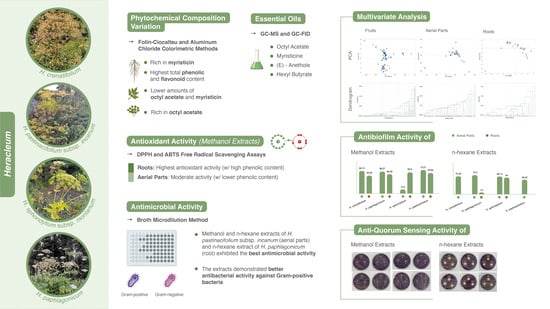
Bioactivities and Chemotaxonomy of Four Heracleum Species: A Comparative Study Across Plant Parts
Abstract
1. Introduction
2. Results and Discussion
2.1. Determination of Total Phenolic and Total Flavonoid Content
2.2. Essential Oils of Heracleum Genus
2.2.1. Essential Oils Derived from Fruits
2.2.2. Essential Oils Derived from Aerial Parts
2.2.3. Essential Oils Derived from Roots
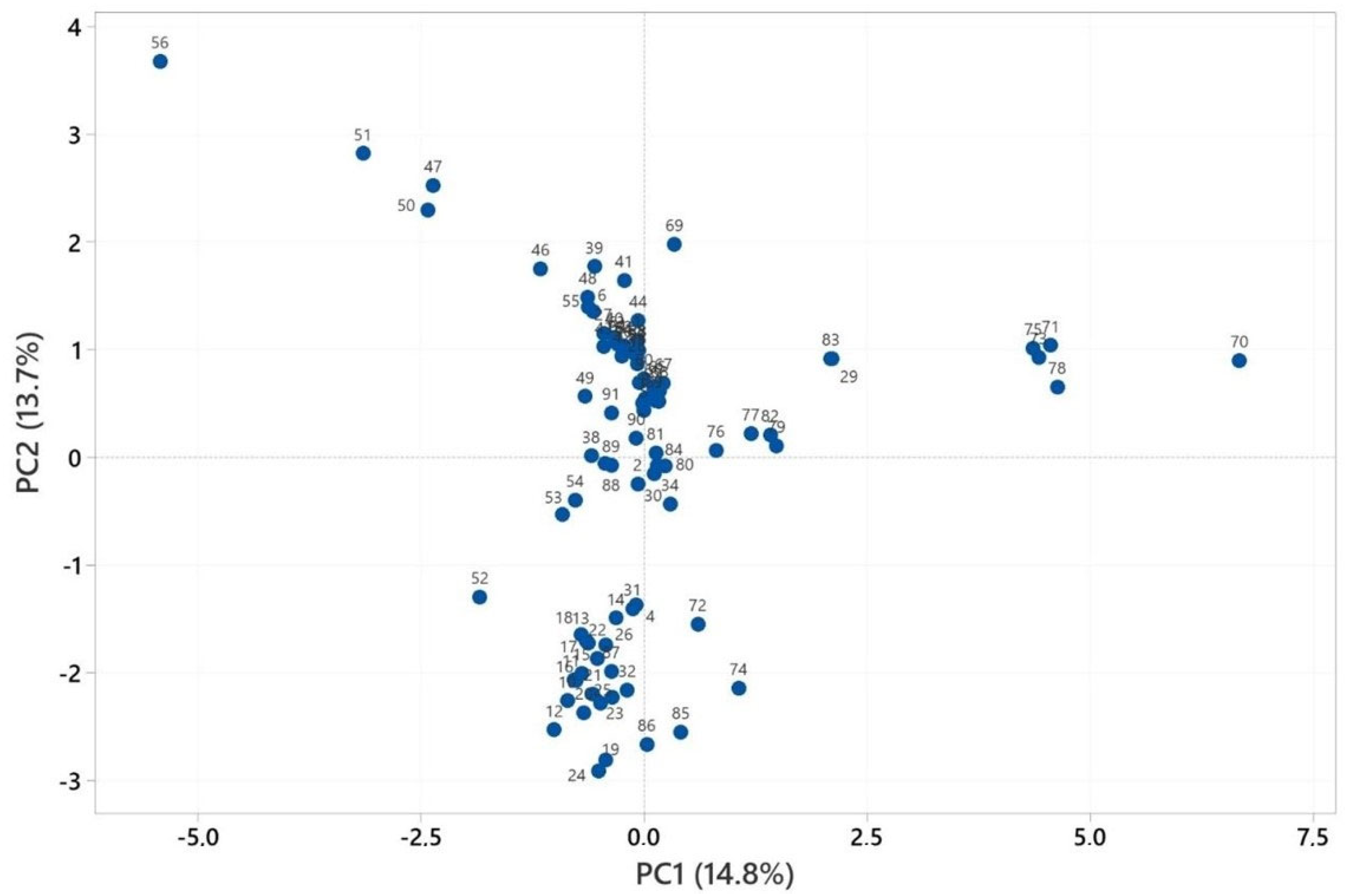
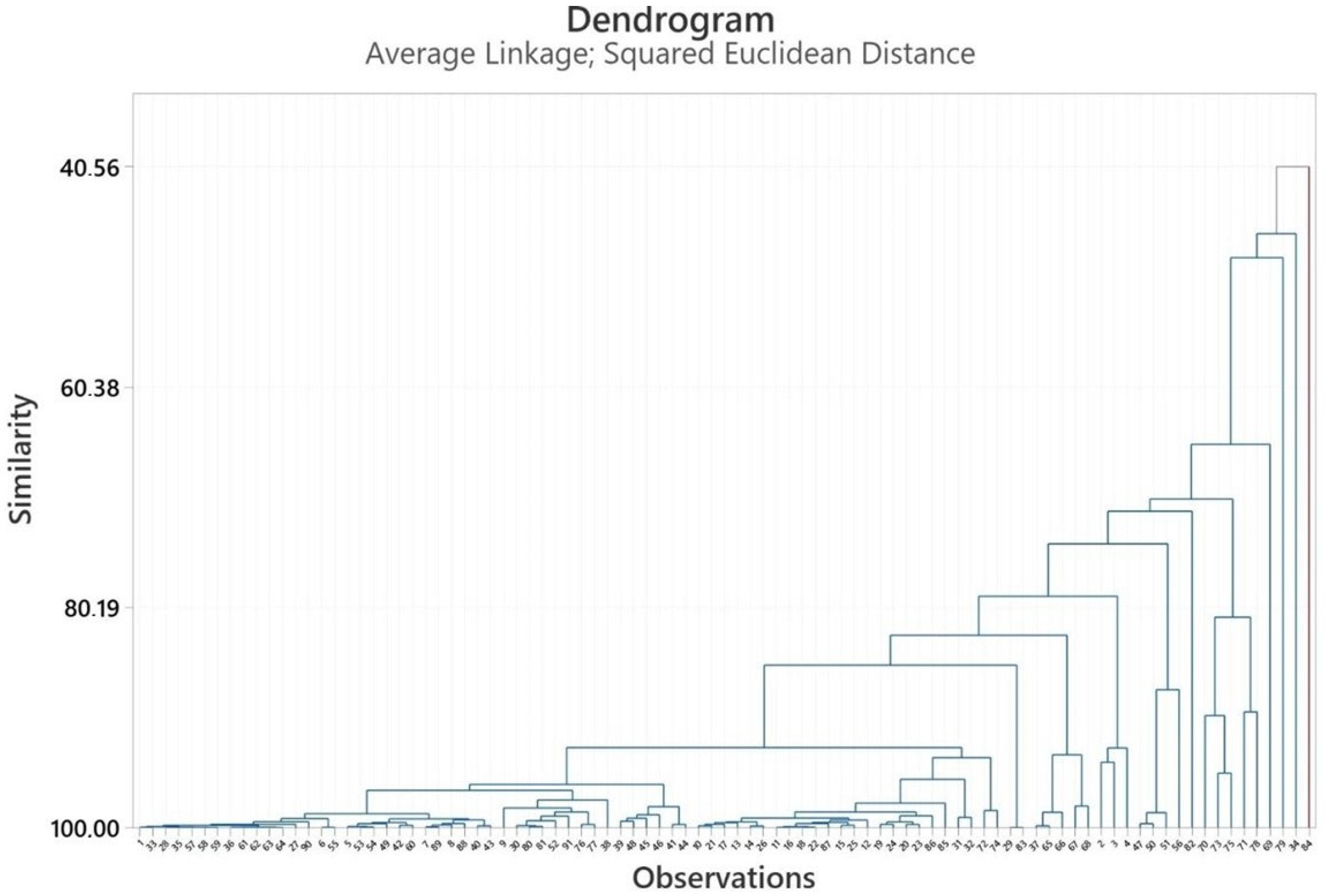
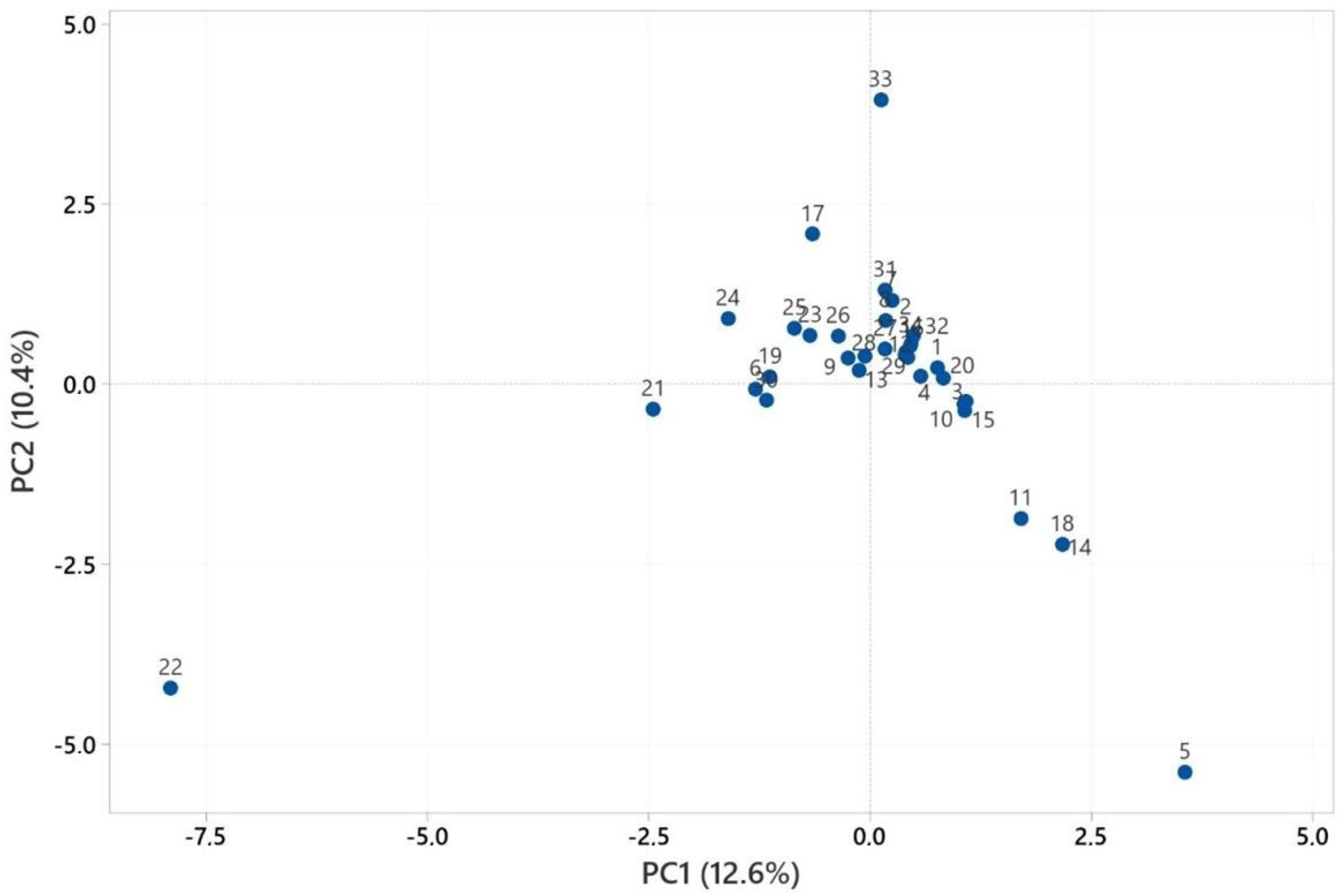
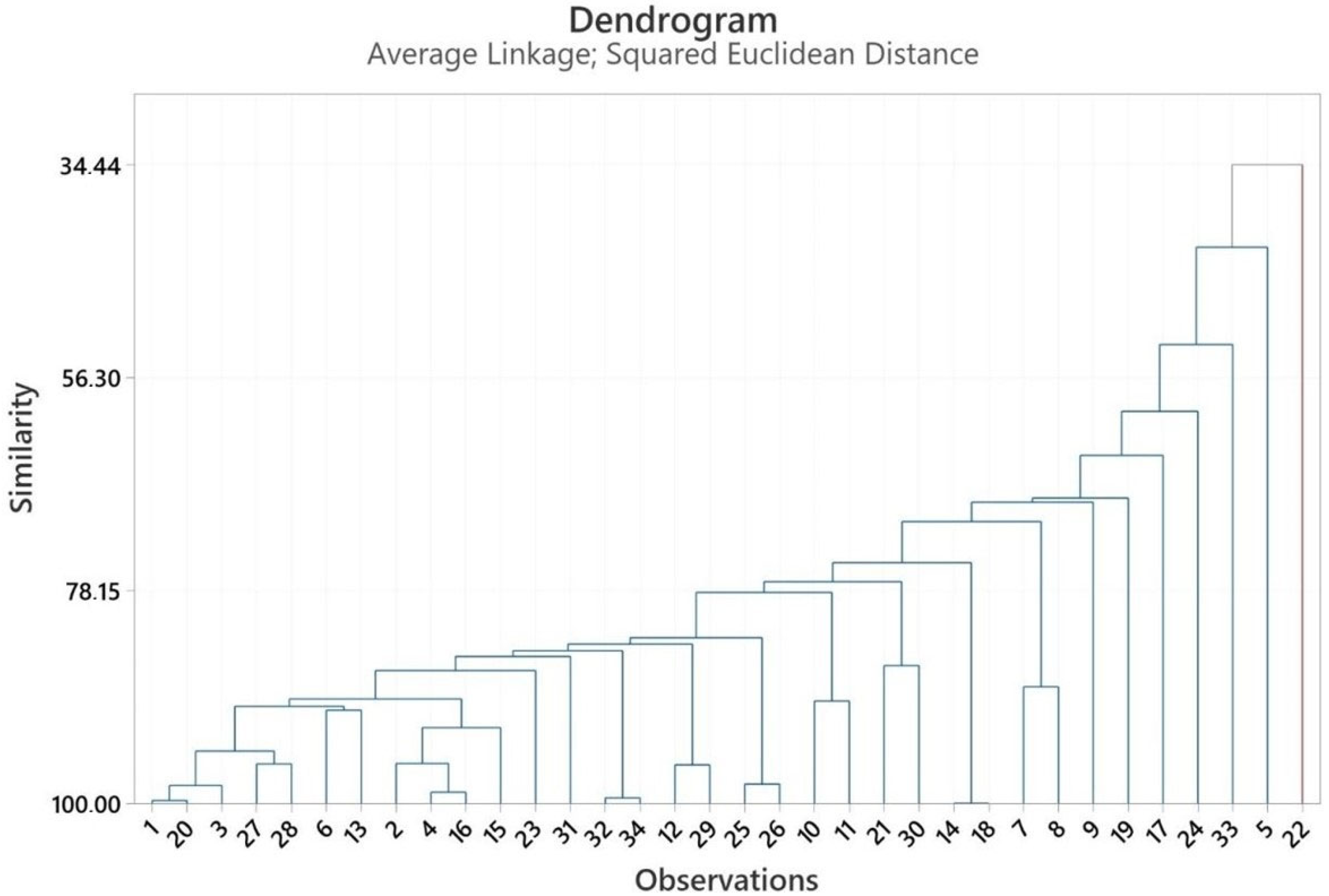
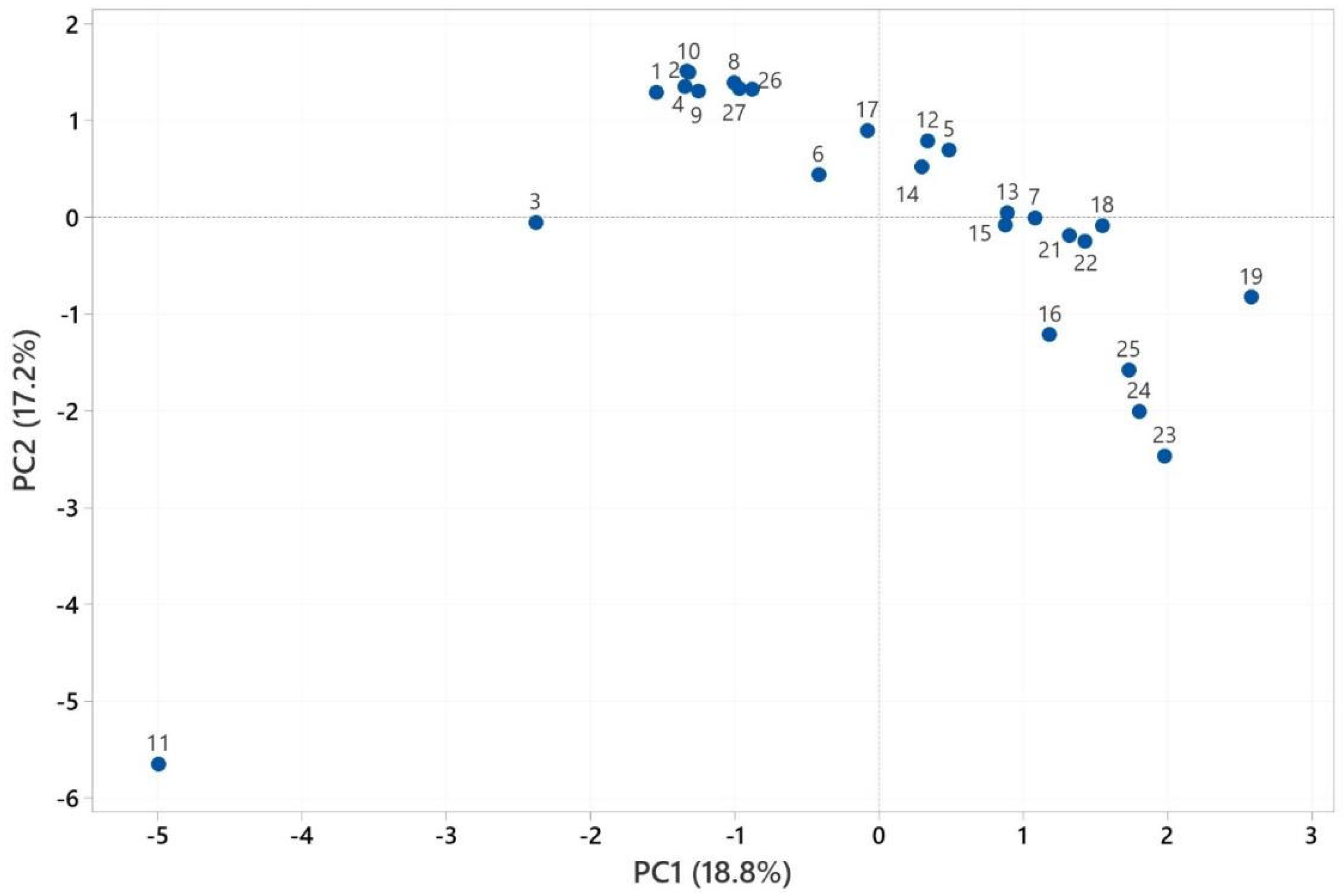
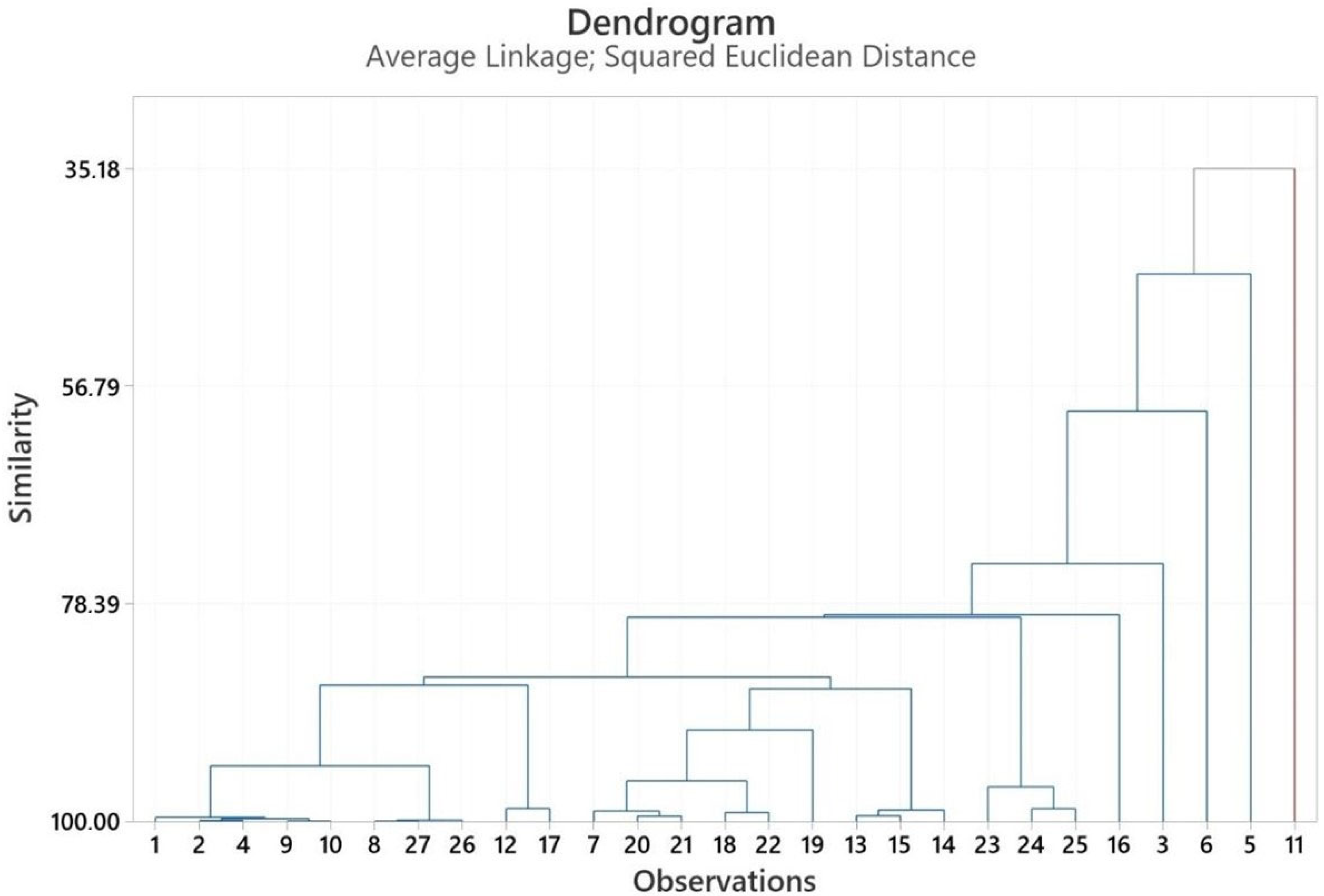
2.3. Multivariate Analysis of Heracleum Genus
2.4. Antioxidant Activity
2.5. Antimicrobial Activity
2.6. Antibiofilm Activity
2.7. Anti-Quorum Sensing Activity
3. Materials and Methods
3.1. Plant Material
3.2. Preparation of Extracts
3.2.1. Methanol Extract
3.2.2. n-Hexane Extract
3.3. Phytochemical Composition
3.3.1. Determination of Total Phenolic Compounds
3.3.2. Determination of Total Flavonoids
3.4. Essential Oils
3.4.1. Isolation of the Essential Oils
3.4.2. GC–MS Analysis
3.4.3. GC–FID Analysis
3.4.4. Identification of the Volatile Compounds
3.4.5. Statistical Analysis
3.5. Antioxidant Activity
3.5.1. DPPH Free Radical Scavenging Activity
3.5.2. ABTS Free Radical Scavenging Activity
3.6. Antimicrobial Activity
3.7. Antibiofilm Activity
3.8. Anti-Quorum Sensing Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Das, G.; Das, S.; Talukdar, A.D.; Venil, C.K.; Bose, S.; Banerjee, S.; Shin, H.-S.; Gutierrez-Grijalva, E.P.; Heredia, J.B.; Patra, J.K. Pharmacology and ethnomedicinal potential of selected plants species from Apiaceae (Umbelliferae). Comb. Chem. High. Throughput Screen. 2023, 26, 256–288. [Google Scholar] [PubMed]
- Al-Jaboury, K.R. Checklist of the Umbelliferae family in the herbarium of Iraq natural history museum. GSC Biol. Pharm. Sci. 2021, 15, 177–181. [Google Scholar]
- Kaval, U.; Tonçer, Ö. Apiaceae Lindley familyasına dâhil bitkilerin antibakteriyal, antifungal ve antiviral aktiviteleri üzerine bir derleme. Batman Univ. J. Life Sci. 2020, 10, 163–182. [Google Scholar]
- Singh, V.; Jain, D.K. Taxonomy of Angiosperms, 6th ed.; Rastogi Publications: Meerut, India, 2007; p. 298. [Google Scholar]
- Pimenov, M.G.; Leonov, M.V. The Asian Umbelliferae biodiversity database (ASIUM) with particular reference to South-West Asian taxa. Turk. J. Bot. 2004, 28, 139–145. [Google Scholar]
- Bakış, Y.; Babaç, M.T.; Uslu, E. Updates and improvements of Turkish Plants Data Service (TÜBİVES). In Proceedings of the 6th International Symposium on Health Informatics and Bioinformatics (HIBIT), İzmir, Türkiye, 2–5 May 2011. [Google Scholar]
- Güner, A.; Aslan, S.; Ekim, T.; Vural, M.; Babaç, M.T. Türkiye Bitkileri Listesi (Damarlı Bitkiler); Nezahat Gökyiğit Botanik Bahçesi ve Flora Araştırmaları Derneği Yayını: İstanbul, Türkiye, 2012. [Google Scholar]
- Gürbüz, P. Flavonoid glycosides from Heracleum pastinaca Fenzl. Turk. J. Pharm. Sci. 2019, 16, 191–195. [Google Scholar]
- Butola, J.S.; Samant, S.S.; Vashistha, R.K.; Malik, A.R. Propagation and cultivation techniques for Heracleum candicans Wall.: A himalayan medicinal resource in Peril. In Medicinal Plants and Sustainable Development; Nova Sciences Publishers, Inc.: New Delhi, India, 2011; pp. 65–76. [Google Scholar]
- Yazlık, A. Heracleum (Apiaceae) taksonlarının Türkiye’de dağılımı, çevresel ve sosyoekonomik etkileri ve önemi. KFBD 2021, 11, 544–556. [Google Scholar]
- Bizim Bitkiler. Available online: www.bizimbitkiler.org.tr (accessed on 7 December 2024).
- Güneş Özkan, N.; Yazlık, A.; Jabran, K. Düzce’de doğal olarak yayılış yapan Heracleum L. taksonları, habitatları ve bu habitatların floristik kompozisyonu. Eurasian J. Forest Sci. 2020, 8, 264–284. [Google Scholar]
- Habibi, Z.; Eshaghi, R.; Mohammadi, M.; Yousefi, M. Chemical composition and antibacterial activity of essential oil of Heracleum rechingeri Manden from Iran. Nat. Prod. Res. 2010, 24, 1013–1017. [Google Scholar]
- Hosseinzadeh, Z.; Ramazani, A.; Razzaghi-Asl, N. Plants of the genus Heracleum as a source of coumarin and furanocoumarin. J. Chem. Rev. 2019, 1, 78–98. [Google Scholar]
- Kuljanabhagavad, T.; Sriubolmas, N.; Ruangrungsi, N. Chemical composition and antimicrobial activity of essential oil from Heracleum siamicum. J. Health Res. 2010, 24, 55–60. [Google Scholar]
- Bahadori, M.B.; Dinparast, L.; Zengin, G. The genus Heracleum: A comprehensive review on its phytochemistry, pharmacology, and ethnobotanical values as a useful herb. Compr. Rev. Food Sci. Food Saf. 2016, 15, 1018–1039. [Google Scholar] [PubMed]
- Mazandarani, M.; Makari, S.; Bajian, G.R.; Moghaddam, P.Z.; Abrodi, M. Evaluation of phytochemical and antioxidant activity in different parts of Heracleum gorganicum Rech. F. in Golestan province of Iran. Iran. J. Plant Physiol. 2012, 2, 381–386. [Google Scholar]
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar]
- World Health Organization. Implementation of the Global Action Plan on Antimicrobial Resistance. 2017. Available online: https://www.who.int/publications/i/item/9789240074668 (accessed on 27 November 2024).
- Önder, A.; Rızvanoğlu, S.S.; Gündoğdu, E.F.; Demirci, B.; Eryılmaz, M. Chemical composition and antimicrobial, anti-biofilm, and anti-quorum sensing activities of Mentha longifolia subsp. typhoides essential oil. Plant Biosyst. 2024, 158, 1085–1092. [Google Scholar]
- Çiçek Polat, D.; Gümüşok, S.; Rızvanoğlu, S.S.; Eryılmaz, M. Bioactivities of Cotinus coggygria and its HPLC-DAD phenolic profiles. Plant Biosyst. 2023, 157, 1061–1066. [Google Scholar]
- Junejo, B.; Eryilmaz, M.; Rizvanoglu, S.S.; Palabiyik, I.M.; Ghumro, T.; Mallah, A.; Solangi, A.R.; Hyder, S.I.; Maleh, H.K.; Dragoi, E.N. Pharmacological assessment of Co3O4, CuO, NiO and ZnO nanoparticles via antibacterial, anti-biofilm and anti-quorum sensing activities. Water Sci. Technol. 2023, 87, 2840–2851. [Google Scholar]
- Koriem, K.M.M.; Asaad, G.F.; Megahed, H.A.; Zahran, H.; Arbid, M.S. Evaluation of the antihyperlipidemic, anti-inflammatory, analgesic, and antipyretic activities of ethanolic extract of Ammi majus Seeds in albino rats and mice. Int. J. Toxicol. 2012, 31, 294–300. [Google Scholar]
- Kumar, D.; Bhat, Z.A.; Kumar, V.; Shah, M.Y. Coumarins from Angelica archangelica Linn. and their effects on anxiety-like behavior. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 40, 180–186. [Google Scholar]
- Lino, C.; Taveira, M.; Viana, G.; Matos, F. Analgesic and antiinflammatory activities of Justicia pectoralis Jacq and its main constituents: Coumarin and umbelliferone. Phytother. Res. 1997, 11, 211–215. [Google Scholar]
- Chu, S.S.; Cao, J.; Liu, Q.Z.; Du, S.S.; Deng, Z.W.; Liu, Z.L. Chemical composition and insecticidal activity of Heracleum moellendorffii Hance essential oil. Chemija 2012, 23, 108–112. [Google Scholar]
- Chauhan, R.S.; Nautiyal, M.C.; Tava, A.; Cecotti, R. Essential oil composition from leaves of Heracleum candicans Wall.: A sustainable method for extraction. J. Essent. Oil Res. 2014, 26, 130–132. [Google Scholar]
- Uysal, A.; Özer, Ö.Y.; Zengin, G.; Stefanucci, A.; Mollica, A.; Picot-Allain, C.M.N.; Mahomoodally, M.F. Multifunctional approaches to provide potential pharmacophores for the pharmacy shelf: Heracleum sphondylium L. subsp. ternatum (Velen.) Brummitt. Comput. Biol. and Chem. 2019, 78, 64–73. [Google Scholar]
- Ocal, M.; Altunoglu, Y.C.; Angeloni, S.; Mustafa, A.M.; Caprioli, G.; Zengin, G.; Paksoy, M.Y.; Baloglu, M.C. Comparative content, biological and anticancer activities of Heracleum humile extracts obtained by ultrasound-assisted extraction method. Chem. Biodivers. 2022, 19, e202101040. [Google Scholar]
- Ghavam, M. Heracleum persicum Desf. ex Fisch., C.A. Mey. & Avé-Lall. fruit essential oil: Content, antimicrobial activity and cytotoxicity against ovarian cancer cell line. BMC Complement. Med. Ther. 2023, 23, 87. [Google Scholar]
- Wu, Z.; Zhong, A.; Shen, P.; Zhu, J.; Li, L.; Xia, G.; Zang, H. Chemical constituents, antioxidant and antibacterial activities of essential oil from the flowering aerial parts of Heracleum moellendorffii Hance. Cogent Food Agric. 2024, 10, 2325198. [Google Scholar]
- İşcan, G.; Demirci, F.; Kürkçüoǧlu, M.; Kıvanç, M.; Can Başer, K. The bioactive essential oil of Heracleum sphondylium L. subsp. ternatum (Velen.) Brummitt. Z. Für Naturforschung C 2003, 58, 195–200. [Google Scholar]
- Firuzi, O.; Asadollahi, M.; Gholami, M.; Javidnia, K. Composition and biological activities of essential oils from four Heracleum species. Food Chem. 2010, 122, 117–122. [Google Scholar]
- Matejic, J.S.; Dzamic, A.M.; Mihajilov-Krstev, T.; Ristic, M.S.; Randelovic, V.N.; Krisovej, Z.D.; Marin, P.D. Chemical composition, antioxidant and antimicrobial properties of essential oil and extracts from Heracleum sphondylium L. J. Essent. Oil Bear. Pl. 2016, 19, 944–953. [Google Scholar]
- Hajhashemi, V.; Sajjadi, S.E.; Heshmati, M. Anti-inflammatory and analgesic properties of Heracleum persicum essential oil and hydroalcoholic extract in animal models. J. Ethnopharmacol. 2009, 124, 475–480. [Google Scholar]
- Radjabian, T.; Salimi, A.; Rahmani, N.; Shockravi, A.; Mozaffarian, V. Essential oil composition of some wild populations of Heracleum persicum Desf. Ex Fischer growing in Iran. J. Essent. Oil Bear. Pl. 2013, 16, 841–849. [Google Scholar]
- Radjabian, T.; Salimi, A.; Rahmani, N. Essential-oil composition of the fruits of six Heracleum L. species from Iran: Chemotaxonomic significance. Chem. Biodivers. 2014, 11, 1945–1953. [Google Scholar]
- Usjak, L.J.; Drobac, M.M.; Niketić, M.S.; Petrović, S.D. Chemosystematic significance of essential oil constituents and furanocoumarins of underground parts and fruits of nine Heracleum L. taxa from Southeastern Europe. Chem. Biodivers. 2018, 15, e1800412. [Google Scholar]
- Jahodová, Š.; Trybush, S.; Pyšek, P.; Wade, M.; Karp, A. Invasive species of Heracleum in Europe: An insight into genetic relationships and invasion history. Divers. Distrib. 2007, 13, 99–114. [Google Scholar]
- Tkachenko, K.G. Essential oils from leaves of several Heracleum species growing in Leningrad oblast. Chem. Nat. Compd. 2010, 46, 319–321. [Google Scholar]
- Usjak, L.; Petrović, S.; Drobac, M.; Soković, M.; Stanojković, T.; Ćirić, A.; Niketić, M. Chemical composition and bioactivity of the essential oils of Heracleum pyrenaicum subsp. pollinianum and Heracleum orphanidis. Nat. Prod. Commun. 2016, 11, 1934578X1601100428. [Google Scholar]
- Tkachenko, K.G. Composition of the essential oils of Heracleum stevenii Manden. J. Essent. Oil Res. 1994, 6, 535–537. [Google Scholar]
- George, V.; Chacko, S.; Sethuraman, M.G. Chemical composition of the essential oil from the rhizomes of Heracleum candolleanum. J. Essent. Oil Res. 2001, 13, 80–81. [Google Scholar]
- Karuppusamy, S.; Muthuraja, G. Chemical composition and antioxidant activity of Heracleum sprengelianum (Wight and Arnott) essential oils growing wild in peninsular India. Iran. J. Pharm. Res. 2011, 10, 769. [Google Scholar]
- Fan, S.; Chang, J.; Zong, Y.; Hu, G.; Jia, J. GC-MS analysis of the composition of the essential oil from Dendranthema indicum var. aromaticum using three extraction methods and two columns. Molecules 2018, 23, 576. [Google Scholar]
- Özek, T.; Özek, G.; Baser, K.H.C.; Duran, A. Comparison of the essential oils of three endemic Turkish Heracleum species obtained by different isolation techniques. J. Essent. Oil Res. 2005, 17, 605–610. [Google Scholar]
- Fierascu, R.C.; Padure, I.M.; Avramecu, S.M.; Ungureanu, C.; Bunghez, R.I.; Ortan, A.; Dinu-pirvu, C.; Fierascu, I.; Soare, L.C. Preliminary assessment of the antioxidant, antifungal and germination inhibitory potential of Heracleum sphondylium L. (APIACEAE). Farmacia 2016, 64, 3. [Google Scholar]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [PubMed]
- Khameneh, B.; Eskin, N.A.M.; Iranshahy, M.; Fazly Bazzaz, B.S. Phytochemicals: A promising weapon in the Arsenal against antibiotic-resistant bacteria. Antibiotics 2021, 10, 1044. [Google Scholar] [CrossRef] [PubMed]
- Lobiuc, A.; Pavăl, N.-E.; Mangalagiu, I.I.; Gheorghiță, R.; Teliban, G.-C.; Amăriucăi-Mantu, D.; Stoleru, V. Future antimicrobials: Natural and functionalized phenolics. Molecules 2023, 28, 1114. [Google Scholar] [CrossRef] [PubMed]
- Biharee, A.; Sharma, A.; Kumar, A.; Jaitak, V. Antimicrobial flavonoids as a potential substitute for overcoming antimicrobial resistance. Fitoterapia 2020, 146, 104720. [Google Scholar]
- Nikaido, H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 2003, 67, 593–656. [Google Scholar]
- Miklasińska-Majdanik, M.; Kępa, M.; Wojtyczka, R.D.; Idzik, D.; Wąsik, T.J. Phenolic compounds diminish antibiotic resistance of Staphylococcus aureus clinical strains. Int. J. Environ. Res. Public Health 2018, 15, 2321. [Google Scholar] [CrossRef]
- Buruk, K.; Sokmen, A.; Aydin, F.; Erturk, M. Antimicrobial activity of some endemic plants growing in the Eastern Black Sea Region, Turkey. Fitoterapia 2006, 77, 388–391. [Google Scholar]
- Cushnie, T.P.; Lamb, A.J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar]
- Xie, Y.; Yang, W.; Tang, F.; Chen, X.; Ren, L. Antibacterial activities of flavonoids: Structure-activity relationship and mechanism. Curr. Med. Chem. 2015, 22, 132–149. [Google Scholar]
- Bouhrour, N.; Nibbering, P.H.; Bendali, F. Medical device-associated biofilm infections and multidrug-resistant pathogens. Pathogens 2024, 13, 393. [Google Scholar] [CrossRef] [PubMed]
- Büyüktuncel, E. Main spectrophotometric methods for the determination of total phenolic content and antioxidant capacity. Marmara Pharm. J. 2013, 17, 93–103. [Google Scholar]
- Demir, F.; Aliyazıcıoğlu, Y.; Demir, S. Asidifikasyon işleminin Laurocerasus officinalis ekstraktlarının antioksidan özellikleri üzerine etkisinin incelenmesi. El-Cezeri 2017, 4, 365–373. [Google Scholar]
- Keskin, S.; Sirin, Y.; Cakir, H.E.; Kaya, G.; Keskin, M. Phenolic composition and antioxidant properties of Spiraea nipponica. Int. J. Sci. Technol. Res. 2019, 5, 87–92. [Google Scholar]
- European Pharmacopoeia, 5th ed.; Council of Europe: Strasbourg, France, 2005; Volume 1, p. 217.
- Demirci, B.; Kırcı, D.; Öztürk, G.; Demirci, F. Effect of extraction time on Origanum onites L. infusions and essential oils—biological evaluation, statistical principal component and hierarchial cluster analyses. Chem. Biodivers. 2022, 19, e202200482. [Google Scholar]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar]
- CLSI Standard M07; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed. Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2018.
- CLSI Standard M27; Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, 4th ed. Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2017.
- Maggi, F.; Quassinti, L.; Bramucci, M.; Lupidi, G.; Petrelli, D.; Vitali, L.A.; Papa, F.; Vittori, S. Composition and biological activities of hogweed [Heracleum sphondylium L. subsp. ternatum (Velen.) Brummitt] essential oil and its main components octyl acetate and octyl butyrate. Nat. Prod. Res. 2014, 28, 1354–1363. [Google Scholar]
- Pirbalouti, A.G.; Sedaghat, L.; Hamedi, B.; Tirgir, F. Chemical composition and antioxidant activity of essential oils of three endemic medicinal plants of Iran. Bangladesh J. Bot. 2014, 42, 327–332. [Google Scholar]
- Akcin, A.; Seyis, F.; Akcin, T.A.; Cayci, Y.T.; Coban, A.Y. Chemical composition and antimicrobial activity of the essential oil of endemic Heracleum platytaenium Boiss. from Turkey. J. Essent. Oil Bear. Pl. 2013, 16, 166–171. [Google Scholar]
- Özkırım, A.; Keskin, N.; Kürkçüoğlu, M.; Başer, K.H.C. Evaluation of some essential oils as alternative antibiotics against American foulbrood agent Paenibacillus larvae on honey bees Apis mellifera L. J. Essent. Oil Res. 2012, 24, 465–470. [Google Scholar] [CrossRef]
- Kuljanabhagavad, T.; Sriubolmas, N.; Ruangrungsi, N. Chemical composition, antibacterial and antifungal activities of essential oil from Heracleum siamicum Craib. Pharm. Chem. J. 2011, 45, 178–182. [Google Scholar]
- Tosun, F.; Kızılay, Ç.A.; Erol, K.; Kılıç, F.S.; Kürkçüoğlu, M.; Başer, K.H.C. Anticonvulsant activity of furanocoumarins and the essential oil obtained from the fruits of Heracleum crenatifolium. Food Chem. 2008, 107, 990–993. [Google Scholar] [CrossRef]
- John, A.J.; Karunakaran, V.P.; George, V.; Sethuraman, M.G. Chemical composition of leaf and fruit oils of Heracleum candolleanum. J. Essent. Oil Res. 2007, 19, 358–359. [Google Scholar] [CrossRef]
- Iscan, G.; Ozek, T.; Ozek, G.; Duran, A.; Baser, K.H.C. Essential oils of three species of Heracleum. anticandidal activity. Chem. Nat. Compd. 2004, 40, 544–547. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Kürkçüoglu, M.; Adigüzel, N.; Aytaç, Z.; Joulain, D.; Laurent, R. Composition of the essential oil of Heracleum paphlagonicum Czeczott. J. Essent. Oil Res. 2000, 12, 385–386. [Google Scholar] [CrossRef]
- Mishyna, M.; Laman, N.; Prokhorov, V.; Maninang, J.S.; Fujii, Y. Identification of octanal as plant growth inhibitory volatile compound released from Heracleum sosnowskyi fruit. Nat. Prod. Commun. 2015, 10, 771–774. [Google Scholar] [CrossRef]
- Özdemir, F.G.G.; Tosun, B.; Şanlı, A.; Karadoğan, T. Bazı Apiaceae uçucu yağlarının Meloidogyne incognita (Kofoid & White, 1919) Chitwood, 1949 (Nematoda: Meloidogynidae)’ya karşı nematoksik etkisi. Ege Üniv Ziraat Fak Derg 2022, 59, 529–539. [Google Scholar]
- Hasheminya, S.M.; Dehghannya, J. Chemical composition, antioxidant, antibacterial, and antifungal properties of essential oil from wild Heracleum rawianum. Biocatal. Agric. Biotechnol. 2021, 31, 101913. [Google Scholar]
- Yılmaz, A.; Çiftçi, V. Determination of essential oil composition of naturally growing Heracleum platytaenium L. plant in Bolu ecological conditions. ISPEC J. Agric. Sci. 2021, 5, 344–349. [Google Scholar]
- Shokri, H.; Sharifzadeh, A.; Tamai, I.A. Anti-Candida zeylanoides activity of some Iranian plants used in traditional medicine. J. Mycol. Med. 2012, 22, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Guleria, S.; Saini, R.; Jaitak, V.; Kaul, V.K.; Lal, B.; Rahi, P.; Gulati, A.; Singh, B. Composition and antimicrobial activity of the essential oil of Heracleum thomsonii (Clarke) from the cold desert of the western Himalayas. Nat. Prod. Res. 2011, 25, 1250–1260. [Google Scholar] [CrossRef] [PubMed]
- Li Wei, L.W.; Chen LinLin, C.L.; Wu Chun, W.C.; Xin JiaYing, X.J. Analysis of the essential oil from seed of Heracleum moellendorffii Hance cultivated in northeast China. Asian J. Chem. 2013, 25, 4701–4702. [Google Scholar] [CrossRef]
- Miladinović, D.L.; Ilić, B.S.; Mihajilov-Krstev, T.M.; Nikolić, D.M.; Cvetković, O.G.; Marković, M.S.; Miladinović, L.C. Antibacterial activity of the essential oil of Heracleum sibiricum. Nat. Prod. Commun. 2013, 8, 1309–1311. [Google Scholar] [CrossRef]
- Torbati, M.; Nazemiyeh, H.; Lotfipour, F.; Asnaashari, S.; Nemati, M.; Fathiazad, F. Composition and antibacterial activity of Heracleum transcaucasicum and Heracleum anisactis aerial parts essential oil. Adv. Pharm. Bull. 2013, 3, 415–418. [Google Scholar]
- Papageorgiou, V.P.; Ochir, G.; Motl, O.; Argyriadou, N.; Dunkel, H. Composition of the essential oil from Heracleum dissectum. J. Nat. Prod. 1985, 48, 851–853. [Google Scholar] [CrossRef]
- Sefidkon, F.; Dabiri, M.; Mohammad, N. Analysis of the oil of Heracleum persicum L. (leaves and flowers). J. Essent. Oil Res. 2002, 14, 295–297. [Google Scholar] [CrossRef]


| Plant Extracts | DPPH Radical Scavenging Activity (IC50 Value (mg/mL) ± SD b) | ABTS Free Radical Scavenging Activity (TEAC a mg TE/g Extract ± SD b) | |
|---|---|---|---|
| H. crenatifolium | aerial parts | 0.216 ± 0.0007 | 155.622 ± 3.47 |
| roots | 0.765 ± 0.015 | 143.574 ± 3.47 | |
| H. paphlagonicum | aerial parts | 0.275 ± 0.001 | 157.63 ± 3.47 |
| roots | 0.242 ± 0.004 | 199.799 ± 6.95 | |
| H. sphondylium subsp. montanum | aerial parts | 0.246 ± 0.002 | 115.461 ± 6.95 |
| roots | 0.416 ± 0.004 | 282.128 ± 6.95 | |
| H. pastinacifolium subsp. incanum | aerial parts | 1.077 ± 0.007 | 79.317 ± 3.47 |
| roots | 0.77 ± 0.003 | 135.542 ± 6.024 | |
| Gallic acid | 0.001 ± 0.0001 | - | |
| Plant Extracts | Gram-Positive Bacteria | Gram-Negative Bacteria | Fungus | ||||
|---|---|---|---|---|---|---|---|
| S. aureus | S. aureus (MRSA) | E. coli | K. pneumoniae | P. aeruginosa | C. albicans | ||
| Aerial parts (Methanol) | H. crenatifolium | 1.25 | 1.25 | - | - | - | - |
| H. paphlagonicum | 1.25 | 1.25 | - | - | - | - | |
| H. sphondylium subsp. montanum | 1.25 | 1.25 | - | - | - | - | |
| H. pastinacifolium subsp. incanum | 1.25 | 0.625 | 5 | - | - | 5 | |
| Roots (Methanol) | H. crenatifolium | 2.5 | 2.5 | - | - | - | 10 |
| H. paphlagonicum | 1.25 | 1.25 | - | - | - | 10 | |
| H. sphondylium subsp. montanum | 1.25 | 1.25 | 10 | - | - | 5 | |
| H. pastinacifolium subsp. incanum | 2.5 | 2.5 | - | - | - | 5 | |
| Aerial parts (n-Hexane) | H. crenatifolium | - | - | - | - | - | - |
| H. paphlagonicum | 1.25 | 1.25 | - | - | - | - | |
| H. sphondylium subsp. montanum | 1.25 | 1.25 | - | - | - | - | |
| H. pastinacifolium subsp. incanum | 0.625 | 0.625 | - | - | - | 10 | |
| Roots (n-Hexane) | H. paphlagonicum | 0.625 | 0.625 | - | - | - | - |
| H. sphondylium subsp. montanum | 1.25 | 5 | - | - | - | - | |
| Ciprofloxacin | <0.00025 | 0.0005 | <0.00025 | <0.00025 | <0.00025 | NT | |
| Gentamicin | 0.0005 | <0.00025 | 0.0005 | <0.00025 | 0.0005 | NT | |
| Fluconazole | NT | NT | NT | NT | NT | 0.00156 | |
| 5% DMSO | - | - | - | - | - | - | |
| Plant | Locality | Date of Collection | Herbarium Number |
|---|---|---|---|
| H. crenatifolium | Konya; Hadim 810–820 m 37.0282, 32.6936 | 15.07.2023 | AEF 31001 |
| H. paphlagonicum | Kastamonu; Ilgaz Dağı 1800–1850 m 41.0772, 33.7299 | 22.07.2023 | AEF 30999 |
| H. sphondylium subsp. montanum | Ankara; Çamlıdere 1100–1150 m 40.5436, 32.5636 | 23.07.2023 | AEF 31000 |
| H. pastinacifolium subsp. incanum | Karabük; Keltepe 1950–2000 m 41.0866, 32.4623 | 05.08.2023 | AEF 30998 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kose, T.I.; Yardimci, G.B.; Kirci, D.; Polat, D.C.; Demirci, B.; Eryilmaz, M.; Kilic, C.S. Bioactivities and Chemotaxonomy of Four Heracleum Species: A Comparative Study Across Plant Parts. Pharmaceuticals 2025, 18, 576. https://doi.org/10.3390/ph18040576
Kose TI, Yardimci GB, Kirci D, Polat DC, Demirci B, Eryilmaz M, Kilic CS. Bioactivities and Chemotaxonomy of Four Heracleum Species: A Comparative Study Across Plant Parts. Pharmaceuticals. 2025; 18(4):576. https://doi.org/10.3390/ph18040576
Chicago/Turabian StyleKose, Tugce Ince, Gamze Benli Yardimci, Damla Kirci, Derya Cicek Polat, Betul Demirci, Mujde Eryilmaz, and Ceyda Sibel Kilic. 2025. "Bioactivities and Chemotaxonomy of Four Heracleum Species: A Comparative Study Across Plant Parts" Pharmaceuticals 18, no. 4: 576. https://doi.org/10.3390/ph18040576
APA StyleKose, T. I., Yardimci, G. B., Kirci, D., Polat, D. C., Demirci, B., Eryilmaz, M., & Kilic, C. S. (2025). Bioactivities and Chemotaxonomy of Four Heracleum Species: A Comparative Study Across Plant Parts. Pharmaceuticals, 18(4), 576. https://doi.org/10.3390/ph18040576