Ferulic Acid Alleviates Radiation-Induced Immune Damage by Acting on JAK/STAT Signaling Pathway
Abstract
1. Introduction
2. Results
2.1. Main Text
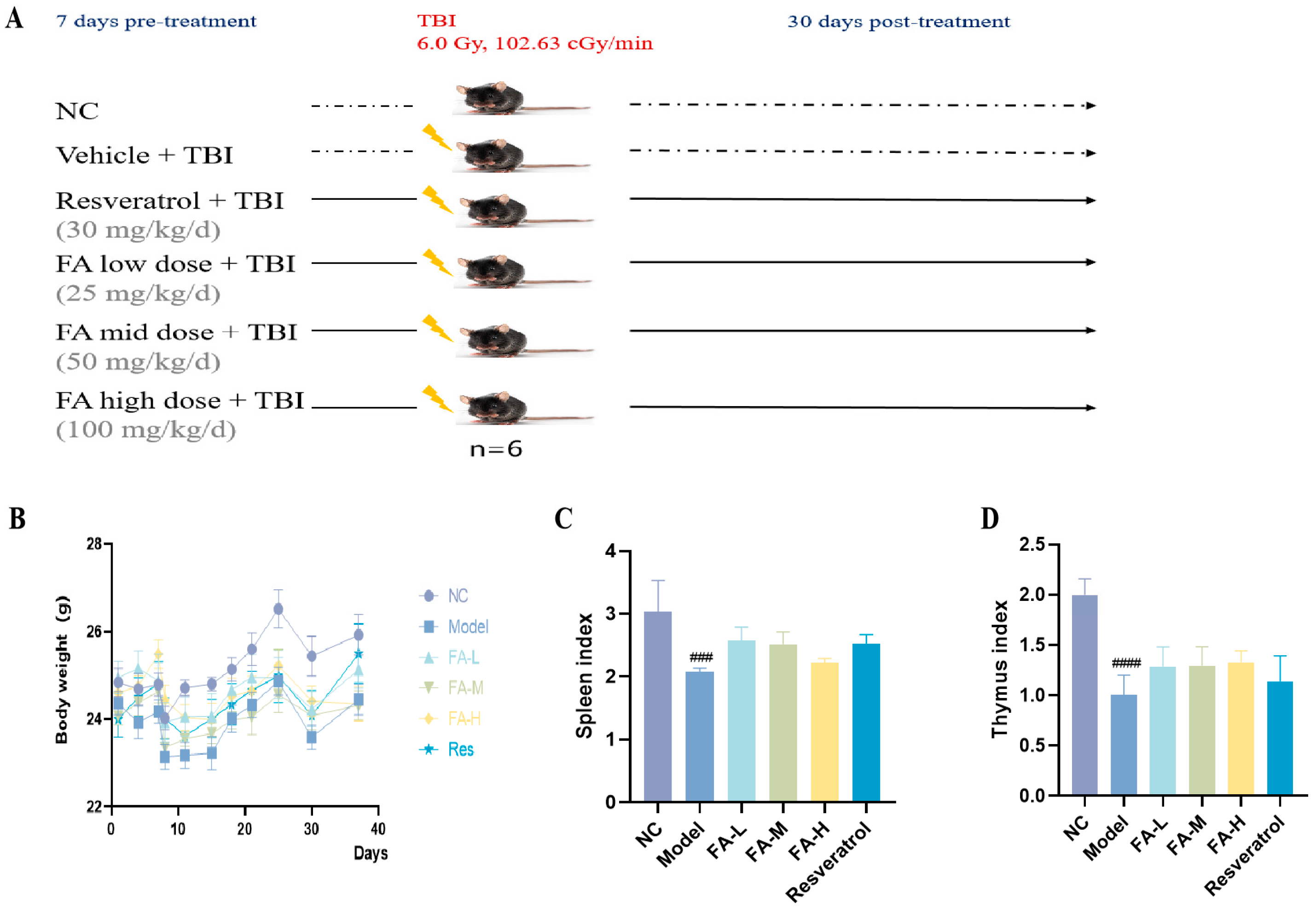
2.1.1. FA Promoted the Recovery of Body Weight and Organ Index of Irradiated Mice
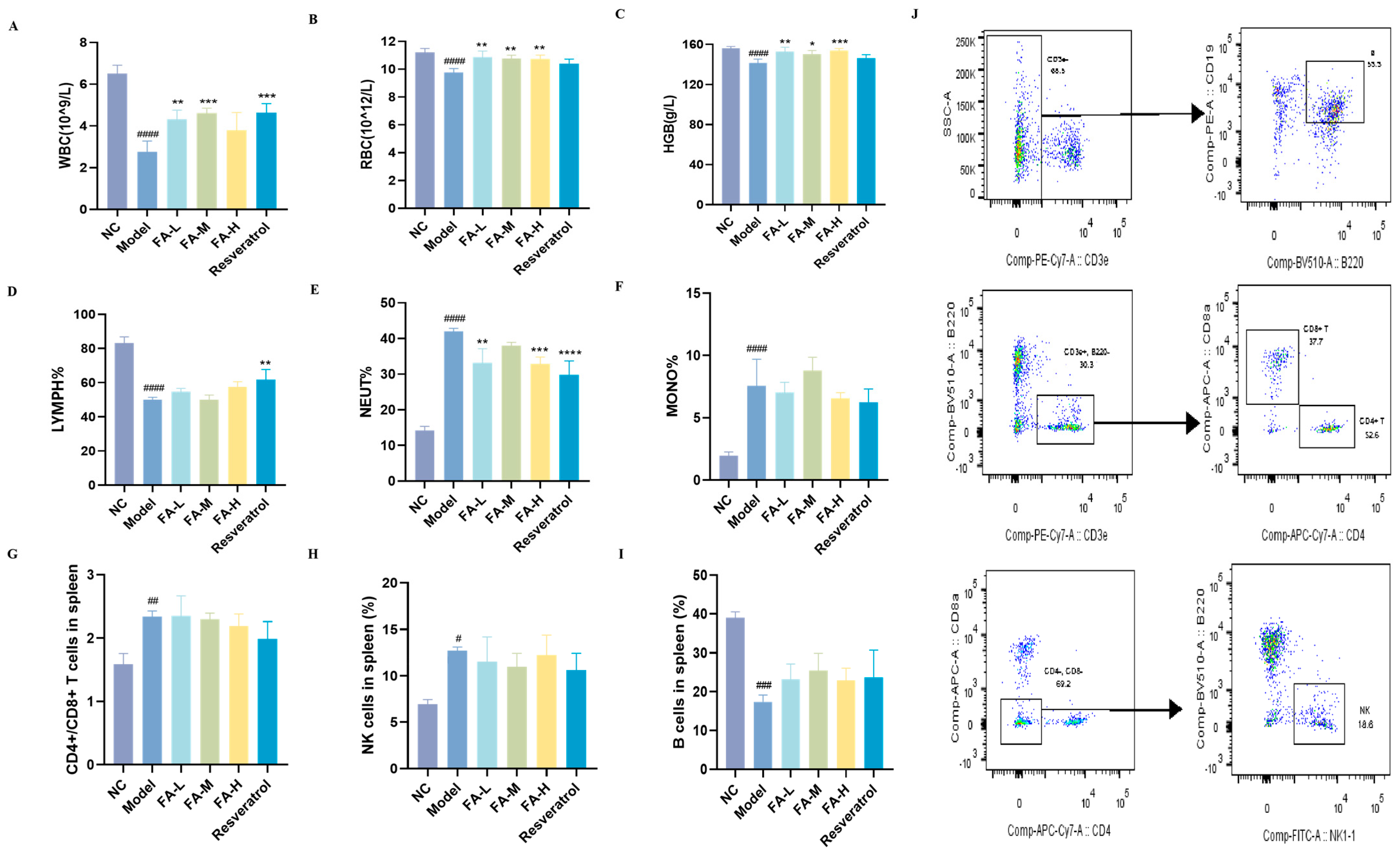
2.1.2. FA Alleviated the Imbalance of Blood/Spleen Immunity in Irradiated Mice
2.1.3. FA Promoted the Recovery of Hematopoietic Stem and Progenitor Cells (HSPCs) in BM after Irradiation
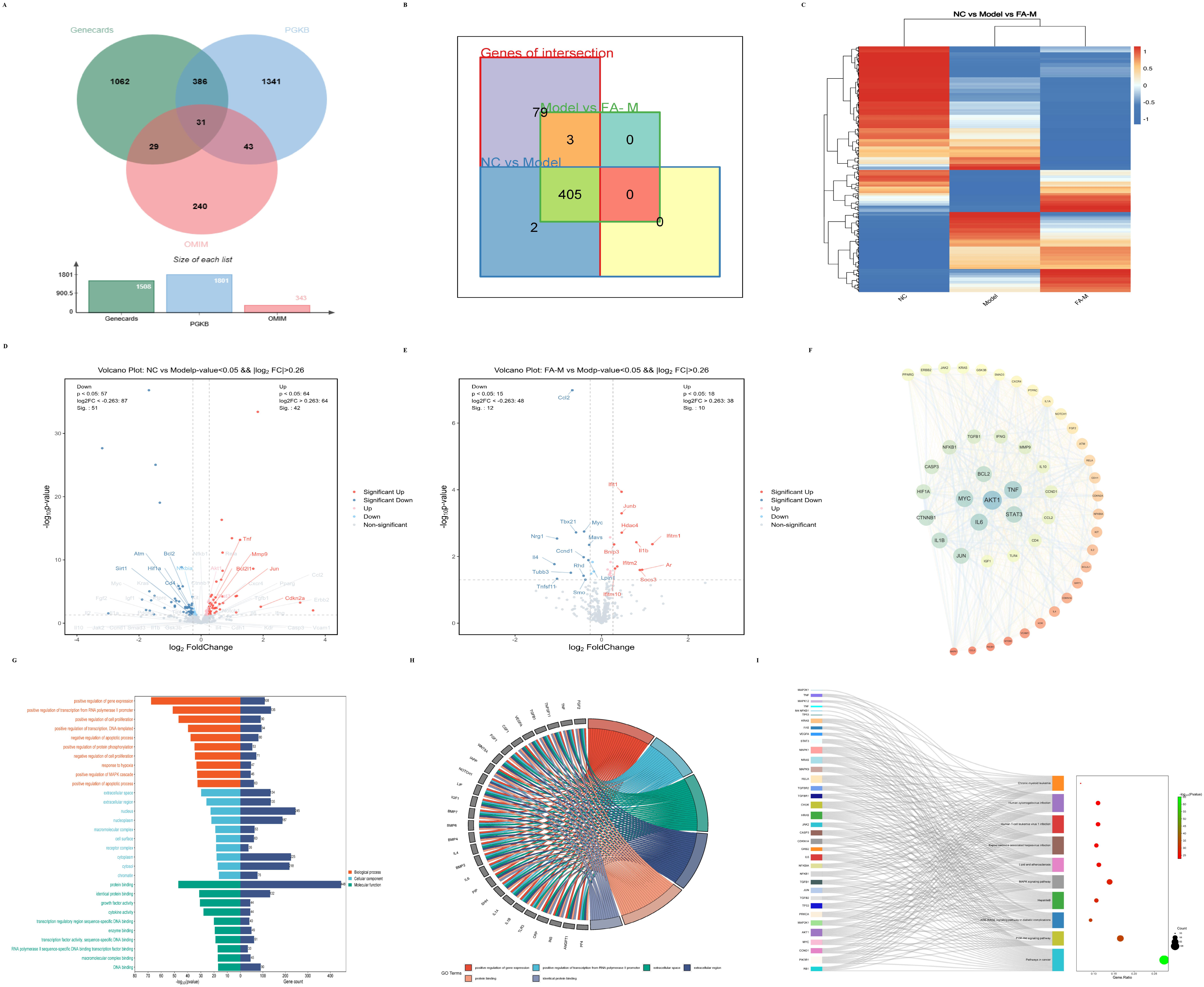
2.1.4. Network Pharmacology to Screen the Core Targets and Signaling Pathways Related to Aging in the Differential Genes
2.1.5. Molecular Docking Results of FA with JAK1 and JAK2
2.1.6. FA Reduced Oxidative Stress and Inflammation in Serum and BM of Irradiated Mice
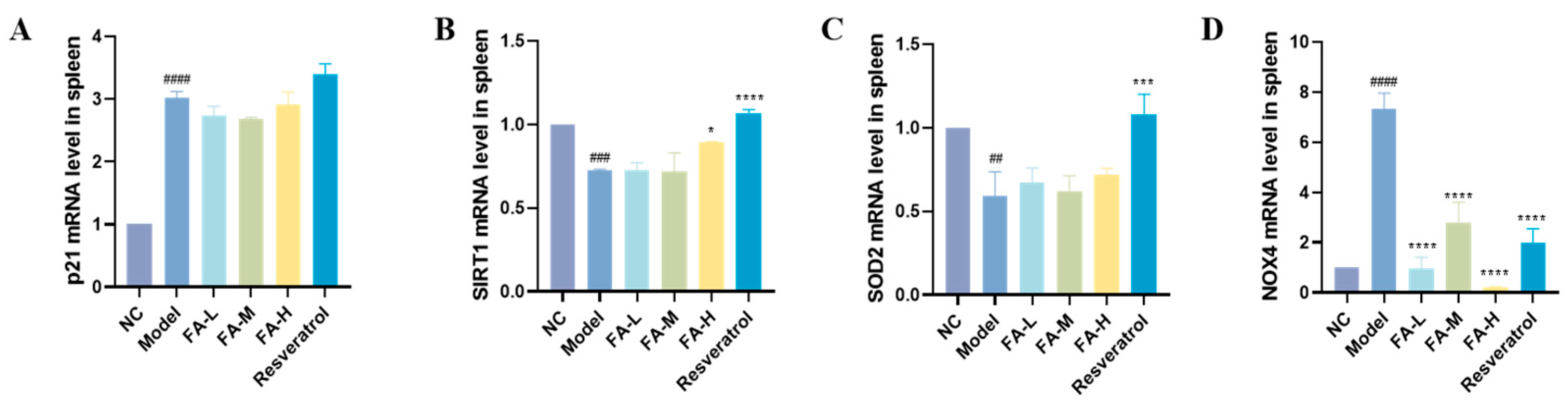
2.1.7. FA Increased SIRT1 Gene and Decreased NOX4 Gene Expression in Spleen
2.1.8. FA Reduced the Pathological Damage to the Spleen, Femur, and Thymus in Irradiated Mice
2.1.9. Other Possible Functions by Which FA Attenuated Immune Injury
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Animals and IR
4.3. Blood Cell Counts
4.4. Calculation of Organ Index
4.5. Flow Cytometry Analysis of HSPCs in BM and Immune Cells in Spleen
4.6. Transcriptome Sequencing
4.7. Network Pharmacology
4.8. Molecular Docking
4.9. Biochemical and Cytokine Analysis in Mouse Serum and BM
4.10. Quantitative Real-Time PCR (qRT-PCR)
4.11. Hematoxylin–Eosin (H&E) Staining
4.12. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liao, W.; Liu, C.; Yang, K.; Chen, J.; Wu, Y.; Zhang, S.; Yu, K.; Wang, L.; Ran, L.; Chen, M.; et al. Aged hematopoietic stem cells entrap regulatory T cells to create a prosurvival microenvironment. Cell. Mol. Immunol. 2023, 20, 1216–1231. [Google Scholar] [CrossRef] [PubMed]
- Cassatt, D.R.; Winters, T.A.; Prabhudas, M. Immune Dysfunction from Radiation Exposure. Radiat. Res. 2023, 200, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Ji, C.; Zheng, C.; Wu, X.-Y.; He, W.-J.; Li, W.-Z.; Yin, H. Research Progress on the Anti-ionizing Radiation Activity of Active Natural Products. Food Sci. 2023, 44, 390–400. [Google Scholar]
- Tyuryaeva, I.; Lyublinskaya, O. Expected and Unexpected Effects of Pharmacological Antioxidants. Int. J. Mol. Sci. 2023, 24, 9303. [Google Scholar] [CrossRef] [PubMed]
- Moldogazieva, N.T.; Mokhosoev, I.M.; Mel’nikova, T.I.; Porozov, Y.B.; Terentiev, A.A. Oxidative Stress and Advanced Lipoxidation and Glycation End Products (ALEs and AGEs) in Aging and Age-Related Diseases. Oxidative Med. Cell. Longev. 2019, 2019, 3085756. [Google Scholar] [CrossRef]
- Zhai, Y.; Wang, T.; Fu, Y.; Yu, T.; Ding, Y.; Nie, H. Ferulic Acid: A Review of Pharmacology, Toxicology, and Therapeutic Effects on Pulmonary Diseases. Int. J. Mol. Sci. 2023, 24, 8011. [Google Scholar] [CrossRef]
- Ma, Z.C.; Hong, Q.; Wang, Y.G.; Tan, H.L.; Xiao, C.R.; Liang, Q.D.; Wang, D.G.; Gao, Y. Ferulic acid protects lymphocytes from radiation-predisposed oxidative stress through extracellular regulated kinase. Int. J. Radiat. Biol. 2011, 87, 130–140. [Google Scholar] [CrossRef]
- Wagle, S.; Sim, H.J.; Bhattarai, G.; Choi, K.-C.; Kook, S.-H.; Lee, J.-C.; Jeon, Y.-M. Supplemental Ferulic Acid Inhibits Total Body Irradiation-Mediated Bone Marrow Damage, Bone Mass Loss, Stem Cell Senescence, and Hematopoietic Defect in Mice by Enhancing Antioxidant Defense Systems. Antioxidants 2021, 10, 1209. [Google Scholar] [CrossRef]
- Chen, Y.; Shen, J.; Zhang, X.; Gao, W.; Cao, Q.; Yan, F.; Xue, C. Protective effects of ferulic acid against ionizing radiation-induced oxidative damage in rat lens through activating Nrf2 signal pathway. Int. J. Ophthalmol. 2023, 16, 687–693. [Google Scholar] [CrossRef]
- Shao, L.; Feng, W.; Li, H.; Gardner, D.; Luo, Y.; Wang, Y.; Liu, L.; Meng, A.; Sharpless, N.E.; Zhou, D. Total body irradiation causes long-term mouse BM injury via induction of HSC premature senescence in an Ink4a- and Arf-independent manner. Blood 2014, 123, 3105–3115. [Google Scholar] [CrossRef]
- Li, W.; Wang, X.; Dong, Y.; Huo, Q.; Yue, T.; Wu, X.; Lu, L.; Zhang, J.; Zhao, Y.; Dong, H.; et al. Nicotinamide riboside intervention alleviates hematopoietic system injury of ionizing radiation-induced premature aging mice. Aging Cell 2023, 22, e13976. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Qi, J.; Babon, J.J.; Cao, L.; Fan, G.; Lang, J.; Zhang, J.; Mi, P.; Kobe, B.; Wang, F. The JAK-STAT pathway: From structural biology to cytokine engineering. Signal Transduct. Target. Ther. 2024, 9, 221. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, M.; Ge, Y.; Wang, X. SIRT1 and aging related signaling pathways. Mech. Ageing Dev. 2020, 187, 111215. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.Y.; Xie, C.N.; Xie, J.Y.; Gao, Z.-W.; Fei, X.-W.; Hong, E.-H.; Chen, W.-J.; Chen, Y.-Z. Knockdown of NADPH oxidase 4 reduces mitochondrial oxidative stress and neuronal pyroptosis following intracerebral hemorrhage. Neural Regen. Res. 2023, 18, 1734–1742. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, S.; Zeng, X.; Lin, D.; Li, Y.; Gui, L.; Lin, M.J. Revealing the pathogenic changes of PAH based on multiomics characteristics. J. Transl. Med. 2019, 17, 231. [Google Scholar] [CrossRef]
- Mu, H.; Sun, J.; Li, L.; Yin, J.; Hu, N.; Zhao, W.; Ding, D.; Yi, L. Ionizing radiation exposure: Hazards, prevention, and biomarker screening. Environ. Sci. Pollut. Res. Int. 2018, 25, 15294–15306. [Google Scholar] [CrossRef]
- Kim, J.H.; Brown, S.L.; Gordon, M.N. Radiation-induced senescence: Therapeutic opportunities. Radiat. Oncol. 2023, 18, 10. [Google Scholar] [CrossRef]
- Fox-Fisher, I.; Shemer, R.; Dor, Y. Epigenetic liquid biopsies: A novel putative biomarker in immunology and inflammation. Trends Immunol. 2023, 44, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Katona, M.; Gladwin, M.T.; Straub, A.C. Flipping off and on the Redox Switch in the Microcirculation. Annu. Rev. Physiol. 2023, 85, 165–189. [Google Scholar] [CrossRef]
- Wigerblad, G.; Kaplan, M.J. Neutrophil extracellular traps in systemic autoimmune and autoinflammatory diseases. Nat. Rev. Immunol. 2023, 23, 274–288. [Google Scholar] [CrossRef]
- Toto, A.; Wild, P.; Graille, M.; Turcu, V.; Crézé, C.; Hemmendinger, M.; Sauvain, J.-J.; Bergamaschi, E.; Guseva Canu, I.; Hopf, N.B. Urinary Malondialdehyde (MDA) Concentrations in the General Population—A Systematic Literature Review and Meta-Analysis. Toxics 2022, 10, 160. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Wu, L.; Yao, H.; Zhao, L. Catalase-Like Nanozymes: Classification, Catalytic Mechanisms, and Their Applications. Small 2022, 18, e2203400. [Google Scholar] [CrossRef] [PubMed]
- Covarrubias, A.J.; Perrone, R.; Grozio, A.; Verdin, E. NAD+ metabolism and its roles in cellular processes during ageing. Nat. Rev. Mol. Cell Biol. 2021, 22, 119–141. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, C.; Zhang, W.; Wang, Y.; Qian, P.; Huang, H. Inflammation and aging: Signaling pathways and intervention therapies. Signal Transduct. Target. Ther. 2023, 8, 239. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Schulte, B.A.; Larue, A.C.; Ogawa, M.; Zhou, D. Total body irradiation selectively induces murine hematopoietic stem cell senescence. Blood 2006, 107, 358–366. [Google Scholar] [CrossRef]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef]
- Fasouli, E.S.; Katsantoni, E. JAK-STAT in Early Hematopoiesis and Leukemia. Front. Cell Dev. Biol. 2021, 9, 669363. [Google Scholar] [CrossRef]
- Grosse, L.; Wagner, N.; Emelyanov, A.; Molina, C.; Lacas-Gervais, S.; Wagner, K.-D. Defined p16High Senescent Cell Types Are Indispensable for Mouse Healthspan. Cell Metab. 2020, 32, 87–99.e6. [Google Scholar] [CrossRef]
- Xu, C.; Wang, L.; Fozouni, P.; Evjen, G.; Chandra, V.; Jiang, J.; Lu, C.; Nicastri, M.; Bretz, C.; Winkler, J.D.; et al. SIRT1 is downregulated by autophagy in senescence and ageing. Nat. Cell Biol. 2020, 22, 1170–1179. [Google Scholar] [CrossRef]
- Zhang, H.; Zhai, Z.; Wang, Y.; Zhang, J.; Wu, H.; Wang, Y.; Li, C.; Li, D.; Lu, L.; Wang, X.; et al. Resveratrol ameliorates ionizing irradiation-induced long-term hematopoietic stem cell injury in mice. Free Radic. Biol. Med. 2013, 54, 40–50. [Google Scholar] [CrossRef]
- Wilson, N.K.; Kent, D.G.; Buettner, F.; Shehata, M.; Macaulay, I.C.; Calero-Nieto, F.J.; Castillo, M.S.; Oedekoven, C.A.; Diamanti, E.; Schulte, R.; et al. Combined Single-Cell Functional and Gene Expression Analysis Resolves Heterogeneity within Stem Cell Populations. Cell Stem Cell 2015, 16, 712–724. [Google Scholar] [CrossRef]

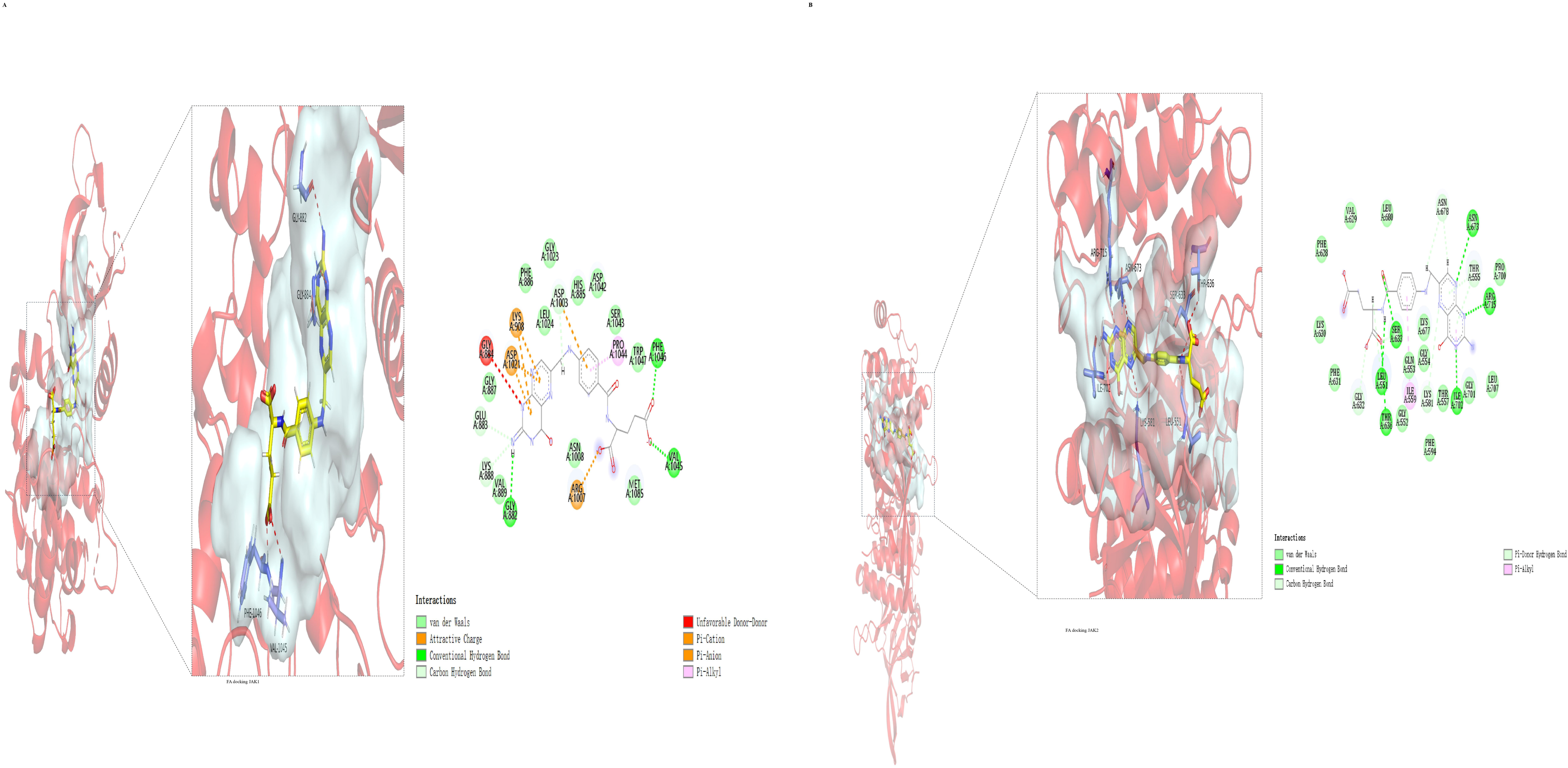



| Name | LibDock Score | Protein and Ligand Interaction |
|---|---|---|
| JAK1 | 139.437 | Conventional Hydrogen Bond (PHE, VAL, GLY); Carbon Hydrogen Bond (ASP, GLU, LYS) |
| JAK2 | 157.567 | Conventional Hydrogen Bond (ASN, ARG, SER, LEU, THR, ILE); Carbon Hydrogen Bond (ASN, THR, LYS, GLY) |
| Antibody | Clone | Conjugate | Source | LOT |
|---|---|---|---|---|
| Hematopoietic Lin cocktail | - | EF450 | eBioscience | 88-7772-72 |
| CD117 | ACK2 | PE-Cy7 | eBioscience | 25-1172-82 |
| LY-6/E | D7 | PE | eBioscience | 12-5981-82 |
| CD34 | RMA34 | FITC | eBioscience | 11-0341-85 |
| CD127 | A7R34 | APC | eBioscience | 17-1271-82 |
| CD48 | HM48-1 | APC-EF780 | eBioscience | 47-0481-82 |
| CD150 | TC15-12F12.2 | BV786 | Biolegend | 115937 |
| CD16/32 | S17011E | BV510 | Biolegend | 101333 |
| CD19 | HIB19 | PE | eBioscience | 12-0199-42 |
| CD45R | RA3-6B2 | EF506 | eBioscience | 69-0452-82 |
| CD3e | 145-2C11 | PE-Cy7 | eBioscience | 25-0031-82 |
| CD4 | GK1.5 | APC-EF780 | eBioscience | 47-0041-82 |
| CD8a | 53-6.7 | APC | eBioscience | 17-0081-82 |
| NK1.1 | PK136 | FITC | eBioscience | 11-5941-82 |
| 7-AAD | - | - | Biolegend | 420404 |
| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| β-actin | CCTCACTGTCCACCTTCCA | GGGTGTAAAACGCAGCTCA |
| p21 | CCTGGTGATGTCCGACCTG | CCATGAGCGCATCGCAATC |
| SIRT1 | TTGGCACCGATCCTCGAAC | CCCAGCTCCAGTCAGAACTAT |
| SOD2 | ATTAACGCGCAGATCATGCA | TGTCCCCCACCATTGAACTT |
| NOX4 | GATTTCTGGACCTTTGTGCCTTT | TGATGGTGACAGGTTTGTTGCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, M.; Ye, A.; Zhang, H.; Chen, J.; Yang, T.; Wei, X.; Gao, Y.; Ma, Z. Ferulic Acid Alleviates Radiation-Induced Immune Damage by Acting on JAK/STAT Signaling Pathway. Pharmaceuticals 2024, 17, 1175. https://doi.org/10.3390/ph17091175
Huang M, Ye A, Zhang H, Chen J, Yang T, Wei X, Gao Y, Ma Z. Ferulic Acid Alleviates Radiation-Induced Immune Damage by Acting on JAK/STAT Signaling Pathway. Pharmaceuticals. 2024; 17(9):1175. https://doi.org/10.3390/ph17091175
Chicago/Turabian StyleHuang, Mingyue, Anping Ye, Haoyu Zhang, Junru Chen, Tingyu Yang, Xue Wei, Yue Gao, and Zengchun Ma. 2024. "Ferulic Acid Alleviates Radiation-Induced Immune Damage by Acting on JAK/STAT Signaling Pathway" Pharmaceuticals 17, no. 9: 1175. https://doi.org/10.3390/ph17091175
APA StyleHuang, M., Ye, A., Zhang, H., Chen, J., Yang, T., Wei, X., Gao, Y., & Ma, Z. (2024). Ferulic Acid Alleviates Radiation-Induced Immune Damage by Acting on JAK/STAT Signaling Pathway. Pharmaceuticals, 17(9), 1175. https://doi.org/10.3390/ph17091175





