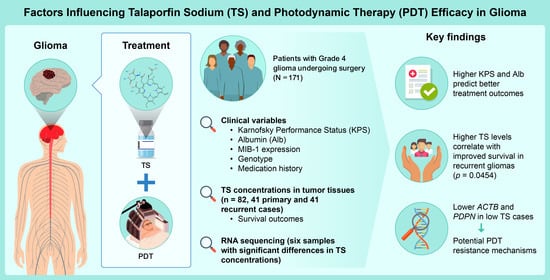
Prognostic Factors and Talaporfin Sodium Concentration in Photodynamic Therapy for Recurrent Grade 4 Glioma
Abstract
1. Introduction
2. Results
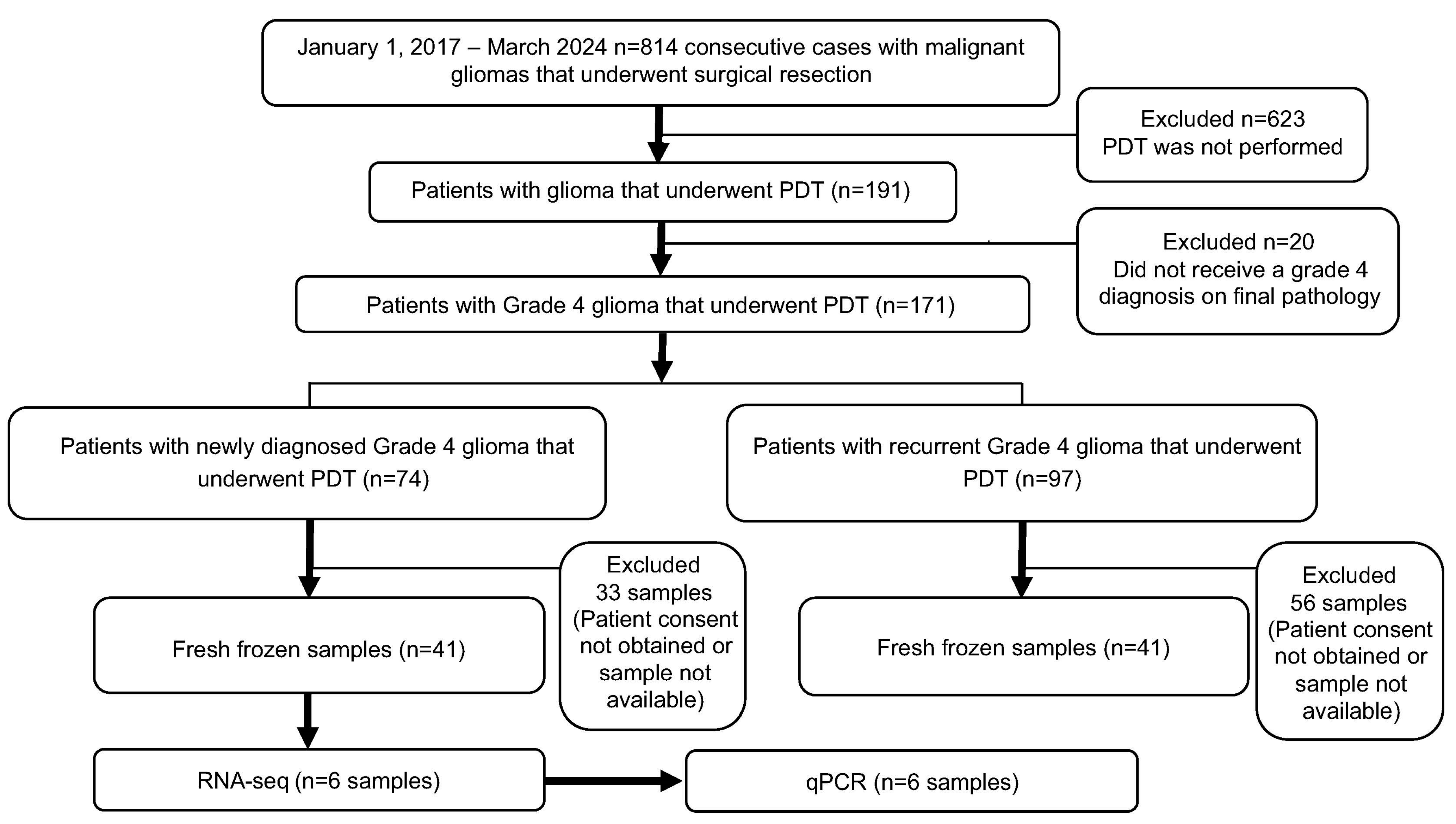
2.1. Clinical Characteristics of Patients Who Underwent PDT
2.2. Identification of Risk Factors in Patients Who Underwent PDT
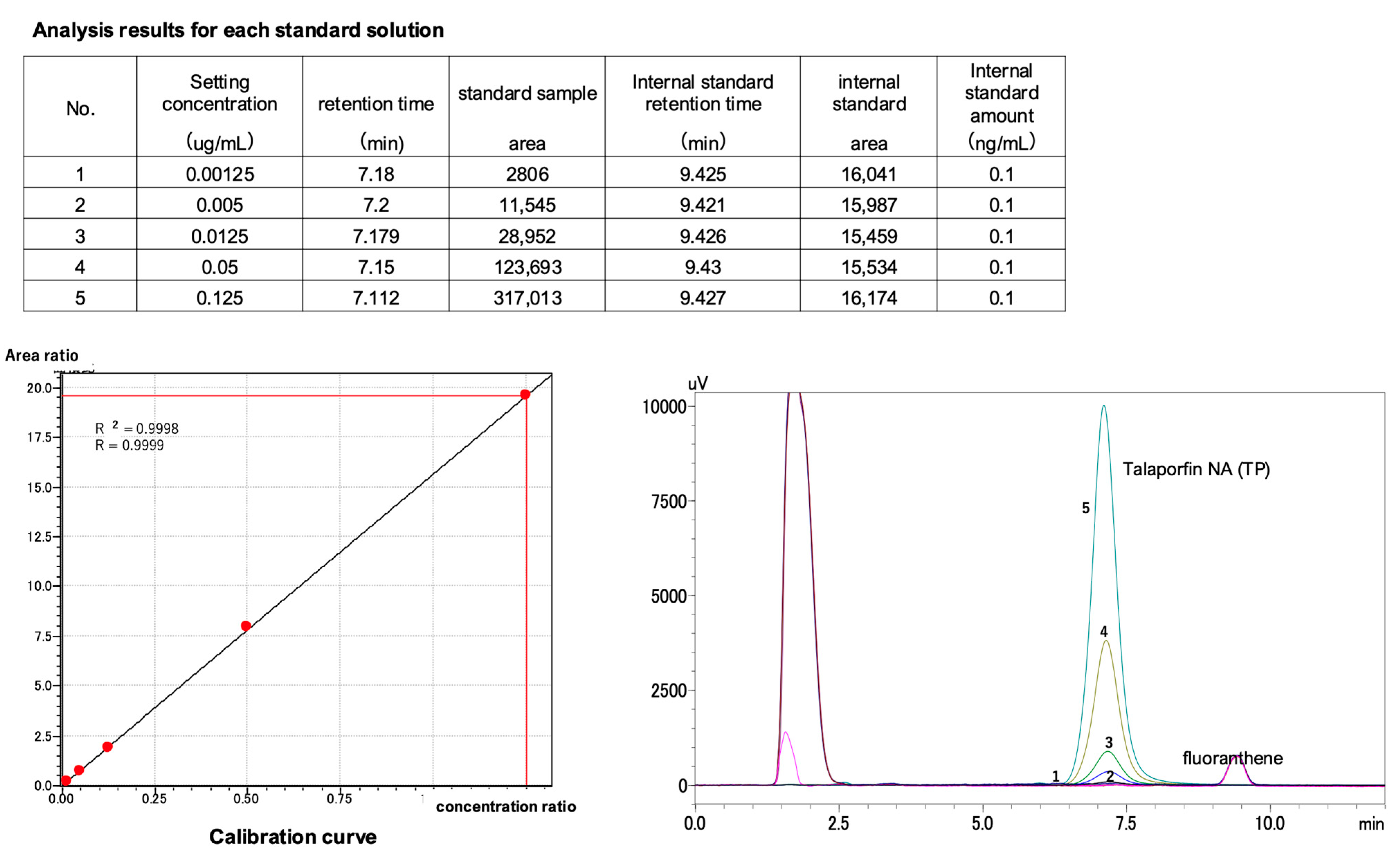
2.3. Calibration Curve for TS Concentration Measurement Using HPLC (Internal Standard Method)
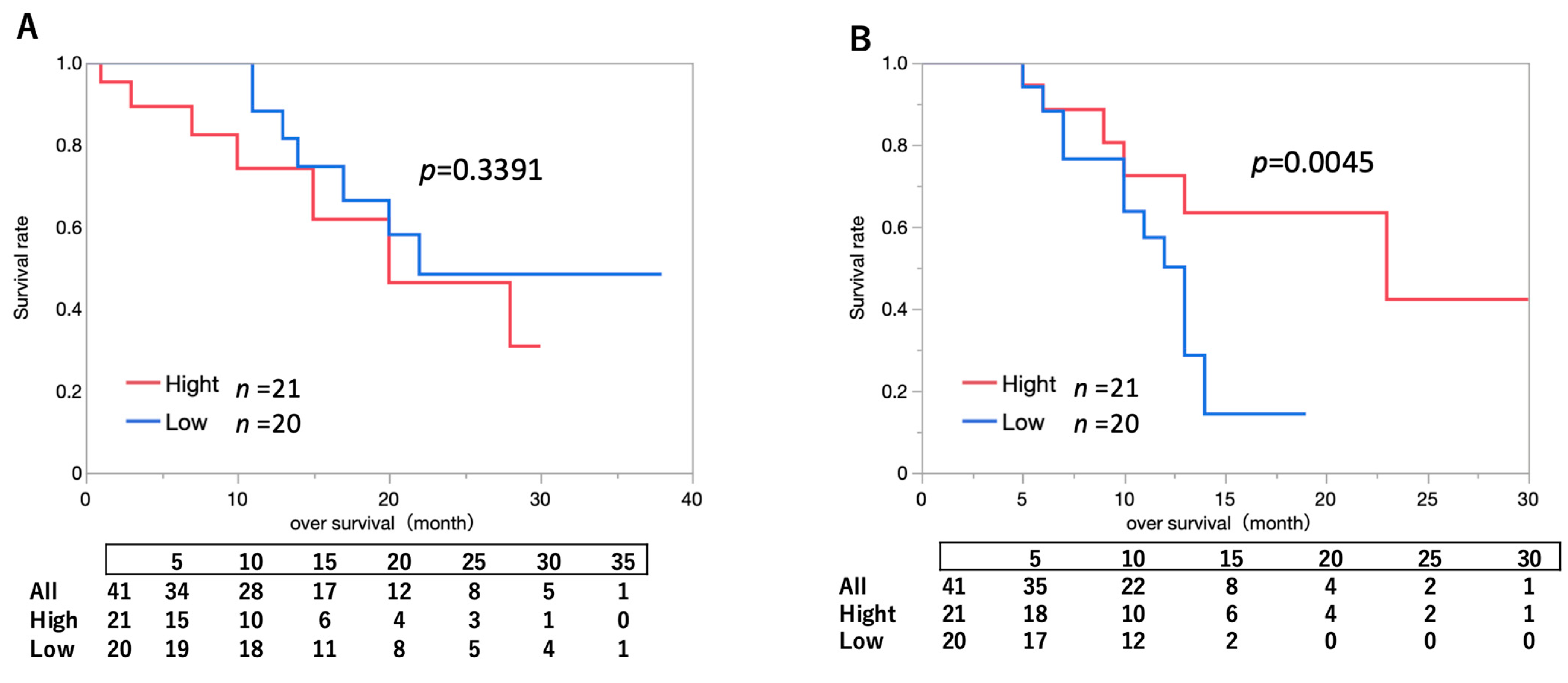
2.4. Impact of Intratumoral Drug Concentration on Survival Outcomes in Patients Who Underwent PDT
2.5. Analysis of Clinical Factors in the High vs. Low Intratumoral TS Concentration Groups
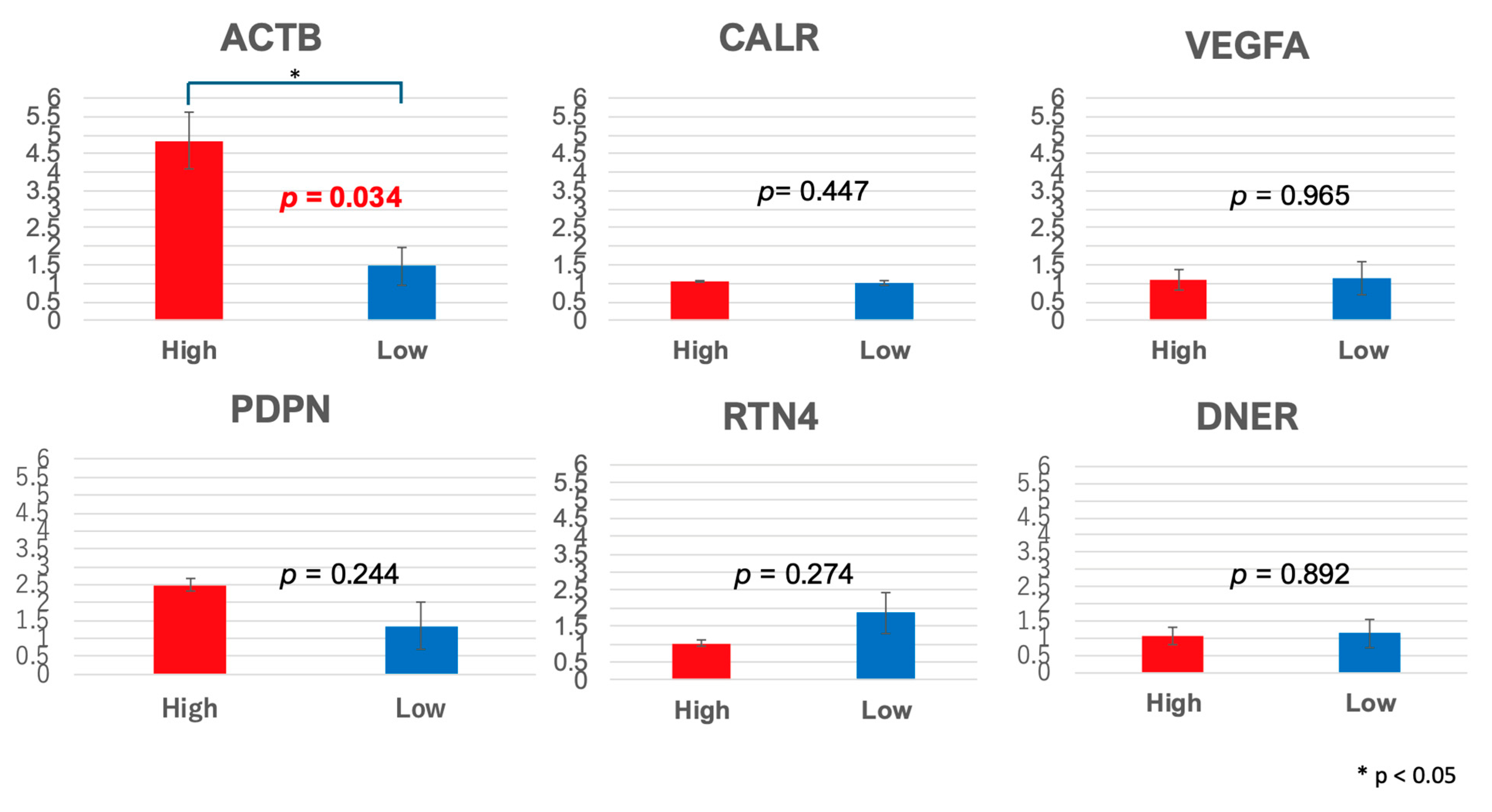
2.6. RNA-Seq Analysis Identified Differential Gene Expression Associated with TS Concentration
3. Discussion
3.1. Main Results
3.2. Factors Influencing the Effectiveness of TS
3.2.1. Prognostic Factors
3.2.2. Relationship Between Intratumoral TS Concentration and Survival
3.2.3. Drug Uptake and Intracellular Dynamics in PDT
3.2.4. Gene Expression Analysis
3.3. Summary
3.4. Limitations
4. Materials and Methods
4.1. Patient Selection
4.2. Data Collection
4.2.1. Risk Factor Analysis
4.2.2. Relationship Between Intratumoral TS Concentration and Survival
4.3. PDT
4.3.1. Photosensitizer: TS
4.3.2. Intraoperative TS-PDT Protocol
4.4. Measurement of TS Concentration
4.4.1. Sample Preparation
4.4.2. High-Performance Liquid Chromatography (HPLC) and Settings
4.4.3. Calibration Curve
4.5. RNA-seq, RNA Extraction, and Library Preparation
4.6. Real-Time PCR
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BBB | Blood–Brain Barrier |
| GBM | Glioblastoma |
| HPLC | High-Performance Liquid Chromatography |
| HpD | Hematoporphyrin Derivative |
| KPS | Karnofsky Performance Status |
| LDL | Low-Density Lipoprotein |
| OS | Overall Survival |
| PBR | Peripheral Benzodiazepine Receptor |
| PDT | Photodynamic Therapy |
| qPCR | Quantitative PCR |
| ROS | Reactive Oxygen Species |
| TS | Talaporfin Sodium |
References
- Ostrom, Q.T.; Gittleman, H.; Liao, P.; Rouse, C.; Chen, Y.; Dowling, J.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro-Oncology 2014, 16 (Suppl. 4), iv1–iv63. [Google Scholar] [CrossRef]
- Thakkar, J.P.; Dolecek, T.A.; Horbinski, C.; Ostrom, Q.T.; Lightner, D.D.; Barnholtz-Sloan, J.S.; Villano, J.L. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1985–1996. [Google Scholar] [CrossRef]
- Scott, J.G.; Bauchet, L.; Fraum, T.J.; Nayak, L.; Cooper, A.R.; Chao, S.T.; Suh, J.H.; Vogelbaum, M.A.; Peereboom, D.M.; Zouaoui, S.; et al. Recursive partitioning analysis of prognostic factors for glioblastoma patients aged 70 years or older. Cancer 2012, 118, 5595–5600. [Google Scholar] [CrossRef] [PubMed]
- Young, R.M.; Jamshidi, A.; Davis, G.; Sherman, J.H. Current trends in the surgical management and treatment of adult glioblastoma. Ann. Transl. Med. 2015, 3, 121. [Google Scholar] [PubMed]
- Chen, L.; Mao, Y. Gross total resection plays a leading role in survival of patients with glioblastoma multiforme. World Neurosurg. 2014, 82, e105–e107. [Google Scholar] [CrossRef]
- Muragaki, Y.; Akimoto, J.; Maruyama, T.; Iseki, H.; Ikuta, S.; Nitta, M.; Maebayashi, K.; Saito, T.; Okada, Y.; Kaneko, S.; et al. Phase II clinical study on intraoperative photodynamic therapy with talaporfin sodium and semiconductor laser in patients with malignant brain tumors. J. Neurosurg. 2013, 119, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Akimoto, J. Photodynamic therapy for malignant brain tumors. Neurol. Med. Chir. 2016, 56, 151–157. [Google Scholar] [CrossRef]
- Nitta, M.; Muragaki, Y.; Maruyama, T.; Iseki, H.; Komori, T.; Ikuta, S.; Saito, T.; Yasuda, T.; Hosono, J.; Okamoto, S.; et al. Role of photodynamic therapy using talaporfin sodium and a semiconductor laser in patients with newly diagnosed glioblastoma. J. Neurol. Surg. 2018, 131, 1361–1368. [Google Scholar] [CrossRef]
- Bellnier, D.A.; Ho, Y.K.; Pandey, R.K.; Missert, J.R.; Dougherty, T.J. Distribution and elimination of Photofrin II in mice. Photochem. Photobiol. 1989, 50, 221–228. [Google Scholar] [CrossRef]
- Perria, C.; Capuzzo, T.; Cavagnaro, G.; Datti, R.; Francaviglia, N.; Rivano, C.; Tercero, V.E. Fast attempts at the photodynamic treatment of human gliomas. J. Neurosurg. Sci. 1980, 24, 119–129. [Google Scholar]
- Eljamel, M.S. Brain photodiagnosis (PD), fluorescence guided resection (FGR) and photodynamic therapy (PDT): Past, present and future. Photodiagn. Photodyn. Ther. 2008, 5, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W.; Novotny, A.; Stepp, H.; Goetz, C.; Bise, K.; Reulen, H.J. Fluorescence-guided resection of glioblastoma multiforme by using 5-aminolevulinic acid-induced porphyrins: A prospective study in 52 consecutive patients. J. Neurosurg. 2000, 93, 1003–1013. [Google Scholar] [CrossRef]
- Usuda, J.; Tsutsui, H.; Honda, H.; Ichinose, S.; Ishizumi, T.; Hirata, T.; Inoue, T.; Ohtani, K.; Maehara, S.; Imai, K.; et al. Photodynamic therapy for lung cancers based on novel photodynamic diagnosis using talaporfin sodium (NPe6) and autofluorescence bronchoscopy. Lung Cancer 2007, 58, 317–323. [Google Scholar] [CrossRef]
- Delgado-López, P.D.; Corrales-García, E.M. Survival in glioblastoma: A review on the impact of treatment modalities. Clin. Transl. Oncol. 2016, 18, 1062–1071. [Google Scholar] [CrossRef]
- Johansson, A.; Faber, F.; Kniebühler, G.; Stepp, H.; Sroka, R.; Egensperger, R.; Beyer, W.; Kreth, F.W. Protoporphyrin IX fluorescence and photobleaching during interstitial photodynamic therapy of malignant gliomas for early treatment prognosis. Lasers Surg. Med. 2013, 45, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Kessel, D. Pharmacokinetics of N-aspartyl chlorin e6 in cancer patients. J. Photochem. Photobiol. B 1997, 39, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Kessel, D. Interactions between N-aspartyl chlorin e6, detergent micelles and plasma lipoproteins. Photochem. Photobiol. 1995, 61, 646–649. [Google Scholar] [CrossRef]
- Ogawa, E.; Motohashi, S.; Ito, A.; Arai, T. Effects of albumin binding on photocytotoxicity of extracellular photosensitization reaction using talaporfin sodium to rat myocardial cells. Photodiagn. Photodyn. Ther. 2015, 12, 252–257. [Google Scholar] [CrossRef]
- Kobayashi, T.; Nitta, M.; Shimizu, K.; Saito, T.; Tsuzuki, S.; Fukui, A.; Koriyama, S.; Kuwano, A.; Komori, T.; Masui, K.; et al. Therapeutic options for recurrent glioblastoma-efficacy of talaporfin sodium mediated photodynamic therapy. Pharmaceutics 2022, 14, 353. [Google Scholar] [CrossRef]
- Gupta, S.; Dwarakanath, B.S.; Muralidhar, K.; Jain, V. Cellular uptake, localization and photodynamic effects of haematoporphyrin derivative in human glioma and squamous carcinoma cell lines. J. Photochem. Photobiol. B 2003, 69, 107–120. [Google Scholar] [CrossRef]
- Woodburn, K.W.; Vardaxis, N.J.; Hill, J.S.; Kaye, A.H.; Reiss, J.A.; Phillips, D.R. Evaluation of porphyrin characteristics required for photodynamic therapy. Photochem. Photobiol. 1992, 55, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The blood–brain barrier and blood-tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 2020, 20, 26–41. [Google Scholar] [CrossRef]
- Ou, A.; Yung, W.K.A.; Majd, N. Molecular mechanisms of treatment resistance in glioblastoma. Int. J. Mol. Sci. 2020, 22, 351. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Facchina, S.L.; Hirsch, D.J.; Song, S.Y.; Dillahey, L.F.; Williams, J.R.; Snyder, S.H. Photodynamic tumor therapy: Mitochondrial benzodiazepine receptors as a therapeutic target. Mol. Med. 1998, 4, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Nitta, M.; Komori, T.; Maruyama, T.; Yasuda, T.; Fujii, Y.; Masamune, K.; Kawamata, T.; Maehara, T.; Muragaki, Y. Intraoperative photodynamic diagnosis using talaporfin sodium simultaneously applied for photodynamic therapy against malignant glioma: A prospective clinical study. Front. Neurol. 2018, 9, 24. [Google Scholar] [CrossRef]
- Ishikawa, T.; Kajimoto, Y.; Inoue, Y.; Ikegami, Y.; Kuroiwa, T. Critical role of ABCG2 in ALA-photodynamic diagnosis and therapy of human brain tumor. Adv. Cancer Res. 2015, 125, 197–216. [Google Scholar]
- Usuda, J.; Tsunoda, Y.; Ichinose, S.; Ishizumi, T.; Ohtani, K.; Maehara, S.; Ono, S.; Tsutsui, H.; Ohira, T.; Okunaka, T.; et al. Breast cancer resistant protein (BCRP) is a molecular determinant of the outcome of photodynamic therapy (PDT) for centrally located early lung cancer. Lung Cancer 2010, 67, 198–204. [Google Scholar] [CrossRef]
- Gu, Y.; Tang, S.; Wang, Z.; Cai, L.; Lian, H.; Shen, Y.; Zhou, Y. A pan-cancer analysis of the prognostic and immunological role of β-actin (ACTB) in human cancers. Bioengineered 2021, 12, 6166–6185. [Google Scholar] [CrossRef]
- Sharma, B.; Agriantonis, G.; Shafaee, Z.; Twelker, K.; Bhatia, N.D.; Kuschner, Z.; Arnold, M.; Agcon, A.; Dave, J.; Mestre, J.; et al. Role of podoplanin (PDPN) in advancing the progression and metastasis of glioblastoma multiforme (GBM). Cancers 2024, 16, 4051. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Li, J.; Liang, J.; Ren, X.; Yun, D.; Liu, J.; Fan, J.; Zhang, Y.; Zhang, J.; et al. PDPN contributes to constructing immunosuppressive microenvironment in IDH wildtype glioma. Cancer Gene Ther. 2023, 30, 345–357. [Google Scholar] [CrossRef]
- Tu, Z.; Wang, C.; Hu, Q.; Tao, C.; Fang, Z.; Lin, L.; Lei, K.; Luo, M.; Sheng, Y.; Long, X.; et al. Protein disulfide-isomerase A4 confers glioblastoma angiogenesis promotion capacity and resistance to anti-angiogenic therapy. J. Exp. Clin. Cancer Res. 2023, 42, 77. [Google Scholar] [CrossRef]
- Yu, M.O.; Song, N.H.; Park, K.J.; Park, D.H.; Kim, S.H.; Chae, Y.S.; Chung, Y.G.; Chi, S.G.; Kang, S.H. Romo1 is associated with ROS production and cellular growth in human gliomas. J. Neurooncol. 2015, 121, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Zheng, W.; Liu, X.; Zhou, Y.; Jin, X.; Xiao, Y.; Bai, Y.; Pan, Y.; Zhang, J.; Shao, C. SDC1-TGM2-FLOT1-BHMT complex determines radiosensitivity of glioblastoma by influencing the fusion of autophagosomes with lysosomes. Theranostics 2023, 13, 3725–3743. [Google Scholar] [CrossRef]
- Reiners, J.J., Jr.; Caruso, J.A.; Mathieu, P.; Chelladurai, B.; Yin, X.M.; Kessel, D. Release of cytochrome c and activation of pro-caspase-9 following lysosomal photodamage involves Bid cleavage. Cell Death Differ. 2002, 9, 934–944. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.M.; Zhu, T.C.; Ong, Y.H.; Finlay, J.C.; Dimofte, A.; Singhal, S.; Glatstein, E.; Cengel, K.A. Infrared navigation system for light dosimetry during pleural photodynamic therapy. Phys. Med. Biol. 2020, 65, 075006. [Google Scholar] [CrossRef] [PubMed]
- Efendiev, K.; Alekseeva, P.; Linkov, K.; Shiryaev, A.; Pisareva, T.; Gilyadova, A.; Reshetov, I.; Voitova, A.; Loschenov, V. Tumor fluorescence and oxygenation monitoring during photodynamic therapy with chlorin e6 photosensitizer. Photodiagn. Photodyn. Ther. 2024, 45, 103969. [Google Scholar] [CrossRef]
- Finlayson, L.; McMillan, L.; Suveges, S.; Steele, D.; Eftimie, R.; Trucu, D.; Brown, C.T.A.; Eadie, E.; Hossain-Ibrahim, K.; Wood, K. Simulating photodynamic therapy for the treatment of glioblastoma using Monte Carlo radiative transport. J. Biomed. Opt. 2024, 29, 025001. [Google Scholar] [CrossRef]
- Usui, K.; Hayashizaki, Y.; Hashiyada, M.; Funayama, M. Rapid drug extraction from human whole blood using a modified QuEChERS extraction method. Leg. Med. 2012, 14, 286–296. [Google Scholar] [CrossRef]

| Newly Diagnosed | Recurrence | p-Value | |
|---|---|---|---|
| No. of cases | 74 | 97 | |
| Age, in years, mean (SD) | 57.5 ± 14.5 | 47.6 ± 14.0 | <0.001 |
| Male/Female sex | 42 (56.8%)/32 (43.2%) | 70 (72.1%)/27 (27.8%) | 0.0360 |
| KPS median (min–max) | 70 (40–100) | 70 (20–90) | 0.6428 |
| Alb median (min–max) | 3.2 (1.9–4.3) | 3.5 (2.3–4.1) | <0.001 |
| T-Cho median (min–max) | 202 (141–279) | 201 (105–395) | 0.8703 |
| Benzodiazepine | 7 (10.0%)/66 (90.0%) | 10 (10.3%)/87 (89.7%) | 0.8767 |
| Smoking | 19 (26.4%)/53 (73.6%) | 40 (41.2%)/56 (58.3%) | 0.0385 |
| Pathology | |||
| Glioblastoma, IDH-wildtype | 70 (94.6%) | 61 (62.9%) | |
| Glioblastoma, IDH-mutant | 3 (4.1%) | 23 (23.7%) | <0.001 |
| others | 1 (1.3%) | 13 (13.4%) | |
| Localization | |||
| Temporal | 31 (41.9%)/43 (58.1%) | 27 (27.8%)/70 (72.2%) | 0.0548 |
| Frontal | 29 (39.2%)/45 (60.8%) | 40 (41.2%)/57 (58.8%) | 0.7867 |
| Parietal | 13 (17.6%)/61 (82.4%) | 18 (18.6%)/79 (81.4%) | 0.8678 |
| Occipital | 5 (6.8%)/69 (93.2%) | 4 (4.1%)/93 (95.9%) | 0.4475 |
| Right/Left (Bilateral) | 43/41 (10) | 52/50 (5) | 0.5571 |
| Gene | |||
| IDH (−/+) | 71 (95.9%)/3 (4.1%) | 72 (75.8%)/23 (24.2%) | 0.0001 |
| P53 (−/+) | 52 (70.3%)/22 (29.7%) | 58 (61.1%)/37 (38.9%) | 0.2107 |
| ATRX (intact/loss/none) | 62(87.3%)/9 (12.7%)/3 | 67(71.2%)/27 (28.7%)/3 | 0.0114 |
| MIB-1 (mean ± SD) | 24.0 ± 11.3 | 20.8 ± 13.2 | 0.1025 |
| Newly Diagnosed | Recurrence | |||||||
|---|---|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | |
| Age | 1.02 (0.99–1.04) | 0.26 | 1.02 (0.99–1.05) | 0.27 | 1.02 (0.99–1.04) | 0.26 | 1.02 (0.99–1.05) | 0.27 |
| Male vs. female sex | 1.35 (0.62–2.94) | 0.44 | 1.35 (0.62–2.94) | 0.44 | ||||
| KPS | 0.98 (0.95–1.00) | 0.097 | 0.96 (0.93–0.99) | 0.01 | 0.98 (0.95–1.00) | 0.097 | 0.96 (0.93–0.99) | 0.01 |
| Alb | 1.51 (0.51–4.48) | 0.45 | 3.68 (1.05–13.76) | 0.047 | 1.51 (0.51–4.48) | 0.45 | 3.68 (1.05–13.76) | 0.047 |
| Total cholesterol | 1.00 (0.98–1.01) | 0.59 | 1.00 (0.98–1.01) | 0.59 | ||||
| Benzodiazepine | 2.36 (0.81–6.88) | 0.15 | 3.06 (0.96–9.76) | 0.059 | 2.36 (0.81–6.88) | 0.15 | 3.06 (0.96–9.76) | 0.059 |
| Smoking | 1.57 (0.70–3.53) | 0.29 | 1.58 (0.67–3.73) | 0.3 | 1.57 (0.70–3.53) | 0.29 | 1.58 (0.67–3.73) | 0.3 |
| Localization | ||||||||
| Temporal vs. not temporal | 0.50 (0.22–1.11) | 0.079 | 0.50 (0.22–1.11) | 0.079 | ||||
| Frontal vs. not frontal | 1.14 (0.51–2.56) | 0.75 | 1.14 (0.51–2.56) | 0.75 | ||||
| Parietal vs. not parietal | 1.32 (0.53–3.31) | 0.56 | 1.32 (0.53–3.31) | 0.56 | ||||
| Newly Diagnosed | Newly Diagnosed | |||||
|---|---|---|---|---|---|---|
| Mean TS Concentration ≤0.16333 | Mean TS Concentration >0.16333 | Mean TS Concentration ≤0.0883 | Mean TS Concentration >0.0883 | |||
| n = 21 51.2 (%) | n = 20 48.8 (%) | p-Value | n = 21 51.2 (%) | n = 20 48.8 (%) | p-Value | |
| Male vs. female | 13 (31.7%)/8 (19.5%) | 13 (31.7%)/7 (17.1%) | 0.837 | 13 (31.7%)/8 (19.5%) | 14 (34.2%)/6 (14.6%) | 0.5843 |
| Age, mean ± SD | 60.7 ± 12.2 | 61.3 ± 11.9 | 0.884 | 47.6 ± 14.2 | 53.6 ± 10.5 | 0.5843 |
| Left vs. right (excluded bilateral case) | 11 (31.2%)/7 (20.0%) | 6 (17.1%)/11 (31.4%) | 0.1245 | 10 (26.3%)/9 (23.7%) | 6 (15.8%)/13 (34.2%) | 0.1869 |
| Pathological diagnosis | ||||||
| Mean MIB-1 ± SD | 28.9 ± 11.9 | 22.1 ± 11.5 | 0.0607 | 21.3 ± 15.4 | 22.0 ± 15.5 | 0.8886 |
| ATRX intact vs. loss | 20 (51.2%)/0 (0%) | 18 (46.2%)/1 (2.6%) | 0.226 | 12 (30.8%)/7 (18.0%) | 16 (39.0%)/4 (9.7%) | 0.2407 |
| P53 (+) vs. (−) | 4 (9.76%)/17 (41.4%) | 6 (14.6%)/14 (34.2%) | 0.4134 | 6 (15.0%)/14 (35.0%) | 7 (17.0%)/13 (31.7%) | 0.7356 |
| IDH (+) vs. (−) | 0 (0%)/21 (51.2%) | 1 (2.4%)/19 (46.3%) | 0.226 | 5 (12.5%)/15 (37.5%) | 4 (9.7%)/16 (39.0%) | 0.7047 |
| Part | ||||||
| Temporal vs. not temporal | 7 (17.1%)/14 (34.1%) | 9 (22.0%)/11 (26.8%) | 0.4435 | 8 (19.5%)/13 (31.7%) | 5 (12.2%)/15 (36.6%) | 0.366 |
| Frontal vs. not frontal | 11 (26.8%)/10 (24.4%) | 8 (19.5%)/12 (29.3%) | 0.4261 | 7 (17.1%)/14 (34.1%) | 8 (19.5%)/12 (29.3%) | 0.6577 |
| Parietal vs. not parietal | 2 (4.9%)/19 (46.3%) | 2 (4.9%)/18 (43.9%) | 0.959 | 5 (12.2%)/16 (39.0%) | 5 (12.2%)/15 (36.6%) | 0.9293 |
| Gene | Primer Sequence (5′-3′) | |
|---|---|---|
| ACTB | Forward | TGGCACCCAGCACAATGAA |
| Reverse | CTAAGTCATAGTCCGCCTAGAAGCA | |
| CALR | Forward | CGAGCCTGCCGTCTACTTCA |
| Reverse | CCGTAGAACTTGCCGGAACTG | |
| VEGFA | Forward | TCACAGGTACAGGGATGAGGACAC |
| Reverse | CAAAGCACAGCAATGTCCTGAAG | |
| PDPN | Forward | ATGCCAGGTGCCGAAGATG |
| Reverse | TGAAGTTGGCAGATCCTCGATG | |
| RTN4 | Forward | TGCTGCATCTGAGCCTGTGA |
| Reverse | CTCTTGACCAGCCGAAATAGTGTTA | |
| DNER | Forward | GGCAACGGTGACACTGCCTA |
| Reverse | TGGCATTCCCACAGGCAATA | |
| GAPDH | Forward | GCACCGTCAAGGCTGAGAAC |
| Reverse | TGGTGAAGACGCCAGTGGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onodera, M.; Kitahara, S.; Sato, Y.; Kawamata, T.; Muragaki, Y.; Masamune, K. Prognostic Factors and Talaporfin Sodium Concentration in Photodynamic Therapy for Recurrent Grade 4 Glioma. Pharmaceuticals 2025, 18, 583. https://doi.org/10.3390/ph18040583
Onodera M, Kitahara S, Sato Y, Kawamata T, Muragaki Y, Masamune K. Prognostic Factors and Talaporfin Sodium Concentration in Photodynamic Therapy for Recurrent Grade 4 Glioma. Pharmaceuticals. 2025; 18(4):583. https://doi.org/10.3390/ph18040583
Chicago/Turabian StyleOnodera, Mikoto, Shuji Kitahara, Yasuto Sato, Takakazu Kawamata, Yoshihiro Muragaki, and Ken Masamune. 2025. "Prognostic Factors and Talaporfin Sodium Concentration in Photodynamic Therapy for Recurrent Grade 4 Glioma" Pharmaceuticals 18, no. 4: 583. https://doi.org/10.3390/ph18040583
APA StyleOnodera, M., Kitahara, S., Sato, Y., Kawamata, T., Muragaki, Y., & Masamune, K. (2025). Prognostic Factors and Talaporfin Sodium Concentration in Photodynamic Therapy for Recurrent Grade 4 Glioma. Pharmaceuticals, 18(4), 583. https://doi.org/10.3390/ph18040583