Close Cardiovascular Monitoring during the Early Stages of Treatment for Patients Receiving Immune Checkpoint Inhibitors
Abstract
1. Introduction
2. Results
2.1. Study Population
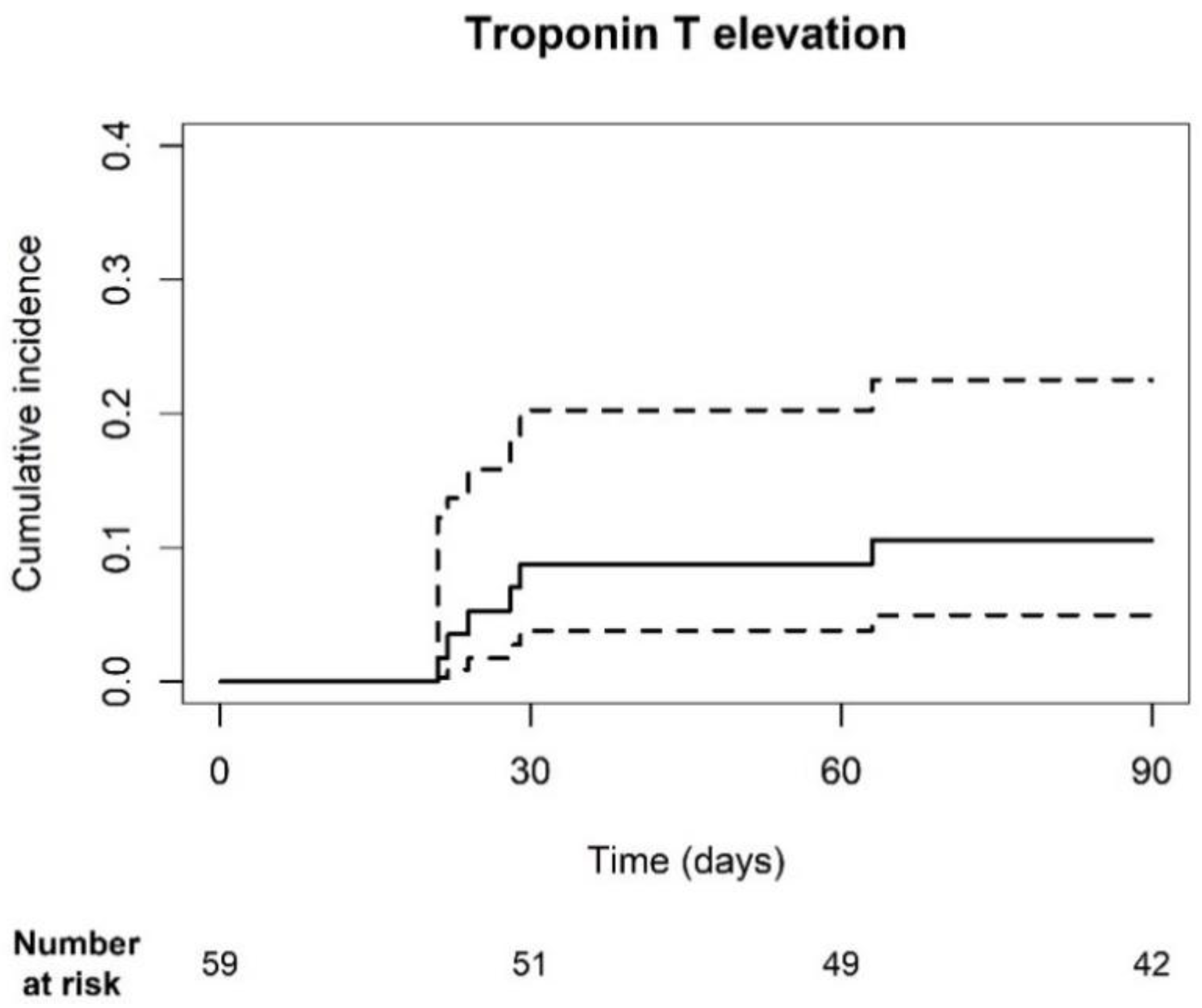
2.2. Cardiac Biomarkers: Hs-TnT, Hs-TnI, and NT-ProBNP
2.3. Agreement between hs-TnI and hs-TnT Elevations
2.4. Echocardiography Parameters at Baseline and Three Months
3. Discussion
4. Methods
4.1. Study Population
4.2. Medical History and Biochemical Parameters
4.3. Three-Dimensional Transthoracic Echocardiography
4.4. Study Endpoints
- The incidence of hs-TnI and NT-proBNP above the ULN;
- Evolution of TTE parameters;
- Association between the evolution of troponin/NT-proBNP and TTE and electrocardiography parameters;
- Agreement between hs-TnT and hs-TnI levels.
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Aren Frontera, O.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthélémy, P.; Porta, C.; George, S.; et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 378, 1277. [Google Scholar] [CrossRef]
- Johnson, D.B.; Nebhan, C.A.; Moslehi, J.J.; Balko, J.M. Immune-checkpoint inhibitors: Long-term implications of toxicity. Nat. Rev. Clin. Oncol. 2022, 19, 254–267. [Google Scholar] [CrossRef]
- Palaskas, N.; Lopez-Mattei, J.; Durand, J.B.; Iliescu, C.; Deswal, A. Immune Checkpoint Inhibitor Myocarditis: Pathophysiological Characteristics, Diagnosis, and Treatment. J. Am. Heart Assoc. 2020, 9, e013757. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, M.; Nielsen, D.; Svane, I.M.; Iversen, K.; Rasmussen, P.V.; Madelaire, C.; Fosbøl, E.; Køber, L.; Gustafsson, F.; Andersson, C.; et al. The risk of cardiac events in patients receiving immune checkpoint inhibitors: A nationwide Danish study. Eur. Heart J. 2021, 42, 1621–1631. [Google Scholar] [CrossRef]
- Totzeck, M.; Lutgens, E.; Neilan, T.G. Are we underestimating the potential for cardiotoxicity related to immune checkpoint inhibitors? Eur. Heart J. 2021, 42, 1632–1635. [Google Scholar] [CrossRef] [PubMed]
- Laenens, D.; Yu, Y.; Santens, B.; Jacobs, J.; Beuselinck, B.; Bechter, O.; Wauters, E.; Staessen, J.; Janssens, S.; Van Aelst, L. Incidence of Cardiovascular Events in Patients Treated with Immune Checkpoint Inhibitors. J. Clin. Oncol. 2022, 40, 3430–3438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, Z.; Qin, S.; Zhu, Y.; Shu, L.; Zuo, Z. Incidence of adverse cardiovascular events associated with immune checkpoint inhibitors and risk factors for left ventricular dysfunction: A single-center prospective clinical study. Front. Cardiovasc. Med. 2023, 10, 1052699. [Google Scholar] [CrossRef]
- Rini, B.I.; Moslehi, J.J.; Bonaca, M.; Schmidinger, M.; Albiges, L.; Choueiri, T.K.; Motzer, R.J.; Atkins, M.B.; Haanen, J.; Mariani, M.; et al. Prospective Cardiovascular Surveillance of Immune Checkpoint Inhibitor-Based Combination Therapy in Patients with Advanced Renal Cell Cancer: Data from the Phase III JAVELIN Renal 101 Trial. J. Clin. Oncol. 2022, 40, 1929–1938. [Google Scholar] [CrossRef]
- Faubry, C.; Faure, M.; Toublanc, A.-C.; Veillon, R.; Lemaître, A.-I.; Vergnenègre, C.; Cochet, H.; Khan, S.; Raherison, C.; Dos Santos, P.; et al. A Prospective Study to Detect Immune Checkpoint Inhibitors Associated with Myocarditis Among Patients Treated for Lung Cancer. Front. Cardiovasc. Med. 2022, 9, 878211. [Google Scholar] [CrossRef]
- Tamura, Y.; Takemura, R.; Yamada, K.; Taniguchi, H.; Iwasawa, J.; Yada, H.; Kawamura, A. Longitudinal Strain and Troponin I Elevation in Patients Undergoing Immune Checkpoint Inhibitor Therapy. JACC CardioOncol. 2022, 4, 673. [Google Scholar] [CrossRef]
- Furukawa, A.; Tamura, Y.; Taniguchi, H.; Kawamura, A.; Nagase, S.; Hayashi, A.; Tada, Y.; Sase, K.; Hatake, K. Prospective screening for myocarditis in cancer patients treated with immune checkpoint inhibitors. J. Cardiol. 2023, 81, 63–67. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Lacchetti, C.; Schneider, B.J.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; Ernstoff, M.S.; Gardner, J.M.; Ginex, P.; et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J. Clin. Oncol. 2018, 36, 1714–1768. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.J.; Naidoo, J.; Santomasso, B.D.; Lacchetti, C.; Adkins, S.; Anadkat, M.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; et al. Management of Immune-Related Adverse Events in Patients Treated with Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J. Clin. Oncol. 2021, 39, 4073–4126. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.A.; Schneider, B.J.; Brahmer, J.; Achufusi, A.; Armand, P.; Berkenstock, M.K.; Bhatia, S.; Budde, L.E.; Chokshi, S.; Davies, M.; et al. Management of Immunotherapy-Related Toxicities, Version 1.2022, NCCN Clinical Practice Guidelines in Oncology. Off. J. Natl. Compr. Cancer Netw. 2022, 20. [Google Scholar] [CrossRef]
- Lyon, A.R.; Lopez-Fernandez, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS)Developed by the task force on cardio-oncology of the European Society of Cardiology (ESC). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef] [PubMed]
- Thuny, F.; Bonaca, M.P.; Cautela, J. What Is the Evidence of the Diagnostic Criteria and Screening of Immune Checkpoint Inhibitor–Induced Myocarditis? JACC CardioOncol. 2022, 4, 624–628. [Google Scholar] [CrossRef]
- National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Common Terminology Criteria for Adverse Events (CTCAE) v5.0. 2017. Available online: http://evs.nci.nih.gov/ftp1/CTCAE/About.html (accessed on 1 June 2024).
- Welsh, P.; Preiss, D.; Shah, A.S.; McAllister, D.; Briggs, A.; Boachie, C.; McConnachie, A.; Hayward, C.; Padmanabhan, S.; Welsh, C.; et al. Comparison between High Sensitivity Cardiac Troponin T and Cardiac Troponin I in a Large General Population Cohort. Clin. Chem. 2018, 64, 1607. [Google Scholar] [CrossRef]
- Escudier, M.; Cautela, J.; Malissen, N.; Ancedy, Y.; Orabona, M.; Pinto, J.; Monestier, S.; Grob, J.-J.; Scemama, U.; Jacquier, A.; et al. Clinical features, management, and outcomes of immune checkpoint inhibitor-related cardiotoxicity. Circulation 2017, 136, 2085–2087. [Google Scholar] [CrossRef]
- Mahmood, S.S.; Fradley, M.G.; Cohen, J.V.; Nohria, A.; Reynolds, K.L.; Heinzerling, L.M.; Sullivan, R.J.; Damrongwatanasuk, R.; Chen, C.L.; Gupta, D.; et al. Myocarditis in Patients Treated with Immune Checkpoint Inhibitors. J. Am. Coll. Cardiol. 2018, 71, 1755–1764. [Google Scholar] [CrossRef]
- Delombaerde, D.; Vervloet, D.; Franssen, C.; Croes, L.; Gremonprez, F.; Prenen, H.; Peeters, M.; Vulsteke, C. Clinical implications of isolated troponinemia following immune checkpoint inhibitor therapy. ESMO Open 2021, 6, 100216. [Google Scholar] [CrossRef] [PubMed]
- Sarocchi, M.; Grossi, F.; Arboscello, E.; Bellodi, A.; Genova, C.; Bello, M.G.D.; Rijavec, E.; Barletta, G.; Rossi, G.; Biello, F.; et al. Serial Troponin for Early Detection of Nivolumab Cardiotoxicity in Advanced Non-Small Cell Lung Cancer Patients. Oncologist 2018, 23, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Petricciuolo, S.; Delle Donne, M.G.; Aimo, A.; Chella, A.; De Caterina, R. Pre-treatment high-sensitivity troponin T for the short-term prediction of cardiac outcomes in patients on immune checkpoint inhibitors. Eur. J. Clin. Investig. 2020, 51, e13400. [Google Scholar] [CrossRef] [PubMed]
- Chuy, K.L.; Oikonomou, E.K.; Postow, M.A.; Callahan, M.K.; Chapman, P.B.; Shoushtari, A.N.; Neilan, T.G.; Fradley, M.G.; Ramanathan, L.V.; Wolchok, J.D.; et al. Myocarditis Surveillance in Patients with Advanced Melanoma on Combination Immune Checkpoint Inhibitor Therapy: The Memorial Sloan Kettering Cancer Center Experience. Oncologist 2019, 24, e196–e197. [Google Scholar] [CrossRef] [PubMed]
- Waliany, S.; Neal, J.W.; Reddy, S.; Wakelee, H.; Shah, S.A.; Srinivas, S.; Padda, S.K.; Fan, A.C.; Colevas, A.D.; Wu, S.M.; et al. Myocarditis Surveillance with High-Sensitivity Troponin I During Cancer Treatment with Immune Checkpoint Inhibitors. JACC CardioOncol. 2021, 3, 137–139. [Google Scholar] [CrossRef] [PubMed]
- Mirabel, M.; Eslami, A.; Thibault, C.; Oudard, S.; Mousseaux, E.; Wahbi, K.; Fabre, E.; Terrier, B.; Marijon, E.; Villefaillot, A.; et al. Adverse myocardial and vascular side effects of immune checkpoint inhibitors: A prospective multimodal cardiovascular assessment. Clin. Res. Cardiol. 2024, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Toublanc, A.-C.; Faure, M.; Verdy, G.; Rabeau, A.; Houard, V.; Veillon, R.; Bardel, C.; Vergnenegre, C.; Dos Santos, P.; Mazieres, J.; et al. Prospective cardiovascular events in patients with advanced thoracic cancer treated with immune checkpoint inhibitor. Eur. J. Cancer 2024, 207, 114191. [Google Scholar] [CrossRef] [PubMed]
- Tamura, Y.; Tamura, Y.; Yamada, K.; Taniguchi, H.; Iwasawa, J.; Yada, H.; Kawamura, A. Routine assessment of cardiotoxicity in patients undergoing long-term immune checkpoint inhibitor therapy. Heart Vessel. 2022, 37, 1859–1865. [Google Scholar] [CrossRef] [PubMed]
- Kurzhals, J.K.; Graf, T.; Boch, K.; Grzyska, U.; Frydrychowicz, A.; Zillikens, D.; Terheyden, P.; Langan, E.A. Serum Troponin T Concentrations Are Frequently Elevated in Advanced Skin Cancer Patients Prior to Immune Checkpoint Inhibitor Therapy: Experience From a Single Tertiary Referral Center. Front. Med. 2021, 8, 665. [Google Scholar] [CrossRef]
- Isawa, T.; Toi, Y.; Sugawara, S.; Taguri, M.; Toyoda, S. Incidence, Clinical Characteristics, and Predictors of Cardiovascular Immune-Related Adverse Events Associated with Immune Checkpoint Inhibitors. Oncologist 2022, 27, e410. [Google Scholar] [CrossRef]
- Waissengein, B.; Abu Ata, B.; Merimsky, O.; Shamai, S.; Wolf, I.; Arnold, J.H.; Bar-On, T.; Banai, S.; Khoury, S.; Laufer-Perl, M. The predictive value of high sensitivity troponin measurements in patients treated with immune checkpoint inhibitors. Clin. Res. Cardiol. 2023, 112, 409–418. [Google Scholar] [CrossRef]
- Nishikawa, T.; Inoue, T.; Otsuka, T.; Kuno, I.; Kukita, Y.; Nakamura, H.; Ikeda, Y.; Yasui, T.; Shioyama, W.; Oka, T.; et al. Prevalence and characteristics of immune checkpoint inhibitor-related myocardial damage: A prospective observational study. PLoS ONE 2022, 17, e0275865. [Google Scholar] [CrossRef]
- Xu, A.; Yuan, M.; Zhan, X.; Zhao, G.; Mu, G.; Wang, T.; Hu, H.; Fu, H. Early detection of immune checkpoint inhibitor-related subclinical cardiotoxicity: A pilot study by using speckle tracking imaging and three-dimensional echocardiography. Front. Cardiovasc. Med. 2022, 17, e0275865. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Fourth Universal Definition of Myocardial Infarction (2018). Circulation 2018, 138, e618–e651. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.-R.; Florido, R.; Lipson, E.J.; Naidoo, J.; Ardehali, R.; Tocchetti, C.G.; Lyon, A.R.; Padera, R.F.; Johnson, D.B.; Moslehi, J. Cardiovascular toxicities associated with immune checkpoint inhibitors. Cardiovasc. Res. 2019, 115, 854–868. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, E895–E1032. [Google Scholar] [CrossRef] [PubMed]
- Ananthan, K.; Lyon, A.R. The Role of Biomarkers in Cardio-Oncology. J. Cardiovasc. Transl. Res. 2020, 13, 431–450. [Google Scholar] [CrossRef]
- Pavo, N.; Raderer, M.; Hülsmann, M.; Neuhold, S.; Adlbrecht, C.; Strunk, G.; Goliasch, G.; Gisslinger, H.; Steger, G.G.; Hejna, M.; et al. Cardiovascular biomarkers in patients with cancer and their association with all-cause mortality. Heart 2015, 101, 1874–1880. [Google Scholar] [CrossRef] [PubMed]
- Welsh, P.; Preiss, D.; Sattar, N. Utility of High-Sensitivity Troponin T and I: Are They the Same?—American College of Cardiology 2019. Available online: https://www.acc.org/Latest-in-Cardiology/Articles/2019/11/21/07/26/Utility-of-High-Sensitivity-Troponin-T-and-I (accessed on 13 September 2023).
- Lehmann, L.H.; Heckmann, M.B.; Bailly, G.; Finke, D.; Procureur, A.; Power, J.R.; Stein, F.; Bretagne, M.; Ederhy, S.; Fenioux, C.; et al. Cardiomuscular Biomarkers in the Diagnosis and Prognostication of Immune Checkpoint Inhibitor Myocarditis. Circulation 2023, 148, 473–486. [Google Scholar] [CrossRef]
- Kalam, K.; Otahal, P.; Marwick, T.H. Prognostic implications of global LV dysfunction: A systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart 2014, 100, 1673–1680. [Google Scholar] [CrossRef]
- Tamura, Y.; Tamura, Y. Usefulness of Longitudinal Strain to Assess Cancer Therapy-Related Cardiac Dysfunction and Immune Checkpoint Inhibitor-Induced Myocarditis. Pharm 2023, 16, 1297. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, E.K.; Kokkinidis, D.G.; Kampaktsis, P.N.; Amir, E.A.; Marwick, T.H.; Gupta, D.; Thavendiranathan, P. Assessment of Prognostic Value of Left Ventricular Global Longitudinal Strain for Early Prediction of Chemotherapy-Induced Cardiotoxicity: A Systematic Review and Meta-analysis. JAMA Cardiol. 2019, 4, 1007. [Google Scholar] [CrossRef] [PubMed]
- Sławiński, G.; Hawryszko, M.; Liżewska-Springer, A.; Nabiałek-Trojanowska, I.; Lewicka, E. Global Longitudinal Strain in Cardio-Oncology: A Review. Cancers 2023, 15, 986. [Google Scholar] [CrossRef]
- McGregor, P.C.; Moura, F.A.; Banchs, J.; Aragam, J.R. Role of myocardial strain imaging in surveillance and management of cancer therapeutics-related cardiac dysfunction: A systematic review. Echocardiography 2021, 38, 314–328. [Google Scholar] [CrossRef] [PubMed]
- Awadalla, M.; Mahmood, S.S.; Groarke, J.D.; Hassan, M.Z.; Nohria, A.; Rokicki, A.; Murphy, S.P.; Mercaldo, N.D.; Zhang, L.; Zlotoff, D.A.; et al. Global Longitudinal Strain and Cardiac Events in Patients with Immune Checkpoint Inhibitor-Related Myocarditis. J. Am. Coll. Cardiol. 2020, 75, 467. [Google Scholar] [CrossRef] [PubMed]
- Pohl, J.; Totzeck, M.; Mincu, R.; Margraf, S.M.; Scheipers, L.; Michel, L.; Mahabadi, A.A.; Zimmer, L.; Rassaf, T.; Hendgen-Cotta, U.B. Right ventricular and atrial strain in patients with advanced melanoma undergoing immune checkpoint inhibitor therapy. ESC Heart Fail. 2022, 9, 3533. [Google Scholar] [CrossRef] [PubMed]
- Mincu, R.; Pohl, J.; Mrotzek, S.; Michel, L.; Hinrichs, L.; Lampe, L.; Rassaf, T.; Totzeck, M. Left ventricular global longitudinal strain reduction in patients with melanoma and extra-cardiac immune-related adverse events during immune checkpoint inhibitor therapy. Eur. Heart J. 2020, 41, ehaa946.3261. [Google Scholar] [CrossRef]
- Delombaerde, D.; De Sutter, J.; Croes, L.; Vervloet, D.; Moerman, V.; Van de Veire, N.; Willems, A.-M.; Wouters, K.; Peeters, M.; Prenen, H.; et al. Extensive CArdioVAscular Characterization and Follow-Up of Patients Receiving Immune Checkpoint Inhibitors: A Prospective Multicenter Study. Pharmaceuticals 2023, 16, 625. [Google Scholar] [CrossRef] [PubMed]
- Douglas, P.S.; Garcia, M.J.; Haines, D.E.; Lai, W.W.; Manning, W.J.; Patel, A.R.; Picard, M.H.; Polk, D.M.; Ragosta, M.; Ward, R.P.; et al. ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 Appropriate use criteria for echocardiography. J. Am. Soc. Echocardiogr. 2011, 24, 229–267. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Patient Cohort N = 59 (%) | |
|---|---|---|
| General | ECOG 0 1 2 3 | 30 (51) 16 (27) 7 (12) 6 (10) |
| Male gender | 45 (76) | |
| Mean age (years) | 68 ± 12 | |
| BMI (kg/m2) | 24.8 ± 4.1 | |
| Race/ethnicity | Caucasian | 59 (100) |
| Primary tumor type | Renal cell carcinoma | 15 (25) |
| Bladder cancer | 16 (27) | |
| Melanoma | 14 (24) | |
| HNSCC | 6 (10) | |
| NSCLC | 2 (3) | |
| Merkel cell carcinoma | 2 (3) | |
| Other | 4 (7) | |
| ICI regimen | Pembrolizumab | 22 (37) |
| Cemiplimab | 1 (2) | |
| Avelumab | 11 (19) | |
| Nivolumab | 10 (17) | |
| Nivolumab + ipilimumab | 15 (25) | |
| Line of systemic treatment ° | 1st line | 33 (56) |
| 2nd line | 25 (42) | |
| ≥3rd line | 1 (2) | |
| Risk factors | Diabetes mellitus | 10 (17) |
| Hypercholesterolemia | 39 (66) | |
| Arterial hypertension | 27 (46) | |
| Smoking status Current smoker Former smoker Never | 8 (14) 29 (49) 22 (37) | |
| Prior chest irradiation | 2 (3) | |
| eGFR < 60 mL/min | 21 (36) | |
| Cardiac history | Coronary artery disease *- | 10/54 (19) |
| Peripheral artery disease - | 9/52 (17) | |
| Stroke - | 5/58 (9) | |
| TIA | 1 (2) | |
| Heart failure | 3 (5) | |
| LVEF < 50% - | 5/54 (9) | |
| Calcium score > 400 +- | 14/52 (27) | |
| Cardiac biomarkers | Hs-TnT (ng/L) Hs-TnI (ng/L) NT-proBNP (pg/mL) | 11.6 [7.9; 19.5] 3.7 [2.5; 9.5] 148.0 [59.9; 413.0] |
| Medication | ACE-I/ARBs | 17 (29) |
| Beta blockers | 22 (37) | |
| Diuretics | 14 (24) | |
| Nitrate | 1 (2) | |
| SGLT2-inhibitors | 2 (3) | |
| Statins | 29 (49) |
| Troponin I. | Baseline < 45.2 ng/L | Baseline ≥ 45.2 ng/L |
|---|---|---|
| 3-month < 45.2 ng/L | 48/49 | 0/49 |
| 3-month ≥ 45.2 ng/L | 0/49 | 1/49 |
| NT-proBNP | Baseline < 125 pg/mL | Baseline ≥ 125 pg/mL |
| 3-month < 125 pg/mL | 21/49 | 5/49 |
| 3-month ≥ 125 pg/mL | 2/49 | 21/49 |
| Baseline (n = 59) | 3 Months (n = 49) | |||
|---|---|---|---|---|
| Troponin | Hs-TnI < 45.2 | Hs-TnI ≥ 45.2 | Hs-TnI < 45.2 | Hs-TnI ≥ 45.2 |
| Hs-TnT < 14.0 ng/L | 36 | 0 | 33 | 0 |
| Hs-TnT ≥ 14.0 ng/L | 20 | 3 | 15 | 1 |
| Parameters. | n | Baseline | 3 Months | p |
|---|---|---|---|---|
| 3D-LVEF (%) | 44 | 56 ± 6 | 56 ± 6 | 0.90 |
| LVEDV (mL) | 43 | 105 [82; 129] | 108 [94; 133] | 0.25 |
| LVESV (mL) | 43 | 45 [34; 59] | 48 [38; 63] | 0.24 |
| GLS (%) 2-chamber (%) 3-chamber (%) 4-chamber (%) | 37 34 33 34 | −17.8 [−18.5; −14.2] −16.92 ± 3.64 −18.0 [−19.0; −14.9] −16.81 ± 3.27 | −17.0 [−18.8; −15.1] −16.84 ± 3.36 −17.0 [−18.8; −16.0] −16.59 ± 3.44 | 0.66 0.91 0.90 0.70 |
| Right ventricular function TAPSE (mm) s’-wave (cm/s) | 48 47 | 23 ± 5 12 ± 3 | 22 ± 4 13 ± 3 | 0.34 0.58 |
| Dimensions Left atrial area (cm2) | 47 | 17 ± 4 | 18 ± 5 | 0.26 |
| Diastolic function E (cm/s) A (cm/s) E/A ratio Deceleration time (ms) Peak e’ velocity of septal wall (cm/s) Peak e’ velocity of lateral wall (cm/s) E/e’ septal wall E/e’ lateral wall MV E/e’ average | 48 47 47 46 49 49 48 48 48 | 62 ± 16 76 ± 20 0.8 [0.7; 0.9] 194 ± 55 8 ± 2 9 ± 3 8 [6; 10] 7 [5; 8] 7 [6; 9] | 63 ± 20 76 ± 17 0.8 [0.6; 1.0] 194 ± 62 8 ± 2 9 ± 3 8 [7; 10] 7 [5; 8] 7 [6; 9] | 0.54 0.94 0.82 0.99 0.96 0.85 0.67 0.86 0.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delombaerde, D.; Vulsteke, C.; Van de Veire, N.; Vervloet, D.; Moerman, V.; Van Calster, L.; Willems, A.-M.; Croes, L.; Gremonprez, F.; De Meulenaere, A.; et al. Close Cardiovascular Monitoring during the Early Stages of Treatment for Patients Receiving Immune Checkpoint Inhibitors. Pharmaceuticals 2024, 17, 965. https://doi.org/10.3390/ph17070965
Delombaerde D, Vulsteke C, Van de Veire N, Vervloet D, Moerman V, Van Calster L, Willems A-M, Croes L, Gremonprez F, De Meulenaere A, et al. Close Cardiovascular Monitoring during the Early Stages of Treatment for Patients Receiving Immune Checkpoint Inhibitors. Pharmaceuticals. 2024; 17(7):965. https://doi.org/10.3390/ph17070965
Chicago/Turabian StyleDelombaerde, Danielle, Christof Vulsteke, Nico Van de Veire, Delphine Vervloet, Veronique Moerman, Lynn Van Calster, Anne-Marie Willems, Lieselot Croes, Félix Gremonprez, Astrid De Meulenaere, and et al. 2024. "Close Cardiovascular Monitoring during the Early Stages of Treatment for Patients Receiving Immune Checkpoint Inhibitors" Pharmaceuticals 17, no. 7: 965. https://doi.org/10.3390/ph17070965
APA StyleDelombaerde, D., Vulsteke, C., Van de Veire, N., Vervloet, D., Moerman, V., Van Calster, L., Willems, A.-M., Croes, L., Gremonprez, F., De Meulenaere, A., Elzo Kraemer, X., Wouters, K., Peeters, M., Prenen, H., & De Sutter, J. (2024). Close Cardiovascular Monitoring during the Early Stages of Treatment for Patients Receiving Immune Checkpoint Inhibitors. Pharmaceuticals, 17(7), 965. https://doi.org/10.3390/ph17070965







