Hexasodium Fytate (SNF472 or CSL525) Inhibits Ectopic Calcification in Various Pseudoxanthoma Elasticum and Calcinosis Cutis Animal Models
Abstract
1. Introduction
2. Results
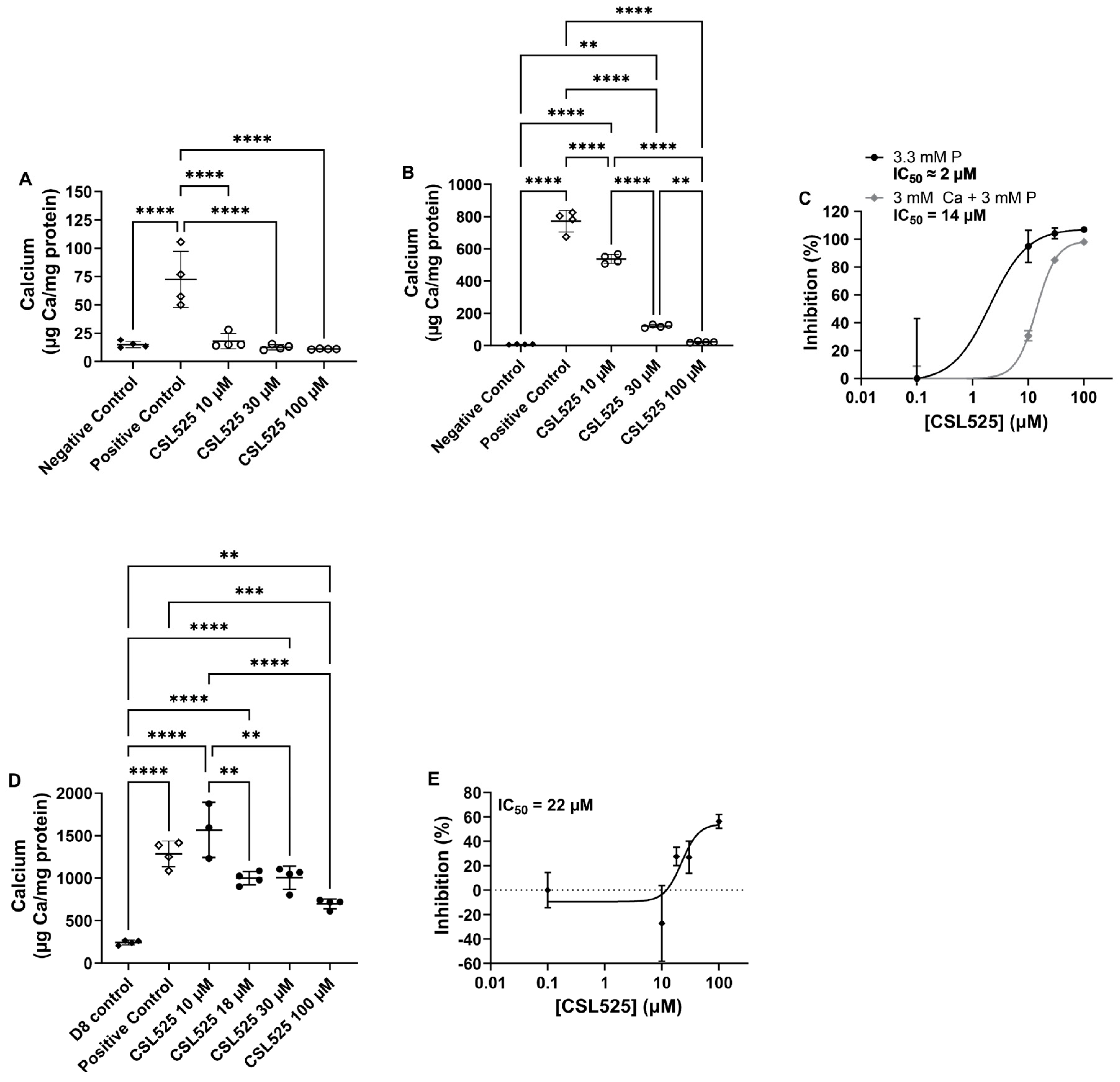
2.1. Human Vascular Smooth Muscle Cells Culture
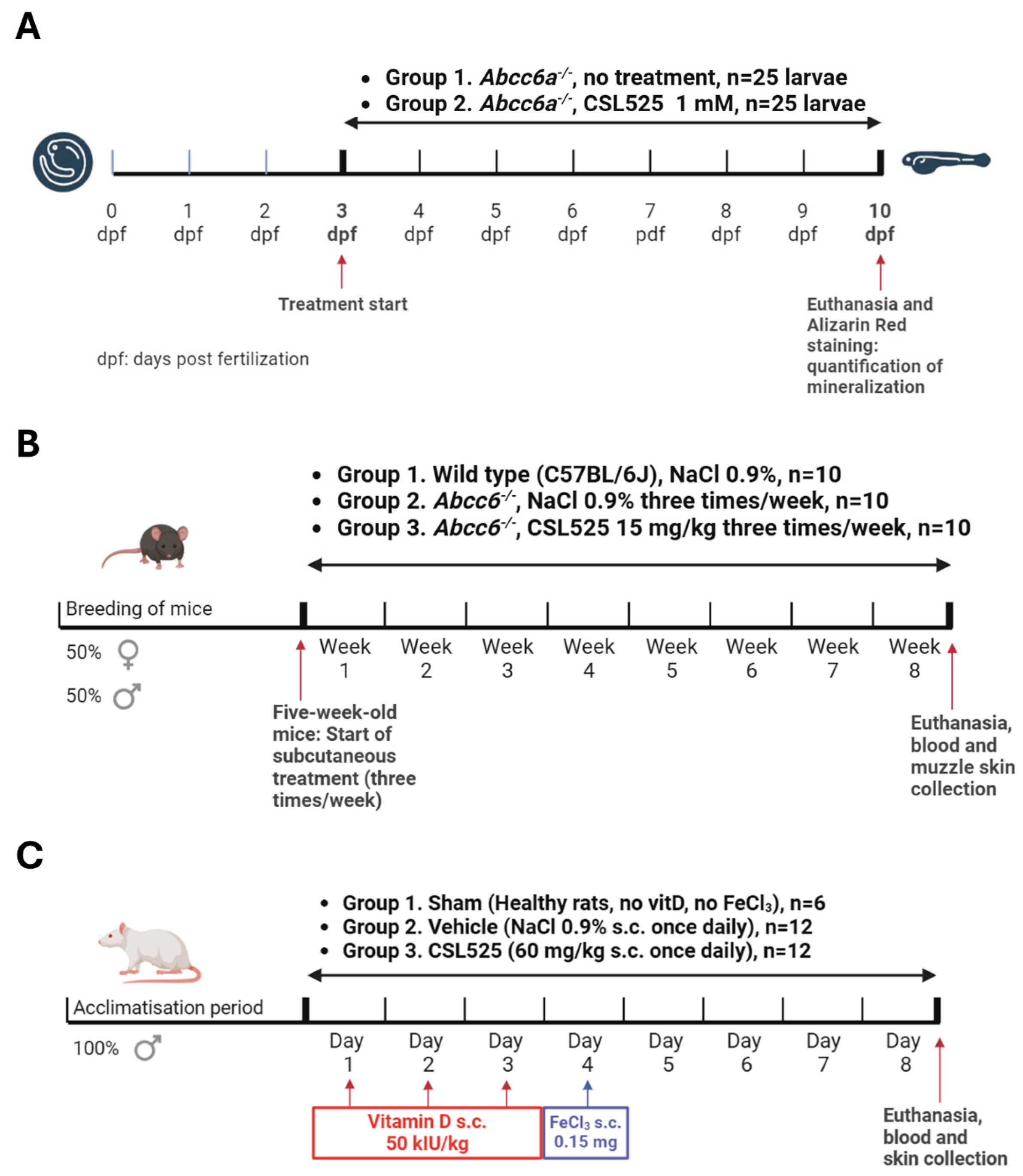
2.2. PXE Zebrafish Model
2.3. PXE Mouse Model
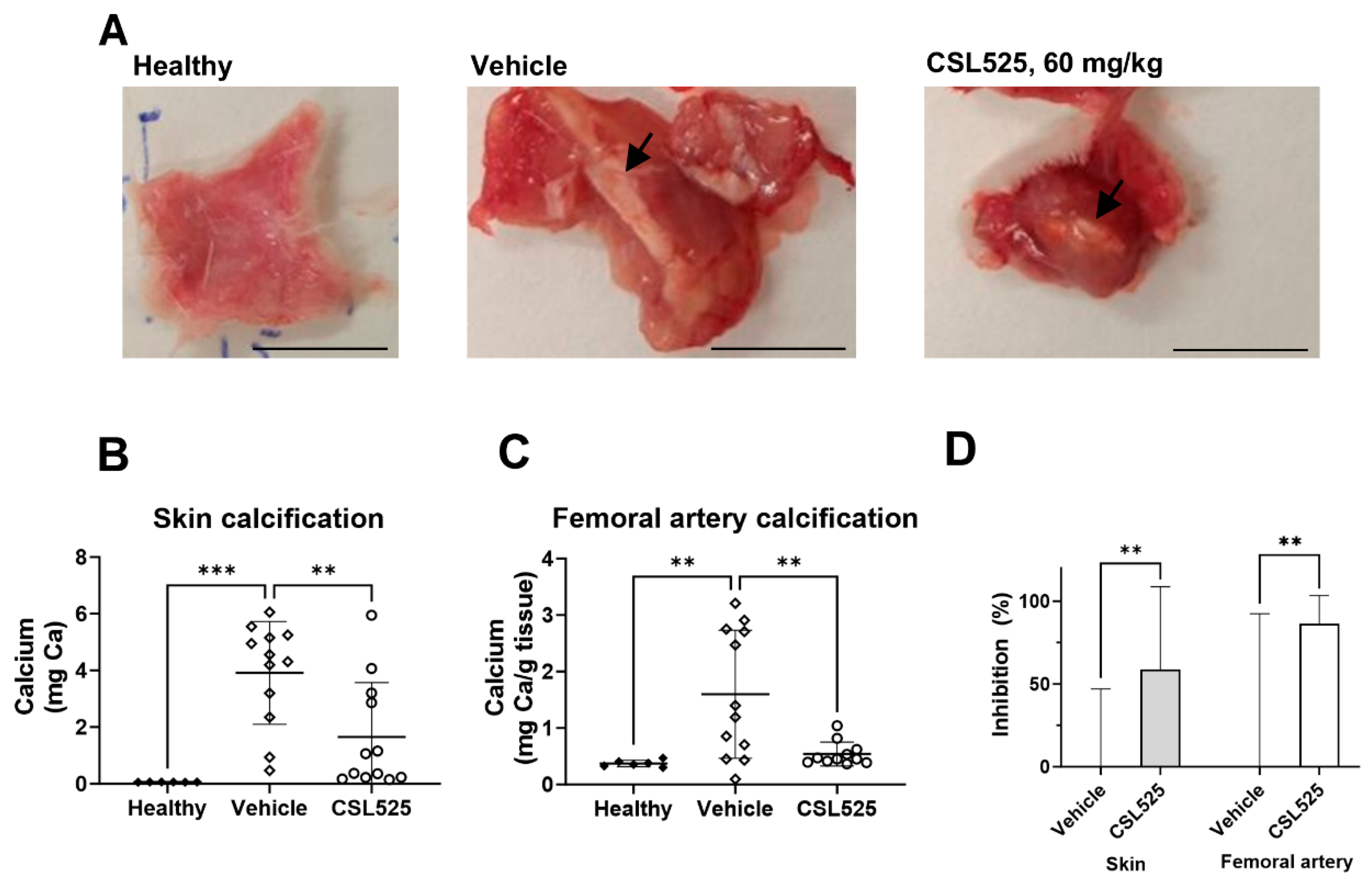
2.4. Calcinosis Cutis Rat Model
3. Discussion
4. Materials and Methods
4.1. Human Vascular Smooth Muscle Cells Culture
4.2. PXE Zebrafish Model
4.3. PXE Mouse Model
4.4. Calcinosis Cutis Rat Model
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shanahan, C.M.; Crouthamel, M.H.; Kapustin, A.; Giachelli, C.M. Arterial Calcification in Chronic Kidney Disease: Key Roles for Calcium and Phosphate. Circ. Res. 2011, 109, 697–711. [Google Scholar] [CrossRef] [PubMed]
- Villa-Bellosta, R. Vascular Calcification: Key Roles of Phosphate and Pyrophosphate. Int. J. Mol. Sci. 2021, 22, 13536. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, S.K.; Stack, A.G.; Levin, N.W.; Hulbert-Shearon, T.; Port, F.K. Association of Elevated Serum PO4, Ca × PO4 Product, and Parathyroid Hormone with Cardiac Mortality Risk in Chronic Hemodialysis Patients. J. Am. Soc. Nephrol. 2001, 12, 2131–2138. [Google Scholar] [CrossRef]
- Jono, S.; McKee, M.D.; Murry, C.E.; Shioi, A.; Nishizawa, Y.; Mori, K.; Morii, H.; Giachelli, C.M. Phosphate Regulation of Vascular Smooth Muscle Cell Calcification. Circ. Res. 2000, 87, E10–E17. [Google Scholar] [CrossRef] [PubMed]
- Villa-Bellosta, R.; Bogaert, Y.E.; Levi, M.; Sorribas, V. Characterization of Phosphate Transport in Rat Vascular Smooth Muscle Cells: Implications for Vascular Calcification. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1030–1036. [Google Scholar] [CrossRef]
- Schlieper, G.; Westenfeld, R.; Brandenburg, V.; Ketteler, M. Inhibitors of Calcification in Blood and Urine. Semin. Dial. 2007, 20, 113–121. [Google Scholar] [CrossRef]
- Nagaset, H.; Woessner, J.F. Matrix Metalloproteinases. J. Biol. Chem. 1999, 274, 21491. [Google Scholar]
- Neven, E.; Dauwe, S.; Broe, M.E.D.; D’Haese, P.C.; Persy, V. Endochondral Bone Formation Is Involved in Media Calcification in Rats and in Men. Kidney Int. 2007, 72, 574–581. [Google Scholar] [CrossRef]
- Neven, E.; Persy, V.; Dauwe, S.; Schutter, T.D.; Broe, M.E.D.; Dhaese, P.C. Chondrocyte Rather than Osteoblast Conversion of Vascular Cells Underlies Medial Calcification in Uremic Rats. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1741–1750. [Google Scholar] [CrossRef]
- El-Abbadi, M.; Giachelli, C.M. Mechanisms of Vascular Calcification. Adv. Chronic Kidney Dis. 2007, 14, 54–66. [Google Scholar] [CrossRef]
- Joubert, P.; Ketteler, M.; Salcedo, C.; Perello, J. Hypothesis: Phytate Is an Important Unrecognised Nutrient and Potential Intravenous Drug for Preventing Vascular Calcification. Med. Hypotheses 2016, 94, 89–92. [Google Scholar] [CrossRef]
- Noordzij, M.; Cranenburg, E.M.; Engelsman, L.F.; Hermans, M.M.; Boeschoten, E.W.; Brandenburg, V.M.; Bos, W.J.W.; Kooman, J.P.; Dekker, F.W.; Ketteler, M.; et al. Progression of Aortic Calcification Is Associated with Disorders of Mineral Metabolism and Mortality in Chronic Dialysis Patients. Nephrol. Dial. Transplant. 2011, 26, 1662–1669. [Google Scholar] [CrossRef] [PubMed]
- Budoff, M.J.; Hokanson, J.E.; Nasir, K.; Shaw, L.J.; Kinney, G.L.; Chow, D.; Demoss, D.; Nuguri, V.; Nabavi, V.; Ratakonda, R.; et al. Progression of Coronary Artery Calcium Predicts All-Cause Mortality. JACC Cardiovasc. Imaging 2010, 3, 1229–1236. [Google Scholar] [CrossRef]
- Sarnak, M.J. Cardiovascular Complications in Chronic Kidney Disease. Am. J. Kidney Dis. 2003, 41, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Mizobuchi, M.; Towler, D.; Slatopolsky, E. Vascular Calcification: The Killer of Patients with Chronic Kidney Disease. J. Am. Soc. Nephrol. 2009, 20, 1453–1464. [Google Scholar] [CrossRef] [PubMed]
- Molina, P.; Molina, M.D.; Pallardó, L.M.; Torralba, J.; Escudero, V.; Álvarez, L.; Peris, A.; Sánchez-Pérez, P.; González-Rico, M.; Puchades, M.J.; et al. Disorders in Bone-Mineral Parameters and the Risk of Death in Persons with Chronic Kidney Disease Stages 4 and 5: The PECERA Study. J. Nephrol. 2021, 34, 1189–1199. [Google Scholar] [CrossRef]
- Marconi, B.; Bobyr, I.; Campanati, A.; Molinelli, E.; Consales, V.; Brisigotti, V.; Scarpelli, M.; Racchini, S.; Offidani, A. Pseudoxanthoma Elasticum and Skin: Clinical Manifestations, Histopathology, Pathomechanism, Perspectives of Treatment. IRDR 2015, 4, 113–122. [Google Scholar] [CrossRef]
- Neidner, K.H. History. Clin. Dermatol. 1988, 6, 1–4. [Google Scholar] [CrossRef]
- Bergen, A.A.B.; Plomp, A.S.; Schuurman, E.J.; Terry, S.; Breuning, M.; Dauwerse, H.; Swart, J.; Kool, M.; Soest, S.V.; Baas, F.; et al. Mutations in ABCC6 Cause Pseudoxanthoma Elasticum. Nat. Genet. 2000, 25, 228–231. [Google Scholar] [CrossRef]
- Ringpfeil, F.; Lebwohl, M.G.; Christiano, A.M.; Uitto, J. Pseudoxanthoma Elasticum: Mutations in the MRP6 Gene Encoding a Transmembrane ATP-Binding Cassette (ABC) Transporter. Proc. Natl. Acad. Sci. USA 2000, 97, 6001–6006. [Google Scholar] [CrossRef]
- Saux, O.L.; Urban, Z.; Tschuch, C.; Csiszar, K.; Bacchelli, B.; Quaglino, D.; Pasquali-Ronchetti, I.; Pope, F.M.; Richards, A.; Terry, S.; et al. Mutations in a Gene Encoding an ABC Transporter Cause Pseudoxanthoma Elasticum. Nat. Genet. 2000, 25, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Ralph, D.; van de Wetering, K.; Uitto, J.; Li, Q. Inorganic Pyrophosphate Deficiency Syndromes and Potential Treatments for Pathologic Tissue Calcification. Am. J. Pathol. 2022, 192, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Kavukcuoglu, N.B.; Li, Q.; Pleshko, N.; Uitto, J. Connective Tissue Mineralization in Abcc6−/− Mice, a Model for Pseudoxanthoma Elasticum. Matrix Biol. 2012, 31, 246–252. [Google Scholar] [CrossRef][Green Version]
- Cozzolino, M.; Maffei Faccioli, F.; Cara, A.; Boni Brivio, G.; Rivela, F.; Ciceri, P.; Magagnoli, L.; Galassi, A.; Barbuto, S.; Speciale, S.; et al. Future Treatment of Vascular Calcification in Chronic Kidney Disease. Expert Opin. Pharmacother. 2023, 24, 2041–2057. [Google Scholar] [CrossRef] [PubMed]
- Bernardor, J.; De Mul, A.; Bacchetta, J.; Schmitt, C.P. Impact of Cinacalcet and Etelcalcetide on Bone Mineral and Cardiovascular Disease in Dialysis Patients. Curr. Osteoporos. Rep. 2023, 21, 193–204. [Google Scholar] [CrossRef]
- Patel, L.; Bernard, L.M.; Elder, G.J. Sevelamer Versus Calcium-Based Binders for Treatment of Hyperphosphatemia in CKD: A Meta-Analysis of Randomized Controlled Trials. Clin. J. Am. Soc. Nephrol. 2016, 11, 232–244. [Google Scholar] [CrossRef]
- Ruospo, M.; Palmer, S.C.; Natale, P.; Craig, J.C.; Vecchio, M.; Elder, G.J.; Strippoli, G.F. Phosphate Binders for Preventing and Treating Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Cochrane Database Syst. Rev. 2018, 2018, CD006023. [Google Scholar] [CrossRef]
- Schlieper, G.; Brandenburg, V.; Ketteler, M.; Floege, J. Sodium Thiosulfate in the Treatment of Calcific Uremic Arteriolopathy. Nat. Rev. Nephrol. 2009, 5, 539–543. [Google Scholar] [CrossRef]
- Pasch, A.; Schaffner, T.; Huynh-Do, U.; Frey, B.M.; Frey, F.J.; Farese, S. Sodium Thiosulfate Prevents Vascular Calcifications in Uremic Rats. Kidney Int. 2008, 74, 1444–1453. [Google Scholar] [CrossRef]
- Djuric, P.; Dimkovic, N.; Schlieper, G.; Djuric, Z.; Pantelic, M.; Mitrovic, M.; Jankovic, A.; Milanov, M.; Kuzmanovic Pficer, J.; Floege, J. Sodium Thiosulphate and Progression of Vascular Calcification in End-Stage Renal Disease Patients: A Double-Blind, Randomized, Placebo-Controlled Study. Nephrol. Dial. Transpl. 2020, 35, 162–169. [Google Scholar] [CrossRef]
- Lomashvili, K.A.; Cobbs, S.; Hennigar, R.A.; Hardcastle, K.I.; O’Neill, W.C. Phosphate-Induced Vascular Calcification: Role of Pyrophosphate and Osteopontin. J. Am. Soc. Nephrol. 2004, 15, 1392–1401. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, W.C.; Lomashvili, K.A.; Malluche, H.H.; Faugere, M.-C.; Riser, B.L. Treatment with Pyrophosphate Inhibits Uremic Vascular Calcification. Kidney Int. 2011, 79, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Price, P.A.; Buckley, J.R.; Williamson, M.K. The Amino Bisphosphonate Ibandronate Prevents Vitamin D Toxicity and Inhibits Vitamin D-Induced Calcification of Arteries, Cartilage, Lungs and Kidneys in Rats. J. Nutr. 2001, 131, 2910–2915. [Google Scholar] [CrossRef]
- Lomashvili, K.A.; Monier-Faugere, M.-C.; Wang, X.; Malluche, H.H.; O’Neill, W.C. Effect of Bisphosphonates on Vascular Calcification and Bone Metabolism in Experimental Renal Failure. Kidney Int. 2009, 75, 617–625. [Google Scholar] [CrossRef]
- Price, P.A.; Faus, S.A.; Williamson, M.K. Bisphosphonates Alendronate and Ibandronate Inhibit Artery Calcification at Doses Comparable to Those That Inhibit Bone Resorption. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Sundberg, J.P.; Levine, M.A.; Terry, S.F.; Uitto, J. The Effects of Bisphosphonates on Ectopic Soft Tissue Mineralization Caused by Mutations in the ABCC6 Gene. Cell Cycle 2015, 14, 1082–1089. [Google Scholar] [CrossRef]
- Dedinszki, D.; Szeri, F.; Kozák, E.; Pomozi, V.; Tőkési, N.; Mezei, T.R.; Merczel, K.; Letavernier, E.; Tang, E.; Le Saux, O.; et al. Oral Administration of Pyrophosphate Inhibits Connective Tissue Calcification. EMBO Mol. Med. 2017, 9, 1463–1470. [Google Scholar] [CrossRef]
- Omarjee, L.; Nitschke, Y.; Verschuere, S.; Bourrat, E.; Vignon, M.-D.; Navasiolava, N.; Leftheriotis, G.; Kauffenstein, G.; Rutsch, F.; Vanakker, O.M.; et al. Severe Early-Onset Manifestations of Pseudoxanthoma Elasticum Resulting from the Cumulative Effects of Several Deleterious Mutations in ENPP1, ABCC6 and HBB: Transient Improvement in Ectopic Calcification with Sodium Thiosulfate. Br. J. Dermatol. 2020, 183, 367–372. [Google Scholar] [CrossRef]
- Clotaire, L.; Rubera, I.; Duranton, C.; Gal, J.; Chamorey, E.; Humeau, H.; Yamani, S.; Chiaverini, C.; Willoteaux, S.; Padovani, B.; et al. The PROPHECI Trial: A Phase II, Double-Blind, Placebo-Controlled, Randomized Clinical Trial for the Treatment of Pseudoxanthoma Elasticum with Oral Pyrophosphate. Trials 2025, 26, 30. [Google Scholar] [CrossRef]
- Brandenburg, V.M.; Sinha, S.; Torregrosa, J.V.; Garg, R.; Miller, S.; Canals, A.Z.; Bahr, D.; Joubert, P.H.; Salcedo, C.; Carroll, K.J.; et al. Improvement in Wound Healing, Pain, and Quality of Life after 12 Weeks of SNF472 Treatment: A Phase 2 Open-Label Study of Patients with Calciphylaxis. J. Nephrol. 2019, 32, 811–821. [Google Scholar] [CrossRef]
- Perelló, J.; Ferrer, M.D.; Pérez, M.d.M.; Kaesler, N.; Brandenburg, V.M.; Behets, G.J.; D’Haese, P.C.; Garg, R.; Isern, B.; Gold, A.; et al. Mechanism of Action of SNF472, a Novel Calcification Inhibitor to Treat Vascular Calcification and Calciphylaxis. Br. J. Pharmacol. 2020, 177, 4400–4415. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.D.; Ketteler, M.; Tur, F.; Tur, E.; Isern, B.; Salcedo, C.; Joubert, P.H.; Behets, G.J.; Neven, E.; D’Haese, P.C.; et al. Characterization of SNF472 Pharmacokinetics and Efficacy in Uremic and Non-Uremic Rats Models of Cardiovascular Calcification. PLoS ONE 2018, 13, e0197061. [Google Scholar] [CrossRef]
- Raggi, P.; Bellasi, A.; Bushinsky, D.; Bover, J.; Rodriguez, M.; Ketteler, M.; Sinha, S.; Salcedo, C.; Gillotti, K.; Padgett, C.; et al. Slowing Progression of Cardiovascular Calcification with SNF472 in Patients on Hemodialysis: Results of a Randomized Phase 2b Study. Circulation 2020, 141, 728–739. [Google Scholar] [CrossRef]
- Sinha, S.; Raggi, P.; Chertow, G.M. SNF472: Mechanism of Action and Results from Clinical Trials. Curr. Opin. Nephrol. Hypertens. 2021, 30, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wei, Z.; Shi, W.; Xing, J.; Zhang, X. SNF472: A Novel Therapeutic Agent for Vascular Calcification and Calciphylaxis. J. Nephrol. 2024, 37, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Smith, E.R.; Tiong, M.K.; Ruderman, I.; Toussaint, N.D. Interventions To Attenuate Vascular Calcification Progression in Chronic Kidney Disease: A Systematic Review of Clinical Trials. J. Am. Soc. Nephrol. 2022, 33, 1011–1032. [Google Scholar] [CrossRef]
- Nigwekar, S.U.; Thadhani, R.; Brandenburg, V.M. Calciphylaxis. N. Engl. J. Med. 2018, 378, 1704–1714. [Google Scholar] [CrossRef]
- Zabirnyk, A.; Ferrer, M.D.; Bogdanova, M.; Pérez, M.M.; Salcedo, C.; Kaljusto, M.-L.; Kvitting, J.-P.E.; Stensløkken, K.-O.; Perelló, J.; Vaage, J. SNF472, a Novel Anti-Crystallization Agent, Inhibits Induced Calcification in an in Vitro Model of Human Aortic Valve Calcification. Vasc. Pharmacol. 2019, 122–123, 106583. [Google Scholar] [CrossRef]
- Abedin, M.; Tintut, Y.; Demer, L.L. Vascular Calcification: Mechanisms and Clinical Ramifications. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1161–1170. [Google Scholar] [CrossRef]
- Giachelli, C.M.; Speer, M.Y.; Li, X.; Rajachar, R.M.; Yang, H. Regulation of Vascular Calcification: Roles of Phosphate and Osteopontin. Circ. Res. 2005, 96, 717–722. [Google Scholar] [CrossRef]
- Oca, A.M.D.; Madueño, J.A.; Martinez-Moreno, J.M.; Guerrero, F.; Muñoz-Castañeda, J.; Rodriguez-Ortiz, M.E.; Mendoza, F.J.; Almaden, Y.; Lopez, I.; Rodriguez, M.; et al. High-Phosphate-Induced Calcification Is Related to SM22α Promoter Methylation in Vascular Smooth Muscle Cells. J. Bone Miner. Res. 2010, 25, 1996–2005. [Google Scholar] [CrossRef] [PubMed]
- Villa-Bellosta, R.; Millan, A.; Sorribas, V. Role of Calcium-Phosphate Deposition in Vascular Smooth Muscle Cell Calcification. Am. J. Physiol. Cell Physiol. 2011, 300, C210–C220. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Curinga, G.; Giachelli, C.M. Elevated Extracellular Calcium Levels Induce Smooth Muscle Cell Matrix Mineralization in Vitro. Kidney Int. 2004, 66, 2293–2299. [Google Scholar] [CrossRef]
- Wynsberghe, J.V.; Vanakker, O.M. Significance of Premature Vertebral Mineralization in Zebrafish Models in Mechanistic and Pharmaceutical Research on Hereditary Multisystem Diseases. Biomolecules 2023, 13, 1621. [Google Scholar] [CrossRef]
- Gils, M.V.; Willaert, A.; Coucke, P.J.; Vanakker, O.M. The Abcc6a Knockout Zebrafish Model as a Novel Tool for Drug Screening for Pseudoxanthoma Elasticum. Front. Pharmacol. 2022, 13, 822143. [Google Scholar] [CrossRef]
- Mackay, E.W.; Apschner, A.; Schulte-Merker, S. Vitamin K Reduces Hypermineralisation in Zebrafish Models of PXE and GACI. Development 2015, 142, 1095–1101. [Google Scholar] [CrossRef]
- Nollet, L.; Gils, M.V.; Willaert, A.; Coucke, P.J.; Vanakker, O.M. Minocycline Attenuates Excessive DNA Damage Response and Reduces Ectopic Calcification in Pseudoxanthoma Elasticum. J. Investig. Dermatol. 2022, 142, 1629–1638.e6. [Google Scholar] [CrossRef]
- Kauffenstein, G.; Chappard, D.; Leftheriotis, G.; Martin, L. ABCC6 Deficiency and Bone Loss: A Double Benefit of Etidronate for Patient Presenting with Pseudoxanthoma Elasticum? Exp. Dermatol. 2022, 31, 1635. [Google Scholar] [CrossRef]
- Perez, M.M.; Ferrer, M.D.; Lazo-Rodriguez, M.; Canals, A.Z.; Banon-Maneus, E.; Campistol, J.M.; Miller, S.; Garg, R.; Gold, A.; Salcedo, C.; et al. A Novel Assay to Measure Calcification Propensity: From Laboratory to Humans. Sci. Rep. 2020, 10, 17578. [Google Scholar] [CrossRef] [PubMed]
- Zabirnyk, A.; Perez, M.D.M.; Blasco, M.; Stensløkken, K.-O.; Ferrer, M.D.; Salcedo, C.; Vaage, J. A Novel Ex Vivo Model of Aortic Valve Calcification. A Preliminary Report. Front. Pharmacol. 2020, 11, 568764. [Google Scholar] [CrossRef]
- Klement, J.F.; Matsuzaki, Y.; Jiang, Q.-J.; Terlizzi, J.; Choi, H.Y.; Fujimoto, N.; Li, K.; Pulkkinen, L.; Birk, D.E.; Sundberg, J.P.; et al. Targeted Ablation of the Abcc6 Gene Results in Ectopic Mineralization of Connective Tissues. Mol. Cell. Biol. 2005, 25, 8299–8310. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, I.J.; Cheng, Z.; Ralph, D.; O’Brien, K.; Flaman, L.; Howe, J.; Thompson, D.; Uitto, J.; Li, Q.; Sabbagh, Y. INZ-701, a Recombinant ENPP1 Enzyme, Prevents Ectopic Calcification in an Abcc6−/− Mouse Model of Pseudoxanthoma Elasticum. Exp. Dermatol. 2022, 31, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, I.J.; Obiri-Yeboah, D.; Stabach, P.R.; Braddock, D.T.; Li, Q. Novel Treatment for PXE: Recombinant ENPP1 Enzyme Therapy. Mol. Ther. 2024, 32, 3815–3820. [Google Scholar] [CrossRef]
- Li, Q.; Huang, J.; Pinkerton, A.B.; Millan, J.L.; van Zelst, B.D.; Levine, M.A.; Sundberg, J.P.; Uitto, J. Inhibition of Tissue-Nonspecific Alkaline Phosphatase Attenuates Ectopic Mineralization in the Abcc6–/– Mouse Model of PXE but Not in the Enpp1 Mutant Mouse Models of GACI. J. Investig. Dermatol. 2019, 139, 360–368. [Google Scholar] [CrossRef]
- Bouderlique, E.; Nollet, L.; Letavernier, E.; Vanakker, O.M. Minocycline Counteracts Ectopic Calcification in a Murine Model of Pseudoxanthoma Elasticum: A Proof-of-Concept Study. Int. J. Mol. Sci. 2022, 23, 1838. [Google Scholar] [CrossRef]
- Jacobs, I.J.; Li, D.; Ivarsson, M.E.; Uitto, J.; Li, Q. A Phytic Acid Analogue INS-3001 Prevents Ectopic Calcification in an Abcc6-/-; Mouse Model of Pseudoxanthoma Elasticum. Exp. Dermatol. 2021, 30, 853–858. [Google Scholar] [CrossRef]
- Schantl, A.E.; Verhulst, A.; Neven, E.; Behets, G.J.; D’Haese, P.C.; Maillard, M.; Mordasini, D.; Phan, O.; Burnier, M.; Spaggiari, D.; et al. Inhibition of Vascular Calcification by Inositol Phosphates Derivatized with Ethylene Glycol Oligomers. Nat. Commun. 2020, 11, 721. [Google Scholar] [CrossRef]
- Bushinsky, D.A.; Raggi, P.; Bover, J.; Ketteler, M.; Bellasi, A.; Rodriguez, M.; Sinha, S.; Garg, R.; Perelló, J.; Gold, A.; et al. Effects of Myo-Inositol Hexaphosphate (SNF472) on Bone Mineral Density in Patients Receiving Hemodialysis: An Analysis of the Randomized, Placebo-Controlled CaLIPSO Study. Clin. J. Am. Soc. Nephrol. 2021, 16, 736–745. [Google Scholar] [CrossRef]
- Price, P.A.; Omid, N.; Than, T.N.; Williamson, M.K. The Amino Bisphosphonate Ibandronate Prevents Calciphylaxis in the Rat at Doses That Inhibit Bone Resorption. Calcif. Tissue Int. 2002, 71, 356–363. [Google Scholar] [CrossRef]
- Grases, F.; Perelló, J.; Isern, B.; Prieto, R.M. Study of a Myo-Inositol Hexaphosphate-Based Cream to Prevent Dystrophic Calcinosis Cutis. Br. J. Dermatol. 2005, 152, 1022–1025. [Google Scholar] [CrossRef]
- Opdebeeck, B.; Neven, E.; Millán, J.L.; Pinkerton, A.B.; D’Haese, P.C.; Verhulst, A. Pharmacological TNAP Inhibition Efficiently Inhibits Arterial Media Calcification in a Warfarin Rat Model but Deserves Careful Consideration of Potential Physiological Bone Formation/Mineralization Impairment. Bone 2020, 137, 115392. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Gils, M.V.; Willaert, A.; Vilder, E.Y.G.D.; Coucke, P.J.; Vanakker, O.M. Generation and Validation of a Complete Knockout Model of Abcc6a in Zebrafish. J. Investig. Dermatol. 2018, 138, 2333–2342. [Google Scholar] [CrossRef]
- Huang, J.; Ralph, D.; Boraldi, F.; Quaglino, D.; Uitto, J.; Li, Q. Inhibition of the DNA Damage Response Attenuates Ectopic Calcification in Pseudoxanthoma Elasticum. J. Investig. Dermatol. 2022, 142, 2140–2148.e1. [Google Scholar] [CrossRef]
- Saeidian, A.H.; Youssefian, L.; Huang, J.; Touati, A.; Vahidnezhad, H.; Kowal, L.; Caffet, M.; Wurst, T.; Singh, J.; Snook, A.E.; et al. Genetic Heterogeneity of Heritable Ectopic Mineralization Disorders in a Large International Cohort. Genet. Med. 2022, 24, 75–86. [Google Scholar] [CrossRef]
- Tur, F.; Tur, E.; Lentheric, I.; Mendoza, P.; Encabo, M.; Isern, B.; Grases, F.; Maraschiello, C.; Perelló, J. Validation of an LC-MS Bioanalytical Method for Quantification of Phytate Levels in Rat, Dog and Human Plasma. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 928, 146–154. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrer, M.D.; Pérez-Ferrer, M.d.M.; Blasco, M.; Jacobs, I.J.; Li, Q.; Vanakker, O.M.; Dangreau, L.; López, A.; Malagraba, G.; Bassissi, F.; et al. Hexasodium Fytate (SNF472 or CSL525) Inhibits Ectopic Calcification in Various Pseudoxanthoma Elasticum and Calcinosis Cutis Animal Models. Pharmaceuticals 2025, 18, 567. https://doi.org/10.3390/ph18040567
Ferrer MD, Pérez-Ferrer MdM, Blasco M, Jacobs IJ, Li Q, Vanakker OM, Dangreau L, López A, Malagraba G, Bassissi F, et al. Hexasodium Fytate (SNF472 or CSL525) Inhibits Ectopic Calcification in Various Pseudoxanthoma Elasticum and Calcinosis Cutis Animal Models. Pharmaceuticals. 2025; 18(4):567. https://doi.org/10.3390/ph18040567
Chicago/Turabian StyleFerrer, Miguel D., Maria del Mar Pérez-Ferrer, Marc Blasco, Ida Joely Jacobs, Qiaoli Li, Olivier M. Vanakker, Lisa Dangreau, Andrea López, Gianluca Malagraba, Firas Bassissi, and et al. 2025. "Hexasodium Fytate (SNF472 or CSL525) Inhibits Ectopic Calcification in Various Pseudoxanthoma Elasticum and Calcinosis Cutis Animal Models" Pharmaceuticals 18, no. 4: 567. https://doi.org/10.3390/ph18040567
APA StyleFerrer, M. D., Pérez-Ferrer, M. d. M., Blasco, M., Jacobs, I. J., Li, Q., Vanakker, O. M., Dangreau, L., López, A., Malagraba, G., Bassissi, F., Perelló, J., & Salcedo, C. (2025). Hexasodium Fytate (SNF472 or CSL525) Inhibits Ectopic Calcification in Various Pseudoxanthoma Elasticum and Calcinosis Cutis Animal Models. Pharmaceuticals, 18(4), 567. https://doi.org/10.3390/ph18040567






