Mechanistic Insights into Drug-Induced Guillain–Barré Syndrome: A Large-Cohort Analysis of the FAERS Database
Abstract
1. Introduction
2. Results
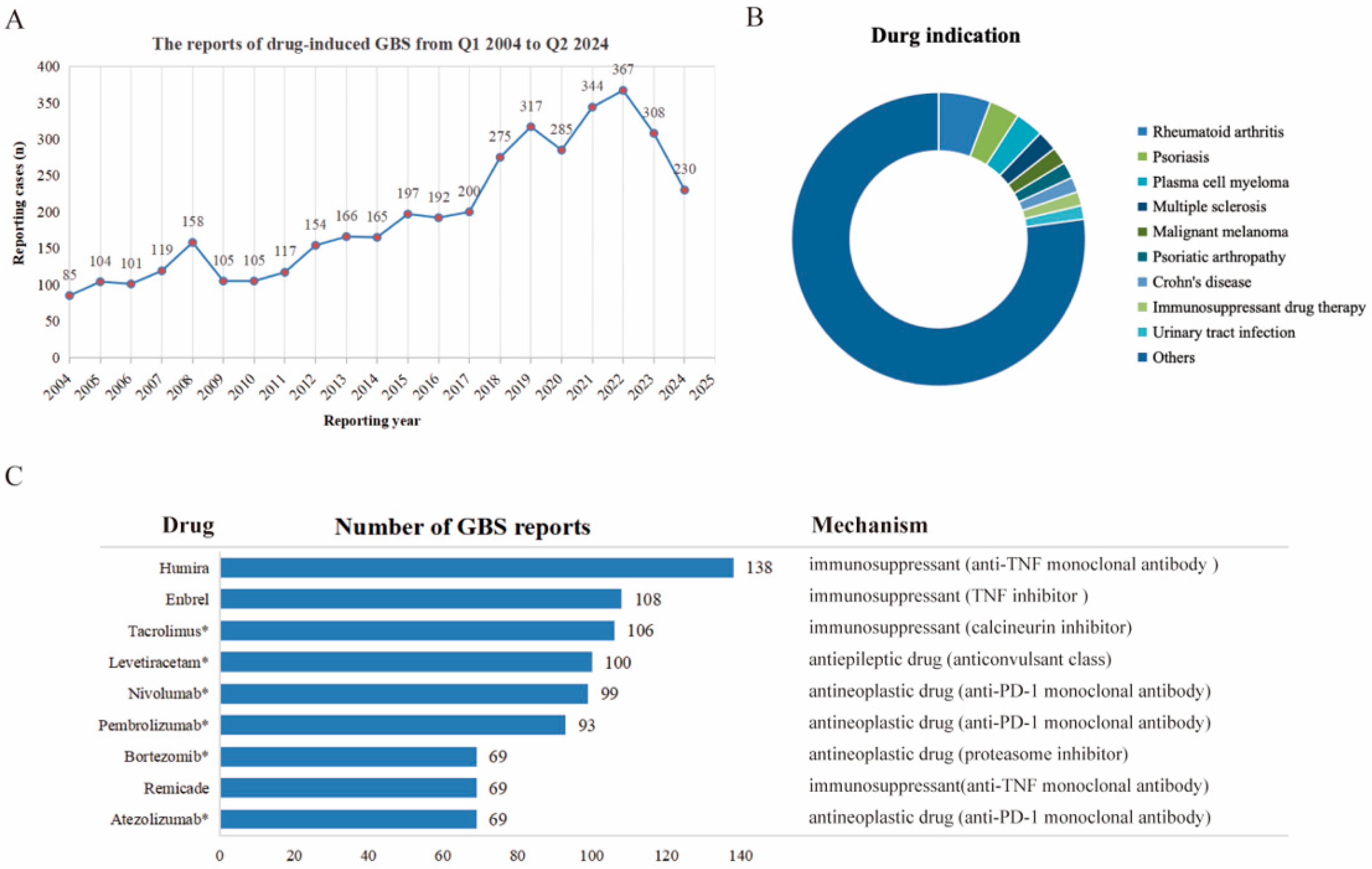
2.1. Descriptive Analysis
2.2. Signal Strength Detection
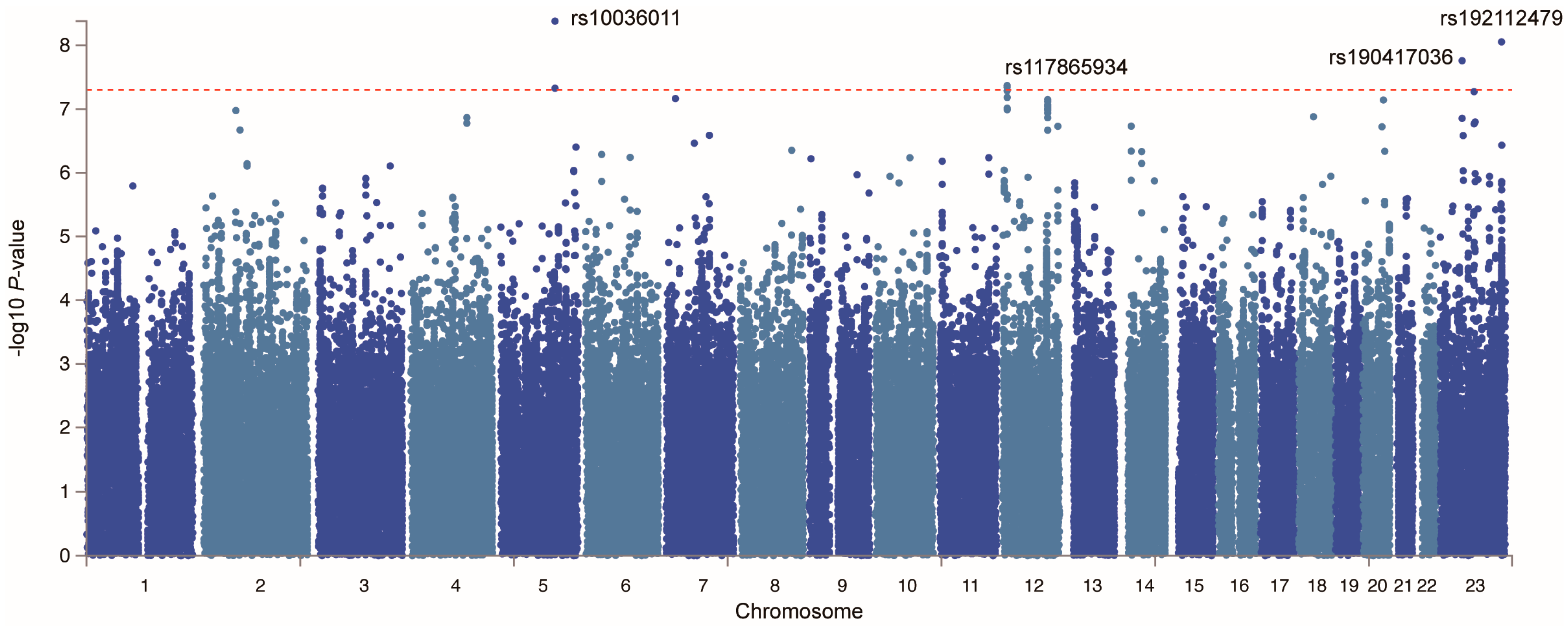
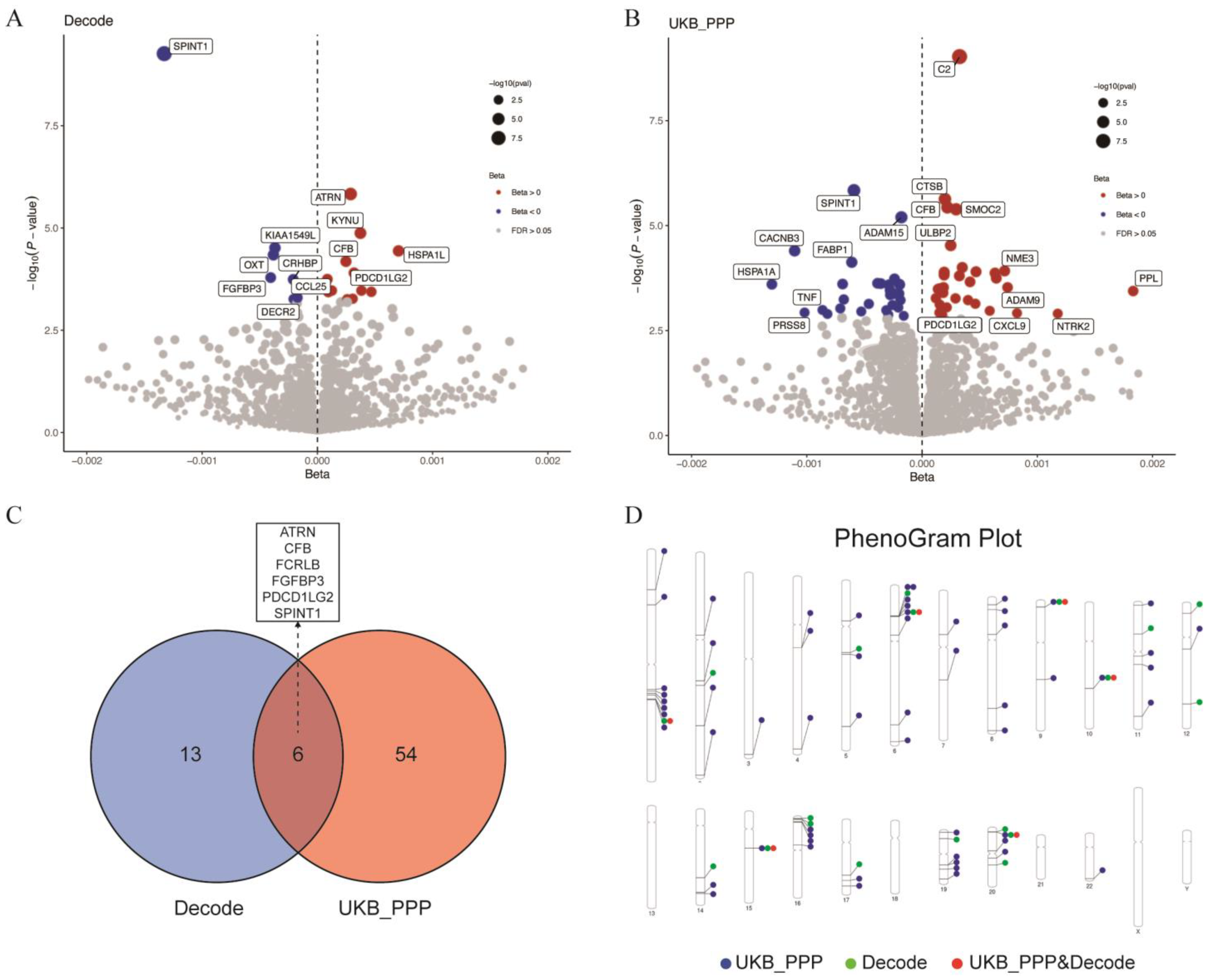
2.3. Potential Susceptibility Proteins for GBS
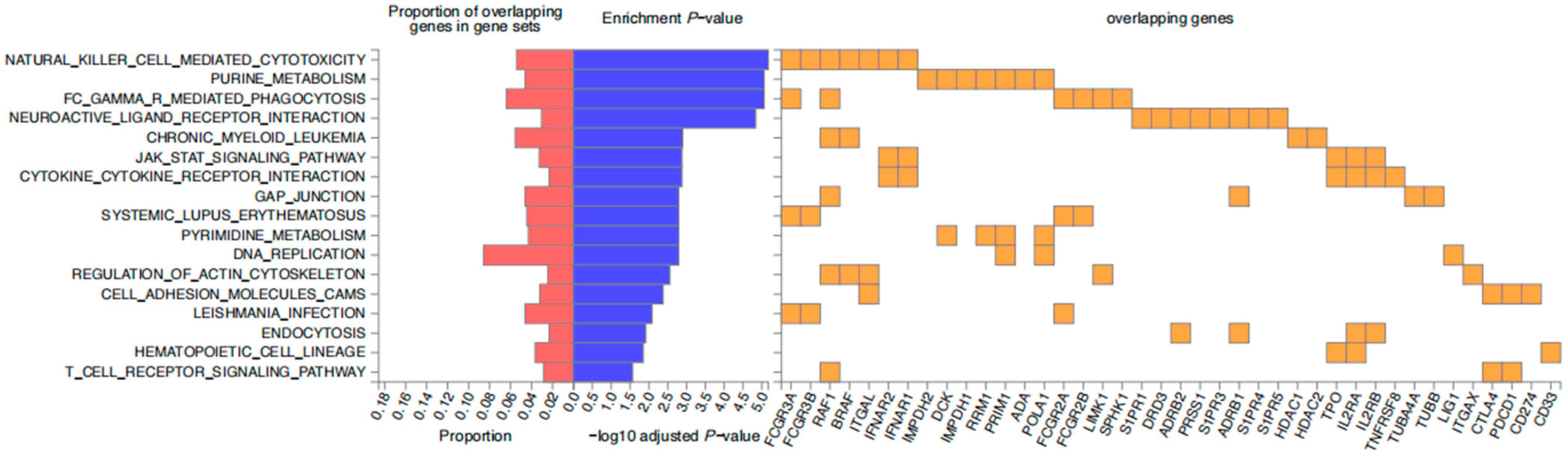
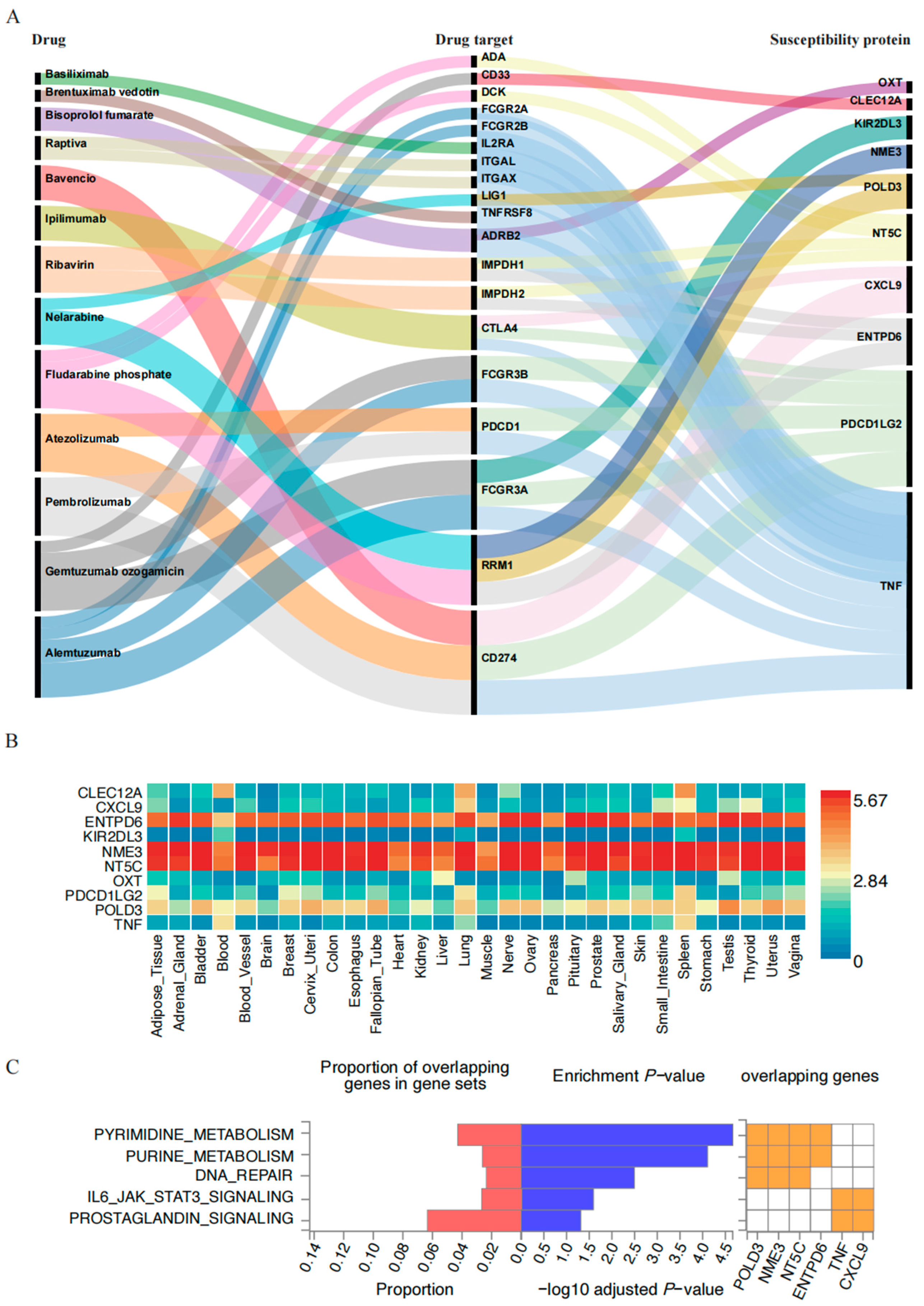
2.4. PPI Network and Enrichment Analysis Results
3. Discussion
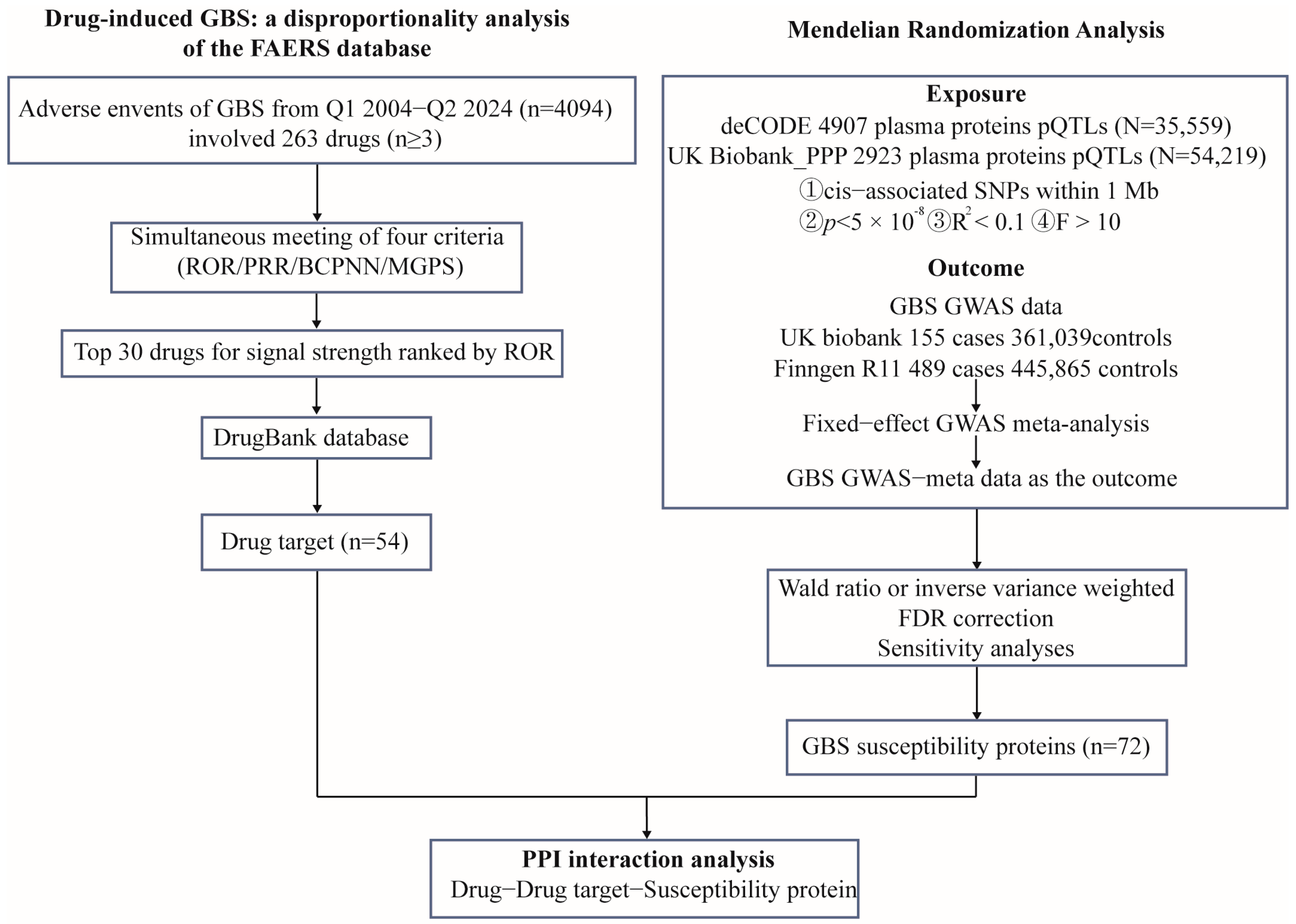
4. Materials and Methods
4.1. Data Sources and Processing
4.2. Mendelian Randomization
4.3. PPI Network and Enrichment Analysis
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GBS | Guillain–Barré Syndrome |
| MR | Mendelian Randomization |
| PPI | Protein–protein Interaction |
| FAERS | Food and Drug Administration Adverse Event Reporting System |
| ROR | Reporting Odds Ratio |
| BCPNN | Bayesian Confidence Propagation Neural Network |
| MGPS | Multi-item Gamma-Poisson Shrinker |
| p-QTLs | protein Quantitative Tait Loci |
| FDR | False Discovery Rate |
| CI | Confidence Interval |
| PRR | Proportional Reporting Ratio |
| TNF | Tumor Necrosis Factor |
| ICIs | Immune Checkpoint Inhibitors |
| RRM1 | Ribonucleotide Reductase M1 |
References
- Wijdicks, E.F.M.; Klein, C.J. Guillain-Barré Syndrome. Mayo Clin. Proc. 2017, 92, 467–479. [Google Scholar] [CrossRef]
- Xu, L.; Zhao, C.; Bao, Y.; Liu, Y.; Liang, Y.; Wei, J.; Liu, G.; Wang, J.; Zhan, S.; Wang, S.; et al. Variation in worldwide incidence of Guillain-Barré Syndrome: A population-based study in urban China and existing global evidence. Front. Immunol. 2024, 15, 1415986. [Google Scholar] [CrossRef]
- Kozyreva, A.A.; Bembeeva, R.T.; Druzhinina, E.S.; Zavadenko, N.N. Guillain-Barre Syndrome in children. Zh. Nevrol. Psikhiatr. Im. S. S. Korsakova 2023, 123, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Haber, P.; DeStefano, F.; Angulo, F.J.; Iskander, J.; Shadomy, S.V.; Weintraub, E.; Chen, R.T. Guillain-Barré Syndrome following influenza vaccination. JAMA 2004, 292, 2478–2481. [Google Scholar] [CrossRef]
- Awong, I.E.; Dandurand, K.R.; Keeys, C.A.; Maung-Gyi, F.A. Drug-associated Guillain-Barré Syndrome: A literature review. Ann. Pharmacother. 1996, 30, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Fagius, J.; Osterman, P.O.; Sidén, A.; Wiholm, B.E. Guillain-Barré Syndrome following zimeldine treatment. J. Neurol. Neurosurg. Psychiatry 1985, 48, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Raschetti, R.; Maggini, M.; Popoli, P.; Caffari, B.; Da Cas, R.; Menniti-Ippolito, F.; Spila-Alegiani, S.; Traversa, G. Gangliosides and Guillain-Barré Syndrome. J. Clin. Epidemiol. 1995, 48, 1399–1405. [Google Scholar] [CrossRef]
- Okuyan, E.; Cakar, M.A.; Dinckal, M.H. Guillain-Barré Syndrome after thrombolysis with streptokinase. Cardiol. Res. Pract. 2010, 2010, 315856. [Google Scholar] [CrossRef]
- Gerriets, V.; Goyal, A.; Khaddour, K. Tumor Necrosis Factor Inhibitors. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025; Available online: http://www.ncbi.nlm.nih.gov/books/NBK482425/ (accessed on 13 March 2025).
- Mikami, T.; Liaw, B.; Asada, M.; Niimura, T.; Zamami, Y.; Green-LaRoche, D.; Pai, L.; Levy, M.; Jeyapalan, S. Neuroimmunological adverse events associated with immune checkpoint inhibitor: A retrospective, pharmacovigilance study using FAERS database. J. Neurooncol. 2021, 152, 135–144. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Zhao, C. Guillain-Barré Syndrome-Like Polyneuropathy Associated with Immune Checkpoint Inhibitors: A Systematic Review of 33 Cases. BioMed Res. Int. 2021, 2021, 9800488. [Google Scholar] [CrossRef]
- Fan, Q.; Hu, Y.; Wang, X.; Zhao, B. Guillain-Barré Syndrome in patients treated with immune checkpoint inhibitors. J. Neurol. 2021, 268, 2169–2174. [Google Scholar] [CrossRef]
- Abrahao, A.; Tenório, P.H.d.M.; Rodrigues, M.; Mello, M.; Nascimento, O.J.M. Guillain-Barré Syndrome and checkpoint inhibitor therapy: Insights from pharmacovigilance data. BMJ Neurol. Open 2024, 6, e000544. [Google Scholar] [CrossRef]
- Zheng, X.; Fang, Y.; Song, Y.; Liu, S.; Liu, K.; Zhu, J.; Wu, X. Is there a causal nexus between COVID-19 infection, COVID-19 vaccination, and Guillain-Barré Syndrome? Eur. J. Med. Res. 2023, 28, 98. [Google Scholar] [CrossRef] [PubMed]
- Malekpour, M.; Khanmohammadi, S.; Meybodi, M.J.E.; Shekouh, D.; Rahmanian, M.R.; Kardeh, S.; Azarpira, N. COVID-19 as a trigger of Guillain-Barré Syndrome: A review of the molecular mechanism. Immun. Inflamm. Dis. 2023, 11, e875. [Google Scholar] [CrossRef]
- Waheed, S.; Bayas, A.; Hindi, F.; Rizvi, Z.; Espinosa, P.S. Neurological Complications of COVID-19: Guillain-Barre Syndrome Following Pfizer COVID-19 Vaccine. Cureus 2021, 13, e13426. [Google Scholar] [CrossRef] [PubMed]
- Braish, J.S.; Kugler, E.; Jabbour, E.; Woodman, K.; Ravandi, F.; Nicholas, S.; Jain, N.; Kantarjian, H.; Sasaki, K. Incidence and Clinical Presentation of Severe Neurotoxicity from Nelarabine in Patients with T-Cell Acute Lymphoblastic Leukemia. Clin. Lymphoma Myeloma Leuk. 2024, 24, 783–788. [Google Scholar] [CrossRef]
- DeAngelo, D.J. Nelarabine for the treatment of patients with relapsed or refractory T-cell acute lymphoblastic leukemia or lymphoblastic lymphoma. Hematol. Oncol. Clin. N. Am. 2009, 23, 1121–1135. [Google Scholar] [CrossRef] [PubMed]
- Alli, N.; Lou-Hing, A.; Bolt, E.L.; He, L. POLD3 as Controller of Replicative DNA Repair. Int. J. Mol. Sci. 2024, 25, 12417. [Google Scholar] [CrossRef]
- Curik, N.; Polivkova, V.; Burda, P.; Koblihova, J.; Laznicka, A.; Kalina, T.; Kanderova, V.; Brezinova, J.; Ransdorfova, S.; Karasova, D.; et al. Somatic Mutations in Oncogenes Are in Chronic Myeloid Leukemia Acquired De Novo via Deregulated Base-Excision Repair and Alternative Non-Homologous End Joining. Front. Oncol. 2021, 11, 744373. [Google Scholar] [CrossRef]
- Mehawej, C.; Chouery, E.; Azar-Atallah, S.; Shebaby, W.; Delague, V.; Mansour, I.; Mustapha, M.; Lefranc, G.; Megarbane, A. POLD3 deficiency is associated with severe combined immunodeficiency, neurodevelopmental delay, and hearing impairment. Clin. Immunol. Orlando Fla 2023, 251, 109326. [Google Scholar] [CrossRef]
- Wu, H.; Huang, X.; Chen, S.; Li, S.; Feng, J.; Zouxu, X.; Xie, Z.; Xie, X.; Wang, X. Comprehensive analysis of the NME gene family functions in breast cancer. Transl. Cancer Res. 2020, 9, 6369–6382. [Google Scholar] [CrossRef]
- Chen, C.-W.; Wang, H.-L.; Huang, C.-W.; Huang, C.-Y.; Lim, W.K.; Tu, I.-C.; Koorapati, A.; Hsieh, S.-T.; Kan, H.-W.; Tzeng, S.-R.; et al. Two separate functions of NME3 critical for cell survival underlie a neurodegenerative disorder. Proc. Natl. Acad. Sci. USA 2019, 116, 566–574. [Google Scholar] [CrossRef]
- Kamakura, K.; Kaida, K.; Kusunoki, S.; Miyamoto, N.; Masaki, T.; Nakamura, R.; Motoyoshi, K.; Fukuda, J. Harmful effects of anti-GalNAc-GD1a antibodies and TNF-alpha on rat dorsal root ganglia. J. Peripher. Nerv. Syst. JPNS 2005, 10, 190–201. [Google Scholar] [CrossRef]
- Hartung, H.P. Immune-mediated demyelination. Ann. Neurol. 1993, 33, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Nyati, K.K.; Prasad, K.N.; Verma, A.; Paliwal, V.K. Correlation of matrix metalloproteinases-2 and -9 with proinflammatory cytokines in Guillain-Barré Syndrome. J. Neurosci. Res. 2010, 88, 3540–3546. [Google Scholar] [CrossRef]
- Solomon, A.J.; Spain, R.I.; Kruer, M.C.; Bourdette, D. Inflammatory neurological disease in patients treated with tumor necrosis factor alpha inhibitors. Mult. Scler. Houndmills Basingstoke Engl. 2011, 17, 1472–1487. [Google Scholar] [CrossRef] [PubMed]
- Stübgen, J.-P. Tumor necrosis factor-alpha antagonists and neuropathy. Muscle Nerve 2008, 37, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Tristano, A.G. Neurological adverse events associated with anti-tumor necrosis factor α treatment. J. Neurol. 2010, 257, 1421–1431. [Google Scholar] [CrossRef]
- Shitara, K.; Ajani, J.A.; Moehler, M.; Garrido, M.; Gallardo, C.; Shen, L.; Yamaguchi, K.; Wyrwicz, L.; Skoczylas, T.; Bragagnoli, A.C.; et al. Nivolumab plus chemotherapy or ipilimumab in gastro-oesophageal cancer. Nature 2022, 603, 942–948. [Google Scholar] [CrossRef]
- Fultang, L.; Panetti, S.; Ng, M.; Collins, P.; Graef, S.; Rizkalla, N.; Booth, S.; Lenton, R.; Noyvert, B.; Shannon-Lowe, C.; et al. MDSC targeting with Gemtuzumab ozogamicin restores T cell immunity and immunotherapy against cancers. EBioMedicine 2019, 47, 235–246. [Google Scholar] [CrossRef]
- Horn, L.; Mansfield, A.S.; Szczęsna, A.; Havel, L.; Krzakowski, M.; Hochmair, M.J.; Huemer, F.; Losonczy, G.; Johnson, M.L.; Nishio, M.; et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2220–2229. [Google Scholar] [CrossRef] [PubMed]
- von Essen, M.R.; Chow, H.H.; Holm Hansen, R.; Buhelt, S.; Sellebjerg, F. Immune reconstitution following alemtuzumab therapy is characterized by exhausted T cells, increased regulatory control of proinflammatory T cells and reduced B cell control. Front. Immunol. 2023, 14, 1249201. [Google Scholar] [CrossRef]
- Li, W.; Mei, M.; Liu, T.; Zhang, S.; Wang, Z.; Suo, Y.; Wang, S.; Liu, Y.; Zhang, N.; Lu, W. Identification of PDCD1 and PDCD1LG2 as Prognostic Biomarkers and Associated with Immune Infiltration in Hepatocellular Carcinoma. Int. J. Gen. Med. 2022, 15, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Esfahani, K.; Buhlaiga, N.; Thébault, P.; Lapointe, R.; Johnson, N.A.; Miller, W.H. Alemtuzumab for Immune-Related Myocarditis Due to PD-1 Therapy. N. Engl. J. Med. 2019, 380, 2375–2376. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.U.; Ahn, K.-S.; Sung, M.H.; Park, J.-W.; Ryu, H.W.; Lee, H.-J.; Hong, S.-T.; Oh, S.-R. Indacaterol inhibits tumor cell invasiveness and MMP-9 expression by suppressing IKK/NF-κB activation. Mol. Cells 2014, 37, 585–591. [Google Scholar] [CrossRef]
- Chang, S.-H.; Tian, X.-B.; Wang, J.; Liu, M.-Q.; Huang, C.-N.; Qi, Y.; Zhang, L.-J.; Gao, C.-L.; Zhang, D.-Q.; Sun, L.-S.; et al. Increased Cerebrospinal Fluid Uric Acid Levels in Guillain-Barré Syndrome. Front. Neurol. 2020, 11, 589928. [Google Scholar] [CrossRef]
- Bansil, S.; Mithen, F.A.; Singhal, B.S.; Cook, S.D.; Rohowsky-Kochan, C. Elevated neopterin levels in Guillain-Barré Syndrome. Further evidence of immune activation. Arch. Neurol. 1992, 49, 1277–1280. [Google Scholar] [CrossRef]
- Benito-León, J.; Porta-Etessam, J. Guillain-Barré Syndrome and allopurinol-induced hypersensitivity. Eur. Neurol. 2001, 45, 186–187. [Google Scholar] [CrossRef]
- Liu, X.; Liu, L.; Zhang, J. Causal role of the pyrimidine deoxyribonucleoside degradation superpathway mediation in Guillain-Barré Syndrome via the HVEM on CD4 + and CD8 + T cells. Sci. Rep. 2024, 14, 27418. [Google Scholar] [CrossRef]
- Shimony, S.; DeAngelo, D.J.; Luskin, M.R. Nelarabine: When and how to use in the treatment of T-cell acute lymphoblastic leukemia. Blood Adv. 2024, 8, 23–36. [Google Scholar] [CrossRef]
- Chun, H.G.; Leyland-Jones, B.; Cheson, B.D. Fludarabine phosphate: A synthetic purine antimetabolite with significant activity against lymphoid malignancies. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1991, 9, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Hess, J.R.; Greenberg, N.A. The role of nucleotides in the immune and gastrointestinal systems: Potential clinical applications. Nutr. Clin. Pract. Off. Publ. Am. Soc. Parenter. Enter. Nutr. 2012, 27, 281–294. [Google Scholar] [CrossRef]
- Rothman, K.J.; Lanes, S.; Sacks, S.T. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol. Drug Saf. 2004, 13, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.J.; Waller, P.C.; Davis, S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol. Drug Saf. 2001, 10, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Bate, A.; Lindquist, M.; Edwards, I.R.; Olsson, S.; Orre, R.; Lansner, A.; De Freitas, R.M. A Bayesian neural network method for adverse drug reaction signal generation. Eur. J. Clin. Pharmacol. 1998, 54, 315–321. [Google Scholar] [CrossRef]
- Szarfman, A.; Machado, S.G.; O’Neill, R.T. Use of screening algorithms and computer systems to efficiently signal higher-than-expected combinations of drugs and events in the US FDA’s spontaneous reports database. Drug Saf. 2002, 25, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Ferkingstad, E.; Sulem, P.; Atlason, B.A.; Sveinbjornsson, G.; Magnusson, M.I.; Styrmisdottir, E.L.; Gunnarsdottir, K.; Helgason, A.; Oddsson, A.; Halldorsson, B.V.; et al. Large-scale integration of the plasma proteome with genetics and disease. Nat. Genet. 2021, 53, 1712–1721. [Google Scholar] [CrossRef]
- Sun, B.B.; Chiou, J.; Traylor, M.; Benner, C.; Hsu, Y.-H.; Richardson, T.G.; Surendran, P.; Mahajan, A.; Robins, C.; Vasquez-Grinnell, S.G.; et al. Plasma proteomic associations with genetics and health in the UK Biobank. Nature 2023, 622, 329–338. [Google Scholar] [CrossRef]
- Turner, S.D. qqman: An R package for visualizing GWAS results using Q-Q and manhattan plots. J. Open Source Softw. 2018, 3, 005165. [Google Scholar] [CrossRef]
- Staley, J. jrs95/gassocplot. Published online 17 February 2025. Available online: https://github.com/jrs95/gassocplot (accessed on 17 March 2025).
- Watanabe, K.; Taskesen, E.; van Bochoven, A.; Posthuma, D. Functional mapping and annotation of genetic associations with FUMA. Nat. Commun. 2017, 8, 1826. [Google Scholar] [CrossRef]
- Bourgault, J.; Abner, E.; Manikpurage, H.D.; Pujol-Gualdo, N.; Laisk, T.; Estonian Biobank Research Team; Gobeil, É.; Gagnon, E.; Girard, A.; Mitchell, P.L.; et al. Proteome-Wide Mendelian Randomization Identifies Causal Links Between Blood Proteins and Acute Pancreatitis. Gastroenterology 2023, 164, 953–965.e3. [Google Scholar] [CrossRef] [PubMed]
- A Practical Guide to Methods Controlling False Discoveries in Computational Biology|Genome Biology|Full Text. Available online: https://genomebiology.biomedcentral.com/articles/10.1186/s13059-019-1716-1 (accessed on 17 March 2025).
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING database in 2021: Customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef] [PubMed]
| Variable | Cases n (%) |
|---|---|
| Sex | |
| Female | 1591 (38.86) |
| Male | 2046 (49.98) |
| Unknown | 457 (11.16) |
| Age | |
| ≤18 | 158 (3.86) |
| 19–40 | 503 (12.29) |
| 41–64 | 1282 (31.31) |
| ≥65 | 1022 (24.96) |
| Unknown | 1129 (27.58) |
| Reporter | |
| Physician | 1679 (41.01) |
| Consumer | 792 (19.35) |
| Others | 1429 (34.90) |
| Unknown | 194 (4.74) |
| Reported countries | |
| United States | 1021 (24.94) |
| France | 266 (6.50) |
| United Kingdom | 201 (4.91) |
| Others | 1598 (39.03) |
| Unknown | 1008 (24.62) |
| Indication | |
| Clear indication | 3377 (82.49) |
| Unknown | 717 (17.51) |
| Drug | Cases | ROR (95% CI) | PRR (95% CI) | χ2 | IC (IC-2SD) | EBGM (EBGM05) |
|---|---|---|---|---|---|---|
| Nelarabine | 16 | 138.91 (84.37, 228.71) | 134.71 (82.53, 219.89) | 2115.68 | 7.07 (6.37) | 134.19 (88.42) |
| Roferon-a | 3 | 60.62 (19.39, 189.51) | 59.81 (19.57, 182.8) | 173.4 | 5.90 (4.47) | 59.77 (23.03) |
| Zerit | 3 | 30.17 (9.69, 93.96) | 29.97 (9.62, 93.41) | 83.98 | 4.90 (3.48) | 29.95 (11.58) |
| Methimazole | 9 | 22.43 (11.64, 43.21) | 22.32 (11.69, 42.62) | 182.94 | 4.48 (3.58) | 22.28 (12.87) |
| Pravastatin sodium | 4 | 22.25 (8.33, 59.47) | 22.15 (8.31, 59.02) | 80.71 | 4.47 (3.20) | 22.13 (9.72) |
| Vinorelbine | 3 | 22.10 (7.10, 68.75) | 21.99 (7.06, 68.54) | 60.09 | 4.46 (3.04) | 21.98 (8.5) |
| Gemtuzumab ozogamicin | 3 | 21.11 (6.79, 65.66) | 21.02 (6.74, 65.51) | 57.16 | 4.39 (2.97) | 21.00 (8.13) |
| Raptiva | 11 | 19.88 (10.99, 35.97) | 19.8 (11.00, 35.65) | 195.82 | 4.30 (3.48) | 19.74 (12.02) |
| Brentuximab vedotin | 29 | 19.72 (13.68, 28.44) | 19.64 (13.53, 28.5) | 509.47 | 4.29 (3.77) | 19.51 (14.36) |
| Basiliximab | 3 | 19.43 (6.25, 60.43) | 19.35 (6.21, 60.31) | 52.19 | 4.27 (2.85) | 19.34 (7.48) |
| Sunitinib malate | 3 | 17.57 (5.65, 54.63) | 17.51 (5.62, 54.57) | 46.67 | 4.13 (2.71) | 17.5 (6.77) |
| Vincristine | 9 | 17.41 (9.04, 33.53) | 17.35 (9.09, 33.13) | 138.38 | 4.11 (3.22) | 17.31 (10.00) |
| Colchicine | 4 | 14.61 (5.47, 39.02) | 14.57 (5.47, 38.82) | 50.51 | 3.86 (2.60) | 14.56 (6.40) |
| Evusheld | 5 | 14.29 (5.94, 34.4) | 14.25 (5.9, 34.42) | 61.53 | 3.83 (2.67) | 14.23 (6.82) |
| Bisoprolol fumarate | 3 | 13.62 (4.38, 42.33) | 13.58 (4.36, 42.33) | 34.96 | 3.76 (2.35) | 13.58 (5.26) |
| Pravastatin | 4 | 12.06 (4.52, 32.2) | 12.03 (4.51, 32.05) | 40.43 | 3.59 (2.32) | 12.02 (5.29) |
| Epzicom | 3 | 11.84 (3.81, 36.79) | 11.81 (3.79, 36.81) | 29.68 | 3.56 (2.14) | 11.80 (4.57) |
| Atezolizumab | 69 | 11.00 (8.67, 13.96) | 10.97 (8.67, 13.88) | 615.02 | 3.43 (3.09) | 10.80 (8.85) |
| Bavencio | 3 | 10.96 (3.53, 34.04) | 10.93 (3.51, 34.07) | 27.06 | 3.45 (2.03) | 10.93 (4.23) |
| Rosuvastatin calcium | 4 | 10.83 (4.06, 28.91) | 10.81 (4.06, 28.8) | 35.58 | 3.43 (2.17) | 10.80 (4.75) |
| Fludarabine phosphate | 19 | 10.75 (6.85, 16.88) | 10.73 (6.84, 16.84) | 166.84 | 3.42 (2.78) | 10.68 (7.32) |
| Rosuvastatin | 9 | 10.51 (5.46, 20.23) | 10.49 (5.49, 20.03) | 77.10 | 3.39 (2.49) | 10.47 (6.05) |
| Alemtuzumab | 9 | 10.32 (5.36, 19.87) | 10.30 (5.39, 19.67) | 75.45 | 3.36 (2.47) | 10.28 (5.94) |
| Ipilimumab | 40 | 10.29 (7.53, 14.06) | 10.27 (7.51, 14.05) | 331.50 | 3.35 (2.90) | 10.18 (7.84) |
| Fingolimod | 4 | 10.12 (3.79, 27.00) | 10.10 (3.79, 26.91) | 32.76 | 3.33 (2.07) | 10.09 (4.44) |
| Bortezomib | 69 | 10.00 (7.88, 12.69) | 9.98 (7.89, 12.63) | 548.19 | 3.30 (2.96) | 9.83 (8.05) |
| Ribavirin | 8 | 9.98 (4.98, 19.98) | 9.96 (5.02, 19.78) | 64.35 | 3.31 (2.37) | 9.94 (5.56) |
| Oxaliplatin | 67 | 9.93 (7.80, 12.64) | 9.91 (7.83, 12.54) | 528.00 | 3.29 (2.94) | 9.76 (7.98) |
| Dabrafenib | 6 | 9.89 (4.44, 22.06) | 9.87 (4.42, 22.05) | 47.79 | 3.30 (2.23) | 9.86 (5.04) |
| Pembrolizumab | 93 | 9.49 (7.73, 11.66) | 9.47 (7.78, 11.52) | 689.07 | 3.21 (2.92) | 9.28 (7.81) |
| Algorithms | Equation | Criteria |
|---|---|---|
| ROR | ROR | a ≥ 3, 95%CI (lower limit) > 1 |
| 95% CI | ||
| PRR | PRR | a ≥ 3, 95%CI (lower limit) > 1, PRR ≥ 2, ≥ 4 |
| 95% CI | ||
| BCPNN | IC | IC-2SD > 0 |
| E(IC) | ||
| V(IC) | ||
| IC-2SD = E(IC) − 2 | ||
| p.s. | ||
| MGPS | EBGM | EBGM05 > 2 |
| 95%CI |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gui, J.; Li, X.; Chu, H.; Zhang, J.; Dong, M.; Zhang, F.; Li, R.; Luo, H.; Gao, K.; Jiang, Y. Mechanistic Insights into Drug-Induced Guillain–Barré Syndrome: A Large-Cohort Analysis of the FAERS Database. Pharmaceuticals 2025, 18, 498. https://doi.org/10.3390/ph18040498
Gui J, Li X, Chu H, Zhang J, Dong M, Zhang F, Li R, Luo H, Gao K, Jiang Y. Mechanistic Insights into Drug-Induced Guillain–Barré Syndrome: A Large-Cohort Analysis of the FAERS Database. Pharmaceuticals. 2025; 18(4):498. https://doi.org/10.3390/ph18040498
Chicago/Turabian StyleGui, Jianxiong, Xiao Li, Hongyuan Chu, Junjiao Zhang, Meiyu Dong, Fan Zhang, Renqiuguo Li, Huaxia Luo, Kai Gao, and Yuwu Jiang. 2025. "Mechanistic Insights into Drug-Induced Guillain–Barré Syndrome: A Large-Cohort Analysis of the FAERS Database" Pharmaceuticals 18, no. 4: 498. https://doi.org/10.3390/ph18040498
APA StyleGui, J., Li, X., Chu, H., Zhang, J., Dong, M., Zhang, F., Li, R., Luo, H., Gao, K., & Jiang, Y. (2025). Mechanistic Insights into Drug-Induced Guillain–Barré Syndrome: A Large-Cohort Analysis of the FAERS Database. Pharmaceuticals, 18(4), 498. https://doi.org/10.3390/ph18040498







