization of Hematopoietic Stem Cells—Definitions, Incidence, Risk Factors, and Impact on Outcome of Autologous Transplantation. Biol. Blood Marrow Transplant. 2010, 16, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.S.; Dimopoulos, M.A.; Sonneveld, P.; Ho, P.J.; Belch, A.; Leiba, M.; Capra, M.; Gomez, D.; Medvedova, E.; Iida, S.; et al. Pharmacokinetics and Exposure–Response Analyses of Daratumumab in Combination Therapy Regimens for Patients with Multiple Myeloma. Adv. Ther. 2018, 35, 1859–1872. [Google Scholar] [CrossRef]
- Kumar, S.; Giralt, S.; Stadtmauer, E.A.; Harousseau, J.L.; Palumbo, A.; Bensinger, W.; Comenzo, R.L.; Lentzsch, S.; Munshi, N.; Niesvizky, R.; et al. Mobilization in myeloma revisited: IMWG consensus perspectives on stem cell collection following initial therapy with thalidomide-, lenalidomide-, or bortezomib-containing regimens. Blood 2009, 114, 1729–1735. [Google Scholar] [CrossRef] [PubMed]
- Giralt, S.; Stadtmauer, E.A.; Harousseau, J.L.; Palumbo, A.; Bensinger, W.; Comenzo, R.L.; Kumar, S.; Munshi, N.C.; Dispenzieri, A.; Kyle, R.; et al. International myeloma working group (IMWG) consensus statement and guidelines regarding the current status of stem cell collection and high-dose therapy for multiple myeloma and the role of plerixafor (AMD 3100). Leukemia 2009, 23, 1904–1912. [Google Scholar] [CrossRef] [PubMed]
- Mohty, M.; Hübel, K.; Kröger, N.; Aljurf, M.; Apperley, J.; Basak, G.W.; Bazarbachi, A.; Douglas, K.; Gabriel, I.; Garderet, L.; et al. Autologous haematopoietic stem cell mobilisation in multiple myeloma and lymphoma patients: A position statement from the European Group for Blood and Marrow Transplantation. Bone Marrow Transpl. 2014, 49, 865–872. [Google Scholar] [CrossRef]
- Zannetti, B.A.; Saraceni, F.; Cellini, C.; Fabbri, E.; Monaco, F.; Guarini, A.; Laszlo, D.; Martino, M.; Olivieri, A.; Imola, M.; et al. Low-Dose Cyclophosphamide versus Intermediate-High-Dose Cyclophosphamide versus Granulocyte Colony-Stimulating Factor Alone for Stem Cell Mobilization in Multiple Myeloma in the Era of Novel Agents: A Multicenter Retrospective Study. Transpl. Cell Ther. 2021, 27, 244.e1–244.e8. [Google Scholar] [CrossRef] [PubMed]
- Awan, F.; Kochuparambil, S.T.; Falconer, D.E.; Cumpston, A.; Leadmon, S.; Watkins, K.; DeRemer, D.; Jillella, A.; Craig, M.; Hamadani, M. Comparable efficacy and lower cost of PBSC mobilization with intermediate-dose cyclophosphamide and G-CSF compared with plerixafor and G-CSF in patients with multiple myeloma treated with novel therapies. Bone Marrow Transpl. 2013, 48, 1279–1284. [Google Scholar] [CrossRef]
- Chaudhary, L.; Awan, F.; Cumpston, A.; Leadmon, S.; Watkins, K.; Tse, W.; Craig, M.; Hamadani, M. Peripheral blood stem cell mobilization in multiple myeloma patients treat in the novel therapy-era with plerixafor and G-CSF has superior efficacy but significantly higher costs compared to mobilization with low-dose cyclophosphamide and G-CSF. J. Clin. Apher. 2013, 28, 359–367. [Google Scholar] [CrossRef]
- Van de Wyngaert, Z.; Nerich, V.; Fouquet, G.; Chretien, M.-L.; Caillot, D.; Azar, N.; Garderet, L.; Lenain, P.; Macro, M.; Bourhis, J.-H.; et al. Cost and efficacy of peripheral stem cell mobilization strategies in multiple myeloma. Bone Marrow Transpl. 2020, 55, 2254–2260. [Google Scholar] [CrossRef] [PubMed]
- Attolico, I.; Strafella, V.; Tarantini, F.; Carluccio, P. Impact of Dara-VTD induction therapy on hematopoietic stem cell collection and engraftment in Multiple Myeloma patients eligible for ASCT: Results of the real-life PRIMULA study [E-poster abstract]. In Proceedings of the 50th Annual Meeting of the European Group for Blood and Marrow Transplantation, Glasgow, UK, 14–17 April 2024. [Google Scholar]
- Costa, L.J.; Alexander, E.T.; Hogan, K.R.; Schaub, C.; Fouts, T.V.; Stuart, R.K. Development and validation of a decision-making algorithm to guide the use of plerixafor for autologous hematopoietic stem cell mobilization. Bone Marrow Transpl. 2011, 46, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Shah, E.E.; Young, R.P.; Wong, S.W.; Damon, L.E.; Wolf, J.L.; Shah, N.D.; Leavitt, A.D.; Loeffler, P.; Martin, T.G. Impact of Plerixafor Use at Different Peripheral Blood CD34+ Thresholds on Autologous Stem Cell Collection in Patients with Multiple Myeloma. Biol. Blood Marrow Transplant. 2020, 26, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Song, Z.; Wang, A.; Srinivasan, S.; Yang, G.; Greco, R.; Theilhaber, J.; Shehu, E.; Wu, L.; Yang, Z.-Y.; et al. Isatuximab Acts through Fc-Dependent, Independent, and Direct Pathways to Kill Multiple Myeloma Cells. Front. Immunol. 2020, 11, 1771. [Google Scholar] [CrossRef] [PubMed]
- Mele, S.; Devereux, S.; Pepper, A.G.; Infante, E.; Ridley, A.J. Calcium-RasGRP2-Rap1 signaling mediates CD38-induced migration of chronic lymphocytic leukemia cells. Blood Adv. 2018, 2, 1551–1561. [Google Scholar] [CrossRef]
- Vaisitti, T.; Aydin, S.; Rossi, D.; Cottino, F.; Bergui, L.; D’Arena, G.; Bonello, L.; Horenstein, A.L.; Brennan, P.; Pepper, C.; et al. CD38 increases CXCL12-mediated signals and homing of chronic lymphocytic leukemia cells. Leukemia 2010, 24, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Dispenzieri, A.; Lacy, M.Q.; Hayman, S.R.; Buadi, F.K.; A Gastineau, D.; Litzow, M.R.; Fonseca, R.; Roy, V.; Rajkumar, S.V.; et al. Impact of lenalidomide therapy on stem cell mobilization and engraftment post-peripheral blood stem cell transplantation in patients with newly diagnosed myeloma. Leukemia 2007, 21, 2035–2042. [Google Scholar] [CrossRef]
- Bhutani, D.; Zonder, J.; Valent, J.; Tageja, N.; Ayash, L.; Deol, A.; Al-Kadhimi, Z.; Abrams, J.; Lum, L.; Ratanatharathorn, V.; et al. Evaluating the effects of lenalidomide induction therapy on peripheral stem cells collection in patients undergoing autologous stem cell transplant for multiple myeloma. Support. Care Cancer 2013, 21, 2437–2442. [Google Scholar] [CrossRef]
- Partanen, A.; Valtola, J.; Silvennoinen, R.; Ropponen, A.; Siitonen, T.; Putkonen, M.; Sankelo, M.; Pelkonen, J.; Mäntymaa, P.; Varmavuo, V.; et al. Impact of lenalidomide-based induction therapy on the mobilization of CD34+ cells, blood graft cellular composition, and post-transplant recovery in myeloma patients: A prospective multicenter study. Transfusion 2017, 57, 2366–2372. [Google Scholar] [CrossRef]
- Chang, D.H.; Liu, N.; Klimek, V.; Hassoun, H.; Mazumder, A.; Nimer, S.D.; Jagannath, S.; Dhodapkar, M.V. Enhancement of ligand-dependent activation of human natural killer T cells by lenalidomide: Therapeutic implications. Blood 2006, 108, 618–621. [Google Scholar] [CrossRef]
- Desikan, K.R.; Tricot, G.; Munshi, N.C.; Anaissie, E.; Spoon, D.; Fassas, A.; Toor, A.; Zangari, M.; Badros, A.; Morris, C.; et al. Preceding chemotherapy, tumour load and age influence engraftment in multiple myeloma patients mobilized with granulocyte colony-stimulating factor alone. Br. J. Haematol. 2001, 112, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Rees, M.J.; Mollee, P.; Ng, J.Y.; Murton, A.; Gonsalves, J.F.; Panigrahi, A.; Beer, H.; Loh, J.; Nguyen, P.; Hunt, S.; et al. The association of mobilising regimen on immune reconstitution and survival in myeloma patients treated with bortezomib, cyclophosphamide and dexamethasone induction followed by a melphalan autograft. Bone Marrow Transpl. 2021, 56, 2152–2159. [Google Scholar] [CrossRef] [PubMed]
- Skerget, M.; Skopec, B.; Zontar, D.; Cernelc, P. Mobilization with cyclophosphamide reduces the number of lymphocyte subpopulations in the leukapheresis product and delays their reconstitution after autologous hematopoietic stem cell transplantation in patients with multiple myeloma. Radiol. Oncol. 2016, 50, 402–408. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bigi, F.; Manzato, E.; Barbato, S.; Talarico, M.; Puppi, M.; Masci, S.; Sacchetti, I.; Restuccia, R.; Iezza, M.; Rizzello, I.; et al. Impact of Anti-CD38 Monoclonal Antibody Therapy on CD34+ Hematopoietic Stem Cell Mobilization, Collection, and Engraftment in Multiple Myeloma Patients—A Systematic Review. Pharmaceuticals 2024, 17, 944. https://doi.org/10.3390/ph17070944
Bigi F, Manzato E, Barbato S, Talarico M, Puppi M, Masci S, Sacchetti I, Restuccia R, Iezza M, Rizzello I, et al. Impact of Anti-CD38 Monoclonal Antibody Therapy on CD34+ Hematopoietic Stem Cell Mobilization, Collection, and Engraftment in Multiple Myeloma Patients—A Systematic Review. Pharmaceuticals. 2024; 17(7):944. https://doi.org/10.3390/ph17070944
Chicago/Turabian StyleBigi, Flavia, Enrica Manzato, Simona Barbato, Marco Talarico, Michele Puppi, Simone Masci, Ilaria Sacchetti, Roberta Restuccia, Miriam Iezza, Ilaria Rizzello, and et al. 2024. "Impact of Anti-CD38 Monoclonal Antibody Therapy on CD34+ Hematopoietic Stem Cell Mobilization, Collection, and Engraftment in Multiple Myeloma Patients—A Systematic Review" Pharmaceuticals 17, no. 7: 944. https://doi.org/10.3390/ph17070944






