Clinical Research on Lysergic Acid Diethylamide (LSD) in Psychiatry and Neuroscience
Abstract
1. Introduction
2. Pharmacology and Mechanistic Foundations of LSD
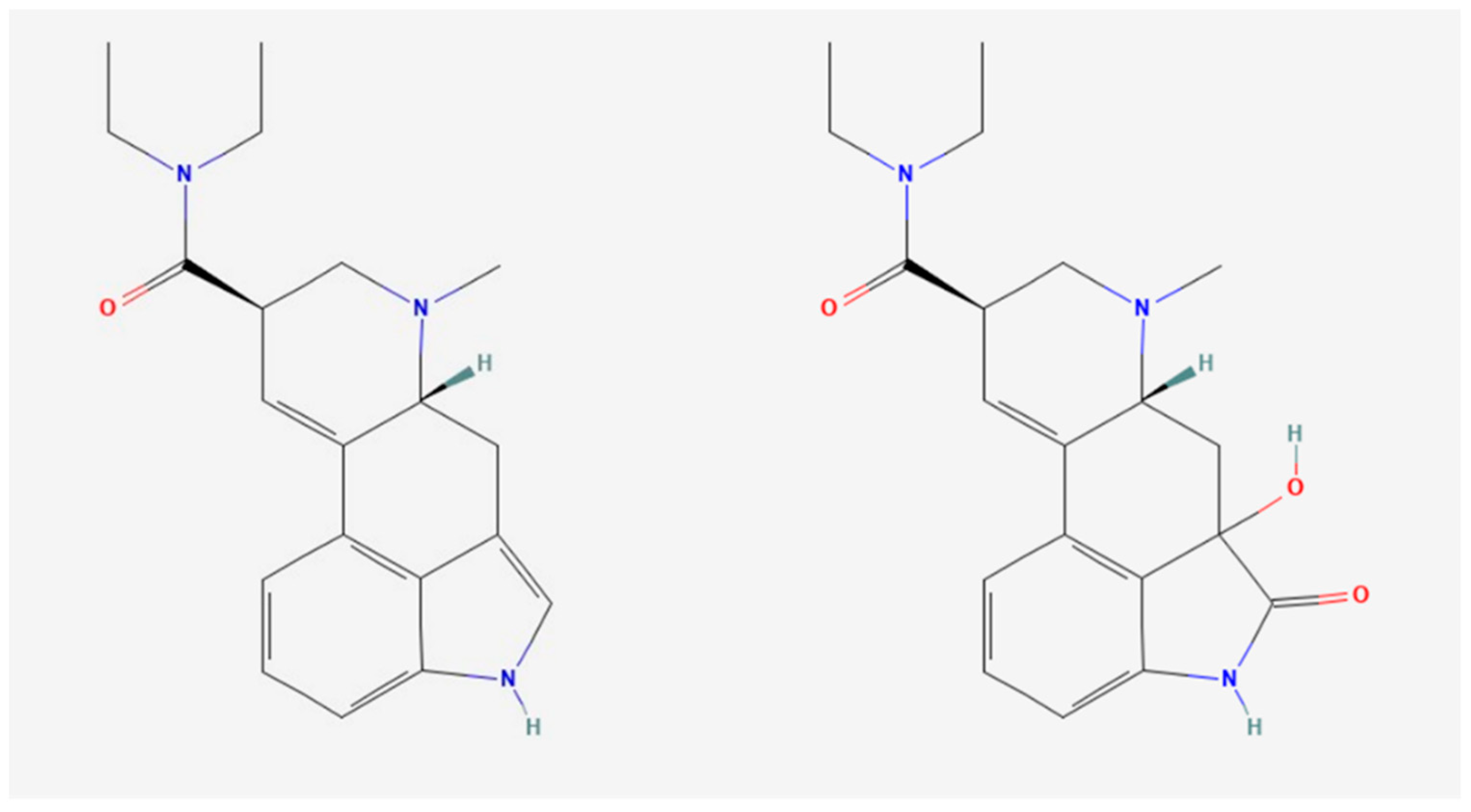
2.1. LSD Metabolism and Pharmacokinetics
2.2. Receptor Binding and Physiological Effects
2.3. Neurophysiological and Brain Connectivity
2.4. Neuroplasticity and Biomarkers
2.5. Genetic Polymorphisms in ADME and Receptor Activity
2.5.1. Cytochrome P450 Polymorphisms and LSD Metabolism
2.5.2. Serotonin Receptor Genetic Variability and LSD Response
2.5.3. Genetic Influence on Drug Responsiveness and Psychopharmacology
3. Therapeutic Applications of LSD
3.1. LSD in Psychotherapy
3.2. LSD for Anxiety, Depression, and End-of-Life Distress
3.3. Alcohol Use Disorder (AUD)
3.4. Microdosing and Mood Disorders
3.5. LSD and Pain Management
3.6. Cognitive Flexibility, Learning, and Behavioral Therapy
3.7. Mystical-Type Experiences and Emotional Processing
3.8. Co-Administration with Other Psychedelics
4. Safety and Risk Management
4.1. Safety and Adverse Effects
4.2. Pharmacological Interactions and Variability in Safety
4.3. Analytical and Forensic Detection
4.4. Risk Management
5. Public Health and Epidemiology
5.1. Population and Use Trends
5.2. Public Health and Demographic
5.3. Nonmedical Use and Associated Risk
6. Research Challenges and Methodological Gaps
6.1. Sample Size and Participant Bias
6.2. Dosage, Context, and Study Design Variability
6.3. Limited Long-Term Data
6.4. Gaps in Mechanistic Understanding
6.5. Lack of Integration with Pharmacogenetics
6.6. Preclinical Clinical Translation Issues
6.7. Regulatory and Ethical Constraints
7. Future Directions and Innovations in LSD Research
7.1. Larger, Inclusive Clinical Trials
7.2. Standardization of Protocols and Dosing
7.3. Long-Term and Longitudinal Research
7.4. Mechanistic Research and Brain Modeling
7.5. Advanced Neuroscientific Approaches: Brain Dynamics and Predictive Models
7.6. Integration of Pharmacogenetics and Personalized Medicine
7.7. Novel LSD Analogs
7.8. LSD Co-Use with Other Substances
7.9. Cross-Disciplinary Collaboration
7.10. Regulatory Advocacy and Public Education
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hofmann, A. LSD: My Problem Child; Oxford University Press: New York, NY, USA, 2013. [Google Scholar]
- Nichols, D.E. Psychedelics. Pharmacol. Rev. 2016, 68, 264–355. [Google Scholar] [PubMed]
- Passie, T.; Halpern, J.H.; Stichtenoth, D.O.; Emrich, H.M.; Hintzen, A. The pharmacology of lysergic acid diethylamide: A review. CNS Neurosci. Ther. 2008, 14, 295–314. [Google Scholar] [CrossRef] [PubMed]
- Grinspoon, L.; Bakalar, J.B. Psychedelic Drugs Reconsidered; Basic Books: New York, NY, USA, 1979; Volume 168. [Google Scholar]
- Nour, M.M.; Carhart-Harris, R.L. Psychedelics and the science of self-experience. Br. J. Psychiatry 2017, 210, 177–179. [Google Scholar] [CrossRef]
- Knight, B.J.; Harbit, R.C.; Smith, J.M. Six-Step Synthesis of (±)-Lysergic Acid. J. Org. Chem. 2023, 88, 2158–2165. [Google Scholar] [CrossRef] [PubMed]
- Dyck, E. ‘Hitting highs at rock bottom’: LSD treatment for alcoholism, 1950–1970. Soc. Hist. Med. 2006, 19, 313–329. [Google Scholar] [CrossRef]
- Oram, M. Efficacy and enlightenment: LSD psychotherapy and the Drug Amendments of 1962. J. Hist. Med. Allied Sci. 2014, 69, 221–250. [Google Scholar] [CrossRef]
- Bonson, K.R.; Murphy, D.L. Alterations in responses to LSD in humans associated with chronic administration of tricyclic antidepressants, monoamine oxidase inhibitors or lithium. Behav. Brain Res. 1996, 73, 229–233. [Google Scholar] [CrossRef]
- Holze, F.; Avedisian, I.; Varghese, N.; Eckert, A.; Liechti, M.E. Role of the 5-HT(2A) Receptor in Acute Effects of LSD on Empathy and Circulating Oxytocin. Front. Pharmacol. 2021, 12, 711255. [Google Scholar] [CrossRef]
- Muller, F.; Dolder, P.C.; Schmidt, A.; Liechti, M.E.; Borgwardt, S. Altered network hub connectivity after acute LSD administration. Neuroimage Clin. 2018, 18, 694–701. [Google Scholar] [CrossRef]
- Preller, K.H.; Razi, A.; Zeidman, P.; Stampfli, P.; Friston, K.J.; Vollenweider, F.X. Effective connectivity changes in LSD-induced altered states of consciousness in humans. Proc. Natl. Acad. Sci. USA 2019, 116, 2743–2748. [Google Scholar] [CrossRef]
- Hirschfeld, T.; Prugger, J.; Majic, T.; Schmidt, T.T. Dose-response relationships of LSD-induced subjective experiences in humans. Neuropsychopharmacology 2023, 48, 1602–1611. [Google Scholar] [CrossRef] [PubMed]
- Liechti, M.E.; Dolder, P.C.; Schmid, Y. Alterations of consciousness and mystical-type experiences after acute LSD in humans. Psychopharmacology 2017, 234, 1499–1510. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.; Jeremiah, A.; Murphy, R.; Sumner, R.; Forsyth, A.; Hoeh, N.; Menkes, D.B.; Evans, W.; Muthukumaraswamy, S.; Sundram, F.; et al. LSD increases sleep duration the night after microdosing. Transl. Psychiatry 2024, 14, 191. [Google Scholar] [CrossRef]
- Donegan, C.J.; Daldegan-Bueno, D.; Sumner, R.; Menkes, D.; Evans, W.; Hoeh, N.; Sundram, F.; Reynolds, L.; Ponton, R.; Cavadino, A.; et al. An open-label pilot trial assessing tolerability and feasibility of LSD microdosing in patients with major depressive disorder (LSDDEP1). Pilot. Feasibility Stud. 2023, 9, 169. [Google Scholar] [CrossRef]
- Family, N.; Hendricks, P.S.; Williams, L.T.; Luke, D.; Krediet, E.; Maillet, E.L.; Raz, S. Safety, tolerability, pharmacokinetics, and subjective effects of 50, 75, and 100 microg LSD in healthy participants within a novel intervention paradigm: A proof-of-concept study. J. Psychopharmacol. 2022, 36, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Holze, F.; Caluori, T.V.; Vizeli, P.; Liechti, M.E. Safety pharmacology of acute LSD administration in healthy subjects. Psychopharmacology 2022, 239, 1893–1905. [Google Scholar] [CrossRef]
- Murphy, R.J.; Sumner, R.; Evans, W.; Ponton, R.; Ram, S.; Godfrey, K.; Forsyth, A.; Cavadino, A.; Krishnamurthy Naga, V.; Smith, T.; et al. Acute Mood-Elevating Properties of Microdosed Lysergic Acid Diethylamide in Healthy Volunteers: A Home-Administered Randomized Controlled Trial. Biol. Psychiatry 2023, 94, 511–521. [Google Scholar] [CrossRef]
- Dolder, P.C.; Schmid, Y.; Haschke, M.; Rentsch, K.M.; Liechti, M.E. Pharmacokinetics and Concentration-Effect Relationship of Oral LSD in Humans. Int. J. Neuropsychopharmacol. 2015, 19, 7. [Google Scholar] [CrossRef]
- Dolder, P.C.; Schmid, Y.; Steuer, A.E.; Kraemer, T.; Rentsch, K.M.; Hammann, F.; Liechti, M.E. Pharmacokinetics and Pharmacodynamics of Lysergic Acid Diethylamide in Healthy Subjects. Clin. Pharmacokinet. 2017, 56, 1219–1230. [Google Scholar] [CrossRef]
- Holze, F.; Duthaler, U.; Vizeli, P.; Muller, F.; Borgwardt, S.; Liechti, M.E. Pharmacokinetics and subjective effects of a novel oral LSD formulation in healthy subjects. Br. J. Clin. Pharmacol. 2019, 85, 1474–1483. [Google Scholar] [CrossRef]
- Luethi, D.; Hoener, M.C.; Krahenbuhl, S.; Liechti, M.E.; Duthaler, U. Cytochrome P450 enzymes contribute to the metabolism of LSD to nor-LSD and 2-oxo-3-hydroxy-LSD: Implications for clinical LSD use. Biochem. Pharmacol. 2019, 164, 129–138. [Google Scholar] [CrossRef]
- 2-Oxo-3-hydroxy-LSD. Available online: https://pubchem.ncbi.nlm.nih.gov/#query=2-Oxo-3-hydroxy-LSD (accessed on 18 March 2025).
- Lysergide. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/5761 (accessed on 18 March 2025).
- Dolder, P.C.; Liechti, M.E.; Rentsch, K.M. Development and validation of an LC-MS/MS method to quantify lysergic acid diethylamide (LSD), iso-LSD, 2-oxo-3-hydroxy-LSD, and nor-LSD and identify novel metabolites in plasma samples in a controlled clinical trial. J. Clin. Lab. Anal. 2018, 32, 8. [Google Scholar] [CrossRef] [PubMed]
- Wachelko, O.; Nowak, K.; Tusiewicz, K.; Zawadzki, M.; Szpot, P. A highly sensitive UHPLC-MS/MS method for determining 15 designer LSD analogs in biological samples with application to stability studies. Analyst 2025, 150, 290–308. [Google Scholar] [CrossRef]
- Darke, S.; Duflou, J.; Peacock, A.; Farrell, M.; Hall, W.; Lappin, J. A retrospective study of the characteristics and toxicology of cases of lysergic acid diethylamide (LSD)- and psilocybin-related death in Australia. Addiction 2024, 119, 1564–1571. [Google Scholar] [CrossRef] [PubMed]
- Brandt, S.D.; Kavanagh, P.V.; Westphal, F.; Stratford, A.; Odland, A.U.; Klein, A.K.; Dowling, G.; Dempster, N.M.; Wallach, J.; Passie, T.; et al. Return of the lysergamides. Part VI: Analytical and behavioural characterization of 1-cyclopropanoyl-d-lysergic acid diethylamide (1CP-LSD). Drug Test. Anal. 2020, 12, 812–826. [Google Scholar] [CrossRef] [PubMed]
- Brandt, S.D.; Kavanagh, P.V.; Westphal, F.; Elliott, S.P.; Wallach, J.; Stratford, A.; Nichols, D.E.; Halberstadt, A.L. Return of the lysergamides. Part III: Analytical characterization of N6-ethyl-6-norlysergic acid diethylamide (ETH-LAD) and 1-propionyl ETH-LAD (1P-ETH-LAD). Drug Test. Anal. 2017, 9, 1641–1649. [Google Scholar] [CrossRef]
- Schindler, E.A.; Dave, K.D.; Smolock, E.M.; Aloyo, V.J.; Harvey, J.A. Serotonergic and dopaminergic distinctions in the behavioral pharmacology of (±)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) and lysergic acid diethylamide (LSD). Pharmacol. Biochem. Behav. 2012, 101, 69–76. [Google Scholar] [CrossRef]
- McKenna, D.J.; Nazarali, A.J.; Hoffman, A.J.; Nichols, D.E.; Mathis, C.A.; Saavedra, J.M. Common receptors for hallucinogens in rat brain: A comparative autoradiographic study using [125I]LSD and [125I]DOI, a new psychotomimetic radioligand. Brain Res. 1989, 476, 45–56. [Google Scholar] [CrossRef]
- Schmid, Y.; Enzler, F.; Gasser, P.; Grouzmann, E.; Preller, K.H.; Vollenweider, F.X.; Brenneisen, R.; Muller, F.; Borgwardt, S.; Liechti, M.E. Acute Effects of Lysergic Acid Diethylamide in Healthy Subjects. Biol. Psychiatry 2015, 78, 544–553. [Google Scholar] [CrossRef]
- Kavanagh, P.V.; Westphal, F.; Pulver, B.; Elliott, S.P.; Stratford, A.; Halberstadt, A.L.; Brandt, S.D. Analytical and behavioral characterization of 1-dodecanoyl-LSD (1DD-LSD). Drug Test. Anal. 2025, 17, 101–109. [Google Scholar] [CrossRef]
- Jobst, B.M.; Atasoy, S.; Ponce-Alvarez, A.; Sanjuan, A.; Roseman, L.; Kaelen, M.; Carhart-Harris, R.; Kringelbach, M.L.; Deco, G. Increased sensitivity to strong perturbations in a whole-brain model of LSD. Neuroimage 2021, 230, 117809. [Google Scholar] [CrossRef] [PubMed]
- Hutten, N.; Mason, N.L.; Dolder, P.C.; Theunissen, E.L.; Holze, F.; Liechti, M.E.; Varghese, N.; Eckert, A.; Feilding, A.; Ramaekers, J.G.; et al. Low Doses of LSD Acutely Increase BDNF Blood Plasma Levels in Healthy Volunteers. ACS Pharmacol. Transl. Sci. 2021, 4, 461–466. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, D.; Popic, J.; Enns, J.P.; Inserra, A.; Skalecka, A.; Markopoulos, A.; Posa, L.; Lopez-Canul, M.; He, Q.; Lafferty, C.K.; et al. Lysergic acid diethylamide (LSD) promotes social behavior through mTORC1 in the excitatory neurotransmission. Proc. Natl. Acad. Sci. USA 2021, 118, 9. [Google Scholar] [CrossRef]
- Smedh, K.; Spigset, O.; Allard, P.; Mjorndal, T.; Adolfsson, R. Platelet [3H]paroxetine and [3H]lysergic acid diethylamide binding in seasonal affective disorder and the effect of bright light therapy. Biol. Psychiatry 1999, 45, 464–470. [Google Scholar] [CrossRef]
- Zec, N.; Filiano, J.J.; Panigrahy, A.; White, W.F.; Kinney, H.C. Developmental changes in [3H]lysergic acid diethylamide ([3H]LSD) binding to serotonin receptors in the human brainstem. J. Neuropathol. Exp. Neurol. 1996, 55, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Mehl, E.; Ruther, E.; Redemann, J. Endogenous ligands of a putative LSD-serotonin receptor in the cerebrospinal fluid: Higher level of LSD-displacing factors (LDF) in unmedicated psychotic patients. Psychopharmacology 1977, 54, 9–16. [Google Scholar] [CrossRef]
- Schmid, Y.; Gasser, P.; Oehen, P.; Liechti, M.E. Acute subjective effects in LSD- and MDMA-assisted psychotherapy. J. Psychopharmacol. 2021, 35, 362–374. [Google Scholar] [CrossRef]
- Shagass, C.; Bittle, R.M. Therapeutic effects of LSD: A follow-up study. J. Nerv. Ment. Dis. 1967, 144, 471–478. [Google Scholar] [CrossRef]
- Beutler, B.D.; Shinozuka, K.; Tabaac, B.J.; Arenas, A.; Cherian, K.; Evans, V.D.; Fasano, C.; Muir, O.S. Psychedelic Therapy: A Primer for Primary Care Clinicians-Lysergic Acid Diethylamide (LSD). Am. J. Ther. 2024, 31, e104–e111. [Google Scholar] [CrossRef]
- Krebs, T.S.; Johansen, P.O. Lysergic acid diethylamide (LSD) for alcoholism: Meta-analysis of randomized controlled trials. J. Psychopharmacol. 2012, 26, 994–1002. [Google Scholar] [CrossRef]
- Meinhardt, M.W.; Gungor, C.; Skorodumov, I.; Mertens, L.J.; Spanagel, R. Psilocybin and LSD have no long-lasting effects in an animal model of alcohol relapse. Neuropsychopharmacology 2020, 45, 1316–1322. [Google Scholar] [CrossRef]
- Daldegan-Bueno, D.; Donegan, C.J.; Forsyth, A.; Sumner, R.L.; Murphy, R.J.; Menkes, D.B.; Evans, W.; Hoeh, N.; Sundram, F.; Reynolds, L.M.; et al. LSDDEP2: Study protocol for a randomised, double-dummy, triple-blind, active placebo-controlled, parallel groups trial of LSD microdosing in patients with major depressive disorder. Trials 2024, 25, 560. [Google Scholar] [CrossRef] [PubMed]
- Ramaekers, J.G.; Hutten, N.; Mason, N.L.; Dolder, P.; Theunissen, E.L.; Holze, F.; Liechti, M.E.; Feilding, A.; Kuypers, K.P. A low dose of lysergic acid diethylamide decreases pain perception in healthy volunteers. J. Psychopharmacol. 2021, 35, 398–405. [Google Scholar] [CrossRef]
- Faramarzi, A.; Fooladi, M.; Yousef Pour, M.; Khodamoradi, E.; Chehreh, A.; Amiri, S.; Shavandi, M.; Sharini, H. Clinical utility of fMRI in evaluating of LSD effect on pain-related brain networks in healthy subjects. Heliyon 2024, 10, e34401. [Google Scholar] [CrossRef] [PubMed]
- Kanen, J.W.; Luo, Q.; Rostami Kandroodi, M.; Cardinal, R.N.; Robbins, T.W.; Nutt, D.J.; Carhart-Harris, R.L.; den Ouden, H.E.M. Effect of lysergic acid diethylamide (LSD) on reinforcement learning in humans. Psychol. Med. 2023, 53, 6434–6445. [Google Scholar] [CrossRef] [PubMed]
- Zeifman, R.J.; Kettner, H.; Pagni, B.A.; Mallard, A.; Roberts, D.E.; Erritzoe, D.; Ross, S.; Carhart-Harris, R.L. Co-use of MDMA with psilocybin/LSD may buffer against challenging experiences and enhance positive experiences. Sci. Rep. 2023, 13, 13645. [Google Scholar] [CrossRef]
- Bazid, A.H.; Wasfy, M.; Fawzy, M.; Nayel, M.; Abdelmegeid, M.; Thabet, R.Y.; Yong, H.S.; El-Sayed, M.M.; Magouz, A.; Badr, Y. Emergency vaccination of cattle against lumpy skin disease: Evaluation of safety, efficacy, and potency of MEVAC((R)) LSD vaccine containing Neethling strain. Vet. Res. Commun. 2023, 47, 767–777. [Google Scholar] [CrossRef]
- Family, N.; Maillet, E.L.; Williams, L.T.J.; Krediet, E.; Carhart-Harris, R.L.; Williams, T.M.; Nichols, C.D.; Goble, D.J.; Raz, S. Safety, tolerability, pharmacokinetics, and pharmacodynamics of low dose lysergic acid diethylamide (LSD) in healthy older volunteers. Psychopharmacology 2020, 237, 841–853. [Google Scholar] [CrossRef]
- Anderson, L.J.; Henley, W.; Wyatt, K.M.; Nikolaou, V.; Hughes, D.A.; Waldek, S.; Logan, S. Long-term effectiveness of enzyme replacement therapy in adults with Gaucher disease: Results from the NCS-LSD cohort study. J. Inherit. Metab. Dis. 2014, 37, 953–960. [Google Scholar] [CrossRef]
- Anderson, L.J.; Henley, W.; Wyatt, K.M.; Nikolaou, V.; Waldek, S.; Hughes, D.A.; Lachmann, R.H.; Logan, S. Effectiveness of enzyme replacement therapy in adults with late-onset Pompe disease: Results from the NCS-LSD cohort study. J. Inherit. Metab. Dis. 2014, 37, 945–952. [Google Scholar] [CrossRef]
- Anderson, L.J.; Henley, W.; Wyatt, K.M.; Nikolaou, V.; Waldek, S.; Hughes, D.A.; Pastores, G.M.; Logan, S. Long-term effectiveness of enzyme replacement therapy in children with Gaucher disease: Results from the NCS-LSD cohort study. J. Inherit. Metab. Dis. 2014, 37, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.J.; Wyatt, K.M.; Henley, W.; Nikolaou, V.; Waldek, S.; Hughes, D.A.; Pastores, G.M.; Logan, S. Long-term effectiveness of enzyme replacement therapy in Fabry disease: Results from the NCS-LSD cohort study. J. Inherit. Metab. Dis. 2014, 37, 969–978. [Google Scholar] [CrossRef]
- Larsen, J.K. LSD treatment in Scandinavia: Emphasizing indications and short-term treatment outcomes of 151 patients in Denmark. Nord. J. Psychiatry 2017, 71, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.G.; Gelkopf, M.; Oyffe, I.; Finkel, B.; Katz, S.; Sigal, M.; Weizman, A. LSD-induced hallucinogen persisting perception disorder treatment with clonidine: An open pilot study. Int. Clin. Psychopharmacol. 2000, 15, 35–37. [Google Scholar] [CrossRef]
- Lev-Ran, S.; Feingold, D.; Goodman, C.; Lerner, A.G. Comparing triggers to visual disturbances among individuals with positive vs negative experiences of hallucinogen-persisting perception disorder (HPPD) following LSD use. Am. J. Addict. 2017, 26, 568–571. [Google Scholar] [CrossRef] [PubMed]
- Kopra, E.I.; Ferris, J.A.; Rucker, J.J.; McClure, B.; Young, A.H.; Copeland, C.S.; Winstock, A.R. Adverse experiences resulting in emergency medical treatment seeking following the use of lysergic acid diethylamide (LSD). J. Psychopharmacol. 2022, 36, 956–964. [Google Scholar] [CrossRef]
- Kopra, E.I.; Ferris, J.A.; Winstock, A.R.; Kuypers, K.P.; Young, A.H.; Rucker, J.J. Investigation of self-treatment with lysergic acid diethylamide and psilocybin mushrooms: Findings from the Global Drug Survey 2020. J. Psychopharmacol. 2023, 37, 733–748. [Google Scholar] [CrossRef]
- Walsh, C.A.; Gorfinkel, L.; Shmulewitz, D.; Stohl, M.; Hasin, D.S. Use of Lysergic Acid Diethylamide by Major Depression Status. JAMA Psychiatry 2024, 81, 89–96. [Google Scholar] [CrossRef]
- Yockey, R.A.; Vidourek, R.A.; King, K.A. Trends in LSD use among US adults: 2015-2018. Drug Alcohol. Depend. 2020, 212, 108071. [Google Scholar] [CrossRef]
- De Gregorio, D.; Aguilar-Valles, A.; Preller, K.H.; Heifets, B.D.; Hibicke, M.; Mitchell, J.; Gobbi, G. Hallucinogens in Mental Health: Preclinical and Clinical Studies on LSD, Psilocybin, MDMA, and Ketamine. J. Neurosci. 2021, 41, 891–900. [Google Scholar] [CrossRef]
- Inserra, A.; Piot, A.; De Gregorio, D.; Gobbi, G. Lysergic Acid Diethylamide (LSD) for the Treatment of Anxiety Disorders: Preclinical and Clinical Evidence. CNS Drugs 2023, 37, 733–754. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, A.; Purvin, V. Persistent palinopsia following ingestion of lysergic acid diethylamide (LSD). Arch. Ophthalmol. 1996, 114, 47–50. [Google Scholar] [CrossRef]
- Alper, K.; Dong, B.; Shah, R.; Sershen, H.; Vinod, K.Y. LSD Administered as a Single Dose Reduces Alcohol Consumption in C57BL/6J Mice. Front. Pharmacol. 2018, 9, 994. [Google Scholar] [CrossRef]
- Brandt, S.D.; Kavanagh, P.V.; Westphal, F.; Stratford, A.; Elliott, S.P.; Dowling, G.; Wallach, J.; Halberstadt, A.L. Return of the lysergamides. Part V: Analytical and behavioural characterization of 1-butanoyl-d-lysergic acid diethylamide (1B-LSD). Drug Test. Anal. 2019, 11, 1122–1133. [Google Scholar] [CrossRef] [PubMed]
- Conway, C.N.; Baker, L.E. Lysergic Acid Diethylamide Produces Anxiogenic Effects in the Rat Light/Dark Test and Elevated Plus Maze. Psi Chi J. Psychol. Res. 2022, 27, 10. [Google Scholar] [CrossRef]
- Bedford, P.; Hauke, D.J.; Wang, Z.; Roth, V.; Nagy-Huber, M.; Holze, F.; Ley, L.; Vizeli, P.; Liechti, M.E.; Borgwardt, S.; et al. The effect of lysergic acid diethylamide (LSD) on whole-brain functional and effective connectivity. Neuropsychopharmacology 2023, 48, 1175–1183. [Google Scholar] [CrossRef]
- Maltarollo, V.G.; Honorio, K.M.; Emery, F.S.; Ganesan, A.; Trossini, G.H. Hologram quantitative structure-activity relationship and comparative molecular interaction field analysis of aminothiazole and thiazolesulfonamide as reversible LSD1 inhibitors. Future Med. Chem. 2015, 7, 1381–1394. [Google Scholar] [CrossRef]
- Jang, M.; Kim, J.; Han, I.; Yang, W. Simultaneous determination of LSD and 2-oxo-3-hydroxy LSD in hair and urine by LC-MS/MS and its application to forensic cases. J. Pharm. Biomed. Anal. 2015, 115, 138–143. [Google Scholar] [CrossRef]
- Meert, T.F.; de Haes, P.; Janssen, P.A. Risperidone (R 64 766), a potent and complete LSD antagonist in drug discrimination by rats. Psychopharmacology 1989, 97, 206–212. [Google Scholar] [CrossRef]
- Abraham, H.D.; Duffy, F.H. EEG coherence in post-LSD visual hallucinations. Psychiatry Res. 2001, 107, 151–163. [Google Scholar] [CrossRef]
- Leonard, J.B.; Anderson, B.; Klein-Schwartz, W. Does getting high hurt? Characterization of cases of LSD and psilocybin-containing mushroom exposures to national poison centers between 2000 and 2016. J. Psychopharmacol. 2018, 32, 1286–1294. [Google Scholar] [CrossRef] [PubMed]
- Adamska, I.; Finc, K. Effect of LSD and music on the time-varying brain dynamics. Psychopharmacology 2023, 240, 1601–1614. [Google Scholar] [CrossRef] [PubMed]
- Hendricks, P.S.; Copes, H.; Family, N.; Williams, L.T.; Luke, D.; Raz, S. Perceptions of safety, subjective effects, and beliefs about the clinical utility of lysergic acid diethylamide in healthy participants within a novel intervention paradigm: Qualitative results from a proof-of-concept study. J. Psychopharmacol. 2022, 36, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.J.; Sumner, R.L.; Evans, W.; Menkes, D.; Lambrecht, I.; Ponton, R.; Sundram, F.; Hoeh, N.; Ram, S.; Reynolds, L.; et al. MDLSD: Study protocol for a randomised, double-masked, placebo-controlled trial of repeated microdoses of LSD in healthy volunteers. Trials 2021, 22, 302. [Google Scholar] [CrossRef]
- Liu, S.F.; Tu, D.S. On the applications of Fisher’s least significant difference (LSD) procedure in three-arm clinical trials with survival endpoints. Drug Inf. J. 2008, 42, 81–91. [Google Scholar] [CrossRef]
| Study Type | Agents Used and Doses | Key Findings | References |
|---|---|---|---|
| Randomized controlled trials (RCTs) | - LSD microdosing (10 μg every third day) - High-dose LSD (50–200 μg) - MEVAC® vaccine for lumpy skin disease (LSDV) - Ongoing Phase 2b trial (LSDDEP2) for major depressive disorder | - LSD microdosing increased sleep duration but had no effect on sleep stages or physical activity. - High-dose LSD was well tolerated in controlled settings, with effects lasting up to 12 h and no significant long-term adverse effects. - MEVAC® vaccine was safe and effective in cattle. - No clinical outcomes reported yet from LSDDEP2 trial. | [15,17,18,46,48,51,52] |
| Longitudinal cohort studies | - Enzyme replacement therapy (ERT) for Gaucher, Pompe, and Fabry diseases | - Improved blood markers and mobility in Gaucher and Pompe diseases. - In Fabry disease, ERT reduced left ventricular mass index and proteinuria risk in adults but had no significant effects in children. | [53,54,55,56] |
| Retrospective studies | - LSD interactions with antidepressants - LSD-related deaths - Historical LSD psychotherapy - HPPD cases | - Tricyclic antidepressants and lithium increased LSD effects, while monoamine oxidase inhibitors reduced them. - Most LSD-related deaths were trauma-related, with self-harm cases involving physical means. - Historical LSD psychotherapy had mixed outcomes—some short-term improvements but poor long-term results. - HPPD symptoms persisted for years in some cases. | [7,9,28,57,58,59] |
| Cross-sectional studies/surveys | - General population LSD use trends - Self-treatment with psychedelics - MDMA co-administration with LSD/psilocybin | - LSD use increased, particularly among individuals with depression and younger adults. - Self-treatment was common, with both positive (improved mood, insight) and negative effects (anxiety, distress). - MDMA co-administration reduced negative experiences (e.g., fear, grief) during psychedelic use. | [50,60,61,62,63] |
| Meta-analyses/systematic reviews | - LSD for anxiety in life-threatening illnesses - LSD for alcohol use disorder - LSD dose–response - Comparative efficacy of psychedelics (LSD, psilocybin, MDMA, ketamine) | - LSD showed long-term benefits for anxiety in life-threatening illnesses. - Significantly improved alcohol use disorder outcomes. - Effects plateaued at ~100 µg (higher doses do not necessarily enhance effects). - Distinct therapeutic potentials identified among LSD, psilocybin, MDMA, and ketamine. | [13,43,44,64,65] |
| Case series/case reports | - LSD-induced visual disturbances (HPPD, palinopsia) - Clonidine for HPPD treatment | - HPPD symptoms persisted in some LSD users. - Clonidine reduced HPPD symptoms in certain cases. | [58,66] |
| Animal studies | - LSD (50 μg/kg) in mice - LSD analogs (1P-ETH-LAD, 1CP-LSD) - LSD in rats (behavioral effects) - LSD-induced social behaviors in mice | - LSD reduced alcohol consumption in mice. - Certain LSD analogs functioned as prodrugs, converting to LSD or ETH-LAD in vivo. - LSD transiently increased anxiety-like behaviors in rats. - LSD promoted social behaviors via 5-HT2A and AMPA receptor activation. | [29,37,67,68,69] |
| Pharmacokinetic/pharmacodynamic studies | - LSD (5–200 μg) - LSD BDNF effects (5–20 μg) | - First-order elimination kinetics with peak plasma concentrations at ~1.4–1.7 h. - Subjective effects lasted 8–12 h. - Low doses (5–20 μg) increased BDNF levels, suggesting neuroplasticity effects. | [10,20,21,22,26,36] |
| Neuroimaging/fMRI studies | - LSD effects on brain connectivity - LSD and pain perception - LSD and emotional empathy | - LSD increased whole-brain connectivity while reducing local coherence in specific regions. - Altered pain perception by reducing activity in pain-processing areas. - Enhanced emotional empathy, correlating with thalamic activity and changes in the default mode network (DMN). | [11,12,33,35,48,49,70] |
| Computational/modeling studies | - LSD-induced brain dynamics - Machine learning and LSD - LSD1 enzyme inhibition (cancer research) | - LSD shifted brain dynamics further from equilibrium, increasing response flexibility. - Machine learning identified LSD-induced connectivity changes with 91.11% accuracy. - Computational models were developed for LSD1 enzyme inhibition, targeting potential cancer therapies. | [32,35,71] |
| Analytical/forensic studies | - LSD analog detection (ETH-LAD, 1P-ETH-LAD, 1CP-LSD) - LSD stability in biological samples | - Novel LSD analogs identified, with some acting as prodrugs. - New analytical methods improved LSD detection in hair, urine, and plasma. - Sodium fluoride (NaF) storage minimized LSD degradation in biological samples. | [27,29,30,72] |
| Psychopharmacology/cognitive studies | - LSD and cognitive flexibility - LSD and reinforcement learning - LSD and pain perception - LSD receptor interactions (serotonin-mediated) | - LSD increased cognitive flexibility and altered reinforcement learning by enhancing learning rates for both reward and punishment. - LSD’s effects were mediated primarily by serotonin receptors. - Risperidone effectively blocked LSD’s effects. - LSD reduced pain perception in controlled settings. | [47,49,73] |
| Disorder/Condition | Key Findings | References |
|---|---|---|
| Hallucinogen-persisting perception disorder (HPPD) | EEG studies in HPPD patients showed widespread reduced cortical coherence in the eyes-open state and increased occipital coherence upon eye closure, suggesting visual cortex dysregulation. Clonidine treatment reduced HPPD symptoms in some patients. LSD-induced palinopsia persisted for years in rare cases. | [58,66,74] |
| Mood disorders (depression, anxiety, seasonal affective disorder—SAD) | LSD-assisted psychotherapy provided sustained benefits for anxiety in life-threatening illnesses and showed potential in depression treatment. A Phase 2b trial (LSDDEP2) is ongoing to evaluate LSD microdosing for major depressive disorder, but clinical outcomes have not yet been reported. Bright light therapy for seasonal affective disorder (SAD) was correlated with changes in serotonin receptor binding. | [16,38,43,46,65] |
| Substance use disorders (alcoholism, addiction, relapse prevention) | A meta-analysis showed that a single LSD dose significantly reduced alcohol misuse in clinical settings. In animal studies, LSD reduced alcohol consumption in mice at a 50 μg/kg dose but did not prevent relapses in addiction models. | [44,45,67] |
| Neurodegenerative/neuromuscular disorders (Gaucher, Fabry, Pompe, MPS, NPC) | Long-term enzyme replacement therapy (ERT) improved platelet counts, hemoglobin levels, organ function, and mobility in Gaucher and Pompe diseases. In Fabry disease, ERT provided benefits in adults (e.g., reduced left ventricular mass index and proteinuria risk) but had no significant effects in children. | [53,54,55,56] |
| Pain disorders (chronic pain, analgesia, migraine) | LSD reduced activity in pain-processing brain regions, including the anterior cingulate cortex, thalamus, and insula. A low dose of LSD (20 μg) increased pain tolerance in a Cold Pressor Test without inducing a full psychedelic experience. | [47,48] |
| Psychosis/schizophrenia | LSD-displacing factors in cerebrospinal fluid (CSF) were elevated in unmedicated psychotic patients and correlated with symptom improvement after antipsychotic treatment. LSD’s effects were blocked by risperidone, confirming its interaction with serotonin and dopamine receptors. | [40,73] |
| Cognitive/executive functioning disorders | LSD increased cognitive flexibility, improved reinforcement learning rates for rewards and punishments, and reduced stimulus stickiness, indicating heightened exploration. These findings suggest potential therapeutic applications for cognitive disorders. | [49] |
| Cardiovascular/autonomic disorders | LSD binding was highest in fetal brainstem regions involved in cardiovascular and respiratory regulation. A small number of LSD-related cardiovascular deaths were reported, but most fatalities were trauma-related. | [28,39] |
| Forensic/toxicology Studies (LSD-related deaths, emergency treatment, poisoning) | LSD-related deaths were primarily trauma-induced, with a low risk of acute toxicity. LSD cases resulted in more hospital admissions than psilocybin cases. New forensic detection methods improved LSD identification in biological samples, with sodium fluoride storage minimizing LSD degradation. | [27,28,60,61,75] |
| Psychopharmacology/neuroplasticity | LSD increased brain-derived neurotrophic factor (BDNF) levels, suggesting potential neuroplasticity effects. Music significantly influenced LSD-induced brain dynamics, highlighting its role in psychedelic therapy settings. | [36,76] |
| Public health and LSD usage trends | LSD use increased significantly among young adults, individuals with depression, and those with higher education. Changing social attitudes and increased accessibility were identified as potential factors influencing usage patterns. | [62,63] |
| Category | Key Findings | References |
|---|---|---|
| Acute safety profile | LSD was generally well tolerated in controlled settings, producing dose-dependent increases in heart rate, blood pressure, and transient psychological effects. No severe or long-term adverse effects were observed in controlled trials. | [17,18] |
| Adverse effects and risks | The most commonly reported adverse effects of LSD include anxiety, panic, confusion, and agitation, often influenced by set and setting. Severe effects, such as hyperthermia, seizures, and cardiovascular complications, were rare but more likely in cases involving polysubstance use. | [28,60,61,75] |
| Emergency medical treatment (EMT) risk | Approximately 1% of LSD users sought emergency medical treatment (EMT) within a year, with a per-event risk of 0.2%. Psychological distress, including anxiety and panic, was the most common reason for hospital visits. | [60] |
| HPPD and visual disturbances | Persistent visual disturbances, including HPPD and palinopsia, have been reported in some LSD users, sometimes lasting for years. EEG studies in HPPD patients suggest reduced cortical coherence in the eyes-open state and increased occipital coherence upon eye closure, indicating cortical dysregulation. Clonidine has shown potential in reducing HPPD symptoms in some patients. | [58,66,74] |
| Potential for addiction or dependence | LSD does not produce compulsive drug-seeking behavior typical of addictive substances. No evidence of physical dependence or withdrawal symptoms has been reported in controlled studies. | [18] |
| Psychological risks | While some individuals experience profound positive effects, LSD may cause transient anxiety or psychological distress, particularly in those with underlying psychiatric conditions. Individuals with pre-existing psychiatric disorders may be at increased risk for prolonged or severe adverse psychological reactions. Further research is needed to determine LSD’s effects on psychosis vulnerability. | [65,73] |
| Research Area | Key Findings | References |
|---|---|---|
| Neuroplasticity and cognitive effects | LSD enhances cognitive flexibility and may promote brain plasticity by increasing BDNF levels. These effects suggest potential applications in cognitive and neuropsychiatric disorders. | [36,49] |
| LSD and pain management | LSD altered pain perception by reducing activity in pain-processing brain regions, including the anterior cingulate cortex, thalamus, and insula. A low dose of LSD (20 μg) increased pain tolerance in a Cold Pressor Test, supporting its potential as an analgesic. | [47,48] |
| LSD and therapy | Clinical trials suggest that LSD-assisted therapy may offer long-term benefits for anxiety in life-threatening illnesses, alcohol use disorder, and depression. Further research is needed to optimize treatment protocols. | [43,44,65] |
| Emerging research: LSD microdosing | Ongoing studies are investigating whether LSD microdosing can improve mood, creativity, and cognition. Preliminary findings suggest transient mood-enhancing effects but no significant long-term changes in overall mood or cognition. | [19,46,78] |
| Emerging research: psychedelics in neurological disorders | LSD’s effects on brain networks, neuroplasticity, and serotonin receptor activity suggest potential therapeutic applications for neurological disorders. However, no direct evidence currently supports its use in neurodegenerative conditions such as Parkinson’s or Alzheimer’s. Further research is needed. | [36,39] |
| Interdisciplinary research: psychedelics and social behavior | LSD has been shown to enhance social behavior in animals and increase emotional empathy in humans. These effects are mediated through serotonin 5-HT2A and AMPA receptor activation. Further research is needed to determine its potential therapeutic applications. | [10,37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omidian, H.; Omidian, A. Clinical Research on Lysergic Acid Diethylamide (LSD) in Psychiatry and Neuroscience. Pharmaceuticals 2025, 18, 499. https://doi.org/10.3390/ph18040499
Omidian H, Omidian A. Clinical Research on Lysergic Acid Diethylamide (LSD) in Psychiatry and Neuroscience. Pharmaceuticals. 2025; 18(4):499. https://doi.org/10.3390/ph18040499
Chicago/Turabian StyleOmidian, Hossein, and Alborz Omidian. 2025. "Clinical Research on Lysergic Acid Diethylamide (LSD) in Psychiatry and Neuroscience" Pharmaceuticals 18, no. 4: 499. https://doi.org/10.3390/ph18040499
APA StyleOmidian, H., & Omidian, A. (2025). Clinical Research on Lysergic Acid Diethylamide (LSD) in Psychiatry and Neuroscience. Pharmaceuticals, 18(4), 499. https://doi.org/10.3390/ph18040499











