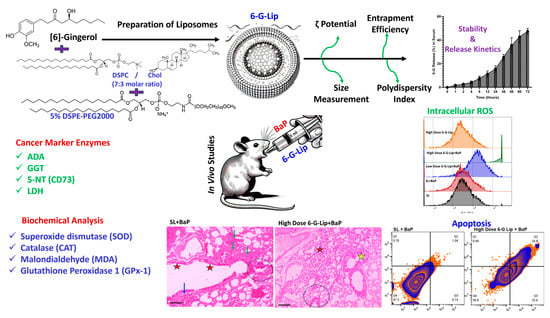
Lipid-Based Nanoformulations of [6]-Gingerol for the Chemoprevention of Benzo[a] Pyrene-Induced Lung Carcinogenesis: Preclinical Evidence
Abstract
:1. Introduction
2. Results
2.1. Characterization of Liposomes
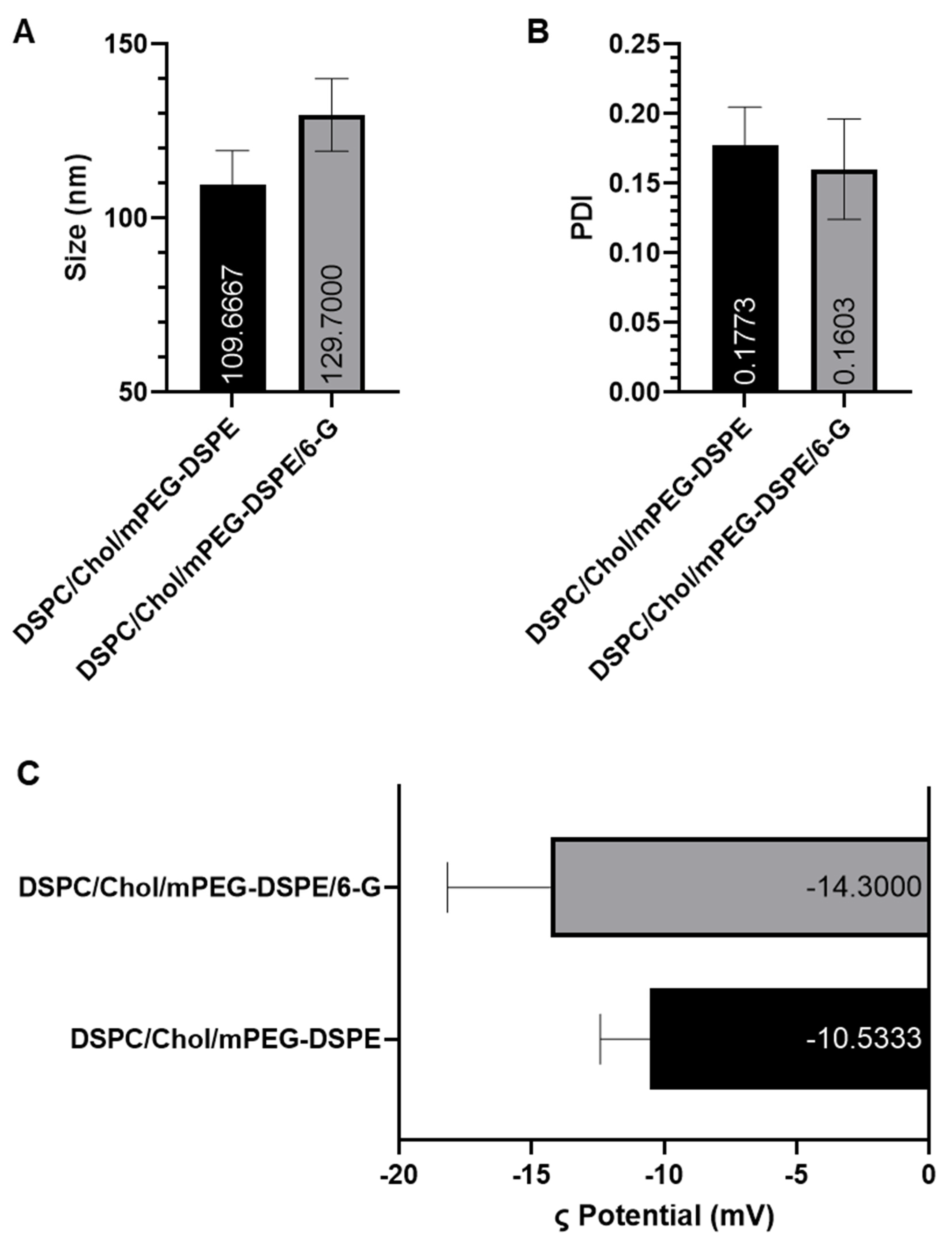
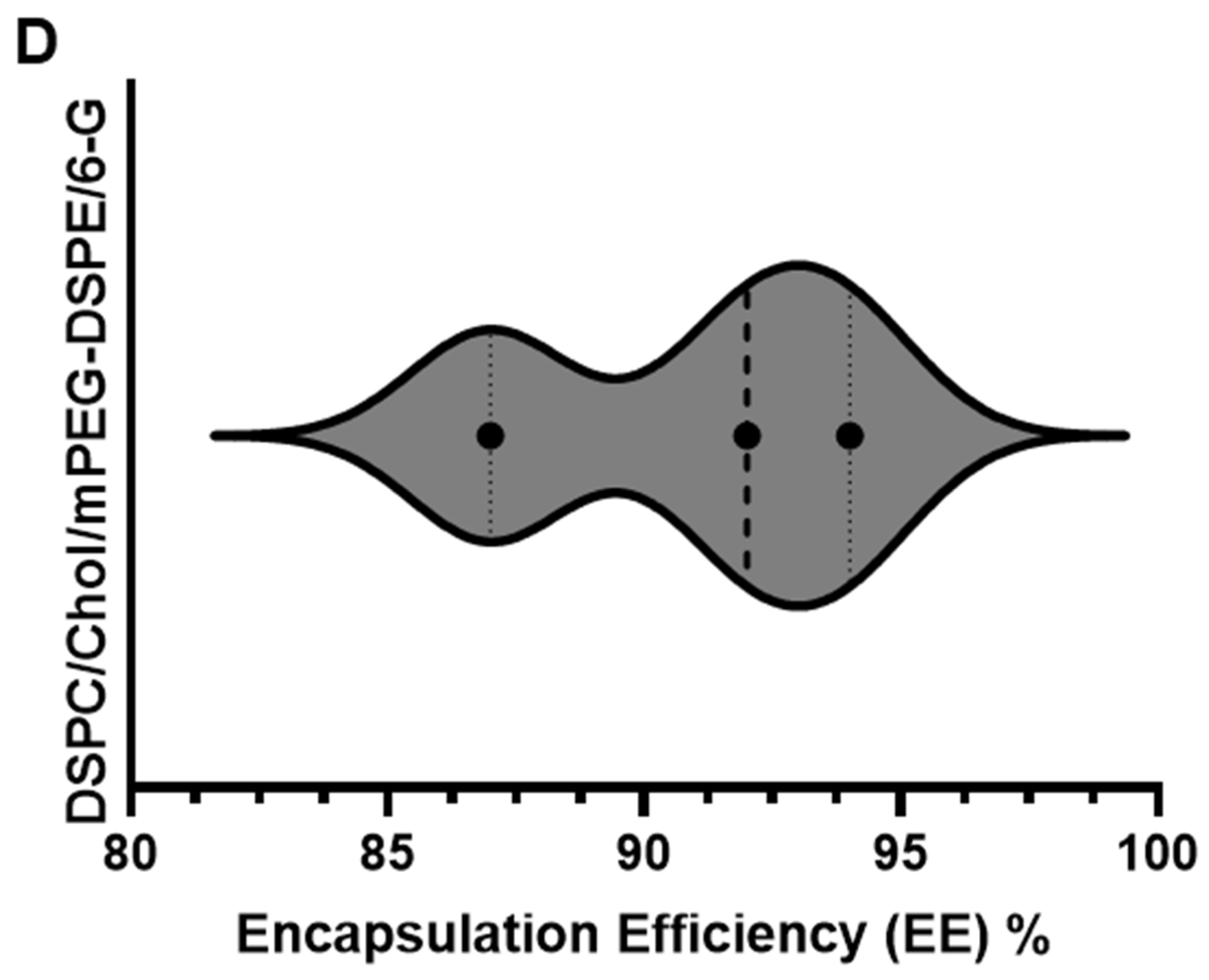
2.1.1. Particle Size, Polydispersity Index (PDI), Zeta Potential, and Encapsulation Efficiency (EE)
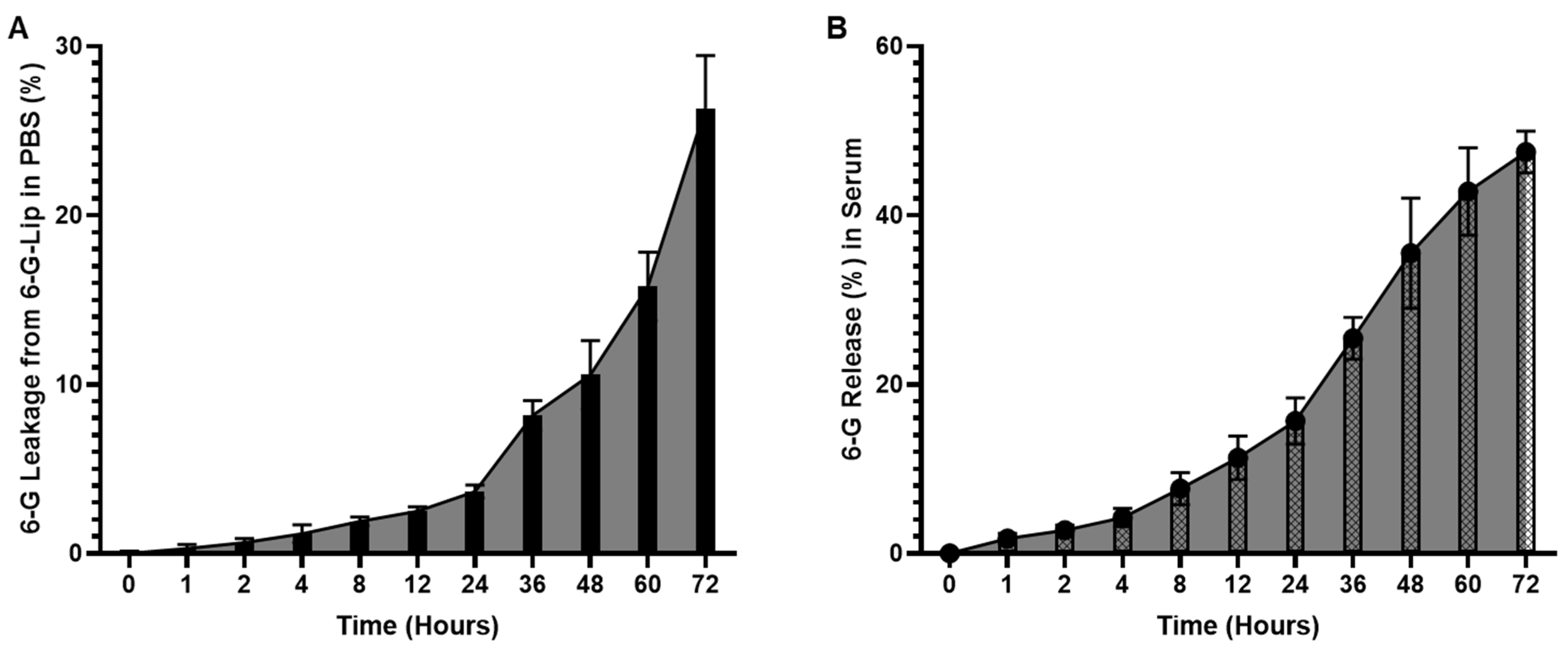
2.1.2. In Vitro Stability and Release Kinetics
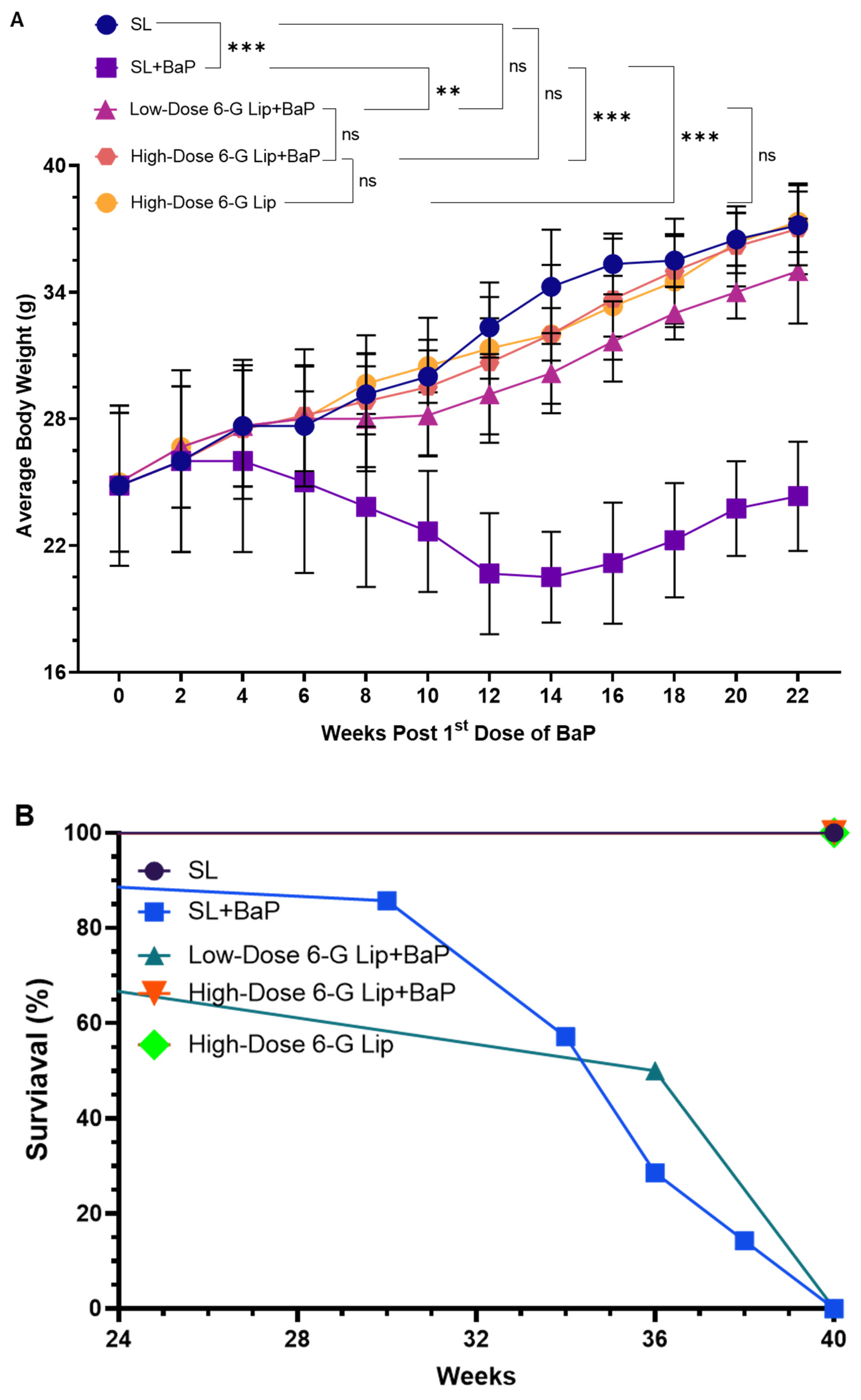
2.2. Protective Effects of 6-G-Lip on BaP-Induced Body Weight Changes and Survival Rates
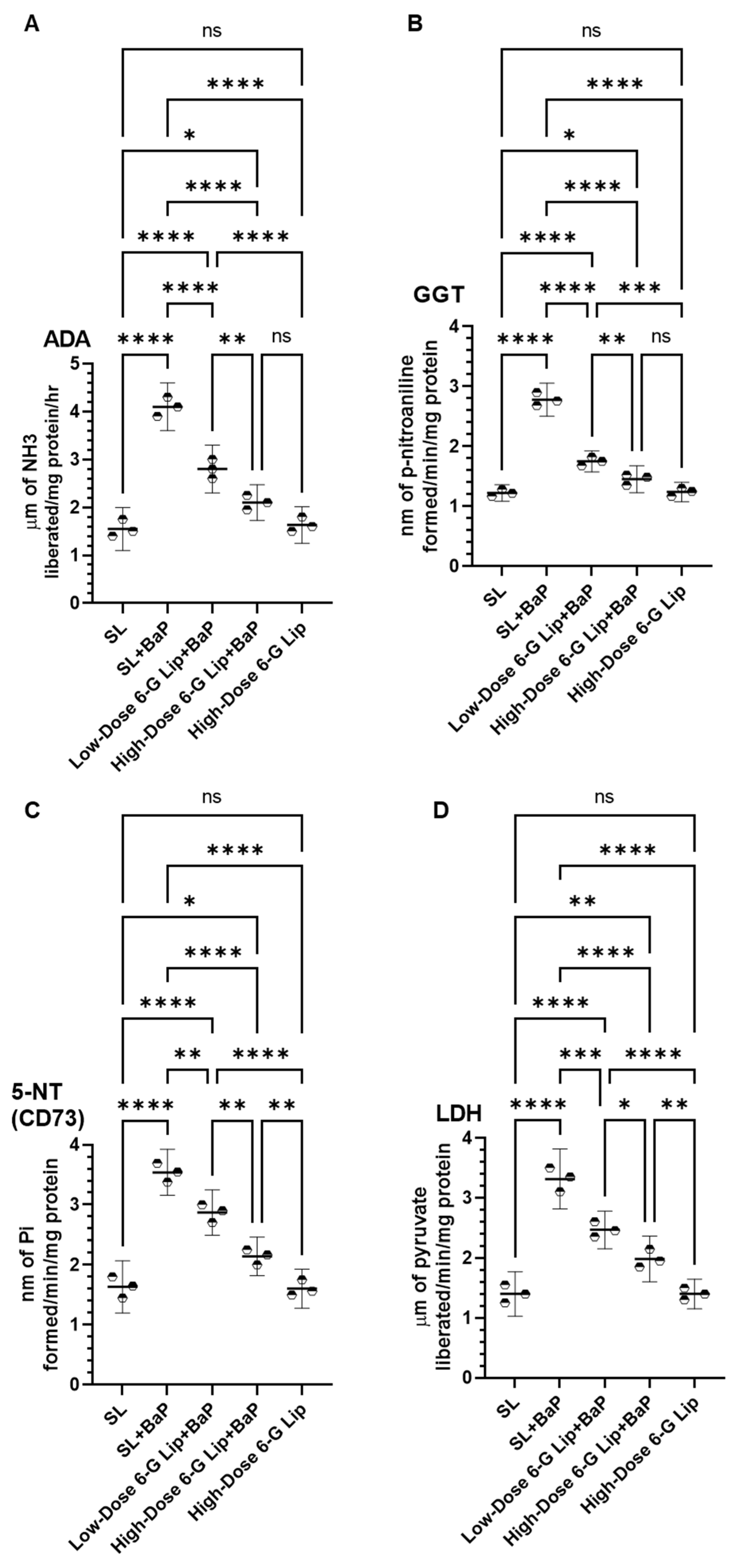
2.3. Modulation of Cancer Marker Enzymes in Serum
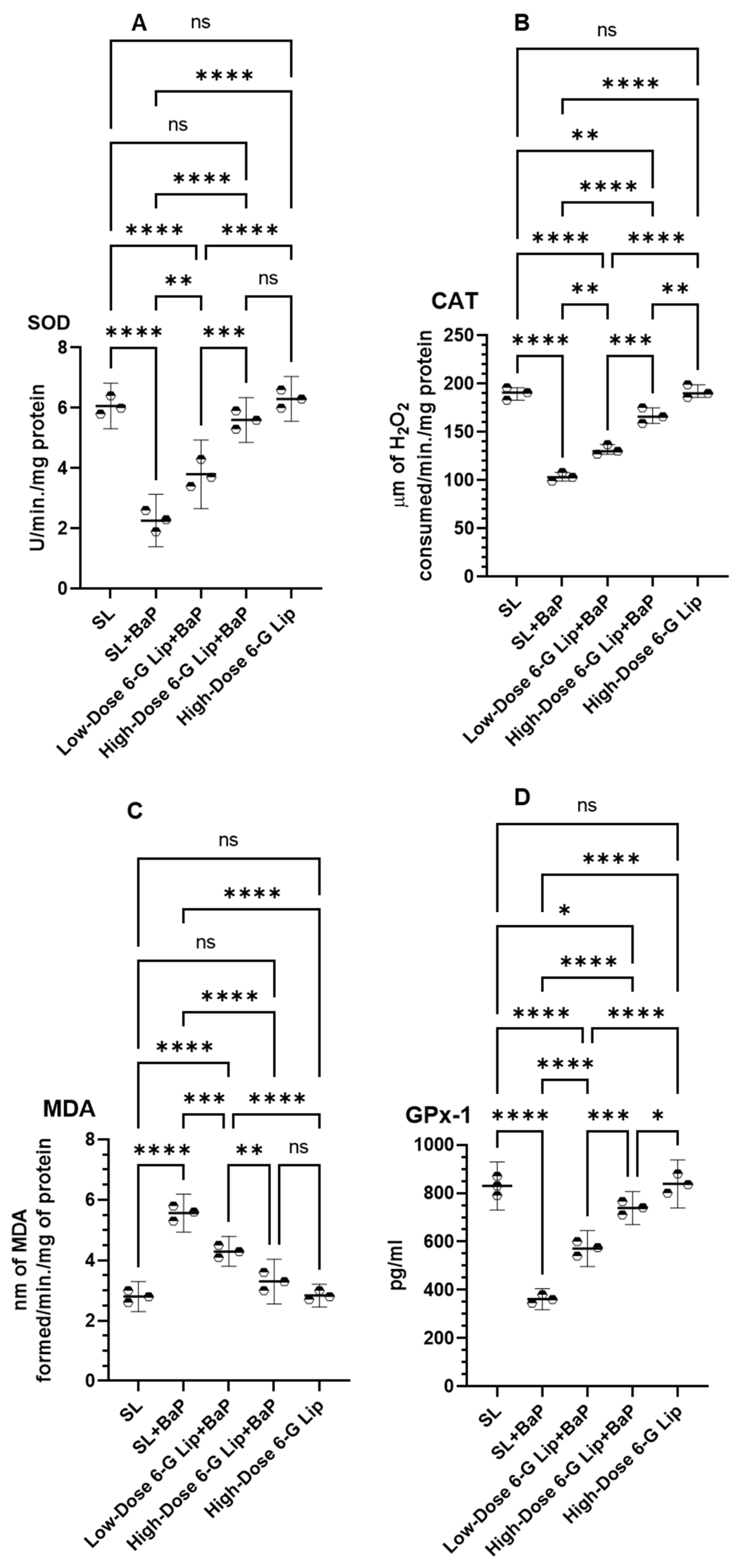
2.4. Antioxidant Enzyme Activity and Lipid Peroxidation
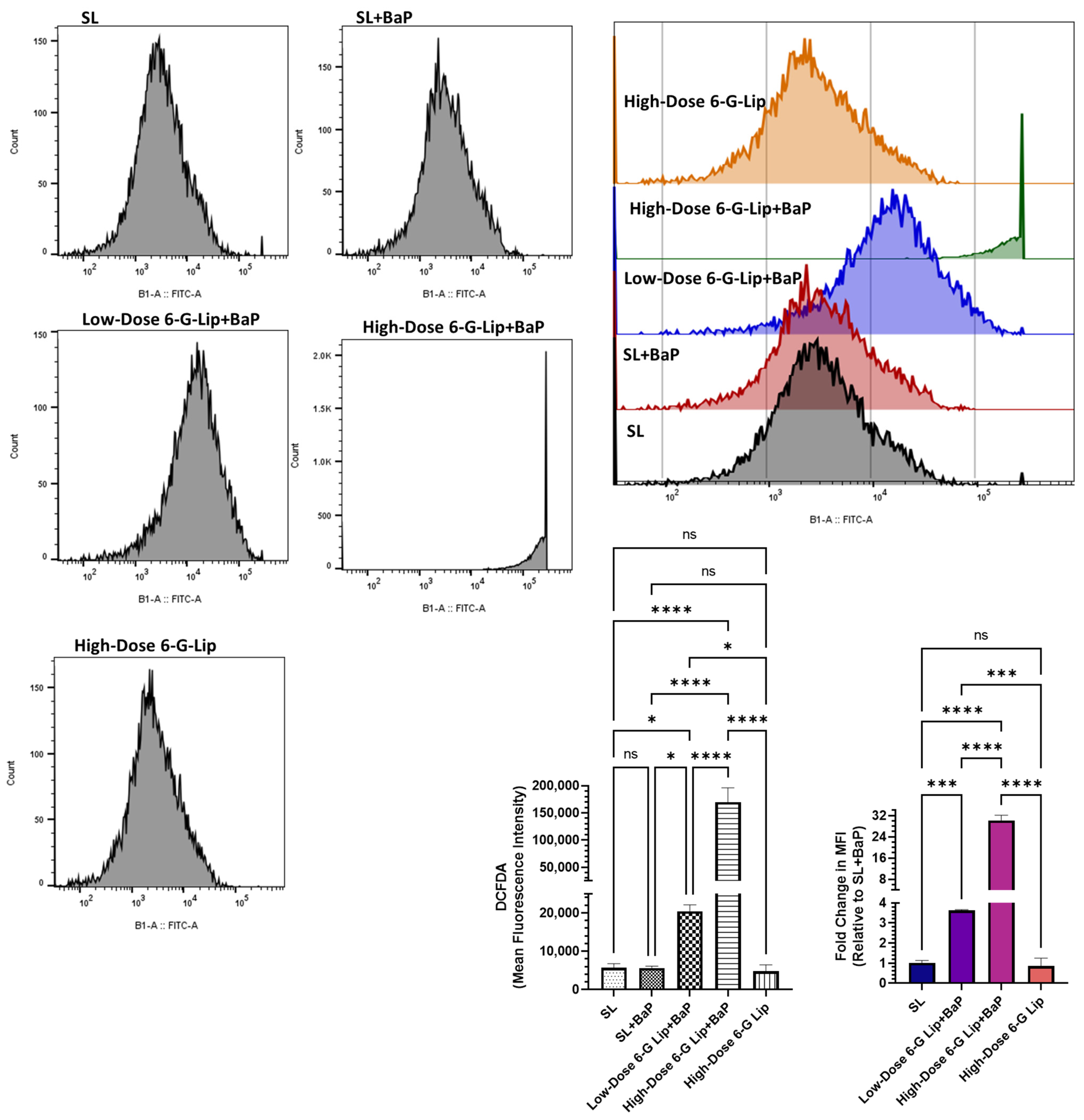
2.5. Reactive Oxygen Species (ROS) Analysis via Flow Cytometry
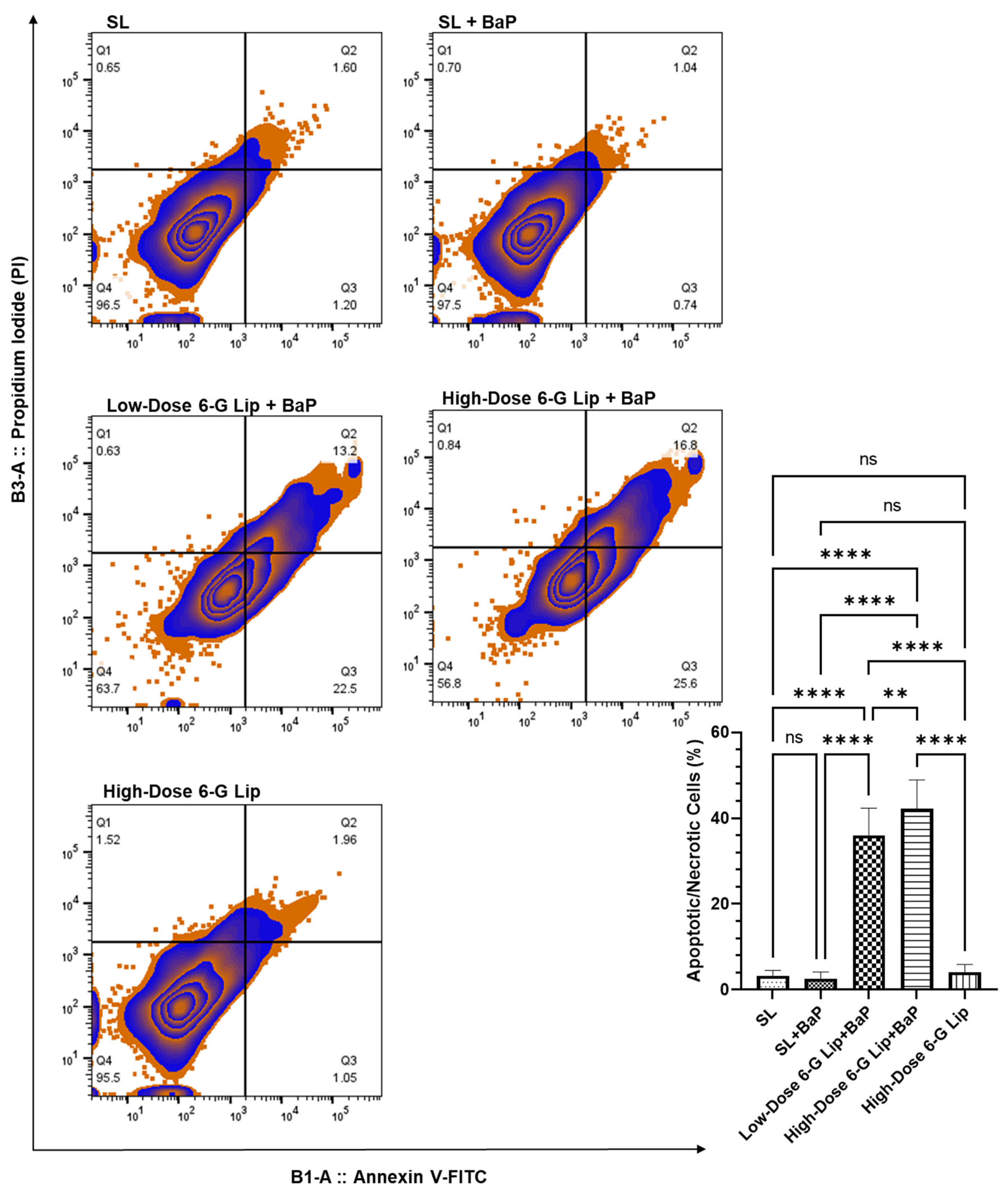
2.6. Induction of Apoptosis in Lung Cells
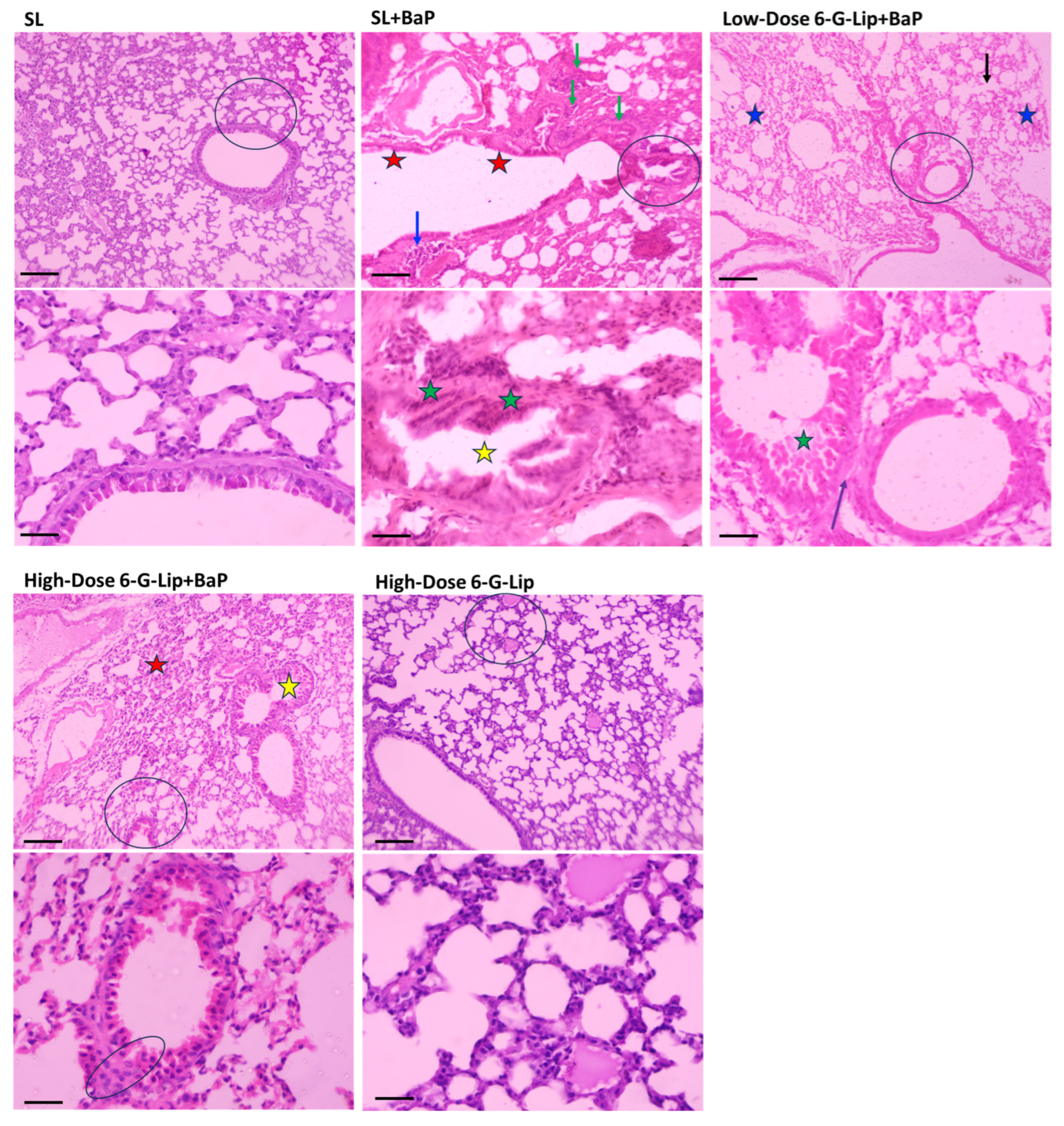
2.7. Histopathological Assessment of Lung Tissues
3. Discussions
4. Materials and Methods
4.1. Reagents
4.2. Preparation of 6-Gingerol-Loaded Liposomes
4.3. Characterization of Liposomes
4.4. In Vitro Stability and Release Studies
4.5. Animal Studies
4.5.1. Ethical Considerations and Animal Handling
4.5.2. Experimental Design
4.5.3. Study of Average Body Weight (ABW) and Survival Rate
4.5.4. Biochemical and Histological Analyses
Biochemical Marker Evaluation
Antioxidant Enzyme Activity
Histopathological Assessment of Lung Tissues
4.5.5. Apoptosis and ROS Assays
Apoptosis Analysis
Measurement of Intracellular ROS Production
4.5.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A.l. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Thai, A.A.; Solomon, B.J.; Sequist, L.V.; Gainor, J.F.; Heist, R.S. Lung cancer. Lancet 2021, 398, 535–554. [Google Scholar] [CrossRef]
- Carroll, R.; Bortolini, M.; Calleja, A.; Munro, R.; Kong, S.; Daumont, M.J.; Penrod, J.R.; Lakhdari, K.; Lacoin, L.; Cheung, W.Y. Trends in treatment patterns and survival outcomes in advanced non-small cell lung cancer: A Canadian population-based real-world analysis. BMC Cancer 2022, 22, 255. [Google Scholar] [CrossRef]
- Basse, C.; Carton, M.; Milder, M.; Geiss, R.; Du Rusquec, P.; Daniel, C.; Massiani, M.; Liwartowski, A.; Girard, N. Real-World Survival Impact of New Treatment Strategies for Lung Cancer: A 2000–2020 French Cohort. Cancers 2024, 16, 2768. [Google Scholar] [CrossRef] [PubMed]
- Jazieh, A.; AlGhamdi, M.; AlGhanem, S.; AlGarni, M.; AlKattan, K.; AlRujaib, M.; AlMaimi, M.; Babelli, O.M.; AlShehri, S.; AlQahtani, R.; et al. Saudi lung cancer prevention and screening guidelines. Ann. Thorac. Med. 2018, 13, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Maghfoor, I.; Perry, M.C. Lung Cancer. Ann. Saudi Med. 2005, 25, 1–12. [Google Scholar] [CrossRef]
- Ramadan, M.; Alhusseini, N.; Samhan, L.; Samhan, S.; Abbad, T. Tobacco control policies implementation and future lung cancer incidence in Saudi Arabia. A population-based study. Prev. Med. Rep. 2023, 36, 102439. [Google Scholar] [CrossRef]
- Jazieh, A.R.; Algwaiz, G.; Alshehri, S.M.; Alkattan, K. Lung Cancer in Saudi Arabia. J. Thorac. Oncol. 2019, 14, 957–962. [Google Scholar] [CrossRef]
- Alamri, S.A.; Alzahrani, M.M.; Alamri, A.A.; Khalifa, W.W.; Alsulami, R.Y.; Bardesi, J.; Salah, W.; Zakariah, A.F. Understanding the public knowledge, attitude, and practice toward screening and risk factors of lung cancer in Saudi Arabia: A cross-sectional study. Ann. Thorac. Med. 2024, 19, 275–283. [Google Scholar] [CrossRef]
- Straif, K.; Baan, R.; Grosse, Y.; Secretan, B.; El Ghissassi, F.; Cogliano, V. Carcinogenicity of polycyclic aromatic hydrocarbons. Lancet Oncol. 2005, 6, 931–932. [Google Scholar] [CrossRef]
- Akhmetova, D.A.; Kozlov, V.V.; Gulyaeva, L.F. New Insight into the Role of AhR in Lung Carcinogenesis. Biochemistry 2022, 87, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Gao, H.; Zhang, X.; Liu, X.; Chen, J.; Liao, X.; Zhang, H.; Huang, Q. Aryl hydrocarbon receptor mediates benzo[a]pyrene-induced metabolic reprogramming in human lung epithelial BEAS-2B cells. Sci. Total Environ. 2021, 756, 144130. [Google Scholar] [CrossRef]
- Chen, J.; Yakkundi, P.; Chan, W.K. Down-Regulation of p23 in Normal Lung Epithelial Cells Reduces Toxicities From Exposure to Benzo[a]pyrene and Cigarette Smoke Condensate via an Aryl Hydrocarbon Receptor-Dependent Mechanism. Toxicol. Sci. 2019, 167, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Kometani, T.; Yoshino, I.; Miura, N.; Okazaki, H.; Ohba, T.; Takenaka, T.; Shoji, F.; Yano, T.; Maehara, Y. Benzo[a]pyrene promotes proliferation of human lung cancer cells by accelerating the epidermal growth factor receptor signaling pathway. Cancer Lett. 2009, 278, 27–33. [Google Scholar] [CrossRef]
- Tan, Z.; Chang, X.; Puga, A.; Xia, Y. Activation of mitogen-activated protein kinases (MAPKs) by aromatic hydrocarbons: Role in the regulation of aryl hydrocarbon receptor (AHR) function. Biochem. Pharmacol. 2002, 64, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Hecht, S.S. Approaches to chemoprevention of lung cancer based on carcinogens in tobacco smoke. Environ. Health Perspect. 1997, 105, 955–963. [Google Scholar] [CrossRef]
- Kasala, E.R.; Bodduluru, L.N.; Barua, C.C.; Sriram, C.S.; Gogoi, R. Benzo(a)pyrene induced lung cancer: Role of dietary phytochemicals in chemoprevention. Pharmacol. Rep. 2015, 67, 996–1009. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, C.; Yang, Z.; Zhang, G.; Wu, P.; Luo, Y.; Zeng, Q.; Wang, L.; Xue, Q.; Zhang, Y.; et al. m6A regulators as predictive biomarkers for chemotherapy benefit and potential therapeutic targets for overcoming chemotherapy resistance in small-cell lung cancer. J. Hematol. Oncol. 2021, 14, 190. [Google Scholar] [CrossRef]
- Quigley, M.; Brada, M.; Heron, C.; Horwich, A. Severe lung toxicity with a weekly low dose chemotherapy regimen in patients with non-Hodgkin’s lymphoma. Hematol. Oncol. 1988, 6, 319–324. [Google Scholar] [CrossRef]
- Vahid, B.; Marik, P.E. Pulmonary Complications of Novel Antineoplastic Agents for Solid Tumors. Chest 2008, 133, 528–538. [Google Scholar] [CrossRef]
- Polański, J.; Świątoniowska-Lonc, N.; Kołaczyńska, S.; Chabowski, M. Diet as a Factor Supporting Lung Cancer Treatment—A Systematic Review. Nutrients 2023, 15, 1477. [Google Scholar] [CrossRef] [PubMed]
- Furlan, V.; Bren, U. Protective Effects of [6]-Gingerol Against Chemical Carcinogens: Mechanistic Insights. Int. J. Mol. Sci. 2020, 21, 695. [Google Scholar] [CrossRef] [PubMed]
- Sp, N.; Kang, D.Y.; Lee, J.-M.; Bae, S.W.; Jang, K.-J. Potential Antitumor Effects of 6-Gingerol in p53-Dependent Mitochondrial Apoptosis and Inhibition of Tumor Sphere Formation in Breast Cancer Cells. Int. J. Mol. Sci. 2021, 22, 4660. [Google Scholar] [CrossRef] [PubMed]
- Wala, K.; Szlasa, W.; Sauer, N.; Kasperkiewicz-Wasilewska, P.; Szewczyk, A.; Saczko, J.; Rembialkowska, N.; Kulbacka, J.; Baczynsca, D. Anticancer Efficacy of 6-Gingerol with Paclitaxel against Wild Type of Human Breast Adenocarcinoma. Molecules 2022, 27, 2693. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, H.; Liu, T.; Yang, W.; Lv, W.; He, D.; Guo, P.; Li, L. 6-Gingerol induces cell-cycle G1-phase arrest through AKT-GSK 3β-cyclin D1 pathway in renal-cell carcinoma. Cancer Chemother. Pharmacol. 2020, 85, 379–390. [Google Scholar] [CrossRef]
- Zhang, H.; Kim, E.; Yi, J.; Hai, H.; Kim, H.; Park, S.; Lim, S.; Kim, S.; Jang, S.; Kim, K.; et al. [6]-Gingerol Suppresses Oral Cancer Cell Growth by Inducing the Activation of AMPK and Suppressing the AKT/mTOR Signaling Pathway. In Vivo 2021, 35, 3193–3201. [Google Scholar] [CrossRef]
- Khan, A.; Alhumaydhi, F.A.; Alwashmi, A.S.S.; Allemailem, K.S.; Alsahli, M.A.; Alrumaihi, F.A.; Almatroudi, A.; Mobark, M.A.; Mousa, A.; Khan, M.A. Diallyl Sulfide-Mediated Modulation of the Fatty Acid Synthase (FASN) Leads to Cancer Cell Death in BaP-Induced Lung Carcinogenesis in Swiss Mice. J. Inflamm. Res. 2020, 13, 1075–1087. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, N.; Mi, S. Plasma pharmacokinetics and tissue distribution of [6]-gingerol in rats. Biopharm. Drug Dispos. 2008, 29, 529–537. [Google Scholar] [CrossRef]
- Dymek, M.; Sikora, E. Liposomes as biocompatible and smart delivery systems—The current state. Adv. Colloid Interface Sci. 2022, 309, 102757. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Saindane, D.; Prajapati, B.G. Liposomal Drug Delivery and Its Potential Impact on Cancer Research. Anticancer Agents Med. Chem. 2022, 22, 2671–2683. [Google Scholar] [CrossRef]
- Fidan, Y.; Muçaj, S.; Timur, S.S.; Gürsoy, R.N. Recent advances in liposome-based targeted cancer therapy. J. Liposome Res. 2024, 34, 316–334. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.C.; Moreira, J.N.; Sousa Lobo, J.M. Current Insights on Lipid-Based Nanosystems 2023. Pharmaceuticals 2023, 16, 1700. [Google Scholar] [CrossRef]
- Tenchov, R.; Sasso, J.M.; Zhou, Q.A. PEGylated Lipid Nanoparticle Formulations: Immunological Safety and Efficiency Perspective. Bioconjug. Chem. 2023, 34, 941–960. [Google Scholar] [CrossRef]
- Khan, A.; Alsahli, M.A.; Aljasir, M.A.; Maswadeh, H.; Mobark, M.A.; Azam, F.; Allemailem, K.S.; Alrumaihi, F.; Alhumaydhi, F.A.; Alwashmi, A.S.S.; et al. Safety, Stability, and Therapeutic Efficacy of Long-Circulating TQ-Incorporated Liposomes: Implication in the Treatment of Lung Cancer. Pharmaceutics 2022, 14, 153. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Shukla, Y.; Kalra, N.; Alam, M.; Ahmad, M.G.; Hakim, S.R.; Owais, M. Potential of diallyl sulfide bearing pH-sensitive liposomes in chemoprevention against DMBA-induced skin papilloma. Mol. Med. 2007, 13, 443–451. [Google Scholar] [CrossRef]
- Alrumaihi, F.; Khan, M.A.; Babiker, A.Y.; Alsaweed, M.; Azam, F.; Allemailem, K.S.; Almatroudi, A.A.; Ahamad, S.R.; Alsuhaymi, N.; Alsugoor, M.H.; et al. The Effect of Liposomal Diallyl Disulfide and Oxaliplatin on Proliferation of Colorectal Cancer Cells: In Vitro and In Silico Analysis. Pharmaceutics 2022, 14, 236. [Google Scholar] [CrossRef]
- Alrumaihi, F.; Khan, M.A.; Babiker, A.Y.; Alsaweed, M.; Azam, F.; Allemailem, K.S.; Almatroudi, A.A.; Ahamad, S.R.; Alsugoor, M.H.; Alharbi, K.N.; et al. Lipid-Based Nanoparticle Formulation of Diallyl Trisulfide Chemosensitizes the Growth Inhibitory Activity of Doxorubicin in Colorectal Cancer Model: A Novel In Vitro, In Vivo and In Silico Analysis. Molecules 2022, 27, 2192. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Alsahli, M.A.; Aljasir, M.A.; Maswadeh, H.; Mobark, M.A.; Azam, F.; Allemailem, K.S.; Alrumaihi, F.; Alhumaydhi, F.A.; Almatroudi, A.A.; et al. Experimental and Theoretical Insights on Chemopreventive Effect of the Liposomal Thymoquinone Against Benzo[a]pyrene-Induced Lung Cancer in Swiss Albino Mice. J. Inflamm. Res. 2022, 15, 2263–2280. [Google Scholar] [CrossRef]
- Li, M.; Du, C.; Guo, N.; Teng, Y.; Meng, X.; Sun, H.; Li, S.; Yu, P.; Galons, H. Composition design and medical application of liposomes. Eur. J. Med. Chem. 2019, 164, 640–653. [Google Scholar] [CrossRef]
- Ahmed, K.S.; Hussein, S.A.; Ali, A.H.; Korma, S.A.; Lipeng, Q.; Jinghua, C. Liposome: Composition, characterisation, preparation, and recent innovation in clinical applications. J. Drug Target. 2019, 27, 742–761. [Google Scholar] [CrossRef]
- Anderson, M.; Omri, A. The effect of different lipid components on the in vitro stability and release kinetics of liposome formulations. Drug Deliv. 2008, 11, 33–39. [Google Scholar] [CrossRef]
- Crommelin, D.J. Influence of lipid composition and ionic strength on the physical stability of liposomes. J. Pharm. Sci. 1984, 73, 1559–1563. [Google Scholar] [CrossRef]
- Khan, A.; Aljarbou, A.N.; Aldebasi, Y.H.; Allemailem, K.S.; Alsahli, M.A.; Khan, S.; Alruwetei, A.M.; Khan, M.A. Fatty Acid Synthase (FASN) siRNA-Encapsulated-Her-2 Targeted Fab’-Immunoliposomes for Gene Silencing in Breast Cancer Cells. Int. J. Nanomed. 2020, 15, 5575–5589. [Google Scholar] [CrossRef] [PubMed]
- Briuglia, M.-L.; Rotella, C.; McFarlane, A.; Lamprou, D.A. Influence of cholesterol on liposome stability and on in vitro drug release. Drug Deliv. Transl. Res. 2015, 5, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Magarkar, A.; Dhawan, V.; Kallinteri, P.; Viitala, T.; Elmowafy, M.; Róg, T.; Bunker, A. Cholesterol level affects surface charge of lipid membranes in saline solution. Sci. Rep. 2014, 4, 5005. [Google Scholar] [CrossRef] [PubMed]
- Papahadjopoulos, D.; Cowden, M.; Kimelberg, H. Role of cholesterol in membranes. Effects on phospholipid-protein interactions, membrane permeability and enzymatic activity. Biochim. Biophys. Acta 1973, 330, 8–26. [Google Scholar] [CrossRef]
- Riaz, M.; Riaz, M.; Zhang, X.; Lin, C.; Wong, K.; Chen, X.; Zhang, G.; Lu, A.; Yang, Z. Surface Functionalization and Targeting Strategies of Liposomes in Solid Tumor Therapy: A Review. Int. J. Mol. Sci. 2018, 19, 195. [Google Scholar] [CrossRef]
- Liu, Y.; Castro Bravo, K.M.; Liu, J. Targeted liposomal drug delivery: A nanoscience and biophysical perspective. Nanoscale Horiz. 2021, 6, 78–94. [Google Scholar] [CrossRef]
- Sayan, M.; Ozkan, D.; Kankoc, A.; Tombul, I.; Celik, A.; Kurul, I.C.; Tastepe, A.I. IS GAMMA-GLUTAMYL TRANSFERASE A PROGNOSTIC INDICATOR FOR EARLY-STAGE LUNG CANCER TREATED SURGICALLY? Wiad. Lek. 2021, 74, 1804–1808. [Google Scholar] [CrossRef]
- Nikkhoo, B.; Sigari, N.; Ghaderi, B.; Afkhamzadeh, A.; Azadi, N.-A.; Mohsenpour, B.; Fathi, F.; Abdi, M. Diagnostic Utility of Adenosine Deaminase in Serum and Bronchoalveolar Lavage Fluid for Screening Lung Cancer in Western Iran. J. Med. Biochem. 2013, 32, 109–115. [Google Scholar] [CrossRef]
- Gao, Z.; Liu, C.; Yang, L.; Chen, H.; Yang, L.; Zhang, H.; Dong, K. CD73 Severed as a Potential Prognostic Marker and Promote Lung Cancer Cells Migration via Enhancing EMT Progression. Front. Genet. 2021, 12, 728200. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Hanai, J.; Ren, J.-G.; Kats, L.; Burgess, K.; Bhargava, P.; Signoretti, S.; Billiard, J.; Duffy, K.J.; Grant, A.; et al. Targeting Lactate Dehydrogenase-A Inhibits Tumorigenesis and Tumor Progression in Mouse Models of Lung Cancer and Impacts Tumor-Initiating Cells. Cell Metab. 2014, 19, 795–809. [Google Scholar] [CrossRef]
- Corti, A.; Franzini, M.; Paolicchi, A.; Pompella, A. Gamma-glutamyltransferase of Cancer Cells at the Crossroads of Tumor Progression, Drug Resistance and Drug Targeting. Anticancer Res. 2010, 30, 1169–1181. [Google Scholar] [PubMed]
- Kocianova, E.; Piatrikova, V.; Golias, T. Revisiting the Warburg Effect with Focus on Lactate. Cancers 2022, 14, 6028. [Google Scholar] [CrossRef]
- Jurisic, V.; Radenkovic, S.; Konjevic, G. The Actual Role of LDH as Tumor Marker, Biochemical and Clinical Aspects. In Advances in Cancer Biomarkers; Springer: Dordrecht, The Netherlands, 2015; pp. 115–124. [Google Scholar] [CrossRef]
- Lian, W.; Jiang, D.; Lin, W.; Jiang, M.; Zhang, Y.; Wang, H.; Zhao, L. Dual role of CD73 as a signaling molecule and adenosine-generating enzyme in colorectal cancer progression and immune evasion. Int. J. Biol. Sci. 2024, 20, 137–151. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug. Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, R.; Weng, S.; Xu, H.; Zhang, Y.; Chen, S.; Liu, S.; Ba, Y.; Zhou, Z.; Luo, P.; et al. Multifaceted role of redox pattern in the tumor immune microenvironment regarding autophagy and apoptosis. Mol. Cancer 2023, 22, 130. [Google Scholar] [CrossRef]
- Marengo, B.; Nitti, M.; Furfaro, A.L.; Colla, R.; Ciucis, C.; De Marinari, U.M.; Pronzato, M.A.; Traverso, N.; Domenicotti, C. Redox Homeostasis and Cellular Antioxidant Systems: Crucial Players in Cancer Growth and Therapy. Oxid. Med. Cell. Longev. 2016, 2016, 6235641. [Google Scholar] [CrossRef]
- Galadari, S.; Rahman, A.; Pallichankandy, S.; Thayyullathil, F. Reactive oxygen species and cancer paradox: To promote or to suppress? Free Radic. Biol. Med. 2017, 104, 144–164. [Google Scholar] [CrossRef]
- Wang, Y.; Qi, H.; Liu, Y.; Duan, C.; Liu, X.; Xia, T.; Chen, D.; Piao, H.; Liu, H. The double-edged roles of ROS in cancer prevention and therapy. Theranostics 2021, 11, 4839–4857. [Google Scholar] [CrossRef] [PubMed]
- Thangavelu, P.; Sundaram, V.; Gunasekaran, K.; Mujyambere, B.; Raju, S.; Kannan, A.; Arasu, A.; Krishna, K.; Ramamoorthi, J.; Ramasamy, S.; et al. Development of optimized novel liposome loaded with 6-gingerol and assessment of its therapeutic activity against NSCLC In vitro and In vivo experimental models. Chem. Phys. Lipids 2022, 245, 105206. [Google Scholar] [CrossRef] [PubMed]
- Alshaikh, F.; Al-Samydai, A.; Issa, R.; Alshaer, W.; Alqaraleh, M.; Al-Halaseh, L.K.; Alsanabarah, A.; Ghanim, B.Y.; Azzam, K.M.A.; Qinna, N.A. Encapsulation of gingerol into nanoliposomes: Evaluation of in vitro anti-inflammatory and anti-cancer activity. Biomed. Chromatogr. 2024, 38, e5899. [Google Scholar] [CrossRef] [PubMed]
- Cigarroa-Mayorga, O.E. Tuning the size stability of MnFe2O4 nanoparticles: Controlling the morphology and tailoring of surface properties under the hydrothermal synthesis for functionalization with myricetin. Ceram. Int. 2021, 47, 32397–32406. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alrumaihi, F.; Babiker, A.Y.; Khan, A. Lipid-Based Nanoformulations of [6]-Gingerol for the Chemoprevention of Benzo[a] Pyrene-Induced Lung Carcinogenesis: Preclinical Evidence. Pharmaceuticals 2025, 18, 574. https://doi.org/10.3390/ph18040574
Alrumaihi F, Babiker AY, Khan A. Lipid-Based Nanoformulations of [6]-Gingerol for the Chemoprevention of Benzo[a] Pyrene-Induced Lung Carcinogenesis: Preclinical Evidence. Pharmaceuticals. 2025; 18(4):574. https://doi.org/10.3390/ph18040574
Chicago/Turabian StyleAlrumaihi, Faris, Ali Yousif Babiker, and Arif Khan. 2025. "Lipid-Based Nanoformulations of [6]-Gingerol for the Chemoprevention of Benzo[a] Pyrene-Induced Lung Carcinogenesis: Preclinical Evidence" Pharmaceuticals 18, no. 4: 574. https://doi.org/10.3390/ph18040574
APA StyleAlrumaihi, F., Babiker, A. Y., & Khan, A. (2025). Lipid-Based Nanoformulations of [6]-Gingerol for the Chemoprevention of Benzo[a] Pyrene-Induced Lung Carcinogenesis: Preclinical Evidence. Pharmaceuticals, 18(4), 574. https://doi.org/10.3390/ph18040574