Insights into Immune Exhaustion in Chronic Hepatitis B: A Review of Checkpoint Receptor Expression
Abstract
1. Introduction
1.1. Hepatitis B Virus Infection: A Global Health Challenge in Need of Targeted Interventions
1.2. Hepatitis B Transmission and Strategies for Disease Eradication
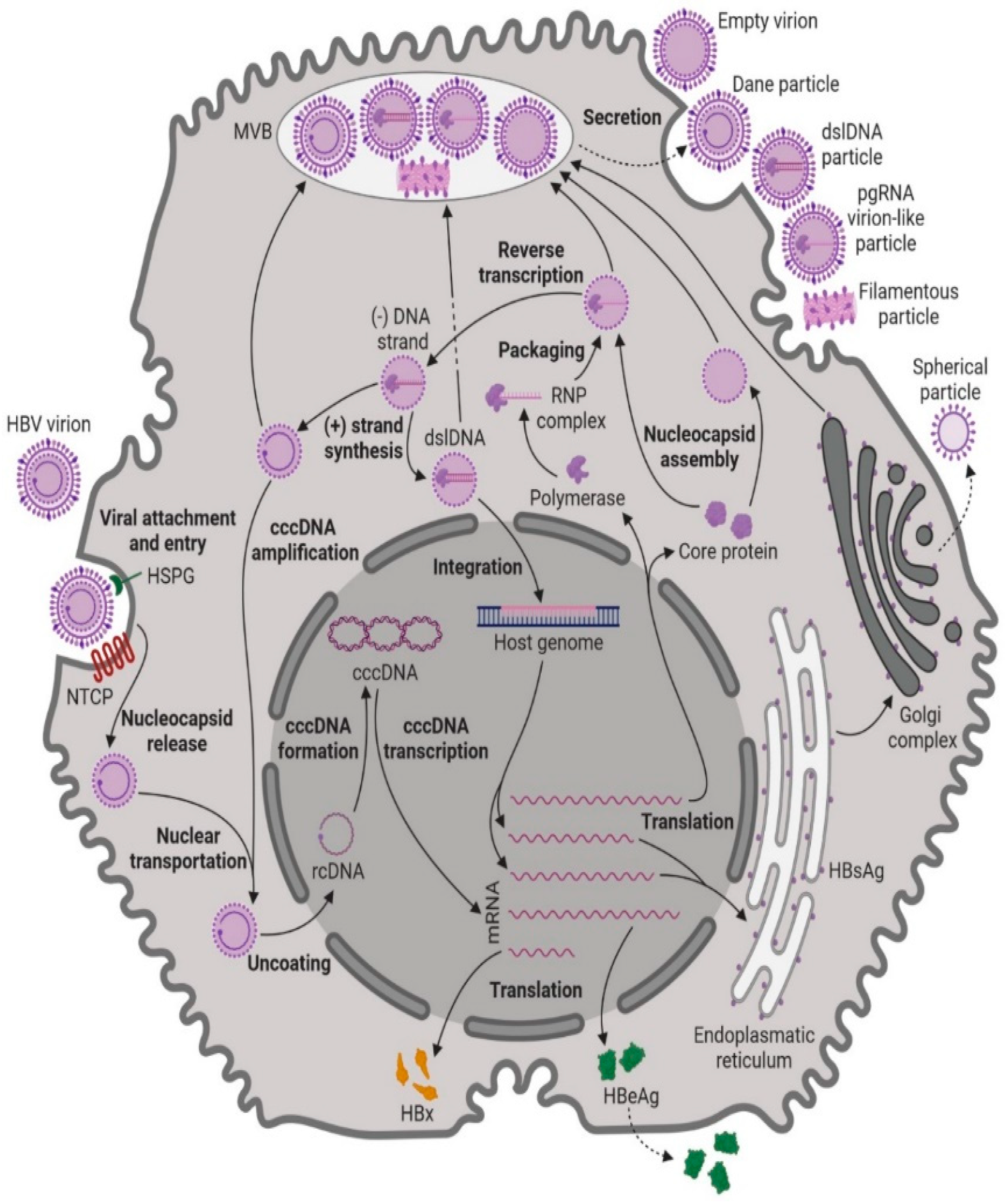
1.3. Hepatitis B Virus: Pathogenesis, Replication, and Genotypes
1.4. CHB Disease Course
1.5. Treatment
2. HBV and the Immune System
2.1. HBV Infection Natural Control
2.2. HBV Persistency
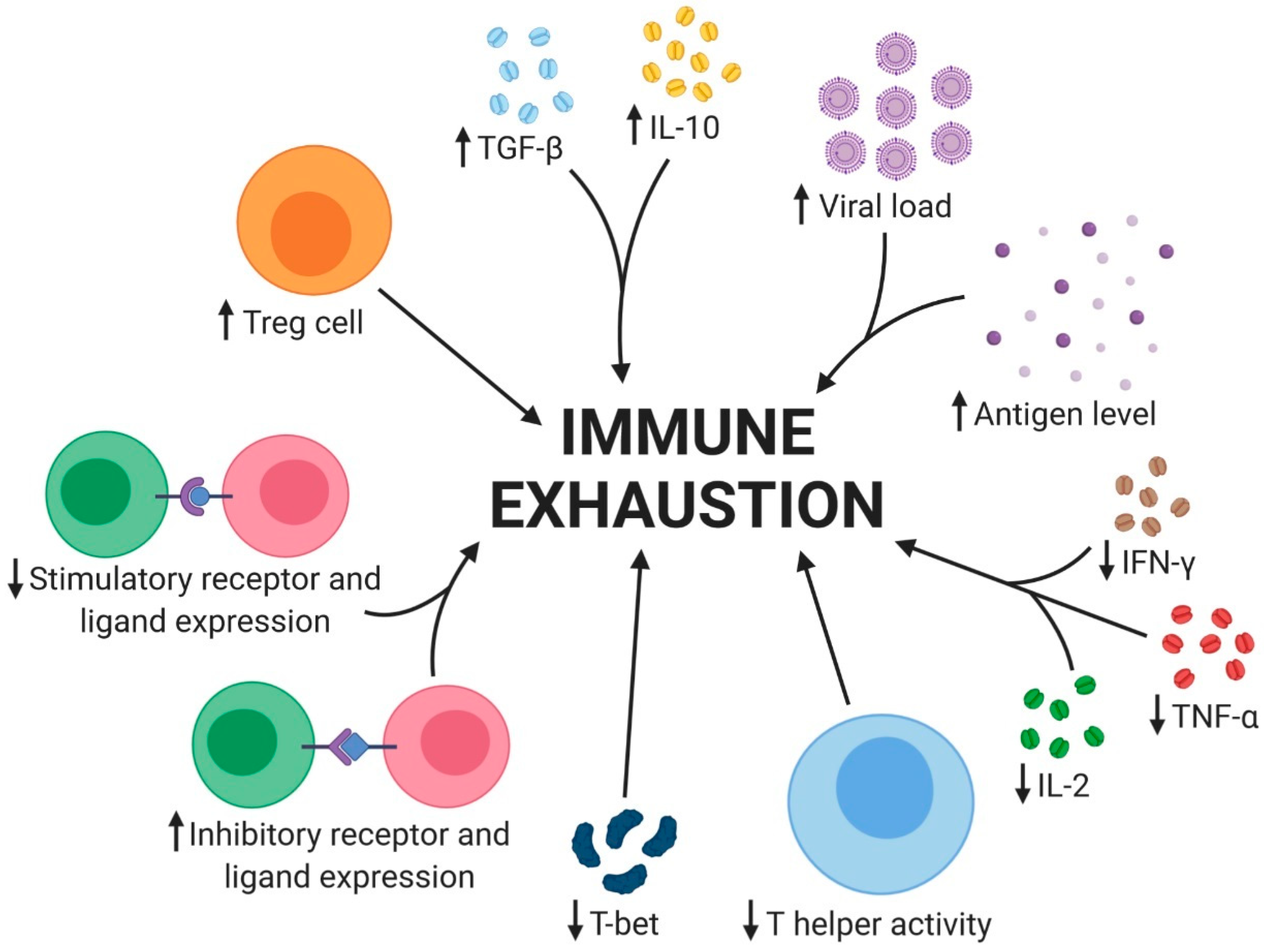
2.3. Immune Cell Exhaustion
2.3.1. Soluble Factors
2.3.2. Cell Types
NK Cells
Monocytes and Macrophages
Myeloid Derived Suppressive Cells
Dendritic Cells
Regulatory T Cells
T Cells
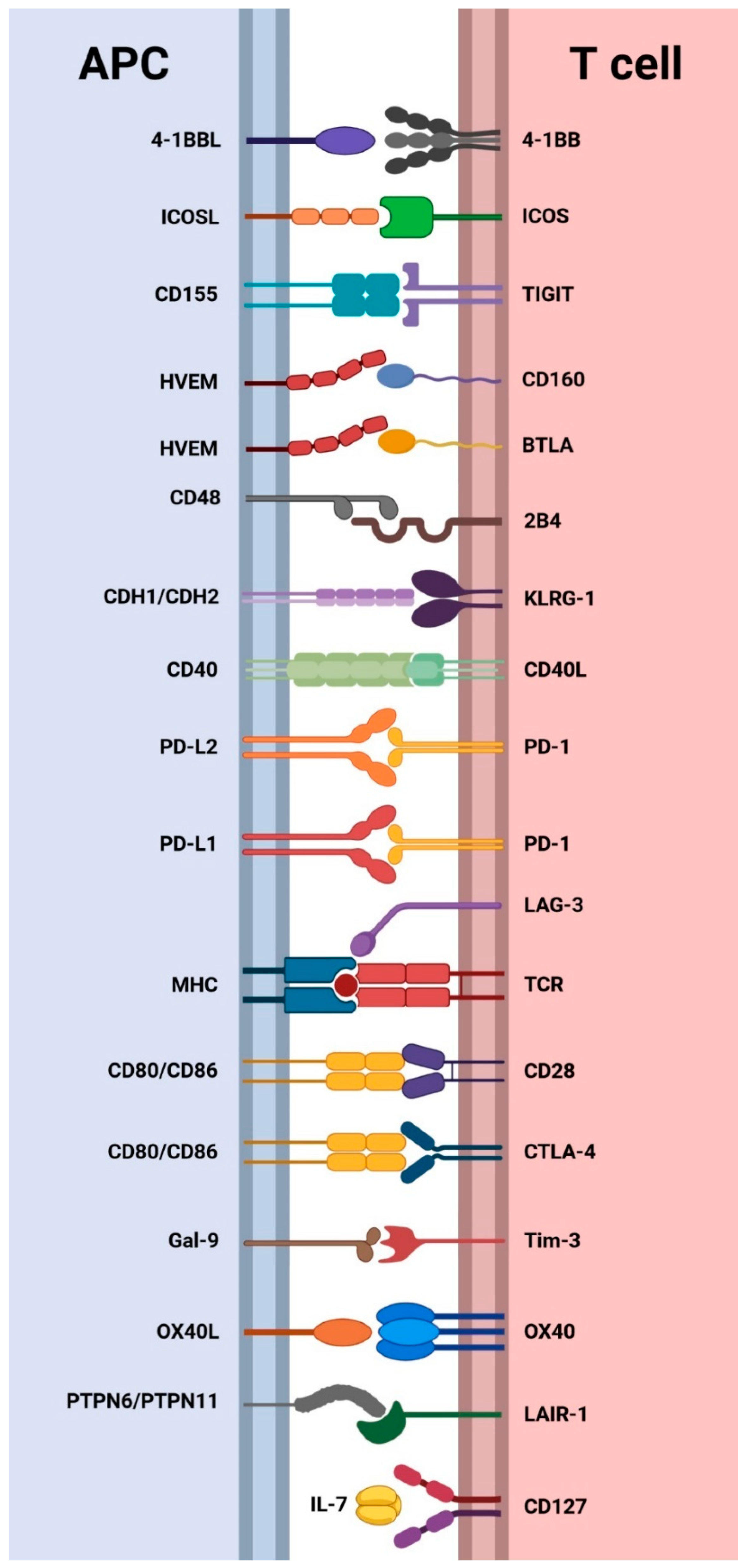
2.3.3. Cell Surface Receptors
3. Expression of Cell Surface Co-Inhibitory Receptors in CHB
3.1. CTLA-4, CD28, CD80, and CD86
3.2. PD-1, PD-L1, and PD-L2
3.3. LAG-3
3.4. 2B4 and CD48
3.5. KLRG-1
3.6. TIGIT
3.7. BTLA and CD160
3.8. Tim-3
3.9. LAIR-1
4. Expression of Cell Surface Co-Stimulatory Receptors in CHB
4.1. CD127
4.2. CD40 and CD40L
4.3. ICOS and ICOSL
4.4. 4-1BB and 4-1BBL
4.5. OX40 and OX40L
5. Immune Checkpoint Inhibitors in HBV-Related Hepatocellular Carcinoma
6. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- WHO. Hepatitis B Fact Sheet. 2016. Available online: https://www.who.int/en/news-room/fact-sheets/detail/hepatitis-b (accessed on 25 September 2023).
- WHO. Global Hepatitis Report 2024; WHO: Geneva, Switzerland, 2024. [Google Scholar]
- Caviglia, G.P.; Ciancio, A.; Rizzetto, M. A Review of HDV Infection. Viruses 2022, 14, 1749. [Google Scholar] [CrossRef] [PubMed]
- UN. Transforming Our World: The 2030 Agenda for Sustainable Development. 2015. Available online: https://sdgs.un.org/2030agenda (accessed on 25 September 2023).
- Seto, W.K.; Lo, Y.R.; Pawlotsky, J.M.; Yuen, M.F. Chronic hepatitis B virus infection. Lancet 2018, 392, 2313–2324. [Google Scholar] [CrossRef] [PubMed]
- WHO. Hepatitis B vaccines: WHO position paper, July 2017—Recommendations. Vaccine 2019, 37, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.; Revill, P. Overview of hepatitis B viral replication and genetic variability. J. Hepatol. 2016, 64 (Suppl. S1), S4–S16. [Google Scholar] [CrossRef] [PubMed]
- Valaydon, Z.S.; Locarnini, S.A. The virological aspects of hepatitis B. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 257–264. [Google Scholar] [CrossRef]
- Lucifora, J.; Protzer, U. Attacking hepatitis B virus cccDNA—The holy grail to hepatitis B cure. J. Hepatol. 2016, 64, S41–S48. [Google Scholar] [CrossRef]
- EASL EAftSotL. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol. 2017, 67, 370–398. [Google Scholar] [CrossRef]
- Glebe, D.; Urban, S.; Knoop, E.V.; Cag, N.; Krass, P.; Grun, S.; Bulavaite, A.; Sasnauskas, K.; Gerlich, W.H. Mapping of the hepatitis B virus attachment site by use of infection-inhibiting preS1 lipopeptides and tupaia hepatocytes. Gastroenterology 2005, 129, 234–245. [Google Scholar] [CrossRef]
- Hayes, C.N.; Zhang, Y.; Makokha, G.N.; Hasan, M.Z.; Omokoko, M.D.; Chayama, K. Early events in hepatitis B virus infection: From the cell surface to the nucleus. J. Gastroenterol. Hepatol. 2016, 31, 302–309. [Google Scholar] [CrossRef]
- Rabe, B.; Delaleau, M.; Bischof, A.; Foss, M.; Sominskaya, I.; Pumpens, P.; Cazenave, C.; Castroviejo, M.; Kann, M. Nuclear entry of hepatitis B virus capsids involves disintegration to protein dimers followed by nuclear reassociation to capsids. PLoS Pathog. 2009, 5, e1000563. [Google Scholar] [CrossRef]
- Bruss, V. Hepatitis B virus morphogenesis. World J. Gastroenterol. 2007, 13, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Himmelsbach, K.; Ren, H.; Boller, K.; Hildt, E. Subviral Hepatitis B Virus Filaments, like Infectious Viral Particles, Are Released via Multivesicular Bodies. J. Virol. 2015, 90, 3330–3341. [Google Scholar] [CrossRef] [PubMed]
- Junker-Niepmann, M.; Bartenschlager, R.; Schaller, H. A short cis-acting sequence is required for hepatitis B virus pregenome encapsidation and sufficient for packaging of foreign RNA. EMBO J. 1990, 9, 3389–3396. [Google Scholar] [CrossRef] [PubMed]
- Habig, J.W.; Loeb, D.D. Sequence identity of the direct repeats, DR1 and DR2, contributes to the discrimination between primer translocation and in situ priming during replication of the duck hepatitis B virus. J. Mol. Biol. 2006, 364, 32–43. [Google Scholar] [CrossRef][Green Version]
- Tuttleman, J.S.; Pourcel, C.; Summers, J. Formation of the Pool of Covalently Close Circular Viral DNA in Hepadnavirus-Infected. Cell 1986, 47, 451–460. [Google Scholar] [CrossRef]
- Staprans, S.; Loeb, D.D.; Ganem, D. Mutations Affecting Hepadnavirus Plus-Strand DNA Synthesis Dissociate Primer Cleavage from Translocation and Reveal the Origin of Linear Viral DNA. J. Virol. 1991, 65, 1255–1262. [Google Scholar] [CrossRef]
- An, P.; Xu, J.; Yu, Y.; Winkler, C.A. Host and Viral Genetic Variation in HBV-Related Hepatocellular Carcinoma. Front. Genet. 2018, 9, 261. [Google Scholar] [CrossRef]
- Lin, C.L.; Kao, J.H. Hepatitis B virus genotypes and variants. Cold Spring Harb. Perspect. Med. 2015, 5, a021436. [Google Scholar] [CrossRef]
- Sunbul, M. Hepatitis B virus genotypes: Global distribution and clinical importance. World J. Gastroenterol. 2014, 20, 5427–5434. [Google Scholar] [CrossRef]
- Kennedy, P.T.F.; Sandalova, E.; Jo, J.; Gill, U.; Ushiro-Lumb, I.; Tan, A.T.; Naik, S.; Foster, G.R.; Bertoletti, A. Preserved T-cell function in children and young adults with immune-tolerant chronic hepatitis B. Gastroenterology 2012, 143, 637–645. [Google Scholar] [CrossRef]
- Tsai, K.N.; Kuo, C.F.; Ou, J.J. Mechanisms of Hepatitis B Virus Persistence. Trends Microbiol. 2018, 26, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Yuen, M.F.; Wong, D.K.; Fung, J.; Ip, P.; But, D.; Hung, I.; Lau, K.; Yuen, J.C.; Lai, C.L. HBsAg Seroclearance in chronic hepatitis B in Asian patients: Replicative level and risk of hepatocellular carcinoma. Gastroenterology 2008, 135, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Jafri, S.M.; Lok, A.S. Antiviral therapy for chronic hepatitis B. Clin. Liver Dis. 2010, 14, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Buti, M.; Gane, E.; Seto, W.K.; Chan, H.L.; Chuang, W.L.; Stepanova, T.; Hui, A.J.; Lim, Y.S.; Mehta, R.; Janssen, H.L.; et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of patients with HBeAg-negative chronic hepatitis B virus infection: A randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol. Hepatol. 2016, 1, 196–206. [Google Scholar] [CrossRef]
- Lai, C.L.; Wong, D.; Ip, P.; Kopaniszen, M.; Seto, W.K.; Fung, J.; Huang, F.Y.; Lee, B.; Cullaro, G.; Chong, C.K.; et al. Reduction of covalently closed circular DNA with long-term nucleos(t)ide analogue treatment in chronic hepatitis B. J. Hepatol. 2017, 66, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Marcellin, P.; Gane, E.; Buti, M.; Afdhal, N.; Sievert, W.; Jacobson, I.M.; Washington, M.K.; Germanidis, G.; Flaherty, J.F.; Aguilar Schall, R.; et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: A 5-year open-label follow-up study. Lancet 2013, 381, 468–475. [Google Scholar] [CrossRef]
- Wong, G.L.; Chan, H.L.; Mak, C.W.; Lee, S.K.; Ip, Z.M.; Lam, A.T.; Iu, H.W.; Leung, J.M.; Lai, J.W.; Lo, A.O.; et al. Entecavir treatment reduces hepatic events and deaths in chronic hepatitis B patients with liver cirrhosis. Hepatology 2013, 58, 1537–1547. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Lin, J.-T.; Ho, H.J.; Su, C.-W.; Lee, T.-Y.; Wang, S.-Y.; Wu, C.; Wu, J.-C. Association of Nucleos(t)ide Analogue Therapy with Reduced Risk of Hepatocellular Carcinoma in Patients with Chronic Hepatitis B—A Nationwide Cohort Study. Gastroenterology 2014, 147, 143–151.e145. [Google Scholar] [CrossRef] [PubMed]
- Bourliere, M.; Rabiega, P.; Ganne-Carrie, N.; Serfaty, L.; Marcellin, P.; Barthe, Y.; Thabut, D.; Guyader, D.; Hezode, C.; Picon, M.; et al. Effect on HBs antigen clearance of addition of pegylated interferon alfa-2a to nucleos(t)ide analogue therapy versus nucleos(t)ide analogue therapy alone in patients with HBe antigen-negative chronic hepatitis B and sustained undetectable plasma hepatitis B virus DNA: A randomised, controlled, open-label trial. Lancet Gastroenterol. Hepatol. 2017, 2, 177–188. [Google Scholar] [CrossRef]
- Bertoletti, A.; Gehring, A.J. Immune therapeutic strategies in chronic hepatitis B virus infection: Virus or inflammation control? PLoS Pathog. 2013, 9, e1003784. [Google Scholar] [CrossRef]
- Bertoletti, A.; Ferrari, C. Innate and adaptive immune responses in chronic hepatitis B virus infections: Towards restoration of immune control of viral infection. Postgrad. Med. J. 2013, 89, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Guidotti, L.G.; Chisari, F.V. Immunobiology and Pathogenesis of Viral Hepatitis. Annu. Rev. Pathol. Mech. Dis. 2006, 1, 23–61. [Google Scholar] [CrossRef]
- Lobaina, Y.; Michel, M.L. Chronic hepatitis B: Immunological profile and current therapeutic vaccines in clinical trials. Vaccine 2017, 35, 2308–2314. [Google Scholar] [CrossRef]
- Jameson, S.C.; Masopust, D. Diversity in T Cell Memory: An Embarrassment of Riches. Immunity 2009, 31, 859–871. [Google Scholar] [CrossRef]
- Protzer, U.; Maini, M.K.; Knolle, P.A. Living in the liver: Hepatic infections. Nat. Rev. Immunol. 2012, 12, 201–213. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Q.; Zhao, P.; Hu, X.; Jiang, Y. Effects of entecavir on peripheral blood lymphocyte profiles in chronic hepatitis B patients with suboptimal responses to adefovir. Clin. Exp. Pharmacol. Physiol. 2014, 41, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Wieland, S.F.; Chisari, F.V. Stealth and cunning: Hepatitis B and hepatitis C viruses. J. Virol. 2005, 79, 9369–9380. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Chen, Z.J. Intrinsic antiviral immunity. Nat. Immunol. 2012, 13, 214–222. [Google Scholar] [CrossRef]
- Niu, C.; Livingston, C.M.; Li, L.; Beran, R.K.; Daffis, S.; Ramakrishnan, D.; Burdette, D.; Peiser, L.; Salas, E.; Ramos, H.; et al. The Smc5/6 Complex Restricts HBV when Localized to ND10 without Inducing an Innate Immune Response and Is Counteracted by the HBV X Protein Shortly after Infection. PLoS ONE 2017, 12, e0169648. [Google Scholar] [CrossRef]
- Wang, H.; Ryu, W.S. Hepatitis B virus polymerase blocks pattern recognition receptor signaling via interaction with DDX3: Implications for immune evasion. PLoS Pathog. 2010, 6, e1000986. [Google Scholar] [CrossRef]
- Kumar, M.; Jung, S.Y.; Hodgson, A.J.; Madden, C.R.; Qin, J.; Slagle, B.L. Hepatitis B virus regulatory HBx protein binds to adaptor protein IPS-1 and inhibits the activation of beta interferon. J. Virol. 2011, 85, 987–995. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Mao, A.; Li, C.; Li, Y.; Tien, P. Hepatitis B virus X protein suppresses virus-triggered IRF3 activation and IFN-beta induction by disrupting the VISA-associated complex. Cell Mol. Immunol. 2010, 7, 341–348. [Google Scholar] [CrossRef]
- Wu, J.; Meng, Z.; Jiang, M.; Pei, R.; Trippler, M.; Broering, R.; Bucchi, A.; Sowa, J.P.; Dittmer, U.; Yang, D.; et al. Hepatitis B virus suppresses toll-like receptor-mediated innate immune responses in murine parenchymal and nonparenchymal liver cells. Hepatology 2009, 49, 1132–1140. [Google Scholar] [CrossRef] [PubMed]
- Wherry, E.J. T cell exhaustion. Nat. Immunol. 2011, 12, 492–499. [Google Scholar] [CrossRef]
- Blackburn, S.D.; Wherry, E.J. IL-10, T cell exhaustion and viral persistence. Trends Microbiol. 2007, 15, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Paul, W.E. CD4 T cells: Fates, functions, and faults. Blood 2008, 112, 1557–1569. [Google Scholar] [CrossRef] [PubMed]
- Brooks, D.G.; Trifilo, M.J.; Edelmann, K.H.; Teyton, L.; McGavern, D.B.; Oldstone, M.B. Interleukin-10 determines viral clearance or persistence in vivo. Nat. Med. 2006, 12, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Li, M.O.; Wan, Y.Y.; Sanjabi, S.; Robertson, A.K.; Flavell, R.A. Transforming growth factor-beta regulation of immune responses. Annu. Rev. Immunol. 2006, 24, 99–146. [Google Scholar] [CrossRef]
- Tinoco, R.; Alcalde, V.; Yang, Y.; Sauer, K.; Zuniga, E.I. Cell-intrinsic transforming growth factor-beta signaling mediates virus-specific CD8+ T cell deletion and viral persistence in vivo. Immunity 2009, 31, 145–157. [Google Scholar] [CrossRef]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J. Control Release 2004, 100, 5–28. [Google Scholar] [CrossRef]
- Nanjappa, S.G.; Kim, E.H.; Sur, M. Immunotherapeutic effects of IL-7 during a chronic viral infection in mice. Blood 2016, 117, 5123–5132. [Google Scholar] [CrossRef] [PubMed]
- Elsaesser, H.; Sauer, K.; Brooks, D.G. IL-21 is required to control chronic viral infection. Science 2009, 324, 1569–1572. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Biron, C.A.; Brossay, L. NK cells and NKT cells in innate defense against viral infections. Curr. Opin. Immunol. 2001, 13, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Martin-Fontecha, A.; Thomsen, L.L.; Brett, S.; Gerard, C.; Lipp, M.; Lanzavecchia, A.; Sallusto, F. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat. Immunol. 2004, 5, 1260–1265. [Google Scholar] [CrossRef] [PubMed]
- Stelma, F.; de Niet, A.; Tempelmans Plat-Sinnige, M.J.; Jansen, L.; Takkenberg, R.B.; Reesink, H.W.; Kootstra, N.A.; van Leeuwen, E.M. Natural Killer Cell Characteristics in Patients with Chronic Hepatitis B Virus (HBV) Infection Are Associated with HBV Surface Antigen Clearance After Combination Treatment with Pegylated Interferon Alfa-2a and Adefovir. J. Infect. Dis. 2015, 212, 1042–1051. [Google Scholar] [CrossRef] [PubMed]
- Li, T.Y.; Yang, Y.; Zhou, G.; Tu, Z.K. Immune suppression in chronic hepatitis B infection associated liver disease: A review. World J. Gastroenterol. 2019, 25, 3527–3537. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhai, N.; Wang, Z.; Song, H.; Yang, Y.; Cui, A.; Li, T.; Wang, G.; Niu, J.; Crispe, I.N.; et al. Regulatory NK cells mediated between immunosuppressive monocytes and dysfunctional T cells in chronic HBV infection. Gut 2018, 67, 2035–2044. [Google Scholar] [CrossRef] [PubMed]
- Artis, D.; Spits, H. The biology of innate lymphoid cells. Nature 2015, 517, 293–301. [Google Scholar] [CrossRef]
- Soto, E.; Ostroff, G. Glucan Particles as Carriers of Nanoparticles for Macrophage-Targeted Delivery. In Nanomaterials for Biomedicine; ACS Symposium Series. 1119; American Chemical Society: Washington, DC, USA, 2012; Volume 1119, pp. 57–79. [Google Scholar]
- Gabrilovich, D.I. Myeloid-Derived Suppressor Cells. Cancer Immunol. Res. 2017, 5, 3–8. [Google Scholar] [CrossRef]
- Fang, Z.; Li, J.; Yu, X.; Zhang, D.; Ren, G.; Shi, B.; Wang, C.; Kosinska, A.D.; Wang, S.; Zhou, X.; et al. Polarization of Monocytic Myeloid-Derived Suppressor Cells by Hepatitis B Surface Antigen Is Mediated via ERK/IL-6/STAT3 Signaling Feedback and Restrains the Activation of T Cells in Chronic Hepatitis B Virus Infection. J. Immunol. 2015, 195, 4873–4883. [Google Scholar] [CrossRef] [PubMed]
- Pallett, L.J.; Gill, U.S.; Quaglia, A.; Sinclair, L.V.; Jover-Cobos, M.; Schurich, A.; Singh, K.P.; Thomas, N.; Das, A.; Chen, A.; et al. Metabolic regulation of hepatitis B immunopathology by myeloid-derived suppressor cells. Nat. Med. 2015, 21, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Prendergast, G.C.; Smith, C.; Thomas, S.; Mandik-Nayak, L.; Laury-Kleintop, L.; Metz, R.; Muller, A.J. Indoleamine 2,3-dioxygenase pathways of pathogenic inflammation and immune escape in cancer. Cancer Immunol. Immunother. 2014, 63, 721–735. [Google Scholar] [CrossRef] [PubMed]
- Bronte, V.; Brandau, S.; Chen, S.H.; Colombo, M.P.; Frey, A.B.; Greten, T.F.; Mandruzzato, S.; Murray, P.J.; Ochoa, A.; Ostrand-Rosenberg, S.; et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 2016, 7, 12150. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Cui, M.; Lv, Z.; Lu, J.; Zhang, X.; Zhao, Z.; Wang, Y.; Gao, L.; Tsuji, N.M.; Yan, H. Expression and significance of peripheral myeloid-derived suppressor cells in chronic hepatitis B patients. Clin. Res. Hepatol. Gastroenterol. 2018, 42, 462–469. [Google Scholar] [CrossRef]
- Tanne, A.; Bhardwaj, N. Dendritic Cells. In Kelley and Firestein’s Textbook of Rheumatology; Elsevier: Amsterdam, The Netherlands, 2017; Volume 1, pp. 126–144.e126. [Google Scholar] [CrossRef]
- Gehring, A.J.; D’Angelo, J.A. Dissecting the dendritic cell controversy in chronic hepatitis B virus infection. Cell. Mol. Immunol. 2015, 12, 283–291. [Google Scholar] [CrossRef]
- Beckebaum, S.; Cicinnati, V.R.; Zhang, X.; Ferencik, S.; Frilling, A.; Grosse-Wilde, H.; Broelsch, C.E.; Gerken, G. Hepatitis B virus-induced defect of monocyte-derived dendritic cells leads to impaired T helper type 1 response in vitro: Mechanism for viral immune escape. Immunology 2003, 109, 487–495. [Google Scholar] [CrossRef]
- Peng, G.; Li, S.; Wu, W.; Sun, Z.; Chen, Y.; Chen, Z. Circulating CD4+ CD25+ regulatory T cells correlate with chronic hepatitis B infection. Immunology 2008, 123, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Lorentzen, C.L.; Haanen, J.B.; Met, Ö.; Svane, I.M. Clinical advances and ongoing trials of mRNA vaccines for cancer treatment. Lancet Oncol. 2022, 23, e450–e458. [Google Scholar] [CrossRef]
- Stoop, J.N.; van der Molen, R.G.; Baan, C.C.; van der Laan, L.J.; Kuipers, E.J.; Kusters, J.G.; Janssen, H.L. Regulatory T cells contribute to the impaired immune response in patients with chronic hepatitis B virus infection. Hepatology 2005, 41, 771–778. [Google Scholar] [CrossRef]
- TrehanPati, N.; Kotillil, S.; Hissar, S.S.; Shrivastava, S.; Khanam, A.; Sukriti, S.; Mishra, S.K.; Sarin, S.K. Circulating Tregs correlate with viral load reduction in chronic HBV-treated patients with tenofovir disoproxil fumarate. J. Clin. Immunol. 2011, 31, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Fu, J.; Jin, L.; Zhang, H.; Zhou, C.; Zou, Z.; Zhao, J.; Zhang, B.; Shi, M.; Ding, X.; et al. Circulating and Liver Resident CD4+CD25+ Regulatory T Cells Actively Influence the Antiviral Immune Response and Disease Progression in Patients with Hepatitis B. J. Immunol. 2006, 177, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Zajac, A.J.; Blattman, J.N.; Murali-Krishna, K.; Sourdive, D.J.D.; Suresh, M.; Altman, J.D.; Ahmed, R. Viral Immune Evasion Due to Persistence of Activated T Cells Without Effector Function. J. Exp. Med. 1998, 188, 2205–2213. [Google Scholar] [CrossRef]
- McKinney, E.F.; Smith, K.G.C. Metabolic exhaustion in infection, cancer and autoimmunity. Nat. Immunol. 2018, 19, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Fuller, M.J.; Zajac, A.J. Ablation of CD8 and CD4 T cell responses by high viral loads. J. Immunol. 2003, 170, 477–486. [Google Scholar] [CrossRef]
- Wherry, E.J.; Blattman, J.N.; Murali-Krishna, K.; van der Most, R.; Ahmed, R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 2003, 77, 4911–4927. [Google Scholar] [CrossRef]
- Rotte, A.; D’Orazi, G.; Bhandaru, M. Nobel committee honors tumor immunologists. J. Exp. Clin. Cancer Res. 2018, 37, 262. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Agata, Y.; Shibahara, K.; Honjo, T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992, 11, 3887–3895. [Google Scholar] [CrossRef] [PubMed]
- Peach, R.J.; Bajorath, J.; Naemura, J.; Leytze, G.; Greene, J.; Aruffo, A.; Linsley, P.S. Both Extracellular Immunoglobin-like Domains of CD80 Contain Residues Critical for Binding T Cell Surface Receptors CTLA-4 and CD28. J. Biol. Chemestry 1995, 270, 21181–21187. [Google Scholar] [CrossRef]
- Chatzigeorgiou, A.; Lyberi, M.; Chatzilymperis, G.; Nezos, A.; Kamper, E. CD40/CD40L signaling and its implication in health and disease. Biofactors 2009, 35, 474–483. [Google Scholar] [CrossRef]
- Virgin, H.W.; Wherry, E.J.; Ahmed, R. Redefining chronic viral infection. Cell 2009, 138, 30–50. [Google Scholar] [CrossRef] [PubMed]
- Fisicaro, P.; Valdatta, C.; Massari, M.; Loggi, E.; Biasini, E.; Sacchelli, L.; Cavallo, M.C.; Silini, E.M.; Andreone, P.; Missale, G.; et al. Antiviral intrahepatic T-cell responses can be restored by blocking programmed death-1 pathway in chronic hepatitis B. Gastroenterology 2010, 138, 682–693.e4. [Google Scholar] [CrossRef]
- Stamper, C.C.; Zhang, Y.; Tobin, J.F.; Erbe, D.V.; Ikemizu, S.; Davis, S.J.; Stahl, M.L.; Seehra, J.; Somers, W.S.; Mosyak, L. Crystal structure of the B7-1/CTLA-4 complex that inhibits human immune responses. Nature 2001, 410, 608–611. [Google Scholar] [CrossRef]
- Azuma, M.; Ito, D.; Yagita, H.; Okumura, K.; Phillips, J.H.; Lanier, L.L.; Somoza, C. B70 antigen is a second ligand for CTLA-4 and CD28. Nature 1993, 366, 76–79. [Google Scholar] [CrossRef]
- Krummel, M.F.; Allison, J.P. CD28 and CTLA-4 Have Opposing Effects on the Response of T ceils to Stimulation. J. Exp. Med. 1995, 182, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Schneider, H.; Valk, E.; Leung, R.; Rudd, C.E. CTLA-4 activation of phosphatidylinositol 3-kinase (PI 3-K) and protein kinase B (PKB/AKT) sustains T-cell anergy without cell death. PLoS ONE 2008, 3, e3842. [Google Scholar] [CrossRef]
- Rotte, A. Combination of CTLA-4 and PD-1 blockers for treatment of cancer. J. Exp. Clin. Cancer Res. 2019, 38, 255. [Google Scholar] [CrossRef] [PubMed]
- Leach, D.R.; Krummel, M.F.; Allison, J.P. Enhancement of Antitumor Immunity by CTLA-4 Blockade. Science 1996, 271, 1734–1736. [Google Scholar] [CrossRef]
- Tang, Z.S.; Hao, Y.H.; Zhang, E.J.; Xu, C.L.; Zhou, Y.; Zheng, X.; Yang, D.L. CD28 family of receptors on T cells in chronic HBV infection: Expression characteristics, clinical significance and correlations with PD-1 blockade. Mol. Med. Rep. 2016, 14, 1107–1116. [Google Scholar] [CrossRef]
- Bengsch, B.; Martin, B.; Thimme, R. Restoration of HBV-specific CD8+ T cell function by PD-1 blockade in inactive carrier patients is linked to T cell differentiation. J. Hepatol. 2014, 61, 1212–1219. [Google Scholar] [CrossRef]
- Fisicaro, P.; Valdatta, C.; Massari, M.; Loggi, E.; Ravanetti, L.; Urbani, S.; Giuberti, T.; Cavalli, A.; Vandelli, C.; Andreone, P.; et al. Combined blockade of programmed death-1 and activation of CD137 increase responses of human liver T cells against HBV, but not HCV. Gastroenterology 2012, 143, 1576–1585.e4. [Google Scholar] [CrossRef] [PubMed]
- Raziorrouh, B.; Heeg, M.; Kurktschiev, P.; Schraut, W.; Zachoval, R.; Wendtner, C.; Wächtler, M.; Spannagl, M.; Denk, G.; Ulsenheimer, A.; et al. Inhibitory Phenotype of HBV-Specific CD4+ T-Cells Is Characterized by High PD-1 Expression but Absent Coregulation of Multiple Inhibitory Molecules. PLoS ONE 2014, 9, e105703. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, Q.; Shen, H.; Lu, X.; Sun, B. Genetic and phenotypic difference in CD8+ T cell exhaustion between chronic hepatitis B infection and hepatocellular carcinoma. J. Med. Genet. 2019, 56, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Qiu, Z.F.; Li, T.S. Parallel decline of CD8+CD38+ lymphocytes and viremia in treated hepatitis B patients. World J. Gastroenterol. 2011, 17, 2191–2198. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kong, H.; Tian, L.; Zhu, Q.; Wang, Y.; Dong, Y.; Ni, Q.; Chen, Y. Changes of costimulatory molecule CD28 on circulating CD8+ T cells correlate with disease pathogenesis of chronic hepatitis B. Biomed. Res. Int. 2014, 2014, 423181. [Google Scholar] [CrossRef]
- Yang, Z.; Lei, Y.; Chen, C.; Ren, H.; Shi, T. Roles of the programmed cell death 1, T cell immunoglobulin mucin-3, and cluster of differentiation 288 pathways in the low reactivity of invariant natural killer T cells after chronic hepatitis B virus infection. Arch. Virol. 2015, 160, 2535–2545. [Google Scholar] [CrossRef] [PubMed]
- Said, E.A.; Al-Reesi, I.; Al-Riyami, M.; Al-Naamani, K.; Al-Sinawi, S.; Al-Balushi, M.S.; Koh, C.Y.; Al-Busaidi, J.Z.; Idris, M.A.; Al-Jabri, A.A. Increased CD86 but Not CD80 and PD-L1 Expression on Liver CD68+ Cells during Chronic HBV Infection. PLoS ONE 2016, 11, e0158265. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Chen, H.; Xie, Y.; Fei, R.; Cong, X.; Jiang, D.; Wang, S.; Wei, L.; Wang, Y. Dendritic cells from chronic hepatitis B patients can induce HBV antigen-specific T cell responses. World J. Gastroenterol. 2004, 10, 1578–1582. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Zou, H.; Wang, S. Hepatitis B e Antigen Seroconversion Is Related with the Function of Dendritic Cells in Chronic Hepatitis B Virus Infection. Gastroenterol. Res. Pract. 2014, 2014, 413952. [Google Scholar] [CrossRef]
- Lu, G.F.; Tang, F.A.; Zheng, P.Y.; Yang, P.C.; Qi, Y.M. Entecavir up-regulates dendritic cell function in patients with chronic hepatitis B. World J. Gastroenterol. 2008, 14, 1617–1621. [Google Scholar] [CrossRef]
- Wang, F.; Xing, L.; Liu, M.; Zhu, C.; Liu, H.; Wang, H.; Lei, Z. Dysfunction of peripheral blood dendritic cells from patients with chronic hepatitis B virus infection. World J. Gastroenterol. 2001, 7, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Tang, Z.; Han, J.; Xi, M.; Feng, J.; Zang, G. Expression of ICAM-1, HLA-DR, and CD80 on peripheral circulating CD1α DCs induced in vivo by IFN- α in patients with chronic hepatitis B. World J. Gastroenterol. 2006, 12, 1447–1451. [Google Scholar] [CrossRef] [PubMed]
- Tjwa, E.T.; van Oord, G.W.; Biesta, P.J.; Boonstra, A.; Janssen, H.L.; Woltman, A.M. Restoration of TLR3-activated myeloid dendritic cell activity leads to improved natural killer cell function in chronic hepatitis B virus infection. J. Virol. 2012, 86, 4102–4109. [Google Scholar] [CrossRef] [PubMed]
- van der Molen, R.G.; Sprengers, D.; Binda, R.S.; de Jong, E.C.; Niesters, H.G.; Kusters, J.G.; Kwekkeboom, J.; Janssen, H.L. Functional impairment of myeloid and plasmacytoid dendritic cells of patients with chronic hepatitis B. Hepatology 2004, 40, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Shang, Q.; Chen, X.; Nie, W.; Zou, Z.; Huang, A.; Meng, M.; Jin, L.; Xu, R.; Zhang, J.-Y.; et al. Reversal of B-cell hyperactivation and functional impairment is associated with HBsAg seroconversion in chronic hepatitis B patients. Cell. Mol. Immunol. 2015, 12, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, Z.; Chen, W.; Zhang, Z.; Li, Y.; Shi, M.; Zhang, J.; Chen, L.; Wang, S.; Wang, F. B7-H1 Up-Regulation on Myeloid Dendritic Cells Significantly Suppresses T Cell Immune Function in Patients with Chronic Hepatitis B. J. Immunol. 2007, 178, 6634–6641. [Google Scholar] [CrossRef]
- Peng, M.; Chen, M.; Ling, N.; Xu, H.; Qing, Y.; Ren, H. Novel vaccines for the treatment of chronic HBV infection based on mycobacterial heat shock protein 70. Vaccine 2006, 24, 887–896. [Google Scholar] [CrossRef]
- Xiang, X.X.; Zhou, X.Q.; Wang, J.X.; Xie, Q.; Cai, X.; Yu, H.; Zhou, H.J. Effects of CpG-ODNs on phenotype and function of monocyte-derived dendritic cells in chronic hepatitis B. World J. Gastroenterol. 2011, 17, 4825–4830. [Google Scholar] [CrossRef]
- Duan, X.; Zhuang, H.; Wang, M.; Li, H.; Liu, J.; Wang, F. Decreased numbers and impaired function of circulating dendritic cell subsets in patients with chronic hepatitis B infection (R2). J. Gastroenterol. Hepatol. 2005, 2004, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Kunitani, H.; Shimizu, Y.; Murata, H.; Higuchi, K.; Watanabe, A. Phenotypic analysis of circulating and intrahepatic dendritic cell subsets in patients with chronic liver diseases. J. Hepatol. 2002, 32, 734–741. [Google Scholar] [CrossRef]
- Li, M.-H.; Zhang, L.; Zhang, D.; Cao, W.-H.; Qi, T.-L.; Hao, H.-X.; Wang, X.-Y.; Ran, C.-P.; Qu, X.-J.; Liu, S.-A.; et al. Plasmacytoid Dendritic Cell Function and Cytokine Network Profiles in Patients with Acute or Chronic Hepatitis B Virus Infection. Chin. Med. J. 2018, 131, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Martinet, J.; Dufeu–Duchesne, T.; Bruder Costa, J.; Larrat, S.; Marlu, A.; Leroy, V.; Plumas, J.; Aspord, C. Altered Functions of Plasmacytoid Dendritic Cells and Reduced Cytolytic Activity of Natural Killer Cells in Patients with Chronic HBV Infection. Gastroenterology 2012, 143, 1586–1596.e1588. [Google Scholar] [CrossRef] [PubMed]
- Oliviero, B.; Cerino, A.; Varchetta, S.; Paudice, E.; Pai, S.; Ludovisi, S. Enhanced B-cell differentiation and reduced proliferative capacity in chronic hepatitis C and chronic hepatitis B virus infections. J. Hepatol. 2011, 55, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Pei, H.; Huang, B.; Yang, R.L.; Wu, H.Y.; Zhu, X.; Zhu, L. Overexpression of Fc receptor-like 1 associated with B-cell activation during hepatitis B virus infection. Braz. J. Med. Biol. Res. 2012, 45, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Tseng, B.S.; Otsuji, M.; Gorski, K.; Huang, X.; Slansky, J.E.; Pai, S.I.; Shalabi, A.; Shin, T.; Pardoll, D.M.; Tsuchiya, H. B7-DC, a New Dendritic Cell Molecule with Potent Costimulatory Properties for T Cells. J. Exp. Med. 2001, 193, 839–846. [Google Scholar] [CrossRef]
- Moreno-Cubero, E.; Larrubia, J.R. Specific CD8(+) T cell response immunotherapy for hepatocellular carcinoma and viral hepatitis. World J. Gastroenterol. 2016, 22, 6469–6483. [Google Scholar] [CrossRef]
- Carreno, B.M.; Bennett, F.; Chau, T.A.; Ling, V.; Luxenberg, D.; Jussif, J.; Baroja, M.L.; Madrenas, J. CTLA-4 (CD152) can inhibit T cell activation by two different mechanisms depending on its level of cell surface expression. J. Immunol. 2000, 165, 1352–1356. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.S.; Gao, Q.T.; Guo, R.W.; Zhang, X.; Zhou, Z.H.; Yu, Z.; Zhu, X.J.; Gao, Y.T.; Sun, X.H.; Gao, Y.Q.; et al. Immunomodulatory Effects of Combination Therapy with Bushen Formula plus Entecavir for Chronic Hepatitis B Patients. J. Immunol. Res. 2019, 2019, 8983903. [Google Scholar] [CrossRef]
- Chen, J.; Wang, X.M.; Wu, X.J.; Wang, Y.; Zhao, H.; Shen, B.; Wang, G.Q. Intrahepatic levels of PD-1/PD-L correlate with liver inflammation in chronic hepatitis B. Inflamm. Res. 2011, 60, 47–53. [Google Scholar] [CrossRef]
- Triebel, F.; Jitsukawa, S.; Baixeras, E.; Roman-Roman, S.; Genevee, C.; Viegas-Pequignot, E.; Hercend, T. LAG-3, a novel lymphocyte activation gene closely related to CD4. J. Exp. Med. 1990, 171, 1393–1405. [Google Scholar] [CrossRef]
- Goldberg, M.V.; Drake, C.G. LAG-3 in Cancer Immunotherapy. In Cancer Immunology and Immunotherapy; Dranoff, G., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 269–278. [Google Scholar]
- Aggarwal, V.; Workman, C.J.; Vignali, D.A.A. LAG-3 as the third checkpoint inhibitor. Nat. Immunol. 2023, 24, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.; Li, X.; Dong, Y.; Wang, Y.; Tian, L.; Lin, S.; Liu, X.; Kong, H.; Chen, Y. Increasing LAG-3 expression suppresses T-cell function in chronic hepatitis B: A balance between immunity strength and liver injury extent. Medicine 2017, 96, e5275. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, S.D.; Shin, H.; Haining, W.N.; Zou, T.; Workman, C.J.; Polley, A.; Betts, M.R.; Freeman, G.J.; Vignali, D.A.A.; Wherry, E.J. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat. Immunol. 2009, 10, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Valiante, N.M.; Trinchieri, G. Identification of a novel signal transduction surface molecule on human cytotoxic lymphocytes. J. Exp. Med. 1993, 178, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Assarsson, E.; Kambayashi, T.; Persson, C.M.; Chambers, B.J.; Ljunggren, H.-G. 2B4/CD48-Mediated Regulation of Lymphocyte Activation and Function 1. J. Immunol. 2005, 175, 2045–2049. [Google Scholar] [CrossRef]
- Cooksley, H.; Riva, A.; Katzarov, K.; Hadzhiolova-Lebeau, T.; Pavlova, S.; Simonova, M.; Williams, R.; Chokshi, S. Differential Expression of Immune Inhibitory Checkpoint Signatures on Antiviral and Inflammatory T Cell Populations in Chronic Hepatitis B. J. Interferon Cytokine Res. 2018, 38, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Raziorrouh, B.; Schraut, W.; Gerlach, T.; Nowack, D.; Grüner, N.H.; Ulsenheimer, A.; Zachoval, R.; Wächtler, M.; Spannagl, M.; Haas, J.; et al. The immunoregulatory role of CD244 in chronic hepatitis B infection and its inhibitory potential on virus-specific CD8+ T-cell function. Hepatology 2010, 52, 1934–1947. [Google Scholar] [CrossRef] [PubMed]
- Wijaya, R.S.; Read, S.A.; Schibeci, S.; Eslam, M.; Azardaryany, M.K.; El-Khobar, K.; van der Poorten, D.; Lin, R.; Yuen, L.; Lam, V.; et al. KLRG1+ natural killer cells exert a novel antifibrotic function in chronic hepatitis B. J. Hepatol. 2019, 71, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Tessmer, M.S.; Fugere, C.; Stevenaert, F.; Naidenko, O.V.; Chong, H.J.; Leclercq, G.; Brossay, L. KLRG1 binds cadherins and preferentially associates with SHIP-1. Int. Immunol. 2007, 19, 391–400. [Google Scholar] [CrossRef]
- Yu, X.; Harden, K.; Gonzalez, L.C.; Francesco, M.; Chiang, E.; Irving, B.; Tom, I.; Ivelja, S.; Refino, C.J.; Clark, H.; et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat. Immunol. 2009, 10, 48–57. [Google Scholar] [CrossRef]
- Zong, L.; Peng, H.; Sun, C.; Li, F.; Zheng, M.; Chen, Y.; Wei, H.; Sun, R.; Tian, Z. Breakdown of adaptive immunotolerance induces hepatocellular carcinoma in HBsAg-tg mice. Nat. Commun. 2019, 10, 221. [Google Scholar] [CrossRef]
- Schuch, A.; Alizei, E.S.; Heim, K.; Wieland, D.; Kiraithe, M.M.; Kemming, J.; Llewellyn-Lacey, S.; Sogukpinar, Ö.; Ni, Y.; Urban, S.; et al. Phenotypic and functional differences of HBV core-specific versus HBV polymerase-specific CD8+ T cells in chronically HBV-infected patients with low viral load. Gut 2019, 68, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Freeman, G.J. The CD160, BTLA, LIGHT/HVEM pathway: A bidirectional switch regulating T-cell activation. Immunol. Rev. 2009, 229, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.L.; Murphy, K.M. Slow Down and Survive: Enigmatic Immunoregulation by BTLA and HVEM. Annu. Rev. Immunol. 2010, 28, 389–411. [Google Scholar] [CrossRef] [PubMed]
- Hurchla, M.A.; Sedy, J.R.; Gavrielli, M.; Drake, C.G.; Murphy, T.L.; Murphy, K.M. B and T Lymphocyte Attenuator Exhibits Structural and Expression Polymorphisms and Is Highly Induced in Anergic CD4+ T Cells 1. J. Immunol. 2005, 174, 3377–3385. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zheng, Y.; Mao, R.; Su, Z.; Zhang, J. BTLA/HVEM Signaling: Milestones in Research and Role in Chronic Hepatitis B Virus Infection. Front. Immunol. 2019, 10, 617. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.M.; Nelson, C.A.; Šedý, J.R. Balancing co-stimulation and inhibition with BTLA and HVEM. Nat. Rev. Immunol. 2006, 6, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Subudhi, S.K.; Anders, R.A.; Lo, J.; Sun, Y.; Blink, S.; Wang, Y.; Wang, J.; Liu, X.; Mink, K.; et al. The role of herpesvirus entry mediator as a negative regulator of T cell–mediated responses. J. Clin. Investig. 2005, 115, 711–717. [Google Scholar] [CrossRef] [PubMed]
- del Rio, M.L.; Lucas, C.L.; Buhler, L.; Rayat, G.; Rodriguez-Barbosa, J.I. HVEM/LIGHT/BTLA/CD160 cosignaling pathways as targets for immune regulation. J. Leukoc. Biol. 2009, 87, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Nie, X.; Li, L.; Hu, L.; Wu, B.; Lin, J.; Jiang, C.; Wang, H.; Wang, X.; Shen, Q. B and T lymphocyte attenuator is highly expressed on intrahepatic T cells during chronic HBV infection and regulates their function. J. Gastroenterol. 2013, 48, 1362–1372. [Google Scholar] [CrossRef]
- Song, H.-F.; Chen, X.-J.; Tang, P.-J.; Xu, P.; Huang, Z.-Y.; Wang, X.-F. Clinical significance of BTLA and HVEM expression on circulating CD4+ T and CD8+ T cells in chronic hepatitis B virus infection. Viral Immunol. 2022, 35, 291–302. [Google Scholar] [CrossRef]
- Wang, H.; Wu, B.; Li, L.; Hu, L.; Lin, J.; Jiang, C.; Cai, G.; Shen, Q. Hepatic expansion of virus-specific CD8(+)BTLA(+) T cells with regulatory properties in chronic hepatitis B virus infection. Cell Immunol. 2017, 311, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Nan, X.-P.; Zhang, Y.; Yu, H.-T.; Li, Y.; Sun, R.-L.; Wang, J.-P.; Bai, X.-F. Circulating CD4+CD25high Regulatory T Cells and Expression of PD-1 and BTLA on CD4+ T Cells in Patients with Chronic Hepatitis B Virus Infection. Viral Immunol. 2010, 23, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gao, L.F.; Liang, X.H.; Ma, C.H. Role of Tim-3 in hepatitis B virus infection: An overview. World J. Gastroenterol. 2016, 22, 2294–2303. [Google Scholar] [CrossRef]
- Sabatos, C.A.; Chakravarti, S.; Cha, E.; Schubart, A.; Sánchez-Fueyo, A.; Zheng, X.X.; Coyle, A.J.; Strom, T.B.; Freeman, G.J.; Kuchroo, V.K. Interaction of Tim-3 and Tim-3 ligand regulates T helper type 1 responses and induction of peripheral tolerance. Nat. Immunol. 2003, 4, 1102–1110. [Google Scholar] [CrossRef]
- Das, M.; Zhu, C.; Kuchroo, V.K. Tim-3 and its role in regulating anti-tumor immunity. Immunol. Rev. 2017, 276, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Wada, J.; Kanwar, Y.S. Identification and characterization of galectin-9, a novel beta-galactoside-binding mammalian lectin. J. Biol. Chem. 1997, 272, 6078–6086. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.B.; Ndhlovu, L.C.; Barbour, J.D.; Sheth, P.M.; Jha, A.R.; Long, B.R.; Wong, J.C.; Satkunarajah, M.; Schweneker, M.; Chapman, J.M.; et al. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J. Exp. Med. 2008, 205, 2763–2779. [Google Scholar] [CrossRef]
- Zhu, C.; Anderson, A.C.; Schubart, A.; Xiong, H.; Imitola, J.; Khoury, S.J.; Zheng, X.X.; Strom, T.B.; Kuchroo, V.K. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat. Immunol. 2005, 6, 1245–1252. [Google Scholar] [CrossRef]
- Liu, S.; Xu, C.; Yang, F.; Zong, L.; Qin, Y.; Gao, Y.; Su, Q.; Li, T.; Li, Y.; Xu, Y. Natural killer cells induce CD8+ T cell dysfunction via Galectin-9/TIM-3 in chronic hepatitis B virus infection. Front. Immunol. 2022, 13, 884290. [Google Scholar] [CrossRef]
- Lebbink, R.J.; de Ruiter, T.; Adelmeijer, J.; Brenkman, A.B.; van Helvoort, J.M.; Koch, M.; Farndale, R.W.; Lisman, T.; Sonnenberg, A.; Lenting, P.J.; et al. Collagens are functional, high affinity ligands for the inhibitory immune receptor LAIR-1. J. Exp. Med. 2006, 203, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Meyaard, L.; Adema, G.J.; Chang, C.; Woollatt, E.; Sutherland, G.R.; Lanier, L.L.; Phillips, J.H. LAIR-1, a novel inhibitory receptor expressed on human mononuclear leukocytes. Immunity 1997, 7, 283–290. [Google Scholar] [CrossRef]
- Lorenz, U. SHP-1 and SHP-2 in T cells: Two phosphatases functioning at many levels. Immunol. Rev. 2009, 228, 342–359. [Google Scholar] [CrossRef]
- Gu, Y.; Bi, Y.; Wei, H.; Li, J.; Huang, Z.; Liao, C.; Liao, W.; Huang, Y. Expression and clinical significance of inhibitory receptor Leukocyte-associated immunoglobulin-like receptor-1 on peripheral blood T cells of chronic hepatitis B patients: A cross-sectional study. Medicine 2021, 100, e26667. [Google Scholar] [CrossRef] [PubMed]
- Cheuk, A.T.C.; Mufti, G.J.; Guinn, B.-a. Role of 4-1BB:4-1BB ligand in cancer immunotherapy. Cancer Gene Ther. 2004, 11, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Carrette, F.; Surh, C.D. IL-7 signaling and CD127 receptor regulation in the control of T cell homeostasis. Semin. Immunol. 2012, 24, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Mackall, C.L.; Fry, T.J.; Gress, R.E. Harnessing the biology of IL-7 for therapeutic application. Nat. Rev. Immunol. 2011, 11, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-T.; Ye, J.; Chen, Y.-B.; Zhang, L.-X.; Huang, J.-X.; Xian, J.-C.; Liu, L.; Peng, H.-L.; Li, L.; Lin, M.; et al. Changes in the Proportions of CD4+T Cell Subsets Defined by CD127 and CD25 Expression during HBV Infection. Immunol. Investig. 2012, 41, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Wherry, E.J.; Barber, D.L.; Kaech, S.M.; Blattman, J.N.; Ahmed, R. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc. Natl. Acad. Sci. USA 2004, 101, 16004–16009. [Google Scholar] [CrossRef]
- Liu, W.; Putnam, A.L.; Zhou, X.-y.; Szot, G.L.; Lee, M.R.; Zhu, S.; Gottlieb, P.A.; Kapranov, P.; Gingeras, T.R.; de St. Groth, B.F.; et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J. Exp. Med. 2006, 203, 1701–1711. [Google Scholar] [CrossRef]
- Shen, H.-H.; Bai, B.-K.; Wang, Y.-Q.; Zhou, G.-D.; Hou, J.; Hu, Y.; Zhao, J.-M.; Li, B.-S.; Huang, H.-L.; Mao, P.-Y. Serum soluble CD40 is associated with liver injury in patients with chronic hepatitis B. Exp. Ther. Med. 2015, 9, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Quezada, S.A.; Jarvinen, L.Z.; Lind, E.F.; Noelle, R.J. CD40/CD154 Interactions at the Interface of Tolerance and Immunity. Annu. Rev. Immunol. 2004, 22, 307–328. [Google Scholar] [CrossRef]
- Marinelli, O.; Nabissi, M.; Morelli, M.B.; Torquati, L.; Amantini, C.; Santoni, G. ICOS-L as a Potential Therapeutic Target for Cancer Immunotherapy. Curr. Protein Pept. Sci. 2018, 19, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Amatore, F.; Gorvel, L.; Olive, D. Inducible Co-Stimulator (ICOS) as a potential therapeutic target for anti-cancer therapy. Expert. Opin. Ther. Targets 2018, 22, 343–351. [Google Scholar] [CrossRef]
- Chester, C.; Sanmamed, M.F.; Wang, J.; Melero, I. Immunotherapy targeting 4-1BB: Mechanistic rationale, clinical results, and future strategies. Blood 2018, 131, 49–57. [Google Scholar] [CrossRef]
- Chester, C.; Ambulkar, S.; Kohrt, H.E. 4-1BB agonism: Adding the accelerator to cancer immunotherapy. Cancer Immunol. Immunother. 2016, 65, 1243–1248. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, F.J.; Wild, K.; Smits, M.; Zoldan, K.; Csernalabics, B.; Flecken, T.; Lang, J.; Ehrenmann, P.; Emmerich, F.; Hofmann, M.; et al. OX40 stimulation and PD-L1 blockade synergistically augment HBV-specific CD4 T cells in patients with HBeAg-negative infection. J. Hepatol. 2019, 70, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, G.; Chen, Y.; Huang, R.; Tian, C.; Li, Y.; Zhao, X.-A.; Wu, C. HBcAg-induced upregulated 4-1BB ligand on B cells contributes to B-cell hyperactivation during chronic hepatitis B infection. J. Med. Virol. 2019, 91, 781–790. [Google Scholar] [CrossRef]
- Buchan, S.L.; Rogel, A.; Al-Shamkhani, A. The immunobiology of CD27 and OX40 and their potential as targets for cancer immunotherapy. Blood 2018, 131, 39–48. [Google Scholar] [CrossRef]
- Webb, G.J.; Hirschfield, G.M.; Lane, P.J.L. OX40, OX40L and Autoimmunity: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2016, 50, 312–332. [Google Scholar] [CrossRef]
- Levrero, M.; Zucman-Rossi, J. Mechanisms of HBV-induced hepatocellular carcinoma. J. Hepatol. 2016, 64 (Suppl. S1), S84–S101. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Llovet, J.M.; Yarchoan, M.; Mehta, N.; Heimbach, J.K.; Dawson, L.A.; Jou, J.H.; Kulik, L.M.; Agopian, V.G.; Mendiratta-Lala, M.; et al. AASLD Practice Guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology 2023, 78, 1922–1965. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hu, C.; Xie, X.; Qi, L.; Li, C.; Li, S. Immune Checkpoint Inhibitors in HBV-Caused Hepatocellular Carcinoma Therapy. Vaccines 2023, 11, 614. [Google Scholar] [CrossRef] [PubMed]
- Astaras, C.; de Micheli, R.; Moura, B.; Hundsberger, T.; Hottinger, A.F. Neurological adverse events associated with immune checkpoint inhibitors: Diagnosis and management. Curr. Neurol. Neurosci. Rep. 2018, 18, 3. [Google Scholar] [CrossRef]
- Blackburn, S.D.; Shin, H.; Freeman, G.J.; Wherry, E.J. Selective expansion of a subset of exhausted CD8 T cells by αPD-L1 blockade. Proc. Natl. Acad. Sci. USA 2008, 105, 15016–15021. [Google Scholar] [CrossRef]
- Kao, J.-H.; Peng, C.-Y. Evaluate the Efficacy and Safety of HLX10 in Chronic Hepatitis B Patients. 2019. Available online: https://www.clinicaltrials.gov/study/NCT04133259?cond=Evaluate%20the%20Efficacy%20and%20Safety%20of%20HLX10%20in%20Chronic%20Hepatitis%20B%20Patients&rank=1 (accessed on 25 September 2023).

| Serum Ag | Antibodies | HBV DNA | ALT | Liver Histology | |
|---|---|---|---|---|---|
| Phase 1: HBeAg-positive chronic HBV infection | HBeAg present | - | High | Normal | Near normal |
| Phase 2: HBeAg-positive chronic hepatitis B | HBeAg present | - | High | High fluctuating | Moderate/severe necroinflammation and fibrosis progression |
| Phase 3: HBeAg-negative chronic HBV infection | - | anti-HBe | Undetectable or low (<2000 IU/mL) | Normal | Minimal necroinflammation and low fibrosis |
| Phase 4: HBeAg-negative chronic hepatitis B | No HBeAg | anti-HBe | >2000 IU/ml | High fluctuating | Necroinflammation and fibrosis |
| Phase 5: HBsAg-negative phase | No HBsAg | anti-HBc | Usually undetectable | Normal |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, J.P.; de Carvalho, A.; Paiva, A.; Borges, O. Insights into Immune Exhaustion in Chronic Hepatitis B: A Review of Checkpoint Receptor Expression. Pharmaceuticals 2024, 17, 964. https://doi.org/10.3390/ph17070964
Costa JP, de Carvalho A, Paiva A, Borges O. Insights into Immune Exhaustion in Chronic Hepatitis B: A Review of Checkpoint Receptor Expression. Pharmaceuticals. 2024; 17(7):964. https://doi.org/10.3390/ph17070964
Chicago/Turabian StyleCosta, João Panão, Armando de Carvalho, Artur Paiva, and Olga Borges. 2024. "Insights into Immune Exhaustion in Chronic Hepatitis B: A Review of Checkpoint Receptor Expression" Pharmaceuticals 17, no. 7: 964. https://doi.org/10.3390/ph17070964
APA StyleCosta, J. P., de Carvalho, A., Paiva, A., & Borges, O. (2024). Insights into Immune Exhaustion in Chronic Hepatitis B: A Review of Checkpoint Receptor Expression. Pharmaceuticals, 17(7), 964. https://doi.org/10.3390/ph17070964






