From Olive Tree to Treatment: Nano-Delivery Systems for Enhancing Oleuropein’s Health Benefits
Abstract
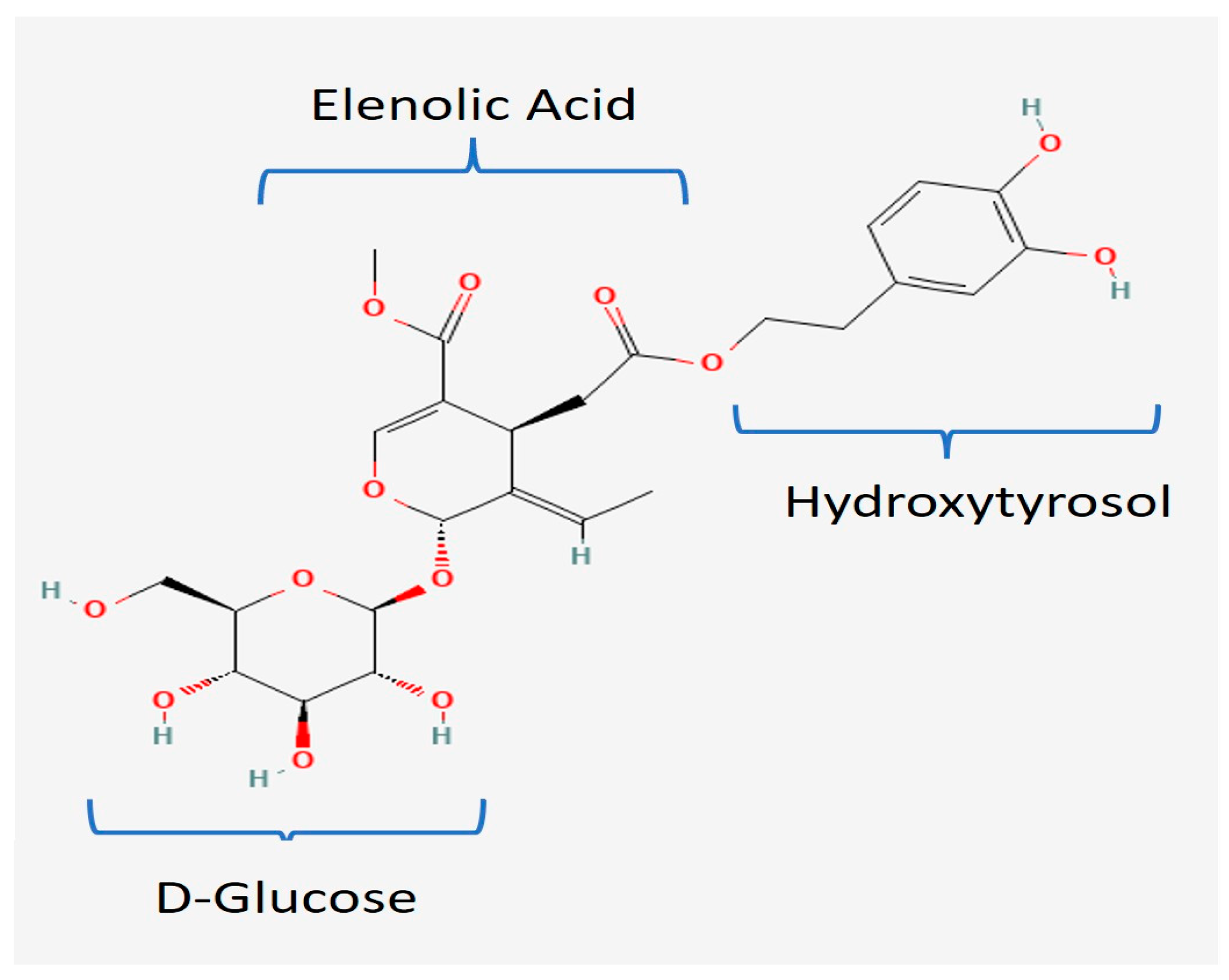
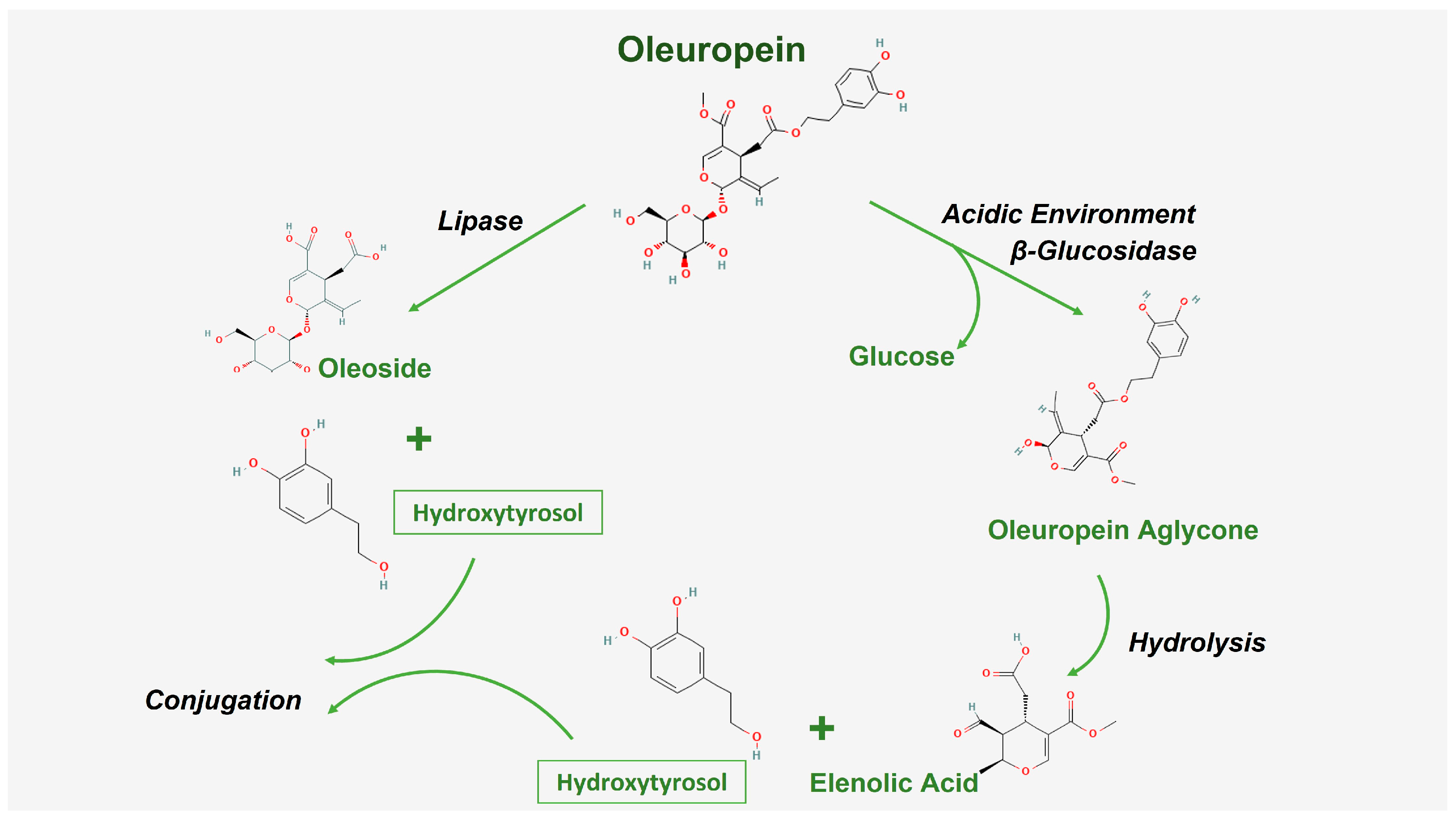
:1. Introduction
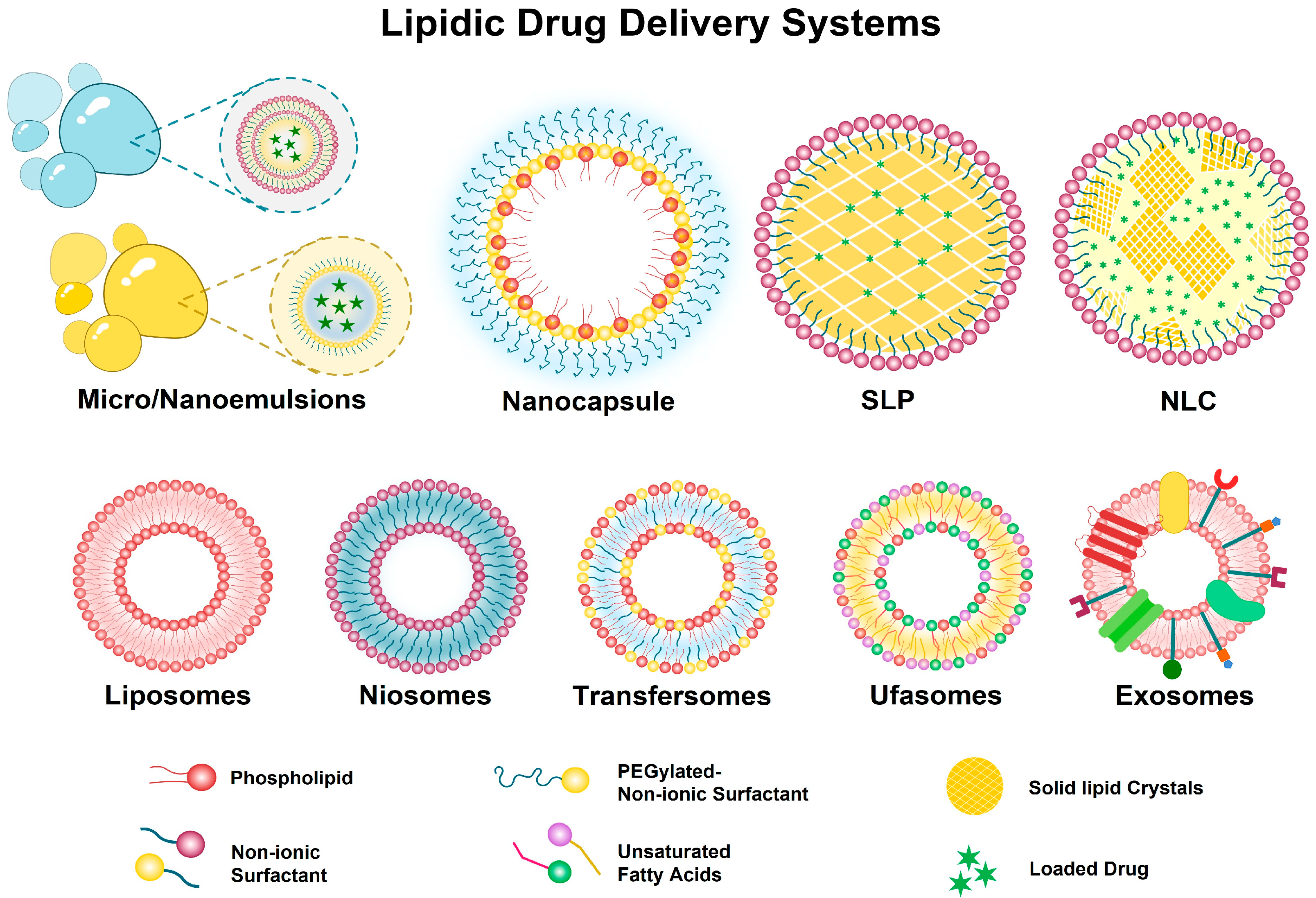
2. Nanocarriers for Delivery of Oleuropein
2.1. Lipid-Based Delivery Systems
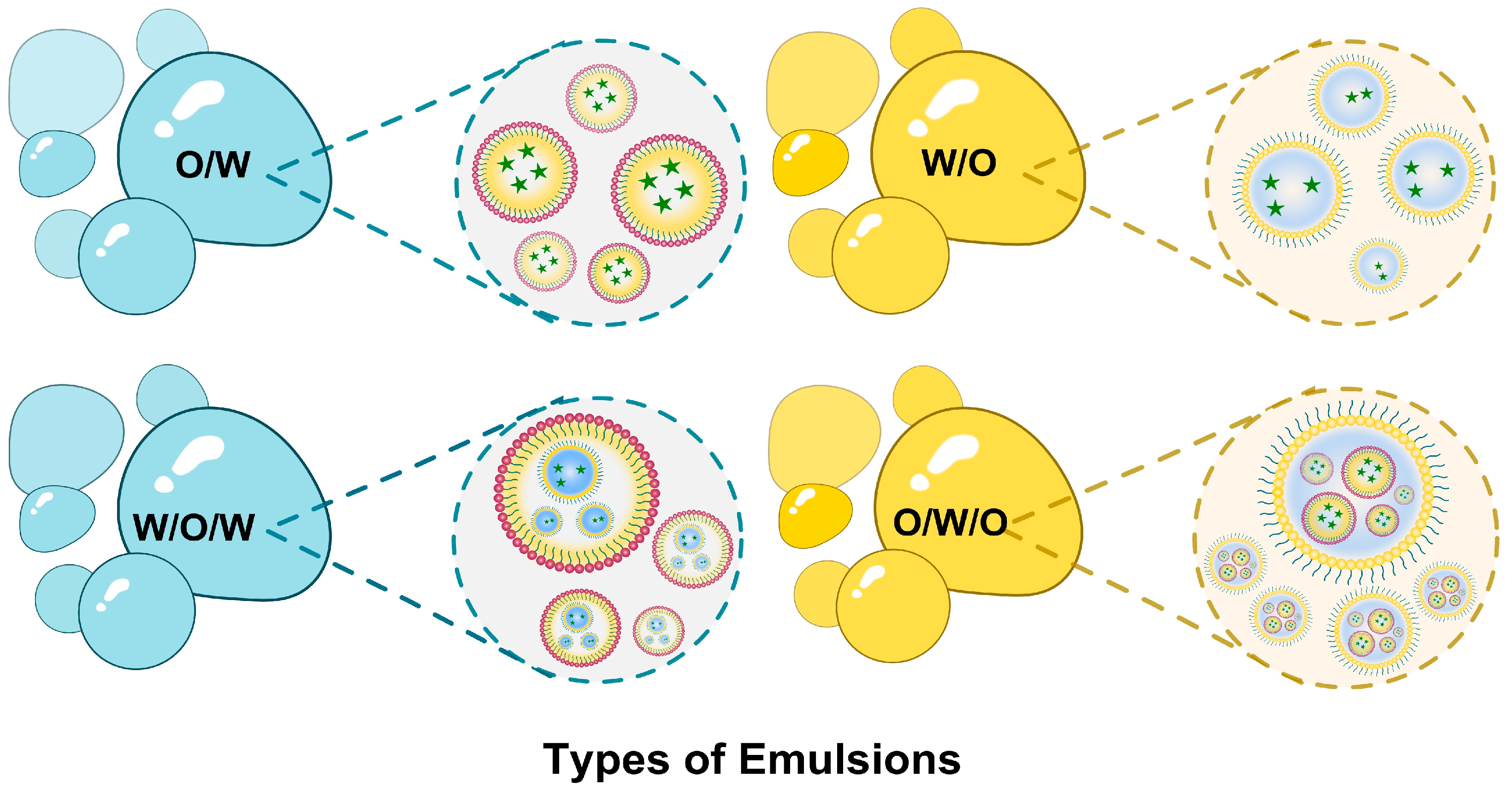
2.1.1. Nano/Microemulsions
2.1.2. Nanostructured Lipid Carriers (NLCs) and Solid Lipid Nanoparticles (SLPs)
2.1.3. Lipidic Nanocapsules
2.2. Vesicular Systems
2.2.1. Liposomes
2.2.2. Niosomes
2.2.3. Transfersomes
2.2.4. Ufasomes (Unsaturated Fatty Acid Liposomes)
2.2.5. Exosome–Liposome Hybrids
2.3. Polymeric Delivery Systems
2.3.1. Cyclodextrin-Based Nanoparticles
2.3.2. Chitosan Nanoparticles
2.3.3. Nanofibers
2.3.4. Inorganic Nanoparticles
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cavaca, L.A.S.; Afonso, C.A.M. Oleuropein: A Valuable Bio-Renewable Synthetic Building Block. Eur. J. Org. Chem. 2018, 2018, 581–589. [Google Scholar] [CrossRef]
- Lama-Muñoz, A.; del Mar Contreras, M.; Espínola, F.; Moya, M.; de Torres, A.; Romero, I.; Castro, E. Extraction of Oleuropein and Luteolin-7-O-Glucoside from Olive Leaves: Optimization of Technique and Operating Conditions. Food Chem. 2019, 293, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Clodoveo, M.L.; Crupi, P.; Annunziato, A.; Corbo, F. Innovative Extraction Technologies for Development of Functional Ingredients Based on Polyphenols from Olive Leaves. Foods 2022, 11, 103. [Google Scholar] [CrossRef]
- Soler-Rivas, C.; Espiń, J.C.; Wichers, H.J. Oleuropein and Related Compounds. J. Sci. Food Agric. 2000, 80, 1013–1023. [Google Scholar] [CrossRef]
- Khalil, A.A.; Rahman, M.M.; Rauf, A.; Islam, M.R.; Manna, S.J.; Khan, A.A.; Ullah, S.; Akhtar, M.N.; Aljohani, A.S.M.; Al Abdulmonem, W.; et al. Oleuropein: Chemistry, Extraction Techniques and Nutraceutical Perspectives—An Update. Crit. Rev. Food Sci. Nutr. 2023, 64, 9933–9954. [Google Scholar] [CrossRef]
- Ansari, M.; Kazemipour, M.; Fathi, S. Development of a Simple Green Extraction Procedure and HPLC Method for Determination of Oleuropein in Olive Leaf Extract Applied to a Multi-Source Comparative Study. JICS 2011, 8, 38–47. [Google Scholar] [CrossRef]
- Rahmanian, N.; Jafari, S.M.; Wani, T.A. Bioactive Profile, Dehydration, Extraction and Application of the Bioactive Components of Olive Leaves. Trends Food Sci. Technol. 2015, 42, 150–172. [Google Scholar] [CrossRef]
- Hassen, I.; Casabianca, H.; Hosni, K. Biological Activities of the Natural Antioxidant Oleuropein: Exceeding the Expectation—A Mini-Review. J. Funct. Foods 2015, 18, 926–940. [Google Scholar] [CrossRef]
- Monteleone, J.I.; Sperlinga, E.; Siracusa, L.; Spagna, G.; Parafati, L.; Todaro, A.; Palmeri, R. Water as a Solvent of Election for Obtaining Oleuropein-Rich Extracts from Olive (Olea europaea) Leaves. Agronomy 2021, 11, 465. [Google Scholar] [CrossRef]
- Otero, D.M.; Lorini, A.; Oliveira, F.M.; da Fonseca Antunes, B.; Oliveira, R.M.; Zambiazi, R.C. Leaves of Olea europaea L. as a Source of Oleuropein: Characteristics and Biological Aspects. Res. Soc. Dev. 2021, 10, e185101321130. [Google Scholar] [CrossRef]
- Tripoli, E.; Giammanco, M.; Tabacchi, G.; Di Majo, D.; Giammanco, S.; La Guardia, M. The Phenolic Compounds of Olive Oil: Structure, Biological Activity and Beneficial Effects on Human Health. Nutr. Res. Rev. 2005, 18, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Özcan, M.M.; Matthäus, B. A review: Benefit and bioactive properties of olive (Olea europaea L.) leaves. Eur. Food Res. Technol. 2017, 243, 89–99. [Google Scholar] [CrossRef]
- de Bock, M.; Thorstensen, E.B.; Derraik, J.G.B.; Henderson, H.V.; Hofman, P.L.; Cutfield, W.S. Human Absorption and Metabolism of Oleuropein and Hydroxytyrosol Ingested as Olive (Olea europaea L.) Leaf Extract. Mol. Nutr. Food Res. 2013, 57, 2079–2085. [Google Scholar] [CrossRef]
- Nediani, C.; Ruzzolini, J.; Romani, A.; Calorini, L. Oleuropein, a Bioactive Compound from Olea europaea L., as a Potential Preventive and Therapeutic Agent in Non-Communicable Diseases. Antioxidants 2019, 8, 578. [Google Scholar] [CrossRef]
- Castejón, M.L.; Montoya, T.; Alarcón-de-la-lastra, C.; Sánchez-hidalgo, M. Potential Protective Role Exerted by Secoiridoids from Olea europaea L. In Cancer, Cardiovascular, Neurodegenerative, Aging-Related, and Immunoinflammatory Diseases. Antioxidants 2020, 9, 149. [Google Scholar] [CrossRef] [PubMed]
- Gervasi, F.; Pojero, F. Use of Oleuropein and Hydroxytyrosol for Cancer Prevention and Treatment: Considerations about How Bioavailability and Metabolism Impact Their Adoption in Clinical Routine. Biomedicines 2024, 12, 502. [Google Scholar] [CrossRef]
- Zheng, S.; Huang, K.; Tong, T. Efficacy and Mechanisms of Oleuropein in Mitigating Diabetes and Diabetes Complications. J. Agric. Food Chem. 2021, 69, 6145–6155. [Google Scholar] [CrossRef] [PubMed]
- Omar, S.H. Oleuropein in Olive and Its Pharmacological Effects. Sci. Pharm. 2010, 78, 133–154. [Google Scholar] [CrossRef]
- Romani, A.; Ieri, F.; Urciuoli, S.; Noce, A.; Marrone, G.; Nediani, C.; Bernini, R. Health Effects of Phenolic Compounds Found in Extra-Virgin Olive Oil, by-Products, and Leaf of Olea europaea L. Nutrients 2019, 11, 1776. [Google Scholar] [CrossRef]
- Gentile, L.; Uccella, N.A.; Sivakumar, G. Oleuropein: Molecular Dynamics and Computation. Curr. Med. Chem. 2017, 24, 4315–4328. [Google Scholar] [CrossRef]
- Markopoulos, C.; Vertzoni, M.; Agalias, A.; Magiatis, P.; Reppas, C. Stability of Oleuropein in the Human Proximal Gut. J. Pharm. Pharmacol. 2009, 61, 143–149. [Google Scholar] [CrossRef]
- Martín-Vertedor, D.; Garrido, M.; Pariente, J.A.; Espino, J.; Delgado-Adámez, J. Bioavailability of Bioactive Molecules from Olive Leaf Extracts and Its Functional Value. Phytother. Res. 2016, 30, 1172–1179. [Google Scholar] [CrossRef]
- Galmés, S.; Reynés, B.; Palou, M.; Palou-March, A.; Palou, A. Absorption, Distribution, Metabolism, and Excretion of the Main Olive Tree Phenols and Polyphenols: A Literature Review. J. Agric. Food Chem. 2021, 69, 5281–5296. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Tzounis, X.; Dessì, M.A.; Deiana, M.; Debnam, E.S.; Visioli, F.; Spencer, J.P.E. The Fate of Olive Oil Polyphenols in the Gastrointestinal Tract: Implications of Gastric and Colonic Microflora-Dependent Biotransformation. Free Radic. Res. 2006, 40, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Lopez de las Hazas, M.-C.; Pinol, C.; Macia, A.; Romero, M.-P.; Pedret, A.; Sola, R.; Rubio, L.; Motilva, M.-J. Differential absorption and metabolism of hydroxytyrosol and its precursors oleuropein and secoiridoids. J. Funct. Food 2016, 22, 53–63. [Google Scholar] [CrossRef]
- Edgecombe, S.C.; Stretch, G.L.; Hayball, P.J. Oleuropein, an Antioxidant Polyphenol from Olive Oil, Is Poorly Absorbed from Isolated Perfused Rat Intestine. J. Nutr. 2000, 130, 2996–3002. [Google Scholar] [CrossRef]
- Carrera-Gonzalez, M.P.; Ramirez-Exposito, M.J.; Mayas, M.D.; Martinez-Martos, J.M. Protective role of oleuropein and its metabolite hydroxytyrosol on cancer. Trends Food Sci. Technol. 2013, 31, 92–99. [Google Scholar] [CrossRef]
- Ashraf, A.; Mahmoud, P.A.; Reda, H.; Mansour, S.; Helal, M.H.; Michel, H.E.; Nasr, M. Silymarin and Silymarin Nanoparticles Guard against Chronic Unpredictable Mild Stress Induced Depressive-like Behavior in Mice: Involvement of Neurogenesis and NLRP3 Inflammasome. J. Psychopharmacol. 2019, 33, 615–631. [Google Scholar] [CrossRef]
- Shaaban, M.; Nasr, M.; Tawfik, A.A.; Fadel, M.; Sammour, O. Novel Bergamot Oil Nanospanlastics Combined with PUVB Therapy as a Clinically Translatable Approach for Vitiligo Treatment. Drug Deliv. Transl. Res. 2019, 9, 1106–1116. [Google Scholar] [CrossRef]
- Liu, H.; Jin, X.; Liu, S.; Liu, X.; Pei, X.; Sun, K.; Li, M.; Wang, P.; Chang, Y.; Wang, T.; et al. Recent advances in self-targeting natural product-based nanomedicines. J. Nanobiotechnology 2025, 23, 31. [Google Scholar] [CrossRef]
- Zeng, M.; Guo, D.; Fernández-Varo, G.; Zhang, X.; Fu, S.; Ju, S.; Yang, H.; Liu, X.; Wang, Y.C.; Zeng, Y.; et al. The Integration of Nanomedicine with Traditional Chinese Medicine: Drug Delivery of Natural Products and Other Opportunities. Mol. Pharm. 2023, 20, 886–904. [Google Scholar] [CrossRef] [PubMed]
- Rafique, A.; Amjad, F.; Janjua, M.R.S.A.; Naqvi, S.A.R.; Hassan, S.U.; Abdullah, H.; Nazir, M.S.; Ali, Z.; Alshihri, A.A.; Momenah, M.A.; et al. Chia seed-mediated fabrication of ZnO/Ag/Ag2O nanocomposites: Structural, antioxidant, anticancer, and wound healing studies. Front. Chem. 2024, 12, 1405385. [Google Scholar] [CrossRef] [PubMed]
- Manocha, S.; Dhiman, S.; Grewal, A.S.; Guarve, K. Nanotechnology: An Approach to Overcome Bioavailability Challenges of Nutraceuticals. J. Drug Deliv. Sci. Technol. 2022, 72, 103418. [Google Scholar] [CrossRef]
- Singh, A.R.; Desu, P.K.; Nakkala, R.K.; Kondi, V.; Devi, S.; Alam, M.S.; Hamid, H.; Athawale, R.B.; Kesharwani, P. Nanotechnology-Based Approaches Applied to Nutraceuticals. Drug Deliv. Transl. Res. 2022, 12, 485–499. [Google Scholar] [CrossRef]
- Patel, D.; Solanki, J.; Kher, M.M.; Azagury, A. A Review: Surface Engineering of Lipid-Based Drug Delivery Systems. Small 2024, 20, 2401990. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, X.; Liu, Y.; Yang, G.; Falconer, R.J.; Zhao, C.X. Lipid Nanoparticles for Drug Delivery. Adv. Nanobiomed Res. 2022, 2, 2100109. [Google Scholar] [CrossRef]
- Kale, S.N.; Deore, S.L. Emulsion Micro Emulsion and Nano Emulsion: A Review. Sys Rev. Pharm. 2016, 8, 39–47. [Google Scholar] [CrossRef]
- Goodarzi, F.; Zendehboudi, S. A Comprehensive Review on Emulsions and Emulsion Stability in Chemical and Energy Industries. Can. J. Chem. Eng. 2019, 97, 281–309. [Google Scholar] [CrossRef]
- Anton, N.; Vandamme, T.F. Nano-Emulsions and Micro-Emulsions: Clarifications of the Critical Differences. Pharm. Res. 2011, 28, 978–985. [Google Scholar] [CrossRef]
- Wilson, R.J.; Li, Y.; Yang, G.; Zhao, C.X. Nanoemulsions for Drug Delivery. Particuology 2022, 64, 85–97. [Google Scholar] [CrossRef]
- Heidari, F.; Jafari, S.M.; Ziaiifar, A.M.; Malekjani, N. Stability and Release Mechanisms of Double Emulsions Loaded with Bioactive Compounds; a Critical Review. Adv. Colloid. Interface Sci. 2022, 299, 102567. [Google Scholar] [CrossRef] [PubMed]
- Gharehbeglou, P.; Jafari, S.M.; Homayouni, A.; Hamishekar, H.; Mirzaei, H. Fabrication of Double W1/O/W2 Nano-Emulsions Loaded with Oleuropein in the Internal Phase (W1) and Evaluation of Their Release Rate. Food Hydrocoll. 2019, 89, 44–55. [Google Scholar] [CrossRef]
- Gharehbeglou, P.; Sarabandi, K.; Akbarbaglu, Z.; Jafari, S.M. Predictive Modeling of Oleuropein Release from Double Nanoemulsions: An Analytical Study Comparing Intelligent Models and Monte Carlo Simulation. J. Agric. Food Res. 2024, 17, 101261. [Google Scholar] [CrossRef]
- El-Gogary, R.I.; Ragai, M.H.; Moftah, N.; Nasr, M. Oleuropein as a Novel Topical Antipsoriatic Nutraceutical: Formulation in Microemulsion Nanocarrier and Exploratory Clinical Appraisal. Expert. Opin. Drug Deliv. 2021, 18, 1523–1532. [Google Scholar] [CrossRef]
- Al-Karaki, R.; Awadallah, A.; Tawfeek, H.M.; Nasr, M. Preparation, Characterization and Cytotoxic Activity of New Oleuropein Microemulsion Against HCT-116 Colon Cancer Cells. Pharm. Chem. J. 2020, 53, 1118–1121. [Google Scholar] [CrossRef]
- Beloqui, A.; Solinís, M.Á.; Rodríguez-Gascón, A.; Almeida, A.J.; Préat, V. Nanostructured Lipid Carriers: Promising Drug Delivery Systems for Future Clinics. Nanomedicine 2016, 12, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Khosa, A.; Reddi, S.; Saha, R.N. Nanostructured Lipid Carriers for Site-Specific Drug Delivery. Biomed. Pharmacother. 2018, 103, 598–613. [Google Scholar] [CrossRef]
- Salvi, V.R.; Pawar, P. Nanostructured Lipid Carriers (NLC) System: A Novel Drug Targeting Carrier. J. Drug Deliv. Sci. Technol. 2019, 51, 255–267. [Google Scholar] [CrossRef]
- Èller, R.H.M.; Èder, K.M.; Gohla, S. Solid Lipid Nanoparticles (SLN) for Controlled Drug Delivery—A Review of the State of the Art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- Naseri, N.; Valizadeh, H.; Zakeri-Milani, P. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Structure Preparation and Application. Adv. Pharm. Bull. 2015, 5, 305–313. [Google Scholar] [CrossRef]
- Palagati, S.; Sv, S.; Kesavan, B.R. Application of Computational Tools for the Designing of Oleuropein Loaded Nanostructured Lipid Carrier for Brain Targeting through Nasal Route. DARU J. Pharm. Sci. 2019, 27, 695–708. [Google Scholar] [CrossRef] [PubMed]
- Sucharitha, P.; Satyanarayana, S.V.; Reddy, K.B. In Vitro-In Vivo Characterization of Oleuropein Loaded Nanostructured Lipid Carriers in the Treatment of Streptococcus Pneumoniae Induced Meningitis. Asian J. Pharm. 2019, 13, 151. [Google Scholar]
- Huguet-Casquero, A.; Moreno-Sastre, M.; López-Méndez, T.B.; Gainza, E.; Pedraz, J.L. Encapsulation of Oleuropein in Nanostructured Lipid Carriers: Biocompatibility and Antioxidant Efficacy in Lung Epithelial Cells. Pharmaceutics 2020, 12, 429. [Google Scholar] [CrossRef]
- Huguet-Casquero, A.; Xu, Y.; Gainza, E.; Pedraz, J.L.; Beloqui, A. Oral Delivery of Oleuropein-Loaded Lipid Nanocarriers Alleviates Inflammation and Oxidative Stress in Acute Colitis. Int. J. Pharm. 2020, 586, 119515. [Google Scholar] [CrossRef]
- Nasr, M.; Abdel-Hamid, S. Lipid Based Nanocapsules: A Multitude of Biomedical Applications. Curr. Pharm. Biotechnol. 2015, 16, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Huynh, N.T.; Passirani, C.; Saulnier, P.; Benoit, J.P. Lipid Nanocapsules: A New Platform for Nanomedicine. Int. J. Pharm. 2009, 379, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Al-Karaki, R.; Awadallah, A.; Saleh, M.; Nasr, M. Novel Oleuropein Nanocapsular Formulation: Preparation, Characterization and Anticolon Cancer Activity. J. Appl. Pharm. 2019, 11, 1. [Google Scholar] [CrossRef]
- Aldalaen, S.; Nasr, M.; El-Gogary, R.I. Angiogenesis and Collagen Promoting Nutraceutical-Loaded Nanovesicles for Wound Healing. J. Drug Deliv. Sci. Technol. 2020, 56, 101548. [Google Scholar] [CrossRef]
- El-Zaafarany, G.M.; Nasr, M. Insightful Exploring of Advanced Nanocarriers for the Topical/Transdermal Treatment of Skin Diseases. Pharm. Dev. Technol. 2021, 26, 1136–1157. [Google Scholar] [CrossRef]
- Jain, S.; Jain, V.; Mahajan, S.C. Lipid Based Vesicular Drug Delivery Systems. Adv. Pharm. 2014, 2014, 574673. [Google Scholar] [CrossRef]
- Escudé Martinez de Castilla, P.; Tong, L.; Huang, C.; Sofias, A.M.; Pastorin, G.; Chen, X.; Storm, G.; Schiffelers, R.M.; Wang, J.W. Extracellular Vesicles as a Drug Delivery System: A Systematic Review of Preclinical Studies. Adv. Drug Deliv. Rev. 2021, 175, 113801. [Google Scholar] [CrossRef] [PubMed]
- Limongi, T.; Susa, F.; Marini, M.; Allione, M.; Torre, B.; Pisano, R.; Di Fabrizio, E. Lipid-Based Nanovesicular Drug Delivery Systems. Nanomaterials 2021, 11, 3391. [Google Scholar] [CrossRef] [PubMed]
- Bonechi, C.; Donati, A.; Tamasi, G.; Pardini, A.; Rostom, H.; Leone, G.; Lamponi, S.; Consumi, M.; Magnani, A.; Rossi, C. Chemical Characterization of Liposomes Containing Nutraceutical Compounds: Tyrosol, Hydroxytyrosol and Oleuropein. Biophys. Chem. 2019, 246, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Nassir, A.M.; Ibrahim, I.A.A.; Md, S.; Waris, M.; Tanuja; Ain, M.R.; Ahmad, I.; Shahzad, N. Surface Functionalized Folate Targeted Oleuropein Nano-Liposomes for Prostate Tumor Targeting: In Vitro and in Vivo Activity. Life Sci. 2019, 220, 136–146. [Google Scholar] [CrossRef]
- Li, W.; Chountoulesi, M.; Antoniadi, L.; Angelis, A.; Lei, J.; Halabalaki, M.; Demetzos, C.; Mitakou, S.; Skaltsounis, L.A.; Wang, C. Development and Physicochemical Characterization of Nanoliposomes with Incorporated Oleocanthal, Oleacein, Oleuropein and Hydroxytyrosol. Food Chem. 2022, 384, 132470. [Google Scholar] [CrossRef]
- Al-Jammal, A.; Bigdeli, M.R.; Mortazavi Moghadam, F. PH-Sensitive Oleuropein-Loaded Niosome: Efficient Treatment for Metastatic Brain Tumors in Initial Steps in-Vivo. OpenNano 2022, 8, 100095. [Google Scholar] [CrossRef]
- Sklenarova, R.; Allaw, M.; Perra, M.; Castangia, I.; Frankova, J.; Luis Pedraz, J.; Letizia Manca, M.; Manconi, M. Co-Delivering of Oleuropein and Lentisk Oil in Phospholipid Vesicles as an Effective Approach to Modulate Oxidative Stress, Cytokine Secretion and Promote Skin Regeneration. Eur. J. Pharm. Biopharm. 2023, 185, 126–136. [Google Scholar] [CrossRef]
- Allaw, M.; Manca, M.L.; Gómez-Fernández, J.C.; Pedraz, J.L.; Terencio, M.C.; Sales, O.D.; Nacher, A.; Manconi, M. Oleuropein Multicompartment Nanovesicles Enriched with Collagen as a Natural Strategy for the Treatment of Skin Wounds Connected with Oxidative Stress. Nanomedicine 2021, 16, 2363–2376. [Google Scholar] [CrossRef]
- Cristiano, M.C.; Froiio, F.; Mancuso, A.; Cosco, D.; Dini, L.; Di Marzio, L.; Fresta, M.; Paolino, D. Oleuropein-Laded Ufasomes Improve the Nutraceutical Efficacy. Nanomaterials 2021, 11, 105. [Google Scholar] [CrossRef]
- Mahmood, T.H.; Al-Samydai, A.; Al Sulaibi, M.; Alqaraleh, M.; Abed, A.I.; Shalan, N.; Alsanabrah, A.; Alsotari, S.T.; Nsairat, H.; Alshaer, W. Development of Pegylated Nano-Phytosome Formulation with Oleuropein and Rutin to Compare Anti-Colonic Cancer Activity with Olea europaea Leaves Extract. Chem. Biodivers. 2023, 20, e202300534. [Google Scholar] [CrossRef]
- Aliakbari, F.; Marzookian, K.; Parsafar, S.; Hourfar, H.; Nayeri, Z.; Fattahi, A.; Raeiji, M.; Boroujeni, N.N.; Otzen, D.E.; Morshedi, D. The Impact of HUC MSC-Derived Exosome-Nanoliposome Hybrids on α-Synuclein Fibrillation and Neurotoxicity. Sci. Adv. 2024, 10, eadl3406. [Google Scholar] [CrossRef]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef] [PubMed]
- Szejtli, J. Introduction and General Overview of Cyclodextrin Chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar] [CrossRef] [PubMed]
- Poulson, B.G.; Alsulami, Q.A.; Sharfalddin, A.; El Agammy, E.F.; Mouffouk, F.; Emwas, A.H.; Jaremko, L.; Jaremko, M. Cyclodextrins: Structural, Chemical, and Physical Properties, and Applications. Polysaccharides 2022, 3, 1. [Google Scholar] [CrossRef]
- Burgalassi, S.; Zucchetti, E.; Birindelli, E.; Tampucci, S.; Chetoni, P.; Monti, D. Ocular Application of Oleuropein in Dry Eye Treatment: Formulation Studies and Biological Evaluation. Pharmaceuticals 2021, 14, 1151. [Google Scholar] [CrossRef]
- Jafernik, K.; Ładniak, A.; Blicharska, E.; Czarnek, K.; Ekiert, H.; Wiącek, A.E.; Szopa, A. Chitosan-Based Nanoparticles as Effective Drug Delivery Systems—A Review. Molecules 2023, 28, 1963. [Google Scholar] [CrossRef]
- Wang, Y.; Mei, L.; Zhao, S.; Xing, X.; Wu, G. Effect of Chitosan-Oleuropein Nanoparticles on Dentin Collagen Cross-Linking. Technol. Health Care 2023, 31, 647–659. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Y.; Chen, Y.; Xing, X.; Wang, Y.; Wu, G. Evaluation of Chitosan-Oleuropein Nanoparticles on the Durability of Dentin Bonding. Drug Des. Dev. Ther. 2023, 17, 167–180. [Google Scholar] [CrossRef]
- Abd-Allah, H.; Youshia, J.; Abdel Jaleel, G.A.; Hassan, A.; El Madani, M.; Nasr, M. Gastroprotective Chitosan Nanoparticles Loaded with Oleuropein: An In Vivo Proof of Concept. Pharmaceutics 2024, 16, 153. [Google Scholar] [CrossRef]
- Duan, X.; Chen, H.; Guo, C. Polymeric Nanofibers for Drug Delivery Applications: A Recent Review. J. Mater. Sci. Mater. Med. 2022, 33, 78. [Google Scholar] [CrossRef]
- Jiffrin, R.; Razak, S.I.A.; Jamaludin, M.I.; Hamzah, A.S.A.; Mazian, M.A.; Jaya, M.A.T.; Nasrullah, M.Z.; Majrashi, M.; Theyab, A.; Aldarmahi, A.A.; et al. Electrospun Nanofiber Composites for Drug Delivery: A Review on Current Progresses. Polymers 2022, 14, 3725. [Google Scholar] [CrossRef] [PubMed]
- Ercelik, M.; Tekin, C.; Parin, F.N.; Mutlu, B.; Dogan, H.Y.; Tezcan, G.; Aksoy, S.A.; Gurbuz, M.; Yildirim, K.; Bekar, A.; et al. Co-Loading of Temozolomide with Oleuropein or Rutin into Polylactic Acid Core-Shell Nanofiber Webs Inhibit Glioblastoma Cell by Controlled Release. Int. J. Biol. Macromol. 2023, 253, 126722. [Google Scholar] [CrossRef] [PubMed]
- Ulker Turan, C.; Derviscemaloglu, M.; Guvenilir, Y. Enzymatically Synthesized Lactone-Based Copolymer and Gelatin Nanofibrous Blends Loaded with an Olive Leaf Phenolic Compound. Mater. Today Commun. 2024, 38, 108215. [Google Scholar] [CrossRef]
- Erdogan, I.; Demir, M.; Bayraktar, O. Olive Leaf Extract as a Crosslinking Agent for the Preparation of Electrospun Zein Fibers. J. Appl. Polym. Sci. 2015, 132, 41338. [Google Scholar] [CrossRef]
- Bayraktar, O.; Balta, A.B.; Bayraktar, G.B. Adsorption/Desorption and Biofunctional Properties of Oleuropein Loaded on Different Types of Silk Fibroin Matrices. Maced. J. Chem. Chem. Eng. 2017, 36, 153–165. [Google Scholar] [CrossRef]
- Chandrakala, V.; Aruna, V.; Angajala, G. Review on Metal Nanoparticles as Nanocarriers: Current Challenges and Perspectives in Drug Delivery Systems. Emergent Mater. 2022, 5, 1593–1615. [Google Scholar] [CrossRef]
- Genc, N.; Yildiz, I.; Chaoui, R.; Erenler, R.; Temiz, C.; Elmastas, M. Biosynthesis, Characterization and Antioxidant Activity of Oleuropein-Mediated Silver Nanoparticles. Inorg. Nano Met. Chem. 2020, 51, 411–419. [Google Scholar] [CrossRef]
- Pirković, A.; Lazić, V.; Spremo-Potparević, B.; Živković, L.; Topalović, D.; Kuzman, S.; Antić-Stanković, J.; Božić, D.; Jovanović Krivokuća, M.; Nedeljković, J.M. Comparative Analysis of Ag NPs Functionalized with Olive Leaf Extract and Oleuropein and Toxicity in Human Trophoblast Cells and Peripheral Blood Lymphocytes. Mutagenesis 2023, 38, 169–181. [Google Scholar] [CrossRef]
- Jimenez-Ruiz, A.; Prado-Gotor, R.; Fernández-Bolaños, J.G.; González-Benjumea, A.; Carnerero, J.M. Encased Gold Nanoparticle Synthesis as a Probe for Oleuropein Self-Assembled Structure Formation. Materials 2021, 14, 50. [Google Scholar] [CrossRef]
- Barzegar, F.; Zaefizadeh, M.; Yari, R.; Salehzadeh, A. Synthesis of Nano-Paramagnetic Oleuropein to Induce KRAS over-Expression: A New Mechanism to Inhibit AGS Cancer Cells. Medicina (Kaunas) 2019, 55, 388. [Google Scholar] [CrossRef]
- Mahdavi Niyaki, Z.; Salehzadeh, A.; Peymani, M.; Zaefizadeh, M. Exploring the Therapeutic Potential of Fe3O4@Glu-Oleuropein Nanoparticles in Targeting KRAS Pathway-Regulating LncRNAs in Colorectal Cancer Cells. Biol. Trace Elem. Res. 2024, 202, 3073–3085. [Google Scholar] [CrossRef] [PubMed]
- Mehdinejad, S.; Peymani, M.; Salehzadeh, A.; Zaefizadeh, M. Genetic Insights and Therapeutic Potential for Colorectal Cancer: Mutation Analysis of KRAS Gene and Efficacy of Oleuropein-Conjugated Iron Oxide Nanoparticles. Naunyn Schmiedebergs Arch. Pharmacol. 2024, 397, 8771–8783. [Google Scholar] [CrossRef] [PubMed]
- Erbaş, E.; Gelen, V.; Kara, H.; Gedikli, S.; Yeşildağ, A.; Özkanlar, S.; Akarsu, S.A. Silver Nanoparticles Loaded with Oleuropein Reduce Doxorubicin-Induced Testicular Damage by Regulating Endoplasmic Reticulum Stress, and Apoptosis. Biol. Trace Elem. Res. 2024, 202, 4687–4698. [Google Scholar] [CrossRef] [PubMed]
- Manzano, M.; Vallet-Regí, M. Mesoporous Silica Nanoparticles for Drug Delivery. Adv. Funct. Mater. 2020, 30, 1902634. [Google Scholar] [CrossRef]
- Park, S.M.; Kim, D.Y.; Lee, K.H.; Shin, Y., II; Han, S.C.; Kwon, S.M. Anti-Tumor Efficacy of Oleuropein-Loaded ZnO/Au Mesoporous Silica Nanoparticle in 5-FU-Resistant Colorectal Cancer Cells. Int. J. Nanomed. 2024, 19, 2675–2690. [Google Scholar] [CrossRef]
| Disease | Mechanism of Action | Ref. |
|---|---|---|
| Cancer |
| [5,15,16] |
| Obesity |
| [5,8,10] |
| Diabetes |
| [5,17] |
| Atherosclerosis |
| [5,15,18] |
| Alzheimer |
| [5,15,18,19] |
| Parkinson |
| [5,15,18] |
| Composition (Solid and Liquid Lipids) | PS (nm) | ZP (mV) | EE (%) | Status of Investigation | Main Findings | Ref. |
|---|---|---|---|---|---|---|
| Tefose® and Capmul® | 169.5 | −27 | 98.4 |
|
| [51] |
| Precirol® ATO 5 and Campul® | 357.8 | −40.1 | 53.42–89.4 |
|
| [52] |
| Precirol® ATO 5 and olive oil | 150 | −21 | 99.12 |
|
| [53] |
| Precirol® ATO 5 and olive oil | 141.2 ± 12.3 | −25.3 ± 2.7 | 99.95 |
|
| [54] |
| Composition | PS (nm) | ZP (mV) | EE (%) | Status of Investigation | Main Findings | Ref. |
|---|---|---|---|---|---|---|
| Zwitterionic liposomes | 106.1 ± 7 | −19.9 ± 9.7 | 30.2 ± 1.6 |
|
| [63] |
| Surface functionalized folate PEG-liposomes | 184.2 ± 9.16 | 1.41 ± 0.24 | 63.52 ± 4.15 |
|
| [64] |
| Liposomes | (BFD) 115 ± 3.2 (AFD) 165.4 ± 1.4 | (BFD) 23.1 ± 1.2 (AFD) 15.8 ± 0.2 | 50–60 |
|
| [65] |
| pH-sensitive niosomes | 85 ± 7.381 | 1.38 ± 0.074 | 96.89 ± 0.241 |
|
| [66] |
| Transfersomes | (BFD) 141 ± 19 (AFD) 149 ± 10 | (BFD) −65 ± 5 (AFD) −63 | (BFD) 88 ± 1 (AFD) 67 ± 2 |
|
| [67] |
| Hyalurosomes | (BFD) 146 ± 25 (AFD) 104 ± 6 | (BFD) −64 ± 2 (AFD) −59 | (BFD) 90 ± 2 (AFD) 67 ± 5 | |||
| Hyalutransfersomes | (BFD) 153 ± 22 (AFD) 101 ± 11 | (BFD) −63 ± 6 (AFD) −67 | (BFD) 89 ± 1 (AFD) 68 ± 2 | |||
| Collagen glytransfersomes | 113 ± 6 | −41 ± 9 | 92 ± 7 |
|
| [68] |
| Ufasomes | 199 ± 1 | −42 ± 1 | 89 ± 2 |
|
| [69] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasr, M.; Katary, S.H. From Olive Tree to Treatment: Nano-Delivery Systems for Enhancing Oleuropein’s Health Benefits. Pharmaceuticals 2025, 18, 573. https://doi.org/10.3390/ph18040573
Nasr M, Katary SH. From Olive Tree to Treatment: Nano-Delivery Systems for Enhancing Oleuropein’s Health Benefits. Pharmaceuticals. 2025; 18(4):573. https://doi.org/10.3390/ph18040573
Chicago/Turabian StyleNasr, Maha, and Salma H. Katary. 2025. "From Olive Tree to Treatment: Nano-Delivery Systems for Enhancing Oleuropein’s Health Benefits" Pharmaceuticals 18, no. 4: 573. https://doi.org/10.3390/ph18040573
APA StyleNasr, M., & Katary, S. H. (2025). From Olive Tree to Treatment: Nano-Delivery Systems for Enhancing Oleuropein’s Health Benefits. Pharmaceuticals, 18(4), 573. https://doi.org/10.3390/ph18040573






