Therapeutic Potential of Hexahydrocurcumin in the Regeneration and Protection of the Retinal Pigment Epithelium
Abstract
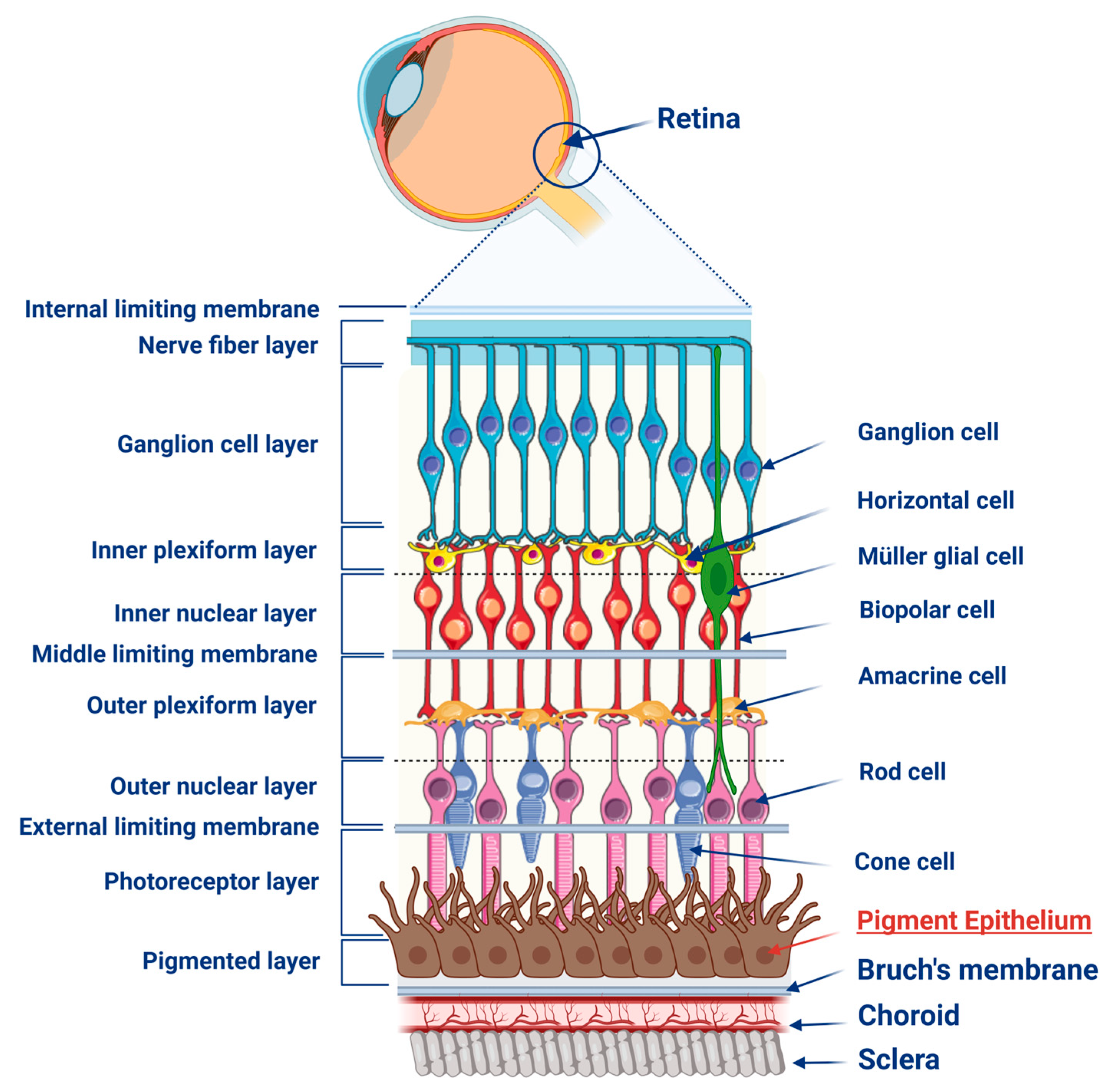
:1. Retinal Pigment Epithelium
2. Diseases Associated with the Degradation of the RPE
2.1. Age-Related Macular Degeneration
2.2. Diabetic Retinopathy
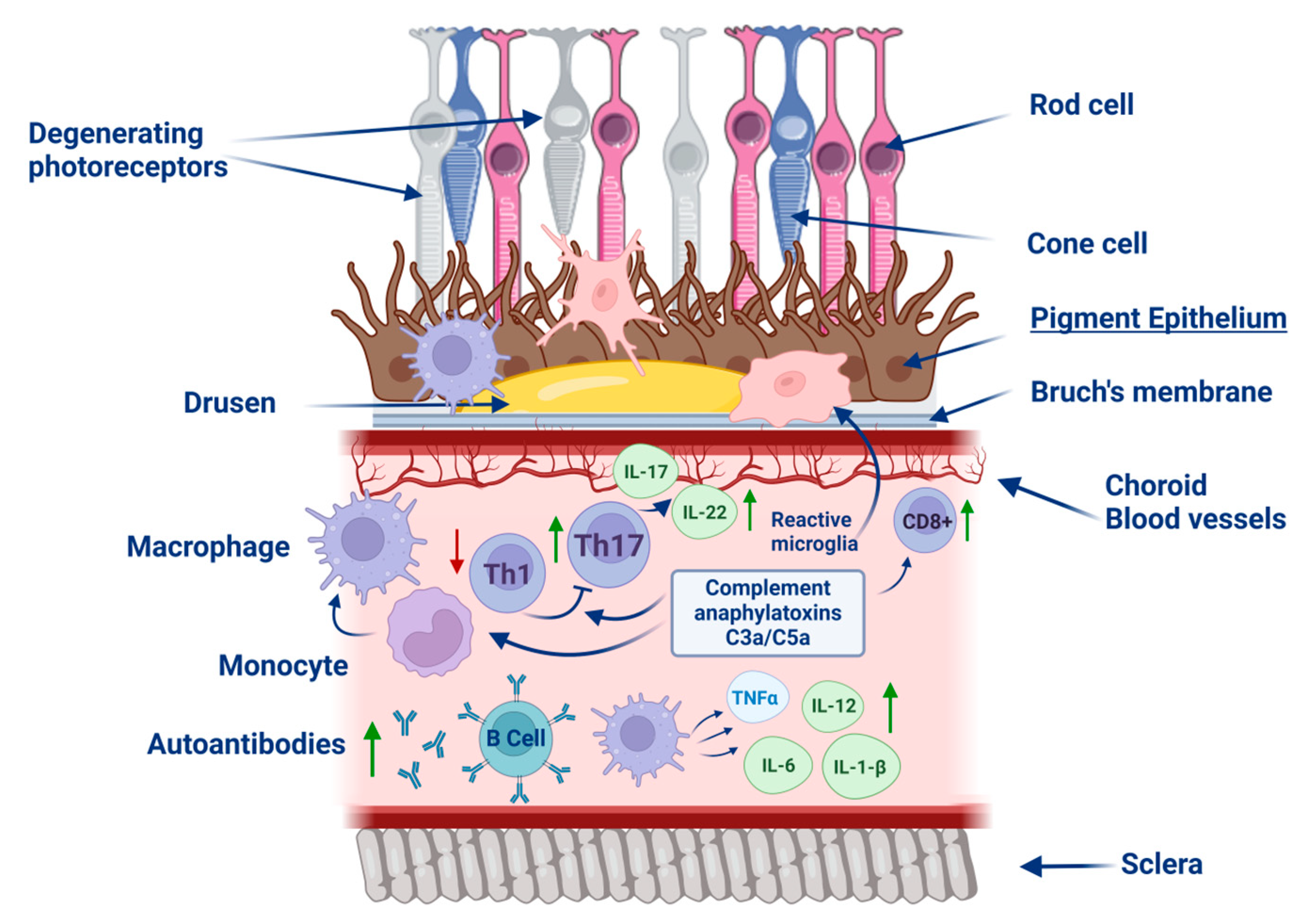
3. Pathology of RPE Degradation
4. Curcuminoids—Biological Properties and Therapeutic Potential
4.1. Curcumin
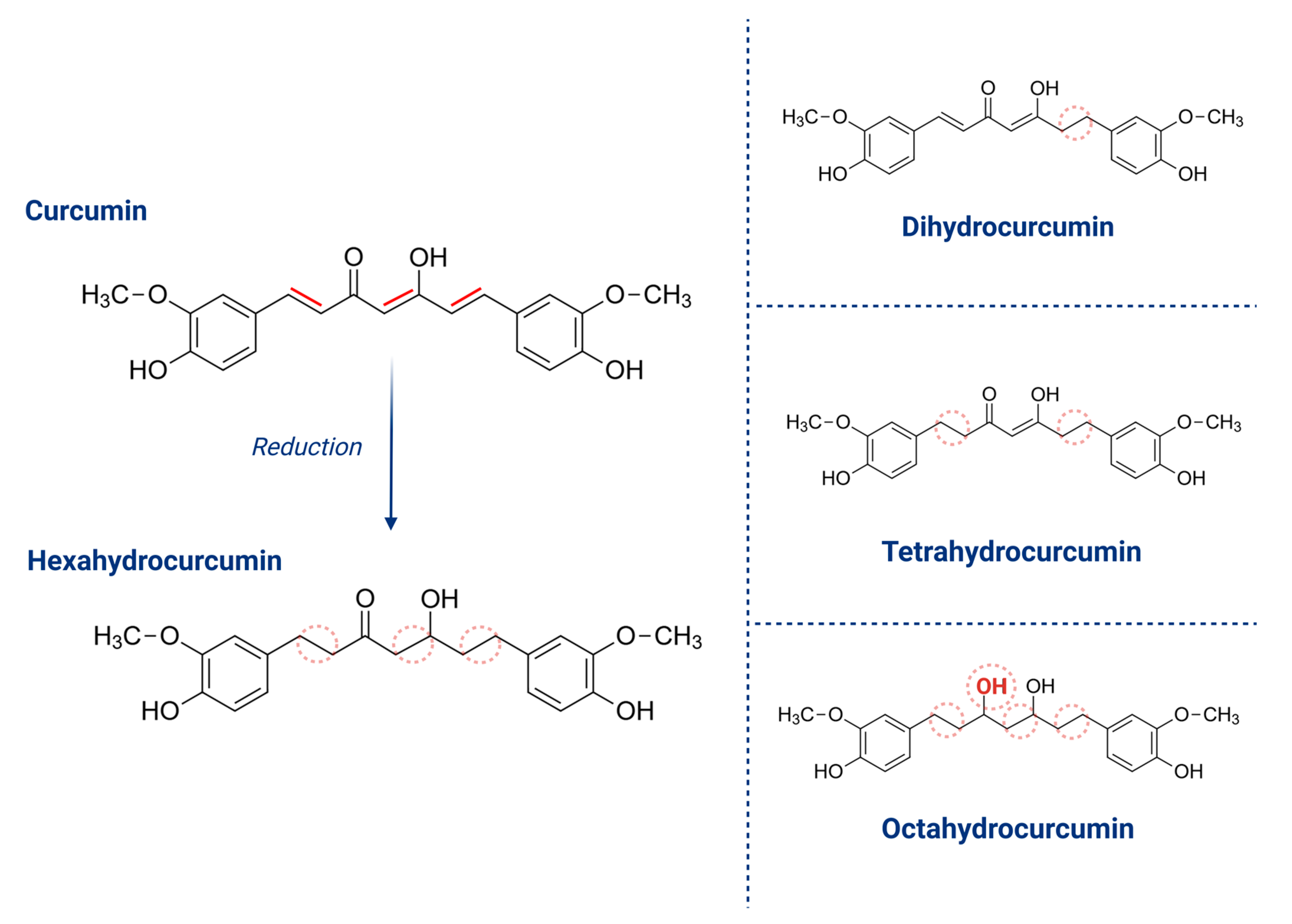
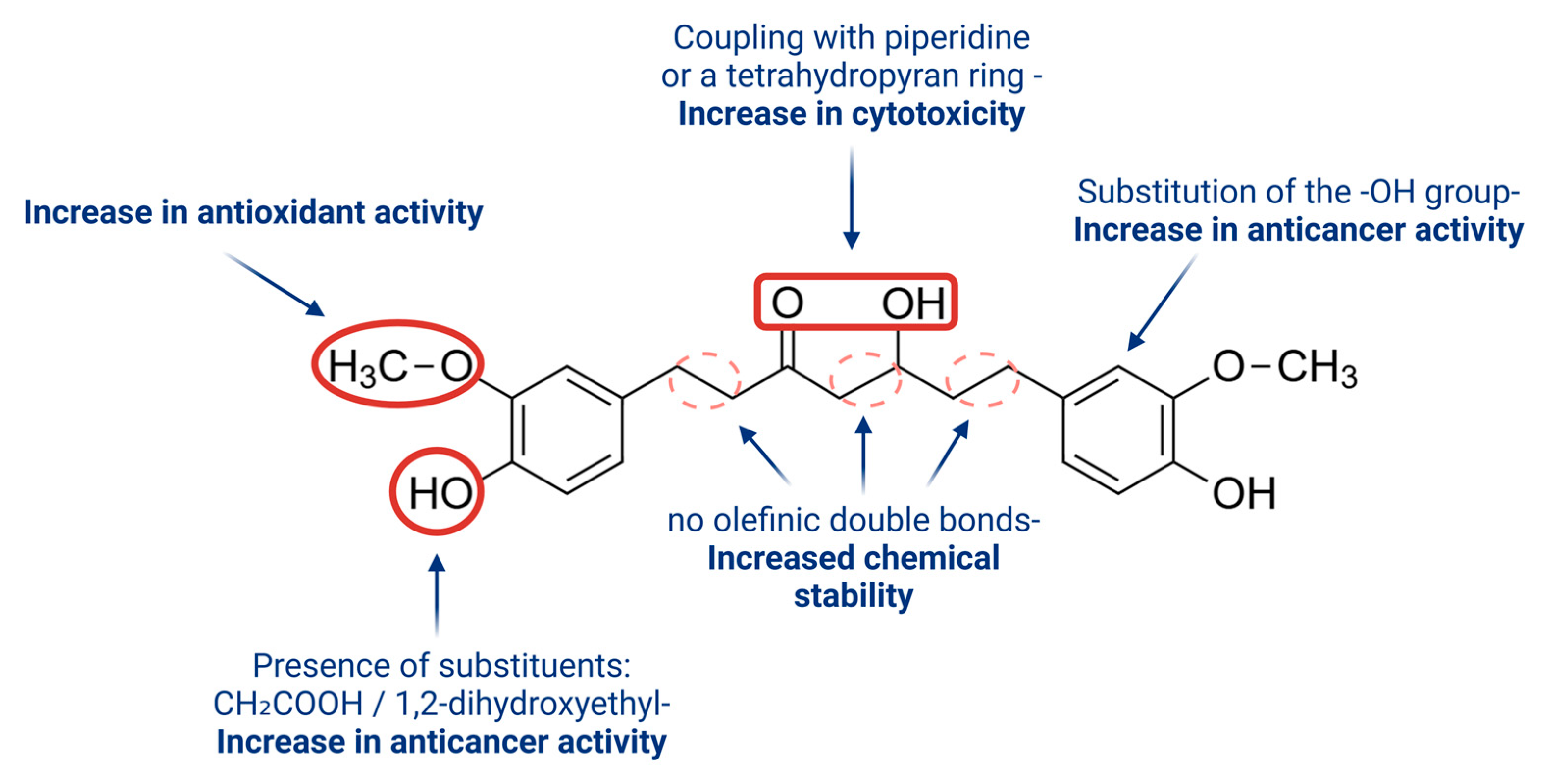
4.2. Hexahydrocurcumin
5. In Vitro and In Vivo Studies of Hexahydrocurcumin
6. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 4-HNE | 4-hydroxynonenal |
| AMD | age-related macular degeneration |
| BCCAO | bilateral common carotid artery occlusion |
| bFGF | basic fibroblast growth factor |
| BRB | blood-retina barrier |
| C3 | complement component 3 |
| CCH | chronic cerebral hypoperfusion |
| CCL2 | C-C motif chemokine ligand 2 |
| CEP | carboxyethylpyrrole |
| COL1 | type I collagen |
| CorNV | corneal neovascularization |
| COX-2 | cyclooxygenase-2 |
| CP | classical pathway |
| DME | diabetic macular edema |
| DR | diabetic retinopathy |
| ER | endoplasmic reticulum |
| GPx | glutathione peroxidase |
| GSH | glutathione |
| GST | glutathione-S-transferase |
| HHC | hexahydrocurcumin |
| HIV | human immunodeficiency virus |
| ICAM-1 | intercellular adhesion molecule-1 |
| IL | interleukin |
| iNOS | inducible nitric oxide synthase |
| JAK | janus kinases |
| LC3-II | microtubule-associated protein 1A/1B-light chain 3 II |
| LOX | lipoxygenase |
| MAC | membrane attack complex |
| MAPK | mitogen-activated protein kinases |
| MCP-1 | monocyte chemoattractant protein-1 |
| MDA | malondialdehyde |
| MIP | macrophage inhibitory protein |
| MMP-9 | matrix metalloproteinase-9 |
| mtDNA | mitochondrial DNA |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NOX | nicotinamide adenine dinucleotide phosphate oxidases |
| NPDR | non-proliferative diabetic retinopathy |
| NRF2 | nuclear factor erythroid 2-related factor 2 |
| PDR | proliferative diabetic retinopathy |
| POS | photoreceptor outer segments |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| RPE | retinal pigment epithelium |
| SOD1 | superoxide dismutase 1 |
| TGF-β1 | transforming growth factor β |
| TNF-α | tumor necrosis factor-alpha |
| UPR | unfolded protein response |
| VCAM-1 | Vascular Cell Adhesion Molecule-1 |
| VEGF | vascular endothelial growth factor |
| Wnt | suppression of Wingless |
| XO | xanthine oxidase |
References
- Strauss, O. The Retinal Pigment Epithelium. In Webvision: The Organization of the Retina and Visual System; Kolb, H., Fernandez, E., Jones, B., Nelson, R., Eds.; University of Utah Health Sciences Center: Salt Lake City, UT, USA, 1995. [Google Scholar]
- Kaufmann, M.; Han, Z. RPE Melanin and Its Influence on the Progression of AMD. Ageing Res. Rev. 2024, 99, 102358. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.; Wang, J.-N.; Cao, Q.-C.; Sun, R.-X.; Zhu, H.-J.; Zhang, Y.-R.; Ji, J.-D.; Liu, Q.-H. m6A Modification of circSPECC1 Suppresses RPE Oxidative Damage and Maintains Retinal Homeostasis. Cell Rep. 2022, 41, 111671. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-M.; Fan, B.; Li, Y.-L.; Zuo, Z.-Y.; Li, G.-Y. Oxidative Stress-Involved Mitophagy of Retinal Pigment Epithelium and Retinal Degenerative Diseases. Cell. Mol. Neurobiol. 2023, 43, 3265–3276. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.-S.; Zheng, M.; Zhang, A.Y.; Han, Z. Melanin-like Nanoparticles as an Alternative to Natural Melanin in Retinal Pigment Epithelium Cells and Their Therapeutic Effects against Age-Related Macular Degeneration. ACS Nano 2022, 16, 19412–19422. [Google Scholar] [CrossRef] [PubMed]
- Cell Types of the Human Retina and Its Organoids at Single-Cell Resolution. Cowan et al—EGA European Genome-Phenome Archive. Available online: https://ega-archive.org/studies/EGAS00001004561 (accessed on 3 March 2025).
- Masland, R.H. The Fundamental Plan of the Retina. Nat. Neurosci. 2001, 4, 877–886. [Google Scholar] [CrossRef]
- Masland, R.H. The Neuronal Organization of the Retina. Neuron 2012, 76, 266–280. [Google Scholar] [CrossRef]
- Somasundaran, S.; Constable, I.J.; Mellough, C.B.; Carvalho, L.S. Retinal Pigment Epithelium and Age-Related Macular Degeneration: A Review of Major Disease Mechanisms. Clin. Exp. Ophthalmol. 2020, 48, 1043–1056. [Google Scholar] [CrossRef]
- Tong, Y.; Wu, Y.; Ma, J.; Ikeda, M.; Ide, T.; Griffin, C.T.; Ding, X.-Q.; Wang, S. Comparative Mechanistic Study of RPE Cell Death Induced by Different Oxidative Stresses. Redox Biol. 2023, 65, 102840. [Google Scholar] [CrossRef]
- Burkholder, B.M.; Jabs, D.A. Uveitis for the non-ophthalmologist. BMJ 2021, 372, m4979. [Google Scholar] [CrossRef]
- Kalogeropoulos, D.; Sakkas, H.; Mohammed, B.; Vartholomatos, G.; Malamos, K.; Sreekantam, S.; Kanavaros, P.; Kalogeropoulos, C. Ocular toxoplasmosis: A review of the current diagnostic and therapeutic approaches. Int. Ophthalmol. 2022, 42, 295–321. [Google Scholar] [CrossRef]
- Rong, S.; Yu, X.; Wiggs, J.L. Genetic Basis of Pigment Dispersion Syndrome and Pigmentary Glaucoma: An Update and Functional Insights. Genes 2024, 15, 142. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Tian, M.; Wang, Y.; Ma, H.; Zhou, Y.; Jiang, X.; Liu, Y. Updates on RPE Cell Damage in Diabetic Retinopathy (Review). Mol. Med. Rep. 2023, 28, 185. [Google Scholar] [CrossRef] [PubMed]
- Jarrett, S.G.; Boulton, M.E. Consequences of Oxidative Stress in Age-Related Macular Degeneration. Mol. Asp. Med. 2012, 33, 399–417. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.H.; Patel, B.; Wilmot, E.G.; Amoaku, W.M. Diabetic Retinopathy for the Non-Ophthalmologist. Clin. Med. 2022, 22, 112–116. [Google Scholar] [CrossRef]
- Tonade, D.; Kern, T.S. Photoreceptor Cells and RPE Contribute to the Development of Diabetic Retinopathy. Prog. Retin. Eye Res. 2021, 83, 100919. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Blasiak, J.; Liton, P.; Boulton, M.; Klionsky, D.J.; Sinha, D. Autophagy in Age-Related Macular Degeneration. Autophagy 2023, 19, 388–400. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
- Yang, Y.-C.; Chien, Y.; Yarmishyn, A.A.; Lim, L.-Y.; Tsai, H.-Y.; Kuo, W.-C.; Tsai, P.-H.; Yang, S.-H.; Hong, S.-I.; Chen, S.-J.; et al. Inhibition of Oxidative Stress-Induced Epithelial-Mesenchymal Transition in Retinal Pigment Epithelial Cells of Age-Related Macular Degeneration Model by Suppressing ERK Activation. J. Adv. Res. 2024, 60, 141–157. [Google Scholar] [CrossRef]
- Tan, T.-E.; Wong, T.Y. Diabetic Retinopathy: Looking Forward to 2030. Front. Endocrinol. 2022, 13, 1077669. [Google Scholar] [CrossRef]
- Tatsumi, T. Current Treatments for Diabetic Macular Edema. Int. J. Mol. Sci. 2023, 24, 9591. [Google Scholar] [CrossRef]
- Zhang, C.; Gu, L.; Xie, H.; Liu, Y.; Huang, P.; Zhang, J.; Luo, D.; Zhang, J. Glucose Transport, Transporters and Metabolism in Diabetic Retinopathy. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 166995. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Cano, M.; Ebrahimi, K.; Wang, L.; Handa, J.T. The Impact of Oxidative Stress and Inflammation on RPE Degeneration in Non-Neovascular AMD. Prog. Retin. Eye Res. 2017, 60, 201–218. [Google Scholar] [CrossRef] [PubMed]
- Cornebise, C.; Perus, M.; Hermetet, F.; Vallas-Fonavet, J.; Richard, T.; Aires, V.; Delmas, D. Red Wine Extract Prevents Oxidative Stress and Inflammation in ARPe-19 Retinal Cells. Cells 2023, 12, 1408. [Google Scholar] [CrossRef] [PubMed]
- Harju, N. Regulation of oxidative stress and inflammatory responses in human retinal pigment epithelial cells. Acta Ophthalmol. 2022, 100 (Suppl. 273), 3–59. [Google Scholar] [CrossRef]
- Kushwah, N.; Bora, K.; Maurya, M.; Pavlovich, M.C.; Chen, J. Oxidative Stress and Antioxidants in Age-Related Macular Degeneration. Antioxidants 2023, 12, 1379. [Google Scholar] [CrossRef]
- Bungau, S.; Abdel-Daim, M.M.; Tit, D.M.; Ghanem, E.; Sato, S.; Maruyama-Inoue, M.; Yamane, S.; Kadonosono, K. Health Benefits of Polyphenols and Carotenoids in Age-Related Eye Diseases. Oxid. Med. Cell. Longev. 2019, 2019, 9783429. [Google Scholar] [CrossRef]
- Lykkesfeldt, J. Malondialdehyde as Biomarker of Oxidative Damage to Lipids Caused by Smoking. Clin. Chim. Acta 2007, 380, 50–58. [Google Scholar] [CrossRef]
- Cano, M.; Wang, L.; Wan, J.; Barnett, B.P.; Ebrahimi, K.; Qian, J.; Handa, J.T. Oxidative Stress Induces Mitochondrial Dysfunction and a Protective Unfolded Protein Response in RPE Cells. Free Radic. Biol. Med. 2014, 69, 1–14. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Sheu, S.-J.; Liu, W.; Hsu, Y.-T.; He, C.-X.; Wu, C.-Y.; Chen, K.-J.; Lee, P.-Y.; Chiu, C.-C.; Cheng, K.-C. Retinal Protective Effect of Curcumin Metabolite Hexahydrocurcumin against Blue Light-Induced RPE Damage. Phytomedicine 2023, 110, 154606. [Google Scholar] [CrossRef]
- Grimm, C.; Remé, C.E.; Rol, P.O.; Williams, T.P. Blue Light’s Effects on Rhodopsin: Photoreversal of Bleaching in Living Rat Eyes. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3984–3990. [Google Scholar] [PubMed]
- Grimm, C.; Remé, C.E. Light Damage Models of Retinal Degeneration. In Retinal Degeneration; Weber, B.H.F., Langmann, T., Eds.; Methods in Molecular Biology; Humana: New York, NY, USA, 2019; Volume 1834, pp. 167–178. ISBN 978-1-4939-8668-2. [Google Scholar] [CrossRef]
- Kuse, Y.; Ogawa, K.; Tsuruma, K.; Shimazawa, M.; Hara, H. Damage of Photoreceptor-Derived Cells in Culture Induced by Light Emitting Diode-Derived Blue Light. Sci. Rep. 2014, 4, 5223. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, X.; Yang, J.; Hong, Z.; Wu, Y.; Xie, Y.; Wang, G. Mechanisms of Blue Light-Induced Eye Hazard and Protective Measures: A Review. Biomed. Pharmacother. 2020, 130, 110577. [Google Scholar] [CrossRef]
- Talens-Estarelles, C.; García-Marqués, J.V.; Cervino, A.; García-Lázaro, S. Use of Digital Displays and Ocular Surface Alterations: A Review. Ocul. Surf. 2021, 19, 252–265. [Google Scholar] [CrossRef]
- Guymer, R.H.; Campbell, T.G. Age-related mascular degeneration. Lancet 2023, 401, 1459–1472. [Google Scholar] [CrossRef] [PubMed]
- Llorián-Salvador, M.; de la Fuente, A.G.; McMurran, C.E.; Dashwood, A.; Dooley, J.; Liston, A.; Penalva, R.; Dombrowski, Y.; Stitt, A.W.; Fitzgerald, D.C. Regulatory T Cells Limit Age-Associated Retinal Inflammation and Neurodegeneration. Mol. Neurodegener. 2024, 19, 32. [Google Scholar] [CrossRef]
- Choudhary, M.; Malek, G. CD68: Potential Contributor to Inflammation and RPE Cell Dystrophy. Adv. Exp. Med. Biol. 2023, 1415, 207–213. [Google Scholar] [CrossRef]
- Huang, Y.; Cao, S.; Zhang, Q.; Zhang, H.; Fan, Y.; Qiu, F.; Kang, N. Biological and Pharmacological Effects of Hexahydrocurcumin, a Metabolite of Curcumin. Arch. Biochem. Biophys. 2018, 646, 31–37. [Google Scholar] [CrossRef]
- Bharti, K.; den Hollander, A.I.; Lakkaraju, A.; Sinha, D.; Williams, D.S.; Finnemann, S.C.; Bowes-Rickman, C.; Malek, G.; D’Amore, P.A. Cell Culture Models to Study Retinal Pigment Epithelium-Related Pathogenesis in Age-Related Macular Degeneration. Exp. Eye Res. 2022, 222, 109170. [Google Scholar] [CrossRef] [PubMed]
- Kuźmińska, J.; Sobczak, A.; Wierzchowski, M.; Gośliński, T.; Jelińska, A. Efekty działania fitoestrogenów w nowotworach hormonozależnych. Farmacja 2021, 14, 140–145. [Google Scholar] [CrossRef]
- Pandey, A.; Chaturvedi, M.; Mishra, S.; Kumar, P.; Somvanshi, P.; Chaturvedi, R. Reductive Metabolites of Curcumin and Their Therapeutic Effects. Heliyon 2020, 6, e05469. [Google Scholar] [CrossRef]
- Zia, A.; Farkhondeh, T.; Pourbagher-Shahri, A.M.; Samarghandian, S. The Role of Curcumin in Aging and Senescence: Molecular Mechanisms. Biomed. Pharmacother. 2021, 134, 111119. [Google Scholar] [CrossRef]
- Sadeghi, M.; Dehnavi, S.; Asadirad, A.; Xu, S.; Majeed, M.; Jamialahmadi, T.; Johnston, T.P.; Sahebkar, A. Curcumin and Chemokines: Mechanism of Action and Therapeutic Potential in Inflammatory Diseases. Inflammopharmacology 2023, 31, 1069–1093. [Google Scholar] [CrossRef] [PubMed]
- Rohilla, M.; Rishabh Bansal, S.; Garg, A.; Dhiman, S.; Dhankhar, S.; Saini, M.; Chauhan, S.; Alsubaie, N.; Batiha, G.E.; Albezrah, N.K.A.; et al. Discussing pathologic mechanisms of Diabetic retinopathy & therapeutic potentials of curcumin and β-glucogallin in the management of Diabetic retinopathy. Biomed. Pharmacother. 2023, 169, 115881. [Google Scholar] [CrossRef]
- Yang, J.; Miao, X.; Yang, F.J.; Cao, J.F.; Liu, X.; Fu, J.L.; Su, G.F. Therapeutic potential of curcumin in diabetic retinopathy. Int. J. Mol. Med. 2021, 47, 75. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanyam, M.; Koteswari, A.A.; Kumar, R.S.; Monickaraj, S.F.; Maheswari, J.U.; Mohan, V. Curcumin-induced inhibition of cellular reactive oxygen species generation: Novel therapeutic implications. J. Biosci. 2003, 28, 715–721. [Google Scholar] [CrossRef]
- Joshi, P.; Joshi, S.; Semwal, D.K.; Verma, K.; Dwivedi, J.; Sharma, S. Role of curcumin in ameliorating hypertension and associated conditions: A mechanistic insight. Mol. Cell. Biochem. 2022, 477, 2359–2385. [Google Scholar] [CrossRef] [PubMed]
- Bianchetti, G.; Clementi, M.E.; Sampaolese, B.; Serantoni, C.; Abeltino, A.; De Spirito, M.; Sasson, S.; Maulucci, G. Metabolic Imaging and Molecular Biology Reveal the Interplay between Lipid Metabolism and DHA-Induced Modulation of Redox Homeostasis in RPE Cells. Antioxidants 2023, 12, 339. [Google Scholar] [CrossRef]
- Chaiyasaeng, W.; Hongwiset, D.; Tocharus, C.; Punyawudho, B.; Tocharus, J.; Chaichompoo, W.; Rojsitthisak, P.; Pabuprapap, W.; Yingyongnarongkul, B.E.; Suksamrarn, A. Comparative Pharmacokinetics and Tissue Distribution of Hexahydrocurcumin Following Intraperitoneal vs Oral Administration in Mice Using LC-MS/MS. ACS Omega 2024, 9, 41032–41042. [Google Scholar] [CrossRef]
- Kuo, C.-N.; Chen, C.-H.; Chen, S.-N.; Huang, J.-C.; Lai, L.-J.; Lai, C.-H.; Hung, C.-H.; Lee, C.-H.; Chen, C.-Y. Anti-Angiogenic Effect of Hexahydrocurcumin in Rat Corneal Neovascularization. Int. Ophthalmol. 2018, 38, 747–756. [Google Scholar] [CrossRef]
- Panthiya, L.; Tocharus, J.; Onsa-Ard, A.; Chaichompoo, W.; Suksamrarn, A.; Tocharus, C. Hexahydrocurcumin Ameliorates Hypertensive and Vascular Remodeling in L-NAME-Induced Rats. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166317. [Google Scholar] [CrossRef]
- Jearjaroen, P.; Thangwong, P.; Tocharus, C.; Chaichompoo, W.; Suksamrarn, A.; Tocharus, J. Hexahydrocurcumin Attenuated Demyelination and Improved Cognitive Impairment in Chronic Cerebral Hypoperfusion Rats. Inflammopharmacology 2024, 32, 1531–1544. [Google Scholar] [CrossRef] [PubMed]
- Sudarshan, K.; Yarlagadda, S.; Sengupta, S. Recent Advances in the Synthesis of Diarylheptanoids. Chem. Asian J. 2024, 19. [Google Scholar] [CrossRef] [PubMed]
- Sudarshan, K.; Perumal, G.; Aidhen, I.S.; Doble, M. Synthesis of Unsymmetrical Linear Diarylheptanoids and their Enantiomers and Antiproliferative Activity Studies. Eur. J. Org. Chem. 2018, 2018, 6379–6387. [Google Scholar] [CrossRef]
| Research Title | Intervention/Treatment | Time Frame | Enrollment | Phase | Status | NCT Number |
|---|---|---|---|---|---|---|
| Effect of Oral Curcumin Supplementation in Dry Age-related Macular Degeneration (AMD) Patients | Drug: Longvida Curcumin Other: Placebo | baseline, 3-month, 6-month, 12-month timepoints | 10 (Actual) | Early Phase 1 | Completed | NCT04590196 |
| A Study to Evaluate the Safety and Efficacy of RQC for AMD | Drug: 100 mg Resveratrol, 120 mg Quercetin, 1000 mg Curcumin (RQC) Drug: 1000 mg Curcumin | 24 months | 150 (Estimated) | Phase 2 | Unknown status | NCT05062486 |
| Anti-inflammatory Effect of Curcumin, Homotaurine, Vitamin D3 on Human Vitreous in Patients With Diabetic Retinopathy | Drug: 0.5 µM i 1 µM Curcumin, 100 µM Homotaurine, 50 nM Vitamin D3 Other: control | 7 days | 25 (Actual) | - | Completed | NCT04378972 |
| Aspect | Hexahydrocurcumin (HHC) | Curcumin | Take-Home Messages |
|---|---|---|---|
| Chemical structure | Reduced form of curcumin (saturated bonds, lacks double bonds) | Natural polyphenol with conjugated double bonds and a diketone moiety | Structural modification improves stability and bioavailability |
| Chemical stability | Higher—resistant to oxidation, light, and alkaline conditions | Unstable—degrades in light, heat, and alkaline environments | HHC is significantly more stable than curcumin |
| Bioavailability | Higher—better absorption and systemic presence | Very low (<1%) | HHC is more efficiently absorbed, enhancing its therapeutic potential |
| Antioxidant properties | Strong—often stronger than curcumin | Strong | HHC maintains or surpasses curcumin’s antioxidant capacity |
| Anti-inflammatory activity | Effective—comparable or superior to curcumin | Proven anti-inflammatory effects | HHC may provide more potent or sustained anti-inflammatory effects |
| Anticancer potential | Promising—in vitro studies show activity | Well-documented anticancer properties | Further studies needed, but HHC shows strong preliminary potential |
| Neuroprotective effects | High—protects neurons and combats oxidative stress | Present, but limited by low bioavailability | HHC may offer better support in neurodegenerative conditions |
| Therapeutic application | Potentially broader—due to improved pharmacokinetics | Known uses limited by poor stability and absorption | HHC is a strong candidate for next-generation curcumin-based therapeutics |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowak, I.; Kubina, R.; Strzałka-Mrozik, B. Therapeutic Potential of Hexahydrocurcumin in the Regeneration and Protection of the Retinal Pigment Epithelium. Pharmaceuticals 2025, 18, 554. https://doi.org/10.3390/ph18040554
Nowak I, Kubina R, Strzałka-Mrozik B. Therapeutic Potential of Hexahydrocurcumin in the Regeneration and Protection of the Retinal Pigment Epithelium. Pharmaceuticals. 2025; 18(4):554. https://doi.org/10.3390/ph18040554
Chicago/Turabian StyleNowak, Ilona, Robert Kubina, and Barbara Strzałka-Mrozik. 2025. "Therapeutic Potential of Hexahydrocurcumin in the Regeneration and Protection of the Retinal Pigment Epithelium" Pharmaceuticals 18, no. 4: 554. https://doi.org/10.3390/ph18040554
APA StyleNowak, I., Kubina, R., & Strzałka-Mrozik, B. (2025). Therapeutic Potential of Hexahydrocurcumin in the Regeneration and Protection of the Retinal Pigment Epithelium. Pharmaceuticals, 18(4), 554. https://doi.org/10.3390/ph18040554







